Introduction
Neuroendocrine neoplasms (NENs) originate from
peptidergic neurons and neuroendocrine cells in the body, with the
gastrointestinal tract and pancreas being the most prevalent
anatomical sites, accounting for >50% of all neuroendocrine
tumors (NETs) (1). A previous study
has shown that the incidence of NENs in the United States increased
from 1.09 per 100,000 in 1973 to 5.25 per 100,000 in 2004,
representing a 3.8-fold rise (2).
Another study indicated that among gastrointestinal NENs reported
in China from 1957 to 2011, pancreatic NETs were the most common,
followed by those in the rectum, appendix, stomach, colorectum,
jejunoileum and duodenum, respectively (3). Based on the World Health Organization
(WHO) 2010 classification, NETs of the digestive system are mainly
divided into three categories: i) NET; ii) neuroendocrine carcinoma
(NEC); and iii) mixed adenoendocrine carcinoma (MANEC) (4). According to the European NET Society
(ENETS) grading method for NENs of the gastrointestinal tract,
well-differentiated tumors are classified as low grade (G1) and
intermediate grade (G2), while poorly differentiated tumors are
classified as high-grade (G3) (5).
Accurate grading requires classifying poorly differentiated NENs,
with features such as abnormal cell morphology and active
proliferation, as high-grade, whereas well-differentiated tumors
are histologically defined by features such as uniform nuclear
structures (6).
Colorectal NEC (CRNEC), a relatively uncommon
malignancy, includes tumors classified, as per the WHO 2010
criteria, as NET, NEC and MANEC. Currently, the main treatment
strategy involves surgical removal followed by chemotherapy. Most
NEC cases in the colon and rectum are of the large cell type (75%),
whereas small cell NEC predominates in the anus (7). NEC cells are positive for at least one
neuroendocrine immunohistochemical marker, such as AE1/AE3,
chromogranin A, CD56, Ki-67 or synaptophysin (Syn) (8,9).
Research shows that CRNEC exhibits high aggressiveness, with
hepatic metastases present in 40–50% of cases upon initial
detection (10). One study has
reported that well-differentiated G3 NETs exhibit a mean Ki-67
index of 30%, significantly lower than the 80% seen in poorly
differentiated pancreatic NECs in pancreatic G3 NENs, with a median
overall survival (OS) of 99 months versus 17 months, respectively
(11).
To the best of our knowledge, studies focusing on
CRNEC remain scarce. Factors such as surgical resection and the use
of adjuvant chemotherapy have been associated with improved
survival in CRNEC. Therefore, the current study conducted a
comparative analysis between histopathological factors and survival
outcomes for patients with CRNEC, and aimed to provide insights in
the clinical management of patients diagnosed with CRNEC.
Materials and methods
Patient selection
The present single-center retrospective study
enrolled 157 consecutive patients with CRNEC receiving treatment at
Harbin Medical University Cancer Hospital (Harbin, China) from
January 2000 to December 2019. There were 102 male and 55 female
patients; and the median age was 55 years (age range, 59–81 years).
WHO 2019 centralized reclassification standards were used to ensure
the diagnostic homogeneity of the patients enrolled over this
period (4). Complete
clinicopathological data and follow-up information were recorded
for the enrolled patients. The study protocol received approval
from the Institutional Review Board of Harbin Medical University
Cancer Hospital (approval no. 82271845; October 10, 2019) and
strictly adhered to the ethical principles of The Declaration of
Helsinki (1964) and subsequent amendments. Before treatment, the
enrolled patients provided written informed consent for the use of
their data in research.
Inclusion criteria and exclusion
criteria
The inclusion criteria were as follows: i)
Pathologically confirmed diagnosis of CRNEC; ii) age ≥18 years with
adequate Eastern Cooperative Oncology Group performance status (≥2)
(12) to tolerate surgical or other
treatments (including chemotherapy and radiotherapy); iii) complete
clinicopathological records and treatment information; and iv)
maintained protocol adherence with follow-up. The exclusion
criteria were as follows: i) Patients with idiopathic inflammatory
bowel diseases of chronic evolution, notably Crohn's disease and
ulcerative colitis; ii) patients with uncontrolled comorbidities or
systemic illnesses; iii) patients with poor treatment adherence or
unwillingness to comply with therapeutic protocols; and iv)
patients with missing follow-up information.
Study variables
According to the WHO 2019 Classification of
Digestive System Tumors (5th edition) (4), these neoplasms were categorized into
five histological categories based on tumor size, number of lymph
nodes affected by cancer and distant metastasis status: i) NET-G1:
Typical carcinoid tumors with well-differentiated morphology and
low proliferative activity [Ki-67 index <3%; mitotic count
<2/10 high-power fields (HPFs)]; ii) NET-G2:
Moderately-differentiated tumors exhibiting intermediate-grade
features (Ki-67 index 3–20%; mitotic count 2–20/10 HPFs); iii)
NEC-G3: Poorly-differentiated high-grade malignancies, including
small cell carcinoma and large cell NEC subtypes (Ki-67 index
>20%; mitotic count >20/10 HPFs); iv) MANEC: Biphasic tumors
containing both gland-forming adenocarcinoma and neuroendocrine
components (each constituting ≥30% of the lesion); and v)
hyperplastic and pre-neoplastic lesions: Non-invasive proliferation
with malignant potential.
According to the ENETS 2017 guidelines (13), based on the Ki-67 index and mitotic
figures, the neoplasms were assigned three differentiation grades
as follows: i) Low-grade (G1): Ki-67 index ≤2% or mitotic count
<2/10 HPFs; ii) intermediate-grade (G2): Ki-67 index 3–20% or
mitotic count 2–20/10 HPFs; and iii) high-grade (G3): Ki-67 index
>20% or mitotic count >20/10 HPFs.
AE1/AE3 expression
AE1/AE3 expression levels were detected and
evaluated by the Department of Pathology (Harbin Medical University
Cancer Hospital). Pathological formalin-fixed paraffin-embedded
tissues from the enrolled patients were obtained. The manual
immunohistochemistry staining assay was followed according to the
standard protocols: i) The CRNEC tissues were fixed with 4%
paraformaldehyde solution at 4°C for 24 h and embedded in 10%
paraffin at room temperature for 48 h; ii) paraffin blocks were
sliced at 60–70°C, and dewaxed twice in xylene (10 min each) and in
a gradient series of alcohol (100, 95, 85 and 70%) for 5 min; iii)
antigen retrieval; iv) endogenous peroxidase activity was blocked
by incubating the samples in 0.3% hydrogen peroxide prepared in
methanol for 30 min at room temperature; v) tissues were blocked
with 10% goat serum albumin (Beyotime Institute of Biotechnology)
for 30 min at room temperature; vi) tissues were incubated with an
anti-AE1/AE3 primary antibody (1:500; cat. no. 67306S; Cell
Signaling Technology, Inc.) overnight at 4°C and with a
HRP-conjugated anti-mouse secondary antibody (1:200; cat. no.
8125S; Cell Signaling Technology) for 30 min at room temperature;
vii) color rendering using diaminobenzidine (1:20; cat. no. P0202;
Beyotime Institute of Biotechnology); viii) recombinant staining of
the cell nucleus with hematoxylin (cat. no. 14166S; Cell Signaling
Technology, Inc.); ix) dehydration and sealing in an alcohol
gradient (95, 95 and 100%) for 3 min and in xylene for 3 min; and
x) microscopic examination of three fields using the Aperio Image
Scope system (version 12.3.3; Leica Biosystems).
The expression of AE1/AE3 was obtained by
immunohistochemistry, authors that evaluated the density and
intensity of stained cells were blinded to the clinicopathological
data. The density of AE1/AE3-positively stained cells was scored as
follows: i) 0: <1% stained; ii) 1: 1–10%; iii) 2: 11–50%; vi) 3:
51–75%; and v) 4: 76–100%. The staining intensity of
AE1/AE3-positivity was scored as follows: i) 0: No staining; ii) 1:
Light yellow staining; iii) 2: Brown-yellow staining; and iv) 3:
Yellowish-brown staining. The total AE1/AE3 expression score was
calculated by multiplying the density score (0–4) and the intensity score (0–3), yielding a composite score ranging from
0 to 12. In the present study, patients were separated into two
groups based on AE1/AE3 expression: AE1/AE3 negative expression
(scores <1 based on stained cell density and intensity) and
AE1/AE3 positive expression (scores ≥1).
Follow-up
Patients were regularly followed up through medical
records (outpatient or inpatient), telephone interviews and
scheduled clinical visits. Follow-up assessments included: i)
Clinical symptom observation; ii) routine hematological tests (for
example, complete blood count, blood biochemistry and tumor
markers); and iii) imaging examinations (CT or MRI). Postoperative
surveillance was conducted every 3–6 months. If tumor progression,
recurrence or metastasis was suspected during follow-up, a repeat
biopsy was performed for reassessment and re-grading. Disease free
survival (DFS) was defined as the time from initial diagnosis to
recurrence or to the last follow-up. OS was defined as the time
from initial diagnosis to death or to the last follow-up.
