Introduction
Colorectal cancer (CRC) is one of the most common
malignancies. According to the 2022 cancer report in China, CRC
remains the second most prevalent cancer and ranks fourth in
cancer-associated mortalities (1).
Despite advances in diagnostic technologies and the ongoing
development of therapeutic strategies, including chemotherapy and
targeted therapy, the prognosis for metastatic CRC (mCRC) remains
poor with a 5-year survival rate of <15% (2). Due to the limited options for
later-line treatment and the deterioration of the physical
condition of patients following multiple treatment lines,
identifying effective, low-toxicity therapy for subsequent lines
remains a major focus of current research. At present, the standard
later-line treatments recommended by the Chinese Society of
Clinical Oncology Diagnosis and Treatment Guidelines include
fruquintinib, regorafenib and TAS-102 (3).
Immune checkpoint inhibitors (ICIs), such as
programmed death-1 (PD-1) inhibitors, have recently transformed the
landscape of clinical oncology. The KEYNOTE-016 study demonstrated
that pembrolizumab provides durable antitumor activity and fewer
treatment-related adverse events (AEs) supporting it as an
efficacious first-line therapy in patients with microsatellite
instability-high (MSI-H) or mismatch repair-deficient mCRC
(4). However, ~95% of patients with
mCRC are classified as microsatellite-stable (MSS)/mismatch repair
proficient (pMMR), characterized by a tumor microenvironment with
limited immune cell infiltration, which results in suboptimal
efficacy of immunotherapy monotherapy (5–8).
Therefore, improving survival outcomes in the MSS/pMMR population
remains a notable challenge in the management of mCRC.
At present, the primary challenge in treatment is
how to modify therapeutic strategies to augment the sensitivity of
patients with MSS-type mCRC to immunotherapy, thereby improving
prognosis. Emerging evidence demonstrates that the combination of
vascular endothelial growth factor receptor (VEGFR) inhibitors with
ICIs has a synergistic antitumor effect (9). Fruquintinib, a highly selective
tyrosine kinase inhibitor (TKI) developed independently in China,
specifically targets VEGFR-1, VEGFR-2 and VEGFR-3, effectively
suppressing tumor angiogenesis (10). The global, multicenter FRESCO-2
trial demonstrated that fruquintinib significantly improved
outcomes compared with the placebo, with a median progression-free
survival (PFS) of 3.7 months and a median overall survival (OS) of
7.4 months (11). Based on these
findings, fruquintinib was approved by the US. Food and Drug
Administration (FDA) for the treatment of recurrent mCRC on
November 8, 2023 (12).
Moreover, the combination of fruquintinib and ICIs
has shown promising therapeutic potential for CRC in both
preclinical and clinical studies. Li et al (13) demonstrated that the combination of
fruquintinib and sintilimab more effectively inhibited the growth
of colorectal tumors and significantly extended survival compared
with monotherapy with either agent in a mouse xenograft model.
Mechanistically, this combination therapy targeted tumor
angiogenesis and modulated the tumor immune microenvironment,
enhancing T-cell infiltration and thereby suppressing tumor
progression. The findings of a study reported by Li et al
(13) suggest a synergistic effect
between TKI and PD-1 inhibitors, providing a solid theoretical
foundation for the treatment of CRC. Clinically, Guo et al
(14) reported a median OS of 20.0
months and a median PFS of 6.9 months in patients with MSS/pMMR
mCRC receiving fruquintinib plus sintilimab, significantly
extending OS. Further evidence from a propensity score-matched
retrospective study presented at the 2024 American Society of
Clinical Oncology Gastrointestinal Cancer Symposium (15) confirmed that fruquintinib plus a
PD-1 inhibitor significantly improved PFS in patients with MSS/pMMR
mCRC. Similarly, several small-scale retrospective studies have
demonstrated that combining fruquintinib with other ICIs yields
superior efficacy compared with fruquintinib monotherapy in
patients with MSS/pMMR mCRC, with manageable AEs, indicating
promising clinical potential (16,17).
However, existing studies have notable limitations.
Most trials focus on the combined effects of a single PD-1
inhibitor and fruquintinib. There is a lack of direct comparison
between fruquintinib combined with different ICIs and an
introduction to the side effects of the combination regimens. The
present study systematically analyzed the efficacy and safety of
different ICIs combined with fruquintinib in patients with MSS/pMMR
mCRC. In contrast to the traditional fixed-dose model, the present
study reviewed the drug dose intensity, administration time and
sequence in the real world, which provided specific guidance for
the optimization of the future administration regimen of
immunotherapy combined with fruquintinib. At the same time, the
present study also explored a more efficient and low-toxicity
combination therapy model and further precisely selected the
beneficiary population. The present study supports important
clinical needs and scientific value.
Materials and methods
Study design and patients
The present retrospective study analyzed the medical
data of patients with MSS/pMMR mCRC between January 2022 and
December 2023 at the Jiangsu Cancer Hospital (Nanjing, China).
Patients who were aged 18–75 years with histopathologically or
cytologically confirmed CRC and MSS/pMMR were eligible for the
study. Patients were required to have failed at least two prior
lines of treatment (all patients had received standard first- and
second-line chemotherapy) and to have received fruquintinib plus
PD-1 inhibitors (PD-1 inhibitor combination group) or programmed
death-ligand 1 (PD-L1) inhibitors (PD-L1 inhibitor combination
group). Patients also had to have radiologically confirmed
metastases with at least one radiological-target lesion, a life
expectancy of ≥3 months and an Eastern Cooperative Oncology Group
(ECOG) performance status of 0 or 1 (18). Patients were excluded if they had
received a live vaccine within 28 days before treatment, were
allergic to fruquintinib or ICIs, had active hepatitis B or C, HIV
infection, active tuberculosis, had a history of active autoimmune
disease, had severe liver or kidney dysfunction or other serious
underlying conditions, and had a history of or concurrent untreated
malignancy.
The present study was conducted in compliance with
Good Clinical Practice guidelines and the Declaration of Helsinki
(2013 version) and approved by the ethics committee of the Jiangsu
Cancer Hospital (grant no. KY-2024-071). The requirement for
informed consent was waived because of the retrospective nature of
the present study.
Treatments
Regarding the initial dose of fruquintinib, the
fruquintinib dose in the FRESCO-2 study (11) was used, which is 5 mg daily q28 with
a 1-week break from days 22–28. However, most patients cannot
tolerate a 5 mg daily dose. Therefore, all patients received oral
fruquintinib once daily, with initial doses of 3, 4 or 5 mg.
