Introduction
The liver is one of the most common sites for cancer
metastasis. At initial diagnosis, solid tumors show an ~6.46%
incidence of liver metastasis, accounting for 37.96% of all
metastatic cases, with a median survival time of 4 months (1). According to the European
Neuroendocrine Tumor Society guidelines (2), hepatic metastases are categorized into
three types: Simple pattern, complex pattern and diffuse pattern.
The diffuse pattern is characterized by widespread metastases
occupying 60–70% of the liver.
Patients with a high hepatic metastasis burden often
have poor performance and liver function scores (3,4).
Studies show that Child-Pugh (5)
class C patients receiving standard liver cirrhosis treatment have
a mortality rate three times higher than class B patients (6). British Society of Gastroenterology
guidelines recommend best supportive care for patients with
Child-Pugh class C or Eastern Cooperative Oncology Group (ECOG)
performance status (PS)>2 (7).
In the present study, a patient with castration-resistant prostate
cancer and diffuse hepatic metastasis, of ECOG PS3 score and with
Child-Pugh class C liver function, was treated at the Department of
Oncology, The First People's Hospital of Yibin (Yibin, China).
After careful consideration, TAI was selected to explore its
potential for prolonging survival and improving quality of life in
this high-risk population.
Case report
Medical history
A 66-year-old man was diagnosed with acinar
adenocarcinoma of the prostate at the Department of Urology, The
First People's Hospital of Yibin, in June 2021. The patient was
started on androgen-deprivation therapy in the Outpatient Clinic
administered as 3.75 mg leuprorelin by subcutaneous injection every
4 weeks plus 50 mg bicalutamide orally once daily. After bone
metastases were documented in January 2022, bicalutamide was
replaced with 1,000 mg abiraterone orally once daily together with
5 mg prednisone twice daily. Progressive bone disease prompted
palliative radiotherapy (30 Gy in 10 fractions over 2 weeks)
administered at the Department of Oncology, Affiliated Hospital of
Southwest Medical University, Luzhou, China) in August 2022.
Widespread progression involving multiple liver and lung metastases
