Introduction
Primary squamous cell carcinoma of the thyroid
(PSCCT) is a rare and aggressive malignancy comprising <1% of
all thyroid cancer cases (1) and is
attributed to the normal absence of squamous epithelium in the
thyroid gland under physiological conditions (2,3).
Immunohistochemistry is pivotal for a definitive diagnosis, helping
to distinguish PSCCT from metastatic tumors of other primary
origins (4). Immunohistochemical
results of PSCCT tumor cells showed diffuse positivity for
cytokeratin 5/6 (CK5/6), cytokeratin 19 (CK19), epithelial membrane
antigen (EMA), p53, and p63, while being negative for thyroid
transcription factor-1 (TTF-1), galectin-3, and thyroglobulin (TG)
(5). Clinically, PSCCT typically
manifests as a rapidly enlarging mass in the anterior neck region.
Surgical resection remains the primary treatment modality; however,
no standardized therapeutic guidelines exist owing to its rarity
(6). Consequently, the efficacy of
adjuvant chemotherapy, radiotherapy, and targeted therapy remains
unclear.
The study of SCCT is important in modern medical
research; however, it poses considerable challenges. The low
incidence of PSCCT, coupled with its aggressive nature and
therapeutic challenges, have attracted extensive research interest
(3,4,7,8).
Investigations primarily focus on etiology, pathology and treatment
(7,8). Etiologically, the underlying
pathogenic mechanisms remain unclear, with only a few hypotheses
having been proposed. It may originate from the thyroid follicular
epithelium or develop through squamous metaplasia (9), whereas other studies propose a
potential association with the thyroglossal duct epithelium
(10–12). Pathologically, further detailed
analyses are required regarding the tumor cell microstructure,
molecular markers and tissue relationships. Therapeutically,
surgical resection is the mainstay; nevertheless, standardized
protocols are absent owing to the rarity of the disease.
Consequently, the evaluation and optimization of chemotherapy,
radiotherapy and targeted therapies remain exploratory and
contentious (13). Targeted
therapy, for example, faces challenges such as drug resistance,
limited efficacy and individual variability (14). Furthermore, cytokines play a dual
role in tumor therapy and inflammatory regulation, potentially
enhancing antitumor immunity and influencing the tumor-associated
microenvironment (15). In summary,
regardless of research progress, a comprehensive diagnostic and
therapeutic framework for PSCCT remains elusive. Therefore, the
present study aims to provide an in-depth investigation into the
pathological and therapeutic challenges of PSCCT, with a particular
focus on its molecular biological characteristics and treatment
responses. This objective was accomplished through a detailed
analysis of a representative case report combined with a
comprehensive review of relevant literature. The case report forms
the core of our research, offering concrete clinical and molecular
insights that complement the existing body of knowledge. In the
discussion section, we integrate these observational findings with
current evidence to elucidate the pathogenesis of PSCCT, highlight
novel treatment approaches, and identify potential strategies for
improving patient survival rates and quality of life. The present
study aimed to lay a solid theoretical foundation for the
development of more scientific and rational clinical
guidelines.
Case report
In July 2023, a 66-year-old female patient
discovered a swelling in the left anterior neck, which was
approximately the size of a walnut, without accompanying pain,
compressive symptoms or hyperthyroidism. The patient had a history
of psoriasis, which remained untreated, with no other notable
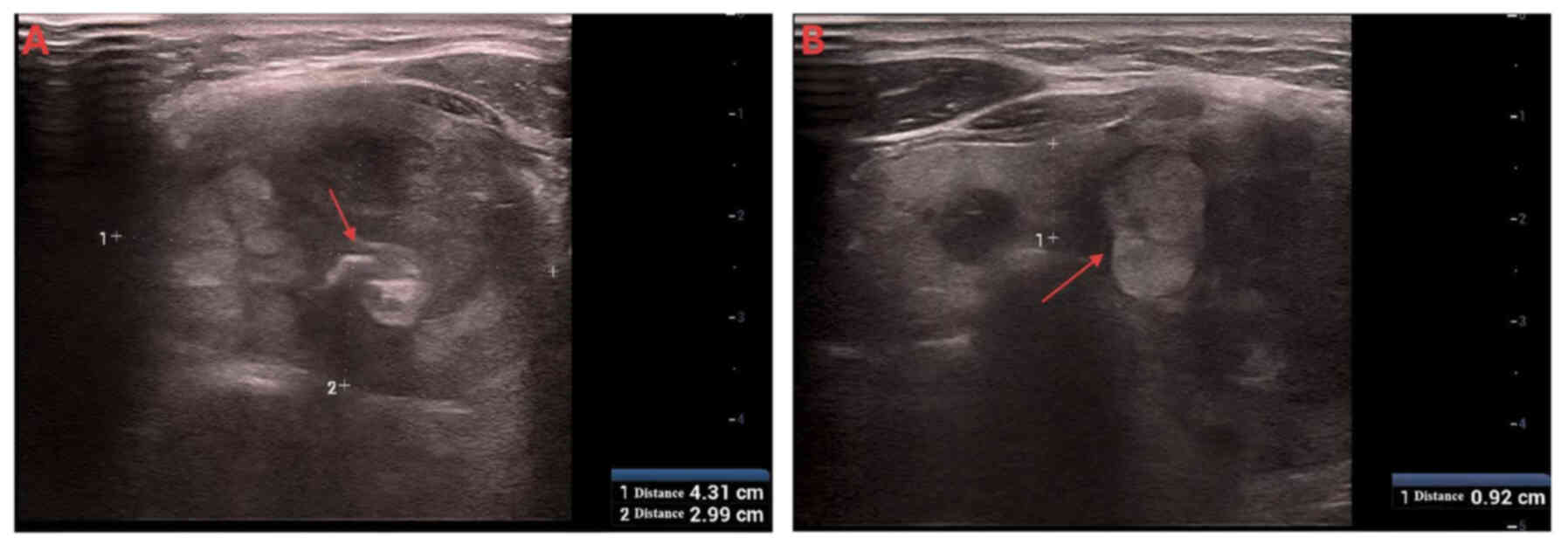
medical conditions. Thyroid ultrasound examination using the Resona
8S system (Mindray) equipped with an L14-5WU transducer (Fig. 1) revealed multiple bilateral thyroid
nodules (Chinese Thyroid Imaging Reporting and Data System-3)
(16) upon referral to Affiliated
Hospital of Shandong Second Medical University (Shandong, China). A
fine-needle aspiration biopsy was recommended for a definitive
diagnosis; however, the patient declined the procedure.
