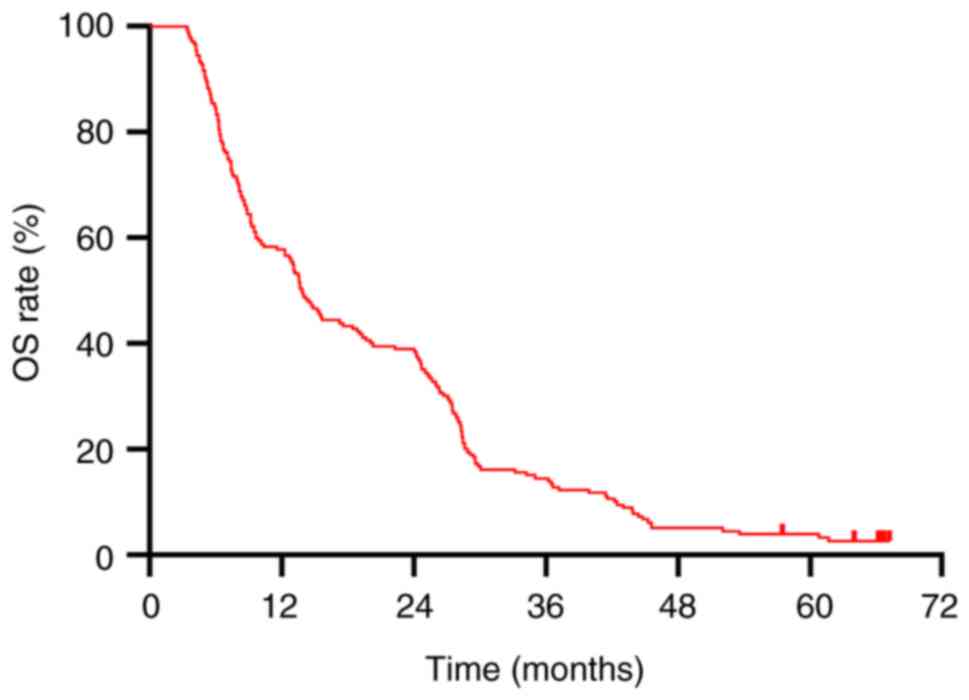
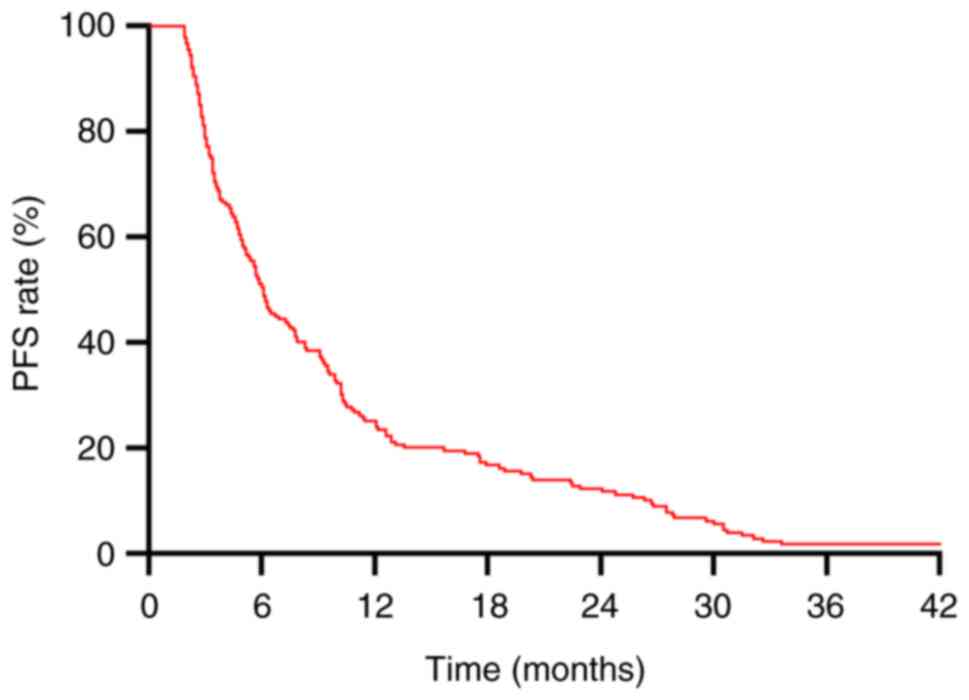
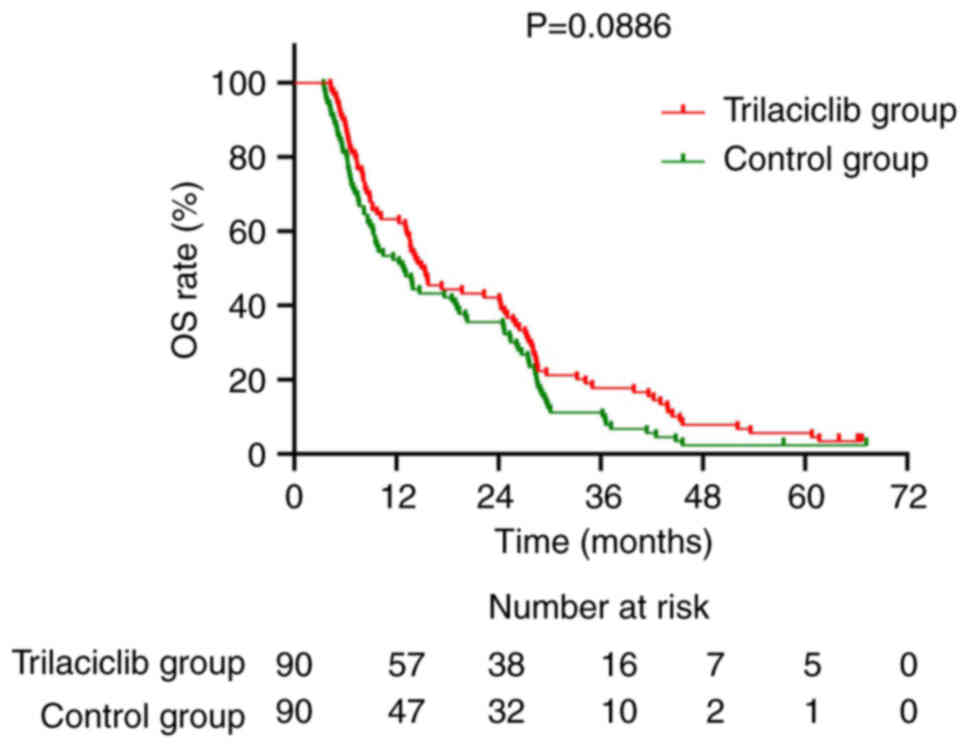
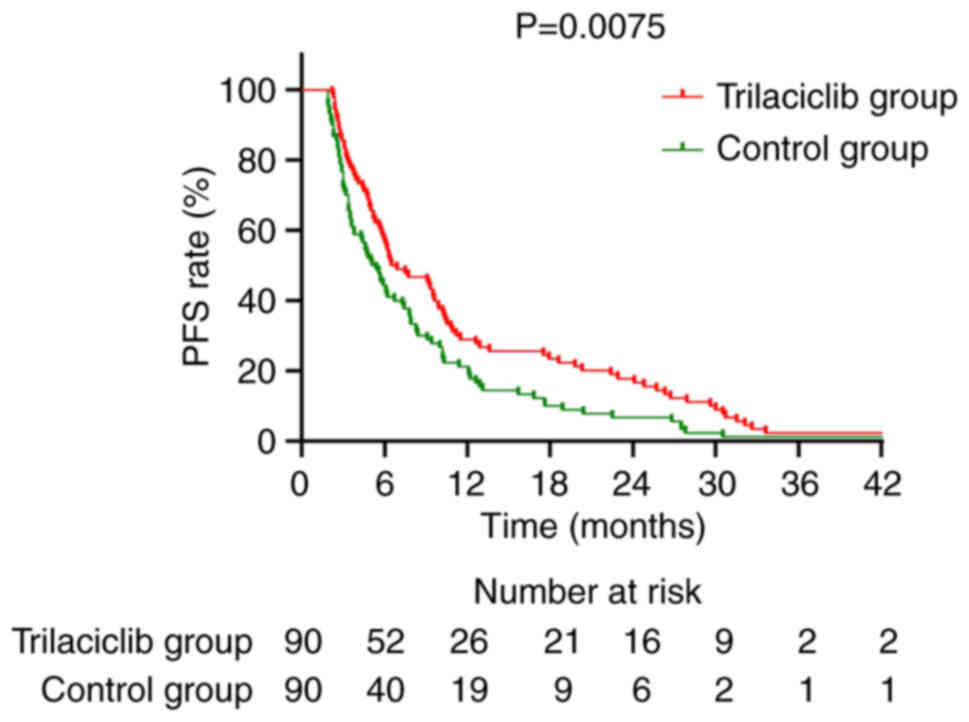