was diagnosed in December 2022; 120 mg docetaxel was intravenously
administered 3 days later (on day 1) and repeated 25 days later in
January 2023, but the disease continued to advance.
In February 2023, the patient visited the Department
of Oncology, The First People's Hospital of Yibin due to diffuse
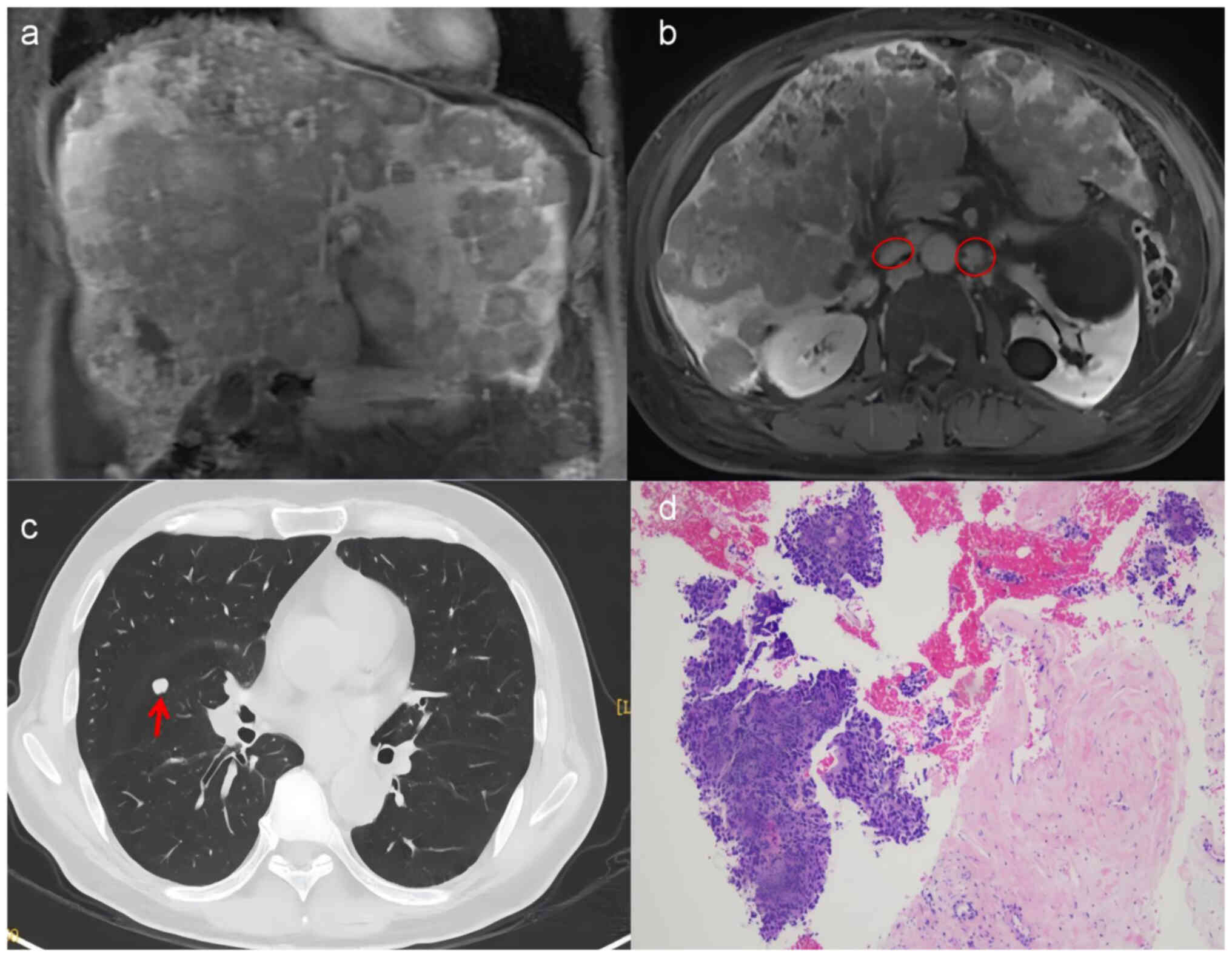
liver metastasis. Magnetic resonance imaging showed diffuse liver
metastasis (Fig. 1A) and
retroperitoneal lymph node metastasis (Fig. 1B), and computed tomography showed
lung metastasis (Fig. 1C). Tumor
marker levels were elevated as follows: Neuron-specific enolase
(NSE), 370 ng/ml (normal reference, ≤20.77 ng/ml); carcinoembryonic
antigen (CEA), 458.62 ng/ml (normal reference, <5.0 ng/ml);
carbohydrate antigen 19-9 (CA19-9), 394.09 U/ml (normal reference,
<36 U/ml); total tPSA, 19.60 ng/ml (normal reference, <4
ng/ml); free PSA, 11.89 ng/ml (normal reference, <1 ng/ml). The
α-fetoprotein level was 2.8 ng/ml (normal reference, <8.3 ng/ml)
and the squamous-cell carcinoma antigen level was 1.1 ng/ml
(reference, <1.5 ng/ml), which were within normal limits. Liver
function assessment showed Child-Pugh class C10 with the following
results: Total bilirubin, 49.3 µmol/l (normal reference, <21
µmol/l); albumin, 22.6 g/l (normal reference, <35 g/l);
prothrombin time, 15.9 sec (normal reference, 10–14 sec); moderate
ascites; and no hepatic encephalopathy. Liver biopsy showed diffuse
small- to medium-sized tumor cells without definite glandular
differentiation and significant fibrotic reaction (Fig. 1D). Immunohistochemistry was
performed on 4-µm formalin-fixed, paraffin-embedded sections.
Tissue blocks were fixed in 10% neutral-buffered formalin at room
temperature for 24 h, processed through graded alcohols and xylene,
and embedded in paraffin (Leica Paraplast Plus; Leica Biosystems).
After dewaxing and rehydration, antigen retrieval was carried out
in 0.01 M citrate buffer (pH 6.0) at 95°C for 20 min. Endogenous
peroxidase was quenched with 3% H2O2 for 10
min at room temperature, followed by blocking with 5% normal goat
serum (cat. no. AR0009; Wuhan Boster Biological Technology, Ltd.)
for 30 min at room temperature. Primary antibodies (Table I) were applied overnight at 4°C.
