Introduction
Globally, the burden of cancer incidence and death
is increasing rapidly (1). Cancer
is a complex disease and there is no single treatment that is
effective for all types (2,3). With an estimated 1,414,259 new cases
worldwide each year, prostate cancer (PC) is the second most
frequent malignancy and the top cause of death for men worldwide
(4). The average age at diagnosis
is 66 years old, and PC incidence and mortality rise with age
globally (3). There are 158.3 new
cases diagnosed per 100,000 males, with 293,818 additional cases
projected by 2040 (5). Moreover,
African-American men have been reported to experience higher
incidence rates and roughly double the mortality rate from PC than
Caucasian men. This discrepancy has been explained by social,
environmental and genetic variations (2).
PC that is detected early may not show any symptoms,
often advances slowly and may be treated with little-to-no
intervention. The most frequent symptoms, however, are nocturia,
increased frequency of urine and difficulty urinating, which are
indicative of prostatic enlargement. In more advanced stages of the
disease, patients may experience back discomfort and urine
retention due to metastasis to the axis skeleton, the most common
site of bone metastatic disease (6).
The risk of PC is notably influenced by diet and
exercise. For example, dietary factors are associated with the
observed variations in PC incidence rates between countries and
ethnic groups (7,8). However, the majority of studies
concentrate on the genes implicated in the inherited form of PC as
well as the mutations that take place in the acquired form
(6,7). Therefore, a thorough analysis of PC
epidemiology and risk factors may aid in understanding the
relationship between genetic abnormalities and the function of the
environment in causing these alterations and/or encouraging tumor
progression. These differences in PC incidence imply that the
etiology of PC is markedly influenced by environmental variables.
However, underdiagnosis, disparities in screening techniques and
access to healthcare may also contribute to variations in incidence
(6).
Due to several biological and tumor
microenvironmental factors, PC presents substantial treatment
challenges. For example, prostate tumors often have a low tumor
mutational burden, meaning that the immune system targets fewer
neoantigens (9). Furthermore,
immunosuppressive cells, such regulatory T cells, tumor-associated
macrophages and myeloid-derived suppressor cells, are present in
the PC immunosuppressive tumor microenvironment. The normal immune
response against cancer cells is hampered by these cells, which
produce a ‘cold’ tumor environment with limited immune cell
penetration (10). Furthermore,
although androgen deprivation therapy is initially successful,
certain patients experience resistance to the treatment, which can
result in metastatic castration-resistant PC. Changes in the tumor
microenvironment and immune evasion strategies are associated with
this development (11).
Using immune checkpoint inhibitors, cancer vaccines,
chimeric antigen receptor T-cell treatment and combination
medicines, immunotherapy offers potential treatments by using the
immune system to target and kill cancer cells. By reducing
antitumor immune responses or modifying tumor immunogenicity,
several immune system components cooperate to either protect the
host against necessary tumor progression, enhance tumor escape, or
both (12). This process is known
as cancer immuno-altering (12,13).
Immune spots, which are resistant cell surface receptors that
regulate the suppression or activation of immune reactions, serve
an important role in different phases of the immune responses. For
example, both PD-1 (Programmed Death-1) and cytotoxic
T-Lymphocyte-Associated Protein 4 CTLA4, prevent overactivation of
the immune system once binding to their ligands (14). The optimum outcome for controlling
tumors is immune system activation (13). In addition to surgery, chemotherapy,
radiation and targeted medicines, cancer immunotherapy has become a
crucial therapeutic approach. Furthermore, cancer immunotherapy has
demonstrated encouraging outcomes with several cancer types and can
be used in conjunction with other treatments. Consequently,
immunotherapy has been reported to have notable benefits: In one
study (15) immunotherapy improved
lung cancer treatment PD-1 inhibitor to standard chemotherapy,
increased objective response rate and progression free survival. In
addition, another study showed that combining immunotherapy with
radiation enhance anti-tumor immune response through increasing the
release of tumor antigen following radiation (16).
A subset of lymphocytes, known as natural killer
(NK) cells, is essential to the innate immune response against
malignancies and infections (17,18).
NK cells are able to recognize and target cancerous and
contaminated cells (19,20), and ~90% of the circulating NK cell
population is made up of CD56 and CD16 cells, which cause cell
lysis by releasing cytolytic granules that include granzyme and
perforin. Together, NK cells and interferon-γ (IFN-γ) cause target
cells to undergo apoptosis (21,22).
For aberrant cells, NK cells increase antibody-mediated
cytotoxicity and cytotoxic T lymphocytes act in a similar manner
(23).
NK cells help to defend the body against foreign
(pathogens) and endogenous (cancer) threats. Extensive research has
been performed with the goal of identifying an efficient method for
targeting impacted cells by utilizing NK cell pathways (24) (Fig.
1). However, notwithstanding the promising results of this
research, tumor cells develop a defense mechanism that thwarts the
cytotoxicity caused by NK cells (25,26).
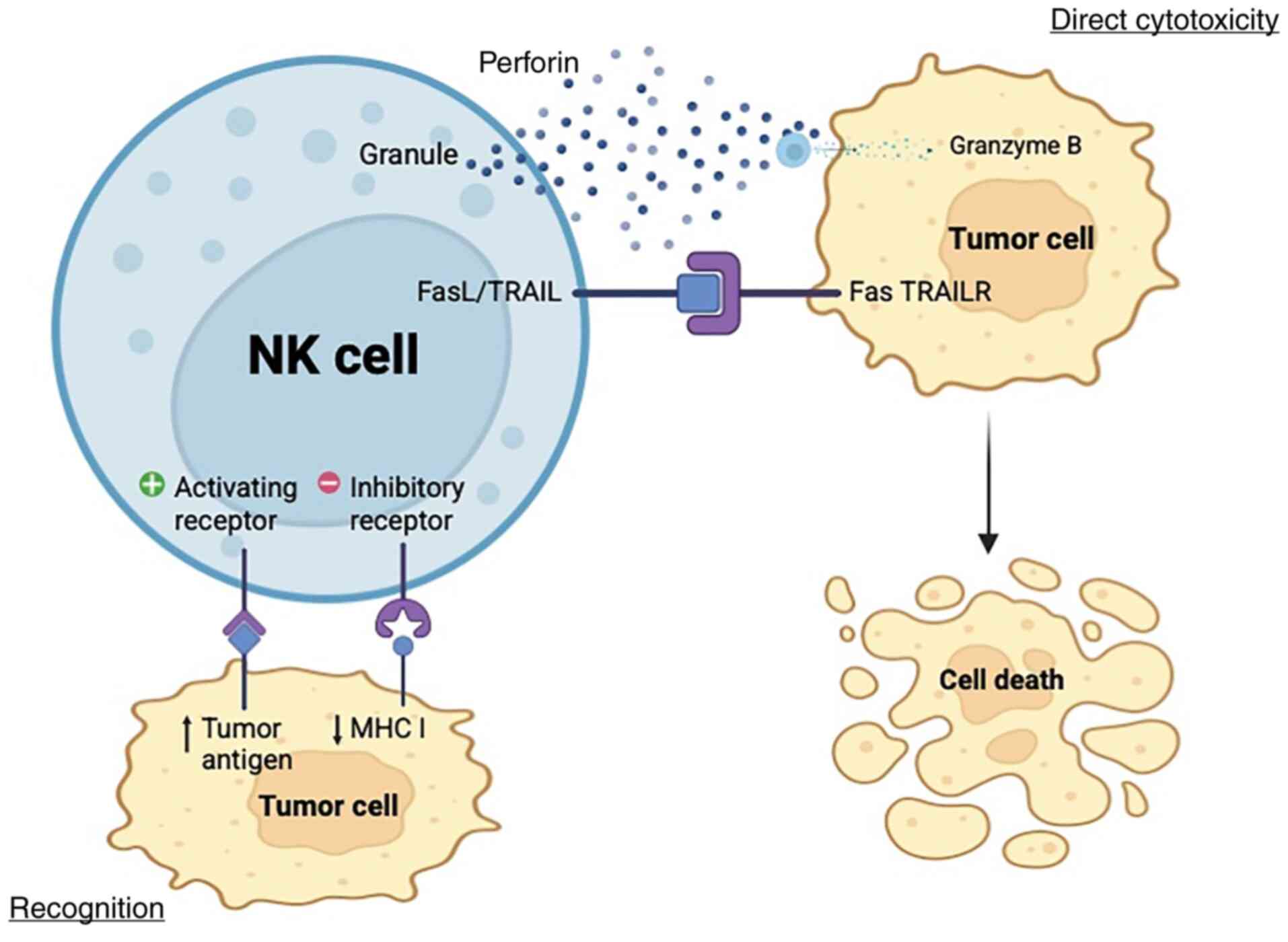 | Figure 1.Cytotoxicity mechanism of NK cells.