Statistical analysis
All statistical analyses were performed using SPSS
19.0 (IBM Corp.) and GraphPad Prism 8.0 (Dotmatics). χ2
test and Fisher's exact test was used to assess associations
between categorical variables and multiple groups. Kaplan-Meier
estimates were generated to analyze survival, and differences
between survival curves were assessed by log-rank test. Univariate
and multivariate Cox proportional hazards models were fitted to
identify the potential prognostic factors. Significant variables
from the univariate analysis (P<0.05) were included in the
multivariate Cox regression model. To address potential confounding
from variations in treatment strategies (such as postoperative
chemotherapy and radiotherapy), these factors were included as
covariates in the multivariate models. The multivariate analyses
formed the basis for constructing prognostic nomograms for DFS and
OS probabilities. Nomogram validation comprised calculation of the
C-index and the generation of calibration curves. A two-tailed
P<0.05 was considered to indicate a statistically significant
difference.
Results
Study population and
characteristics
Overall, 157 patients with CRNEC were enrolled onto
the present study, including 102 men (65%) and 55 women (35%). The
average age of enrolled patients was 54.89±12.86 years, ranging
from 26 to 82 years old. The average body mass index (BMI) was
24.11±3.22 kg/m2, ranging from 17.10 to 32.10
kg/m2. The primary site of 32 cases (20.4%) was the
colon, while the primary site for the other 125 cases (79.6%) was
the rectum. During the study period, regarding the
tumor-node-metastasis (TNM) stage of patients (14), there were four cases (2.5%) with
Tis, 63 cases (40.1%) with stage I, five cases (3.2%) with stage
II, 28 cases (17.8%) with stage III, 43 cases (27.4%) with stage IV
and 14 cases (8.9%) with an unknown stage. The gross type of CRNECs
included elevated type in 72 cases (45.9%), ulcerative type in 22
cases (14.0%) and infiltrative type in 63 cases (40.1%). Lymph node
dissection was performed in 46.1% (59/128) of surgically treated
patients. All enrolled patients were followed-up, and the last
follow-up date was October 2024. There were 39 cases (24.8%) with
distant metastasis and 15 cases (9.6%) with recurrence during the
follow-up time. The clinicopathological characteristics of the
patients are summarized in Table
SI.
Histological types in patients with
CRNEC
Based on the WHO 2019 Classification of Digestive
System Tumors (5th edition) (4),
the pathological types included in the present study were as
follows: i) 82 cases (52.2%) with NET-G1; ii) 13 cases (8.3%) with
NET-G2; iii) 54 cases (34.4%) with NEC-G3; and iv) eight cases
(5.1%) with MANEC.
The clinicopathological characteristics of patients
with different histological subtypes of CRNEC are detailed in
Table I. In the current study, the
total number of lymph nodes includes both metastatic and
non-metastatic lymph nodes, whereas the positive number of lymph
nodes includes only metastatic lymph nodes. Regarding the
clinicopathological characteristics of the patients, the
pathological subtypes were significantly associated with age,
surgical history, primary site, gross type, tumor size,
differentiation, total number of lymph node, positive number of
lymph node, TNM stage, perineural invasion, vascular tumor
thrombus, AE1/AE3, postoperative chemotherapy and postoperative
radiotherapy (all P<0.05).
 | Table I.Clinicopathological characteristics
of patients with colorectal neuroendocrine carcinoma of different
pathological subtypes. |
Table I.
Clinicopathological characteristics
of patients with colorectal neuroendocrine carcinoma of different
pathological subtypes.
| Parameters | Group | NET-G1 | NET-G2 | NEC-G3 | MANEC | P-value |
|---|
| Total | - | 82 | 13 | 54 | 8 |
|
| Sex | Male | 53 (64.6) | 7 (53.8) | 39 (72.2) | 3 (37.5) | 0.330 |
|
| Female | 29 (35.4) | 6 (46.2) | 15 (27.8) | 5 (62.5) |
|
| Body mass index,
kg/m2 | ≤24.49 | 41 (50.0) | 4 (30.8) | 27 (50.0) | 5 (62.5) | 0.669 |
|
| >24.49 | 41 (50.0) | 9 (69.2) | 27 (50.0) | 3 (37.5) |
|
| Age, years | ≤55 | 46 (56.1) | 9 (69.2) | 17 (31.5) | 3 (37.5) | 0.029 |
|
| >55 | 36 (43.9) | 4 (30.8) | 37 (68.5) | 5 (62.5) |
|
| Surgical
history | No | 66 (80.5) | 7 (53.8) | 47 (87.0) | 5 (62.5) | 0.005 |
|
| Yes | 16 (19.5) | 6 (46.2) | 7 (13.0) | 3 (37.5) |
|
| Hypertension | No | 64 (78.0) | 9 (69.2) | 51 (94.4) | 7 (87.5) | 0.076 |
|
| Yes | 18 (22.0) | 4 (30.8) | 3 (5.6) | 1 (12.5) |
|
| Diabetes | No | 71 (86.6) | 12 (92.3) | 51 (94.4) | 7 (87.5) | 0.5675 |
|
| Yes | 11 (13.4) | 1 (7.7) | 3 (5.6) | 1 (12.5) |
|
| Primary site | Colon | 6 (7.3) | 3 (23.1) | 19 (35.2) | 4 (50.0) | <0.001 |
|
| Rectum | 76 (92.7) | 10 (76.9) | 35 (64.8) | 4 (50.0) |
|
| Gross type | Elevated type | 55 (67.1) | 4 (30.8) | 11 (20.4) | 2 (25.0) | <0.001 |
|
| Ulcerative
type | 2 (2.4) | 0 (0.0) | 18 (33.3) | 2 (25.0) |
|
|
| Infiltrative
type | 25 (30.5) | 9 (69.2) | 25 (46.3) | 4 (50.0) |
|
| Tumor size, cm | ≤2 | 66 (80.5) | 6 (46.2) | 8 (14.8) | 1 (12.5) | <0.001 |
|
| >2 | 3 (3.7) | 4 (30.8) | 28 (51.9) | 6 (75.0) |