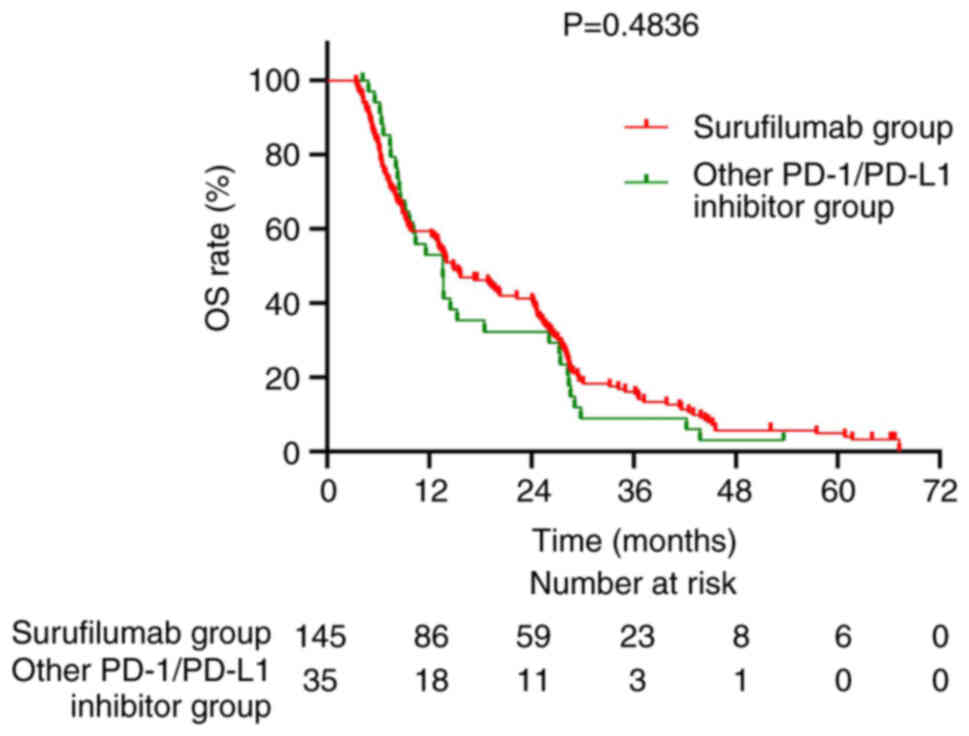
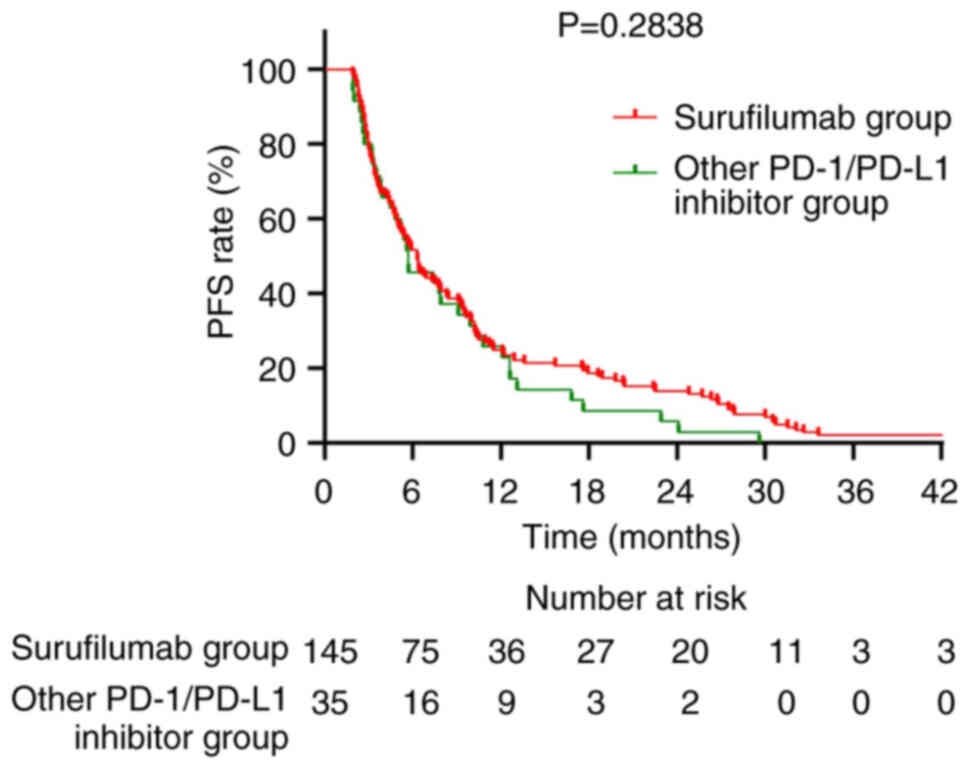
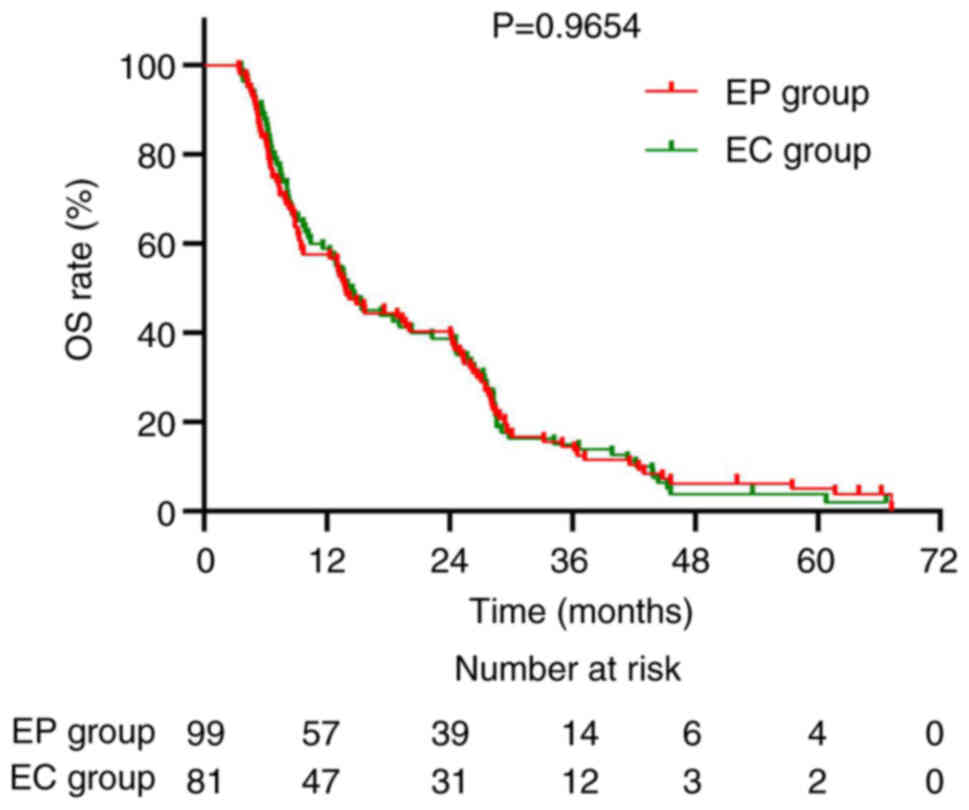
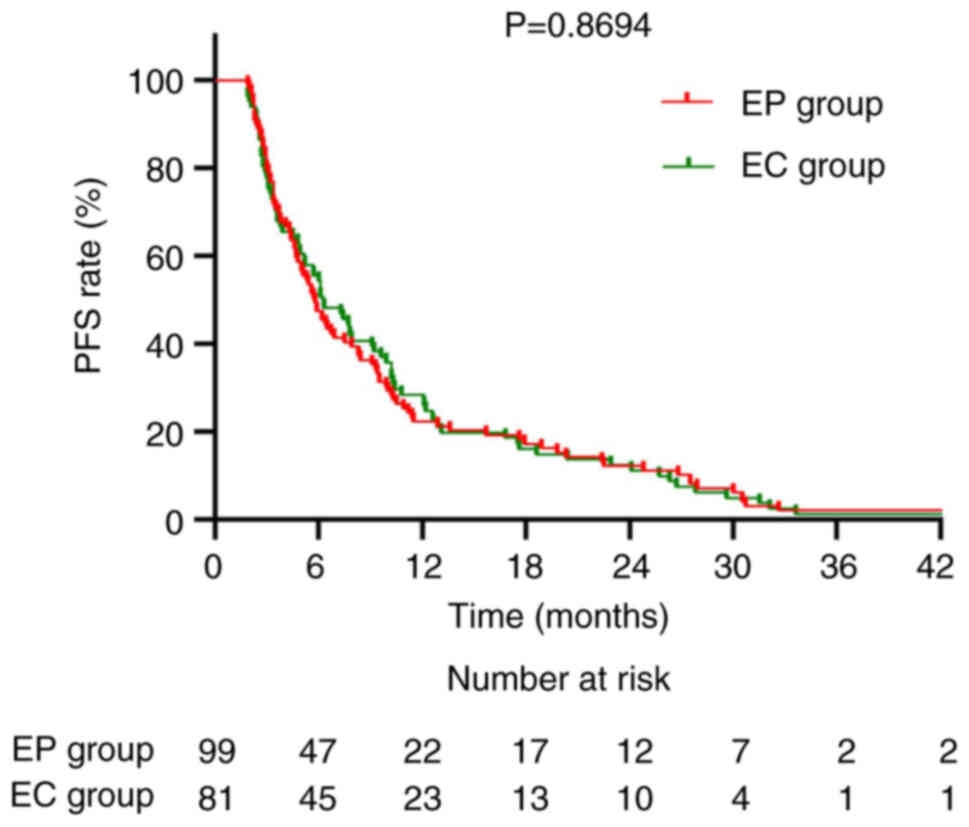