Treatment continued for 2 weeks, followed by a 1-week break, with
each cycle lasting 21 days. The actual initial dose was mainly
selected by clinicians after a full assessment in combination with
the physical condition, body surface area, previous chemotherapy
response, and liver and kidney functions of the patient. Regarding
the issue of drug administration time, a Ib/II study conducted by
Guo et al (14) was
referenced; compared with the treatment group taking 3 mg
fruquintinib daily, the group taking 5 mg/day fruquintinib for 2
weeks and then having a 1-week break showed a marked improvement in
efficacy. The present treatment continued for 2 weeks, followed by
a 1-week break, with each cycle lasting 21 days. If the patient
experienced intolerance, the dose could be adjusted and treatment
discontinued if intolerance persisted.
In the PD-1 inhibitor combination group, the PD-1
inhibitors camrelizumab (200 mg), sintilimab (200 mg), tislelizumab
(200 mg), toripalimab (240 mg) or serplulimab (300 mg) were chosen
by the physician based on the condition of the patient, with
intravenous infusion on day 1 of each 21-day cycle. In the PD-L1
inhibitor combination group, envafolimab (200 mg) was administered
by subcutaneous injection on days 1 and 15 of each 28-day cycle.
All ICIs were continued until disease progression or severe
intolerance.
Outcomes and assessment
Tumor responses were evaluated every 2 cycles
through computed tomography (CT) in accordance with the Response
Evaluation Criteria in Solid Tumors guidelines (version 1.1)
(19). The tumor responses
monitored included complete response (CR), partial response (PR),
stable disease (SD) and progressive disease (PD). Efficacy outcomes
included objective response rate (ORR), disease control rate (DCR)
and PFS. The ORR was defined as the proportion of patients with CR
or PR. The DCR was the proportion of patients who achieved CR, PR
or SD. The DCR time node in the present study was 3 months. The PFS
was calculated as the time from treatment initiation to first
disease progression or death from any cause. All AEs occurring
during the treatment in both groups were collected and evaluated
according to the Common Terminology Criteria for Adverse Events
version 5.0 (20).
Statistical analysis
All statistical analyses were conducted using SPSS
version 22.0 (IBM Corp.) Baseline categorical variables were
compared using the χ2 test. Survival curves were
generated using the Kaplan-Meier method, with group differences
assessed by the log-rank test. Univariate and multivariate analyses
were performed using the Cox proportional hazards model to identify
independent prognostic factors. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
A total of 78 patients with mCRC were included, with
47 (60.3%) males and 31 (39.7%) females and a median age of 57
years. Subsequently, 63 patients received fruquintinib plus a PD-1
inhibitor (20 sintilimab, 13 tislelizumab, 11 camrelizumab, 9
toripalimab or 10 serplulimab) and 15 received fruquintinib plus a
PD-L1 inhibitor (envafolimab). In the PD-1 inhibitor combination
group, 10, 23 and 30 patients received initial doses of
fruquintinib at 3, 4 and 5 mg, respectively, while in the PD-L1
inhibitor combination group, two, 9 and four patients received
initial doses of 3, 4 and 5 mg, respectively. There were no
significant differences in the baseline characteristics between the
two groups (P>0.05; Table
I).
 | Table I.Baseline characteristics of the
included patients. |
Table I.
Baseline characteristics of the
included patients.
| Characteristic | PD-1 inhibitor
combination group (n=63) | PD-L1 inhibitor
combination group (n=15) | χ2 | P-value |
|---|
| Sex |
|
| 1.43 | 0.231 |
| Male | 40 (63.5) | 7 (46.7) |
|
|
|
Female | 23 (36.5) | 8 (53.3) |
|
|
| Age |
|
| 2.03 | 0.154 |
|
<60 | 38 (60.3) | 6 (40.0) |
|
|
| ≥60 | 25 (39.7) | 9 (60.0) |
|
|
| ECOG performance
status |
|
| 0.37 | 0.542 |
| 0 | 24 (38.1) | 7 (46.7) |
|
|
| 1 | 39 (61.9) | 8 (53.3) |
|
|
| Degree of
differentiation |
|
| 4.44 | 0.109 |
| High | 2 (3.2) | 3 (20.0) |
|
|
|
Middle | 44 (69.8) | 9 (60.0) |
|
|
| Low | 17 (27.0) | 3 (20.0) |
|
|
| Tumor location |
|
| 0.32 | 0.571 |
| Left | 49 (77.8) | 10 (66.7) |
|
|
|
Right | 14 (22.2) | 5 (33.3) |
|
|
| Number of
metastases |
|
| 0.03 | 0.872 |
|
<3 | 50 (79.4) | 11 (73.3) |
|
|
| ≥3 | 13 (20.6) | 4 (26.7) |
|
|
| Presence of liver
metastases | 37 (58.7) | 8 (53.3) | 0.15 | 0.704 |
| Presence of lung
metastases | 31 (50.8) | 5 (66.7) | 1.23 | 0.268 |
| Number of treatment
lines |
|
| 0.99 | 0.319 |
|
>3 | 19 (30.2) | 2 (13.3) |
|
|
| 3 | 44 (69.8) | 13 (86.7) |
|
|
| Baseline
carcinoembryonic antigen |
|
| 0.56 | 0.452 |
|
<5 | 32 (50.8) | 6 (40.0) |
|
|
| ≥5 | 31 (49.2) | 9 (60.0) |
|
|
| BRAF
status |
|
| 0.53 | 0.872 |
|
Wild-type | 43 (68.3) | 10 (66.7) |
|
|
|
Mutated | 3 (4.8) | 0 (0.0) |
|
|
|
Unknown | 17 (27.0) | 5 (33.3) |
|
|
| KRAS
status |
|
| 2.64 | 0.267 |
|
Wild-type | 21 (33.3) | 2 (13.3) |
|
|
|
Mutated | 25 (39.7) | 8 (53.3) |
|
|
|
Unknown | 17 (27.0) | 5 (33.3) |
|
|
| Prior antitumor
treatment |
|
|
|
|
| TKI
therapy | 13 (20.6) | 3 (20.0) | 0.003 | 0.956 |
|
Radiotherapy | 20 (31.7) | 8 (53.3) | 2.45 | 0.117 |
|
Surgery | 51 (81.0) | 14 (93.3) | 0.59 | 0.441 |
| Initial dose of
fruquintinib |
|
| 2.90 | 0.234 |
| 3
mg | 10 (15.9) | 2 (13.3) |
|
|
| 4
mg | 23 (36.5) | 9 (60.0) |
|
|
| 5
mg | 30 (47.6) | 4 (26.7) |
|
|
Efficacy
In the PD-1 inhibitor combination group, 5 (7.9%)
patients had a PR and 42 (66.7%) patients had an SD, achieving an
ORR of 7.9% and a DCR of 74.6% (Table
II). In comparison, the PD-L1 inhibitor combination group
included 3 patients (20.0%) with PR and 6 patients (40.0%) with SD,
yielding an ORR of 20.0% and a DCR of 60.0% (Table II). No statistically significant
differences were observed between the PD-1 and PD-L1 inhibitor
combination group in ORR (7.9 vs. 20.0%; P=0.363) or DCR (74.6 vs.