|
|
| Unknown | 13 (15.9) | 3 (23.1) | 18 (33.3) | 1 (12.5) |
|
| Differentiation
grade | Low-grade (G1) | 80 (97.6) | 0 (0.0) | 1 (1.9) | 2 (25.0) | <0.001 |
|
| Intermediate- |
|
|
|
|
|
|
| grade (G2) | 1 (1.2) | 13 (100.0) | 1 (1.9) | 1 (12.5) |
|
|
| High-grade
(G3) | 1 (1.2) | 0 (0.0) | 52 (96.3) | 5 (62.5) |
|
| Total number of
lymph node | ≤18 | 10 (12.2) | 6 (46.2) | 19 (35.2) | 2 (25.0) | <0.001 |
|
| >18 | 4 (4.9) | 2 (15.4) | 11 (20.4) | 5 (62.5) |
|
|
| Unknown | 68 (82.9) | 5 (38.5) | 24 (44.4) | 1 (12.5) |
|
| Positive number of
lymph node | ≤5 | 11 (13.4) | 8 (61.5) | 17 (31.5) | 4 (50.0) | <0.001 |
|
| >5 | 3 (3.7) | 0 (0.0) | 13 (24.1) | 3 (37.5) |
|
|
| Unknown | 68 (82.9) | 5 (38.5) | 24 (44.4) | 1 (12.5) |
|
| TNM stage | Tis | 2 (2.4) | 1 (7.7) | 1 (1.9) | 0 (0.0) | <0.001 |
|
| I | 57 (69.5) | 1 (7.7) | 4 (7.4) | 1 (12.5) |
|
|
| II | 1 (1.2) | 1 (7.7) | 3 (5.6) | 0 (0.0) |
|
|
| III | 6 (7.3) | 2 (15.4) | 17 (31.5) | 3 (37.5) |
|
|
| IV | 6 (7.3) | 5 (38.5) | 28 (51.9) | 4 (50.0) |
|
|
| Unknown | 10 (12.2) | 3 (23.1) | 1 (1.9) | 0 (0.0) |
|
| Perineural
invasion | No | 80 (97.6) | 11 (84.6) | 49 (90.7) | 5 (62.5) | 0.006 |
|
| Yes | 2 (2.4) | 2 (15.4) | 5 (9.3) | 3 (37.5) |
|
| Vascular tumor
thrombus | No | 81 (98.8) | 10 (76.9) | 38 (70.4) | 5 (62.5) | <0.001 |
|
| Yes | 1 (1.2) | 3 (23.1) | 16 (29.6) | 3 (37.5) |
|
| AE1/AE3 | Negative | 29 (35.4) | 6 (46.2) | 47 (87.0) | 4 (50.0) | <0.001 |
|
| Positive | 53 (64.6) | 7 (53.8) | 7 (13.0) | 4 (50.0) |
|
| Postoperative
chemotherapy | No | 79 (96.3) | 8 (61.5) | 27 (50.0) | 5 (62.5) | <0.001 |
|
| Yes | 3 (3.7) | 5 (38.5) | 27 (50.0) | 3 (37.5) |
|
| Postoperative
radiotherapy | No | 82 (100.0) | 13 (100.0) | 48 (88.9) | 7 (87.5) | 0.024 |
|
| Yes | 0 (0.0) | 0 (0.0) | 6 (11.1) | 1 (12.5) |
|
The mean DFS and OS durations stratified by
pathological subtypes were as follows: i) In the NET-G1 group, DFS
was 65.63 months (95% CI, 51.70–71.43) and OS was 103.00 months
(95% CI, 92.43–109.80); ii) in the NET-G2 group, DFS was 25.20
months (95% CI, 15.20–54.60) and OS was 61.73 months (95% CI,
48.80–91.13); iii) in the NEC-G3 group, DFS was 24.87 months (95%
CI, 13.50–34.57) and OS was 50.45 months (95% CI, 25.90–71.10); and
iv) in the MANEC group, DFS was 42.32 months (95% CI, 16.53–92.17)
and OS was 70.22 months (95% CI, 16.53–128.70). Comparative
analysis revealed statistically significant differences in both DFS
(χ2=40.110, P<0.0001) and OS (χ2=38.290,
P<0.0001) among the four pathological subtypes. The survival
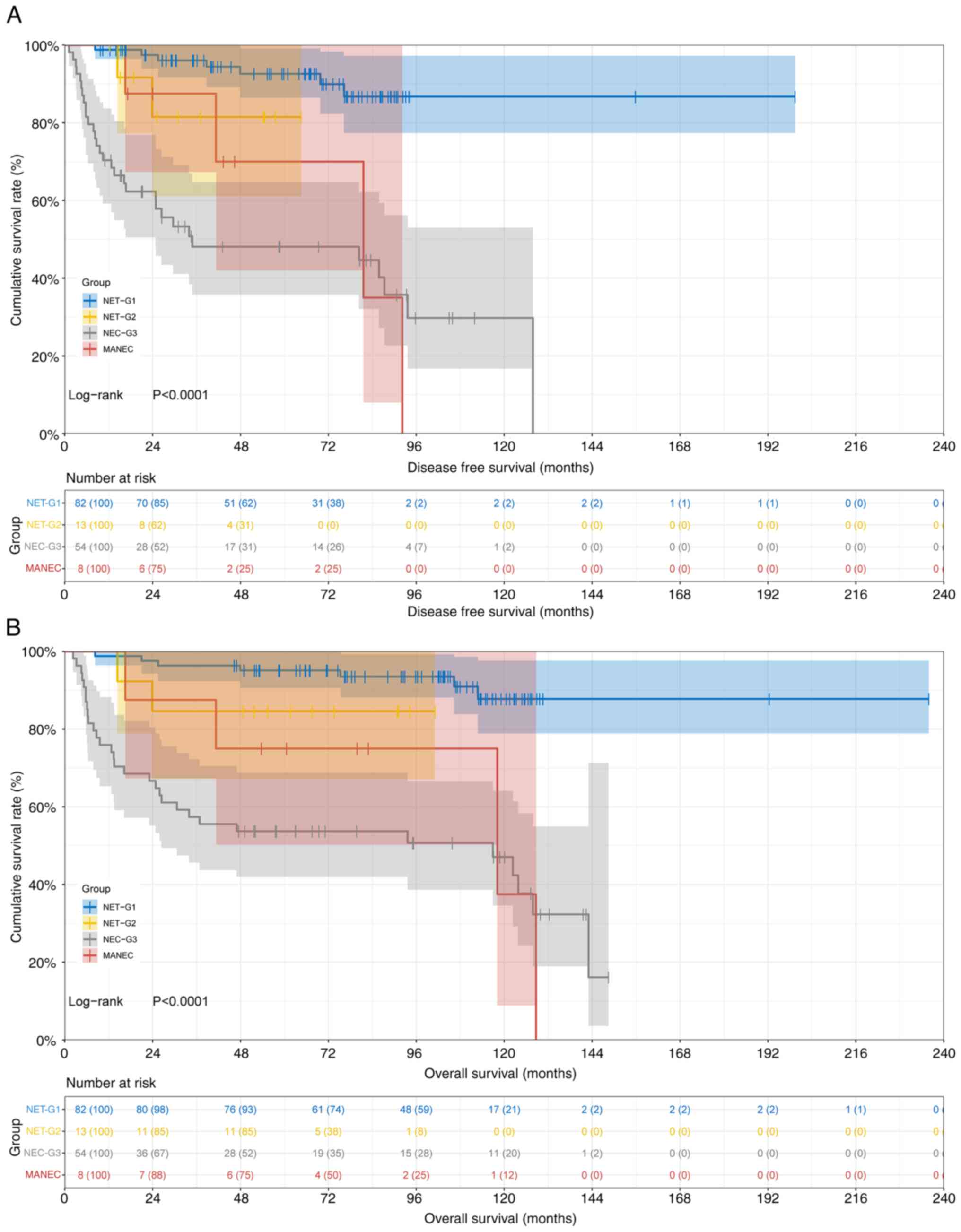
curves are presented in Fig. 1.
Respectively, the 1-, 3- and 5-year survival rates
of DFS and OS stratified by pathological subtypes were as follows:
i) In the NET-G1 group, 0.988 (95% CI, 0.964–1.000), 0.960 (95% CI,
0.917–1.000) and 0.926 (95% CI, 0.865–0.991) for DFS, and 0.988
(95% CI: 0.964–1.000), 0.963 (95% CI: 0.924–1.000), 0.951 (95% CI,
0.905–0.999) for OS; ii) in the NET-G2 group, 1.000 (95% CI,
1.000–1.000), 0.815 (95% CI, 0.611–1.000) and 0.815 (95% CI,
0.611–1.000) for DFS, and 1.000 (95% CI, 1.000–1.000), 0.846 (95%
CI, 0.671–1.000) and 0.846 (95% CI, 0.671–1.000) for OS; iii) in
the NEC-G3 group, 0.704 (95% CI, 0.592–0.837), 0.481 (95% CI,
0.358–0.647) and 0.481 (95% CI, 0.358–0.647) for DFS, and 0.759
(95% CI, 0.653–0.882), 0.574 (95% CI, 0.456–0.722) and 0.537 (95%
CI, 0.419–0.688) for OS; and iv) in the MANEC group, 1.000 (95% CI,
1.000–1.000), 0.875 (95% CI, 0.673–1.000) and 0.700 (95% CI,
0.420–1.000) for DFS, and 1.000 (95% CI, 1.000–1.000), 0.875 (95%
CI, 0.673–1.000) and 0.750 (95% CI, 0.503–1.000) for OS. The
analysis revealed significantly higher 1-, 3- and 5-year DFS and OS
rates for NET-G1 and NET-G2 groups compared with the NEC-G3 group,
with the MANEC group showing intermediate outcomes. The prognosis
was strongly associated with pathological subtype. These findings
highlight the prognostic heterogeneity among the CRNEC subtypes,
with NET-G1 exhibiting the most favorable outcomes, underscoring
the importance of precise histological classification for clinical
management.
Differentiation in patients with
CRNEC
According to ENETS 2017 guidelines, the
differentiation types included in the present study were as
follows: i) 83 cases (52.9%) with low-grade (G1) CRNEC; ii) 16
cases (10.2%) with intermediate-grade (G2) CRNEC; and iii) 58 cases
(36.9%) with high-grade (G3) CRNEC. The clinicopathological
characteristics of patients with CRNEC according to differentiation
subtypes are detailed in Table II.
Regarding the clinicopathological characteristics of the patients,
the differentiation subtypes were associated with age, surgical
history, primary site, gross type, tumor size, histological type,
total lymph node, positive lymph node, TNM stage, vascular tumor
thrombus, AE1/AE3 and postoperative chemotherapy (P<0.05).
 | Table II.Clinicopathological characteristics
of patients with colorectal neuroendocrine carcinoma of
differentiation types. |
Table II.
Clinicopathological characteristics
of patients with colorectal neuroendocrine carcinoma of
differentiation types.
