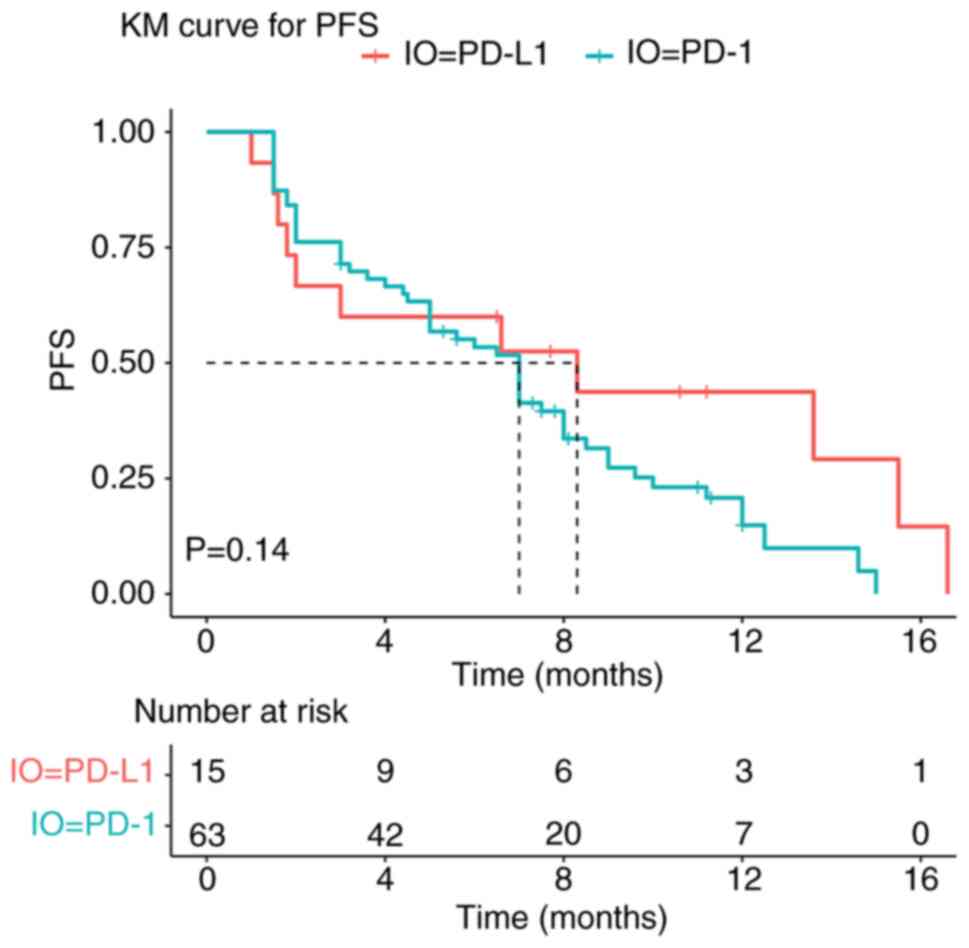
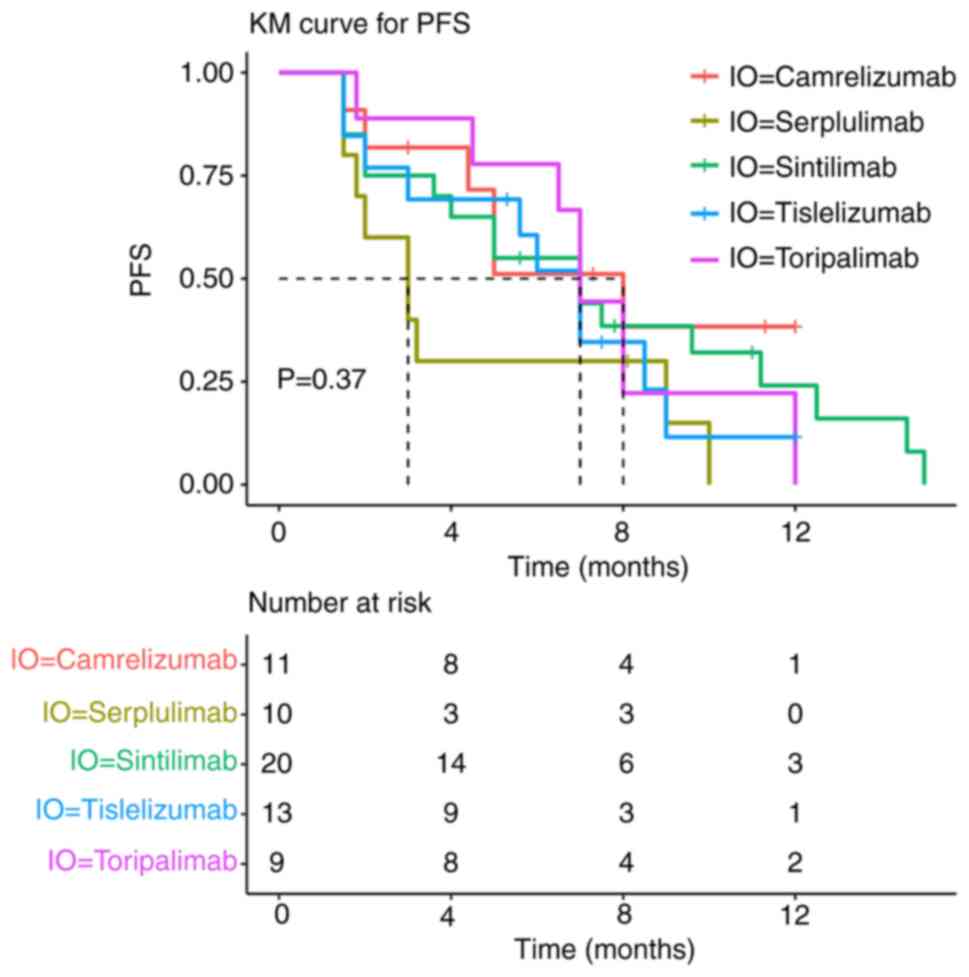
|
1
|
Han B, Zheng R, Zeng H, Wang S, Sun K,
Chen R, Li L, Wei W and He J: Cancer incidence and mortality in
China, 2022. J Natl Cancer Cent. 4:47–53. 2024.PubMed/NCBI
|
|
2
|
Lichtenstern CR, Ngu RK, Shalapour S and
Karin M: Immunotherapy, inflammation and colorectal cancer. Cells.
9:6182020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chinese Society Of Clinical Oncology Csco
Diagnosis and Treatment Guidelines For Colorectal Cancer Working
Group, . Chinese society of clinical oncology (CSCO) diagnosis and
treatment guidelines for colorectal cancer 2018 (english version).
Chin J Cancer Res. 31:117–134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Diaz LA Jr, Shiu KK, Kim TW, Jensen BV,
Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs
P, et al: Pembrolizumab versus chemotherapy for microsatellite
instability-high or mismatch repair-deficient metastatic colorectal
cancer (KEYNOTE-177): Final analysis of a randomised, open-label,
phase 3 study. Lancet Oncol. 23:659–670. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koopman M, Kortman GA, Mekenkamp L,
Ligtenberg MJ, Hoogerbrugge N, Antonini NF, Punt CJ and van Krieken
JH: Deficient mismatch repair system in patients with sporadic
advanced colorectal cancer. Br J Cancer. 100:266–273. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bever KM and Le DT: An expanding role for
immunotherapy in colorectal cancer. J Natl Compr Canc Netw.
