Introduction
Hepatocellular carcinoma (HCC) is the third leading
cause of cancer-associated mortality, with an incidence of ~0.8
million cases in 2022, worldwide (1,2).
Although, surgical resection, transplantation and local ablation
are the standard treatment approaches for patients with early-stage
HCC, nearly half of patients are diagnosed with advanced-stage
disease at initial presentation (3). Systemic therapy is recommended for
patients with advanced HCC, among which sorafenib is the standard
first-line treatment for over a decade (3,4).
However, recent advances in tyrosine kinase inhibitors (TKIs) have
reshaped the therapeutic landscape of advanced HCC, and its
combination with immunotherapy may improve the prognosis of these
patients (4,5).
Apatinib is an oral TKI that inhibits tumor
angiogenesis, and has shown promising efficacy when combined with
programmed cell death protein 1 (PD-1) inhibitors in patients with
advanced HCC (6–8). For example, a previous study reported
an objective response rate (ORR) of 50.0% and a disease control
rate (DCR) of 91.3% in patients with advanced HCC treated with
apatinib combined with a PD-1 inhibitor (7). Additionally, in another study,
patients with advanced HCC who received apatinib plus PD-1
inhibitor showed a median progression-free survival (PFS) of 6.7
months and a median overall survival (OS) of 22.5 months (8). Therefore, apatinib plus PD-1 inhibitor
has become a promising treatment strategy for advanced HCC.
Camrelizumab, a high-affinity immunoglobulin G4k
monoclonal antibody of PD-1, has demonstrated promising efficacy in
treating patients with advanced HCC (9,10).
Previously, several clinical trials have suggested that these
patients can benefit from camrelizumab combined with apatinib
(11,12). For example, the RESCUE trial
reported an ORR of 34.3%, and a 12-month OS rate of 74.7% in
patients with advanced HCC who were treated with camrelizumab plus
apatinib as first-line therapy. Additionally, an ORR of 22.5% and a
12-month OS rate of 68.2% was reported in patients who received
this regimen as a second-line treatment (11). Additionally, the CARES-310 trial
revealed that camrelizumab combined with apatinib as first-line
therapy for patients with advanced HCC could result in an ORR of
25.4%, a DCR of 78.3%, a median PFS of 5.6 months and a median OS
of 23.8 months (12). However,
real-world evidence on camrelizumab plus apatinib in patients with
advanced HCC is limited, and therefore further validation is
needed. Furthermore, prognostic factors affecting treatment
outcomes have not been fully elucidated.
Therefore, the present real-world study aimed to
evaluate the efficacy and safety of camrelizumab plus apatinib in
patients with advanced HCC, as well as to identify potential
prognostic factors associated with treatment outcome in these
patients.
Materials and methods
Patients
The present retrospective, single-arm study aimed to
evaluate the efficacy and tolerability of camrelizumab combined
with apatinib in patients with advanced HCC. The inclusion criteria
were as follows: i) Patients diagnosed with advanced HCC, which was
defined as Barcelona Clinic Liver Cancer stage C (13); ii) age >18 years; iii) treated
with camrelizumab plus apatinib; iv) available complete treatment
response data based on imaging examinations; v) complete follow-up
data, including disease status and survival data; vi) no history of
organ transplantation; and vii) no history of autoimmune disease
requiring systemic therapy. The exclusion criteria were the
following: i) Patients suffering from liver malignancies other than
HCC, such as intrahepatic cholangiocarcinoma or metastatic liver
cancer; ii) patients diagnosed with hematological malignancies or
other primary cancers; iii) Eastern Cooperative Oncology Group
performance status (ECOG PS) of >2; and iv) uncontrolled or
severe comorbidities, such as heart failure and chronic renal
failure. Patients were enrolled from the Handan Central Hospital
between March 2020 and January 2024. The study protocol was
approved by the Institutional Review Board of the Handan Central
Hospital (approval no. 2024029). Written informed consent was
obtained from all patients or their family members.
Data collection
All data were extracted from electronic medical
records and included demographic characteristics, treatment
history, such as surgery or transarterial chemoembolization (TACE),
ECOG PS, disease-related information, treatment details, treatment
response data and follow-up outcomes. Treatment lines were counted
based on systematic therapy only, while prior locoregional
therapies (such as TACE) did not affect the line-counting of
systematic treatments. Treatment response was assessed every 3
months using computed tomography scan or magnetic resonance
imaging. The imaging findings were reviewed centrally and
categorized according to the response evaluation criteria in solid
tumors criteria (14). The
follow-up data included the timing and occurrence of disease
progression and death. PFS and OS rates were estimated based on
follow-up data. PFS was defined as the time from treatment
initiation to the first disease progression or all-cause mortality.
OS was defined as the time from treatment initiation to mortality
from any cause. The median follow-up duration was 10.0 months
(range, 1.0–54.0 months). Finally, the adverse events were recorded
by clinicians and screened by investigators based on the Common
Terminology Criteria for Adverse Events version 5.0 (available at
http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5×7.pdf).