| Parameters | Group | N | G1 | G2 | G3 | P-value |
|---|
| Total | - | 157 | 83 | 16 | 58 |
|
| Sex | Male | 102 (65.0) | 55 (66.3) | 8 (50.0) | 39 (67.2) | 0.413 |
|
| Female | 55 (35.0) | 28 (33.7) | 8 (50.0) | 19 (32.8) |
|
| Body mass index,
kg/m2 | ≤24.49 | 77 (49.0) | 40 (48.2) | 6 (37.5) | 31 (53.4) | 0.515 |
|
| >24.49 | 80 (51.0) | 43 (51.8) | 10 (62.5) | 27 (46.6) |
|
| Age, years | ≤55 | 75 (47.8) | 46 (55.4) | 10 (62.5) | 19 (32.8) | 0.014 |
|
| >55 | 82 (52.2) | 37 (44.6) | 6 (37.5) | 39 (67.2) |
|
| Surgical
history | No | 125 (79.6) | 68 (81.9) | 7 (43.8) | 50 (86.2) | 0.001 |
|
| Yes | 32 (20.4) | 15 (18.1) | 9 (56.2) | 8 (13.8) |
|
| Hypertension | No | 131 (83.4) | 66 (79.5) | 12 (75.0) | 53 (91.4) | 0.095a |
|
| Yes | 26 (16.6) | 17 (20.5) | 4 (25.0) | 5 (8.6) |
|
| Diabetes | No | 141 (89.8) | 73 (88.0) | 14 (87.5) | 54 (93.1) | 0.583a |
|
| Yes | 16 (10.2) | 10 (12.0) | 2 (12.5) | 4 (6.9) |
|
| Primary site | Colon | 32 (20.4) | 7 (8.4) | 3 (18.8) | 22 (37.9) |
<0.001a |
|
| Rectum | 125 (79.6) | 76 (91.6) | 13 (81.2) | 36 (62.1) |
|
| Gross type | Elevated type | 72 (45.9) | 56 (67.5) | 5 (31.2) | 11 (19.0) |
<0.001a |
|
| Ulcerative
type | 22 (14.0) | 2 (2.4) | 0 (0.0) | 20 (34.5) |
|
|
| Infiltrative
type | 63 (40.1) | 25 (30.1) | 11 (68.8) | 27 (46.6) |
|
| Tumor size, cm | ≤2 | 81 (51.6) | 67 (80.7) | 7 (43.8) | 7 (12.1) |
<0.001a |
|
| >2 | 41 (26.1) | 5 (6.0) | 5 (31.2) | 31 (53.4) |
|
|
| Unknown | 35 (22.3) | 11 (13.3) | 4 (25.0) | 20 (34.5) |
|
| Histological
type | NET-G1 | 82 (52.2) | 80 (96.4) | 1 (6.2) | 1 (1.7) |
<0.001a |
|
| NET-G2 | 13 (8.3) | 0 (0.0) | 13 (81.2) | 0 (0.0) |
|
|
| NEC-G3 | 54 (34.4) | 1 (1.2) | 1 (6.2) | 52 (89.7) |
|
|
| MANEC | 8 (5.1) | 2 (2.4) | 1 (6.2) | 5 (8.6) |
|
| Total number
of | ≤18 | 37 (23.6) | 11 (13.3) | 7 (43.8) | 19 (32.8) |
<0.001a |
| lymph node | >18 | 22 (14.0) | 6 (7.2) | 3 (18.8) | 13 (22.4) |
|
|
| Unknown | 98 (62.4) | 66 (79.5) | 6 (37.5) | 26 (44.8) |
|
| Positive number
of | ≤5 | 40 (25.5) | 12 (14.5) | 9 (56.2) | 19 (32.8) |
<0.001a |
| lymph node | >5 | 19 (12.1) | 5 (6.0) | 1 (6.2) | 13 (22.4) |
|
|
| Unknown | 98 (62.4) | 66 (79.5) | 6 (37.5) | 26 (44.8) |
|
| TNM stage | Tis | 4 (2.5) | 2 (2.4) | 1 (6.2) | 1 (1.7) |
<0.001a |
|
| I | 63 (40.1) | 59 (71.1) | 1 (6.2) | 3 (5.2) |
|
|
| II | 5 (3.2) | 1 (1.2) | 1 (6.2) | 3 (5.2) |
|
|
| III | 28 (17.8) | 8 (9.6) | 3 (18.8) | 17 (29.3) |
|
|
| IV | 43 (27.4) | 4 (4.8) | 7 (43.8) | 32 (55.2) |
|
|
| Unknown | 14 (8.9) | 9 (10.8) | 3 (18.8) | 2 (3.4) |
|
| Perineural
invasion | No | 145 (92.4) | 80 (96.4) | 14 (87.5) | 51 (87.9) | 0.085a |
|
| Yes | 12 (7.6) | 3 (3.6) | 2 (12.5) | 7 (12.1) |
|
| Vascular tumor
thrombus | No | 134 (85.4) | 79 (95.2) | 13 (81.2) | 42 (72.4) | 0.001a |
|
| Yes | 23 (14.6) | 4 (4.8) | 3 (18.8) | 16 (27.6) |
|
| AE1/AE3 | Negative | 86 (54.8) | 30 (36.1) | 7 (43.8) | 49 (84.5) | <0.001 |
|
| Positive | 71 (45.2) | 53 (63.9) | 9 (56.2) | 9 (15.5) |
|
| Postoperative
chemotherapy | No | 119 (75.8) | 80 (96.4) | 8 (50.0) | 31 (53.4) |
<0.001a |
|
| Yes | 38 (24.2) | 3 (3.6) | 8 (50.0) | 27 (46.6) |
|
| Postoperative
radiotherapy | No | 150 (95.5) | 82 (98.8) | 15 (93.8) | 53 (91.4) | 0.095a |
|
| Yes | 7 (4.5) | 1 (1.2) | 1 (6.2) | 5 (8.6) |
|
The mean DFS and OS durations stratified by
differentiation subtypes were as follows: i) In the low-grade (G1)
group, DFS was 65.73 months (95% CI, 55.33–71.43) and OS was 103.30
months (95% CI, 92.90–109.80); ii) in the intermediate-grade (G2)
group, DFS was 24.57 months (95% CI, 14.40–54.33) and OS was 58.58
months (95% CI, 46.97–90.87); and iii) in the high-grade (G3)
group, DFS was 25.67 months (95% CI, 16.23–34.83), OS was 51.90
months (95% CI, 26.43–71.10). Comparative analysis revealed
statistically significant differences in both DFS
(χ2=42.910; P<0.0001) and OS (χ2=41.220;
P<0.0001) among the three differentiation subtypes. The survival
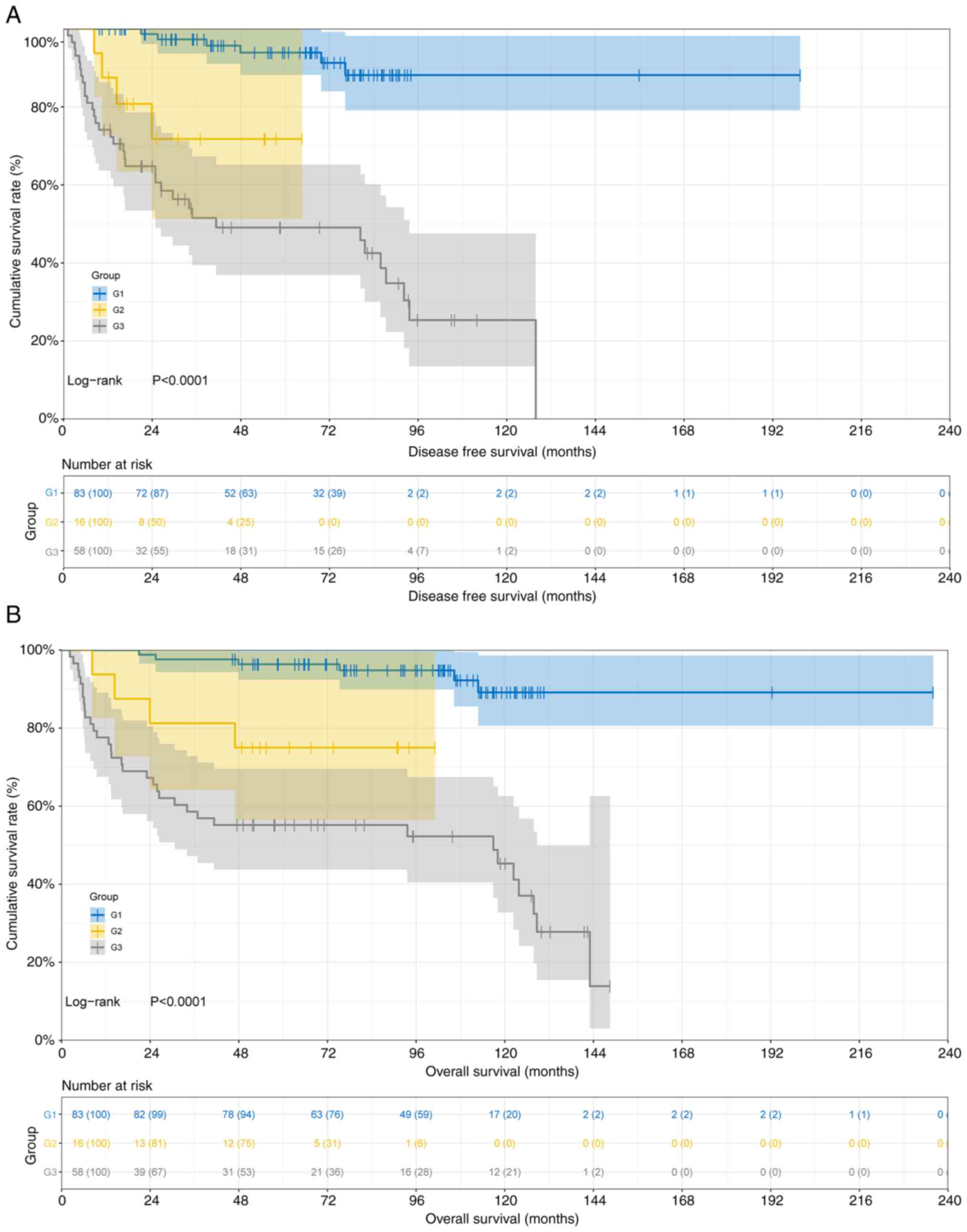
curves are presented in Fig. 2.
Respectively, the 1-, 3- and 5-year survival rates
of DFS and OS stratified by pathological subtypes were as follows:
i) in the low-grade (G1) group, 100.0% (95% CI, 100.0–100.0), 97.3%
(95% CI, 93.7–100.0) and 93.9% (95% CI, 88.2–99.9) for DFS, and
100.0% (95% CI, 100.0–100.0), 97.6% (95% CI, 94.3–100.0) and 96.4%
(95% CI, 92.4–100.0) for OS; ii) in the intermediate-grade (G2)
group, 87.5% (95% CI 72.7–100.0), 71.8% (95% CI, 51.4–100.0) and
71.8% (95% CI, 51.4–100.0) for DFS, and 93.8% (95% CI, 82.6–100.0),
81.2% (95% CI, 64.2–100.0) and 75.0% (95% CI, 56.5–99.5) for OS;
and 3) in the high-grade (G3) group, 74.1% (95% CI, 63.7–86.3),
51.6% (95% CI, 39.5–67.3) and 49.1% (95% CI, 37.0–65.2) for DFS,
and 77.6% (95% CI, 67.6–89.1), 58.6% (95% CI, 47.2–72.8) and 55.2%
(95% CI, 43.7–69.6) for OS. The significant survival differences
(P<0.0001) among the differentiation grades emphasized that
high-grade (G3) tumors require aggressive therapeutic strategies,
while low-grade (G1) tumors may benefit from less intensive
interventions.