After 2 months, the patient returned with marked
enlargement of the mass, accompanied by increased neck swelling and
dyspnea. The patient was readmitted with a provisional diagnosis of
‘thyroid mass (nature to be determined)’. The patient underwent a
surgical intervention under general anesthesia in September 2023.
Preoperative blood routine tests showed no significant
abnormalities; preoperative thyroid function tests indicated
anti-thyroglobulin (TG) antibody level at 320 IU/ml (reference
range, ≤115 IU/ml), with all other parameters normal. Postoperative
re-examination of six thyroid function items yielded the following
results: Free triiodothyronine, 4.26 pmol/l (reference range,
3.1–6.8 pmol/l); thyrotropin receptor antibody <0.8 IU/l
(reference range, ≤1.75 IU/l); free thyroxine, 15.60 pmol/l
(reference range, 11.97–21.88 pmol/l); anti-TG antibody, 87.60
IU/ml (reference range, ≤115 IU/ml); thyroid-stimulating hormone,
3.93 µIU/ml (reference range, 0.27–4.2 µIU/ml); and anti-thyroid
peroxidase antibody, 19.40 IU/ml (reference range, ≤34 IU/ml). The
preoperative cardiac enzyme profile showed the following results:
LDH, 239.0 U/l (reference range, 120–250 U/l); creatine kinase,
43.0 U/l (reference range, 40–200 U/l); CK-MB, 14.00 U/l (reference
range, <25 U/l); and α-hydroxybutyrate dehydrogenase, 191.0 U/l
(reference range, 95–250 U/l), with all the indicators within the
normal ranges.
Intraoperative examination showed a firm, 6.0×5.0-cm
mass in the left thyroid lobe, with invasion into the anterior
cervical muscles. The right lobe contained a well-circumscribed,
regularly shaped, firm, 1.5×1.0-cm nodule at its lower pole.
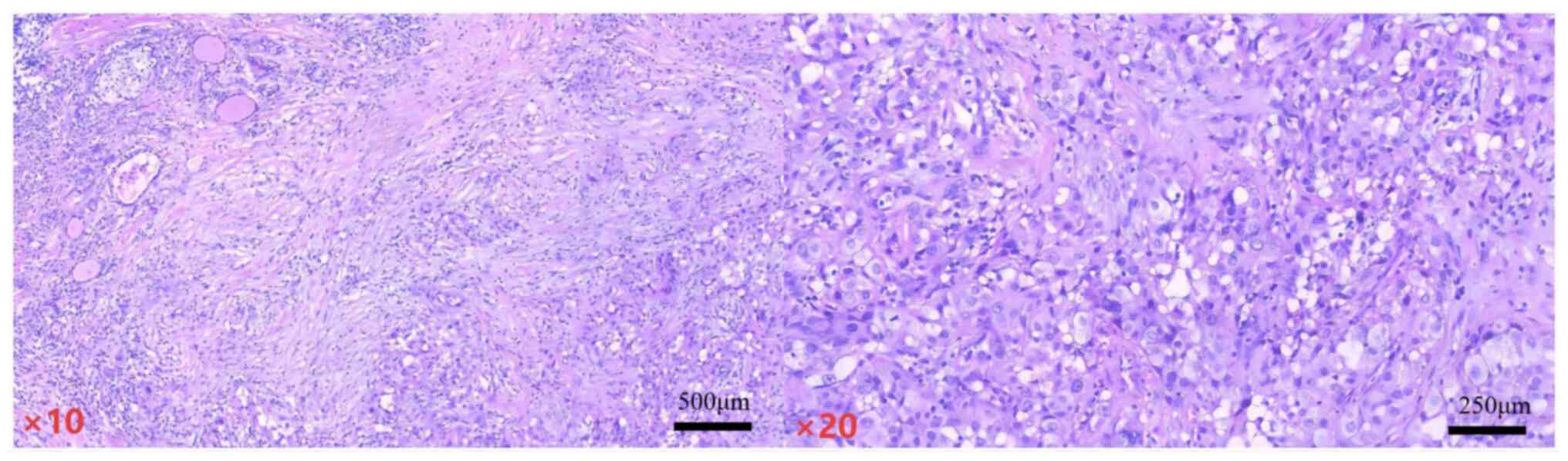
Sections were prepared at a freezing temperature of −20°C with a
thickness of 5 µm. Hematoxylin and eosin staining was subsequently
performed at 26°C for 10 min. The stained sections were examined
using an optical microscope at magnifications of 10× and 20×.)