Sections were then incubated with HRP-conjugated secondary
antibodies (goat anti-mouse/rabbit; cat. no. PV-6000; ready-to-use;
Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) for 30 min at
room temperature. Color was developed with DAB (cat. no. ZLI-9018;
Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) and
counterstained with hematoxylin at room temperature for 2–3 min.
Slides were examined and images were captured with an Olympus BX53
light microscope (Olympus Corporation); representative images were
acquired at ×400 magnification. The results were as follows: Pan
cytokeratin(+), p504s(weak+), PSA(−), chromogranin A (CgA)(−),
CD56(−), synaptophysin (Syn)(−), hepatocytes(−), glutamine
synthetase(−), glypican-3(−) and Ki-67(+, 50%) (data not shown; all
results related to immunohistochemical findings described in this
manuscript are based on official pathology reports, not retrievable
image files). The pathological diagnosis was of a poorly
differentiated carcinoma. A subsequent departmental
multidisciplinary-team (MDT) review, integrating morphology,
immunoprofile and treatment history, concluded that a diagnosis of
treatment-related neuroendocrine prostate carcinoma with mixed
adenocarcinoma-neuroendocrine features (2022 WHO Classification of
Neuroendocrine Neoplasms) (8),
pending RB transcriptional corepressor 1 (RB1)/tumor protein p53
(TP53) confirmation. The patient declined the collection of
additional tissue for next-generation sequencing. In addition, the
MDT recommended active therapy, given the recognized
chemosensitivity of small-cell neuroendocrine carcinoma, absence of
variceal bleeding or hepatic encephalopathy, and the patient's wish
for treatment. Initial management consisted of 10 g human albumin
(i.v.) once daily, 200 mg magnesium isoglycyrrhizinate (i.v.) once
daily for hepatoprotection, 250 ml compound amino-acid injection
(14AA-SF) (21.2 g) (i.v.) once daily combined with 1,000 ml 5%
glucose (50 g) (i.v.) once daily for nutritional support; these
measures failed to improve the patient's condition and repeat
assessment confirmed a persistent Child-Pugh score of C10.
Consequently, as the diffuse tumor burden rendered systemic
intravenous chemotherapy likely to deliver sub-therapeutic hepatic
drug concentrations and since the high risk of hepatic failure
precluded both standard-dose systemic chemotherapy and
transcatheter arterial chemoembolization (TACE), neither modality
was offered. Accordingly, TAI was initiated on day 17 of admission
(February 2023). The first cycle, delivered under local
anaesthesia, comprised 120 mg etoposide plus 400 mg carboplatin on
day 1 (80% of standard protocol), and five additional identical
cycles were subsequently scheduled. On the night after surgery, the
patient experienced hypoxemia (oxygen saturation of ~90%) and
hypotension (86/54 mmHg); oxygen supplementation and intravenous
saline promptly restored cardiopulmonary stability. On the second
postoperative day, the patient experienced acute hepatic injury
[total bilirubin, 70.3 µmol/l; aspartate aminotransferase, 206 U/l
(reference range, 15–35 U/l)] that resolved after hepatoprotective
therapy with 200 mg magnesium isoglycyrrhizinate (i.v.) once daily
plus 465 mg polyene phosphatidylcholin (i.v.) once daily. The
patient completed six cycles of the same TAI regimen and remained
on anti-androgen maintenance consisting of 1,000 mg abiraterone
orally once daily plus 3.75 mg leuprorelin subcutaneously every 4
weeks.
 | Table I.Primary antibodies used. |
Table I.
Primary antibodies used.
