Introduction
Sarcomas are uncommon malignancies, accounting for
approximately 1% of adult cancers and 15% of pediatric cancers
(1). Sarcomas are not only
uncommon, but also vary in their locations, features, and subtypes;
there are more than 100 distinct soft tissue sarcoma (STS) subtypes
that have been classified so far (2,3). Due
to these complexities, STS presents significant diagnostic
challenges and the prognosis remains poor despite advancements in
treatment like radiation therapy and chemotherapy. Between 2000 and
2018, the 5-year survival rate for STS with distant metastasis was
only 16.7% (4). Therefore, it is
essential to explore novel therapeutic approaches for treatment of
STS patients.
Recent studies have highlighted the crucial role of
DNA damage repair (DDR) pathways in both cancer development and
treatment (5–8). DDR contributes to the maintenance of
genomic stability by repairing DNA damage, and its dysfunction can
lead to oncogenesis, ultimately promoting the development of cancer
(5). Moreover, therapies such as
chemotherapy and radiotherapy induce DNA damage to eliminate cancer
cells, with tumors harboring mutations in DNA repair genes often
displaying increased sensitivity to such treatments due to their
reduced DNA repair efficiency (6).
SAMHD1 and p-ATM are key regulators of the DDR pathway (9–12).
Their roles in DNA repair and their potential as therapeutic
targets in cancer warrant further investigation.
SAMHD1 is primarily known for its role as a
deoxynucleoside triphosphate triphosphohydrolase (dNTPase),
preventing abnormal DNA re-synthesis during the DNA end-joining
process (10). When SAMHD1 function
is impaired, this process is disrupted, leading to genomic
instability, which ultimately contributes to cancer development
(11). One cancer type associated
with this mechanism is chronic lymphocytic leukemia (CLL) (11). Research on the impact of SAMHD1
expression on cancer prognosis is ongoing across various cancers
(13–16). In colorectal cancer, patients with
decreased SAMHD1 expression have been shown to have a poor
prognosis, which is hypothesized to be due to the dNTPase activity,
which inhibits tumor cell proliferation and promotes apoptosis
(13). Conversely, a recent study
in breast cancer reported that tumors expressing SAMHD1 exhibited
shorter progression-free and overall survival following
chemotherapy (14). This study
suggested that SAMHD1 depletion reduced interleukin signaling,
potentially altering immune cell infiltration (14).
ATM is essential for the repair of DNA double-strand
breaks, regulating cell cycle checkpoints, and triggering apoptosis
(9,17). Upon DNA damage, ATM is recruited to
the site of damage and is activated through autophosphorylation,
forming p-ATM, which subsequently preserves genomic integrity by
regulating various cellular processes (18). ATM has long been recognized as a key
tumor suppressor in the context of tumorigenesis (19,20).
However, several studies have revealed that ATM signaling
paradoxically supports tumor progression in certain biological
settings, indicating a more complex and context-dependent role in
cancer development. Several studies have provided evidence
supporting the oncogenic role of ATM in cancer progression, as
outlined below. In melanoma patients, both high expression and loss
of p-ATM have been identified as markers of poor survival (21). This is likely because overactivation
of p-ATM-related signaling pathways also promotes tumorigenesis,
while the loss of p-ATM undermines genome maintenance, leading to
cancer progression (21,22). In pancreatic cancer, decreased p-ATM
expression has been linked not only to poor prognosis but also to
the upregulation of anti-apoptotic genes such as BCL-2/BAD,
contributing to gemcitabine resistance (17).
Several studies have demonstrated that the DDR
activity of SAMHD1 is dependent on ATM-mediated signaling, either
through direct phosphorylation or indirect modulation of its
stability and recruitment (23–25).
Conversely, loss of SAMHD1 has been associated with aberrant ATM
pathway activation, leading to increased genomic instability and
tumorigenesis (26). Collectively,
these findings suggest a functional interplay between SAMHD1 and
p-ATM, particularly in the context of genome integrity under
replicative stress or genotoxic insult (23–26).
Despite increasing interest in the role of DDR
components in cancer biology, the clinical significance of SAMHD1
and p-ATM expression in STS remains poorly characterized. To date,
no comprehensive studies have evaluated the prognostic or
pathological implications of SAMHD1 and p-ATM expression
specifically in STS. Given their pivotal roles in genome
maintenance and the emerging evidence of their functional
interaction in the DDR network, investigating the expression
patterns of SAMHD1 and p-ATM in STS might provide valuable insights
into tumor behavior and patient outcomes. Therefore, in this study,
we evaluated the expression of SAMHD1 and p-ATM in STS tissues and
assessed their associations with clinicopathological parameters and
patient prognosis.
Materials and methods
Ethical approval
This study received approval from the Institutional
Review Board of Jeonbuk National University Hospital (IRB number:
2024-04-026-001) and was conducted in compliance with the
Declaration of Helsinki. The requirement for written informed
consent was waived by the IRB due to the retrospective nature of
the study and the use of anonymized data.
Patients and samples
A total of 133 patients with STS were included in
this study. The patient selection was based on the following
inclusion criteria: i) Patients histopathologically diagnosed with
STS; ii) patients who underwent surgical resection at Jeonbuk
National University Hospital between January 2000 and November
2022; iii) availability of formalin-fixed paraffin-embedded (FFPE)
tissue blocks suitable for tissue microarray (TMA) construction;
and iv) availability of complete clinicopathological and follow-up
data from medical records. The exclusion criteria were: i) Cases
with insufficient or poor-quality tissue material for TMA analysis;
and ii) patients with incomplete clinical data or those lost to
follow-up.
Histological types of STS included in this study are
listed in Table I.
Clinicopathologic information was obtained by reviewing medical
records. Factors included sex, age, site, T category, lymph node
metastasis, M category, histologic grade, tumor differentiation,
mitotic count, and tumor necrosis. Histologic slides were reviewed
according to the WHO classification of tumors of soft tissue and
bone tumor (27) and graded using
the FNCLCC (French Fédération Nationale des Centres de Lutte Contre
le Cancer) grading system (28). T
category and M category were classified with reference to the 8th
edition of the American Joint Committee Cancer Staging System
(29).
 | Table I.Expression status of SAMHD1 and p-ATM
according to the histological type of soft tissue sarcoma. |
Table I.
Expression status of SAMHD1 and p-ATM
according to the histological type of soft tissue sarcoma.