(Fig. 2) revealed a poorly
differentiated carcinoma in the left thyroid and a nodular goiter
with focal papillary hyperplasia of the follicular epithelium and
cholesterol crystal deposition in the right thyroid. Consequently,
the patient underwent a radical left thyroidectomy for the
carcinoma and a right lobectomy for the nodular goiter. No
metastatic carcinoma was found in all nine examined central lymph
nodes in the postoperative pathology. Immunohistochemical (IHC)
analysis yielded the following results: CK19(+); thyroid
transcription factor-1(−); TG(−); carcinoembryonic antigen(−);
galectin-3(−); cyclin D1(−); p53(mutant type); epithelial membrane
antigen(+); CK5/6(+) and Ki-67(+)(~60%). The following primary
antibodies were employed: CK19 (OriGene Technologies, Inc.; cat.
no. ZM-0074), galectin-3 (ZSGB-BIO, Cat#: ZM-0143), CEA (ZSGB-BIO,
Cat#: ZM-0062), EMA (cat. no. ZM-0095), TG (ZSGB-BIO, Cat#:
ZM-0241), Ki-67 (ZSGB-BIO, cat. no. ZM-0166), TTF-1 (Gene Tech,
Cat#: GT-2180), p53 (Gene Tech, Cat#: GT-2095), CK5/6 (Gene Tech,
Cat#: GT-2438), and cyclin D1 (RMA-1125). All antibodies were
ready-to-use and required no dilution. Incubation was carried out
at 37°C for 30 min. For detection, DAB Titan Super (Fuzhou Maixin
Biotech, Cat#: TT-0805) was used as a ready-to-use,
high-sensitivity labeled anti-mouse/rabbit IgG polymer. The
staining reaction was developed at 25°C for 30 min. Finally, the
specimens were examined using optical microscopes at 10×
magnification (Fig. 3). Based on
the clinical presentation and pathological findings, the diagnosis
was confirmed as Primary squamous thyroid cancer. The following
recommendations were made, following multidisciplinary
consultations involving nuclear medicine, radiation oncology and
medical oncology teams: i) Comprehensive systemic evaluation
including contrast-enhanced magnetic resonance imaging (MRI) of the
brain and cervical spine, along with whole-body MRI, to establish
accurate clinical staging, rule out secondary malignancies and
provide baseline tumor assessment; ii) molecular profiling,
including programmed death-ligand 1 (PD-L1) expression and BRAF/RAS
mutation testing; and iii) implementation of combination
therapeutic strategies (including potential immunotherapy or
chemotherapy) based on comprehensive diagnostic workup. The patient
had routinely taken 200 mcg levothyroxine sodium tablets each day
in the postoperative period until December 2024.
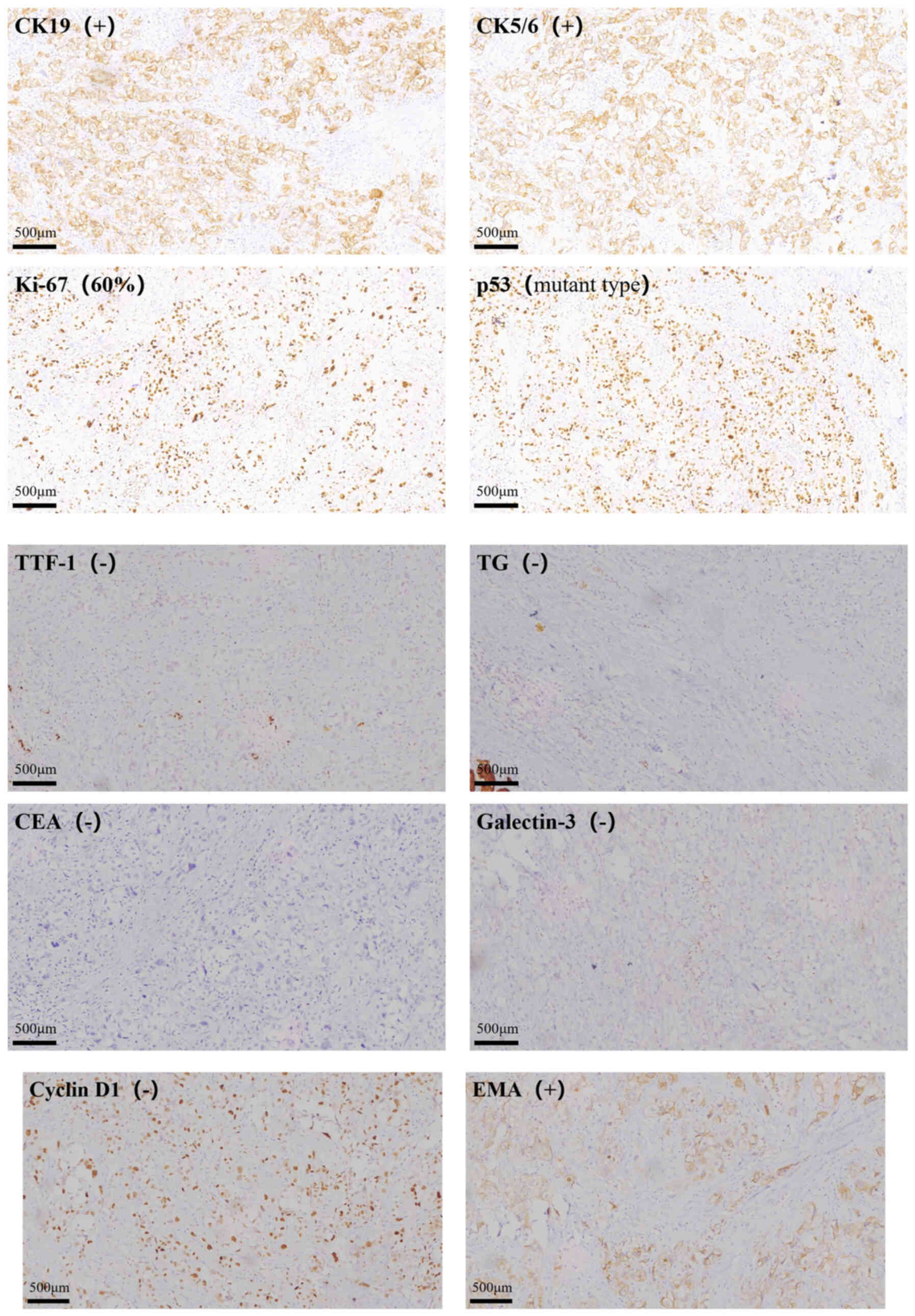 | Figure 3.Immunohistochemical analysis of,
CK5/6, Ki-67, p53, TTF-1, TG, CEA, Galectin-3, Cyclin D1 and EMA
expression in primary squamous cell carcinoma of the thyroid; CK,
cytokeratin; TTF, thyroid transcription factor-1; TG,