Related prognostic factors identified
by Cox regression analysis
According to univariate Cox regression analysis,
BMI, age, alcohol drinking, histological type and differentiation
were the related factors affecting DFS in patients CRNEC. The
multivariate analysis indicated that BMI, age and differentiation
were the potential prognostic factors affecting DFS in patients
with CRNEC (Table SII). Based on
univariate Cox regression analysis, sex, age, diabetes and
differentiation were significantly related to OS in patients with
CRNEC (Table SIII). The
multivariate analysis identified that sex, age and differentiation
were the potential prognostic factors affecting OS in patients with
CRNEC. The identification of age and differentiation as the
potential prognostic factors for DFS and OS provided a foundation
for risk stratification and personalized treatment planning in
patients with CRNEC.
Establishing a prognostic
nomogram
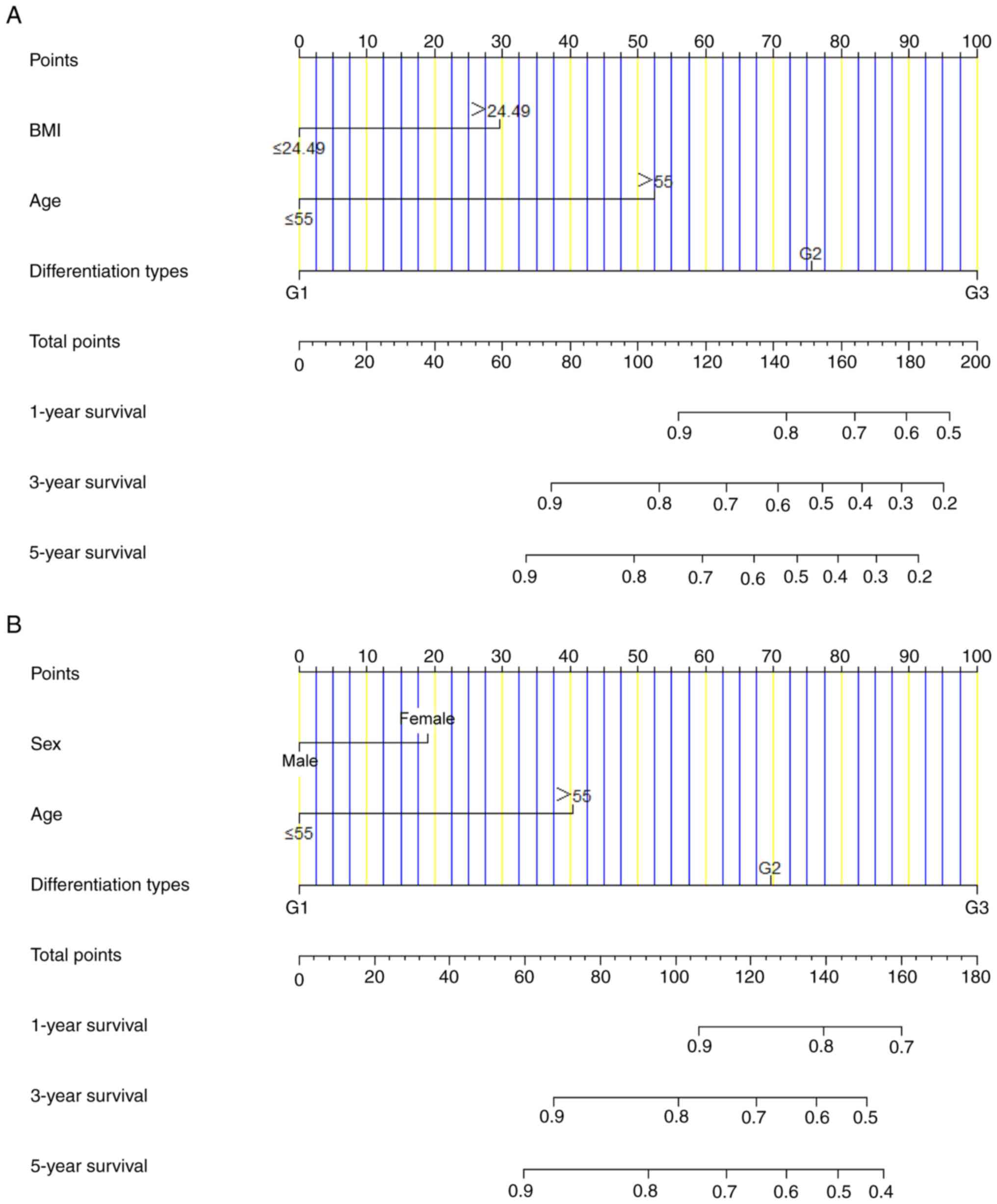
Based on the univariate and multivariate Cox
regression analyses, nomograms were developed to predict 1-, 3- and
5-year DFS and OS in patients with CRNEC. The parameters with
P<0.05, including BMI, age and differentiation were selected via
multivariate analyses to construct a nomogram prognostic model of
DFS (Fig. 3A). The C-index for this
nomogram prognostic model predicting DFS was 0.843 (95% CI,
0.665–0.936). Moreover, the parameters with P<0.05, including
sex, age and differentiation were selected to comprise the nomogram
prognostic model of OS (Fig. 3B).
The C-index for this nomogram prognostic model predicting OS was
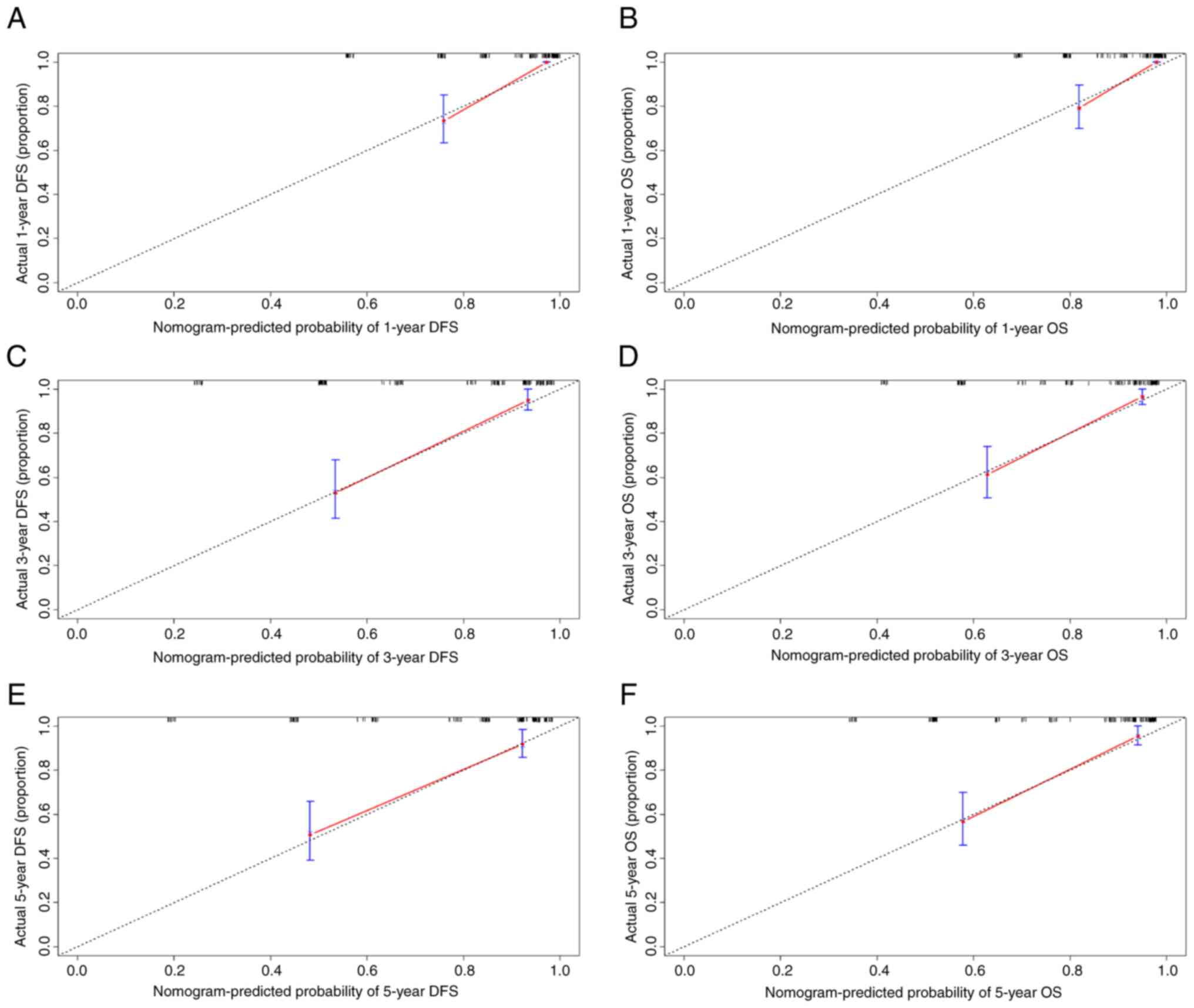
0.819 (95% CI, 0.644–0.919). Calibration curves with 1,000
bootstrap iterations quantified the precision of the nomograms in
estimating DFS and OS probabilities at 1-, 3- and 5-year using the
performance of the Cox regression model. The predicted and observed
survival probabilities showed strong concordance across different
time points (1-, 3- and 5-year), as evidenced by calibration curves
closely aligning with the 45° reference line (Fig. 4A-F). The high C-index values and
well-calibrated curves demonstrated the robust spredictive accuracy
of the nomogram, offering clinicians a practical tool for
individualized prognostic assessment.