Treatment information
Patients received camrelizumab at a dose of 200 mg
intravenously for 3 weeks, combined with apatinib at a dose of 250
mg taken orally once daily. In addition, several patients underwent
TACE according to their own willingness, disease status and
physician recommendation.
Statistical analysis
All statistical analyses were performed using SPSS
software (version 26.0; IBM Corp.). Post hoc statistical power
analysis using PASS software (version 15.0.5; NCSS LLC) was
performed. Descriptive statistics were applied to summarize the
results. PFS and OS in patients with advanced HCC were calculated
using Kaplan-Meier curves. The Log-rank test was used to compare
Kaplan-Meier curves between subgroups. Univariate and multivariate
Cox regression analyses were carried out to identify prognostic
factors associated with PFS and OS. All variables in the univariate
analysis were incorporated into the multivariate model using the
enter method. A Fisher's exact test was used to compare ORR and DCR
between subgroups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical features
The mean age of the 39 patients included in the
present study was 59.4±11.4 years. A total of 8 women (20.5%) and
31 men (79.5%) were enrolled. Among all patients, 13 (33.3%)
presented with portal vein invasion and 17 (43.6%) with
extrahepatic metastasis. A total of 29 patients (74.4%) received
camrelizumab + apatinib as first-line therapy, while 10 patients
(25.6%) were treated with this regimen as second- or later-line
therapy. The detailed patient characteristics are listed in
Table I.
 | Table I.Clinical characteristics of patients
with advanced HCC. |
Table I.
Clinical characteristics of patients
with advanced HCC.
| Characteristic | Patients with
advanced HCC (n=39) |
|---|
| Age (mean ± SD),
years | 59.4±11.4 |
| Sex |
|
|
Female | 8 (20.5) |
| Male | 31 (79.5) |
| HBV history | 27 (69.2) |
| Liver cirrhosis
history | 23 (59.0) |
| A history of surgery
or TACE | 18 (46.2) |
| ECOG PS |
|
| 1 | 38 (97.4) |
| 2 | 1 (2.6) |
| Maximum tumor
diameter (median IQR), cm | 5.0 (4.0–9.3) |
| Maximum tumor
diameter ≥10 cm | 8 (20.5) |
| Portal vein
invasion | 13 (33.3) |
| Extrahepatic
metastasis | 17 (43.6) |
| ALB (median IQR),
g/l | 68.6 (57.1–72.2) |
| Abnormal ALB | 28 (71.8) |
| TBIL (median IQR),
µmol/l | 16.4 (13.8–38.3) |
| Abnormal TBIL | 23 (59.0) |
| ALT (median IQR),
U/l | 36.0 (26.0–51.0) |
| Abnormal ALT | 26 (66.7) |
| AST (median IQR),
U/l | 63.0
(34.0–114.0) |
| Abnormal AST | 12 (30.8) |
| AFP (median IQR),
ng/ml | 24.2
(3.9–1210.0) |
| AFP ≥200 ng/ml | 14 (35.9) |
| Number of TACEs |
|
| 0 | 29 (74.4) |
| 1 | 8 (20.5) |
| 2 | 2 (5.1) |
| Treatment line of
systematic therapy |
|
|
First-line | 29 (74.4) |
| Second
or later | 10 (25.6) |
Treatment response
Among all patients, 10 (25.6%) achieved a complete
response, 4 (10.3%) reached partial response, 18 (46.2%) had stable
disease and 7 (17.9%) exhibited progressive disease. The ORR and
DCR were 35.9 and 82.1%, respectively (Table II).
 | Table II.Treatment response of patients with
advanced HCC. |
Table II.
Treatment response of patients with
advanced HCC.