thyroglobulin; CEA, carcinoembryonic antigen; EMA, epithelial
membrane antigen. |
In October 2023, the patient underwent PD-L1 testing
at another hospital. IHC analysis was employed to assess protein
expression levels, utilizing the PD-L1 IHC 22C3 pharmDx assay kit
(Dako North America Inc.; cat. no. CH5.3 SK006). This kit
qualitatively detects PD-L1 protein in neutral buffered
formalin-fixed, paraffin-embedded tumor tissue. The test results
indicated high PD-L1 expression (combined positive score=50)
(17). Radiotherapy combined with
immunotherapy was recommended to the patient according to the
multidisciplinary team. The patient underwent six cycles of
chemotherapy combined with immunotherapy in the Department of
Oncology of Affiliated Hospital of Shandong Second Medical
University between November 2023 and February 2024. The regimen
specifically included 200 mg tislelizumab on day 0 as
immunotherapy, and 200 mg albumin-bound paclitaxel on days 1 and 8
+ 450 mg carboplatin on day 2 as systemic chemotherapy,
supplemented with adjunctive medications including hepatoprotective
magnesium isoglycyrrhizinate Injection (200 mg, days 1 and 8,
intravenous infusion), and antiemetics Ondansetron Injection (8 mg,
days 1 and 8, intravenous) plus Aprepitant Capsules (125 mg, days 1
and 8, orally), which was tolerated well. The patient commenced
radiotherapy in March 2024. The prescribed dose to achieve the
planning target volume, including the tumor bed and bilateral neck
drainage areas, was 50 Gy/2 Gy/25 fractions. Following this, five
fractions were administered to the local tumor bed during the
treatment period. The patient experienced a sore and dry throat,
accompanied by difficulty in eating, consistent with
radioactive-induced mucositis. Radiotherapy was temporarily
suspended, and parenteral nutrition was provided owing to poor oral
intake. A routine blood examination revealed leukopenia, with a
white blood cell count of 1.96×109/l (reference range,
3.5–9.5×109/l). The condition improved following
leukopoietic therapy. Subsequently, the patient refused to continue
radiotherapy after completing 16 sessions. The patient returned for
monthly follow-up visits, with the last follow-up in June 2024.
Postoperative thyroid changes were noted on imaging, while neck CT
revealed no signs of recurrence. The tumor response was assessed as
complete remission. The patient has reported no discomfort and
maintains an excellent mental status. In this case, following
radical surgery, the patient was treated with paclitaxel +
platinum-based chemotherapy combined with tislelizumab
immunotherapy, along with localized radiotherapy. At the last
follow-up, the patient exhibited stable vital signs with no
recurrence or metastasis.