Subgroup analysis for AE1/AE3
According to the results of Tables I and II, AE1/AE3 was significantly associated
with histological type and differentiation. There were 71 cases
with positive expression of AE1/AE3, and 86 cases with negative
expression of AE1/AE3; representative immunohistochemistry images
are shown in Fig. S1. The
clinicopathological characteristics of patients with CRNEC split
according to AE1/AE3 expression are detailed in Table SIV. Regarding the
clinicopathological characteristics of the patients, AE1/AE3
expression was associated with gross type, tumor size, histological
type, differentiation, TNM stage and postoperative chemotherapy
(P<0.05).
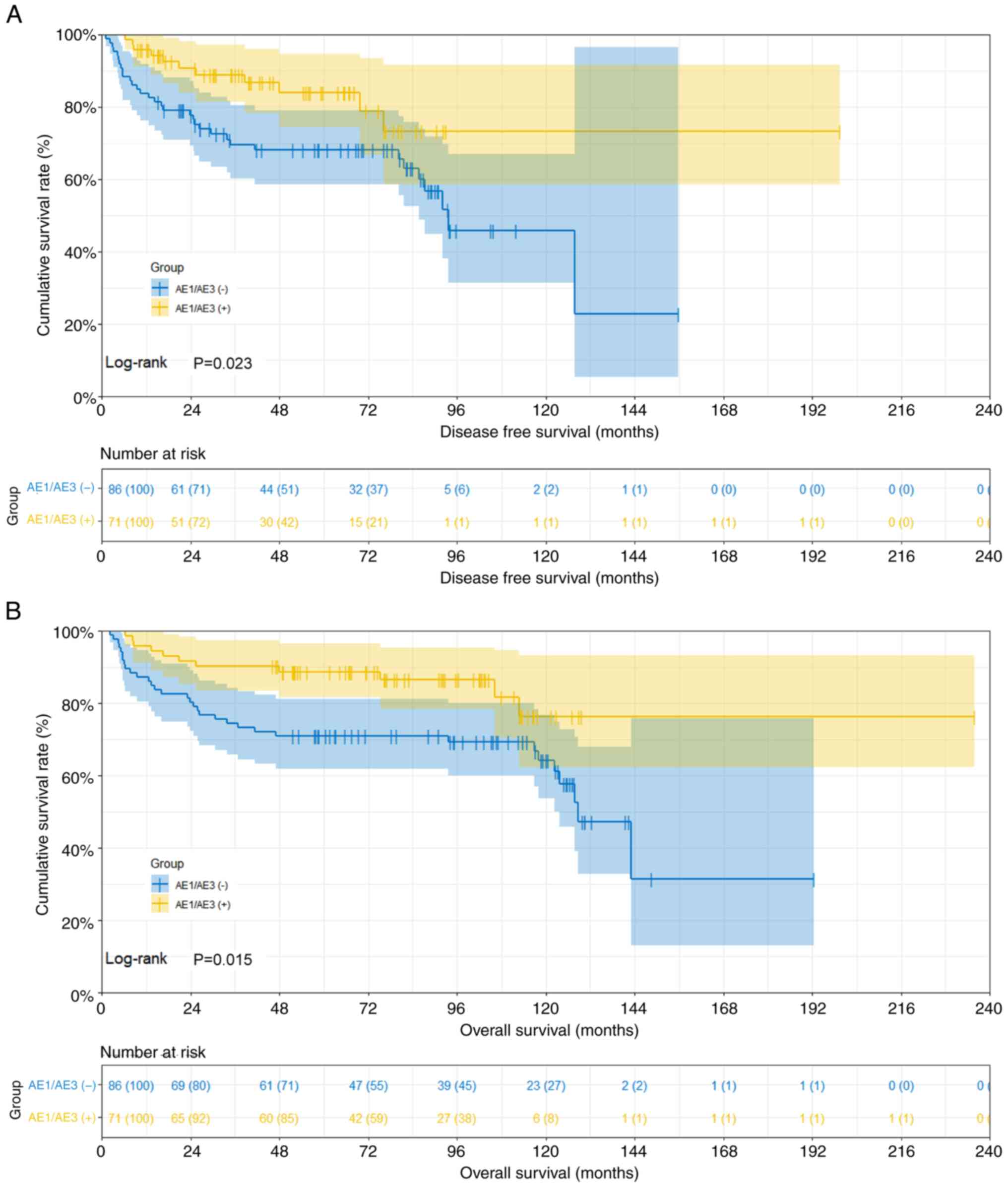
The mean DFS and OS durations stratified by AE1/AE3
expression were as follows: i) In patients with AE1/AE3(−), DFS was
40.57 months (95% CI, 31.00–56.37) and OS was 79.23 months (95% CI,
67.53–96.33); ii) in patients with AE1/AE3(+), DFS was 53.02 months
(95% CI, 29.60–70.23) and OS was 92.25 months (95% CI,
63.03–106.80). Comparative analysis revealed statistically
significant differences in both DFS and OS among AE1/AE3 expression
groups (DFS: χ2=5.162, P=0.023; OS: χ2=6.681,
P=0.015). The survival curves are presented in Fig. 5.
The 1-, 3- and 5-year survival rates of DFS and OS
stratified by AE1/AE3 expression were as follows: i) In patients
with AE1/AE3(−), 83.7% (95% CI, 76.3–91.9), 69.5% (95% CI,
60.2–80.4) and 68.0% (95% CI, 58.5–79.1) for DFS, and 87.2% (95%
CI, 80.4–94.6), 74.4% (95% CI, 65.7–84.2) and 70.9% (95% CI,
62.0–81.2) for OS, respectively; and ii) in patients with
AE1/AE3(+), 95.8% (95% CI, 91.2–100.0), 88.9% (95% CI, 81.4–97.1)
and 83.8% (95% CI, 74.4–94.6) for DFS, and 95.8% (95% CI,
91.2–100.0), 90.1% (95% CI, 83.5–97.3) and 88.7% (95% CI,
81.6–96.4) for OS, respectively (Fig.
5). The association of AE1/AE3 positivity with prolonged
survival suggested its potential role as a favorable biomarker for
tumor biology, which could aid in diagnostic and therapeutic
decision-making.
Subgroup analysis for age
Based on the univariate and multivariate Cox
regression analyses, age was indicated as a potential prognostic
factor affecting DFS and OS in patients with CRNEC. The median age
of enrolled patients was 55 years; according to the median age,
there were 75 patients aged ≤55 years and 82 cases patients aged
>55 years. Notably, patients >55 years old had a poorer
prognosis than those aged ≤55 years (DFS: χ2=11.620;
P=0.0007; OS: χ2=12.450; P=0.0005) (Fig. S2).
Discussion
CRNEC is a relatively rare malignancy, accounting
for 20–30% of all gastroenteropancreatic NENs (GEP-NENs) and <2%
of all colorectal cancers. A majority of CRNECs are non-functional,
exhibiting non-specific symptomatology indistinguishable from
colorectal adenocarcinoma, including abdominal pain, altered bowel
habits and hematochezia (15).
CRNECs are typically more aggressive than conventional colorectal
carcinoma, with higher rates of metastasis and poorer survival
outcomes (16). With advancements
in diagnostic technologies, the improvement of living standards and
increased health awareness, the detection rate of CRNEC has notably
risen in recent years (17). The
diagnosis of CRNEC primarily relies on colonoscopy, biopsy and
pathology after surgery, with definitive confirmation requiring
immunohistochemical evidence of neuroendocrine markers, such as
Syn, chromogranin A and neuron-specific enolase (18). Surgical resection is the main
treatment for CRNEC, while chemotherapy serves a role as a crucial
adjuvant treatment, particularly for tumors with Ki-67 >5%,
utilizing regimens including doxorubicin, etoposide and
fluorouracil (19). By contrast,
conventional colorectal carcinoma is primarily managed with surgery
and chemotherapy regimens such as FOLFOX, CAPEOX or FOLFIRI
(20).
The present study analyzed the clinicopathological
characteristics of 157 cases of CRNEC, including 32 cases of
colonic NETs and 125 cases of rectal NETs. According to data from
China, rectal NENs are the predominant type of gastrointestinal
NETs, and the incidence rate has increased in recent years
(21). Studies have also
demonstrated that tumor stage and tumor size are the critical
prognostic factors for CRNEC (22,23).
