Introduction
Cancer remains a major global health problem, with
an estimated 19.3 million new cases and ~10 million deaths reported
worldwide in 2020 alone (1). The
2024 report of the American Cancer Society projected that 2,001,140
new cancer cases would be diagnosed in the United States in 2024,
with an estimated 611,720 deaths (2). Globally, cancer has become the second
leading cause of death in the past 100 years (3). Stomach, colorectal, esophageal,
pancreatic, ovarian, cervical, kidney, breast, lung, prostate,
thyroid and other cancers threaten human health in several ways
(4–14). Early detection and timely
intervention are crucial for patient prognosis, and early diagnosis
and treatment have always been the guiding principles in managing
such diseases (15). The detection
rate of tumors has increased with the advancements in diagnostic
techniques. However, most tumors are detected at an advanced stage,
resulting in most patients missing the opportunity for surgical
resection treatment. Therefore, determining the target for tumor
biotherapy is an essential step in cancer treatment (16,17).
Biomarkers are molecules in the blood, other body
fluids or tissues that serve as markers of normal or abnormal
processes, conditions or diseases, and can be used to monitor how
the body responds to therapy for a certain disease or condition
(16). Biomarkers can enable
precise and individualized treatment for patients with tumors,
thereby opening novel avenues for treating highly heterogeneous
neoplastic lesions (18). Classical
tumor markers, such as carcinoembryonic antigen, cancer antigen 125
and cancer antigen 19-9, have been widely used in clinical practice
and can serve as indicators of biological processes, states or
conditions to determine the occurrence, development and prognosis
of cancer. However, these markers may also be expressed in nontumor
tissues under the influence of certain factors (19). Therefore, the identification of
sensitive and specific tumor biomarkers is critical for cancer
treatment.
Discoidin protein domain receptor (DDR) tyrosine
kinases belong to the tyrosine kinase receptor family, which is
subdivided into 20 subfamilies based on the homology of
ligand-binding extracellular domain features. Abnormalities in
these tyrosine kinases are associated with several diseases,
including cancer, chronic inflammation and fibrosis (20,21).
DDR1 is predominantly expressed in epithelial cells of different
tissues, whereas DDR2 is expressed in fibroblasts, myofibroblasts,
smooth muscle cells, chondrocytes and other mesenchymal cells
(22). In addition, their
interaction with MAPK, PI3K, JAK/STAT and Rho-GTPases can induce
signal transduction to activate ERK1/2, Akt, cytokine and
RhoA-signaling pathways. The DDR2 receptor serves a role in
cytoskeletal dynamics, cell proliferation, differentiation, cell
survival, adhesion, migration, metabolism, cytokine signaling and
immune response (23). In a
previous study, the expression of DDR2 on tumor-associated
fibroblasts increased the hardness of tumor tissues (24).
The present review summarizes the latest research
progress on DDR2, its role in cancer and its underlying mechanisms
of action, including its abnormal expression in cancer and its
prognostic value. Additionally, the current status of global drug
and future design prospects for DDR2 are also reviewed.
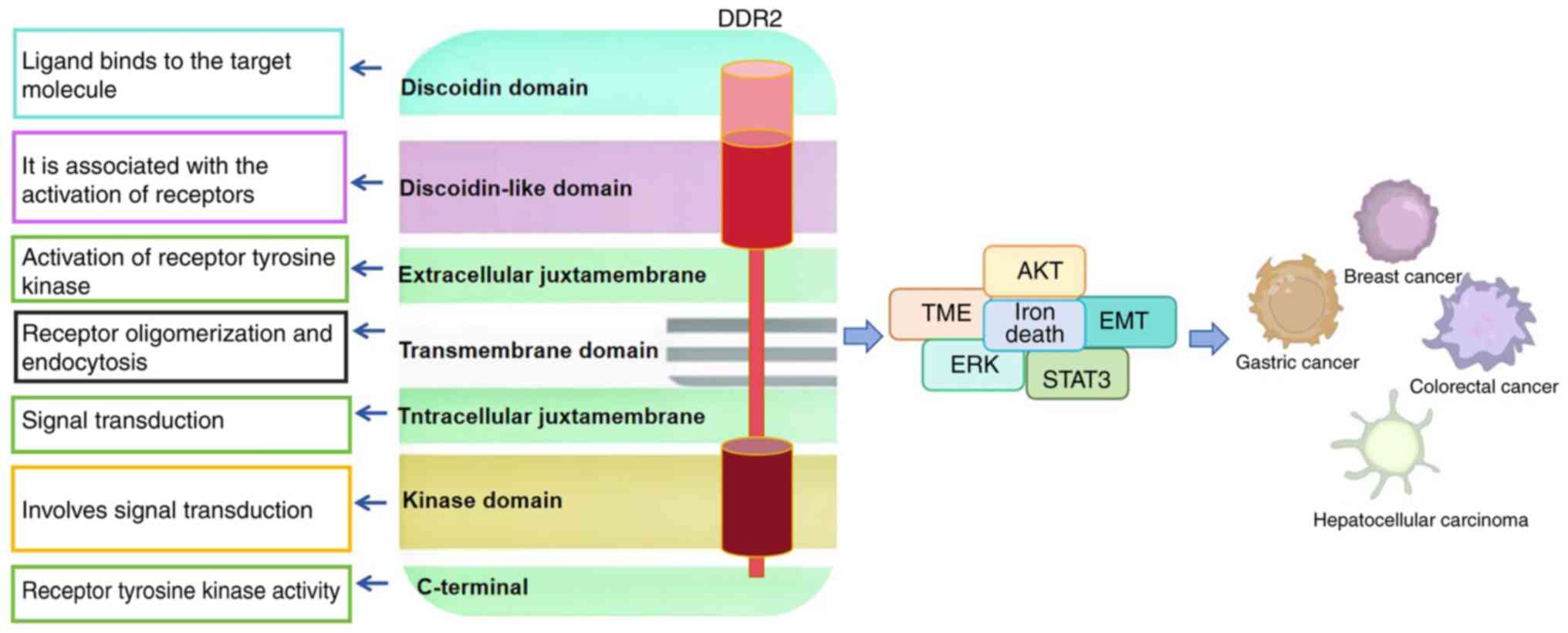
Structure and function of DDR2
The molecular structure of DDR includes an
extracellular binding domain, a transmembrane domain and an
intracellular kinase domain. The extracellular binding domain is
composed of a discoidin (DS) domain and a DS-like domain for
collagen binding (25). The DDR2
gene is located on human chromosome 1 (1q23.3) and consists of 19
exons, of which exons 4-19 are transcribed into a mRNA transcript
that is then translated to produce the DDR2 protein product.
Connective tissue cells originating from the embryonic mesoderm can
be stimulated and activated by collagen types I, II, III and X, and
they participate in several physiological and pathological
processes. The extracellular binding region of DDR2 consists of
N-terminal DS domains that can bind to collagen, DS-like domains
and extracellular phagocyte membrane regions, providing N- and
O-glycosylation sites and MMP cleavage sites. N- and
O-glycosylation sites are important sites for the glycosylation of
DDR2. The glycosylation modification of DDR2 can enhance its
signal-conduction ability (26).
The MMP cleavage sites of DDR2 are associated with the mutual
regulation between DDR2 and MMPs (27). MMPs can cleave several substrates,
including cell surface receptors. Therefore, the presence of MMP
cleavage sites in DDR2 can lead to the cleavage and inactivation of
DDR2 by MMPs, thereby regulating the activity of DDR2 (28). DDR2 can act in conjunction with
myosin IIA to regulate the adhesion and traction of collagen and
condense collagen fibrils into a denser arrangement, thereby
reshaping the generation and arrangement of collagen fibers
(29).
Expression of DDR2 in human single
cells
For further analysis, the present study mapped the
DDR2 in tissues, cells and organs using the Human Protein Atlas
(HPA) database (www.proteinatlas.org), which integrates proteomics,
transcriptomics and systems biology data. The results revealed that
DDR2 was most abundant in nontumor cells in the single-celled
expression cluster (https://www.proteinatlas.org/ENSG00000162733-DDR2/cell+line).
Furthermore, the RNA expression levels of DDR2 in several tissues
were summarized using the HPA database (https://www.proteinatlas.org/ENSG00000162733-DDR2/tissue).
Research progress of DDR2 in solid
tumors
DDR2 is abnormally expressed in numerous human solid
tumors and has been associated with tumorigenesis. The current
research results of DDR2 in several solid tumors are summarized in
Table I.
 | Table I.Research progress of discoidin domain
receptor tyrosine kinase 2 in several solid tumors. |
Table I.