60%; P=0.418). Moreover, there was no significant difference in
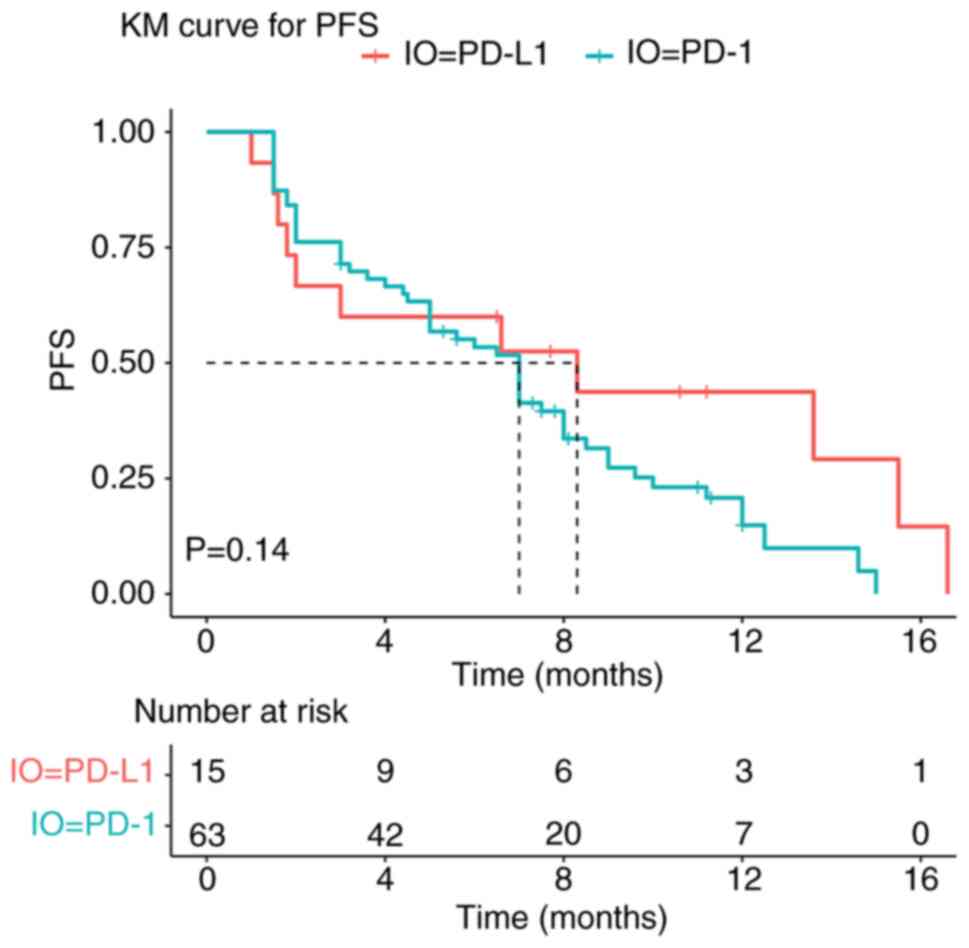
median PFS between the two groups (7.0 vs. 8.3 months; P=0.14;
Fig. 1).
 | Table II.Efficacy between the PD-1 and PD-L1
inhibitor combination groups. |
Table II.
Efficacy between the PD-1 and PD-L1
inhibitor combination groups.
| Response | PD-1 inhibitor
combination group (n=63) | PD-L1 inhibitor
combination group (n=15) | χ2 | P-value |
|---|
| Objective
response |
|
|
|
|
| CR | 0 (0.0) | 0 (0.0) | Not applicable | >0.999 |
| PR | 5 (7.9) | 3 (20.0) | 0.83 | 0.363 |
| SD | 42 (66.7) | 6 (40.0) | 3.64 | 0.056 |
| PD | 16 (25.4) | 6 (40.0) | 1.28 | 0.259 |
| ORR | 5 (7.9) | 3 (20.0) | 0.83 | 0.363 |
| DCR | 47 (74.6) | 9 (60.0) | 0.66 | 0.418 |
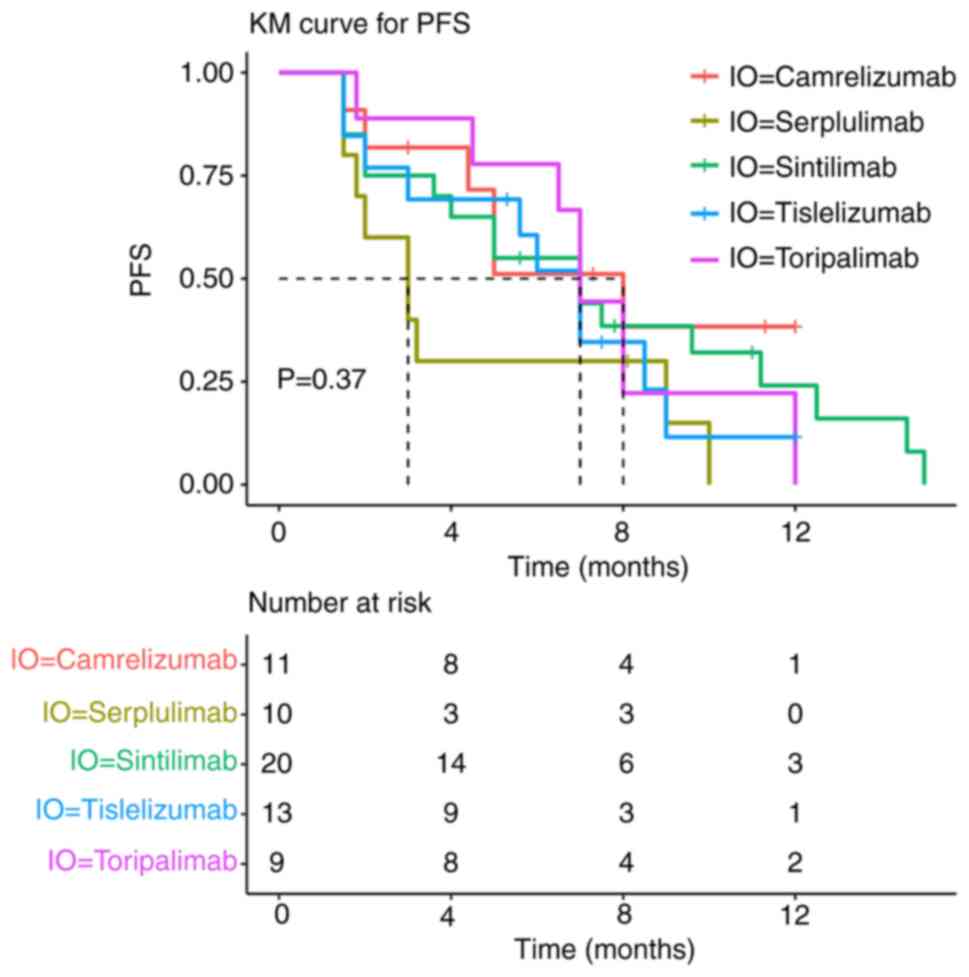
In the PD-1 inhibitor combination group, five PD-1
inhibitors were administered. Subgroup analysis showed no
statistically significant differences in PFS between patients
treated with sintilimab, tislelizumab, camrelizumab, toripalimab or
serplulimab (P=0.37; Fig. 2).