By evaluating the net input of activating and inhibitory signals
from target cells through NK cell surface receptors, NK cells
discriminate healthy cells from target cells. However, several
tumors develop mechanisms to escape NK cell immune responses by
modifying their cell surface molecules, such as low immunogenicity.
Following the identification of the target cell by NK cells, the
stimulation domain is activated. This is initiated by the secretion
of a cytolytic enzyme, known as perforin, stored in NK cell
vesicles. Perforin creates pores in the cell membrane of the target
cell, allowing the passage of granzymes into the target cell and
starting protein digestion (cytolysis). NK cells express TRAIL and
FasL, which interact with TRAIL receptors and FAS ligands in cancer
cells, respectively. This interaction results in cell death,
signaling complex stimulating apoptosis. NK, natural killer; TRAIL,
TNF-related apoptosis inducing ligand; TRAILR, TRAIL receptor; MHC,
major histocompatibility complex; FasL, FAS ligand. Created in
BioRender.com. |
Research is investigating the use of NK cells to
treat (27–29) PC. NK cells can directly target and
eliminate PC cells, resulting in direct tumor cytotoxicity.
Moreover, research has reported that, both in vitro and
in vivo, genetically modified NK cells, such as
prostate-specific membrane antigen (PSMA)-targeted chimeric antigen
receptor-NK cells, have strong cytotoxic effects on PC cells. They
can specifically identify and eliminate PSMA-expressing PC cells,
providing a targeted therapy strategy. Therefore, these altered NK
cells may be employed as an ‘off-the-shelf’ therapeutic option,
enabling a sustainable and economical treatment technique (27).
Additionally, PC cells usually develop defenses
against immune detection, such as by producing soluble NK cell
receptor D (NKG2D) ligands to obstruct the recognition of NK cells.
However, NK cells can adapt to these challenges, and strategies are
being developed to make them more effective against these tumor
escape tactics (28). Investigating
NK cell function against PC is challenging as they produce TGF-β,
IL-10 and regulatory T cells, inhibiting NK activation, and have
low infiltration of NK cells making it hard to study their direct
interaction with PC. PC is a useful model for researching NK
cell-mediated therapy, for example due to the immune
microenvironment dynamics, in which PC tumors are distinguished by
a distinct immune milieu that consists of both immuno-suppressive
and immune-activating components (29–32).
As studies have reported that NK cells in patients with PC
frequently have compromised cytotoxic function, this environment
offers a realistic setting for researching how NK cells interact
with tumor cells and the factors determining their efficacy
(29,33). This malfunction provides a clear
target for therapeutic intervention trials targeted at restoring NK
cell activity and is associated with the course of the disease.
Furthermore, research employing preclinical models has reported
that NK cells are capable of efficiently inhibiting the
proliferation of PC cells resistant to castration. These results
highlight how effective NK cell-based treatments can be in treating
advanced PC (30). Nevertheless, PC
uses several strategies to avoid immune monitoring, especially from
NK cells. These tactics include modulating the expression of NK
cell receptors, secreting immunosuppressive substances and changing
the expression of ligands (28). PC
cells can decrease the expression of ligands such NKG2D and DNAX
accessory molecule 1 that are recognized by NK cell receptors.
Moreover, immunosuppressive cytokines such as TGF-β are abundant in
the tumor microenvironment. The release of TGF-β causes the
activating receptors of NK cells, including NK cell P30-related
protein and NKG2D, to be downregulated, which reduces the cytotoxic
effects (31). Furthermore, PC can
cause NK cells to exhibit a fatigued phenotype, which is typified
by decreased activating receptor expression and decreased cytotoxic
activity. This fatigue is associated with NK cells expressing more
inhibitory receptors, such as T cell immunoglobulin mucin 3 and
programmed cell death protein 1 (PD-1), which results in weakened
antitumor responses (32).
Additionally, programmed death-ligand 1 (PD-L1) expression on
cancer cells may be upregulated in PC with hypoxic circumstances.
Immune evasion is facilitated by the inhibition of the cytotoxic
function of NK cells caused by the interaction between PD-L1 and
its receptor PD-1 (34).
Finally, due to their ability to treat numerous
diseases, several plants are regarded as useful therapeutic
instruments in a wide range of medical disorders. Moreover,
different medicinal plants have been used worldwide to alleviate
the symptoms of several illnesses for millennia (35). One well-known example with a wide
range of applications is Nigella sativa, also known as dark
cumin. For two millennia, populations around the world have
utilized this plant, a dicotyledon of the Ranunculaceae
family, as a snack, spice and nutritional supplement (36). Thymoquinone (TQ), the main active
ingredient in the black seeds, has garnered interest in both
traditional medicine and contemporary therapeutic research
(37,38). Therefore, the present study aimed to
assess how TQ, which comprises ~40% of Nigella sativa
(38), affects the cytotoxic
activity of NK cells against the PC3-RFP cell line. The results
could aid in comprehending the potential therapeutic impact of TQ
on NK cells for cell-based immunotherapy.
Materials and methods
Blood collection
Blood samples were collected at King Abdulaziz
University Hospital (Jeddah, Saudi Arabia) between August and
November 2021 from 10 healthy volunteers six male, four female and
aged 25–45 years in EDTA tubes (BD Vacutainer) and used for NK cell
isolation. All experiments were performed at King Faisal Specialist
Hospital and Research Centre (Jeddah, Saudi Arabia). Inclusion
criteria for enrolled participants include general good health with
no chronic diseases, recent infections and immunosuppressive
medication or vaccination. Exclusion criteria were smoking,
pregnancy or breastfeeding, autoimmune disease and malignancy.
MACS positive isolation
NK cells were purified by positive selection of CD56
cells (CD56 MicroBeads, human, 130-050-401, Miltenyi biotec) and
MACS column (130-042-201, Miltenyi biotec, USA) was used according
to the manufacturer's instruction.
Cell lines and culture
Transfected PC3 cells with red fluorescence (PC3-RFP
cells) with the pDSRed-monomer-Hyg-C1 plasmid to produce
hygromycin-resistant cells that express RFP were provided by
AlShaibi et al (39). The
cells were stored at King Fahd Medical Research Center (Jeddah,
Saudi Arabia) and used in the present study.