Discussion
SCCT was previously classified as a separate entity
in the World Health Organization Classification of Tumors. However,
it is now considered a subtype of interstitial thyroid carcinoma
(18). The thyroid gland typically
lacks squamous epithelium, and squamous carcinoma accounts for
<1% of all thyroid malignancies. The pathogenesis of PSCCT
remains controversial and inconclusive, as squamous epithelial
cells are absent in the thyroid gland. Currently, more scholars
believe that there is a high possibility that the tissue origin of
PSCCT is a direct transformation of follicular cells. This is
supported by findings such as the detection of BRAF gene mutations
in PSCCT specimens (5).
Additionally, the origin may be derived from remnants of the
thyroglossal duct epithelium (19).
Secondary SCCT may arise via infiltration and metastasis from
neighboring structures such as the larynx, trachea and esophagus.
The ‘squamous epithelial hyperplasia theory’ reveals that SCC
develops as a degeneration secondary to squamous epithelial
hyperplasia of the thyroid tissue, possibly occurring at the
expense of underlying histological abnormalities of the thyroid
gland (20). Surgical resection is
the primary treatment for PSCCT, with postoperative chemotherapy
and radiotherapy serving as adjuvant therapies. However, due to
PSCCT poor response to radiotherapy, relative resistance to
chemotherapy, and the ineffectiveness of radioactive iodine
ablation, more effective treatment strategies are needed to improve
patient survival (2,13). Studies (21,22)
have found that the multi-receptor tyrosine kinase inhibitor
lenvatinib may potentially extend the survival of patients with
PSCCT patients, though further research is required to support this
finding. Additionally, PD-L1 immunotherapy for PSCCT has garnered
attention, with certain studies (23–25)
suggesting that anti-PD-L1 immunotherapy could be an effective
option for patients affected by the most aggressive forms of
thyroid cancer. However, one study reported that in a patient with
lung metastases, the primary thyroid lesion gradually enlarged
during pembrolizumab treatment, while the lung lesions
progressively diminished (26).
Lactate dehydrogenase (LDH) is a valuable enzyme biomarker in
patients with cancer (27). The
present cardiac enzyme profile showed the following results: LDH,
239.0 U/l (reference range, 120–250 U/l); creatine kinase), 43.0
U/l (reference range, 40–200 U/l); CK-MB, 14.00 U/l (reference
range, <25 U/l); and α-hydroxybutyrate dehydrogenase, 191.0 U/l
(reference range, 95–250 U/l), with all the indicators within the
normal ranges. The patient's preoperative thyroid function showed
that the anti-TG antibody level was 320 IU/ml, and there was a
history of untreated psoriasis, suggesting that autoantibodies and
inflammatory stimuli may have led to repeated damage to the thyroid
follicular cells. PSCCT involves the lymph nodes in 59% of cases,
and distant metastases occur in 26% of cases. The reported median
survival time of patients is 8 months (2). There is no systematic treatment for
PSCCT; however, radical surgery is the mainstay of treatment. SCCT
can easily invade surrounding tissues and organs, as the lesions
are not completely removed in numerous cases, when surgery is
performed alone. Therefore, adjuvant local radiotherapy
post-surgery is an accepted treatment method (28). Studies have shown that surgical
treatment can be associated with complications, such as
hypoparathyroidism, triggering hypocalcemia, recurrent laryngeal
nerve injury, cervical hematoma and wound infection (29–31).
Studies have shown that high Ki-67 expression and p53 high
expression are associated with a poor patient prognosis and an
increased risk of local recurrence post-surgery (32–35).
The incidence of TP53 mutations varies between sporadic cancers: it
ranges from 38% to 50% in ovarian, esophageal, colorectal, head and
neck, laryngeal, and lung cancers, while being only about 5% in
primary leukemias, sarcomas, testicular cancers, malignant
melanomas, and cervical cancers (36). Due to the high expression of PD-L1,
blocking the programmed cell death protein 1/PD-L1 immune
checkpoint pathway has become an important therapeutic tool for
various tumors by restoring the antitumor activity of T cells. The
treatment has been more widely used in non-small cell lung cancer,
melanoma, bladder cancer, SCC of the head and neck, triple-negative
breast cancer and gastric cancer (37). In the present study, following the
patient's IHC and genetic test results, six cycles of tislelizumab
immunotherapy were administered combined with chemotherapy and
adjuvant radiotherapy, based on the initial radical surgery.
However, the patient refused to continue radiotherapy after 16
sessions owing to the severe side effects of radiotherapy, which
were found to be intolerable. With a high degree of malignancy,
PSCCT has unique clinical, pathological and molecular features. It
is important to recognize this unique variant of thyroid cancer,
perform possible curative surgical resection and conduct further
genomic studies to reveal its molecular pathogenesis. In the 3rd
and 4th editions of the World Health Organization (WHO)
classification of endocrine tumors, squamous cell carcinoma of the
thyroid was categorized as a distinct entity among thyroid
neoplasms; however, in the 5th edition of the WHO classification of
thyroid tumors, it has been reclassified as a subtype of anaplastic
thyroid carcinoma (2,13,38,39).