| Antibody | Clone | Catalog no. | Dilution | Supplier |
|---|
| Pan-CK | AE1/AE3 | CCM-0960 | 1:50 | Celnovte
Biotechnology Co., Ltd. |
| p504s | C7H4 | CAM-0201 | 1:200 | Celnovte
Biotechnology Co., Ltd. |
| PSA | C2C12 | CPM-0404 | 1:200 | Celnovte
Biotechnology Co., Ltd. |
| CgA | C1E8 | CCM-0852 | 1:200 | Celnovte
Biotechnology Co., Ltd. |
| CD56 | C5A2 | CCM-0662 | 1:200 | Celnovte
Biotechnology Co., Ltd. |
| Syn | C9D11 | CSM-0250 | 1:200 | Celnovte
Biotechnology Co., Ltd. |
| Hepatocyte | MX119 | MAB-1034 | Ready-to-use | Fuzhou Maixin Biotech
Co., Ltd. |
| GS | C6E12 | CGM-0191 | Ready-to-use | Celnovte
Biotechnology Co., Ltd. |
| Glypican-3 | 1C12 | CCM-0200 | Ready-to-use | Celnovte
Biotechnology Co., Ltd. |
| Ki-67 | ARC-5050-01 | C1CR-0034 | 1:200 | Celnovte
Biotechnology Co., Ltd. |
After treatment, efficacy was evaluated according to
the Response Evaluation Criteria in Solid Tumors version 1.1.
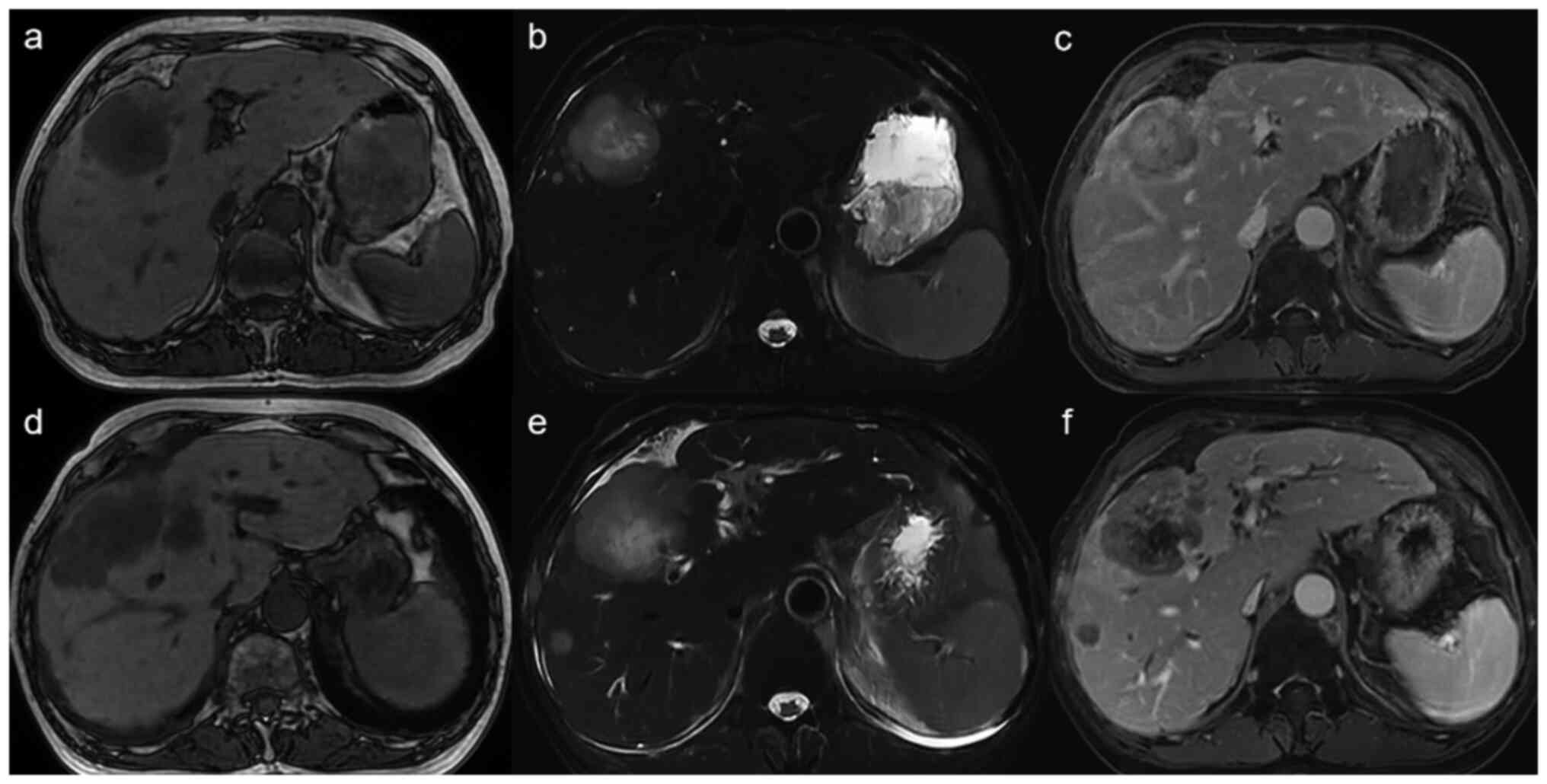
Baseline MRI had demonstrated innumerable
T1-hypointense/T2-hyperintense hepatic nodules with peripheral rim
enhancement on arterial-phase T1-weighted images (Fig. 2A-C). Post-therapy MRI showed >30%
reduction in overall hepatic tumour burden, with smaller and less
conspicuous nodules and markedly diminished contrast enhancement
(Fig. 2D-F), consistent with a
partial response. Concurrently, the ECOG PS score decreased to 1,
liver function recovered to Child-Pugh A5 and the patient regained
full self-care ability. Subsequently, after the tumor progressed
again, with follow-up until September 2024, the patient died at
home. The progression-free survival time from TAI was 11.05 months
and the overall survival time was 18.87 months.