| Outcome | Patients with
advanced HCC (n=39) |
|---|
| Response |
|
| CR | 10 (25.6) |
| PR | 4 (10.3) |
| SD | 18 (46.2) |
| PD | 7 (17.9) |
| ORR | 14 (35.9) |
| DCR | 32 (82.1) |
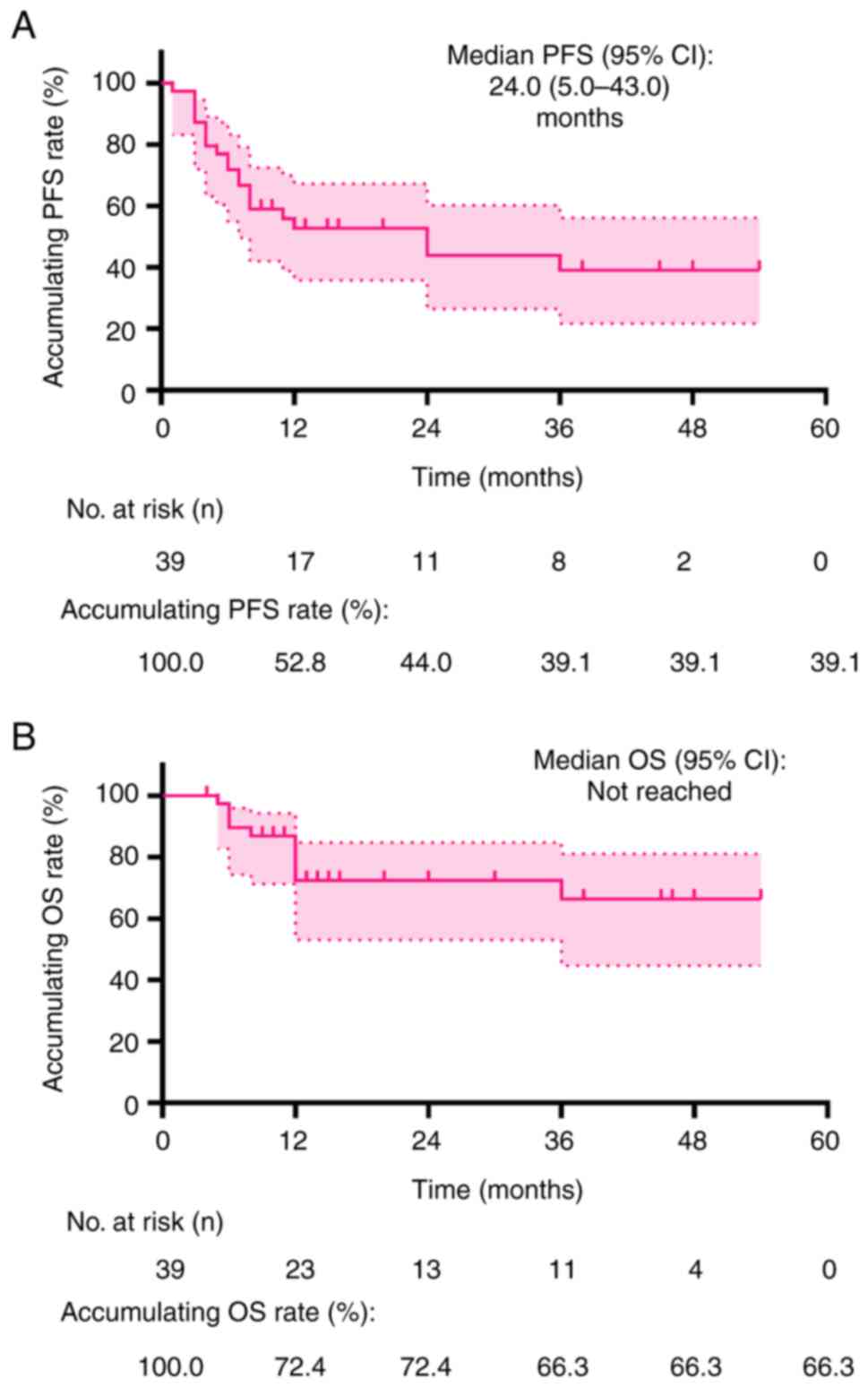
PFS and OS
The median PFS was 24.0 months [95% confidence
interval (CI): 5.0–43.0 months]. The accumulated PFS rates at 12,
24 and 36 months were 52.8, 44.0 and 39.1%, respectively (Fig. 1A). Furthermore, the median OS was
not reached during the follow-up period. The cumulative OS rates at
12, 24 and 36 months were 72.4, 72.4 and 66.3%, respectively
(Fig. 1B).
Subgroup analysis
The subgroup analysis was performed based on use of
TACE or not and demonstrated that the ORR, DCR, PFS and OS were
similar between patients with and without TACE (all P>0.05;
Table SI).
Factors affecting PFS and OS
Univariate Cox regression analysis revealed that
abnormal aspartate aminotransferase levels (yes vs. no) were
associated with higher risk of disease progression or mortality
[hazard ratio (HR), 2.461; P=0.044]. However, in multivariate Cox
regression analysis, none of the evaluated clinical characteristics
were associated with PFS (all P>0.05; Table III). Furthermore, regarding OS,
univariate Cox regression analysis revealed no associations between
OS and clinical features (all P>0.05). By contrast, multivariate
Cox regression analysis identified that extrahepatic metastasis
(yes vs. no) was independently associated with higher mortality
risk (HR, 9.217; P=0.049; Table
IV).
 | Table III.Cox regression analyses on PFS in
patients with advanced hepatocellular carcinoma. |
Table III.