According to the 5th edition of the WHO Classification of Digestive
System Tumors (2019), NETs can be classified as NET-G1, NET-G2,
NEC-G3 and MANEC (24). In the
present study, of all pathological types included, there were 82
cases graded as NET-G1, 13 cases of NET-G2, 54 cases of NEC-G3 and
eight cases of MANEC. The results of the current study demonstrated
that the different pathological types were associated with age,
gross types, differentiation and AE1/AE3. Additional analysis of
prognostic outcomes in patients with CRNEC indicated that the
NET-G1 pathological subtype was associated with significantly
prolonged DFS and OS, as well as improved prognosis, when compared
with the other pathological subtypes. The results of the present
study align with prior evidence, which demonstrated that patients
with pancreatic NET-G1/2 had a significantly longer survival time
compared with that of patients with poorly differentiated NEC
(P=0.002) (25). Punekar et
al (26) revealed that patients
with NET-G1/2 had an improved OS rate than those with NEC-G3 and
MANEC in colorectal NETs in the SEER database.
The potential mechanisms are that NEC-G3 and MANEC
are associated with larger tumors with more aggressive histological
features, and more metastatic sites compared with NET-G1/G2. NECs
are genomically distinct entities characterized by obligate
inactivation of the TP53 and Rb/p16 tumor suppressor pathways
(27). Tanaka et al
(28) observed that MANEC, albeit
rare, has a highly aggressive clinical course, and that
curative-intent surgery with adjuvant chemotherapy could markedly
prolong survival in localized cases.
The present study also analyzed the
clinicopathological features among the various differentiation
types in patients with CRNEC. Regarding differentiation types,
there were 83 low-grade (G1) cases, 16 intermediate-grade (G2)
cases and 58 high-grade (G3) cases. The present study revealed that
the differentiation types were related to age, primary site,
histological type, TNM stage and AE1/AE3 expression. Subsequent
analysis of the outcomes of patients with CRNEC revealed that the
low-grade (G1) subtype was associated with markedly extended DFS
and OS durations and greater prognostic results compared with THE
alternative differentiation classifications. Pommergaard et
al (29) demonstrated that
surgical resection of primary tumors provided favorable long-term
survival in locoregional high-grade GEP-NENs and mixed
neuroendocrine-non-neuroendocrine neoplasms (MiNENs). Holmager
et al (30) demonstrated
that high-grade MiNENs have a neuroendocrine and a
non-neuroendocrine component that is associated with aggressive
biological behavior and unfavorable clinical outcomes. Another
study indicated that high-grade CRNECs exhibit rapid disease
progression, and are characterized by limited therapeutic
responsiveness and low survival rates (31). Moreover, prognostic analysis
revealed that patients with high-grade NEC without metastatic
disease have an adenocarcinoma component within their tumor, or
their response to chemotherapy is associated with modestly improved
clinical outcomes. Alese et al (32) also demonstrated that patients with
high-grade pancreatic NETs exhibited a significantly shorter median
OS (6.0 months) than those with other high-grade gastrointestinal
NECs (9.9 months). The survival data of the present study indicated
that the prognosis of high-grade (G3) tumors was the poorest
compared with low-grade (G1) or intermediate-grade (G1) tumors,
which was consistent with the aforementioned studies.
Notably, the present study revealed that AE1/AE3
expression was related to histological type and differentiation.
According to immunohistochemistry, there were 71 cases with
positive expression of AE1/AE3 and 86 cases with negative
expression of AE1/AE3 in the current study. Subsequent analysis of
the outcomes of patients with CRNEC revealed that AE1/AE3(+)
expression was associated with markedly extended DFS and OS
durations and improved prognostic results compared with AE1/AE3 (−)
expression. AE1/AE3 expression was associated with
well-differentiated CRNEC and may indicate favorable outcomes,
while loss of expression was frequently observed in poorly
differentiated CRNEC. An AE1/AE3 cocktail is the gold standard for
detecting cytokeratin expression in immunohistochemistry. AE1
recognizes high (K10, K14-16) and low (K19) molecular weight
keratins, while AE3 targets type II high (K1-6) and low (K7-8)
molecular weight keratins, and their downregulated expression is a
hallmark of high-grade carcinomas with aberrant differentiation
(33). Badzio et al
(34) demonstrated that patients
with small cell lung cancer undergoing pulmonary resection with
high AE1/AE3 immunoreactivity demonstrated a superior median OS
compared with patients with low expression (24.7 months vs. 13.8
months; P=0.019). Vasilevska et al (35) revealed that AE1/AE3 immunoreactivity
was predominantly observed in moderately differentiated endometrial
carcinoma, and the clinical implication of AE1/AE3 might aid in the
diagnosis of early-stage endocrine carcinoma as well as aiding the
detection of micrometastases, leading to improved survival
outcomes. In addition, this previous study demonstrated that
AE1/AE3 negativity coincided with epithelial-mesenchymal transition
activation and aggressive behavior of the carcinoma, such as rapid
cell proliferation and metastasis (35). Although this has, to the best of our
knowledge, not yet been studied in CRNEC, similar mechanisms may
explain the observation in the present study that negative
expression of AE1/AE3 in tumors indicated higher rates of vascular
invasion and metastasis.
The present study investigated the potential
prognostic factors of CRNEC and developed a prognostic nomogram
model for DFS and OS. Univariate and multivariate analyses revealed
that BMI, age and differentiation were the potential independent
predictors of DFS, while sex, age and differentiation emerged as
significant determinants of OS. Differentiation grade stratifies
survival, with high-grade (G3) tumors predicting adverse outcomes
and reduced OS. Moreover, the present study constructed a
multivariate-derived prognostic nomogram that could provide higher
accuracy in predicting 1-, 3- and 5-year survival probabilities
than single conventional prognostic markers. Calibration analysis
demonstrated agreement between predicted and observed 1-, 3- and
5-year survival probabilities in patients with CRNEC, with the
model-derived curve closely aligning with the ideal reference
line.
Multivariate modeling confirmed age as a potential
prognostic factor for DFS and OS in CRNEC. In the present study,
patients >55 years old had poorer prognosis than those ≤55 years
(P<0.001). Lal et al (36) demonstrated that individuals with
large bowel carcinoids were more likely to be elderly (age >65
years) (OR 2.17; CI, 2.05–2.31; P<0.0001), and age was
identified as a potential risk factor (35). Another study also indicated that age
≥56 years (HR, 7.434; 95% CI, 1.334–41.443; P=0.022) was an
independent prognostic factor via multivariate analyses in
colorectal NETs (37).
Age-dependent therapeutic disparities likely contribute to these
findings, as younger patients demonstrated greater utilization of
different treatment modalities.
The present study had several limitations that
should be acknowledged. Firstly, this investigation employed a
single-center retrospective design, and thus has some inherent
limitations, including potential selection bias and residual
confounding factors despite multivariate adjustments. Secondly, the
conducted nomograms were derived from a restricted set of
covariates and require external validation in independent cohorts
to confirm generalizability. Thirdly, the low incidence of CRNEC
inherently limited the cohort size. Therefore, prospective
multicenter validation studies with larger samples are warranted to
confirm these preliminary findings.
In conclusion, the present study established a
nomogram to predict the prognosis of patients with CRNEC with good
prediction efficacy. Adverse prognostic factors for predicting DFS
and OS time include being aged >55 years and being diagnosed
with high-grade differentiation subtypes (including G2 and G3).
High-grade CRNECs represent highly aggressive malignancies
associated with an unfavorable clinical outcome. In addition,
AE1/AE3 may be helpful for improving the diagnostic accuracy of
patients with CRNEC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HL conceptualized and supervised the study, and was
project administrator. MD and SS acquired, analyzed and interpreted
the data. XL designed the methodology. MD and HL wrote the original
draft, and reviewed and edited the manuscript. MD and HL confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The study protocol received approval from the
Institutional Review Board of Harbin Medical University Cancer
Hospital (approval no. 82271845) and adhered strictly to the
ethical principles of the Declaration of Helsinki (1964) and
subsequent amendments. Before treatment, the enrolled patients
provided written informed consent for the use of their data in
research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mollazadegan K, Welin S and Crona J:
Systemic treatment of gastroenteropancreatic neuroendocrine
carcinoma. Curr Treat Options Oncol. 22:682021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yao JC, Hassan M, Phan A, Dagohoy C, Leary
C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A and Evans
DB: One hundred years after ‘carcinoid’: Epidemiology of and
prognostic factors for neuroendocrine tumors in 35,825 cases in the
United States. J Clin Oncol. 26:3063–3072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu IC, Chu YY, Wang YK, Tsai CL, Lin JC,
Kuo CH, Shih HY, Chung CS, Hu ML, Sun WC, et al:
Clinicopathological features and outcome of esophageal
neuroendocrine tumor: A retrospective multicenter survey by the
digestive endoscopy society of Taiwan. J Formos Med Assoc.
120:508–514. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagtegaal ID, Odze RD, Klimstra D, Paradis
V, Rugge M, Schirmacher P, Washington KM, Carneiro F and Cree IA;
WHO Classification of Tumours Editorial Board, : The 2019 WHO
classification of tumours of the digestive system. Histopathology.