It is characterized by highly aggressive behavior and poor patient
prognosis. The diagnosis of SCCT is difficult due to the
insufficiency of reported cases nationally and internationally.
Conversely, it needs to be differentiated from thyroid carcinoma,
undifferentiated thyroid carcinoma and metastatic carcinoma of
neighboring upper digestive organs (40). Undifferentiated thyroid cancer has a
high rate of distant metastasis; therefore, more patients receive
chemotherapy; its regimen includes Adriamycin alone or in
combination with cisplatin and 5-fluorouracil. However, it is
inefficient, and complete eradication with chemotherapy alone has
not been reported. Recently, a number of new chemotherapeutic
agents or regimens have been used for the treatment of
undifferentiated thyroid cancer, and their efficacy remains under
evaluation (41). PSCCT is a highly
aggressive malignancy, often detected at advanced stages and
associated with a poor prognosis, with a median survival time of
6–9 months and a 1-year survival rate of 33.3% (2,13,40–42).
Age >60 years, lymph node metastasis and tumor size >7 cm are
risk factors affecting patient survival (43). The patient in this case was
diagnosed at 66 years of age, with a maximum tumor diameter of only
6 cm-both of which are significant factors affecting survival
rates. However, due to early detection, precise treatment, and the
administration of tislelizumab, the patient exhibited neither lymph
node metastasis nor distant metastasis. The tumor was completely
surgically resected with negative margins, and the patient has now
survived for over one year. This outcome stands in stark contrast
to the reported one-year survival rate of merely 33.3% in the
literature, underscoring how early detection, diagnosis, and
treatment can significantly improve patient survival. It also
suggests that tislelizumab may contribute to increased survival
rates. In conclusion, the present study reported a case of PSCCT,
reviewing its surgical and subsequent course of treatment. When
discussing its pathogenesis and current therapeutic approaches,
PSCCT is shown to be rare and highly malignant, with limited
treatment options, all contributing to a poor prognosis.
Consequently, early detection is important for radical surgical
resection. In addition, more genomic studies are needed to reveal
the molecular pathogenesis and identify novel targeted therapies.
This includes immunotherapy, owing to the cancer being refractory
to current chemotherapeutic modalities.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SD, XW, ZZ, LF and QX confirm the authenticity of
all the raw data. SD was responsible for conceptualization, writing
the original draft, patient diagnosis and treatment. XW analyzed
and interpreted data. ZZ wrote and revised the manuscript and
analyzed patient data. LF was responsible for reviewing and editing
the manuscript, supervision, patient diagnosis and treatment. QX
designed the study design, acquisition of medical images, and
reviewed and approved the final version for publication. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participants
Not applicable.
Patient consent for publication
Written informed consent for the publication of this
case report was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PSCCT
|
primary squamous cell carcinoma of the
thyroid
|
|
CK
|
cytokeratin
|
|
TG
|
thyroglobulin
|
|
MRI
|
magnetic resonance imaging
|
|
LDH
|
lactate dehydrogenase
|
References
|
1
|
Lu Z, Lijun C, Dezhuan D and Hongyi C:
Primary squamous cell carcinoma of the thyroid: A systematic
review. Asian J Surg. 45:1016–1017. 2022. View Article : Google Scholar
|
|
2
|
Lam AKY: Squamous cell carcinoma of
thyroid: A unique type of cancer in World Health Organization
classification. Endocr Relat Cancer. 27:R177–R192. 2020. View Article : Google Scholar
|
|
3
|
Chen C, Xu Q, Deng Y, Peng J, He X and Liu
L: Ultrasound combined with contrast-enhanced ultrasound in the
diagnosis of primary squamous cell carcinoma of the thyroid: A case
report and literature review. Oncol Lett. 29:1312025. View Article : Google Scholar
|
|
4
|
Liu J and Li S: Primary squamous cell
carcinoma of the thyroid: A case report and literature review.
Asian J Surg. 47:2469–2471. 2024. View Article : Google Scholar
|
|
5
|
Ko YS, Hwang TS, Han HS, Lim SD, Kim WS
and Oh SY: Primary pure squamous cell carcinoma of the thyroid:
Report and histogenic consideration of a case involving a BRAF
mutation. Pathol Int. 62:43–48. 2012. View Article : Google Scholar
|
|
6
|
Lévay B, Kiss A, Oberna F, Slezák A and
Tóth E: Primary squamous cell carcinoma of the thyroid gland. Orv
Hetil. 164:1556–1559. 2023.(In Hungarian). View Article : Google Scholar
|
|
7
|
Yang S, Li C, Shi X, Ma B, Xu W, Jiang H,
Liu W, Ji Q and Wang Y: Primary squamous cell carcinoma in the
thyroid gland: A population-based analysis using the SEER database.
World J Surg. 43:1249–1255. 2019. View Article : Google Scholar
|
|
8
|
Ou D, Ni C, Yao J, Lai M, Chen C, Zhang Y,
Jiang T, Qian T, Wang L and Xu D: Clinical analysis of 13 cases of
primary squamous-cell thyroid carcinoma. Front Oncol.