|
|
| SAMHD1
expression | p-ATM
expression |
|---|
|
|
|
|
|
|---|
| Histological
type | No. | Positive, n
(%) | P-value | Positive, n
(%) | P-value |
|---|
| Leiomyosarcoma | 23 | 16 (69.6) | 0.011 | 12 (52.2) | 0.368 |
| Synovial
sarcoma | 20 | 9 (45.0) | >0.999 | 12 (60.0) | 0.143 |
| Undifferentiated
sarcoma | 15 | 10 (66.7) | 0.096 | 10 (66.7) | 0.095 |
| Myxoid
liposarcoma | 13 | 3 (23.1) | 0.144 | 1 (7.7) | 0.007 |
|
Myxofibrosarcoma | 8 | 3 (37.5) | >0.999 | 0 (0.0) | 0.010 |
| Well-differentiated
liposarcoma | 8 | 2 (25.0) | 0.300 | 2 (25.0) | 0.465 |
| Angiosarcoma | 7 | 3 (42.9) | >0.999 | 5 (71.4) | 0.239 |
| Malignant
peripheral nerve sheath tumor | 6 | 5 (83.3) | 0.088 | 3 (50.0) | >0.999 |
| Ewing sarcoma | 6 | 2 (33.3) | 0.693 | 3 (50.0) | >0.999 |
| Adult
fibrosarcoma | 6 | 2 (33.3) | 0.693 | 3 (50.0) | >0.999 |
| Low-grade
myofibroblastic sarcoma | 5 | 0 (0.0) | 0.066 | 0 (0.0) | 0.068 |
| Alveolar
rhabdomyosarcoma | 4 | 3 (75.0) | 0.322 | 3 (75.0) | 0.317 |
| Dedifferentiated
liposarcoma | 3 | 1 (33.3) | >0.999 | 1 (33.3) | >0.999 |
| Embryonal
rhabdomyosarcoma | 2 | 0 (0.0) | 0.503 | 0 (0.0) | 0.504 |
| Pleomorphic
rhabdomyosarcoma | 2 | 0 (0.0) | 0.503 | 1 (50.0) | >0.999 |
| Epithelioid
sarcoma | 2 | 0 (0.0) | 0.503 | 1 (50.0) | >0.999 |
| Pleomorphic
liposarcoma | 1 | 0 (0.0) | >0.999 | 0 (0.0) | >0.999 |
| Spindle cell
rhabdomyosarcoma | 1 | 0 (0.0) | >0.999 | 0 (0.0) | >0.999 |
| Extraskeletal
myxoid chondrosarcoma | 1 | 0 (0.0) | >0.999 | 1 (100.0) | 0.436 |
Immunohistochemical staining and
scoring
We constructed a TMA using paraffin-embedded tissue
blocks obtained from surgical specimens of 133 STS patients. Two
cores, each 3.0 mm in size, were collected from non-necrotic,
non-degenerative areas of tumors. TMA tissue sections were
deparaffinized, followed by antigen retrieval performed in pH 6.0
antigen retrieval solution (DAKO, Glostrup, Denmark) using a
microwave oven for 20 min. Primary antibodies for SAMHD1 (1:50,
PA5-21515, Invitrogen, Waltham, MA) and p-ATM (1:50, sc-47739,
Santa Cruz Biotechnology, Santa Cruz, CA) were incubated with the
TMA tissue section overnight at 4°C.
Two pathologists (KMK and YJK) who were blinded to
the clinicopathologic information of the patients assessed
immunohistochemical staining under a multi-view microscope (Nikon
Eclipse 80i; Nikon, Tokyo, Japan) by consensus. Both SAMHD1 and
p-ATM were expressed mostly in nuclei (Fig. 1). As previous studies on the
immunohistochemical expression of SAMHD1 and p-ATM have primarily
focused on nuclear staining patterns, our analysis was likewise
based on nuclear localization (13,14,17,21).
To evaluate staining, we first identified the region with the
highest density of positive cells under low magnification and then
counted positive cells per high-power field (HPF), with a maximum
of 50 cells per field. Finally, we calculated the average by
summing the counts from each TMA section. The diameter of HPF was
625 µm. and the area of one HPF was 306,796 µm2.
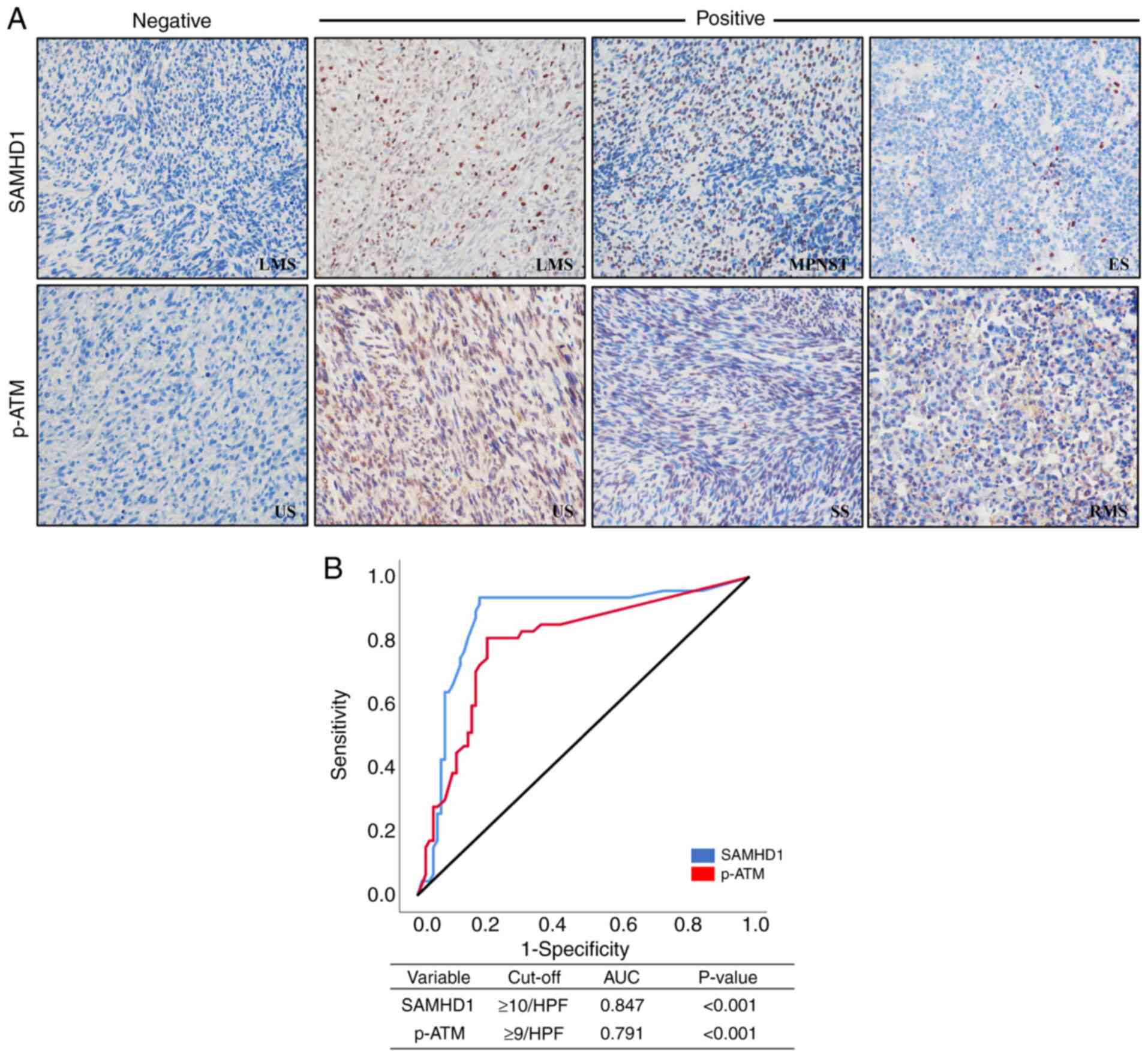 | Figure 1.(A) Immunohistochemistry of SAMHD1
and p-ATM in various STSs (original magnification, ×400). (B)
Receiver operating characteristic curve analysis to determine
cut-off points for the levels of nuclear SAMHD1 (blue line) and
nuclear p-ATM (red line). The cut-off points indicate the point of
the highest AUC to predict the death of patients with STS. AUC,
area under the curve; ES, Ewing sarcoma; HPF, high-power field;
LMS, leiomyosarcoma; MPNST, malignant peripheral nerve sheath
tumor; p-ATM, phosphorylated ataxia-telangiectasia mutated; RMS,
rhabdomyosarcoma; SAMHD1, SAM domain and HD domain-containing
protein 1; SS, synovial sarcoma; STS, soft tissue sarcoma; US,
undifferentiated sarcoma. |
Statistical analysis
Patients were categorized into positive and negative
subgroups based on the immunohistochemical expression levels of
SAMHD1 and p-ATM. Cutoff values for both markers to identify the
threshold with the highest prognostic accuracy for predicting
patient death were established through receiver operating
characteristic (ROC) curve analysis. The end date of follow-up was
either the date of patient death or the last contact date by
December 2022. Prognostic outcomes were evaluated by calculating
overall survival (OS) and progression-free survival (PFS). In the
OS analysis, death specifically due to STS was considered an event,
while cases where patients were alive at their last follow-up or
died from other causes were censored. In the PFS analysis, relapse
and metastasis of STS and death due to STS were treated as events.