PC3-RFP cells were cultured in high-glucose DMEM
(cat. no. 11965118; Thermo Fisher Scientific, Inc.), with sodium
pyruvate L-glutamine and Phenol Red, and supplemented with 10%
penicillin-streptomycin (10,000 U/ml; cat. no. 15140122; Thermo
Fisher Scientific, Inc.). NK cells were cultured in NK MACS Medium
(cat. no. 130-092-657; Miltneyi Biotec B.V. & Co. KG),
supplemented with 20% FBS (cat. no. 12103C; Sigma-Aldrich; Merck
KGaA) and 0.1 mM β-mercaptoethanol (Sigma-Aldrich; Merck KGaA),
IL-2 (human animal-component free, recombinant, expressed in E.
coli, ≥98%; cat. no. SRP3085; Sigma-Aldrich; Merck KGaA). The cells
were routinely maintained in humidified incubator at 37°C and 5%
CO2.
TQ preparation
TQ (cat. no. 274666-1G; Sigma-Aldrich; Merck KGaA)
was prepared by dissolving 4.926 mg TQ crystal in 10 µl anhydrous
dimethyl sulfoxide (≥99.9%; cat. no. 276855; Sigma-Aldrich; Merck
KGaA) and 9,990 µl NK medium to yield a stock solution. The final
DMSO concentration in all TQ working solutions (50 and 25 µM) was
0.1%. Vehicle controls containing 0.1% DMSO in NK medium without TQ
were included in all experiments.
Cell cytotoxicity of TQ on tumor
(PC3-RFP) and NK cells
Cell cytotoxicity was analyzed using the
CytoTox® 96 Non-Radioactive Cytotoxicity Assay kit
(Promega Corporation). Briefly, PC3-RFP was plated at a density of
15×103 cells per well in a 96-well flat-bottom plate and
incubated overnight at room temperature. Subsequently, NK cells
were co-cultured at an effector cell/target cell ratio of 1:2 in
the presence of different concentrations (25 and 50 µM) of TQ for 5
h. CytoTox 96 lysis buffer was added and incubated for 45 min at
room temperature. CytoTox 96 reagent was then added to each well
and incubated for 30 min at room temperature in the dark, and then
the stop solution was added. The absorbance was measured at 680 and
490 nm.
Flow cytometry
Following positive selection of NK cells were
stained with CD56-PE (DAKO; Agilent Technologies, Inc.) at 25°C for
30 min in the dark. The cells were then analyzed using flow
cytometry (Novocyte Flow Cytometer; Agilent Technologies, Inc.).
Phycoerythrin (PE) detection channel was the analyte detector,
CD56-PE was the analyte reporter, and NovoExpress software (version
1.6.3; Agilent Technologies, Inc.)used for data acquisition and
analysis.
ELISA
PC-3-RFP and NK cells (1:2; 15–30×103)
were cocultured in the presence of TQ for 5 h at 37°C. Cell-free
supernatants were harvested and ELISA was used for detection of
IFN-α (Human IFN-α ELISA Kit; cat. no. BMS216 Invitrogen™; Thermo
Fisher Scientific, Inc.), granzyme B [Human granzymes B (Gzms-B)
ELISA kit; cat. no. E0899Hu; Shanghai Korain Biotech Co., Ltd.] and
perforin [Human Perforin/Pore-forming protein (PF/PFP) ELISA Kit;
cat. no. E0070Hu; Shanghai Korain Biotech Co., Ltd.] were used.
Absorbance was measured at 450 nm using a microplate reader.
Statistical analysis
The data were analyzed using GraphPad Prism 9 (IBM
Corp.) and the results are presented as the mean ± standard
deviation of three independent experiments. Statistical
significance was assessed using one-way analysis of variance and
Tukey's multiple comparison test. Spearman correlation coefficient
was used for correlation tests. P<0.05 was considered to
indicate a statistically significant difference.
Results
Flow cytometry analysis
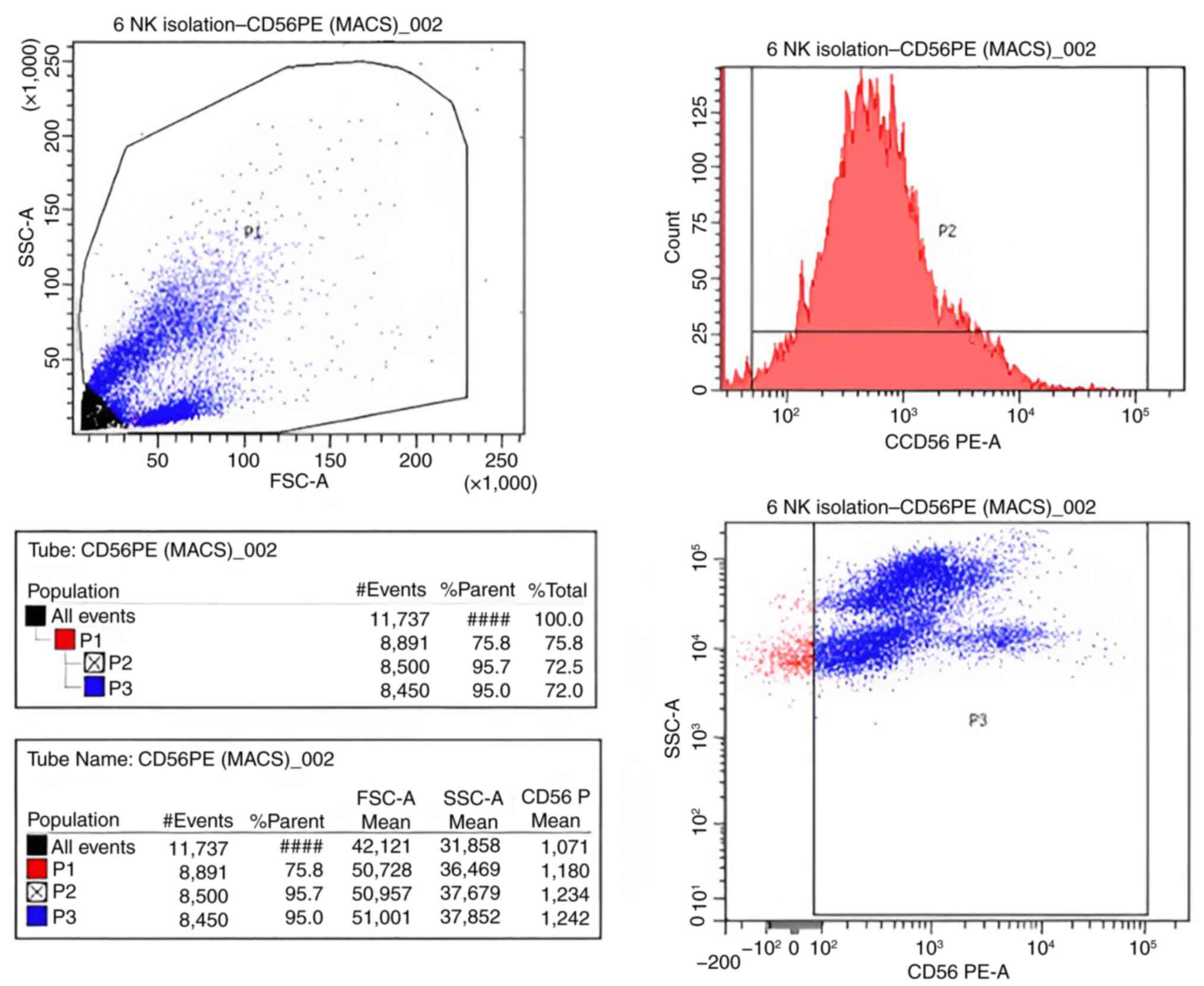
Using flow cytometry, the positivity of CD56 was
determined to assess the purity of the isolated NK cells by
comparison with the defined cutoff values obtained with unstained
control cells. The NK cell purity was 95.7% (Fig. 2).