15:401–410. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chalabi M, Fanchi LF, Dijkstra KK, Van den
Berg JG, Aalbers AG, Sikorska K, Lopez-Yurda M, Grootscholten C,
Beets GL, Snaebjornsson P, et al: Neoadjuvant immunotherapy leads
to pathological responses in MMR-proficient and MMR-deficient
early-stage colon cancers. Nat Med. 26:566–576. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tan AC, Bagley SJ, Wen PY, Lim M, Platten
M, Colman H, Ashley DM, Wick W, Chang SM, Galanis E, et al:
Systematic review of combinations of targeted or immunotherapy in
advanced solid tumors. J Immunother Cancer. 9:e0024592021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun Q, Zhou J, Zhang Z, Guo M, Liang J,
Zhou F, Long J, Zhang W, Yin F, Cai H, et al: Discovery of
fruquintinib, a potent and highly selective small molecule
inhibitor of VEGFR 1, 2, 3 tyrosine kinases for cancer therapy.
Cancer Biol Ther. 15:1635–1645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dasari A, Lonardi S, Garcia-Carbonero R,
Elez E, Yoshino T, Sobrero A, Yao J, García-Alfonso P, Kocsis J,
Cubillo Gracian A, et al: Fruquintinib versus placebo in patients
with refractory metastatic colorectal cancer (FRESCO-2): An
international, multicentre, randomised, double-blind, phase 3
study. Lancet. 402:41–53. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fusco MJ, Casak SJ, Mushti SL, Cheng J,
Christmas BJ, Thompson MD, Fu W, Wang H, Yoon M, Yang Y, et al: FDA
approval summary: fruquintinib for the treatment of refractory
metastatic colorectal cancer. Clin Cancer Res. 30:3100–3104. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Q, Cheng X, Zhou C, Tang Y, Li F, Zhang
B, Huang T, Wang J and Tu S: Fruquintinib enhances the antitumor
immune responses of anti-programmed death receptor-1 in colorectal
cancer. Front Oncol. 12:8419772022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo Y, Zhang W, Ying J, Zhang Y, Pan Y,
Qiu W, Fan Q, Xu Q, Ma Y, Wang G, et al: Phase 1b/2 trial of
fruquintinib plus sintilimab in treating advanced solid tumours:
The dose-escalation and metastatic colorectal cancer cohort in the
dose-expansion phases. Eur J Cancer. 181:26–37. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
An T, Lian Y, Zhou Q, Zhao C, Wang Z and
Zhao R: Fruquintinib with PD-1 inhibitors versus fruquintinib
monotherapy in late-line mCRC: A retrospective cohort study based
on propensity score matching. J Clin Oncol. 42 (Suppl 3):S1392024.
View Article : Google Scholar
|
|
16
|
Yang X, Yin X, Qu X, Guo G, Zeng Y, Liu W,
Jagielski M, Liu Z and Zhou H: Efficacy, safety, and predictors of
fruquintinib plus anti-programmed death receptor-1 (PD-1) antibody
in refractory microsatellite stable metastatic colorectal cancer in
a real-world setting: A retrospective cohort study. J Gastrointest
Oncol. 14:2425–2435. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gou M, Qian N, Zhang Y, Yan H, Si H, Wang
Z and Dai G: Fruquintinib in combination with PD-1 inhibitors in
patients with refractory non-MSI-H/pMMR metastatic colorectal
cancer: A real-world study in China. Front Oncol. 12:8517562022.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Freites-Martinez A, Santana N,
Arias-Santiago S and Viera A: Using the common terminology criteria
for adverse events (CTCAE-version 5.0) to evaluate the severity of
adverse events of anticancer therapies. Actas Dermosifiliogr (Engl
Ed). 112:90–92. 2021.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fukuoka S, Hara H, Takahashi N, Kojima T,
Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, et
al: Regorafenib plus nivolumab in patients with advanced gastric or
colorectal cancer: An open-label, dose-escalation, and
dose-expansion phase Ib trial (REGONIVO, EPOC1603). J Clin Oncol.