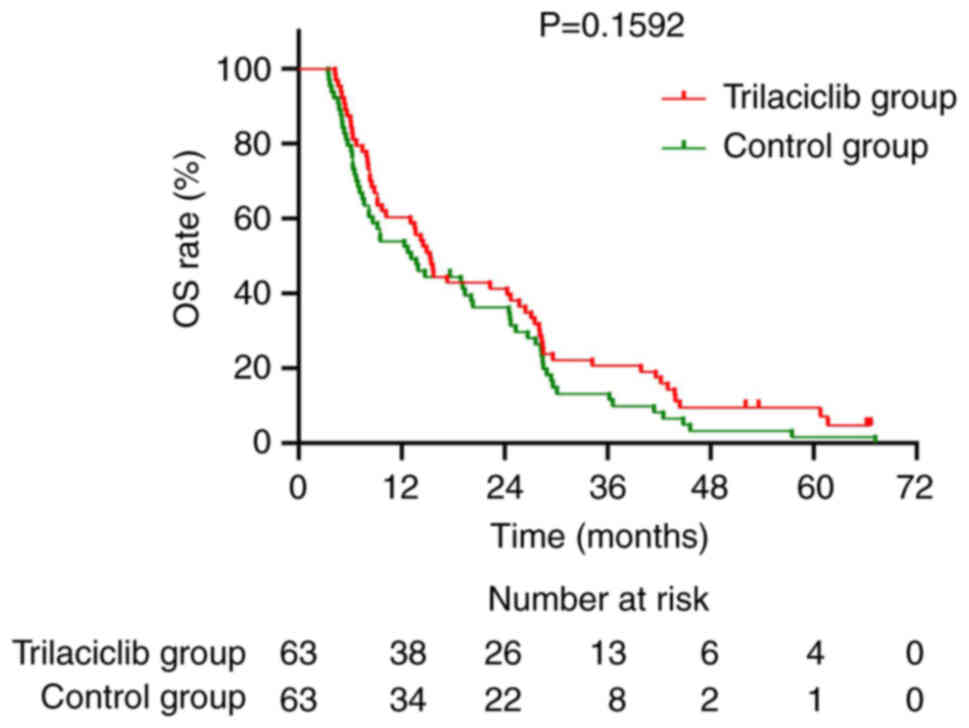
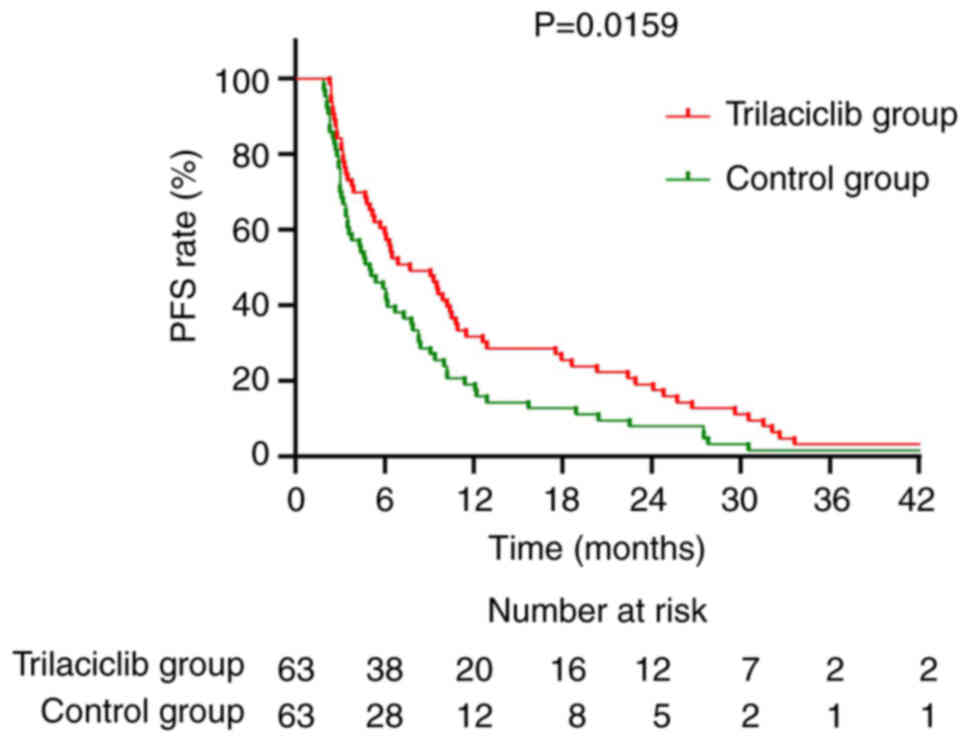
|
1
|
Megyesfalvi Z, Gay CM, Popper H, Pirker R,
Ostoros G, Heeke S, Lang C, Hoetzenecker K, Schwendenwein A,
Boettiger K, et al: Clinical insights into small cell lung cancer:
Tumor heterogeneity, diagnosis, therapy, and future directions. CA
Cancer J Clin. 73:620–652. 2023.PubMed/NCBI
|
|
2
|
Wang Q, Gümüş ZH, Colarossi C, Memeo L,
Wang X, Kong CY and Boffetta P: SCLC: Epidemiology, risk factors,
genetic susceptibility, molecular pathology, screening, and early
detection. J Thorac Oncol. 18:31–46. 2023. View Article : Google Scholar
|
|
3
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33.