Statistical analysis was conducted using SPSS software (IBM,
version 26.0, Armonk, NY). To evaluate differences in SAMHD1 and
p-ATM expression among histological subtypes, Fisher's exact test
was performed for pairwise comparisons between each subtype and the
remainder of the cohort. Kaplan-Meier survival analysis with the
log-rank test was used to compare survival distributions between
groups. For pairwise comparisons between subgroups, P-values were
obtained using the log-rank test and adjusted for multiple testing
using Bonferroni's correction. Cox proportional hazards regression
was used to evaluate the prognosis of STS. Stepwise selection was
employed to include variables independently associated with
survival in the multivariate Cox model. Pearson's chi-square test
assessed the associations between immunohistochemical expression
and clinicopathological factors, while the correlation between
SAMHD1 and p-ATM expression was determined using Pearson's and
Spearman's correlation tests. P-values less than 0.05 were
considered statistically significant.
Results
Expression of SAMHD1 and p-ATM in STS
tissues
Fig. 1 shows the
immunohistochemical staining patterns of SAMHD1 and p-ATM in STS
tissue samples; both these markers exhibited predominantly nuclear
expression (Fig. 1A). ROC curve
analysis, using patient death as a determinant, was employed to
segregate individuals into SAMHD1- and p-ATM-positive and negative
groups. Optimal cutoff values were defined as 10/HPF for SAMHD1 and
9/HPF for p-ATM (Fig. 1B).
Positivity rates of SAMHD1 and p-ATM across various histologic
types of STS are summarized in Table
I.
Correlation between SAMHD1 and p-ATM
expression
Based on previous reports indicating a functional
interaction between SAMHD1 and p-ATM in the DDR pathway, we
analyzed a relationship between their immunohistochemical
expression. Chi-square tests revealed a significant correlation
between SAMHD1 and p-ATM expression when comparing positive and
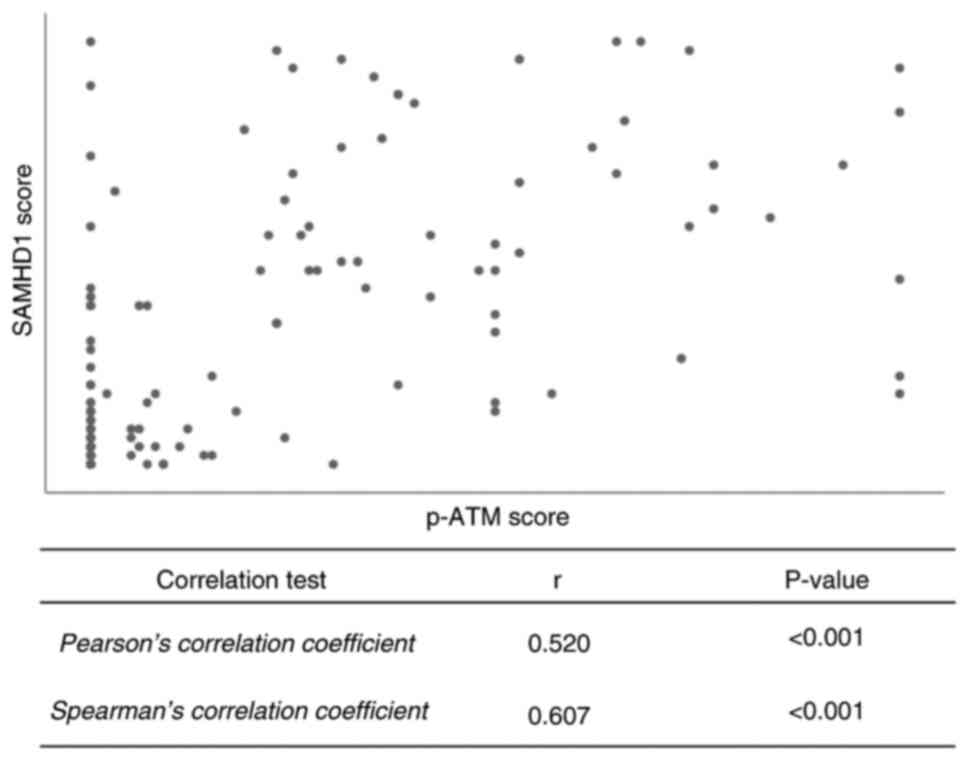
negative expression groups (P<0.001) (Table II). Furthermore, Pearson's
correlation analysis showed a moderate correlation (r=0.520,
P<0.001), and Spearman's correlation revealed a stronger
association (r=0.607, P<0.001) between the immunohistochemical
staining scores of SAMHD1 and p-ATM (Fig. 2).
 | Table II.Association between SAMHD1 and p-ATM
levels. |
Table II.
Association between SAMHD1 and p-ATM
levels.
|
| SAMHD1
expression |
|
|---|
|
|
|
|
|---|
| p-ATM
expression | Positive, n
(%) | Negative, n
(%) | P-value |
|---|
| Positive | 47 (82.5) | 10 (17.5) |
|
| Negative | 13 (17.1) | 63 (82.9) | <0.001 |
Associations of individual and
combined patterns of SAMHD1 and p-ATM expression with
clinicopathological factors
Individual SAMHD1 positivity was associated with
higher histologic grade (P=0.018) (Table III). p-ATM positivity showed
strong correlations with T category (P=0.005) and histologic grade
(P=0.001) (Table III).
 | Table III.Clinicopathologic variables and
levels of SAMHD1 and p-ATM in soft tissue sarcoma. |
Table III.
Clinicopathologic variables and
levels of SAMHD1 and p-ATM in soft tissue sarcoma.
|
|
|
|
|
|
|
|
| Combined
expression |
|
|---|
|
|
| SAMHD1
expression |
| p-ATM
expression |
|
|
|
|---|
|
|
|
|
|
|
|
SAMHD1+/p-ATM+, n
(%) |
SAMHD1+/p-ATM− or
SAMHD−/p-ATM+, n (%) |
SAMHD1−/p-ATM−, n
(%) | P-value |
|---|
|
Characteristics | Total, n | Positive, n
(%) | Negative, n
(%) | P-value | Positive, n
(%) | Negative, n
(%) | P-value |
|---|
| All cases | 133 | 59 (44.4) | 74 (55.6) |
| 57 (42.9) | 76 (57.1) |
| 42 (31.6) | 32 (24.1) | 59 (44.4) |
|
| Sex |
|
|
|
|
|
|
|
|
|
|
|
|
Male | 74 | 32 (43.2) | 42 (56.8) |
| 28 (37.8) | 46 (62.2) |
| 22 (29.7) | 16 (21.6) | 36 (48.6) |
|
|
Female | 59 | 27 (45.8) | 32 (54.2) | 0.771 | 29 (49.2) | 30 (50.8) | 0.190 | 20 (33.9) | 16 (27.1) | 23 (39.0) | 0.526 |
| Age, years |
|
|
|
|
|
|
|
|
|
|
|
|
≤60 | 76 | 34 (44.7) | 42 (55.3) |
| 34 (44.7) | 42 (55.3) |
| 25 (32.9) | 18 (23.7) | 33 (43.4) |
|
|
>60 | 57 | 25 (43.9) | 32 (56.1) | 0.920 | 23 (40.4) | 34 (59.6) | 0.613 | 17 (29.8) | 14 (24.6) | 26 (45.6) | 0.931 |
| Site |
|
|
|
|
|
|
|
|
|
|
|
| Head
and neck | 9 | 4 (44.4) | 5 (55.6) |
| 5 (55.6) | 4 (44.4) |
| 3 (33.3) | 3 (33.3) | 3 (33.3) |
|
| Trunk
and extremities | 105 | 44 (41.9) | 61 (58.1) |
| 43 (41.0) | 62 (59.0) |
| 32 (30.5) | 23 (21.9) | 50 (47.6) |
|
| Abdomen
and thoracic visceral organs | 15 | 10 (66.7) | 5 (33.3) |
| 8 (53.3) | 7 (46.7) |
| 7 (46.7) | 4 (26.7) | 4 (26.7) |
|
|
Retroperitoneum | 4 | 1 (25.0) | 3 (75.0) | 0.274a | 1 (25.0) | 3 (75.0) | 0.585a | 0 (0.0) | 2 (50.0) | 2 (50.0) | 0.321a |