Discussion
The present study reports a case of
castration-resistant prostate cancer with diffuse hepatic
metastasis, severe hepatic dysfunction (Child-Pugh class C10) and
poor PS (ECOG PS 3). The patient showed significant improvement
after TAI with the EC regimen.
Hepatic metastasis in prostate cancer indicates a
poor prognosis. A previous study showed that 20–30% of patients
with metastatic castration-resistant prostate cancer develop
hepatic metastasis during treatment, indicating high aggressiveness
(9). Especially in patients with
rapid progression and elevated NSE, neuroendocrine transformation
should be suspected. Neuroendocrine prostate cancer (NEPC) has an
incidence of 11–17% (10,11); it is characterized by decreased
androgen receptor and PSA expression, and increased NSE, CEA and
CA19-9 levels (12–14). Immunohistochemistry markers such as
CgA, CD56 and Syn are usually positive (15). Mixed pathology is reported in 91.9%
of cases (16). In the present
case, immunohistochemistry showed negative expression of CgA, CD56,
Syn and PSA, with diffuse small- to medium-sized cancer cells, and
significantly elevated NSE, CEA and CA19-9 levels (17). The rapid progression and significant
therapeutic response after NEPC-directed treatment were consistent
with previous studies. Considering the concurrent PSA elevation,
the presence of prostatic acinar adenocarcinoma components could
not be ruled out. Although CgA, Syn and CD56 results were all
negative, the composite evidence of i) treatment-emergent disease
following prolonged androgen deprivation therapy and novel hormonal
therapy, ii) rapid visceral spread, iii) loss of PSA expression
accompanied by markedly elevated NSE, CEA and CA19-9 levels, iv) a
Ki-67 index of 50%, and v) an unmistakable response to
platinum-etoposide, strongly supports the diagnosis of
treatment-related neuroendocrine prostate carcinoma with atypical
marker expression or a mixed adenocarcinoma-neuroendocrine
phenotype. The patient declined genetic testing, leaving the
absence of RB1/TP53 evidence as an unresolved limitation.
Although Child-Pugh class C hepatic dysfunction and
PS 3–4 are often considered treatment contraindications, some
studies suggest that selective treatment can still be considered.
One study showed that patients with Child-Pugh class C who received
TACE had a median survival time of 7.1 months (18). Similarly, two studies on patients
with small cell lung cancer and PS 3–4 showed that chemotherapy
could significantly prolong survival time (19,20).
These findings suggest that selective patients with poor PS time or
severe hepatic dysfunction may still benefit from treatment and
have their survival time extended.
In conclusion, for highly aggressive and
treatment-sensitive tumors such as prostate neuroendocrine
carcinoma, even in the presence of diffuse hepatic
metastasis-induced poor PS and severe hepatic dysfunction,
attempting low-dose local drug therapy can be a viable option.
Otherwise, patients may succumb to rapid tumor progression, hepatic
failure or other complications in a short period of time.