Cytotoxic effect of TQ on NK cells
with PC3-RFP
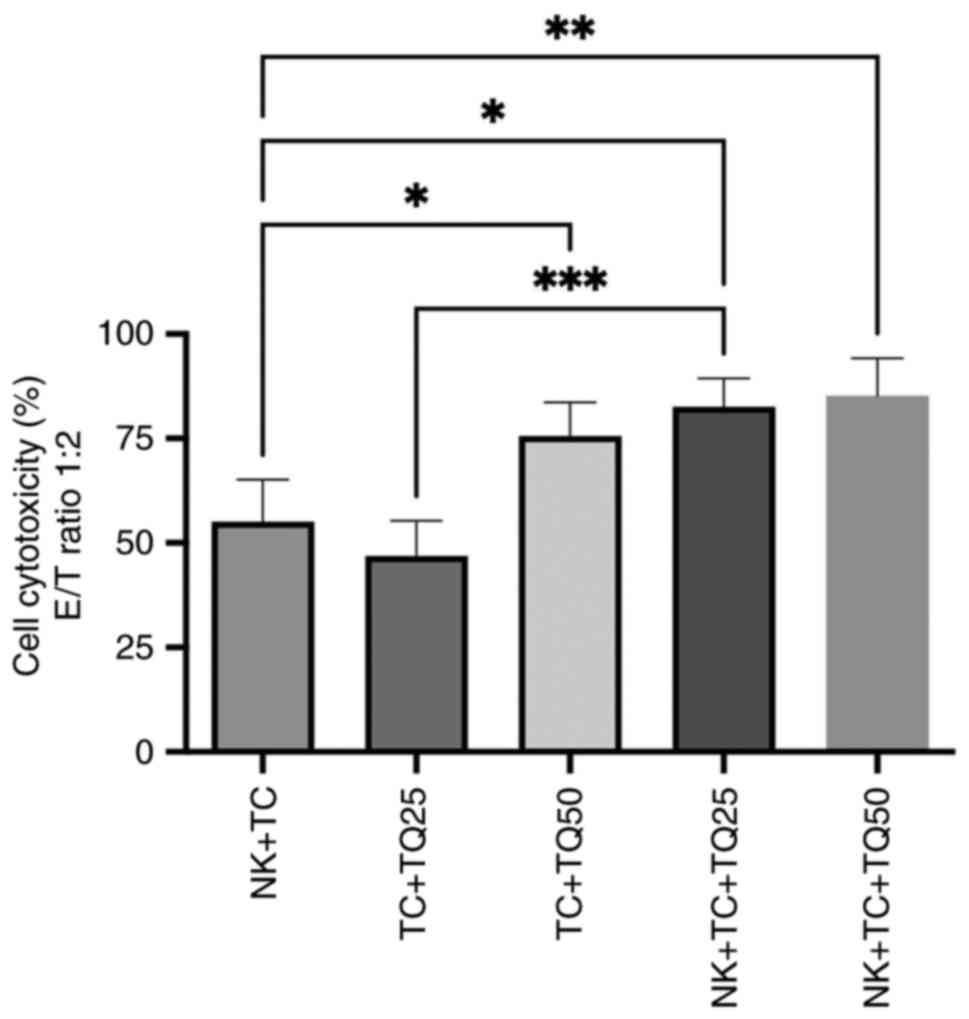
The cytotoxicity of NK cells was evaluated in
co-culture with PC3-RFP cells. The effector (NK cells) and target
(PC3-RFP) ratio was 1:2 and different concentrations of TQ (25 and
50 µM) were added in the culture. In the presence of TQ, there was
a significant increase in NK cell cytotoxicity: Both concentrations
of TQ (25 and 50 µM) significantly upregulated NK cell cytotoxicity
in PC3-RFP cells, with cytotoxicity at 85.35% compared with 55.1%
in the control group of NK cells co-cultured with tumor cells. Cell
cytotoxicity was also significantly increased in tumor cells
treated with both 25 and 50 µM TQ in the presence of NK cells,
compared with tumor cells treated with the same TQ concentrations
in the absence of NK cells (Fig.
3).
Effect of TQ on the activity of NK
cells
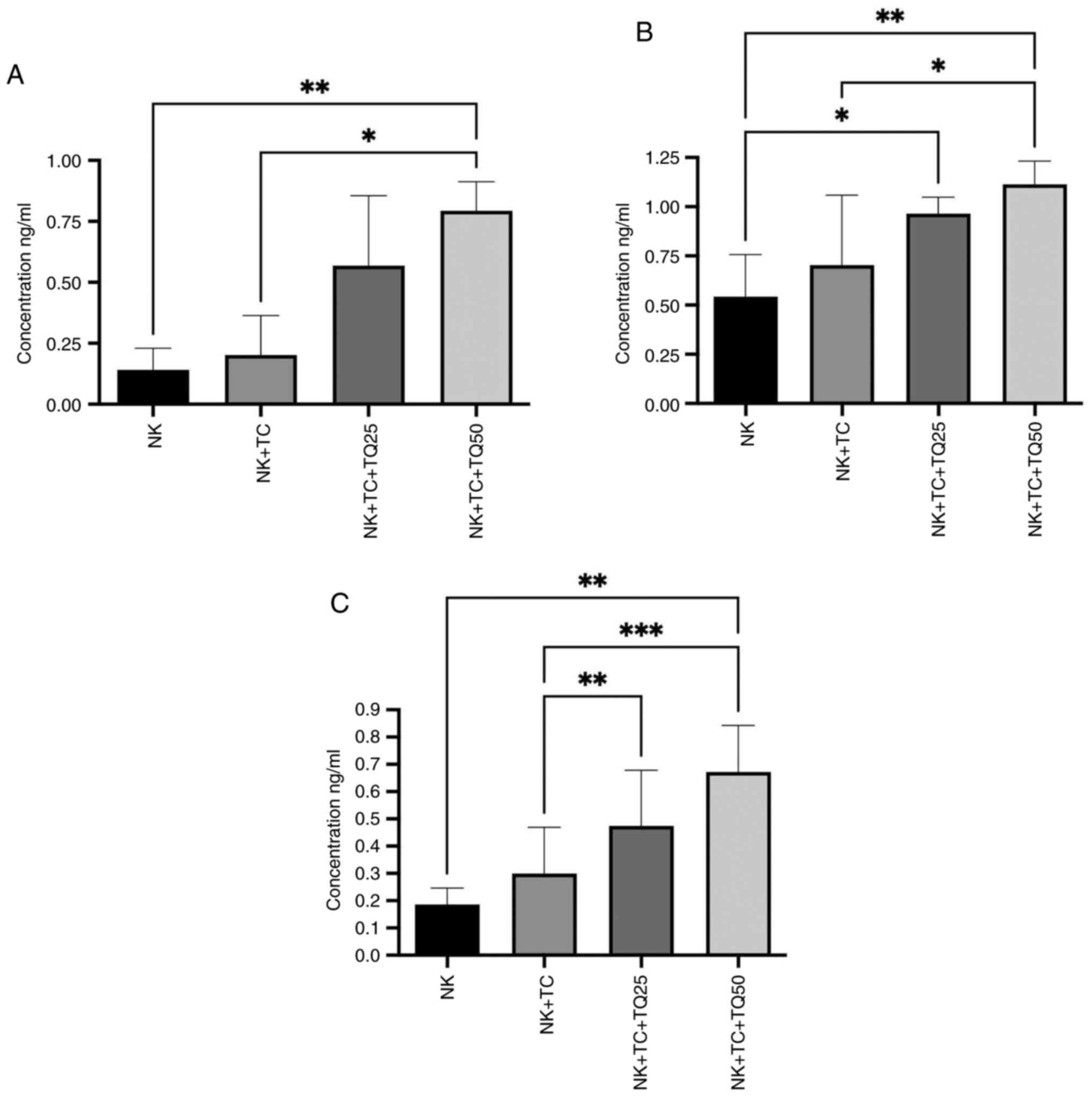
Co-culture of NK cells with PC3-RFP cells
significantly increased the production of perforin, granzyme B and
IFN-α from NK cells compared with NK cells alone using both
concentrations of TQ (25 and 50 µM). NK cells co-cultured with
PC3-RFP cells and treated with 50 µM TQ had significantly increased
production of perforin and granzyme B production compared with the
control NK cells co-cultured with tumor cells. The higher 50 µM TQ
dose increased the production of perforin and granzyme B (0.80 and
1.24 ng/ml, respectively) by the NK cells more than the lower 25 µM
dose (0.50 and 0.76 ng/ml, respectively). Furthermore, IFN-α
production by the NK cells was significantly increased in the
presence of 25 and 50 µM TQ (0.5 and 0.7 ng/ml, respectively)
compared with the control NK cells co-cultured with tumor cells
(0.3 ng/ml), with a greater increase observed in the presence of 50
µM TQ (Fig. 4).