Research progress of discoidin domain
receptor tyrosine kinase 2 in several solid tumors.
| A, Ovarian
cancer |
|---|
|
|---|
| First author,
year | Result | Mechanism | (Refs.) |
|---|
| Schab et al,
2023 | Promotes ovarian
cancer metastasis | Regulates
metabolism and secretion of extracellular matrix proteins | (36) |
| Akinjiyan et
al, 2024 | Promotes tumor
growth | DDR2 expressed by
CAFs promotes collagen production and tumor progression by
regulating arginase activity | (38) |
| Heiserman et
al, 2021 | Promotes tumor
resistance | XIα1 collagen
up-regulates the expression and activity of HSP27 through
DDR2/integrin α1β1-Src-Akt signaling pathway, inducing cisplatin
resistance in ovarian cancer cells | (43) |
|
| B, Breast
cancer |
|
| First author,
year | Result |
Mechanism | (Refs.) |
|
| Corsa et al,
2016 | Promotes breast
cancer metastasis | DDR2 can reshape
the extracellular matrix by affecting TME and promoting breast
cancer metastasis | (45) |
| Lin et al,
2021 | Increases
susceptibility to iron death | DDR2 upregulation
increases susceptibility to iron death in recurrent breast tumors
via the Hippo pathway | (46) |
|
| C, Gastric
cancer |
|
| First author,
year | Result |
Mechanism | (Refs.) |
|
| Kurashige et
al, 2016 | Promotes peritoneal
metastasis of gastric cancer | Dasatinib, a DDR2
inhibitor, reduces peritoneal metastasis in gastric cancer | (54) |
| Wang et al,
2016 | Promotes the growth
of gastric cancer | The mTORC2/Akt
signaling pathway promotes epithelial mesenchymal transformation to
promote the occurrence and development of gastric cancer | (55) |
|
| D, Colorectal
cancer |
|
| First author,
year | Result |
Mechanism | (Refs.) |
|
| Firouzjaei et
al, 2023 | Associated with the
prognosis of colorectal cancer | Analysis using the
GEPIA and GEO databases | (56) |
| Xu et al,
2023 | Promotes metastasis
of colorectal cancer | Regulating
epithelial stromal transformation through activation of AKT
signaling | (60) |
|
| E,
Hepatocellular carcinoma |
|
| First author,
year | Result |
Mechanism | (Refs.) |
|
| Xie et al,
2015 | Promotes the
invasion and migration of hepatocellular cancer cells | Activate ERK signal
and stabilize SNAIL1 | (65) |
| Liu et al,
2024 | Oxaliplatin
resistant | Overexpression of
immunosuppressive checkpoints such as PD-L1 PD-and CD155 via
DDR2/STAT3 positive feedback loops prevents CD8+ T cell-mediated
immunokilling; and the secretion of chemokine CCL20 recruits MDSCs
into the tumor microenvironment, thereby establishing an immune
tolerance environment | (70) |
|
| E,
Hepatocellular carcinoma |
|
| First author,
year | Result |
Mechanism | (Refs.) |
|
| Li et al,
2023 | Resistant to
sorafenib | Sorafenib
resistance is mediated through the NF-κB/c-Rel signaling
pathway | (72) |
| Cai et al,
2022 | Promotes
hepatocellular cancer cell metastasis | Activation of the
DDR2/β-catenin pathway | (75) |
|
| F,
Neuroblastoma |
|
| First author,
year | Result |
Mechanism | (Refs.) |
|
| Rozen et al,
2024 | Control of the
metastasis of neuroblastoma | Sitravatinib blocks
DDR2 to control the metastasis of neuroblastoma | (79) |
|
| G, Thyroid
cancer |
|
| First author,
year | Result |
Mechanism | (Refs.) |
|
| Liang et al,
2017 | DDR2 promotes the
occurrence and growth of thyroid cancer through EMT | Activation of ERK2
increases the protein level of Snail1 and induces EMT in thyroid
papillary carcinoma | (82) |
|
| H, Prostate
cancer |
|
| First author,
year | Result |
Mechanism | (Refs.) |
|
| Yan et al,
2014 | Promotes the bone
metastasis of prostate cancer | By regulating the
phosphorylation and exchange activity of RUNX2, regulating the
expression of PTHrP promotes bone metastasis of prostate
cancer | (87) |
|
| I, Urothelial
carcinoma |
|
| First author,
year | Result |
Mechanism | (Refs.) |
|
| Tsai et al,
2016 | DDR2 is associated
with a poor prognosis of urothelial carcinoma | Urothelial
carcinoma tissue expresses DDR2, and combined with
clinicopathological data, the survival of patients with low DDR2
expression was greater than that of patients with high
expression | (89) |
|
| J,
Melanoma |
|
| First author,
year | Result |
Mechanism | (Refs.) |
|
| Poudel et
al, 2015 | Increases the
migration and invasion of melanoma cells | DDR2 regulates the
production of MMP2/MMP9 in type I collagen response by regulating
ERK and NF-κB signaling pathways, thereby modulating the mechanisms
of cell migration and invasion phenotypes | (92) |
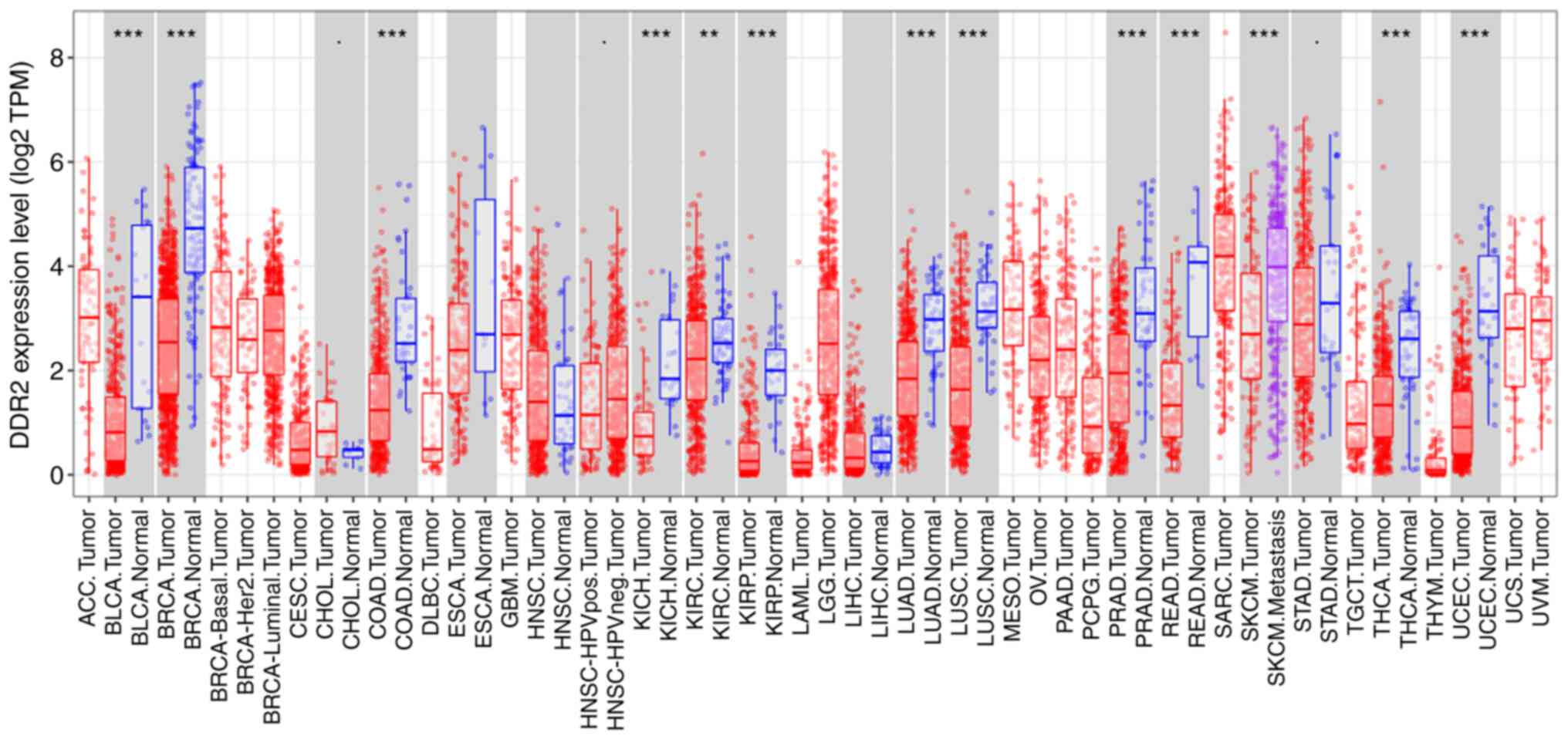
Furthermore, analysis of data from the Tumor Immune
Estimation Resource database (http://cistrome.org/TIMER/) revealed that the DDR2
expression was different across breast duct carcinoma with
subsequent lung adenocarcinoma, breast cancer, colon
adenocarcinoma, kidney chromophobe, kidney renal clear cell
carcinoma, kidney renal papillary cell carcinoma, lung
adenocarcinoma, lung squamous cell carcinoma, prostate
adenocarcinoma, rectal adenocarcinoma, skin cutaneous melanoma,
thyroid carcinoma, uterine corpus endometrial carcinoma and other
cancers (Fig. 1). In addition, a
literature search revealed the involvement of DDR2 in ovarian,
breast, lung, colorectal and other cancers through several pathways
(30–33). Several functions of DDR2 in solid
tumors are presented in Fig. 2.