Cox regression analyses on PFS in
patients with advanced hepatocellular carcinoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
|
|
| 95% CI |
|
| 95% CI |
|---|
|
|
|
|
|
|
|
|
|---|
| Factors | P-value | HR | Lower | Upper | P-value | HR | Lower | Upper |
|---|
| Age (>60 years
vs. ≤60 years) | 0.074 | 0.398 | 0.145 | 1.093 | 0.170 | 0.415 | 0.119 | 1.456 |
| Sex (male vs.
female) | 0.650 | 1.290 | 0.429 | 3.878 | 0.579 | 0.667 | 0.160 | 2.790 |
| HBV history (yes
vs. no) | 0.629 | 1.264 | 0.489 | 3.270 | 0.475 | 2.294 | 0.235 | 22.419 |
| Liver cirrhosis
history (yes vs. no) | 0.518 | 1.339 | 0.552 | 3.249 | 0.877 | 1.179 | 0.148 | 9.377 |
| A history of
surgery or TACE (yes vs. no) | 0.453 | 1.390 | 0.589 | 3.282 | 0.942 | 0.953 | 0.258 | 3.515 |
| Maximum tumor
diameter (≥10 cm vs. <10 cm) | 0.841 | 0.894 | 0.300 | 2.670 | 0.700 | 1.364 | 0.282 | 6.601 |
| Portal vein
invasion (yes vs. no) | 0.549 | 1.310 | 0.541 | 3.170 | 0.098 | 0.329 | 0.088 | 1.229 |
| Extrahepatic
metastasis (yes vs. no) | 0.430 | 1.414 | 0.599 | 3.338 | 0.157 | 2.646 | 0.688 | 10.181 |
| Abnormal ALB (yes
vs. no) | 0.658 | 1.240 | 0.478 | 3.218 | 0.533 | 0.613 | 0.132 | 2.852 |
| Abnormal TBIL (yes
vs. no) | 0.333 | 1.570 | 0.631 | 3.908 | 0.842 | 0.876 | 0.237 | 3.235 |
| Abnormal ALT (yes
vs. no) | 0.342 | 1.585 | 0.613 | 4.096 | 0.388 | 1.856 | 0.456 | 7.551 |
| Abnormal AST (yes
vs. no) | 0.044 | 2.461 | 1.024 | 5.913 | 0.111 | 3.762 | 0.738 | 19.184 |
| AFP (≥200 ng/ml vs.
<200 ng/ml) | 0.391 | 0.660 | 0.256 | 1.704 | 0.113 | 0.315 | 0.075 | 1.317 |
| Treatment line of
systematic therapy (second or later vs. first) | 0.547 | 1.338 | 0.518 | 3.460 | 0.237 | 2.043 | 0.625 | 6.679 |
 | Table IV.Cox regression analyses on OS in
patients with advanced hepatocellular carcinoma. |
Table IV.
Cox regression analyses on OS in
patients with advanced hepatocellular carcinoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
|
|
| 95% CI |
|
| 95% CI |
|---|
|
|
|
|
|
|
|
|
|---|
| Factors | P-value | HR | Lower | Upper | P-value | HR | Lower | Upper |
|---|
| Age (>60 years
vs. ≤60 years) | 0.364 | 0.533 | 0.137 | 2.076 | 0.816 | 0.774 | 0.090 | 6.696 |
| Sex (male vs.
female) | 0.407 | 2.404 | 0.302 | 19.109 | 0.986 | 1.024 | 0.078 | 13.524 |
| HBV history (yes
vs. no) | 0.740 | 0.805 | 0.225 | 2.885 | 0.427 | 3.856 | 0.138 | 107.513 |
| Liver cirrhosis
history (yes vs. no) | 0.855 | 0.890 | 0.254 | 3.120 | 0.491 | 0.347 | 0.017 | 7.053 |
| A history of
surgery or TACE (yes vs. no) | 0.464 | 1.598 | 0.455 | 5.611 | 0.914 | 0.882 | 0.091 | 8.525 |
| Maximum tumor
diameter (≥10 cm vs. <10 cm) | 0.485 | 1.619 | 0.418 | 6.270 | 0.087 | 12.040 | 0.699 | 207.526 |
| Portal vein
invasion (yes vs. no) | 0.766 | 0.813 | 0.208 | 3.174 | 0.067 | 0.084 | 0.006 | 1.192 |
| Extrahepatic
metastasis (yes vs. no) | 0.361 | 1.788 | 0.514 | 6.224 | 0.049 | 9.217 | 1.007 | 84.329 |
| Abnormal ALB (yes
vs. no) | 0.657 | 1.362 | 0.348 | 5.340 | 0.410 | 2.512 | 0.280 | 22.509 |
| Abnormal TBIL (yes
vs. no) | 0.833 | 1.147 | 0.321 | 4.107 | 0.286 | 0.271 | 0.025 | 2.988 |
| Abnormal ALT (yes
vs. no) | 0.589 | 1.452 | 0.374 | 5.632 | 0.835 | 1.292 | 0.115 | 14.483 |
| Abnormal AST (yes
vs. no) | 0.310 | 1.942 | 0.540 | 6.990 | 0.194 | 5.234 | 0.430 | 63.667 |
| AFP (≥200 ng/ml vs.