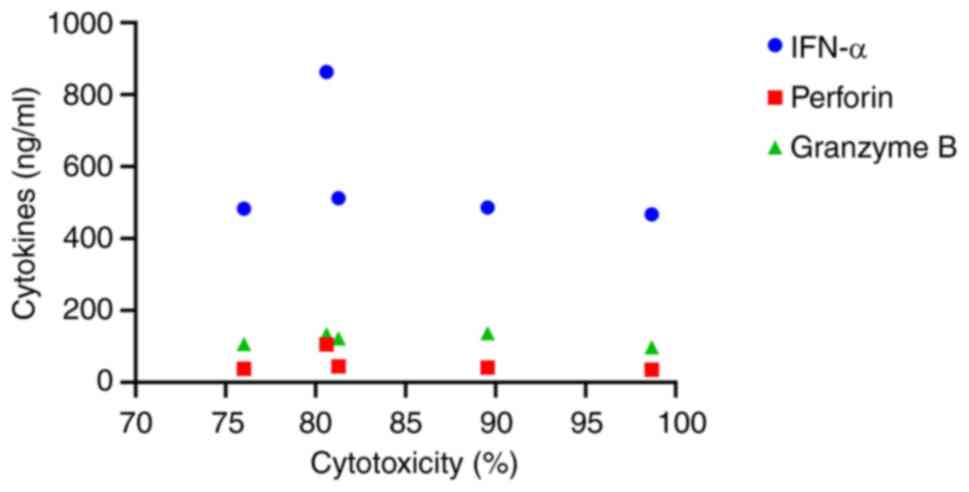
The correlation between individual cytokines and NK
cytotoxicity was analyzed using the Spearman correlation
coefficient. Although there was no significant correlation between
the cytokine concentrations and NK cytotoxicity; NK cells released
higher levels of IFN-α in response to PC3-RFP cells compared to the
other cytokines (Fig. 5).
Discussion
Several studies have reported the immunomodulatory
effect of TQ on immune cells including NK cells (40–43).
The current study focused on investigating the effect of TQ on the
cytotoxicity of NK cells and its anticancer activity against
PC3-RFP cells. The results demonstrated that treating cancer cells
with a high dose of TQ in the presence of NK cells enhanced NK cell
cytotoxicity more than NK cells co-cultured with cancer cells alone
or in cells with a lower dose of TQ Furthermore, NK cells cultured
with cancer cells treated with both TQ concentrations (25 and 50
µM) exhibited considerably increased NK cell cytotoxicity compared
with cancer cells treated with TQ alone. This indicates that the
antitumor effect of TQ is associated with its stimulation of NK
cell function.
Majdalawieh et al (44) assessed how Nigella sativa
extract affected the immune systems of mice and reported that the
extract activated macrophages, increased NK cell antitumor
activity, changed the Th1/Th2 cytokine balance toward a
Th1-dominant response, and markedly increased splenocyte
proliferation. Despite the fact that TQ, a crucial component of
N. sativa, was not isolated, the results indicate that the
extract enhances antitumor immunity by inducing both innate and
adaptive immunological responses, potentially through TQ (44). Furthermore, a study by Sjs et
al (45) produced a
nanomedicine based on TQ and reported that administration of the
nanomedicine to triple negative breast cancer cell line MDA-MB-231
was associated with apoptosis, DNA damage, slowing down of the cell
cycle and prevention of cell division (45). According to these findings, TQ may
be a potential strategy for both preventing and treating cancer
(46,47).
Nigella sativa may affect cancer
pathophysiology via several signaling pathways, such as inducible
nitric oxide synthase, p53, ROS, TNF and caspases. These mechanisms
have been demonstrated by numerous in vitro and in
vivo studies, which suggest that N. sativa concentrates
can be used to treat several malignant tumor types at different
phases of carcinogenesis (40–42).
Additionally, the medicinal plant is well-known for its potent
protective benefits against the development and spread of tumors,
as well as its anti-inflammatory and immune-stimulating properties,
all of which make it a useful supplement to cancer treatment plans.
Its impact on the NK cells may be responsible for these
effects.
It has been reported that TQ combined with ionizing
radiation, such as γ-radiation, has a synergistic lethal effect on
breast cancer cells in vitro (42,43,48–50).
Moreover, according to the present study, TQ administration
directly increased the capacity of NK cells to lyse human PC
(PC3-RFP) cells. TQ functions as an antimetabolic medication and
may inhibit the development of colorectal cancer carcinogenesis by
controlling the glycolytic metabolic pathway and the PI3/AKT axis
(51). Additionally, pretreatment
with TQ-pH-sensitive liposomes (PSL) decreased cancer marker
enzymes, restored the relative weight of the lung and enhanced the
activity of antioxidant enzymes in serum, according to a lung
cancer study that prepared TQ in a particular formula as TQ-PSL to
increase its solubility. Histopathological analysis revealed that
TQ-PSL protected lung tissues by decreasing oxidative stress,
suppressing inflammatory mediators and inducing apoptosis in
pre-cancerous cells (52). TQ
itself may be also useful as a treatment for lung adenocarcinoma as
it has been reported to inhibit tumor cell proliferation, induce
lung cancer cells to undergo apoptosis, markedly reduce TNF and
NF-kB activity, and arrest cells in the S phase of the cell cycle
(53). TQ has also been widely used
in biomaterial treatments (54).
Furthermore, TQ has demonstrated notable anti-hepatocellular
carcinoma potential, modulating the ERK and p38 signaling pathways,
the. Cytotoxic effect of TQ is enhanced by low ERK phosphorylation
or ERK inhibition as elevated p-ERK activity protect hepatocellular
carcinoma from TQ toxic effect (55,56).