DDR2 in the tumorigenesis mechanism
DDR2 in ovarian cancer
Ovarian cancer is a highly prevalent malignant
tumor, and its incidence ranks eighth among female tumors in the
world. Worldwide, 314,000 cases and 207,000 deaths have been
reported annually. Furthermore, the incidence of most cancers is
decreasing in Northern Europe and North America based on age
markers; however, it is on the rise in certain parts of Eastern
Europe and Asia (34,35). In a recent study, Schab et al
(36) reported that the expression
of DDR2 is negatively associated with the survival rate of patients
with ovarian cancer.
DDR2 can facilitate ovarian cancer metastasis and
enhance tumor invasion by regulating the metabolism and secretion
of extracellular matrix (ECM) proteins. Cancer-associated
fibroblasts (CAFs) are major matrix components in the tumor
microenvironment (TME). CAFs exhibit considerable heterogeneity and
plasticity, and have a marked effect on the immune response and
metabolic reprogramming within the TME, thereby influencing tumor
progression (37). Akinjiyan et
al (38) reported that DDR2
expressed by CAFs enhances collagen production and tumor
progression by regulating arginase activity, indicating that DDR2
and arginase in CAFs are likely targets in ovarian cancer. This
process involves the activation of the transcription factor SNAIL1,
which induces epithelial-mesenchymal transition (EMT) of tumor
epithelial cells (39,40). In addition, DDR2 can regulate the
expression of SNAIL1, affect the expression of arginase 1, and
influence the occurrence and development of tumors. Cisplatin is a
well-established chemotherapeutic drug in the treatment of several
human cancers (41), but its
extensive clinical use in treating tumors often leads to chemical
resistance, namely, drug resistance (42). Studies on this drug have received
notable attention. For instance, Heiserman et al (43) reported that type XIα1 collagen
confers cisplatin resistance in ovarian cancer by upregulating heat
shock protein 27 expression and activity through the DDR2/integrin
α1β1-Src-Akt-signaling pathway. Therefore, according to research
findings on the role of DDR2 in ovarian cancer, this receptor can
not only promote the occurrence and metastasis of tumors, but also
increase the drug resistance of tumors through other mechanisms.
Thus, DDR2 is a promising biomarker in ovarian cancer.
DDR2 in breast cancer
Breast cancer is a major health problem among women
worldwide. It is characterized by ‘cold tumors’ exhibiting low
levels of immune cell infiltration, which limits the efficacy of
conventional immunotherapy. At present, research is focused on the
strategy of transforming a ‘cold tumor’ into a ‘hot tumor’ through
the TME (44).
In a recent study on DDR2, Corsa et al
(45) noted that, during the
occurrence, progression and metastasis of breast tumors, both
tumors and tumor-related stromal cells can express DDR2 and reshape
the ECM, thereby altering the TME and promoting breast cancer
metastasis Moreover, in a study by Lin et al (46), DDR2 upregulation was reported to
increase the susceptibility of recurrent breast tumors to
iron-dependent cell death or ferroptosis through the Hippo pathway.
Ferroptosis refers to the iron-dependent lethal accumulation of
membrane lipid peroxides. This process is a form of regulated cell
death that has received attention from the research community since
its proposal by Brent R. Stockwell in 2012 (47). This study experimentally
demonstrated that the high sensitivity of breast tumors to iron is
attributable to the overexpression of DDR2 in breast tumor cells
with mesenchymal characteristics. The study also reported that
increased DDR2 levels may serve as the molecular basis for EMT and
that there is an association between mesenchymal characteristics
and sensitivity to ferroptosis in recurrent breast cancer. This
seemingly forms a closed-loop mechanism. Although increased DDR2
expression promotes breast cancer recurrence, it also enhances
tumor susceptibility to ferroptosis, thus providing new clues for
treating recurrent breast cancer.
Furthermore, triple-negative breast cancer is
characterized by the absence of endocrine therapeutic targets and
human epidermal growth factor receptor 2 blockade, and it has a
poor prognosis (48). Current
research on this disease aims to identify specific molecular and
genetic markers (49). Notably,
DDR2 is expressed in human triple-negative breast tumors and tumor
stroma, potentially providing a new target for the treatment and
diagnosis of this disease (50).
DDR2 in gastric cancer
Gastric cancer, also known as stomach cancer, is a
highly malignant and common digestive tract tumor. Advancements in
surgical techniques and the development of antitumor drugs have
improved the prognosis of patients with gastric cancer. However,
the survival rate and quality of life of these patients are still
lower than those of patients with colorectal cancer, liver cancer
and other digestive tract tumors (51). Peritoneal metastasis (PM) is common
in gastric cancer, and it has been reported that >50% of the
patients have PM at the time of death (52). The 5-year survival rate of patients
with gastric cancer with PM is ~2%, and the median survival time is
3–5 months (53).
A study by Kurashige et al (54) using four gastric cancer cell lines,
reported the association of DDR2 with poor prognosis and peritoneal
dissemination of gastric cancer. It also demonstrated that the DDR2
inhibitor dasatinib can reduce gastric cancer PM. Wang et al
(55) combined the analysis of
immunohistochemistry results and clinicopathological data, and
reported that DDR2 expression was associated with adverse
clinicopathological features in patients with gastric cancer. The
study further demonstrated that the receptor stimulates epithelial
transformation via the mTORC2/Akt-signaling pathway, thereby
promoting the occurrence and development of gastric cancer
(55).
DDR2 in colorectal cancer
Colorectal cancer is the second leading cause of
cancer-related death worldwide. Firouzjaei et al (56), using the Gene Expression Profiling
Interactive Analysis and Gene Expression Omnibus databases,
reported that DDR2 expression was associated with the prognosis of
colorectal cancer. Currently, metastatic colorectal cancer is a
serious clinical problem, and despite having surgery and
chemotherapy as treatment options, it has a poor prognosis.
DDR1 and DDR2 may serve as potential therapeutic
targets for metastatic colorectal cancer (57–59).
In a study by Xu et al (60), high DDR2 expression was associated
with a low survival rate, and patients with a higher expression of
DDR2 had worse overall survival. In addition, DDR2 may promote
colorectal cancer metastasis through the activation of AKT
signaling by regulating epithelial-stromal transformation.
Therefore, the early detection of DDR2 expression in patients with
colorectal cancer may aid in the clinical diagnosis and treatment
of this cancer.
DDR2 in hepatocellular carcinoma
(HCC)
HCC is a primary malignant tumor of the liver and is
usually diagnosed at an advanced stage when the tumor is
unresectable. In such cases, systemic treatment with tyrosine
kinase inhibitors is the primary option (61). Although α-fetoprotein has been used
as a clinical indicator for the diagnosis and prognosis assessment
of liver cancer, the development of new biomarkers remains a focus
of HCC research (62).
Research findings have revealed that downregulating
DDR2 expression can reduce the proliferation and migration of HCC
cells (63,64). Xie et al (65) assessed the expression of DDR2 in the
normal liver LO2 cell line, and the liver cancer SMMC-7721, Huh-7,
HepG2, Hep3B and MHCC97-H5 cell lines, and reported that whilst
DDR2 expression was low in normal liver LO2 cells, it was increased
in the other five HCC cell lines. Additionally, the expression of
DDR2 in the highly aggressive metastatic HCC cell line (MHCC97-H5)
was reported to be notably higher than that in less aggressive HCC
cell lines. Additionally, Cox regression analysis of the clinical
data suggested that DDR2 is an independent prognostic factor for
HCC. Furthermore, DDR2 was reported to promote cell invasion,
migration and EMT by activating ERK signaling, stabilizing SNAIL1
and upregulating the expression of membrane type MMP and MMP2 via
the ERK2/SNAIL1 signaling pathway (65).
Immunotherapy is a valuable approach to HCC
treatment. Programmed cell death protein-1 (PD-1) is a checkpoint
receptor expressed on the surface of several immune cells. PD-L1 is
a natural receptor of PD-1 and is predominantly expressed in tumor
cells. PD-1 and PD-L1 are closely associated with the progression
of human cancer (66). In the liver
of patients of HCC, PD-L1 is mainly expressed in tumor cells,
Kupffer cells and hepatocytes (67). The activation of the STAT3 signaling
pathway can directly and indirectly induce the expression of PD-L1
(68). Oxaliplatin is a commonly
used platinum-based chemotherapeutic drug (69); however, as with several other drugs
used in tumor chemotherapy, drug resistance has become the most
notable problem limiting its effect.
Liu et al (70) reported that DDR2 and STAT3 create an
immunosuppressive microenvironment by upregulating PD-L1 expression
and recruiting myeloid-derived suppressor cells (MDSCs) through a
positive feedback loop, leading to drug resistance in HCC. DDR2 was
found to be highly expressed in drug-resistant HCC, interacts with
STAT3 and promotes STAT3 phosphorylation. In addition, the receptor
increases liver cancer cell proliferation and oxaliplatin
resistance through STAT3 signaling, thereby stimulating HCC
development by increasing DDR2 expression (70). MDSCs are a heterogeneous group of
immature myeloid cells with immunosuppressive activity (71). Oxaliplatin-resistant cells
overexpress immunosuppressive checkpoint proteins such as PD-L1 and
CD155 through DDR2/STAT3-positive feedback loops, thus preventing
CD8+ T-cell-mediated immune killing. Notably,
oxaliplatin-resistant cells secrete the chemokine C-C motif
chemokine ligand 20 (CCL20) to recruit MDSCs into the TME, thereby
establishing an immune-tolerant environment. Sorafenib, a
multikinase inhibitor that promotes apoptosis, alleviates
angiogenesis and inhibits tumor cell proliferation, is the
first-line treatment option for HCC. As a result, the mechanism of
resistance to this drug has attracted extensive research attention
(72,73). DDR2 has been reported to mediate
sorafenib resistance through the NF-κB/c-Rel-signaling pathway
(74). Moreover, as DDR2 has been
reported to induce liver cancer growth and drug resistance, it may
also serve as a link to the mechanism pathway, mediating other
factors to promote the development of liver cancer. For example,
Cai et al (75) reported
that the long noncoding RNA CEBPA-DT promotes liver cancer
metastasis by activating the DDR2/β-catenin signaling through
interaction with heterogeneous nuclear ribonucleoprotein C.