| T category |
|
|
|
|
|
|
|
|
|
|
|
| T 1,
2 | 94 | 39 (41.5) | 55 (58.5) |
| 33 (35.1) | 61 (64.9) |
| 25 (26.6) | 22 (23.4) | 47 (50.0) |
|
| T 3,
4 | 39 | 20 (51.3) | 19 (48.7) | 0.301 | 24 (61.5) | 15 (38.5) | 0.005 | 17 (43.6) | 10 (25.6) | 12 (30.8) | 0.087 |
| LN metastasis |
|
|
|
|
|
|
|
|
|
|
|
|
Absent | 120 | 52 (43.3) | 68 (56.7) |
| 48 (40.0) | 72 (60.0) |
| 38 (31.7) | 27 (22.5) | 55 (45.8) |
|
|
Present | 13 | 7 (53.8) | 6 (46.2) | 0.562a | 6 (46.2) | 7 (53.8) | 0.769a | 4 (30.8) | 5 (38.5) | 4 (30.8) | 0.398a |
| M category |
|
|
|
|
|
|
|
|
|
|
|
| M0 | 123 | 53 (43.1) | 70 (56.9) |
| 50 (40.7) | 73 (59.3) |
| 37 (30.1) | 29 (23.6) | 57 (46.3) |
|
| M1 | 10 | 6 (60.0) | 4 (40.0) | 0.338a | 7 (70.0) | 3 (30.0) | 0.098a | 5 (50.0) | 3 (30.0) | 2 (20.0) | 0.251a |
| Histologic
grade |
|
|
|
|
|
|
|
|
|
|
|
| Grade
1 | 31 | 8 (25.8) | 23 (74.2) |
| 5 (16.1) | 26 (83.9) |
| 4 (12.9) | 5 (16.1) | 22 (71.0) |
|
| Grade 2
and 3 | 102 | 51 (50.0) | 51 (50.0) | 0.018 | 50 (49.0) | 52 (51.0) | 0.001 | 38 (37.3) | 27 (26.5) | 37 (36.3) | 0.003 |
| Tumor
differentiation |
|
|
|
|
|
|
|
|
|
|
|
| 1 | 16 | 8 (50.0) | 8 (50.0) |
| 7 (43.8) | 9 (56.3) |
| 6 (37.5) | 3 (18.8) | 7 (43.8) |
|
| 2 and
3 | 117 | 51 (43.6) | 66 (56.4) | 0.789a | 50 (42.7) | 67 (57.3) |
>0.999a | 36 (30.8) | 29 (24.8) | 52 (44.4) | 0.812a |
| Mitotic count |
|
|
|
|
|
|
|
|
|
|
|
| 0-9/10
HPF | 62 | 27 (43.5) | 35 (56.5) |
| 24 (38.7) | 38 (61.3) |
| 17 (27.4) | 17 (27.4) | 28 (45.2) |
|
| ≥10/10
HPF | 71 | 32 (45.1) | 39 (54.9) | 0.860 | 33 (46.5) | 38 (53.5) | 0.366 | 25 (35.2) | 15 (21.1) | 31 (43.7) | 0.549 |
| Tumor necrosis |
|
|
|
|
|
|
|
|
|
|
|
|
Absent | 71 | 28 (39.4) | 43 (60.6) |
| 25 (35.2) | 46 (64.8) |
| 17 (23.9) | 19 (26.8) | 35 (49.3) |
|
|
Present | 62 | 31 (50.0) | 31 (50.0) | 0.221 | 32 (51.6) | 30 (48.4) | 0.057 | 25 (40.3) | 13 (21.0) | 24 (38.7) | 0.128 |
Additionally, we reclassified patients into three
sub-groups based on SAMHD1 and p-ATM expression levels:
SAMHD1+/p-ATM+, SAMHD1+/p-ATM- or SAMHD1-/p-ATM+, and
SAMHD1-/p-ATM-. Co-expression of SAMHD1 and p-ATM was significantly
associated with histologic grade (P=0.003) (Table III).
Univariate and Kaplan-Meier survival
analyses of individual and combined expression patterns of SAMHD1
and p-ATM for OS and PFS of STS patients
Analysis of various clinical parameters using
univariate methods showed significant correlations between several
factors and patient outcomes. Specifically, T category (P=0.005), M
category (P=0.038) and histologic grade (P=0.013) were
significantly associated with poor OS, along with SAMHD1 expression
(P<0.001), p-ATM expression (P<0.001), and co-expression
pattern of SAMHD1 and p-ATM (P<0.001) (Table IV). In univariate analysis of PFS,
age (P=0.016), M category (P<0.001) and histologic grade
(P=0.007) were significantly associated with shorter PFS. SAMHD1
(P<0.001), p-ATM (P=0.001), and co-expression of SAMHD1 and
p-ATM (P<0.001) also showed correlations with shorter PFS in the
univariate analysis (Table
IV).
 | Table IV.Univariate Cox proportional hazards
regression analysis of OS and PFS in patients with soft tissue
sarcoma. |
Table IV.
Univariate Cox proportional hazards
regression analysis of OS and PFS in patients with soft tissue
sarcoma.
|
| OS | PFS |
|---|
|
|
|
|
|---|
|
Characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex, male (vs.
female) | 0.831
(0.468–1.474) | 0.527 | 0.997
(0.636–1.565) | 0.990 |
| Age, >60 years
(vs. ≤60 years) | 1.141
(0.639–2.037) | 0.656 | 1.742
(1.109–2.736) | 0.016 |
| T category 3, 4
(vs. T1, 2) | 2.3
(1.288–4.107) | 0.005 | 1.514
(0.943–2.431) | 0.086 |
| LN metastasis,
present (vs. absent) | 1.425
(0.638–3.182) | 0.388 | 0.819
(0.394–1.705) | 0.594 |
| M category M1 (vs.
M0) | 2.481
(1.051–5.853) | 0.038 | 7.623
(3.823–15.200) | <0.001 |
| Histologic grade 2
and 3 (vs. grade 1) | 3.237
(1.279–8.910) | 0.013 | 2.344
(1.261–4.357) | 0.007 |
| Mitotic count
≥10/10 HPF (vs. 0–9/10 HPF) | 1.064
(0.599–1.888) | 0.833 | 1.046
(0.669–1.636) | 0.844 |
| Tumor
differentiation 2 and 3 (vs. 1) | 1.280
(0.506–3.239) | 0.602 | 0.961
(0.494–1.869) | 0.907 |
| Tumor necrosis
present (vs. absent) | 1.267
(0.714–2.247) | 0.419 | 1.059
(0.677–1.657) | 0.802 |
| SAMHD1, positive
(vs. negative) | 7.583
(3.536–16.262) | <0.001 | 4.621
(2.805–7.614) | <0.001 |
| p-ATM, positive
(vs. negative) | 7.65
(3.688–15.870) | <0.001 | 2.939
(1.853–4.661) | <0.001 |
| Combined expression
of SAMHD1/p-ATM |
|
|
|
|
|
SAMHD1+/p-ATM−
or SAMHD1−/p-ATM+ | 7.179
(2.001–25.753) | 0.002 | 3.694
(1.926–7.085) | <0.001 |
| (vs.