<200 ng/ml) | 0.434 | 0.538 | 0.114 | 2.539 | 0.080 | 0.077 | 0.004 | 1.355 |
| Treatment line of
systematic therapy (second or later vs. first) | 0.255 | 2.090 | 0.588 | 7.434 | 0.054 | 10.809 | 0.957 | 122.073 |
Adverse events
Adverse events are summarized in Table V. The most common adverse events
were anemia (79.5%), thrombocytopenia (69.2%), leukopenia (64.1%)
and pain (56.4%). Other adverse events included neutropenia
(41.0%), fever (33.3%), hypertension (28.2%), decreased appetite
(23.1%), nausea and vomiting (23.1%), diarrhea (7.7%), fatigue
(7.7%) and reactive cutaneous capillary endothelial proliferation
(RCCEP; 7.7%). All adverse events were of grade 1–2. In addition,
camrelizumab treatment-related adverse events included fever
(17.9%), RCCEP (7.7%), anemia (5.1%), neutropenia (5.1%), diarrhea
(5.1%), thrombocytopenia (2.6%) and fatigue (2.6%). Treatment
modifications, including dose reduction or delay, were required for
1 patient (2.6%) due to toxicity.
 | Table V.Adverse events in patients with
advanced hepatocellular carcinoma. |
Table V.
Adverse events in patients with
advanced hepatocellular carcinoma.
| Adverse event | Total (n=39) | Camrelizumab
treatment-related (n=39) |
|---|
| Anemia | 31 (79.5) | 2 (5.1) |
|
Thrombocytopenia | 27 (69.2) | 1 (2.6) |
| Leukopenia | 25 (64.1) | 0 (0.0) |
| Pain | 22 (56.4) | 0 (0.0) |
| Neutropenia | 16 (41.0) | 2 (5.1) |
| Fever | 13 (33.3) | 7 (17.9) |
| Hypertension | 11 (28.2) | 0 (0.0) |
| Decreased
appetite | 9 (23.1) | 0 (0.0) |
| Nausea and
vomiting | 9 (23.1) | 0 (0.0) |
| Diarrhea | 3 (7.7) | 2 (5.1) |
| Fatigue | 3 (7.7) | 1 (2.6) |
| RCCEP | 3 (7.7) | 3 (7.7) |
Discussion
Systemic therapy still remains the gold standard
treatment approach for advanced HCC, with sorafenib serving as the
standard first-line treatment for a number of years. However, in
previous years, the combination of immune checkpoint inhibitors and
TKIs has shown encouraging efficacy, thus emerging as a potential
treatment option for this type of cancer (4,12,15).
Camrelizumab, as a PD-1 inhibitor, might also exert its antitumor
effect by other mechanisms, such as regulating the immune
infiltration, which is demonstrated by previous studies on tumor
microenvironment-related biomarkers for predicting camrelizumab
efficacy (16,17). Besides, camrelizumab combined with
apatinib potentially exert synergistic effects. Previous studies
demonstrated that apatinib could increase PD-1 expression in
tumor-infiltrating CD4+ cells, thus improving the
efficacy of camrelizumab (18,19).
The present study reports the efficacy and safety of camrelizumab
combined with apatinib in a single center; meanwhile, our study
also reports the prognostic factors, which helps the early
recognition of prognosis to facilitate early intervention and
improve outcome in advanced HCC patients receiving camrelizumab
combined with apatinib.
Clinically, previous studies have evaluated the
efficacy of camrelizumab combined with apatinib in patients with
advanced HCC, reporting ORR of 17.3–45.7%, DCR of 57.7–78.6%,
median PFS of 5.5–5.7 months and 12-month OS rates of 68.2–76.5% in
these patients (11,12,20).
In the present study, an ORR of 35.9%, a DCR of 82.1%, a median PFS
of 24.0 months and a 12-month OS rate of 72.4% was reported for
patients with advanced HCC treated with the combination of
camrelizumab plus apatinib. The results on ORR, DCR and 12-month OS
rate were consistent with those of previous studies, while a
prolonged median PFS was reported (11,12,20).
This discrepancy could be due to differences in baseline patient
characteristics. Notably, in the present study, the proportion of
patients with extrahepatic metastasis was lower (43.6%) compared
with that reported in previous studies (64.3–75.8%) (11,12).
In addition, the proportion of patients with maximum tumor diameter
of ≥10 cm was also lower (20.5%) compared with a previous study
(40.4%) (20). Therefore, the
aforementioned findings suggest that the patients included in the
present study could suffer from a less aggressive disease compared
with the previously published studies (11,12,20).
Notably, in the present study, the complete response
rate was 25.6%, which was markedly higher compared with that
reported in the CARES-310 phase III trial (1.1%). This difference
could be attributed to the fact that in the present study, 25.6% of
patients received TACE, which was not applied in the CARES-310
phase III trial (12). Therefore,
it is hypothesized that TACE could improve the complete response of
camrelizumab plus apatinib therapy in patients with advanced HCC.
However, further studies are required to validate these
findings.