DDR2 in neuroblastoma
Neuroblastoma is a cancer that arises from neural
crest cells and is the most common extracranial solid tumor in
children (76). Research by
Vessella et al (77)
reported that DDR2 is required for the normal proliferation of
neuroblastoma cells and that DDR2 signaling and mechanical sensing
regulate the growth of neuroblastoma cells by several
transcriptomic mechanisms. In neuroblastoma, the action of
sitravatinib, an immunoregulatory multitarget kinase inhibitor
(78), was reported to be mediated
by DDR2. This drug blocks DDR2 to inhibit the metastasis of
neuroblastoma (79).
DDR2 in thyroid cancer
Thyroid cancer is the most common endocrine
malignancy, and most cases are diagnosed early, are highly
differentiated and have a good prognosis (80). Papillary carcinoma accounts for ~80%
of thyroid cancers, and the increase in thyroid cancer incidence
can almost entirely be attributed to the increase in papillary
thyroid cancer (81).
Liang et al (82) studied the mechanism of DDR2 in
thyroid cancer, and reported that, as in other cancers, DDR2
promotes the occurrence and development of thyroid cancer through
EMT. DDR2 specifically activates ERK2 to increase the protein
expression of SNAIL1 to induce EMT in papillary carcinoma.
DDR2 in prostate cancer
Prostate cancer is a type of urologic cancer that
forms in the prostate, with a relatively high incidence worldwide,
and it is the second-most common cancer in men after lung cancer
(83). Although prostate-specific
antigen is widely used as a clinical marker for prostate cancer, it
lacks specificity. Thus, prostate cancer biomarkers continue to be
the focus of current research (84).
Azemikhah et al (85) reported that DDR2 is differentially
expressed in prostate cancer tissues compared with that in
noncancerous prostate tissues. The mRNA expression of DDR2 is
upregulated in advanced prostate cancer and prostatic hyperplasia
tissues. Furthermore, the expression of DDR2 mRNA and protein in
advanced prostate cancer tissues was associated with prognostic
factors. Additionally, an analysis of The Cancer Genome Atlas
database by Huang et al (86) revealed that DDR2 is associated with
disease diagnosis in patients with prostate cancer. A study by Yan
et al (87) also reported
that DDR2 promotes prostate cancer bone metastasis by regulating
the phosphorylation and the exchange activity of RUNX family
transcription factor 2, thereby regulating the expression of
parathyroid hormone-related protein.
DDR2 in urothelial carcinoma
The most common pathological subtype of bladder and
upper urinary tract malignancies is urothelial carcinoma (88). In a large cohort study, DDR2 was
reported to be overexpressed in upper urinary tract urothelial
carcinoma and urinary bladder urothelial carcinoma. When combined
with clinicopathological data, DDR2 was associated with the poor
prognosis of urothelial carcinoma, and the survival of patients
with low expression of DDR2 was reported to be higher than that of
those with high expression (89).
DDR2 in melanoma
Although DDR1 is the primary DDR in the epidermis,
where it is involved in melanocyte homeostasis, DDR2 appears to be
the primary DDR implicated in melanoma (90). In addition, DDR2 controls cell and
tumor proliferation via the MAP kinase pathway in vitro and
in vivo in drug-resistant cells. Therefore, inhibiting DDR2
may represent a novel strategy to combat the resistance mechanism
(91). Poudel et al
(92) reported that DDR2 regulates
the production of MMP2/9 in type I collagen response by regulating
the ERK and NF-κB signaling pathways, thereby modulating cell
migration mechanisms and invasion phenotypes. Therefore, DDR2 is a
receptor tyrosine kinase with notable therapeutic potential for
melanoma (92).
DDR2 and the TME
The TME refers to the noncancerous cells and their
components present in the tumor environment, including fibroblasts,
endothelial cells, neurons, fat cells, adaptive cells and innate
immune cells. This term also refers to the continuous interaction
between tumor cells and the TME, which serves a decisive role in
the cancer development, progression and metastasis, as well as in
the therapeutic response of the tumor (93,94).
The ECM is an important component of the TME. Tumor cells interact
with the ECM to promote cancer cell proliferation, migration,
invasion, angiogenesis and immune escape; thus, the ECM has become
a key target in cancer treatment (95,96).
The ECM is mainly composed of proteoglycans, glycoproteins, matrix
proteins, osteopontin, thrombo-reactive protein and structural
proteins, which undergo dynamic remodeling to maintain the TME
(97,98). DDR2 is uniquely positioned to act as
an ECM sensor and can be activated by ECM collagen-induced binding
protein receptors. Processes such as migration, proliferation and
cytokine secretion are regulated, leading to ECM remodeling and
reconstruction in an unbalanced homeostasis (24,99).
DDR2-expressing CAFs can promote metastasis of ovarian cancer by
influencing ECM remodeling (100).
DDR2 and CAFs
The population of fibroblasts found in both primary
and metastatic cancers is collectively referred to as CAFs. They
are the most abundant cell types in the TME and are the central hub
of cross-communication among several cells in the tumor stroma
(101,102). Aside from being highly
heterogeneous, CAFs are differentially expressed in different tumor
tissues, and several CAF subtypes have been identified in numerous
cancers. Targeted CAF therapy is currently a research hotspot in
antitumor therapy. In CAFs, DDR2 expression is directly associated
with their ability to reshape the ECM (103,104). For example, a previous study
reported that DDR2-expressing CAFs regulate periostin (POSTN)
protein via integrin subunit B1 (ITGB1), promoting ovarian cancer
metastasis. DDR2 and POSTN signal through the PI3K/AKT and Src
pathways and can serve as potential therapeutic targets for ovarian
cancer (105). In a breast cancer
study, DDR expression by CAFs increased the aggressiveness of
breast tumor cells through regulation of the basement membrane and
paracrine signaling. Based on these findings, the study indicated
that the independent tyrosine kinase activity of DDR2 in breast
tumor cells and breast tumor CAFs regulates breast cancer
metastasis. Therefore, adjuvant therapy targeting tyrosine kinase
activity should not only target tumor cells and stromal cells but
also target the tumor stromal cells. Furthermore, drugs that
inhibit tyrosine kinase-dependent and tyrosine kinase-independent
effects are urgently needed (106).
DDR2 and immunotherapy
To date, immune checkpoint-targeted drugs, such as
anti-cytotoxic T lymphocyte-associated protein 4, anti-PD-1 and
anti-PD-L1, as well as other new targeted drugs, have achieved
notable results in several cancer immunotherapies. However,
accumulating evidence suggests that positive response rates remain
low in patients receiving immune checkpoint-targeted drugs and drug
resistance emerges, which is an issue that warrants attention
(107,108). DDR1 and DDR2 have been identified
as potential therapeutic targets for MAPK inhibitor resistance, and
mutations in DDR2 have shown particular efficacy with dasatinib in
squamous cell lung carcinoma (109,110). The tumor immune microenvironment
is composed of tumor cells, immune cells and cytokines. These
components can be classified as antitumor and protumor, and the
interaction between them determines the trend of antitumor immunity
(111). These reported findings
demonstrate that DDR2 is involved in multiple mechanisms mediating
the interactions between tumor cells and immune cells, and thus
immunotherapy targeting DDR2 may provide new perspectives to tumor
therapy.
Co-activation pathway of DDR2 in different
solid tumors
EMT refers to the cellular process through which
epithelial cells lose their properties and gain interstitial
properties to facilitate cell movement. This process is abnormally
activated in human cancers and contributes to enhanced tumor
initiation, cell migration, invasion, metastasis and therapeutic
resistance (112). DDR2 is
expressed in interstitial cells and can be activated by collagen,
thus we hypothesize that DDR2 is associated with organ fibrosis and
EMT. DDR2 can promote tumor metastasis and invasion through EMT in
ovarian cancer, breast cancer, stomach cancer, colorectal cancer,
thyroid cancer and other cancers (37,38,43,53,58,80).
Therefore, as DDR2 can promote tumor metastasis and invasion,
therapies targeting the tumor microenvironment in patients with
cancer with high DDR2 expression may be effective.
Current status of research on drugs and
antibodies targeting DDR2
Due to its potential use in antitumor therapy,
several drugs targeting DDR2 have been developed and used in
clinical trials and research. The present review used the
Pharnexcloud Cloud database (https://www.pharnexcloud.com/) to retrieve information
on DDR2 global clinical trials (Table
II) and drug development (Table
III) (113–118). In addition, the prognostic value
of DDR2 in solid tumors was also summarized in Table IV.
 | Table II.Clinical trials of discoidin domain
receptor tyrosine kinase 2. |
Table II.