SAMHD1−/p-ATM−) |
|
|
|
|
|
SAMHD1+/p-ATM+
(vs. SAMHD1−/p-ATM−) | 22.774
(6.950–74.624) | <0.001 | 6.213
(3.387–11.398) | <0.001 |
Patients with positive SAMHD1 expression faced a
7.583-fold higher risk of death [95% confidential interval (CI)
3.536–16.262, P< 0.001)], as well as a 4.621-fold greater risk
of disease progression or mortality (95% CI; 2.805–7.614,
P<0.001). Patients positive for p-ATM expression exhibited a
7.65-fold (95% CI; 3.688–15.87, P<0.001) elevated risk of death
and a 2.939-fold higher risk of disease progression or mortality
(95% CI; 1.853–4.661, P<0.001) compared to those with negative
expression (Table IV). Regarding
the co-expression of SAMHD1 and p-ATM, STS patients who were
SAMHD1+/p-ATM- or SAMHD1-/p-ATM+ had a 7.179-fold (95% CI;
2.001–25.753) and a 3.694-fold (95% CI; 1.926–7.085) increased risk
of death and progression or death, respectively, compared to STS
patients with the SAMHD1-/p-ATM-expression pattern. STS patients
with the SAMHD1+/p-ATM+ expression pattern exhibited even higher
risks, with a 22.774-fold (95% CI; 6.95–74.624) and a 6.213-fold
(95% CI; 3.387–11.398) increased likelihood of death and relapse or
death, respectively, compared to STS patients with the
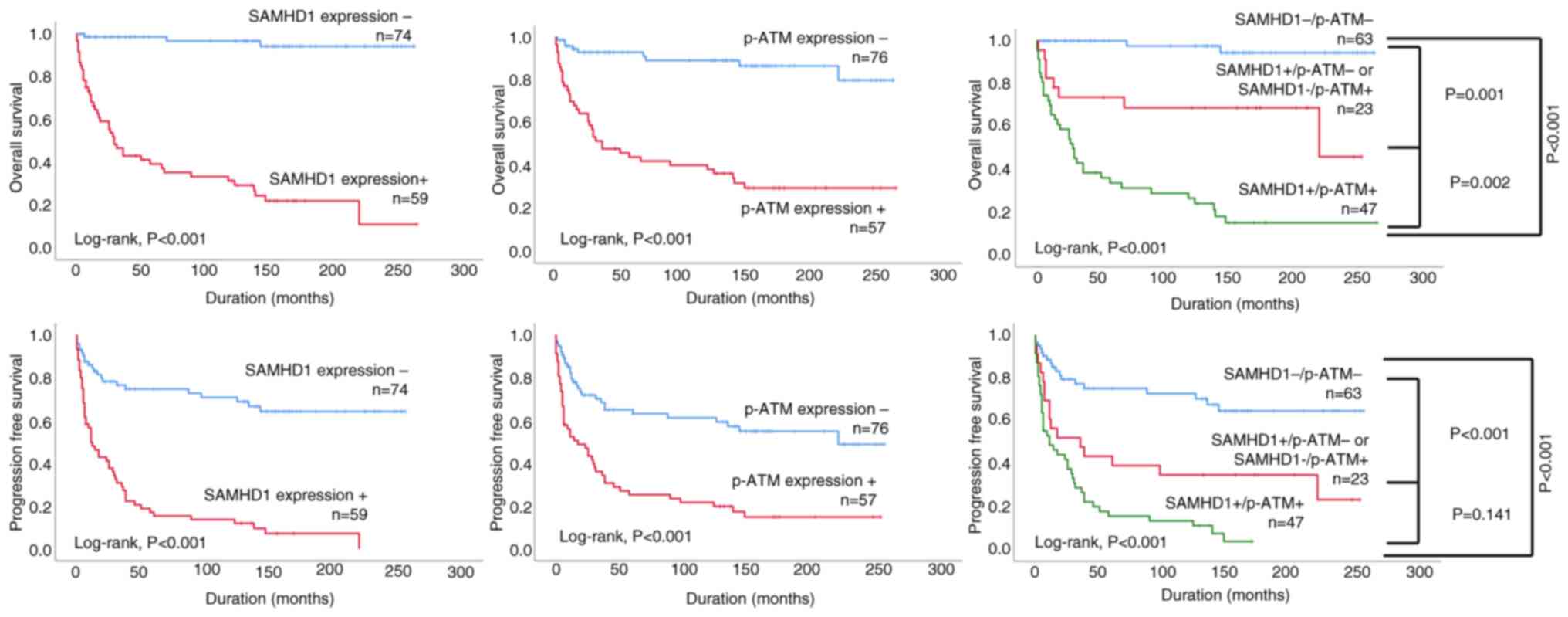
SAMHD1-/p-ATM-expression pattern (Table IV). Kaplan-Meier survival curves
for OS and PFS based on individual and co-expression patterns of
SAMHD1 and p-ATM in STS patients are presented in Fig. 3.
Multivariate survival analysis of
individual and combined expression patterns of SAMHD1 and p-ATM for
OS and PFS of STS patients
To investigate the impact of individual and combined
expression of SAMHD1 and p-ATM on OS and PFS in STS patients, we
performed multivariate survival analyses. Expression patterns were
independently analyzed using two models: Model 1 for individual
expression and Model 2 for co-expression. Each model included the
expression pattern along with other clinicopathological variables
that were statistically significant for OS or PFS in the univariate
analysis. Independent prognostic indicators for OS were SAMHD1
expression, and p-ATM (Table V,
model 1). STS patients with positive SAMHD1 expression had a
4.178-fold higher risk of mortality than those with negative SAMHD1
expression (95% CI; 1.828–9.548, P=0.002) (Table V, model 1). Furthermore, STS
patients with positive p-ATM expression had a 3.420-fold higher
risk of mortality than those with negative p-ATM expression (95%
CI; 1.518–7.704, P=0.003) (Table V,
model 1). Age, M category, and SAMHD1 expression were significant
independent factors associated with PFS in the multivariate
analysis (Table VI, model 1). The
SAMHD1-positive group had a 3.617-fold increased risk of
progression or death (95% CI: 2.154–6.074, <0.001) compared to
the SAMHD1-negative group (Table
VI, model 1).
 | Table V.Multivariate Cox proportional hazards
regression analysis for OS in patients with soft tissue
sarcoma. |
Table V.
Multivariate Cox proportional hazards
regression analysis for OS in patients with soft tissue
sarcoma.
|
| OS |
|---|
|
|
|
|---|
|
Characteristics | HR (95% CI) | P-value |
|---|
| Model
1a |
|
|
| SAMHD1,
positive (vs. negative) | 4.178
(1.828–9.548) | 0.002 |
| p-ATM,
positive (vs. negative) | 3.420
(1.518–7.704) | 0.003 |
| Model
2b |
|
|
|
Combined expression of
SAMHD1/p-ATM |
|
|
|
SAMHD1+/p-ATM−
or SAMHD1−/p-ATM+ (vs.
SAMHD1−/p-ATM−) | 6.588
(1.834–23.670) | 0.004 |
|
SAMHD1+/p-ATM+
(vs. SAMHD1−/p-ATM−) | 18.915
(5.710–62.654) | <0.001 |
 | Table VI.Multivariate Cox proportional hazards
regression analysis for PFS in patients with soft tissue
sarcoma. |
Table VI.
Multivariate Cox proportional hazards
regression analysis for PFS in patients with soft tissue
sarcoma.
|
| PFS |
|---|
|
|
|
|---|
|
Characteristics | HR (95% CI) | P-value |
|---|
| Model
1a |
|
|
| Age,
>60 years (vs. ≤60 years) | 2.038
(1.254–3.313) | 0.004 |
| M
category M1 (vs. M0) | 5.426
(2.575–11.434) | <0.001 |
| SAMHD1,
positive (vs. negative) | 3.617
(2.154–6.074) | <0.001 |
| Model
2b |
|
|
| Age,
>60 years (vs. ≤60 years) | 2.180
(1.345–3.534) | 0.002 |
| M
category M1 (vs. M0) | 6.024
(2.863–12.675) | <0.001 |
| Combined expression
of SAMHD1/p-ATM |
|
|
|
SAMHD1+/p-ATM−
or SAMHD1−/p-ATM+ (vs.