Univariate analysis showed that age, ECOG performance status and
liver metastases were associated with PFS (Table III). Further multivariate analysis
using a COX regression model revealed that an ECOG performance
status of 1 was an independent factor of poor prognosis for PFS in
patients with mCRC treated with fruquintinib plus ICIs (Table III).
 | Table III.Univariate and multivariate analysis
for progression-free survival. |
Table III.
Univariate and multivariate analysis
for progression-free survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex (male vs.
female) | 0.97 | 0.58–1.63 | 0.912 |
|
|
|
| Age (<60 vs.
≥60) | 0.54 | 0.32–0.92 | 0.022 | 0.69 | 0.40–1.19 | 0.180 |
| ECOG performance
status (0 vs. 1) | 0.25 | 0.14–0.46 | <0.001 | 0.28 | 0.15–0.52 | <0.001 |
| Degree of
differentiation |
|
|
|
|
|
|
| Low vs.
high | 1.36 | 0.77–2.39 | 0.293 |
|
|
|
| Low vs.
middle | 1.21 | 0.58–3.20 | 0.703 |
|
|
|
| High
vs. middle | 1.28 | 0.48–3.38 | 0.620 |
|
|
|
| Tumor location
(right vs. left) | 1.14 | 0.64–2.04 | 0.652 |
|
|
|
| Number of
metastases (<3 vs. ≥3) | 1.18 | 0.63–2.23 | 0.603 |
|
|
|
| Liver metastases
(no vs. yes) | 0.55 | 0.33–0.93 | 0.027 | 0.72 | 0.42–1.23 | 0.226 |
| Lung metastases (no
vs. yes) | 1.28 | 0.77–2.13 | 0.349 |
|
|
|
| Number of treatment
lines (3 vs. >3) | 1.45 | 0.81–2.58 | 0.207 |
|
|
|
| Baseline
carcinoembryonic antigen (<5 vs. ≥5) | 0.73 | 0.43–1.22 | 0.227 |
|
|
|
| BRAF
status |
|
|
|
|
|
|
| Unknown
vs. mutated | 1.00 | 0.55–1.80 | 0.989 |
|
|
|
| Unknown
vs. wild-type | 1.43 | 0.34–5.97 | 0.622 |
|
|
|
| Mutated
vs. wild-type | 1.46 | 0.35–6.09 | 0.608 |
|
|
|
| KRAS
status |
|
|
|
|
|
|
| Unknown
vs. mutated | 0.86 | 0.43–1.71 | 0.670 |
|
|
|
| Unknown
vs. wild-type | 0.81 | 0.44–1.48 | 0.491 |
|
|
|
| Mutated
vs. wild-type | 0.44 | 0.43–1.44 | 0.788 |
|
|
|
| Prior TKI drugs
therapy (no vs. yes) | 0.74 | 0.41–1.33 | 0.309 |
|
|
|
| Prior radiotherapy
(no vs. yes) | 1.55 | 0.91–2.65 | 0.109 |
|
|
|
| Prior surgery (no
vs. yes) | 1.64 | 0.86–3.13 | 0.133 |
|
|
|
| Initial dose of
fruquintinib |
|
|
|
|
|
|
| 3 mg
vs. 4 mg | 0.73 | 0.33–1.61 | 0.430 |
|
|
|
| 3 mg
vs. 5 mg | 1.50 | 0.87–2.59 | 0.145 |
|
|
|
| 4 mg
vs. 5 mg | 1.53 | 0.89–2.63 | 0.127 |
|
|
|
| ICIs (PD-1
inhibitors vs. PD-L1 inhibitors) | 1.71 | 0.82–3.54 | 0.151 |
|
|
|
Safety
The AEs during treatment in both groups were
predominantly grade 1–2 according to the Common Terminology
Criteria for Adverse Events version 5.0 (20), with the most common being hand-foot
syndrome (PD-1 inhibitor combination group vs. PD-L1 inhibitor
combination group, 20.6 vs. 33.3%), proteinuria (23.8 vs. 33.3%)
and hypothyroidism (31.7 vs. 26.7%). Notably, grade 3 or worse AEs
were infrequent. Throughout the treatment, no patients in either
group died due to serious AEs, such as life-threatening, permanent
or severe disability or loss of function.
The treatment was discontinued in 2 patients in the
PD-1 inhibitor combination group due to immune-related diabetes and
immune-related hepatic injury, respectively. In the PD-L1 inhibitor
combination group, no patients discontinued treatment for AEs.
Additionally, the incidence of AEs did not differ significantly
between the PD-1 and PD-L1 inhibitor combination group (P>0.05,
Table IV) and the incidence of AEs
did not differ significantly between PD-1 inhibitor combination
groups (P>0.05, Table V).
 | Table IV.Adverse events. |
Table IV.