Clinical trials of discoidin domain
receptor tyrosine kinase 2.
| Project name | Drug name | Recruitment
status |
|---|
| Phase II trial of
dasatinib in subjects with advanced cancers harboring DDR2 mutation
or inactivating B-RAF mutation | Dasatinib | Recruitment
cancellation |
| Phase II trial of
dasatinib in subjects with advanced cancers harboring DDR2 mutation
or inactivating B-RAF mutation | Dasatinib | Terminated |
| An exploratory
clinical trial of PET-MRI application o64Cu-DDR2 vs. 18F-PDG in
preoperative diagnosis of newly diagnosed glioblastoma | - | Not yet
started |
| Prospective,
single-arm, multicenter clinical study of the efficacy and safety
of dasatinib for imatinib treatment failure in fibroids | Dasatinib | Not yet
started |
 | Table III.Current research on drugs targeting
DDR2. |
Table III.
Current research on drugs targeting
DDR2.
| Drug | Research field | Indication | Highest research
and development stage | (Refs.) |
|---|
| CIDD-0108633 | Tumor | Pancreatic ductal
adenocarcinoma | Preclinical | (113) |
| ICP-033 | Tumor | Advanced solid
tumor, colorectal cancer, liver tumor and renal cell tumor | Phase I
clinical | (114) |
| BK-40143 | Neural research
Tumor | Alzheimer's
disease | Preclinical | (115) |
| PB-1 |
| Squamous cell
carcinoma | Preclinical | (116) |
| Dual DDR-1/2
nhibitors | Acute lung injury
and autoinflammatory disease | Respiratory system
and inflammation | Drug discovery | (117) |
| Dasatinib | Acute lymphoblastic
leukemia, chronic myeloid leukemia, glioblastoma, triple-positive
breast cancer, breast cancer, hormone receptor negative breast
cancer, lympho-plasmacytoid lymphoma/immunocytoma, metastatic
breast cancer, metastatic non-small cell lung cancer
andtriple-negative breast cancer | - | Preclinical | (118) |
 | Table IV.Prognostic value of discoidin domain
receptor tyrosine kinase 2 in solid cancers. |
Table IV.
Prognostic value of discoidin domain
receptor tyrosine kinase 2 in solid cancers.
| First author,
year | Cancer | Association with
prognosis | Prognostic
result | (Refs.) |
|---|
| Schab et al,
2023 | Ovarian cancer | Expression of DDR2
is associated with the survival rate of patients | Higher the
expression of DDR2 is associated with a lower survival rate | (36) |
| Lin et al,
2018 | Breast cancer | DDR2 expression is
associated with recurrent breast cancer | Increase of DDR2
expression promotes the recurrence of breast cancer | (46) |
| Kurashige et
al, 2016 | Gastric cancer | DDR2 expression is
associated with adverse types and survival rate of gastric
cancer | Higher expression
of DDR2 is associated with a lower survival rate | (54) |
| Xu et al,
2023 | Colorectal
cancer | DDR2 expression is
associated with the survival rate | Higher expression
of DDR2 is associated with a lower survival rate | (60) |
| Xie et al,
2015 | Hepatocellular
carcinoma | DDR2 is an
independent prognostic factor for hepatocellular carcinoma | DDR2 expression
increases the invasiveness of hepatocellular carcinoma | (65) |
| Tsai et al,
2018 | Urothelial
carcinoma | DDR2 expression is
associated with survival rate | Survival period of
patients with low expression of DDR2 is longer than that of
patients with high expression | (89) |
Strengths of the present review
Table V provides a
comparison between the present review and the study by Trono et
al (119). Moreover, the
present review included multiple studies on DDR2 with the aim of
including more innovations. The strengths of the present review are
as follows: i) The review systematically outlines the role of DDR2
in several solid tumors, such as ovarian cancer, breast cancer,
gastric cancer and liver cancer (a total of 12 categories). The
expression, function and clinical significance of DDR2 in each
cancer type are listed in Table I;
ii) the present review describes the single-cell expression profile
of DDR2 in nontumor cells based on the HPA database, emphasizing
its specific distribution in stromal cells and providing a
cell-type basis for targeted therapy; iii) the present review
describes the current status of clinical trials, drug and antibody
research, and development of anti-DDR2 drugs using databases such
as Pharnexcloud; iv) the present review offers the following
mechanistic innovations: It proposes a new mechanism by which the
DDR2-Hippo pathway regulates ferroptosis, and also explains that,
although high expression of DDR2 promotes breast cancer recurrence,
it also enhances the sensitivity of tumor cells to ferroptosis,
thus providing a new idea for combined targeted therapy. Moreover,
it identifies a new axis of immune resistance in liver cancer,
observing that the DDR2/STAT3/PD-L1 positive feedback loop mediates
oxaliplatin resistance. It also describes that the recruitment of
MDSC by CCL20 creates an immunosuppressive microenvironment that
promotes immune escape; v) the present review identifies a new
target of stromal cell, revealing that in CAFs DDR2 regulates POSTN
through ITGB1 to promote ovarian cancer metastasis, and also
highlights the therapeutic value of targeting the
DDR2-POSTN-PI3K/Akt axis; and vi) the present review focuses on
clinical transformation and unsolved issues by associating DDR2
with chemotherapy resistance (such as cisplatin resistance in
ovarian cancer and sorafenib resistance in liver cancer), proposing
new pathways (such as DDR2/NF-κB/c-Rel signaling) and guiding the
design of combination therapy; and proposing the combination of
DDR2 inhibitors and PD-1/PD-L1 antibodies to reverse ‘cold tumors’
(such as breast cancer) and exploring its potential to affect
T-cell infiltration by regulating collagen arrangement. In summary,
the primary strength of the present review lies in its integration
of multi-cancer clinical data, systematic analysis of drug
resistance mechanisms and comprehensive review of current
therapeutic developments targeting DDR2. It addresses the gaps in
cancer-type coverage in other reviews and aligns more closely with
the practical needs of clinicians and researchers in translational
medicine. Although available studies on DDR2 emphasize mechanistic
explorations, the present review broadens the scope by
incorporating clinical data and cross-cancer analyses, thereby
expanding potential applications. The information provided in the
present review offers notable practical value for clinical
decision-making. Furthermore, the review complements other
investigations in the field and contributes to advancing DDR2
research in both basic science and clinical settings.
 | Table V.Innovative summary. |
Table V.
Innovative summary.
| First author,
year | Research focus | Type of cancer | Mechanism | Treatment
strategy | Data source | (Refs.) |
|---|
| Trono et al,
2024 | Molecular mechanism
of DDR2, micro-environment remodeling and the synergy of
immunotherapy | Breast and ovarian
cancer | DDR2-collagen
positive feedback loop; formation mechanism of invasive
pseudopodia; andkinase-independent function | Novel inhibitor
(WRG-28) and combined immunotherapy | Original
experimental data integration and cBioPortal mutation analysis | (119) |
| Present study | Cancer-specific
effects, clinical transformation and drug resistance mechanisms of
DDR2 | >12 types of
cancer, e.g., gastric cancer, colorectal cancer and hepatocellular
carcinoma | EMT and drug
resistance pathways (STAT3/PD-L1) | Existing drug
applications (dasatinib) and antibody development | Public databases
(TIMER/HPA) and literature reviews | - |
Conclusions and perspectives
Several studies have reported that DDR2 serves
different pivotal roles in numerous types of solid tumor,
especially through ECM and CAFs. It also participates in multiple
mechanisms to promote tumor metastasis and drug resistance. Both
ECM and CAFs, as well as EMT, are a major focus of tumor research.
As the research on DDR2 progresses, biological processes involved
in tumor occurrence and development are likely to be revealed. In
addition, as the potential of DDR2 inhibitors continues to be
investigated through drug development and clinical trials, new
perspectives are likely to emerge in the treatment of tumors.
The present article offers a comprehensive and
insightful review of DDR2 as a promising target in solid tumors. It
examines key aspects, including the structure and function of DDR2,
its expression in several tissues and tumors, and its role in the
tumorigenesis of different types of cancer, such as ovarian, breast
and stomach cancers. This broad coverage is useful for researchers
looking for a comprehensive resource for DDR2 in solid tumors.
Additionally, it bridges the gap between basic research and
clinical application by assessing the current global progress on
DDR2 drugs and antibodies, which is crucial for translating
laboratory findings into potential cancer treatments. Finally, the
present review provides potential new directions for the pursuit of
effective cancer treatments through DDR2-targeted strategies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Regional Science
Foundation Project of the National Natural Science Foundation of
China (grant nos. 82160111 and 82360115) and the Targeted
Exploration Project of the Medical Department of Lanzhou University
(grant no. lzuyxcx-2022-181).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
TL designed and wrote the article and searched for
relevant literature. HGu revised the article and searched for
relevant literature. HGo and YM refined the language. YT and DZ
revised the article. TL and DZ confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li D, Cao D, Sun Y, Cui Y, Zhang Y, Jiang
J and Cao X: The roles of epigallocatechin gallate in the tumor
microenvironment, metabolic reprogramming, and immunotherapy. Front
Immunol. 15:13316412024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49.