12:9562892022. View Article : Google Scholar
|
|
9
|
Sahoo M, Bal CS and Bhatnagar D: Primary
squamous-cell carcinoma of the thyroid gland: New evidence in
support of follicular epithelial cell origin. Diagn Cytopathol.
27:227–231. 2002. View
Article : Google Scholar
|
|
10
|
Hanna E: Squamous cell carcinoma in a
thyroglossal duct cyst (TGDC): Clinical presentation, diagnosis,
and management. Am J Otolaryngol. 17:353–357. 1996. View Article : Google Scholar
|
|
11
|
Goldberg HM and Harvey P: Squamous-cell
cysts of the thyroid with special reference to the aetiology of
squamous epithelium in the human thyroid. Br J Surg. 43:565–569.
1956. View Article : Google Scholar
|
|
12
|
Miyauchi A, Kuma K, Matsuzuka F,
Matsubayashi S, Kobayashi A, Tamai H and Katayama S: Intrathyroidal
epithelial thymoma: An entity distinct from squamous cell carcinoma
of the thyroid. World J Surg. 9:128–135. 1985. View Article : Google Scholar
|
|
13
|
Ding W, Gao X and Ran X: Progress in
diagnosing and treating thyroid squamous cell carcinoma under the
5th edition of WHO classification. Front Endocrinol (Lausanne).
14:12734722024. View Article : Google Scholar
|
|
14
|
Hamidi S and Maniakas A: Recent advances
in anaplastic thyroid cancer management. Curr Opin Endocrinol
Diabetes Obes. 30:259–264. 2023. View Article : Google Scholar
|
|
15
|
Jurisic V: Multiomic analysis of cytokines
in immuno-oncology. Expert Rev Proteomics. 17:663–674. 2020.
View Article : Google Scholar
|
|
16
|
Zhou J, Yin L, Wei X, Zhang S, Song Y, Luo
B, Li J, Qian L, Cui L, Chen W, et al: 2020 Chinese guidelines for
ultrasound malignancy risk stratification of thyroid nodules: The
C-TIRADS. Endocrine. 70:256–279. 2020. View Article : Google Scholar
|
|
17
|
Vranic S and Gatalica Z: PD-L1 testing by
immunohistochemistry in immuno-oncology. Biomol Biomed. 23:15–25.
2023.
|
|
18
|
Gupta S, Guo R and Erickson LA: Anaplastic
thyroid carcinoma, squamous cell carcinoma pattern. Mayo Clin Proc.
97:1584–1585. 2022. View Article : Google Scholar
|
|
19
|
Shah S, Kadakia S, Khorsandi A, Andersen
A, Iacob C and Shin E: Squamous cell carcinoma in a thyroglossal
duct cyst: A case report with review of the literature. Am J
Otolaryngol. 36:460–462. 2015. View Article : Google Scholar
|
|
20
|
Kallel S, Kallel R, Ayadi S and Ghorbel A:
Primary squamous cell carcinoma of the thyroid associated with
papillary thyroid carcinoma and Hashimoto's thyroiditis. Eur Ann
Otorhinolaryngol Head Neck Dis. 135:291–293. 2018. View Article : Google Scholar
|
|
21
|
Yasumatsu R, Sato M, Uchi R, Nakano T,
Hashimoto K, Kogo R, Taura M, Matsuo M, Nakashima T and Nakagawa T:
The treatment and outcome analysis of primary squamous cell
carcinoma of the thyroid. Auris Nasus Larynx. 45:553–557. 2018.
View Article : Google Scholar
|
|
22
|
Kimura-Tsuchiya R, Sasaki E, Nakamura I,
Suzuki S, Kawana S, Okouchi C, Fukushima T, Hashimoto Y, Suzuki S
and Saji S: A case of squamous cell carcinoma of unknown primary
that responded to the multi-tyrosine kinase inhibitor lenvatinib.
Case Rep Oncol. 11:75–80. 2018. View Article : Google Scholar
|
|
23
|
Ulisse S, Tuccilli C, Sorrenti S,
Antonelli A, Fallahi P, D'Armiento E, Catania A, Tartaglia F,
Amabile MI, Giacomelli L, et al: PD-1 ligand expression in
epithelial thyroid cancers: Potential clinical implications. Int J
Mol Sci. 20:14052019. View Article : Google Scholar
|
|
24
|
Paolino G, Pantanowitz L, Barresi V, Pagni
F, Munari E, Moretta L, Brunelli M, Bariani E, Vigliar E, Pisapia
P, et al: PD-L1 evaluation in head and neck squamous cell
carcinoma: Insights regarding specimens, heterogeneity and therapy.
Pathol Res Pract. 226:1536052021. View Article : Google Scholar
|
|
25
|
Torrez M, Braunberger RC, Yilmaz E and
Agarwal S: Primary squamous cell carcinoma of thyroid with a novel
BRAF mutation and High PDL-1 expression: A case report with
treatment implications and review of literature. Pathol Res Pract.