SAMHD1−/p-ATM−) | 3.749
(1.943–7.233) | <0.001 |
|
SAMHD1+/p-ATM+
(vs. SAMHD1−/p-ATM−) | 5.159
(2.773–9.597) | <0.001 |
Only combined SAMHD1 and p-ATM expression was an
independent predictor of OS of STS patients (Table V, model 2). STS patients with the
SAMHD1+/p-ATM-expression pattern or SAMHD1-/p-ATM+ expression
pattern had a 6.588-fold (95% CI; 1.834–23.670, P=0.004) increased
risk of death compared to SAMHD1-/p-ATM-cases, while SAMHD1+/p-ATM+
cases showed a 18.915-fold (95% CI; 5.710–62.654, P<0.001)
higher risk of death (Table V,
model 2). Age, M category and combined expression of SAMHD1 and
p-ATM were independent prognostic factors of PFS in STS patients
(Table VI, model 2). STS patients
with the SAMHD1+/p-ATM- or SAMHD1-/p-ATM+ expression pattern
demonstrated a 3.749-fold (95% CI; 1.943–7.233, P<0.001) greater
risk of death or progression, whereas SAMHD1+/p-ATM+ cases had a
5.159-fold (95% CI; 2.773–9.597, P<0.001) increased risk of
death or progression compared to STS patient with the
SAMHD1-/p-ATM-expression pattern (Table VI, model 2).
Discussion
In this study, we evaluated the immunohistochemical
expression of SAMHD1 and p-ATM in STS tissues and investigated
their associations with clinicopathological features and patient
outcomes. Although previous studies have investigated the
associations between SAMHD1 and p-ATM expression and prognosis in
various cancers (23–25), their prognostic significance in STS
has not been explored. To our knowledge, this is the first study to
evaluate the prognostic impact of SAMHD1 and p-ATM expression
specifically in STS. In the present study, both SAMHD1 and p-ATM
exhibited predominant nuclear localization based on
immunohistochemical staining. We found that both the individual and
combined expression of SAMHD1 and p-ATM were significantly
associated with higher histologic grade and adverse survival
outcomes in STS patients. Multivariate analysis revealed that both
the individual and combined expression of SAMHD1 and p-ATM
independently served as prognostic factors for OS. Additionally,
SAMHD1 expression and the co-expression pattern of SAMHD1 and p-ATM
were independent prognostic factors for PFS. Furthermore, SAMHD1
and p-ATM expression were highly correlated.
SAMHD1, by facilitating DNA end resection at
double-strand breaks, plays important roles in DDR and homologous
recombination (24). Loss or
dysfunction of SAMHD1 can lead to the accumulation of genomic
instability and has been associated with tumorigenesis in several
malignancies (30,31). To date, several studies have
investigated the impact of SAMHD1 expression on cancer progression
across various tumor types, with conflicting results reported
depending on the specific cancer type (13–16).
In contrast to our findings, decreased SAMHD1 expression has been
associated with a poor prognosis in colorectal cancer and diffuse
large B-cell lymphoma (13,32). However, numerous studies have
reported results consistent with ours. For instance, SAMHD1
expression was associated with shorter time-to-progression and OS
in early breast cancer patients treated with neoadjuvant
chemotherapy (14). Additionally,
SAMHD1 acted as a poor prognostic marker in classical Hodgkin
lymphoma (33), aligning with our
findings. Taken together, these divergent findings suggest that the
prognostic role of SAMHD1 varies depending on tumor type,
potentially reflecting cancer-specific differences in biological
function and interaction with the tumor microenvironment.
ATM is activated through autophosphorylation in
response to DNA double-strand breaks, initiating checkpoint
signaling and repair pathways (34). ATM is well recognized for its role
as a tumor suppressor (20).
Ataxia-telangiectasia (A-T) is an inherited autosomal recessive
disorder resulting from germline mutations in the ATM gene
(20,35). Patients with A-T commonly present
with neurological and systemic abnormalities, including ataxia of
the cerebellum, telangiectasias affecting the skin and eyes, immune
dysfunction, and impaired gonadal development (35). In addition to these features,
individuals with A-T face a markedly elevated lifetime cancer risk,
with approximately 38.2% developing malignancies by the age of 40
(35,36). Although ATM is widely recognized as
a tumor suppressor, as previously mentioned, accumulating research
suggests that the underlying signaling pathway can, in certain
biological contexts, contribute to the advancement of cancer
(37). In melanoma, high p-ATM
expression was linked to lower 5-year survival and a more
aggressive phenotype (21) and was
associated with poor locoregional disease-free survival and shorter
disease-specific survival in cervical cancer (38), consistent with our findings.
Importantly, our study is among the first to assess
the co-expression patterns of SAMHD1 and p-ATM in STS. A
moderate-to-strong positive correlation between their expression
was observed, supporting previous reports suggesting functional
crosstalk between these proteins (19,24,39).
SAMHD1 activity has been shown to be modulated by ATM-dependent
phosphorylation, and SAMHD1 deficiency can lead to dysregulated ATM
signaling and genomic instability (39). The strong prognostic value of the
SAMHD1+/p-ATM+ co-expression pattern observed in our study further
supports the biological interdependence of these DDR components and
suggests that their combined assessment enhances prognostic
stratification in STS patients. Considering the findings of the
present study, we propose that the DDR plays a critical role in the
progression of STS.
STSs can be broadly categorized into two genomic
subgroups: i) tumors characterized by extensive copy-number
alterations, chromosomal instability, and a high burden of
structural variants, and ii) translocation-associated tumors with
relatively simple genomes driven by pathognomonic fusion
oncoproteins (1). In the former,
which is common across adult STSs, chronic replication stress and
ongoing DNA damage are intrinsic features of tumor biology
(2,3). In tumors with chromosomal instability,
persistent DDR signaling may act as a protective mechanism by
helping to prevent catastrophic genome collapse, buffering
replication stress, and potentially supporting continued
proliferation under genotoxic pressure (4). Collectively, these activities make
SAMHD1 an attractive tumor promoter and explain why high
SAMHD1/p-ATM expression correlates with an increased grade and
poorer survival in our STS cohort. p-ATM and SAMHD1 indicate active
double-strand break signaling, checkpoint activation, end
resection, and homologous recombination at stalled forks and
breaks, thereby contributing to the maintenance of genome integrity
under stress (5,6). Collectively, these functions suggest
that SAMHD1 may act as a tumor promoter and provide a rationale for
the observed association between high SAMHD1/p-ATM expression,
increased tumor grade, and poorer survival in our STS cohort'.
As mentioned previously, discordant findings have
been reported in other malignancies, such as colorectal cancer. In
a study investigating the role of SAMHD1 in colorectal cancer,
reduced SAMHD1 expression was associated with poor prognosis. The
authors demonstrated that the dNTPase function of SAMHD1 suppresses
cell proliferation and reduces replication errors, thereby
contributing to its tumor-suppressive role. Moreover, mutations
affecting SAMHD1′s catalytic sites were shown to inhibit the
expression of apoptosis-related proteins while upregulating
anti-apoptotic factors such as Bcl-2, ultimately suppressing
apoptosis and promoting uncontrolled proliferation. We propose that
the apparent discrepancy with our study can be explained by the
fact that many STS arise under persistent replication stress and
pronounced structural instability, conditions in which elevated
SAMHD1, together with active ATM signaling, enhances repair
capacity and stress tolerance, thereby supporting tumor persistence
and progression (1,3). Such cancer-type differences give a
reasonable explanation for the different results between our STS
study and colorectal cancer.
DDR is fundamentally recognized as a
tumor-suppressive mechanism (40).
It is activated upon genomic insults such as DNA double-strand
breaks, single-strand breaks, or replication stress and serves to
arrest the cell cycle, initiate DNA repair, or induce apoptosis or
senescence in irreparably damaged cells (40). Through these mechanisms, DDR
prevents the accumulation of mutations and maintains genomic
stability, acting as a safeguard against malignant transformation
(40). However, accumulating
evidence suggests that DDR can also exert oncogenic effects under
certain biological contexts, giving rise to a paradox in its role
in cancer (8). In established
tumors, particularly those experiencing high replication stress,
DDR activity can support cancer cell survival by managing chronic
DNA damage (8). Consequently, an
intact DDR pathway can contribute to tumor progression and be
associated with poor patient prognosis (8). In light of this, the association
between SAMHD1 and p-ATM expression and poor prognosis supports the
possibility that an intact or upregulated DDR pathway contributes
to tumor progression in STS.