TQ and TNF-related apoptosis inducing ligand may cooperate to
trigger apoptosis in hepatocellular carcinoma by mediating DNA
damage, according to Zhang et al (57) TQ also has an effect on other
cancers. For example, TQ markedly increased the amount of reactive
oxygen species (ROS) produced by human pancreatic cancer cells
whilst simultaneously suppressing the migration and proliferation
of cancer cells. Moreover, natural quinones such as TQ may be
effective antimetastatic treatments for pancreatic cancer based on
these findings (58). As TQ
successfully inhibits the fusion of autophagosomes and lysosomes,
cancer cells incur apoptosis. Furthermore, TQ causes apoptosis by
increasing ROS levels (59). TQ may
also function as an immunomodulatory drug that boosts anticancer
immune activity, as reported in other in vitro and in
vivo research (48,59). It has been also reported that TQ may
work in conjunction with chemotherapy and radiation to improve
treatment results and reduce treatment side effects (59,60).
Research by Shimasaki et al (61) indicates that NK cells are essential
for the regulation of tumor development and metastasis. NK cells
contribute to the induction of adaptive anticancer T cell and B
cell responses in addition to their function in the early defenses
against infection and cancer (61,62).
Additionally, NK cells can swiftly eliminate nearby cells that have
surface markers associated with neoplastic transformation (53). The direct immune-stimulating impact
of TQ on NK is well supported by the current data. In the present
study, TQ increased the NK cell production of IFN, which is
consistent with other research (4,47)
which have reported that the cytotoxic effect of NK cells on tumor
cells was increased in the presence of TQ, with increased secretion
of perforin, granzyme B, and IFN-α, and that TQ promoted the
cytotoxic activity of NK cells against breast cancer MCF-7 cells
(63). The results of the present
study also supported earlier research by demonstrating the
inhibitory action of TQ on human PC PC3-RFP cells. Notably, TQ
therapy directly increased the capacity of NK cells to kill PC3-RFP
cancer cells. In the NK + TQ + PC3-RFP group in the present study,
IFN activity increased, indicating higher concentration.
Subsequently, the rise in granzyme B was comparable with earlier
research (60,61,64)
which showed granzyme B trigger apoptosis by cleaving (BID) BH3
Interacting-Domain Death Agonist pro apoptotic protein of Bcl2
family, causing mitochondrial distribution and activating caspases.
This process is crucial for NK cells to induce death in cancer
cells (65). Although BID activity
was not measured in the present study, previous studies
demonstrated the importance of mitochondrial disruption by BID to
reach the lethal effect supporting the importance of granzyme B
mediated pathways in NK cells cytotoxicity on caspases and causing
cell death (64,65). Therefore, granzyme activity
measurements are important in studies on NK cytotoxicity.
Furthermore, according to the findings of the
present study, TQ affects NK cells, which then directly stimulates
the immune system. The TQ-induced immunoreactivity of NK cells
against PC3-RFP cells increased when 50 µM TQ was applied, compared
with 25 µM. This indicated that the stimulation of NK cells by TQ
was associated with its cytotoxic effect on PC3-RFP cells. However,
although certain signaling molecules implicated in producing the TQ
immunostimulatory action in NK cells have been identified, the
specific signaling pathways and molecular targets in these cells
are still unknown. It has been suggested that TQ increases NK
cell-mediated cytotoxicity by upregulating pro-apoptotic markers
such as BAX and BID and downregulating anti-apoptotic proteins such
as BCL-2 and myeloid cell leukemia 1 (66). TQ prevents NF-κB, a transcription
factor implicated in inflammatory reactions, from activating. TQ
also improves NK cell function and lowers pro-inflammatory cytokine
expression by inhibiting NF-κB activation, which helps to
strengthen antitumor immunity (67). Moreover, by upregulating PTEN, TQ
disrupts the PI3K/AKT signaling pathway and reduces AKT activity.
This downregulation enhances NK cell-mediated cytotoxicity and
promotes apoptosis in cancer cells by reducing cell survival and
proliferation signals. Furthermore, antioxidant enzymes are
upregulated when TQ stimulates the Nrf2 pathway. By shielding NK
cells from oxidative stress, this modulation preserves their
functioning and increases their antitumor activity (68). Therefore, more in vitro and
in vivo studies are required to identify the target
receptors and intracellular and extracellular components that have
unique activities in the signal transduction pathways associated
with TQ changes in NK cells.
Despite, the promising results of the capacity of TQ
to increase NK cell cytotoxicity against PC3-RFP PC cells in
vitro, a number of limitations of the present study should be
noted. The in vitro design of an experiment cannot fully
replicate the intricate interactions in the tumor microenvironment,
including tumor-induced immunosuppression, cytokine gradients and
immune cell trafficking. In addition, the study did not explore the
precise molecular mechanisms behind TQ-induced increased NK cell
activity, necessitating further investigation into signaling
pathways, receptor expression changes and gene regulatory networks.
Moreover, the study used PC3-RFP, a single PC cell line; however,
its potential application to other subtypes or primary tumor cells
is uncertain due to its heterogeneity. Finally, the observed
effects may be influenced by the NK cell source and activation
state, with variability in cytotoxic responses potentially
introduced by incomplete donor characterization, isolation
techniques and baseline NK cell activity. Future therapeutic
development depends on determining the ideal concentration range
that maximizes tumor cell killing without compromising NK cell
viability or function.
In conclusion, the present study demonstrated that
TQ, a bioactive substance obtained from Nigella sativa,
significantly increases the cytotoxic activity of NK cells against
PC3-RFP cells. As a potential supplement to immunotherapeutic
approaches in the treatment of PC, the findings imply that TQ may
enhance the innate immune response, specifically by increasing NK
cell-mediated tumor cell lysis. Furthermore, the potential of TQ as
an immunomodulatory agent with anticancer effects is supported by
the observed upregulation of NK cell cytotoxicity in its presence.
Future research should clarify the precise molecular mechanisms by
which TQ improves NK cell function, including its effects on
activation receptors, cytokine production and intracellular
signaling pathways, in order to build on the present findings.
Clinical trials and in vivo research are also necessary to
confirm the therapeutic potential of TQ in a physiological setting
and evaluate its safety and effectiveness when used in conjunction
with currently available immunotherapies. Furthermore,
investigating the impact of TQ on different subtypes of PC and its
association with the tumor microenvironment may elucidate its
suitability for personalized medicine.
Acknowledgements
The authors would like to thank Ms Samar A. Zailaie
(King Faisal Specialist Hospital and Research Centre, Jeddah) for
technical assistance and scientific advice.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
NIT and NuAA contributed to the study design. NoAA
performed the study. NAK and HFA performed the data analysis. NIT,
NuAA and HFA performed the data interpretation. HFA and NoAA
drafted the manuscript. NIT, NoAA, HFA and NuAA revised the
manuscript. All authors critically reviewed the manuscript. NIT and
NuAA confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Biomedical
Ethics Research Committee of King Abdulaziz University Faculty of
Medicine (approval no. No640-20). Written informed consent was
obtained from all subjects involved in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Omran AR: The epidemiological transition:
A theory of the epidemiology of population change. Millbank Mem
Fund Q. 49:509–538. 1971. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Ervik M, Lam F, Laversanne M,
Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I and Bray F;
Global Cancer Observatory, : World. International Agency for
Research on Cancer; Lyon, France: https://gco.iarc.who.int/media/globocan/factsheets/populations/900-world-fact-sheet.pdfDecember
26–2024
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI
|
|
4
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
5
|
Panigrahi GK, Praharaj PP, Kittaka H,
Mridha AR, Black OM, Singh R, Mercer R, van Bokhoven A, Torkko KC,
Agarwal C, et al: Exosome proteomic analyses identify inflammatory
phenotype and novel biomarkers in African American prostate cancer
patients. Cancer Med. 8:1110–1123. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rawla P: Epidemiology of prostate cancer.