The present study also aimed to identify factors
affecting PFS and OS in patients with advanced HCC treated with
camrelizumab plus apatinib. Multivariate Cox regression analyses
demonstrated that extrahepatic metastasis was independently
associated with higher mortality risk in these patients. This
result was consistent with those reported in previous studies
(21,22). However, this index was not
associated with the mortality in the univariate Cox regression
analysis, potentially due to small sample size. No other clinical
factors were associated with PFS or OS in these patients. This
finding could be due to the sample size, which could weaken
statistical power. Furthermore, the subgroup analysis indicated
that the ORR, DCR and PFS were not different between patients with
or without TACE. We hypothesize the potential reasons might be as
follows: i) The small sample size would reduce the statistical
power; and ii) patients who received TACE in addition to
camrelizumab plus apatinib presented with heavier disease burden
such as higher proportion of patients with maximum tumor diameter
≥10 cm (40.0 vs. 13.8%), with portal vein invasion (50.0 vs.
27.6%), and Child-Pugh stage B (50.0 vs. 6.3%) compared with those
without TACE. Hence, the efficacy was not different between
patients with or without TACE.
Previous studies also reported that the most common
adverse events in patients with HCC treated with camrelizumab
combined with apatinib were anemia (20.0–55.8%), thrombocytopenia
(46.3–60.0%) and leukopenia (50.0–57.1%) (11,12,23–25).
Consistently, in the present study, anemia (79.5%),
thrombocytopenia (69.2%) and leukopenia (64.1%) were the most
frequent adverse events in patients with advanced HCC treated with
camrelizumab plus apatinib. By contrast, in the present study, the
incidence of pain was 56.4%, which was higher than that reported in
previous studies (26.8–49.2%) (11,23,25).
This could be again explained by the fact that 25.6% of patients in
the present study underwent TACE, which is commonly associated with
the development of pain (20,26).
The incidences of other adverse events, including decreased
appetite, nausea and vomiting and diarrhea, were comparable with
those observed in previous studies (10,27,28).
These results indicate that camrelizumab combined with apatinib
could be well-tolerated by patients with advanced HCC. However,
further efforts are needed to optimize tolerability. Furthermore,
in previous study, the most common adverse events following
camrelizumab monotherapy includes the reactive cutaneous capillary
endothelial proliferation (67%), proteinuria (23%), and increased
alanine aminotransferase (22%) (10). The incidence of most adverse events
were numerically far lower than the camrelizumab combined with
apatinib therapy, which implied the clinicians to pay attention to
balance the clinical efficacy benefit and the risk of adverse
events when choosing the monotherapy regimen and the combination
regimen.
The present study also has several limitations.
Firstly, the inherent drawback of the retrospective and single-arm
study design should be considered, which could induce several
biases, such as selection bias and incomplete data availability. In
addition, the lack of a control group made it difficult to draw a
solid conclusion. Therefore, a further randomized and prospective
study is needed to eliminate the selection bias in the baseline
characteristics, and a control group should be included to
explicitly evaluate the benefit of camrelizumab. Secondly, post hoc
statistical power analysis using PASS software was performed based
on the observed median PFS in the present study and a previous
study (29), and a test efficacy of
89.6% was achieved when setting the a-value at 0.05. However, the
sample size was still small which limited the statistical power.
Furthermore, due to the short follow-up period, the incidence of
endpoint events was low in the present study and the median OS was
not reached. Consequently, the median OS of patients with advanced
HCC receiving camrelizumab plus apatinib needs more investigation.
Thirdly, only patients with advanced HCC from China were enrolled,
and therefore future studies involving patients across different
geographical regions are warranted to further verify the results.
Finally, previously, in patients with HCC, adjuvant therapy
following the liver transplantation was reported to improve the
prognosis of patients with HCC (30,31).
However, this was not considered in the present study. Future
studies are needed to evaluate whether the combination of liver
transplantation with camrelizumab and apatinib would improve the
survival of patients with advanced HCC.
In conclusion, camrelizumab combined with apatinib
showed satisfactory treatment efficacy and acceptable tolerance in
patients with advanced HCC. Furthermore, extrahepatic metastasis
was independently associated with worse OS in the aforementioned
patients. These findings could provide real-world evidence
supporting the application of camrelizumab plus apatinib in
treating patients advanced HCC. However, due to the small sample
size in the present study, a further study with a larger sample
size is required.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
RQ and ZY conceived and designed the study. LY and
YS collected the data. RA performed the data analysis. YH and HH
contributed to the interpretation of the data. JX analyzed data and
drafted the manuscript, while RQ and ZY provided critical
revisions. RQ and ZY confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Institutional
Review Board of the Handan Central Hospital (approval no. 2024029).
All patients or their families provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vogel A, Meyer T, Sapisochin G, Salem R
and Saborowski A: Hepatocellular carcinoma. Lancet. 400:1345–1362.
2022. View Article : Google Scholar
|
|
2
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.
|
|
3
|
Llovet JM, Kelley RK, Villanueva A, Singal
AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J and
Finn RS: Hepatocellular carcinoma. Nat Rev Dis Primers. 7:62021.