216:1531462020. View Article : Google Scholar
|
|
26
|
Hsieh ML, Besch BM, Peterson JEG and
Henson C: Primary squamous cell carcinoma of the thyroid treated
with concurrent chemoradiation and palliative immunotherapy: A case
report. J Med Case Rep. 16:3642022. View Article : Google Scholar
|
|
27
|
Jurisic V, Radenkovic S and Konjevic G:
The actual role of LDH as tumor marker, biochemical and clinical
aspects. Adv Exp Med Biol. 867:115–124. 2015. View Article : Google Scholar
|
|
28
|
Sniezek JC and Holtel M: Rare tumors of
the thyroid gland. Otolaryngol Clin North Am. 36:107–115. 2003.
View Article : Google Scholar
|
|
29
|
Tolone S, Roberto R, del Genio G,
Brusciano L, Parmeggiani D, Amoroso V, Casalino G, Verde I, Bosco
A, D'Alessandro A, et al: The impact of age and oral calcium and
vitamin D supplements on postoperative hypocalcemia after total
thyroidectomy. A prospective study. BMC Surg. 13 (Suppl 2):S112013.
View Article : Google Scholar
|
|
30
|
Canu GL, Medas F, Cappellacci F, Giordano
ABF, Gurrado A, Gambardella C, Docimo G, Feroci F, Conzo G, Testini
M and Calò PG: Risk of complications in patients undergoing
completion thyroidectomy after hemithyroidectomy for thyroid nodule
with indeterminate cytology: An Italian multicentre retrospective
study. Cancers (Basel). 14:24722022. View Article : Google Scholar
|
|
31
|
Gambardella C, Offi C, Romano RM, De Palma
M, Ruggiero R, Candela G, Puziello A, Docimo L, Grasso M and Docimo
G: Transcutaneous laryngeal ultrasonography: A reliable,
non-invasive and inexpensive preoperative method in the evaluation
of vocal cords motility-a prospective multicentric analysis on a
large series and a literature review. Updates Surg. 72:885–892.
2020. View Article : Google Scholar
|
|
32
|
Dobashi Y, Sakamoto A, Sugimura H, Mernyei
M, Mori M, Oyama T and Machinami R: Overexpression of p53 as a
possible prognostic factor in human thyroid carcinoma. Am J Surg
Pathol. 17:375–381. 1993. View Article : Google Scholar
|
|
33
|
Nishida T, Nakao K, Hamaji M, Nakahara MA
and Tsujimoto M: Overexpression of p53 protein and DNA content are
important biologic prognostic factors for thyroid cancer. Surgery.
119:568–575. 1996. View Article : Google Scholar
|
|
34
|
Kleer CG, Giordano TJ and Merino MJ:
Squamous cell carcinoma of the thyroid: An aggressive tumor
associated with tall cell variant of papillary thyroid carcinoma.
Mod Pathol. 13:742–746. 2000. View Article : Google Scholar
|
|
35
|
Booya F, Sebo TJ, Kasperbauer JL and
Fatourechi V: Primary squamous cell carcinoma of the thyroid:
Report of ten cases. Thyroid. 16:89–93. 2006. View Article : Google Scholar
|
|
36
|
Olivier M, Hollstein M and Hainaut P: TP53
mutations in human cancers: Origins, consequences, and clinical
use. Cold Spring Harb Perspect Biol. 2:a0010082010. View Article : Google Scholar
|
|
37
|
Mirjačić Martinović K, Vuletić A, Tišma
Miletić N, Matković S, Gavrilović D, Ninković A, Jurišić V and
Babović N: Circulating IL-6 is associated with disease progression
in BRAFwt metastatic melanoma patients receiving anti-PD-1 therapy.
J Clin Pathol. 77:343–351. 2024. View Article : Google Scholar
|
|
38
|
Juhlin CC, Mete O and Baloch ZW: The 2022
WHO classification of thyroid tumors: Novel concepts in
nomenclature and grading. Endocr Relat Cancer. 30:e2202932022.
|
|
39
|
Baloch ZW, Asa SL, Barletta JA, Ghossein
RA, Juhlin CC, Jung CK, LiVolsi VA, Papotti MG, Sobrinho-Simões M,
Tallini G and Mete O: Overview of the 2022 WHO classification of
thyroid neoplasms. Endocr Pathol. 33:27–63. 2022. View Article : Google Scholar
|
|
40
|
Cho JK, Woo SH, Park J, Kim MJ and Jeong
HS: Primary squamous cell carcinomas in the thyroid gland: An
individual participant data meta-analysis. Cancer Med. 3:1396–1403.
2014. View
Article : Google Scholar
|
|
41
|
Wu Y, Sun C, Xi Y, Sun R, Fe J, Li X and
Sui J: Treatment and prognosis of squamous cell carcinoma of
thyroid. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
24:241–243. 2010.(In Chinese).
|
|
42
|
Yan W, Chen H, Li J, Zhou R and Su J:
Primary squamous cell carcinoma of thyroid gland: 11 Case reports
and a population-based study. World J Surg Oncol. 20:3522022.
View Article : Google Scholar
|
|
43
|
Kim TY, Kim KW, Jung TS, Kim JM, Kim SW,
Chung KW, Kim EY, Gong G, Oh YL, Cho SY, et al: Prognostic factors
for Korean patients with anaplastic thyroid carcinoma. Head Neck.
29:765–772. 2007. View Article : Google Scholar
|