Adverse events.
|
| Any grade | Grade ≥3 |
|---|
|
|
|
|
|---|
| Adverse event | PD-1 inhibitor
combination group (n=63) | PD-L1 inhibitor
combination group (n=15) | χ2 | P-value | PD-1 inhibitor
combination group (n=63) | PD-L1 inhibitor
combination group (n=15) | χ2 | P-value |
|---|
| Hypertension | 10 (15.9) | 3 (20.0) | NA | >0.999 |
|
|
|
|
| Hand-foot
syndrome | 13 (20.6) | 5 (33.3) | 0.50 | 0.479 | 4 (6.3) | 3 (20.0) | 1.35 | 0.246 |
| Diarrhea | 4 (6.3) | 0 (0.0) | 0.12 | 0.726 |
|
|
|
|
| Fatigue | 7 (11.1) | 4 (26.7) | 1.31 | 0.253 |
|
|
|
|
| Decreased
appetite | 3 (4.8) | 3 (20.0) | 2.11 | 0.147 |
|
|
|
|
|
Nausea/vomiting | 1 (1.6) | 1 (6.7) | 0.04 | 0.834 |
|
|
|
|
| Leukopenia | 7 (11.1) | 2 (13.3) | NA | >0.999 |
|
|
|
|
|
Thrombocytopenia | 5 (7.9) | 1 (6.7) | NA | >0.999 | 2 (3.2) |
| NA | >0.999 |
| Hepatic injury | 1 (1.6) | 1 (6.7) | 0.04 | 0.834 | 1 (1.6) |
| NA | >0.999 |
| Proteinuria | 15 (23.8) | 5 (33.3) | 0.19 | 0.667 | 1 (1.6) | 1 (6.7) | 0.04 | 0.834 |
|
Hyperthyroidism | 3 (4.8) | 0 (0.0) | 0.01 | 0.909 |
|
|
|
|
| Hypothyroidism | 20 (31.7) | 4 (26.7) | 0.01 | 0.943 |
|
|
|
|
| Mucositis oral | 3 (4.8) | 0 (0.0) | 0.01 | 0.909 |
|
|
|
|
| Rash | 3 (4.8) | 1 (6.7) | NA | >0.999 |
|
|
|
|
| Hoarseness | 1 (1.6) | 0 (0.0) | NA | >0.999 |
|
|
|
|
| Muscle
tenderness | 2 (3.2) | 0 (0.0) | NA | >0.999 |
|
|
|
|
| Hyperglycemia | 2 (3.2) | 0 (0.0) | NA | >0.999 | 1 (1.6) |
| 0.43 | 0.512 |
| Infection | 1 (1.6) | 0 (0.0) | NA | >0.999 |
|
|
|
|
 | Table V.PD-1 inhibitor combination group
adverse events (any grade). |
Table V.
PD-1 inhibitor combination group
adverse events (any grade).
| Adverse event | Camrelizumab
(n=11) | Sintilimab
(n=10) | Tislelizumab
(n=9) | Toripalimab
(n=13) | Serplulimab
(n=20) | χ2 | P-value |
|---|
| Hypertension | 4 (36.4) | 2 (20) | 2 (22.2) | 1 (7.7) | 1 (5) | 6.177 | 0.186 |
| Hand-foot
syndrome | 4 (36.4) | 3 (30) | 1 (11.1) | 0 (0.0) | 5 (25) | 8.734 | 0.068 |
| Diarrhea | 0 (0.0) | 1 (10) | 0 (0.0) | 2 (15.4) | 1 (5) | 4.191 | 0.381 |
| Fatigue | 1 (9.1) | 1 (10) | 0 (0.0) | 1 (7.7) | 4 (20) | 3.681 | 0.451 |
| Decreased
appetite | 0 (0.0) | 0 (0) | 1 (11.1) | 1 (7.7) | 1 (5) | 2.851 | 0.583 |
|
Nausea/vomiting | 0 (0.0) | 0 (0) | 0 (0.0) | 1 (7.7) | 0 (0) | 3.991 | 0.407 |
| Leukopenia | 0 (0.0) | 2 (20) | 2 (22.2) | 2 (15.4) | 1 (5) | 5.307 | 0.257 |
|
Thrombocytopenia | 0 (0.0) | 0 (0) | 1 (11.1) | 3 (23.1) | 1 (5) | 6.664 | 0.155 |
| Hepatic injury | 0 (0.0) | 0 (0) | 1 (11.1) | 0 (0.0) | 0 (0) | 3.991 | 0.407 |
| Proteinuria | 5 (45.5) | 3 (30) | 0 (0.0) | 2 (15.4) | 5 (25) | 8.190 | 0.085 |
|
Hyperthyroidism | 0 (0.0) | 1 (10) | 0 (0.0) | 0 (0.0) | 2 (10) | 4.617 | 0.329 |
| Hypothyroidism | 2 (18.2) | 4 (40) | 2 (22.2) | 2 (15.4) | 10 (50) | 6.428 | 0.169 |
| Mucositis oral | 0 (0.0) | 1 (10) | 1 (11.1) | 0 (0.0) | 1 (5) | 3.401 | 0.493 |
| Rash | 1 (9.1) | 0 (0) | 1 (11.1) | 1 (7.7) | 0 (0) | 4.090 | 0.394 |
| Hoarseness | 0 (0.0) | 0 (0) | 0 (0.0) | 1 (7.7) | 0 (0) | 3.219 | 0.522 |
| Muscle
tenderness | 0 (0.0) | 1 (10) | 0 (0.0) | 0 (0.0) | 1 (5) | 3.294 | 0.510 |
| Hyperglycemia | 0 (0.0) | 0 (0) | 0 (0.0) | 1 (7.7) | 1 (5) | 2.744 | 0.601 |
| Infection | 0 (0.0) | 0 (0) | 0 (0.0) | 1 (7.7) | 0 (0) | 3.219 | 0.522 |
The present findings are preliminary and recommend
conducting well-designed prospective cohort studies or multi-center
collaborations in the future, including a larger sample size, to
validate the present findings and draw more conclusive
conclusions.