World J Oncol. 10:63–89. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chan JM, Gann PH and Giovannucci EL: Role
of diet in prostate cancer development and progression. J Clin
Oncol. 23:8152–8160. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Platz EA, Leitzmann MF, Michaud DS,
Willett WC and Giovannucci E: Interrelation of energy intake, body
size, and physical activity with prostate cancer in a large
prospective cohort study. Cancer Res. 63:8542–8548. 2003.PubMed/NCBI
|
|
9
|
Hegde PS and Chen DS: Top 10 challenges in
cancer immunotherapy. Immunity. 52:17–35. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lopez-Bujanda Z and Drake CG:
Myeloid-derived cells in prostate cancer progression: Phenotype and
prospective therapies. J Leukoc Biol. 102:393–406. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beltran H, Rickman DS, Park K, Chae SS,
Sboner A, MacDonald TY, Wang Y, Sheikh KL, Terry S, Tagawa ST, et
al: Molecular characterization of neuroendocrine prostate cancer
and identification of new drug targets. Cancer Discov. 1:487–495.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fridman WH, Pagès F, Sautès-Fridman C and
Galon J: The immune contexture in human tumours: Impact on clinical
outcome. Nat Rev Cancer. 12:298–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Donnell JS, Teng MWL and Smyth MJ:
Cancer immunoediting and resistance to T cell-based immunotherapy.
Nat Rev Clin Oncol. 16:151–167. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Langer CJ, Gadgeel SM, Borghaei H,
Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins
RG, Stevenson JP, Jalal SI, et al: Carboplatin and pemetrexed with
or without pembrolizumab for advanced, non-squamous non-small-cell
lung cancer: A randomised, phase 2 cohort of the open-label
KEYNOTE-021 study. Lancet Oncol. 17:1497–1508. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patel SH, Rimner A and Cohen RB: Combining
immunotherapy and radiation therapy for small cell lung cancer and
thymic tumors. Transl Lung Cancer Res. 6:186–195. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Orange JS and Ballas ZK: Natural killer
cells in human health and disease. Clin Immunol. 118:1–10. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vivier E, Tomasello E, Baratin M, Walzer T
and Ugolini S: Functions of natural killer cells. Nat Immunol.
9:503–510. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doherty DG and O'Farrelly C: Innate and
adaptive lymphoid cells in the human liver. Immunol Rev. 174:5–20.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Joyce JA and Pollard JW:
Microenvironmental regulation of metastasis. Nat Rev Cancer.
9:239–252. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ferlazzo G, Pack M, Thomas D, Paludan C,
Schmid D, Strowig T, Bougras G, Muller WA, Moretta L and Münz C:
Distinct roles of IL-12 and IL-15 in human natural killer cell
activation by dendritic cells from secondary lymphoid organs. Proc
Natl Acad Sci USA. 101:16606–16611. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Romagnani C, Juelke K, Falco M, Morandi B,
D'Agostino A, Costa R, Ratto G, Forte G, Carrega P, Lui G, et al:
CD56brightCD16- killer Ig-like receptor-NK cells display longer
telomeres and acquire features of CD56dim NK cells upon activation.
J Immunol. 178:4947–4955. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cooper MA, Fehniger TA and Caligiuri MA:
The biology of human natural killer-cell subsets. Trends Immunol.
22:633–640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Domaica CI, Sierra JM, Zwirner NW and
Fuertes MB: Immunomodulation of NK cell activity. Methods Mol Biol.
2097:125–136. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Balsamo M, Scordamaglia F, Pietra G,
Manzini C, Cantoni C, Boitano M, Queirolo P, Vermi W, Facchetti F,
Moretta A, et al: Melanoma-associated fibroblasts modulate NK cell
phenotype and antitumor cytotoxicity. Proc Natl Acad Sci USA.
106:20847–20852. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee HH and Cho H: Improved anti-cancer
effect of curcumin on breast cancer cells by increasing the
activity of natural killer cells. J Microbiol Biotechnol.
28:874–882. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Montagner IM, Penna A, Fracasso G,
Carpanese D, Dalla Pietà A, Barbieri V, Zuccolotto G and Rosato A:
Anti-PSMA CAR-engineered NK-92 cells: An off-the-shelf cell therapy
for prostate cancer. Cells. 9:13822020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lundholm M, Schröder M, Nagaeva O, Baranov
V, Widmark A, Mincheva-Nilsson L and Wikström P: Prostate
tumor-derived exosomes down-regulate NKG2D expression on natural
killer cells and CD8+ T cells: Mechanism of immune evasion. PLoS
One. 9:e1089252014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Levy EM, Roberti MP and Mordoh J: Natural
killer cells in human cancer: From biological functions to clinical
applications. Biomed Res Int. 2011:6761982011. View Article : Google Scholar
|
|
30
|
Tian T and Li Z: Targeting Tim-3 in cancer
with resistance to PD-1/PD-L1 blockade. Front Oncol. 11:7311752021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Siemińska I and Baran J: Myeloid-derived
suppressor cells as key players and promising therapy targets in
prostate cancer. Front Oncol. 12:8624162022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Modena A, Ciccarese C, Iacovelli R,
Brunelli M, Montironi R, Fiorentino M, Tortora G and Massari F:
Immune checkpoint inhibitors and prostate cancer: A new frontier?
Oncol Rev. 10:2932016.PubMed/NCBI
|
|
33
|
Kamada T, Togashi Y, Tay C, Ha D, Sasaki
A, Nakamura Y, Sato E, Fukuoka S, Tada Y, Tanaka A, et al: PD-1(+)
regulatory T cells amplified by PD-1 blockade promote
hyperprogression of cancer. Proc Natl Acad Sci USA. 116:9999–10008.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Quatrini L, Mariotti FR, Munari E, Tumino
N, Vacca P and Moretta L: The immune checkpoint PD-1 in natural
killer cells: Expression, function and targeting in tumour
immunotherapy. Cancers (Basel). 12:32852020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Majdalawieh AF and Fayyad MW: Recent
advances on the anti-cancer properties of Nigella sativa, a widely
used food additive. J Ayurveda Integr Med. 7:173–180. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dajani EZ, Shahwan TG and Dajani NE:
Overview of the preclinical pharmacological properties of Nigella
sativa (black seeds): A complementary drug with historical and
clinical significance. J Physiol Pharmacol. 67:801–817.
2016.PubMed/NCBI
|
|
37
|
Bayır AG and Karakaş I: The Role of
Nigella sativa and Its Active Component Thymoquinone in
Cancer Prevention and Treatment: A Review Article. Eurasian J Med
Biol Sci. 1:1–12. 2021.
|
|
38
|
Ramadan MF: Nutritional value, functional
properties and nutraceutical applications of black cumin (Nigella
sativa L.): An overview. Int J Food Sci Technol. 42:1208–1218.
2007. View Article : Google Scholar
|
|
39
|
AlShaibi HF, Ahmed F, Buckle C, Fowles
ACM, Awlia J, Cecchini MG and Eaton CL: The BMP antagonist Noggin
is produced by osteoblasts in response to the presence of prostate
cancer cells. Biotechnol Appl Biochem. 65:407–418. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Randhawa MA and Alghamdi MS: Anticancer
activity of Nigella sativa (black seed)-a review. Am J Chin Med.