2024.PubMed/NCBI
|
|
3
|
Lane DS and Smith RA: Cancer screening:
Patient and population strategies. Med Clin North Am. 107:989–999.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mamun TI, Younus S and Rahman MH: Gastric
cancer-Epidemiology, modifiable and non-modifiable risk factors,
challenges and opportunities: An updated review. Cancer Treat Res
Commun. 41:1008452024.PubMed/NCBI
|
|
5
|
Alessa AM and Khan AS: Epidemiology of
colorectal cancer in Saudi Arabia: A review. Cureus.
16:e645642024.PubMed/NCBI
|
|
6
|
Strzelec B, Chmielewski PP and Kielan W:
Esophageal cancer: Current status and new insights from
inflammatory markers-a brief review. Pol Przegl Chir. 96:83–87.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liang Z, Zheng X, Li M and Liu M:
Improving the prognosis of pancreatic cancer: Insights from
epidemiology, genomic alterations, and therapeutic challenges.
Front Med. 17:1135–1169. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Webb PM and Jordan SJ: Global epidemiology
of epithelial ovarian cancer. Nat Rev Clin Oncol. 21:389–400. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gu Y, Mu Q and Cheng D: Androgens in
cervical cancer: Their role in epidemiology and biology. iScience.
27:1101552024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cirillo L, Innocenti S and Becherucci F:
Global epidemiology of kidney cancer. Nephrol Dial Transplant.
39:920–928. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thomas A, Douglas E, Reis-Filho JS, Gurcan
MN and Wen HY: Metaplastic breast cancer: Current understanding and
future directions. Clin Breast Cancer. 23:775–783. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hasson RM, Bridges CJ, Curley RJ and
Erhunmwunsee L: Access to lung cancer screening. Thorac Surg Clin.
33:353–363. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Culp MB, Soerjomataram I, Efstathiou JA,
Bray F and Jemal A: Recent global patterns in prostate cancer
incidence and mortality rates. Eur Urol. 77:38–52. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L, Feng Q, Wang J, Tan Z, Li Q and
Ge M: Molecular basis and targeted therapy in thyroid cancer:
Progress and opportunities. Biochim Biophys Acta Rev Cancer.
1878:1889282023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Connal S, Cameron JM, Sala A, Brennan PM,
Palmer DS, Palmer JD, Perlow H and Baker MJ: Liquid biopsies: The
future of cancer early detection. J Transl Med. 21:1182023.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou Y, Tao L, Qiu J, Xu J, Yang X, Zhang
Y, Tian X, Guan X, Cen X and Zhao Y: Tumor biomarkers for
diagnosis, prognosis and targeted therapy. Signal Transduct Target
Ther. 9:1322024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Waarts MR, Stonestrom AJ, Park YC and
Levine RL: Targeting mutations in cancer. J Clin Invest.
132:e1549432022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Passaro A, Al Bakir M, Hamilton EG, Diehn
M, André F, Roy-Chowdhuri S, Mountzios G, Wistuba II, Swanton C and
Peters S: Cancer biomarkers: Emerging trends and clinical
implications for personalized treatment. Cell. 187:1617–1635. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zaidi SA, Shahzad F and Batool S: Progress
in cancer biomarkers monitoring strategies using graphene modified
support materials. Talanta. 210:1206692020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen L, Kong X, Fang Y, Paunikar S, Wang
X, Brown JAL, Bourke E, Li X and Wang J: Recent advances in the
role of discoidin domain receptor tyrosine kinase 1 and discoidin
domain receptor tyrosine kinase 2 in breast and ovarian cancer.
Front Cell Dev Biol. 9:7473142021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Agarwal G, Smith AW and Jones B: Discoidin
domain receptors: Micro insights into macro assemblies. Biochim
Biophys Acta Mol Cell Res. 1866:1184962019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Elkamhawy A, Lu Q, Nada H, Woo J, Quan G
and Lee K: The Journey of DDR1 and DDR2 kinase inhibitors as rising
stars in the fight against cancer. Int J Mol Sci. 22:65352021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mariadoss AVA and Wang CZ: Exploring the
cellular and molecular mechanism of discoidin domain receptors
(DDR1 and DDR2) in bone formation, regeneration, and its associated
disease conditions. Int J Mol Sci. 24:148952023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zeltz C, Kusche-Gullberg M, Heljasvaara R
and Gullberg D: Novel roles for cooperating collagen receptor
families in fibrotic niches. Curr Opin Cell Biol. 85:1022732023.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao Y, Zhou J and Li J: Discoidin domain
receptors orchestrate cancer progression: A focus on cancer
therapies. Cancer Sci. 112:962–969. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shimizu T, Kato Y, Sakai Y, Hisamoto N and
Matsumoto K: N-Glycosylation of the Discoidin domain receptor is
required for axon regeneration in caenorhabditis elegans. Genetics.
213:491–500. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Olaso E, Ikeda K, Eng FJ, Xu L, Wang LH,
Lin HC and Friedman SL: DDR2 receptor promotes MMP-2-mediated
proliferation and invasion by hepatic stellate cells. J Clin
Invest. 108:1369–1378. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Juurikka K, Butler GS, Salo T, Nyberg P
and Åström P: The role of MMP8 in cancer: A systematic review. Int
J Mol Sci. 20:45062019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gong H, Xu HM and Zhang DK: Focusing on
discoidin domain receptors in premalignant and malignant liver
diseases. Front Oncol. 13:11236382023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schab AM, Greenwade MM, Stock E,
Lomonosova E, Cho K, Grither WR, Noia H, Wilke D, Mullen MM,
Hagemann AR, et al: Stromal DDR2 promotes ovarian cancer metastasis
through regulation of metabolism and secretion of extracellular
matrix proteins. Mol Cancer Res. 21:1234–1248. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu C, Ying J, Dai M, Peng J and Zhang D:
Co-expression of DDR2 and IFITM1 promotes breast cancer cell
proliferation, migration and invasion and inhibits apoptosis. J
Cancer Res Clin Oncol. 148:3385–3398. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fathi Z, Mousavi SAJ, Roudi R and Ghazi F:
Distribution of KRAS, DDR2, and TP53 gene mutations in lung cancer:
An analysis of Iranian patients. PLoS One. 13:e02006332018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun M and Shen Z: Knockdown of long
non-coding RNA (lncRNA) Colon cancer-associated transcript-1
(CCAT1) suppresses oral squamous cell carcinoma proliferation,
invasion, and migration by inhibiting the discoidin domain receptor
2 (DDR2)/ERK/AKT Axis. Med Sci Monit. 26:e9200202020.PubMed/NCBI
|
|
34
|
Sideris M, Menon U and Manchanda R:
Screening and prevention of ovarian cancer. Med J Aust.
220:264–274. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Veneziani AC, Gonzalez-Ochoa E, Alqaisi H,
Madariaga A, Bhat G, Rouzbahman M, Sneha S and Oza AM:
Heterogeneity and treatment landscape of ovarian carcinoma. Nat Rev
Clin Oncol. 20:820–842. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schab AM, Greenwade MM, Stock E,
Lomonosova E, Cho K, Grither WR, Noia H, Wilke D, Mullen MM,
Hagemann AR, et al: Stromal DDR2 promotes ovarian cancer metastasis
through regulation of metabolism and secretion of extracellular
matrix proteins. Mol Cancer Res. 21:1234–1248. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yuan WC, Zhang JX, Chen HB, Yuan Y, Zhuang
YP, Zhou HL, Li MH, Qiu WL and Zhou HG: A bibliometric and visual
analysis of cancer-associated fibroblasts. Front Immunol.
14:13231152023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Akinjiyan FA, Ibitoye Z, Zhao P, Shriver
LP, Patti GJ, Longmore GD and Fuh KC: DDR2-regulated arginase
activity in ovarian cancer-associated fibroblasts promotes collagen
production and tumor progression. Oncogene. 43:189–201. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Arumi-Planas M, Rodriguez-Baena FJ,
Cabello-Torres F, Gracia F, Lopez-Blau C, Nieto MA and
Sanchez-Laorden B: Microenvironmental Snail1-induced
immunosuppression promotes melanoma growth. Oncogene. 42:2659–2672.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Baulida J and García de Herreros A:
Snail1-driven plasticity of epithelial and mesenchymal cells
sustains cancer malignancy. Biochim Biophys Acta. 1856:55–61.