Acknowledgements
The authors acknowledge that IHC images are
unavailable for inclusion in this manuscript, as the institution
does not archive them.
Funding
Funding was provided by the Yibin University Medical Research
Fund (grant no. 2024YBUYXJJ022), the National Anti-Tumor Drug
Surveillance System of National Cancer Center (grant no.
DSS-YSF-202311) and the Health Commission of Sichuan Provincial
Medical Science and Technology Program (grant no. 24WSXT074).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XCC was responsible for study conception, clinical
data acquisition and interpretation, and drafting of the
manuscript. XQL participated in follow-up data collection and
analysis, literature review and manuscript revision. YL contributed
to clinical data interpretation and critically revised the
manuscript. XZ performed histopathological evaluation of biopsy
specimens and revised the manuscript. CL performed all
trans-arterial infusion procedures. ZPY analyzed and interpreted
MRI/computed tomography images, and critically revised the
manuscript. XCC and ZPY confirm the authenticity of all the raw
data. All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Ethics approval was granted from the Ethics
Committee of the First People's Hospital of Yibin [approval no.
2025 (12)]. Informed written
consent was obtained from the patient to participate in this
study.
Patient consent for publication
Written informed consent for publication of this
case report and any accompanying images was obtained from the
patient after completion of trans-arterial infusion therapy.
Following the patient's death, verbal confirmation of consent was
obtained from the patient's son via telephone.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript or
to generate images, and subsequently, the authors revised and
edited the content produced by the AI tools as necessary, taking
full responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Wang S, Feng Y, Swinnen J, Oyen R, Li Y
and Ni Y: Incidence and prognosis of liver metastasis at diagnosis:
A pan-cancer population-based study. Am J Cancer Res. 10:1477–1517.
2020.
|
|
2
|
Pavel M, Baudin E, Couvelard A, Krenning
E, Öberg K, Steinmüller T, Anlauf M, Wiedenmann B and Salazar R;
Barcelona Consensus Conference participant, : ENETS consensus
guidelines for the management of patients with liver and other
distant metastases from neuroendocrine neoplasms of foregut,
midgut, hindgut, and unknown primary. Neuroendocrinology.
95:157–176. 2012. View Article : Google Scholar
|
|
3
|
Hsu CY, Lee YH, Hsia CY, Huang YH, Su CW,
Lin HC, Lee RC, Chiou YY, Lee FY and Huo TI: Performance status in
patients with hepatocellular carcinoma: Determinants, prognostic
impact, and ability to improve the Barcelona clinic liver cancer
system. Hepatology. 57:112–119. 2013. View Article : Google Scholar
|
|
4
|
Culine S, Kramar A, Saghatchian M, Bugat
R, Lesimple T, Lortholary A, Merrouche Y, Laplanche A and Fizazi K;
French Study Group on Carcinomas of Unknown Primary, : Development
and validation of a prognostic model to predict the length of
survival in patients with carcinomas of an unknown primary site. J
Clin Oncol. 20:4679–4683. 2002. View Article : Google Scholar
|
|
5
|
Pugh RNH, Murray-Lyon IM, Dawson JL,
Pietroni MC and Williams R: Transection of the oesophagus for
bleeding oesophageal varices. Br J Surg. 60:646–649. 1973.
View Article : Google Scholar
|
|
6
|
Conejo I, Guardascione MA, Tandon P,
Cachero A, Castellote J, Abraldes JG, Amitrano L, Genescà J and
Augustin S: Multicenter external validation of risk stratification
criteria for patients with variceal bleeding. Clin Gastroenterol
Hepatol. 16:132–139.e8. 2018. View Article : Google Scholar
|
|
7
|
Suddle A, Reeves H, Hubner R, Marshall A,
Rowe I, Tiniakos D, Hubscher S, Callaway M, Sharma D, See TC, et
al: British society of gastroenterology guidelines for the
management of hepatocellular carcinoma in adults. Gut.
73:1235–1268. 2024. View Article : Google Scholar
|
|
8
|
Rindi G, Mete O, Uccella S, Basturk O, La
Rosa S, Brosens LAA, Ezzat S, de Herder WW, Klimstra DS, Papotti M
and Asa SL: Overview of the 2022 WHO classification of
neuroendocrine neoplasms. Endocr Pathol. 33:115–154. 2022.