39:1075–1091. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schneider-Stock R, Fakhoury IH, Zaki AM,
El-Baba CO and Gali-Muhtasib HU: Thymoquinone: Fifty years of
success in the battle against cancer models. Drug Discov Today.
19:18–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Khan MA, Tania M, Fu S and Fu J:
Thymoquinone, as an anticancer molecule: From basic research to
clinical investigation. Oncotarget. 8:519072017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Khan MA, Tania M, Wei C, Mei Z, Fu S,
Cheng J, Xu J and Fu J: Thymoquinone inhibits cancer metastasis by
downregulating TWIST1 expression to reduce epithelial to
mesenchymal transition. Oncotarget. 6:19580–19591. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Majdalawieh AF, Hmaidan R and Carr RI:
Nigella sativa modulates splenocyte proliferation, Th1/Th2 cytokine
profile, macrophage function and NK anti-tumor activity. J
Ethnopharmacol. 131:268–275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sjs B, Kavithaa K, Poornima A, Haribalan
P, Renukadevi B and Sumathi S: Modulation of gene expression by
thymoquinone conjugated zinc oxide nanoparticles arrested cell
cycle, DNA damage and increased apoptosis in triple negative breast
cancer cell line MDA-MB-231. Drug Dev Ind Pharm. 47:1–19. 2022.
|
|
46
|
Alshaibi HF, Aldarmahi NA, Alkhattabi NA,
Alsufiani HM and Tarbiah NI: Studying the anticancer effects of
thymoquinone on breast cancer cells through natural killer cell
activity. Biomed Res Int. 2022:92186402022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Murphy EM, Centner CS, Bates PJ, Malik MT
and Kopechek JA: Delivery of thymoquinone to cancer cells with
as1411-conjugated nanodroplets. PLoS One. 15:e02334662020.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Peng L, Liu A, Shen Y, Xu HZ, Yang SZ,
Ying XZ, Liao W, Liu HX, Lin ZQ, Chen QY, et al: Antitumor and
anti-angiogenesis effects of thymoquinone on osteosarcoma through
the NF-κB pathway. Oncol Rep. 29:571–578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Waggoner SN, Daniels KA and Welsh RM:
Therapeutic depletion of natural killer cells controls persistent
infection. J Virol. 88:1953–1960. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Krebs P, Barnes MJ, Lampe K, Whitley K,
Bahjat KS, Beutler B, Janssen E and Hoebe K: NK cell-mediated
killing of target cells triggers robust antigen-specific T
cell-mediated and humoral responses. Blood. 113:6593–6602. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Karim S, Burzangi AS, Ahmad A, Siddiqui
NA, Ibrahim IM, Sharma P, Abualsunun WA and Gabr GA: PI3K-AKT
pathway modulation by thymoquinone limits tumor growth and
glycolytic metabolism in colorectal cancer. Int J Mol Sci.
23:23052022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Khan A, Alsahli MA, Aljasir MA, Maswadeh
H, Mobark MA, Azam F, Allemailem KS, Alrumaihi F, Alhumaydhi FA,
Almatroudi AA, et al: Experimental and theoretical insights on
chemopreventive effect of the liposomal thymoquinone against benzo
[a] pyrene-induced lung cancer in swiss albino mice. J Inflamm Res.
15:2263–2280. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mirzaei S, Zarrabi A, Hashemi F, Zabolian
A, Saleki H, Ranjbar A, Saleh SH, Bagherian M, Sharifzadeh SO,
Hushmandi K, et al: Regulation of nuclear factor-KappaB (NF-κB)
signaling pathway by non-coding RNAs in cancer: Inhibiting or
promoting carcinogenesis? Cancer Lett. 509:63–80. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang N and Bevan MJ: CD8+ T cells: Foot
soldiers of the immune system. Immunity. 35:161–168. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ibrahim S, Fahim SA, Tadros SA and Badary
OA: Suppressive effects of thymoquinone on the initiation stage of
diethylnitrosamine hepatocarcinogenesis in rats. J Biochem Mol
Toxicol. 36:e230782022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang B, Ting WJ, Gao J, Kang ZF, Huang CY
and Weng YJ: Erk phosphorylation reduces the thymoquinone toxicity
in human hepatocarcinoma. Environ Toxicol. 36:1990–1998. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang R, Wu T, Zheng P, Liu M, Xu G, Xi M
and Yu J: Thymoquinone sensitizes human hepatocarcinoma cells to
TRAIL-induced apoptosis via oxidative DNA damage. DNA Repair
(Amst). 103:1031172021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Narayanan P, Farghadani R, Nyamathulla S,
Rajarajeswaran J, Thirugnanasampandan R and Bhuwaneswari G: Natural
quinones induce ROS-mediated apoptosis and inhibit cell migration
in PANC-1 human pancreatic cancer cell line. J Biochem Mol Toxicol.
36:e230082022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang M, Du H, Wang L, Yue Y, Zhang P,
Huang Z, Lv W, Ma J, Shao Q, Ma M, et al: Thymoquinone suppresses
invasion and metastasis in bladder cancer cells by reversing EMT
through the Wnt/β-catenin signaling pathway. Chem Biol Interact.
320:1090222020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sutton VR, Davis JE, Cancilla M, Johnstone
RW, Ruefli AA, Sedelies K, Browne KA and Trapani JA: Initiation of
apoptosis by granzyme B requires direct cleavage of bid, but not
direct granzyme B-mediated caspase activation. J Exp Med.
192:1403–1414. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shimasaki N, Jain A and Campana D: NK
cells for cancer immunotherapy. Nat Rev Drug Discov. 19:200–218.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Smyth MJ, Cretney E, Kelly JM, Westwood
JA, Street SE, Yagita H, Takeda K, van Dommelen SL, Degli-Esposti
MA and Hayakawa Y: Activation of NK cell cytotoxicity. Mol Immunol.
42:501–510. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kingdom of Saudi Arabia, Saudi Health
Council, National Cancer Center, Saudi Cancer Registry, . Cancer
Incididence Report Saudi Arabia 2020. https://shc.gov.sa/Arabic/NewNCC/Activities/AnnualReports/2020.pdfJanuary
5–2025
|
|
64
|
Chowdhury D and Lieberman J: Death by a
thousand cuts: Granzyme pathways of programmed cell death. Annu Rev
Immunol. 26:389–420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Young JD, Hengartner H, Podack ER and Cohn
ZA: Purification and characterization of a cytolytic pore-forming
protein from granules of cloned lymphocytes with natural killer
activity. Cell. 44:849–859. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Singh SK, Mishra MK, Lillard JW and Singh
R: Thymoquinone enhanced the tumoricidal activity of NK cells
against lung cancer. J Immunol. 200 (Supplement_1):S124–S125. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sethi G, Ahn KS and Aggarwal BB: Targeting
nuclear factor-κB activation pathway by thymoquinone: role in
suppression of antiapoptotic gene products and enhancement of
apoptosis. Mol Cancer Res. 6:1059–1070. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sadeghi E, Imenshahidi M and Hosseinzadeh
H: Molecular mechanisms and signaling pathways of black cumin
(Nigella sativa) and its active constituent, thymoquinone: A
review. Mol Biol Rep. 50:5439–5454. 2023. View Article : Google Scholar : PubMed/NCBI
|