2015.PubMed/NCBI
|
|
41
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li F, Zheng Z, Chen W, Li D, Zhang H, Zhu
Y, Mo Q, Zhao X, Fan Q, Deng F, et al: Regulation of cisplatin
resistance in bladder cancer by epigenetic mechanisms. Drug Resist
Updat. 68:1009382023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Heiserman JP, Nallanthighal S, Gifford CC,
Graham K, Samarakoon R, Gao C, Sage JJ, Zhang W, Higgins PJ and
Cheon DJ: Heat shock protein 27, a novel downstream target of
collagen type XI alpha 1, synergizes with fatty acid oxidation to
confer cisplatin resistance in ovarian cancer cells. Cancers
(Basel). 13:48552021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang L, Hu Q and Huang T: Breast cancer
treatment strategies targeting the tumor microenvironment: How to
Convert ‘Cold’ Tumors to ‘Hot’ Tumors. Int J Mol Sci. 25:72082024.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Corsa CA, Brenot A, Grither WR, Van Hove
S, Loza AJ, Zhang K, Ponik SM, Liu Y, DeNardo DG, Eliceiri KW, et
al: The action of discoidin domain receptor 2 in basal tumor cells
and stromal Cancer-associated fibroblasts is critical for breast
cancer metastasis. Cell Rep. 15:2510–2523. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lin CC, Yang WH, Lin YT, Tang X, Chen PH,
Ding CC, Qu DC, Alvarez JV and Chi JT: DDR2 upregulation confers
ferroptosis susceptibility of recurrent breast tumors through the
Hippo pathway. Oncogene. 40:2018–2034. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhou Q, Meng Y, Li D, Yao L, Le J, Liu Y,
Sun Y, Zeng F, Chen X and Deng G: Ferroptosis in cancer: From
molecular mechanisms to therapeutic strategies. Signal Transduct
Target Ther. 9:552024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Carvalho FM: Triple-negative breast
cancer: From none to multiple therapeutic targets in two decades.
Front Oncol. 13:12447812023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Varzaru VB, Vlad T, Popescu R, Vlad CS,
Moatar AE and Cobec IM: Triple-negative breast cancer: Molecular
particularities still a challenge. Diagnostics (Basel).
14:18752024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Toy KA, Valiathan RR, Núñez F, Kidwell KM,
Gonzalez ME, Fridman R and Kleer CG: Tyrosine kinase discoidin
domain receptors DDR1 and DDR2 are coordinately deregulated in
triple-negative breast cancer. Breast Cancer Res Treat. 150:9–18.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rao X, Zhang C, Luo H, Zhang J, Zhuang Z,
Liang Z and Wu X: Targeting gastric cancer stem cells to enhance
treatment response. Cells. 11:28282022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li Z, Wang J, Wang Z and Xu Y: Towards an
optimal model for gastric cancer peritoneal metastasis: Current
challenges and future directions. EBioMedicine. 92:1046012023.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yao X, Ajani JA and Song S: Molecular
biology and immunology of gastric cancer peritoneal metastasis.
Transl Gastroenterol Hepatol. 5:572020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kurashige J, Hasegawa T, Niida A,
Sugimachi K, Deng N, Mima K, Uchi R, Sawada G, Takahashi Y, Eguchi
H, et al: Integrated molecular profiling of human gastric cancer
identifies DDR2 as a potential regulator of peritoneal
dissemination. Sci Rep. 6:223712016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang YG, Xu L, Jia RR, Wu Q, Wang T, Wei
J, Ma JL, Shi M and Li ZS: DDR2 induces gastric cancer cell
activities via activating mTORC2 signaling and is associated with
clinicopathological characteristics of gastric cancer. Dig Dis Sci.
61:2272–2283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Firouzjaei AA, Aghaee-Bakhtiari SH, Tafti
A, Sharifi K, Abadi MHJN, Rezaei S and Mohammadi-Yeganeh S: Impact
of curcumin on ferroptosis-related genes in colorectal cancer:
Insights from in-silico and in-vitro studies. Cell Biochem Funct.
41:1488–1502. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ruff SM, Brown ZJ and Pawlik TM: A review
of targeted therapy and immune checkpoint inhibitors for metastatic
colorectal cancer. Surg Oncol. 51:1019932023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lafitte M, Sirvent A and Roche S: Collagen
kinase receptors as potential therapeutic targets in metastatic
colon cancer. Front Oncol. 10:1252020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Beauchemin N: The colorectal tumor
microenvironment: The next decade. Cancer Microenviron. 4:181–185.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Xu X, Duan X, Wang S, Zhang Y, Gao Y, Xu
X, Yeerkenbieke G, Zhou J and Li J: Special issue ‘The advance of
solid tumor research in China’: Discoidin domain receptor 2
promotes colorectal cancer metastasis by regulating epithelial
mesenchymal transition via activating AKT signaling. Int J Cancer.
152:51–65. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chidambaranathan-Reghupaty S, Fisher PB
and Sarkar D: Hepatocellular carcinoma (HCC): Epidemiology,
etiology and molecular classification. Adv Cancer Res. 149:1–61.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Piñero F, Dirchwolf M and Pessôa MG:
Biomarkers in hepatocellular carcinoma: Diagnosis, prognosis and
treatment response assessment. Cells. 9:13702020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wu L, Zhao X, Ma H, Zhang L and Li X:
Discoidin domain receptor 1, a potential biomarker and therapeutic
target in hepatocellular carcinoma. Int J Gen Med. 15:2037–2044.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Park JW, Lee YS, Kim JS, Lee SK, Kim BH,
Lee JA, Lee NO, Kim SH and Hong EK: Downregulation of discoidin
domain receptor 2 decreases tumor growth of hepatocellular
carcinoma. J Cancer Res Clin Oncol. 141:1973–1983. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Xie B, Lin W, Ye J, Wang X, Zhang B, Xiong
S, Li H and Tan G: DDR2 facilitates hepatocellular carcinoma
invasion and metastasis via activating ERK signaling and
stabilizing SNAIL1. J Exp Clin Cancer Res. 34:1012015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Tang Q, Chen Y, Li X, Long S, Shi Y, Yu Y,
Wu W, Han L and Wang S: The role of PD-1/PD-L1 and application of
immune-checkpoint inhibitors in human cancers. Front Immunol.
13:9644422022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hao L, Li S and Deng J: The current status
and future of PD-L1 in liver cancer. Front Immunol. 14:13235812023.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wen Q, Han T, Wang Z and Jiang S: Role and
mechanism of programmed death-ligand 1 in hypoxia-induced liver
cancer immune escape. Oncol Lett. 19:2595–2601. 2020.PubMed/NCBI
|
|
69
|
Kang L, Tian Y, Xu S and Chen H:
Oxaliplatin-induced peripheral neuropathy: Clinical features,
mechanisms, prevention and treatment. J Neurol. 268:3269–3282.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu W, Zhang F, Quan B, Yao F, Chen R, Ren
Z, Dong L and Yin X: DDR2/STAT3 positive feedback loop mediates the
immunosuppressive microenvironment by upregulating PD-L1 and
recruiting MDSCs in Oxaliplatin-resistant HCC. Cell Mol
Gastroenterol Hepatol. 18:1013772024. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Li K, Shi H, Zhang B, Ou X, Ma Q, Chen Y,
Shu P, Li D and Wang Y: Myeloid-derived suppressor cells as
immunosuppressive regulators and therapeutic targets in cancer.
Signal Transduct Target Ther. 6:3622021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Li Q, Chen K, Zhang T, Jiang D, Chen L,
Jiang J, Zhang C and Li S: Understanding sorafenib-induced
ferroptosis and resistance mechanisms: Implications for cancer
therapy. Eur J Pharmacol. 955:1759132023. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tang W, Chen Z, Zhang W, Cheng Y, Zhang B,
Wu F, Wang Q, Wang S, Rong D, Reiter FP, et al: The mechanisms of
sorafenib resistance in hepatocellular carcinoma: Theoretical basis
and therapeutic aspects. Signal Transduct Target Ther. 5:872020.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Liu QQ, Liu YW, Xie YK, Zhang JH, Song CX,
Wang JZ and Xie BH: Amplification of DDR2 mediates sorafenib
resistance through NF-κB/c-Rel signaling in hepatocellular
carcinoma. Cell Biol Int. 45:1906–1916. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Cai Y, Lyu T, Li H, Liu C, Xie K, Xu L, Li
W, Liu H, Zhu J, Lyu Y, et al: LncRNA CEBPA-DT promotes liver
cancer metastasis through DDR2/β-catenin activation via interacting
with hnRNPC. J Exp Clin Cancer Res. 41:3352022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Chung C, Boterberg T, Lucas J, Panoff J,
Valteau-Couanet D, Hero B, Bagatell R and Hill-Kayser CE:
Neuroblastoma. Pediatr Blood Cancer. 68 (Suppl 2):e284732021.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Vessella T, Xiang S, Xiao C, Stilwell M,
Fok J, Shohet J, Rozen E, Zhou HS and Wen Q: DDR2 signaling and
mechanosensing orchestrate neuroblastoma cell fate through
different transcriptome mechanisms. FEBS Open Bio. 14:867–882.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Karam JA, Msaouel P, Haymaker CL, Matin
SF, Campbell MT, Zurita AJ, Shah AY, Wistuba II, Marmonti E, Duose
DY, et al: Phase II trial of neoadjuvant sitravatinib plus
nivolumab in patients undergoing nephrectomy for locally advanced
clear cell renal cell carcinoma. Nat Commun. 14:26842023.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Rozen EJ, Frantz W, Wigglesworth K,
Vessella T, Zhou HS and Shohet JM: Blockade of discoidin domain
receptor signaling with sitravatinib reveals DDR2 as a mediator of
neuroblastoma pathogenesis and metastasis. Mol Cancer Ther.
23:1124–1138. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Agosto Salgado S, Kaye ER, Sargi Z, Chung
CH and Papaleontiou M: Management of advanced thyroid cancer:
Overview, advances, and opportunities. Am Soc Clin Oncol Educ Book.