Discussion
Immunotherapy has become a research hotspot in the
treatment of mCRC, with the combination of ICIs and TKIs being
widely endorsed and established as the mainstream treatment
strategy in later-line therapies. The REGONIVO trial reported an
ORR of >30% in patients with MSS-type mCRC who received
regorafenib plus nivolumab therapy, demonstrating favorable
efficacy along with favorable safety (21). Although studies have shown that the
combination of TKI and ICIs is more effective than TKI monotherapy,
to the best of our knowledge, no studies have yet compared the
efficacy and safety of TKIs plus different ICIs. The present study
retrospectively assessed the efficacy and safety of fruquintinib
plus different ICIs in MSS/pMMR mCRC. The present study aimed to
identify the optimal combination, understand the factors
influencing outcomes and determine the patient populations most
likely to benefit from this therapy. The results showed no
statistically significant differences in ORR (7.9 vs. 20.0%;
P=0.363), DCR (74.6 vs. 60.0%; P=0.418) and median PFS (7.0 vs. 8.3
months; P=0.14) between the PD-1 inhibitor and PD-L1 inhibitor
combination groups. When comparing the ORR and DCR between the two
groups, the DCR was found to be more promising in the PD-1 group,
while the ORR was improved in the PD-L1 group, although neither
difference was statistically significant.
The inconsistency between the DCR and ORR is a
common and well-elucidated phenomenon (22–24).
The most direct source of the inconsistency between the values is
the patients who only achieved SD. These patients are included in
the DCR but not in the ORR. In terms of the mechanism of action, it
takes time to activate the immune system. Thus, the ORR may not be
high at early evaluation, but the proportion of patients achieving
SD may be relatively high (with a higher DCR). In addition, tumors
exhibit heterogeneity and have drug resistance. At present, the
drug-resistance mechanisms of targeted immunotherapy combinations
are not fully understood. From the perspective of the patient
population, patients with a large tumor burden may find it more
difficult to achieve a deep response (CR/PR). The resistance to
treatment may increase with increased previous treatment lines and
the drugs are more likely to control the tumor rather than
significantly shrink it (high proportion of SD). The difference in
the number of cases between the PD-1 and PD-L1 inhibitor
combination group) may also lead to the inconsistency of ORR and
DCR, which is also the limitation of this study.. In the future,
the number of the population will be further expanded, the
follow-up time will be extended and the data will be optimized to
verify and obtain more accurate results.
The findings of the present study were consistent
with another retrospective study reported by Yang et al
(16), which also analyzed the
efficacy and safety of fruquintinib + PD-1 inhibitors in mCRC,
reporting an ORR of 11.4%, a DCR of 84.3% and a median PFS of 5.5
months. The PD-1 inhibitors in the present retrospective study
included sintilimab, tislelizumab, toripalimab and pembrolizumab.
However, due to significant differences in the sample sizes between
groups, subgroup analyses were not performed. A study by Bai et
al (25) evaluated the
fruquintinib + geptanolimab for the treatment of CRC. The results
indicated that, in all evaluable patients with mCRC, the overall
ORR was 26.7%, with an ORR of 33.3% in the recommended phase II
dose (RP2D) group, a DCR of 80.0% and a median PFS of 7.3 months.
For patients with MSS-type mCRC, the ORR was 25.0%, the DCR was 75%
and the median PFS was 5.5 months. The findings of the present
study suggested that fruquintinib plus ICI exhibited manageable
safety and promising antitumor activity in patients with mCRC. In
another retrospective study reported by Gou et al (17), fruquintinib plus PD-1 inhibitors in
45 patients with MSS-type mCRC resulted in a DCR of 62.2%, ORR of
11.1% and median PFS of 3.8 months. The limited efficacy observed
might be attributed to the lack of strict ECOG performance status
criteria for inclusion criteria. Additionally, considering patient
tolerance, the dosing of fruquintinib might be lower in real-world
settings, which could also impact efficacy. The present study
evaluated the PFS of patients treated with fruquintinib plus
different PD-1 inhibitors and observed no statistically significant
differences between the different PD-1 inhibitors. As a result, the
most effective ICIs could not be identified.
The CheckMate-142 (26) and CheckMate 8HW (27) studies both confirmed the favorable
efficacy of PD-1 inhibitors plus cytotoxic T-lymphocyte protein 4
(CTLA-4) inhibitors in MSI-H CRC. Similarly, studies are presently
exploring the efficacy of dual ICI therapy in MSS-type mCRC. In a
phase I clinical trial presented at the 2022 ESMO-GI conference
(28), the combination of the
botensilimab (a CTLA-4 inhibitor) and balstilimab (a PD-1
inhibitor) showed strong clinical activity and sustained efficacy
in patients with MSS mCRC who had previously failed multiple lines
of treatment. Importantly, the treatment was well-tolerated with no
serious AEs, indicating the broad potential of dual immunotherapy
in the management of mCRC. However, the present study did not
include patients receiving the combination of fruquintinib and dual
immunotherapy due to economic limitations, as the high cost of dual
immunotherapy might have been unaffordable for some patients. By
contrast, PD-1 or PD-L1 inhibitors are more cost-effective and
therefore more accessible to patients, while still providing
significant therapeutic benefits.
In the present study, univariate analysis indicated
that age, ECOG performance status and liver metastasis were
associated with PFS. Multivariate Cox regression analysis
identified an ECOG performance status of 1 as an independent risk
factor for PFS. Immunotherapy exerts its effect by activating
immune cells that specifically recognize and eliminate tumor cells,
requiring a sufficient immune cell reserve. Patients with an ECOG
performance status of 0 generally have improved physical conditions
and a stronger immune response. In the CheckMate 153 trial
(29), patients with an ECOG
performance status of 0–1 derived significantly more benefit from
immunotherapy compared with those with a score of 2. Similarly, the
KEYNOTE-177 trial (30) observed
that PD-1 inhibitors did not significantly improve PFS in patients
with an ECOG performance status of 1. Additionally, Fakih et
al (31) found that liver
metastasis is a poor prognostic factor. However, other studies have
shown no association between KRAS mutations, lung
metastasis, liver metastasis and PFS or OS, warranting further
validation in larger clinical trials (32,33).
The most common AEs previously reported with
fruquintinib plus ICIs were hypertension, hand-foot syndrome and
hypothyroidism. In the present study, the most common AEs in the
PD-1 inhibitor combination group were hypothyroidism (31.7%),
proteinuria (23.8%) and hand-foot syndrome (20.6%). In the PD-L1
inhibitor combination group, the most common AEs were proteinuria
(33.3%), hand-foot syndrome (33.3%) and hypothyroidism (26.7%). No
deaths due to serious AEs were reported in either group.