From a clinical standpoint, the identification of
SAMHD1 and p-ATM as independent prognostic markers has important
implications. These molecules could potentially be incorporated
into histopathological assessment to help stratify patients at
higher risk, informing decisions regarding closer monitoring or
individualized therapeutic strategies. In parallel, there has been
increasing interest in targeting components of the DDR as a novel
therapeutic avenue in oncology (7).
Although PARP inhibitors have demonstrated clinical benefit in
tumors harboring homologous recombination deficiencies, the
therapeutic relevance of modulating SAMHD1 or ATM activity in this
context remains to be fully elucidated (26). Further research is warranted to
explore whether inhibition of SAMHD1 or ATM could enhance the
therapeutic efficacy of chemotherapy or radiotherapy in STS.
Our study has several limitations. The retrospective
design and the use of tissue microarrays limit the generalizability
of our findings. Moreover, the synergistic correlation between
SAMHD1 and p-ATM in the current study depends on
immunohistochemical and clinical evidence, and there is no
functional evidence directly supporting it. Further in vitro and in
vivo experiments, such as co-knockdown or overexpression studies,
are warranted to explore the underlying biological mechanism of the
interaction between them and the effects on tumor cell
proliferation, apoptosis, and DNA repair ability. In addition, the
relatively small cohort size and the critical underrepresentation
of certain histological subtypes may limit the statistical power of
our analyses and compromise the generalizability of
subtype-specific conclusions. Larger, multi-institutional studies
are required to validate and extend our findings.
In conclusion, we investigated the expression of
SAMHD1 and p-ATM in patients with STS and found that their
expression is significantly associated with aggressive tumor
characteristics and poor clinical outcomes. Their individual and
combined expression patterns were independent prognostic factors
for OS and PFS. These findings provide new insights into the
molecular pathology of STS and suggest that DDR components such as
SAMHD1 and p-ATM can serve as valuable prognostic biomarkers and
potential therapeutic targets in this challenging-to-treat
malignancy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Fund of Biomedical
Research Institute, Jeonbuk National University Hospital, a grant
from the Korea Health Technology R&D Project through the Korea
Health Industry Development Institute, funded by the Ministry of
Health & Welfare, Republic of Korea (grant no. HR22C1832), and
the National Institute of Health (NIH) research project (project
no. 2024-ER0511-01).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YJK and KMK conceived and designed the study, and
performed the experiments. YJM contributed to the acquisition of
clinical samples, participated in pathological review and assisted
in the interpretation of clinical data. KYJ, ARA, MJC and WSM
analyzed the data. YJM, ARA, MJC and KYJ were involved in
statistical analysis, data visualization and interpretation of the
results. YJK and KMK drafted the manuscript. ARA, HSP, MJC and WSM
verified the authenticity of all the raw data, assisted in data
curation and validation, contributed to the interpretation of the
findings, and critically revised the manuscript for important
intellectual content. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was conducted in accordance with The
Declaration of Helsinki and approved by the Institutional Review
Board of Jeonbuk National University Hospital (approval no.
2024-04-026-001; Jeonju, South Korea). The requirement for consent
for participation was waived due to the retrospective nature of the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
von Mehren M, Kane JM, Bui MM, Choy E,
Connelly M, Dry S, Ganjoo KN, George S, Gonzalez RJ, Heslin MJ, et
al: NCCN guidelines insights: soft tissue sarcoma, version 1.2021.
J Natl Compr Canc Netw. 18:1604–1612. 2020. View Article : Google Scholar
|
|
2
|
Ressing M, Wardelmann E, Hohenberger P,
Jakob J, Kasper B, Emrich K, Eberle A, Blettner M and Zeissig SR:
Strengthening health data on a rare and heterogeneous disease:
Sarcoma incidence and histological subtypes in Germany. BMC Public
Health. 18:2352018. View Article : Google Scholar
|
|
3
|
Nacev BA, Sanchez-Vega F, Smith SA,
Antonescu CR, Rosenbaum E, Shi H, Tang C, Socci ND, Rana S,
Gularte-Mérida R, et al: Clinical sequencing of soft tissue and
bone sarcomas delineates diverse genomic landscapes and potential
therapeutic targets. Nat Commun. 13:34052022. View Article : Google Scholar
|
|
4
|
Rutland CS: Advances in soft tissue and
bone sarcoma. Cancers (Basel). 16:28752024. View Article : Google Scholar
|
|
5
|
Khanna KK and Jackson SP: DNA
double-strand breaks: Signaling, repair and the cancer connection.
Nat Genet. 27:247–254. 2001. View
Article : Google Scholar
|
|
6
|
Moon J, Kitty I, Renata K, Qin S, Zhao F
and Kim W: DNA damage and its role in cancer therapeutics. Int J
Mol Sci. 24:47412023. View Article : Google Scholar
|
|
7
|
Drew Y, Zenke FT and Curtin NJ: DNA damage
response inhibitors in cancer therapy: Lessons from the past,
current status and future implications. Nat Rev Drug Discov.
24:19–39. 2025. View Article : Google Scholar
|
|
8
|
Park SH, Noh SJ, Kim KM, Bae JS, Kwon KS,
Jung SH, Kim JR, Lee H, Chung MJ, Moon WS, et al: Expression of DNA
damage response molecules PARP1, γH2AX, BRCA1, and BRCA2 predicts
poor survival of breast carcinoma patients. Trans Oncol. 8:239–249.
2015. View Article : Google Scholar
|
|
9
|
Ueno S, Sudo T and Hirasawa A: ATM:
Functions of ATM kinase and its relevance to hereditary tumors. Int
J Mol Sci. 23:5232022. View Article : Google Scholar
|
|
10
|
Akimova E, Gassner FJ, Schubert M,
Rebhandl S, Arzt C, Rauscher S, Tober V, Zaborsky N, Greil R and
Geiberger R: SAMHD1 restrains aberrant nucleotide insertions at
repair junctions generated by DNA end joining. Nucleic Acids Res.
49:2598–2608. 2021. View Article : Google Scholar
|
|
11
|
Schott K, Majer C, Bulashevska A, Childs
L, Schmidt MHH, Rajalingam Munder M and König R: SAMHD1 in cancer:
Curse or cure? J Mol Med (Berl). 100:351–372. 2022. View Article : Google Scholar
|
|
12
|
Li Y, Gao Y, Jiang X, Cheng Y, Zhang J, Xu
L, Liu X, Huang Z, Xie C and Gong Y: SAMHD1 silencing cooperates
with radiotherapy to enhance anti-tumor immunity through
IFI16-STING pathway in lung adenocarcinoma. J Transl Med.
20:6282022. View Article : Google Scholar
|
|
13
|
Zhang Z, Li P and Sun P: Expression of
SAMHD1 and its mutation on prognosis of colon cancer. Oncol Lett.
24:3032022. View Article : Google Scholar
|
|
14
|
Gutiérrez-Chamorro L, Felip E, Castellà E,
Quiroga V, Ezeonwumelu IJ, Angelats L, Esteve A, Rerez-Roca L,
Martínez-Cardús A, Fernandez PL, et al: SAMHD1 expression is a
surrogate marker of immune infiltration and determines prognosis
after neoadjuvant chemotherapy in early breast cancer. Cell Oncol
(Dordr). 47:189–208. 2024. View Article : Google Scholar
|
|
15
|
Kim KM, Moon YJ, Park SH, Park HJ, Wang
SI, Park HS, Lee H, Kwon KS, Moon WS, Lee DG, et al: Individual and
combined expression of DNA damage response molecules PARP1, γH2AX,
BRCA1, and BRCA2 predict shorter survival of soft tissue sarcoma
patients. PLoS One. 11:e01631932016. View Article : Google Scholar
|
|
16
|
Jiang H, Li C, Liu Z, Hospital S and Hu R:
Expression and relationship of SAMHD1 with other apoptotic and
autophagic genes in acute myeloid leukemia patients. Acta Haematol.