43:e3897082023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lam AK: Papillary thyroid carcinoma:
Current position in epidemiology, genomics, and classification.
Methods Mol Biol. 2534:1–15. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Liang Z, Xie WJ, Zhao M, Cheng GP and Wu
MJ: DDR2 facilitates papillary thyroid carcinoma epithelial
mesenchymal transition by activating ERK2/Snail1 pathway. Oncol
Lett. 14:8114–8121. 2017.PubMed/NCBI
|
|
83
|
Wilson TK and Zishiri OT: Prostate cancer:
A review of genetics, current biomarkers and personalised
treatments. Cancer Rep (Hoboken). 7:e700162024.PubMed/NCBI
|
|
84
|
Nevo A, Navaratnam A and Andrews P:
Prostate cancer and the role of biomarkers. Abdom Radiol (NY).
45:2120–2132. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Azemikhah M, Ashtiani HA, Aghaei M and
Rastegar H: Evaluation of discoidin domain receptor-2 (DDR2)
expression level in normal, benign, and malignant human prostate
tissues. Res Pharm Sci. 10:356–363. 2015.PubMed/NCBI
|
|
86
|
Huang RH, Ge ZL, Xu G, Zeng QM, Jiang B,
Xiao GC, Xia W, Wu YT and Liao YF: Prognosis and diagnosis of
prostate cancer based on hypergraph regularization sparse least
partial squares regression algorithm. Aging (Albany NY).
16:9599–9624. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yan Z, Jin S, Wei Z, Huilian H, Zhanhai Y,
Yue T, Juan L, Jing L, Libo Y and Xu L: Discoidin domain receptor 2
facilitates prostate cancer bone metastasis via regulating
parathyroid hormone-related protein. Biochim Biophys Acta.
1842:1350–1363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Tang G, Liu J, Qi L and Li Y: The evolving
role of checkpoint inhibitors in the treatment of urothelial
carcinoma. Br J Clin Pharmacol. 89:93–113. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Tsai MC, Li WM, Huang CN, Ke HL, Li CC,
Yeh HC, Chan TC, Liang PI, Yeh BW, Wu WJ, et al: DDR2
overexpression in urothelial carcinoma indicates an unfavorable
prognosis: A large cohort study. Oncotarget. 7:78918–78931. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Cario M: DDR1 and DDR2 in skin. Cell Adh
Migr. 12:386–393. 2018.PubMed/NCBI
|
|
91
|
Sala M, Allain N, Moreau M, Jabouille A,
Henriet E, Abou-Hammoud A, Uguen A, Di-Tommaso S, Dourthe C,
Raymond AA, et al: Discoidin domain receptor 2 orchestrates
melanoma resistance combining phenotype switching and
proliferation. Oncogene. 41:2571–2586. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Poudel B, Lee YM and Kim DK: DDR2
inhibition reduces migration and invasion of murine metastatic
melanoma cells by suppressing MMP2/9 expression through ERK/NF-κB
pathway. Acta Biochim Biophys Sin (Shanghai). 47:292–298. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Xiao Y and Yu D: Tumor microenvironment as
a therapeutic target in cancer. Pharmacol Ther. 221:1077532021.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Fu X, He Y, Li M, Huang Z and Najafi M:
Targeting of the tumor microenvironment by curcumin. Biofactors.
47:914–932. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Huang J, Zhang L, Wan D, Zhou L, Zheng S,
Lin S and Qiao Y: Extracellular matrix and its therapeutic
potential for cancer treatment. Signal Transduct Target Ther.
6:1532021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Jiang Y, Zhang H, Wang J, Liu Y, Luo T and
Hua H: Targeting extracellular matrix stiffness and
mechanotransducers to improve cancer therapy. J Hematol Oncol.
15:342022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yuan Z, Li Y, Zhang S, Wang X, Dou H, Yu
X, Zhang Z, Yang S and Xiao M: Extracellular matrix remodeling in
tumor progression and immune escape: From mechanisms to treatments.
Mol Cancer. 22:482023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Prakash J and Shaked Y: The Interplay
between extracellular matrix remodeling and cancer therapeutics.
Cancer Discov. 14:1375–1388. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Borza CM and Pozzi A: Discoidin domain
receptors in disease. Matrix Biol. 34:185–192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chen Y, McAndrews KM and Kalluri R:
Clinical and therapeutic relevance of cancer-associated
fibroblasts. Nat Rev Clin Oncol. 18:792–804. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhang H, Yue X, Chen Z, Liu C, Wu W, Zhang
N, Liu Z, Yang L, Jiang Q, Cheng Q, et al: Define cancer-associated
fibroblasts (CAFs) in the tumor microenvironment: New opportunities
in cancer immunotherapy and advances in clinical trials. Mol
Cancer. 22:1592023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wright K, Ly T, Kriet M, Czirok A and
Thomas SM: Cancer-associated fibroblasts: Master tumor
microenvironment modifiers. Cancers (Basel). 15:18992023.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yamamoto Y, Kasashima H, Fukui Y, Tsujio
G, Yashiro M and Maeda K: The heterogeneity of cancer-associated
fibroblast subpopulations: Their origins, biomarkers, and roles in
the tumor microenvironment. Cancer Sci. 114:16–24. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Akinjiyan FA, Dave RM, Alpert E, Longmore
GD and Fuh KC: DDR2 expression in Cancer-associated fibroblasts
promotes ovarian cancer tumor invasion and metastasis through
periostin-ITGB1. Cancers (Basel). 14:34822022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Barcus CE, Hwang PY, Morikis V, Brenot A,
Pence P, Clarke M and Longmore GD: Tyrosine kinase-independent
actions of DDR2 in tumor cells and cancer-associated fibroblasts
influence tumor invasion, migration and metastasis. J Cell Sci.
134:jcs2584312021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Tang T, Huang X, Zhang G, Hong Z, Bai X
and Liang T: Advantages of targeting the tumor immune
microenvironment over blocking immune checkpoint in cancer
immunotherapy. Signal Transduct Target Ther. 6:722021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Sun Q, Hong Z, Zhang C, Wang L, Han Z and
Ma D: Immune checkpoint therapy for solid tumours: Clinical
dilemmas and future trends. Signal Transduct Target Ther.
8:3202023. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Labrie M, Brugge JS, Mills GB and
Zervantonakis IK: Therapy resistance: Opportunities created by
adaptive responses to targeted therapies in cancer. Nat Rev Cancer.
22:323–339. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Rammal H, Saby C, Magnien K, Van-Gulick L,
Garnotel R, Buache E, El Btaouri H, Jeannesson P and Morjani H:
Discoidin domain receptors: Potential actors and targets in cancer.
Front Pharmacol. 7:552016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Reger de Moura C, Prunotto M, Sohail A,
Battistella M, Jouenne F, Marbach D, Lebbé C, Fridman R and Mourah
S: Discoidin domain receptors in melanoma: Potential therapeutic
targets to overcome MAPK inhibitor resistance. Front Oncol.
10:17482020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Lv B, Wang Y, Ma D, Cheng W, Liu J, Yong
T, Chen H and Wang C: Immunotherapy: Reshape the tumor immune
microenvironment. Front Immunol. 13:8441422022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Fontana R, Mestre-Farrera A and Yang J:
Update on Epithelial-Mesenchymal plasticity in cancer progression.
Annu Rev Pathol. 19:133–156. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Pharnexcloud Cloud database: CIDD-0108633.
https://data.pharnexcloud.com/1/detail/44/b112ca4087d668785e947a57493d1740?detailTitle=CIDD-0108633November
10–2024
|
|
114
|
Pharnexcloud Cloud database ICP-033.
https://data.pharnexcloud.com/1/detail/44/06d801cb636235b298c40029ad9921e7?detailTitle=ICP-033November
10–2024
|
|
115
|
Pharnexcloud Cloud database BK-40143.
https://data.pharnexcloud.com/1/detail/44/654784daf0b133e42d02214b22cb03a6?detailTitle=BK-40143November
10–2024
|
|
116
|
Pharnexcloud Cloud database PB-1.
https://data.pharnexcloud.com/1/detail/44/6368349d3319f374ddfd35dfd477ea29?detailTitle=PB-1November
10–2024
|
|
117
|
Pharnexcloud Cloud database
Dual-DDR1/2inhibitors. https://data.pharnexcloud.com/1/detail/44/06c9c2f149b73e46fba1487930c5acb8?detailTitle=dual%20DDR-1%2F2%20inhibitors%20%28acute%20lung%20injury%2Finflammation%29November
10–2024
|
|
118
|
Dasatinib. https://data.pharnexcloud.com/1/detail/44/0245952ecff55018e2a459517fdb40e3?detailTitle=dasatinibhttps://data.pharnexcloud.com/1/detail/44/0245952ecff55018e2a459517fdb40e3?detailTitle=dasatinibNovember
10–2024
|
|
119
|
Trono P, Ottavi F and Rosano' L: Novel
insights into the role of Discoidin domain receptor 2 (DDR2) in
cancer progression: A new avenue of therapeutic intervention.
Matrix Biol. 125:31–39. 2024. View Article : Google Scholar : PubMed/NCBI
|