The present study has several limitations. Firstly,
it is a single-center, retrospective analysis, which might
introduce selection bias. Secondly, the administration of five
different PD-1 inhibitors and three initial doses of fruquintinib
in the PD-1 inhibitor combination group might have affected
treatment consistency. Next, the sample size was relatively small
and the follow-up period was relatively short. The reason why the
sample size, especially the PD-L1 inhibitor combination group, is
small is that fruquintinib is only reimbursed by Chinese medical
insurance for third-line colorectal cancer treatment, with no
coverage beyond this line. Immunotherapy drugs are not covered for
colorectal cancer at all. Consequently, few patients can afford the
combination of immunotherapy and fruquintinib. Affordability is
even lower for combinations involving PD-L1 inhibitors due to their
higher cost. Additionally, the present study has limitations in
safety assessment, as the small sample size limits the ability to
detect rare toxicities and the relatively short follow-up time
affects the assessment of long-term toxicities. In the future,
large-scale real-world research should be carried out to confirm
safety, explore biomarkers for toxicity prediction and optimize
adverse reaction management strategies.
Although there was no statistically significant
difference in efficacy between the groups of fruquintinib combined
with PD-1 inhibitor and fruquintinib combined with PD-L1 inhibitor,
it should be noted that the statistical power of this result was
low and it should be regarded as a preliminary finding that needs
to be verified in future larger-scale studies. Similarly, for the
present results where no significant difference was found, the
possibility of clinically relevant differences should not be ruled
out as the lack of significance may be due to insufficient sample
size. Finally, one of the other major limitations of the present
study is the lack of mature OS data. This is mainly because the
follow-up time at the data cut-off was relatively short and some
patients were lost to follow-up. Therefore, it is currently
impossible to evaluate the long-term survival benefits of the
present study based on OS. Against the backdrop of a high risk of
loss to follow-up, the present study considered earlier-occurring
endpoints with more reliable data acquisition (such as PFS or ORR)
as the primary endpoints. The data collection was relatively
complete (loss to follow-up occurred after the PFS event), so the
analysis results were reliable and clinically meaningful. Future
studies should pre-set a more rigorous analysis plan for OS in the
case of a high loss-to-follow-up rate in the protocol. In future
studies, we will be committed to improving the survival follow-up
strategy and investing more resources in designing and implementing
a more powerful survival follow-up strategy.
In conclusion, although no statistically significant
differences in efficacy were observed between the PD-1 inhibitor
combination group and the PD-L1 inhibitor combination group,
fruquintinib plus ICIs improved survival compared with the
previously reported efficacy of fruquintinib monotherapy in mCRC.
Furthermore, this combination therapy did not increase serious AEs,
indicating an acceptable safety profile. Future clinical trials
with larger sample sizes are needed to confirm the findings of the
present study and explore the potential of immunotherapy plus
targeted therapy to establish new treatment strategies for mCRC in
clinical practice.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Jiangsu Provincial Cancer
Hospital 2023 Hospital Science and Technology Development Fund
(grant no. ZL202308).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Author's contributions
YP was responsible for the curation of data, formal
analysis, investigation, validation, visualization, writing the
original draft and reviewing and editing of the manuscript. SL
analyzed data and edited the manuscript. LZ was responsible for the
conceptualization and supervision of the present study. All authors
read and approved the final version of the manuscript. YP, SL and
LZ confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The study was conducted in compliance with Good
Clinical Practice guidelines and the Declaration of Helsinki (2013
version) and was approved by the ethics committee of the Jiangsu
Cancer Hospital (approval no. KY-2024-071). The requirement for
informed consent was waived because of the retrospective nature of
the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Han B, Zheng R, Zeng H, Wang S, Sun K,
Chen R, Li L, Wei W and He J: Cancer incidence and mortality in
China, 2022. J Natl Cancer Cent. 4:47–53. 2024.PubMed/NCBI
|
|
2
|
Lichtenstern CR, Ngu RK, Shalapour S and
Karin M: Immunotherapy, inflammation and colorectal cancer. Cells.
9:6182020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chinese Society Of Clinical Oncology Csco
Diagnosis and Treatment Guidelines For Colorectal Cancer Working
Group, . Chinese society of clinical oncology (CSCO) diagnosis and
treatment guidelines for colorectal cancer 2018 (english version).
Chin J Cancer Res. 31:117–134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Diaz LA Jr, Shiu KK, Kim TW, Jensen BV,
Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs
P, et al: Pembrolizumab versus chemotherapy for microsatellite
instability-high or mismatch repair-deficient metastatic colorectal
cancer (KEYNOTE-177): Final analysis of a randomised, open-label,
phase 3 study. Lancet Oncol. 23:659–670. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koopman M, Kortman GA, Mekenkamp L,
Ligtenberg MJ, Hoogerbrugge N, Antonini NF, Punt CJ and van Krieken
JH: Deficient mismatch repair system in patients with sporadic
advanced colorectal cancer. Br J Cancer. 100:266–273. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bever KM and Le DT: An expanding role for
immunotherapy in colorectal cancer. J Natl Compr Canc Netw.
15:401–410. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chalabi M, Fanchi LF, Dijkstra KK, Van den
Berg JG, Aalbers AG, Sikorska K, Lopez-Yurda M, Grootscholten C,
Beets GL, Snaebjornsson P, et al: Neoadjuvant immunotherapy leads
to pathological responses in MMR-proficient and MMR-deficient
early-stage colon cancers. Nat Med. 26:566–576. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tan AC, Bagley SJ, Wen PY, Lim M, Platten
M, Colman H, Ashley DM, Wick W, Chang SM, Galanis E, et al:
Systematic review of combinations of targeted or immunotherapy in
advanced solid tumors. J Immunother Cancer. 9:e0024592021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun Q, Zhou J, Zhang Z, Guo M, Liang J,
Zhou F, Long J, Zhang W, Yin F, Cai H, et al: Discovery of
fruquintinib, a potent and highly selective small molecule
inhibitor of VEGFR 1, 2, 3 tyrosine kinases for cancer therapy.