143:51–59. 2020. View Article : Google Scholar
|
|
17
|
Xun J, Ohtsuka H, Hirose K, Douchi D,
Nakayama S, Ishida M, Miura T, Ariake K, Mizuma M, Nakagawa K, et
al: Reduced expression of phosphorylated ataxia-telangiectasia
mutated gene is related to poor prognosis and gemcitabine
chemoresistance in pancreatic cancer. BMC Cancer. 23:8352023.
View Article : Google Scholar
|
|
18
|
Stucci LS, Internò V, Tucci M, Perrone M,
Mannavola F, Palmirotta R and Porta C: The ATM gene in breast
cancer: Its relevance in clinical practice. Genes (Basel).
12:7272021. View Article : Google Scholar
|
|
19
|
Schumann T, Ramon SC, Schubert N, Mayo MA,
Hega M, Maser KI, Ada SR, Sydow L, Hajikazemi M, Badstübner M, et
al: Deficiency for SAMHD1 activates MDA5 in a cGAS/STING-dependent
manner. J Exp Med. 220:e202208292023. View Article : Google Scholar
|
|
20
|
Savitsky K, Bar-Shira A, Gilad S, Rotman
G, Ziv Y, Vanagaite L, Tagle DA, Smith S, Uziel T, Sfez S, et al: A
single ataxia telangiectasia gene with a product similar to PI-3
kinase. Science. 268:1749–1753. 1995. View Article : Google Scholar
|
|
21
|
Bhandaru M, Martinka M, McElwee KJ and
Rotte A: Prognostic significance of nuclear phospho-ATM expression
in melanoma. PLoS One. 10:e01346782015. View Article : Google Scholar
|
|
22
|
Stagni V, Oropallo V, Fianco G, Antonelli
M, Cinà I and Barilà D: Tug of war between survival and death:
Exploring ATM function in cancer. Int J Mol Sci. 15:5388–5409.
2014. View Article : Google Scholar
|
|
23
|
Coquel F, Silva MJ, Técher H, Zadorozhny
K, Sharma S, Nieminuszczy J, Mettling C, Dardillac E, Barthe A,
Schmitz AL, et al: SAMHD1 acts at stalled replication forks to
prevent interferon induction. Nature. 557:57–61. 2018. View Article : Google Scholar
|
|
24
|
Daddacha W, Koyen AE, Bastien AJ, Head PE,
Dhere VR, Nabeta GN, Connolly EC, Werner E, Madden MZ, Daly MB, et
al: SAMHD1 promotes DNA end resection to facilitate DNA repair by
homologous recombination. Cell Rep. 20:1921–1935. 2017. View Article : Google Scholar
|
|
25
|
Kapoor-Vazirani P, Rath SK, Liu X, Shu Z,
Bowen NE, Chen Y, Haji-Seyed-Javadi R, Daddacha W, Minten EV,
Danelia D, et al: SAMHD1 deacetylation by SIRT1 promotes DNA end
resection by facilitating DNA binding at double-strand breaks. Nat
Commun. 13:67072022. View Article : Google Scholar
|
|
26
|
Rodríguez-Sánchez A, Quijada-Álamo M,
Pérez-Carretero C, Herrero AB, Arroyo-Barea A, Dávila-Valls J,
Rubio A, de Coca AG, Benito-Sánchez R, Rodríguez-Vicente AE, et al:
SAMHD1 dysfunction impairs DNA damage response and increases
sensitivity to PARP inhibition in chronic lymphocytic leukemia. Sci
Rep. 15:104462025. View Article : Google Scholar
|
|
27
|
World Health Organization (WHO), . Soft
tissue and bone tumours. WHO Classification of Tumours Editorial.
5th Edition. IARC Press; Lyon, France: 2020
|
|
28
|
Coindre J: Histologic grading of adult
soft tissue sarcomas. Verh Dtsch Ges Pathol. 82:59–63. 1998.
|
|
29
|
Byrd DR, Brookland RK, Washington MK,
Gershenwald JE, Compton CC, Hess KR, Sullivan DC and Jessup JM:
AJCC Cancer Staging Manual. Amin MB, Edge SB and Greene FL: Eighth
Edition. Springer; New York, USA: pp. 251–274. 2017
|
|
30
|
Clifford R, Louis T, Robbe P, Ackroyd S,
Burns A, Timbs AT, Colopy GW, Dreau H, Sigaux F, Judde JG, et al:
SAMHD1 is mutated recurrently in chronic lymphocytic leukemia and
is involved in response to DNA damage. Blood. 123:1021–1031. 2014.
View Article : Google Scholar
|
|
31
|
Chen Z, Hu J, Ying S and Xu A: Dual roles
of SAMHD1 in tumor development and chemoresistance to anticancer
drugs. Oncol Lett. 21:4512021. View Article : Google Scholar
|
|
32
|
Daddacha W, Monroe D, Schlafstein AJ,
Withers AE, Thompson EB, Danelia D, Luong NC, Sesay F, Rath SK,
Usoro ER, et al: SAMHD1 expression contributes to doxorubicin
resistance and predicts survival outcomes in diffuse large B-cell
lymphoma patients. NAR Cancer. 6:zcae0072024. View Article : Google Scholar
|
|
33
|
Xagoraris I, Vassilakopoulos TP, Drakos E,
Angelopoulou MK, Panitsas F, Herold N, Medeiros LJ, Giakoumis X,
Pangalis GA and Rassidakis GZ: Expression of the novel tumour
suppressor sterile alpha motif and HD domain-containing protein 1
is an independent adverse prognostic factor in classical Hodgkin
lymphoma. Br J Haematol. 193:488–496. 2021. View Article : Google Scholar
|
|
34
|
Bakkenist CJ and Kastan MB: DNA damage
activates ATM through intermolecular autophosphorylation and dimer
dissociation. Nature. 421:499–506. 2003. View Article : Google Scholar
|
|
35
|
Rothblum-Oviatt C, Wright J, Lefton-Greif
MA, McGrath-Morrow SA, Crawford TO and Lederman HM: Ataxia
telangiectasia: A review. Orphanet J Rare Dis. 11:1592016.
View Article : Google Scholar
|
|
36
|
Suarez F, Mahlaoui N, Canioni D,
Andriamanga C, d'Enghien CD, Brousse N, Jais JP, Fischer A, Hermine
O and Stoppa-Lyonnet D: Incidence, presentation, and prognosis of
malignancies in ataxia-telangiectasia: A report from the French
national registry of primary immune deficiencies. J Clin Oncol.
33:202–208. 2015. View Article : Google Scholar
|
|
37
|
Lee JH: Targeting the ATM pathway in
cancer: Opportunities, challenges and personalized therapeutic
strategies. Cancer Treat Rev. 129:1028082024. View Article : Google Scholar
|
|
38
|
Roossink F, Wieringa HW, Noordhuis MG, ten
Hoor KA, Kok M, Slagter-Menkema L, Hollema H, de Bock GH, Pras E,
de Vries EGE, et al: The role of ATM and 53BP1 as predictive
markers in cervical cancer. Int J Cancer. 131:2056–2066. 2012.
View Article : Google Scholar
|
|
39
|
Maunakea AK, Chepelev I, Cui K and Zhao K:
Intragenic DNA methylation modulates alternative splicing by
recruiting MeCP2 to promote exon recognition. Cell Res.
23:1256–1269. 2013. View Article : Google Scholar
|
|
40
|
Ciccia A and Elledge SJ: The DNA damage
response: Making it safe to play with knives. Mol Cell. 40:179–204.
2010. View Article : Google Scholar
|