View Article : Google Scholar
|
|
9
|
Ni X, Wei Y, Li X, Pan J, Fang B, Zhang T,
Lu Y, Ye D and Zhu Y: From biology to the clinic-exploring liver
metastasis in prostate cancer. Nat Rev Urol. 21:593–614. 2024.
View Article : Google Scholar
|
|
10
|
Aggarwal R, Huang J, Alumkal JJ, Zhang L,
Feng FY, Thomas GV, Weinstein AS, Friedl V, Zhang C, Witte ON, et
al: Clinical and genomic characterization of treatment-emergent
small-cell neuroendocrine prostate cancer: A multi-institutional
prospective study. J Clin Oncol. 36:2492–2503. 2018. View Article : Google Scholar
|
|
11
|
Abida W, Cyrta J, Heller G, Prandi D,
Armenia J, Coleman I, Cieslik M, Benelli M, Robinson D, Van Allen
EM, et al: Genomic correlates of clinical outcome in advanced
prostate cancer. Proc Natl Acad Sci USA. 116:11428–11436. 2019.
View Article : Google Scholar
|
|
12
|
Yao JL, Madeb R, Bourne P, Lei J, Yang X,
Tickoo S, Liu Z, Tan D, Cheng L, Hatem F, et al: Small cell
carcinoma of the prostate: An immunohistochemical study. Am J Surg
Pathol. 30:705–712. 2006. View Article : Google Scholar
|
|
13
|
Aparicio AM, Harzstark AL, Corn PG, Wen S,
Araujo JC, Tu SM, Pagliaro LC, Kim J, Millikan RE, Ryan C, et al:
Platinum-based chemotherapy for variant castrate-resistant prostate
cancer. Clin Cancer Res. 19:3621–3630. 2013. View Article : Google Scholar
|
|
14
|
Szarvas T, Csizmarik A, Fazekas T, Hüttl
A, Nyirády P, Hadaschik B, Grünwald V, Püllen L, Jurányi Z, Kocsis
Z, et al: Comprehensive analysis of serum chromogranin A and
neuron-specific enolase levels in localized and
castration-resistant prostate cancer. BJU Int. 127:44–55. 2021.
View Article : Google Scholar
|
|
15
|
Haffner MC, Morris MJ, Ding CKC, Sayar E,
Mehra R, Robinson B, True LD, Gleave M, Lotan TL, Aggarwal R, et
al: Framework for the pathology workup of metastatic
castration-resistant prostate cancer biopsies. Clin Cancer Res.
31:466–4478. 2025. View Article : Google Scholar
|
|
16
|
Gagnon R, Kish EK, Cook S, Takemura K,
Cheng BYC, Bressler K, Heng DYC, Alimohamed N, Ruether D, Lee-Ying
RM, et al: Real-world clinical outcomes and prognostic factors in
neuroendocrine prostate cancer. Clin Genitourin Cancer.
23:1022742025. View Article : Google Scholar
|
|
17
|
Liu S, Alabi BR, Yin Q and Stoyanova T:
Molecular mechanisms underlying the development of neuroendocrine
prostate cancer. Semin Cancer Biol. 86:57–68. 2022. View Article : Google Scholar
|
|
18
|
Choi TW, Kim HC, Lee JH, Yu SJ, Kang B,
Hur S, Lee M, Jae HJ and Chung JW: The safety and clinical outcomes
of chemoembolization in child-pugh class C patients with
hepatocellular carcinomas. Korean J Radiol. 16:1283–1293. 2015.
View Article : Google Scholar
|
|
19
|
Aida Y, Nakazawa K, Shiozawa T, Ogawa R,
Kiwamoto T, Morishima Y, Sakamoto T, Sekine I and Hizawa N:
Small-Cell lung cancer treatment of newly diagnosed patients with
poor performance status. Case Rep Oncol. 12:613–620. 2019.
View Article : Google Scholar
|
|
20
|
Bahij R, Jeppesen SS, Olsen KE, Halekoh U,
Holmskov K and Hansen O: Outcome of treatment in patients with
small cell lung cancer in poor performance status. Acta Oncol.
58:1612–1617. 2019. View Article : Google Scholar
|