38:2053–2061. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seymour L, Bogaerts J, Perrone A, Ford R,
Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, et
al: iRECIST: Guidelines for response criteria for use in trials
testing immunotherapeutics. Lancet Oncol. 18:e143–e152. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yao JC, Shah MH, Ito T, Bohas CL, Wolin
EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG,
et al: Everolimus for advanced pancreatic neuroendocrine tumors. N
Engl J Med. 364:514–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bai Y, Xu N, An S, Chen W, Gao C and Zhang
D: A phase Ib trial of assessing the safety and preliminary
efficacy of a combination therapy of geptanolimab (GB 226) plus
fruquintinib in patients with metastatic colorectal cancer (mCRC).
J Clin Oncol. 39 (Suppl 15):e155512021. View Article : Google Scholar
|
|
26
|
Overman MJ, McDermott R, Leach JL, Lonardi
S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, et al:
Nivolumab in patients with metastatic DNA mismatch repair-deficient
or microsatellite instability-high colorectal cancer (CheckMate
142): An open-label, multicentre, phase 2 study. Lancet Oncol.
18:1182–1191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Andre T, Elez E, Van Cutsem E, Jensen LH,
Bennouna J, Mendez G, Schenker M, De la Fouchardière C, Limon MJ,
Yoshino T, et al: Nivolumab (NIVO) plus ipilimumab (IPI) vs
chemotherapy (chemo) as first-line (1L) treatment for
microsatellite instability-high/mismatch repair-deficient
(MSI-H/dMMR) metastatic colorectal cancer (mCRC): First results of
the CheckMate 8HW study. J Clin Oncol. 42 (Suppl 3):LBA7682024.
View Article : Google Scholar
|
|
28
|
Bullock A, Grossman J, Fakih M, Lenz H,
Gordon M, Margolin K, Wilky B, Mahadevan D, Trent J, Bockorny B, et
al: LBA O-9 Botensilimab, a novel innate/adaptive immune activator,
plus balstilimab (anti-PD-1) for metastatic heavily pretreated
microsatellite stable colorectal cancer. Ann Oncol. 33 (Suppl
4):S3762022. View Article : Google Scholar
|
|
29
|
Waterhouse DM, Garon EB, Chandler J,
McCleod M, Hussein M, Jotte R, Horn L, Daniel DB, Keogh G, Creelan
B, et al: Continuous versus 1-year fixed-duration nivolumab in
previously treated advanced non-small-cell lung cancer: CheckMate
153. J Clin Oncol. 38:3863–3873. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Andre T, Shiu KK, Kim TW, Jensen BV,
Jensen LH, Punt CJA, Smith DM, Garcia-Carbonero R, Benavides M
Gibbs OP, et al: Pembrolizumab versus chemotherapy for
microsatellite instability-high/mismatch repair deficient
metastatic colorectal cancer: The phase 3 KEYNOTE-177 study. J Clin
Oncol. 38 (Suppl 18):LBA42020. View Article : Google Scholar
|
|
31
|
Fakih M, Raghav KPS, Chang DZ, Bendell JC,
Larson T, Cohn AL, Huyck TK, Cosgrove D, Fiorillo JA, Garbo LE, et
al: Single-arm, phase 2 study of regorafenib plus nivolumab in
patients with mismatch repair-proficient (pMMR)/microsatellite
stable (MSS) colorectal cancer (CRC). J Clin Oncol. 39 (Suppl
15):S35602021. View Article : Google Scholar
|
|
32
|
Albertsmeier M, Riedl K, Stephan AJ, Drefs
M, Schiergens TS, Engel J, Angele MK, Werner J and Guba M: Improved
survival after resection of colorectal liver metastases in patients
with unresectable lung metastases. HPB (Oxford). 22:368–375. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Loupakis F, Cremolini C, Masi G, Lonardi
S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi
R, et al: Initial therapy with FOLFOXIRI and bevacizumab for
metastatic colorectal cancer. N Engl J Med. 371:1609–1618. 2014.
View Article : Google Scholar : PubMed/NCBI
|