View Article : Google Scholar
|
|
4
|
Galle PR, Dufour JF, Peck-Radosavljevic M,
Trojan J and Vogel A: Systemic therapy of advanced hepatocellular
carcinoma. Future Oncol. 17:1237–1251. 2021. View Article : Google Scholar
|
|
5
|
Gordan JD, Kennedy EB, Abou-Alfa GK, Beal
E, Finn RS, Gade TP, Goff L, Gupta S, Guy J, Hoang HT, et al:
Systemic therapy for advanced hepatocellular carcinoma: ASCO
guideline update. J Clin Oncol. 42:1830–1850. 2024. View Article : Google Scholar
|
|
6
|
Tian Z, Niu X and Yao W: Efficacy and
response biomarkers of apatinib in the treatment of malignancies in
China: A review. Front Oncol. 11:7490832021. View Article : Google Scholar
|
|
7
|
Li D, Xu L, Ji J, Bao D, Hu J, Qian Y,
Zhou Y, Chen Z, Li D, Li X, et al: Sintilimab combined with
apatinib plus capecitabine in the treatment of unresectable
hepatocellular carcinoma: A prospective, open-label, single-arm,
phase II clinical study. Front Immunol. 13:9440622022. View Article : Google Scholar
|
|
8
|
Xia WL, Zhao XH, Guo Y, Cao GS, Wu G, Fan
WJ, Yao QJ, Xu SJ, Guo CY, Hu HT and Li HL: Transarterial
chemoembolization combined with apatinib with or without PD-1
inhibitors in BCLC stage C hepatocellular carcinoma: A multicenter
retrospective study. Front Oncol. 12:9613942022. View Article : Google Scholar
|
|
9
|
Song H, Liu X, Jiang L, Li F, Zhang R and
Wang P: Current status and prospects of camrelizumab, A humanized
antibody against programmed cell death receptor 1. Recent Pat
Anticancer Drug Discov. 16:312–332. 2021. View Article : Google Scholar
|
|
10
|
Qin S, Ren Z, Meng Z, Chen Z, Chai X,
Xiong J, Bai Y, Yang L, Zhu H, Fang W, et al: Camrelizumab in
patients with previously treated advanced hepatocellular carcinoma:
A multicentre, open-label, parallel-group, randomised, phase 2
trial. Lancet Oncol. 21:571–580. 2020. View Article : Google Scholar
|
|
11
|
Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J,
Shao G, Zhang Y, Xu L, Yin T, et al: Camrelizumab in combination
with apatinib in patients with advanced hepatocellular carcinoma
(RESCUE): A nonrandomized, open-label, phase II trial. Clin Cancer
Res. 27:1003–1011. 2021. View Article : Google Scholar
|
|
12
|
Qin S, Chan SL, Gu S, Bai Y, Ren Z, Lin X,
Chen Z, Jia W, Jin Y, Guo Y, et al: Camrelizumab plus rivoceranib
versus sorafenib as first-line therapy for unresectable
hepatocellular carcinoma (CARES-310): A randomised, open-label,
international phase 3 study. Lancet. 402:1133–1146. 2023.
View Article : Google Scholar
|
|
13
|
Ayuso C, Rimola J, Vilana R, Burrel M,
Darnell A, García-Criado Á, Bianchi L, Belmonte E, Caparroz C,
Barrufet M, et al: Diagnosis and staging of hepatocellular
carcinoma (HCC): Current guidelines. Eur J Radiol. 101:72–81. 2018.
View Article : Google Scholar
|
|
14
|
Schwartz LH, Seymour L, Litière S, Ford R,
Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, et
al: RECIST 1.1-standardisation and disease-specific adaptations:
Perspectives from the RECIST working group. Eur J Cancer.
62:138–145. 2016. View Article : Google Scholar
|
|
15
|
Peng W, Pan Y, Xie L, Yang Z, Ye Z, Chen
J, Wang J, Hu D, Xu L, Zhou Z, et al: The emerging therapies are
reshaping the first-line treatment for advanced hepatocellular
carcinoma: A systematic review and network meta-analysis. Therap
Adv Gastroenterol. 17:175628482412376312024. View Article : Google Scholar
|
|
16
|
Feng T, Li Q, Zhu R, Yu C, Xu L, Ying L,
Wang C, Xu W, Wang J, Zhu J, et al: Tumor microenvironment
biomarkers predicting pathological response to neoadjuvant
chemoimmunotherapy in locally advanced esophageal squamous cell
carcinoma: Post-hoc analysis of a single center, phase 2 study. J
Immunother Cancer. 12:e0089422024. View Article : Google Scholar
|
|
17
|
Liu Q, Zhang H, Xiao H, Ren A, Cai Y, Liao
R, Yu H, Wu Z and Huang Z: Discovery of novel diagnostic biomarkers
of hepatocellular carcinoma associated with immune infiltration.