Cancer Biol Ther. 15:1635–1645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dasari A, Lonardi S, Garcia-Carbonero R,
Elez E, Yoshino T, Sobrero A, Yao J, García-Alfonso P, Kocsis J,
Cubillo Gracian A, et al: Fruquintinib versus placebo in patients
with refractory metastatic colorectal cancer (FRESCO-2): An
international, multicentre, randomised, double-blind, phase 3
study. Lancet. 402:41–53. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fusco MJ, Casak SJ, Mushti SL, Cheng J,
Christmas BJ, Thompson MD, Fu W, Wang H, Yoon M, Yang Y, et al: FDA
approval summary: fruquintinib for the treatment of refractory
metastatic colorectal cancer. Clin Cancer Res. 30:3100–3104. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Q, Cheng X, Zhou C, Tang Y, Li F, Zhang
B, Huang T, Wang J and Tu S: Fruquintinib enhances the antitumor
immune responses of anti-programmed death receptor-1 in colorectal
cancer. Front Oncol. 12:8419772022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo Y, Zhang W, Ying J, Zhang Y, Pan Y,
Qiu W, Fan Q, Xu Q, Ma Y, Wang G, et al: Phase 1b/2 trial of
fruquintinib plus sintilimab in treating advanced solid tumours:
The dose-escalation and metastatic colorectal cancer cohort in the
dose-expansion phases. Eur J Cancer. 181:26–37. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
An T, Lian Y, Zhou Q, Zhao C, Wang Z and
Zhao R: Fruquintinib with PD-1 inhibitors versus fruquintinib
monotherapy in late-line mCRC: A retrospective cohort study based
on propensity score matching. J Clin Oncol. 42 (Suppl 3):S1392024.
View Article : Google Scholar
|
|
16
|
Yang X, Yin X, Qu X, Guo G, Zeng Y, Liu W,
Jagielski M, Liu Z and Zhou H: Efficacy, safety, and predictors of
fruquintinib plus anti-programmed death receptor-1 (PD-1) antibody
in refractory microsatellite stable metastatic colorectal cancer in
a real-world setting: A retrospective cohort study. J Gastrointest
Oncol. 14:2425–2435. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gou M, Qian N, Zhang Y, Yan H, Si H, Wang
Z and Dai G: Fruquintinib in combination with PD-1 inhibitors in
patients with refractory non-MSI-H/pMMR metastatic colorectal
cancer: A real-world study in China. Front Oncol. 12:8517562022.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Freites-Martinez A, Santana N,
Arias-Santiago S and Viera A: Using the common terminology criteria
for adverse events (CTCAE-version 5.0) to evaluate the severity of
adverse events of anticancer therapies. Actas Dermosifiliogr (Engl
Ed). 112:90–92. 2021.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fukuoka S, Hara H, Takahashi N, Kojima T,
Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, et
al: Regorafenib plus nivolumab in patients with advanced gastric or
colorectal cancer: An open-label, dose-escalation, and
dose-expansion phase Ib trial (REGONIVO, EPOC1603). J Clin Oncol.
38:2053–2061. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seymour L, Bogaerts J, Perrone A, Ford R,
Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, et
al: iRECIST: Guidelines for response criteria for use in trials
testing immunotherapeutics. Lancet Oncol. 18:e143–e152. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yao JC, Shah MH, Ito T, Bohas CL, Wolin
EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG,
et al: Everolimus for advanced pancreatic neuroendocrine tumors. N
Engl J Med. 364:514–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bai Y, Xu N, An S, Chen W, Gao C and Zhang
D: A phase Ib trial of assessing the safety and preliminary
efficacy of a combination therapy of geptanolimab (GB 226) plus
fruquintinib in patients with metastatic colorectal cancer (mCRC).
J Clin Oncol. 39 (Suppl 15):e155512021. View Article : Google Scholar
|
|
26
|
Overman MJ, McDermott R, Leach JL, Lonardi
S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, et al:
Nivolumab in patients with metastatic DNA mismatch repair-deficient
or microsatellite instability-high colorectal cancer (CheckMate
142): An open-label, multicentre, phase 2 study. Lancet Oncol.
18:1182–1191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Andre T, Elez E, Van Cutsem E, Jensen LH,
Bennouna J, Mendez G, Schenker M, De la Fouchardière C, Limon MJ,
Yoshino T, et al: Nivolumab (NIVO) plus ipilimumab (IPI) vs
chemotherapy (chemo) as first-line (1L) treatment for
microsatellite instability-high/mismatch repair-deficient
(MSI-H/dMMR) metastatic colorectal cancer (mCRC): First results of
the CheckMate 8HW study. J Clin Oncol. 42 (Suppl 3):LBA7682024.
View Article : Google Scholar
|
|
28
|
Bullock A, Grossman J, Fakih M, Lenz H,
Gordon M, Margolin K, Wilky B, Mahadevan D, Trent J, Bockorny B, et
al: LBA O-9 Botensilimab, a novel innate/adaptive immune activator,
plus balstilimab (anti-PD-1) for metastatic heavily pretreated
microsatellite stable colorectal cancer. Ann Oncol. 33 (Suppl
4):S3762022. View Article : Google Scholar
|
|
29
|
Waterhouse DM, Garon EB, Chandler J,
McCleod M, Hussein M, Jotte R, Horn L, Daniel DB, Keogh G, Creelan
B, et al: Continuous versus 1-year fixed-duration nivolumab in
previously treated advanced non-small-cell lung cancer: CheckMate
153. J Clin Oncol. 38:3863–3873. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Andre T, Shiu KK, Kim TW, Jensen BV,
Jensen LH, Punt CJA, Smith DM, Garcia-Carbonero R, Benavides M
Gibbs OP, et al: Pembrolizumab versus chemotherapy for
microsatellite instability-high/mismatch repair deficient
metastatic colorectal cancer: The phase 3 KEYNOTE-177 study. J Clin
Oncol. 38 (Suppl 18):LBA42020. View Article : Google Scholar
|
|
31
|
Fakih M, Raghav KPS, Chang DZ, Bendell JC,
Larson T, Cohn AL, Huyck TK, Cosgrove D, Fiorillo JA, Garbo LE, et
al: Single-arm, phase 2 study of regorafenib plus nivolumab in
patients with mismatch repair-proficient (pMMR)/microsatellite
stable (MSS) colorectal cancer (CRC). J Clin Oncol. 39 (Suppl
15):S35602021. View Article : Google Scholar
|
|
32
|
Albertsmeier M, Riedl K, Stephan AJ, Drefs
M, Schiergens TS, Engel J, Angele MK, Werner J and Guba M: Improved
survival after resection of colorectal liver metastases in patients
with unresectable lung metastases. HPB (Oxford). 22:368–375. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Loupakis F, Cremolini C, Masi G, Lonardi
S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi
R, et al: Initial therapy with FOLFOXIRI and bevacizumab for
metastatic colorectal cancer. N Engl J Med. 371:1609–1618. 2014.
View Article : Google Scholar : PubMed/NCBI
|