76:182–188. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Panzuto F, Andrini E, Lamberti G, Pusceddu
S, Rinzivillo M, Gelsomino F, Raimondi A, Bongiovanni A, Davì MV,
Cives M, et al: Sequencing treatments in patients with advanced
well-differentiated pancreatic neuroendocrine tumor (pNET): Results
from a large multicenter Italian cohort. J Clin Med. 13:20742024.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sultana Q, Kar J, Verma A, Sanghvi S, Kaka
N, Patel N, Sethi Y, Chopra H, Kamal MA and Greig NH: A
comprehensive review on neuroendocrine neoplasms: Presentation,
pathophysiology and management. J Clin Med. 12:51382023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shia J, Tang LH, Weiser MR, Brenner B,
Adsay NV, Stelow EB, Saltz LB, Qin J, Landmann R, Leonard GD, et
al: Is nonsmall cell type high-grade neuroendocrine carcinoma of
the tubular gastrointestinal tract a distinct disease entity? Am J
Surg Pathol. 32:719–731. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ho YH, Hsu CY, Yau Li AF and Liang WY:
Colorectal neuroendocrine carcinoma and mixed
neuroendocrine-non-neuroendocrine neoplasm: Prognostic factors and
PD-L1 expression. Hum Pathol. 145:80–85. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tessier-Cloutier B, Kang EY, Alex D,
Stewart CJR, McCluggage WG, Köbel M and Lee CH: Endometrial
neuroendocrine carcinoma and undifferentiated carcinoma are
distinct entities with overlap in neuroendocrine marker expression.
Histopathology. 81:44–54. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gudmundsdottir H, Habermann EB, Vierkant
RA, Starlinger P, Thiels CA, Warner SG, Smoot RL, Truty MJ,
Kendrick ML, Halfdanarson TR, et al: Survival and symptomatic
relief after cytoreductive hepatectomy for neuroendocrine tumor
liver metastases: Long-term follow-up evaluation of more than 500
patients. Ann Surg Oncol. 30:4840–4851. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heetfeld M, Chougnet CN, Olsen IH, Rinke
A, Borbath I, Crespo G, Barriuso J, Pavel M, O'Toole D and Walter
T; Other Knowledge Network Members, : Characteristics and treatment
of patients with G3 gastroenteropancreatic neuroendocrine
neoplasms. Endocr Relat Cancer. 22:657–664. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mischel AM and Rosielle DA: Eastern
cooperative oncology group performance status #434. J Palliat Med.
25:508–510. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Perren A, Couvelard A, Scoazec JY, Costa
F, Borbath I, Delle Fave G, Gorbounova V, Gross D, Grossma A, Jense
RT, et al: Antibes consensus conference participants. Enets
consensus guidelines for the standards of care in neuroendocrine
tumors: Pathology: Diagnosis and prognostic stratification.
Neuroendocrinology. 105:196–200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Emile SH, Horesh N, Garoufalia Z, Gefen R,
Wignakumar A and Wexner SD: Predictors of lymph node metastasis and
survival in radically resected rectal neuroendocrine tumors: A
surveillance, epidemiology, and end results (SEER) database
analysis. Surgery. 176:668–675. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Donofrio CA, Pizzimenti C, Djoukhadar I,
Kearney T, Gnanalingham K and Roncaroli F: Colorectal carcinoma to
pituitary tumour: Tumour to tumour metastasis. Br J Neurosurg.
37:1367–1370. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng K, Duan J, Wang R, Chen H, He H,
Zheng X, Zhao Z, Jing B, Zhang Y, Liu S, et al: Deep learning model
with pathological knowledge for detection of colorectal
neuroendocrine tumor. Cell Rep Med. 5:1017852024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sabella G, Centonze G, Lagano V, Scardino
A, Belli F, Garzone G, Pardo C, Galbiati D, Pusceddu S, Mangogna A,
et al: Unveiling the prognostic role of synaptophysin in
conventional colorectal carcinomas. Neuroendocrinology. 15:632–647.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reina JJ, Serrano R, Codes M, Jiménez E,
Bolaños M, Gonzalez E and Sevilla I: Second primary malignancies in
patients with neuroendocrine tumors. Clin Transl Oncol. 16:921–926.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao W, Jin L, Chen P, Li D, Gao W and
Dong G: Colorectal cancer immunotherapy-Recent progress and future
directions. Cancer Lett. 545:2158162022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang J, Chen H, Zhang J, Wang S, Guan Y,
Gu W, Li J, Zhang X, Li J, Wang X, et al: Molecular features of
gastroenteropancreatic neuroendocrine carcinoma: A comparative
analysis with lung neuroendocrine carcinoma and digestive
adenocarcinomas. Chin J Cancer Res. 36:90–102. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gallo C, Rossi RE, Cavalcoli F, Barbaro F,
Boškoski I, Invernizzi P and Massironi S: Rectal neuroendocrine
tumors: Current advances in management, treatment, and
surveillance. World J Gastroenterol. 28:1123–1138. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chauhan A, Chan K, Halfdanarson TR,
Bellizzi AM, Rindi G, O'Toole D, Ge PS, Jain D, Dasari A, Anaya DA,
et al: Critical updates in neuroendocrine tumors: Version 9
American joint committee on cancer staging system for
gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin.
74:359–367. 2024.PubMed/NCBI
|
|
23
|
Yoshida T, Kamimura K, Hosaka K, Doumori
K, Oka H, Sato A, Fukuhara Y, Watanabe S, Sato T, Yoshikawa A, et
al: Colorectal neuroendocrine carcinoma: A case report and review
of the literature. World J Clin Cases. 7:1865–1875. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rindi G, Mete O, Uccella S, Basturk O, La
Rosa S, Brosens LAA, Ezzat S, de Herder WW, Klimstra DS, Papotti M
and Asa SL: Overview of the 2022 WHO classification of
neuroendocrine neoplasms. Endocr Pathol. 33:115–154. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Basturk O, Yang Z, Tang LH, Hruban RH,
Adsay V, McCall CM, Krasinskas AM, Jang KT, Frankel WL, Balci S, et
al: The high-grade (WHO G3) pancreatic neuroendocrine tumor
category is morphologically and biologically heterogenous and
includes both well differentiated and poorly differentiated
neoplasms. Am J Surg Pathol. 39:683–690. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Punekar SR, Kaakour D, Masri-Lavine L,
Hajdu C, Newman E and Becker DJ: Characterization of a novel entity
of G3 (high-grade well-differentiated) colorectal neuroendocrine
tumors (NET) in the SEER database. Am J Clin Oncol. 43:846–849.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yachida S, Vakiani E, White CM, Zhong Y,
Saunders T, Morgan R, de Wilde RF, Maitra A, Hicks J, Demarzo AM,
et al: Small cell and large cell neuroendocrine carcinomas of the
pancreas are genetically similar and distinct from
well-differentiated pancreatic neuroendocrine tumors. Am J Surg
Pathol. 36:173–184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tanaka T, Kaneko M, Nozawa H, Emoto S,
Murono K, Otani K, Sasaki K, Nishikawa T, Kiyomatsu T, Hata K, et
al: Diagnosis, assessment, and therapeutic strategy for colorectal
mixed adenoneuroendocrine carcinoma. Neuroendocrinology.
105:426–434. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pommergaard HC, Nielsen K, Sorbye H,
Federspiel B, Tabaksblat EM, Vestermark LW, Janson ET, Hansen CP,
Ladekarl M, Garresori H, et al: Surgery of the primary tumour in
201 patients with high-grade gastroenteropancreatic neuroendocrine
and mixed neuroendocrine-non-neuroendocrine neoplasms. J
Neuroendocrinol. 33:e129672021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Holmager P, Langer SW, Kjaer A, Ringholm
L, Garbyal RS, Pommergaard HC, Hansen CP, Federspiel B, Andreassen
M and Knigge U: Surgery in patients with gastro-entero-pancreatic
neuroendocrine carcinomas, neuroendocrine tumors G3 and high grade
mixed neuroendocrine-non-neuroendocrine neoplasms. Curr Treat
Options Oncol. 23:806–817. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Smith JD, Reidy DL, Goodman KA, Shia J and
Nash GM: A retrospective review of 126 high-grade neuroendocrine
carcinomas of the colon and rectum. Ann Surg Oncol. 21:2956–2962.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alese OB, Jiang R, Shaib W, Wu C, Akce M,
Behera M and El-Rayes BF: High-grade gastrointestinal
neuroendocrine carcinoma management and outcomes: A national cancer
database study. Oncologist. 24:911–920. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ordóñez NG: Broad-spectrum
immunohistochemical epithelial markers: A review. Hum Pathol.
44:1195–215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Badzio A, Czapiewski P, Gorczyński A,
Szczepańska-Michalska K, Haybaeck J, Biernat W and Jassem J:
Prognostic value of broad-spectrum keratin clones AE1/AE3 and
CAM5.2 in small cell lung cancer patients undergoing pulmonary
resection. Acta Biochim Pol. 66:111–114. 2019.PubMed/NCBI
|
|
35
|
Vasilevska D, Rudaitis V, Lewkowicz D,
Širvienė D, Mickys U, Semczuk M, Obrzut B and Semczuk A: Expression
patterns of cytokeratins (CK7, CK20, CK19, CK AE1/AE3) in atypical
endometrial hyperplasia coexisting with endometrial cancer. Int J
Mol Sci. 25:90842024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lal P, Saleh MA, Khoudari G, Gad MM,
Mansoor E, Isenberg G and Cooper GS: Epidemiology of large bowel
carcinoid tumors in the USA: A population-based national study. Dig
Dis Sci. 65:269–275. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zheng X, Wu M, Er L, Deng H, Wang G, Jin L
and Li S: Risk factors for lymph node metastasis and prognosis in
colorectal neuroendocrine tumours. Int J Colorectal Dis.
37:421–428. 2022. View Article : Google Scholar : PubMed/NCBI
|