Ann Med. 57:25036452025. View Article : Google Scholar
|
|
18
|
Shigeta K, Datta M, Hato T, Kitahara S,
Chen IX, Matsui A, Kikuchi H, Mamessier E, Aoki S, Ramjiawan RR, et
al: Dual programmed death receptor-1 and vascular endothelial
growth factor receptor-2 blockade promotes vascular normalization
and enhances antitumor immune responses in hepatocellular
carcinoma. Hepatology. 71:1247–1261. 2020. View Article : Google Scholar
|
|
19
|
Ciciola P, Cascetta P, Bianco C, Formisano
L and Bianco R: Combining immune checkpoint inhibitors with
anti-angiogenic agents. J Clin Med. 9:6752020. View Article : Google Scholar
|
|
20
|
Ju S, Zhou C, Yang C, Wang C, Liu J, Wang
Y, Huang S, Li T, Chen Y, Bai Y, et al: Apatinib plus camrelizumab
with/without chemoembolization for hepatocellular carcinoma: A
real-world experience of a single center. Front Oncol.
11:8358892022. View Article : Google Scholar
|
|
21
|
Cai M, Huang W, Huang J, Shi W, Guo Y,
Liang L, Zhou J, Lin L, Cao B, Chen Y, et al: Transarterial
chemoembolization combined with lenvatinib plus PD-1 inhibitor for
advanced hepatocellular carcinoma: A retrospective cohort study.
Front Immunol. 13:8483872022. View Article : Google Scholar
|
|
22
|
Li T, Guo J, Liu Y, Du Z, Guo Z, Fan Y,
Cheng L, Zhang Y, Gao X, Zhao Y, et al: Effectiveness and
tolerability of camrelizumab combined with molecular targeted
therapy for patients with unresectable or advanced HCC. Cancer
Immunol Immunother. 72:2137–2149. 2023. View Article : Google Scholar
|
|
23
|
Yuan G, Cheng X, Li Q, Zang M, Huang W,
Fan W, Wu T, Ruan J, Dai W, Yu W, et al: Safety and efficacy of
camrelizumab combined with apatinib for advanced hepatocellular
carcinoma with portal vein tumor thrombus: A multicenter
retrospective study. Onco Targets Ther. 13:12683–12693. 2020.
View Article : Google Scholar
|
|
24
|
Mei K, Qin S, Chen Z, Liu Y, Wang L and
Zou J: Camrelizumab in combination with apatinib in second-line or
above therapy for advanced primary liver cancer: Cohort A report in
a multicenter phase Ib/II trial. J Immunother Cancer.
9:e0021912021. View Article : Google Scholar
|
|
25
|
Chen D, Chen X, Xu L, Wang Y, Zhu L and
Kang M: Camrelizumab combined with apatinib in the treatment of
patients with hepatocellular carcinoma: A real-world assessment.
Neoplasma. 70:580–587. 2023. View Article : Google Scholar
|
|
26
|
He MK, Zou RH, Wei W, Shen JX, Zhao M,
Zhang YF, Lin XJ, Zhang YJ, Guo RP and Shi M: Comparison of stable
and unstable ethiodized oil emulsions for transarterial
chemoembolization of hepatocellular carcinoma: Results of a
single-center double-blind prospective randomized controlled trial.
J Vasc Interv Radiol. 29:1068–1077.e2. 2018. View Article : Google Scholar
|
|
27
|
Qin S, Li Q, Gu S, Chen X, Lin L, Wang Z,
Xu A, Chen X, Zhou C, Ren Z, et al: Apatinib as second-line or
later therapy in patients with advanced hepatocellular carcinoma
(AHELP): A multicentre, double-blind, randomised,
placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol.
6:559–568. 2021. View Article : Google Scholar
|
|
28
|
Hou Z, Zhu K, Yang X, Chen P, Zhang W, Cui
Y, Zhu X, Song T, Li Q, Li H and Zhang T: Apatinib as first-line
treatment in patients with advanced hepatocellular carcinoma: A
phase II clinical trial. Ann Transl Med. 8:10472020. View Article : Google Scholar
|
|
29
|
Jin ZC, Zhong BY, Chen JJ, Zhu HD, Sun JH,
Yin GW, Ge NJ, Luo B, Ding WB, Li WH, et al: Real-world efficacy
and safety of TACE plus camrelizumab and apatinib in patients with
HCC (CHANCE2211): A propensity score matching study. Eur Radiol.
33:8669–8681. 2023. View Article : Google Scholar
|
|
30
|
Chen ICY, Dungca LBP, Yong CC and Chen CL:
Sequential living donor liver transplantation after liver resection
optimizes outcomes for patients with high-risk hepatocellular
carcinoma. Hepatobiliary Pancreat Dis Int. 24:50–56. 2025.
View Article : Google Scholar
|
|
31
|
Zhu HK, Mou HB, Wang ZY, Zhang W, Zhu D,
Zhong SY, Zheng SS and Zhuang L: Adjuvant chemotherapy improves
post-transplant outcome in patients with hepatocellular carcinoma.
Hepatobiliary Pancreat Dis Int. S1499-3872(25)00093-1. 2025.(Epub
ahead of print). View Article : Google Scholar
|