2022.PubMed/NCBI
|
|
4
|
Schwendenwein A, Megyesfalvi Z, Barany N,
Valko Z, Bugyik E, Lang C, Ferencz B, Paku S, Lantos A, Fillinger
J, et al: Molecular profiles of small cell lung cancer subtypes:
Therapeutic implications. Mol Ther Oncolytics. 20:470–483. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang S, Tang J, Sun T, Zheng X, Li J, Sun
H, Zhou X, Zhou C, Zhang H, Cheng Z, et al: Survival changes in
patients with small cell lung cancer and disparities between
different sexes, socioeconomic statuses and ages. Sci Rep.
7:13392017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spigel DR, Townley PM, Waterhouse DM, Fang
L, Adiguzel I, Huang JE, Karlin DA, Faoro L, Scappaticci FA and
Socinski MA: Randomized phase II study of bevacizumab in
combination with chemotherapy in previously untreated
extensive-stage small-cell lung cancer: Results from the SALUTE
trial. J Clin Onco. 29:2215–2222. 2011. View Article : Google Scholar
|
|
7
|
Gomez-Randulfe I, Leporati R, Gupta B, Liu
S and Califano R: Recent advances and future strategies in
first-line treatment of ES-SCLC. Eur J Cancer. 200:1135812024.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang W, Wu G, Luo W, Lin L, Zhou C, Yao G,
Chen M, Wu X, Chen Z, Ye J, et al: Anlotinib plus oral
fluoropyrimidine S-1 in refractory or relapsed small-cell lung
cancer (SALTER TRIAL): A multicenter, single-arm, phase II trial.
BMC Cancer. 24:11822024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herzog BH, Devarakonda S and Govindan R:
Overcoming chemotherapy resistance in SCLC. J Thorac Oncol.
16:2002–2015. 2021. View Article : Google Scholar
|
|
10
|
Gajra A, Reeves P, O'Brien S, Carey A,
Moore M and Huang H: HSR25-156: Myelosuppression and healthcare
resource utilization in community oncology patients with
limited-stage small cell lung cancer (LS-SCLC) receiving first-line
therapy with or without trilaciclib. J Natl Compr Canc Netw.
23:HSR25–HSR156. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Povsic M, Enstone A, Wyn R, Kornalska K,
Penrod JR and Yuan Y: Real-world effectiveness and tolerability of
small-cell lung cancer (SCLC) treatments: A systematic literature
review (SLR). PLoS One. 14:e02196222019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Epstein RS, Aapro MS, Basu Roy UK, Salimi
T, Krenitsky J, Leone-Perkins ML, Girman C, Schlusser C and
Crawford J: Patient burden and real-world management of
chemotherapy-induced myelosuppression: Results from an online
survey of patients with solid tumors. Adv Ther. 37:3606–3618. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Adams JR, Lyman GH, Djubegovic B,
Feinglass J and Bennett CL: G-CSF as prophylaxis of febrile
neutropenia in SCLC. Expert Opin Pharmacother. 3:1273–1281. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dhillon S: Trilaciclib: First approval.
Drugs. 81:867–874. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lai AY, Sorrentino JA, Dragnev KH, Weiss
JM, Owonikoko TK, Rytlewski JA, Hood J, Yang Z, Malik RK, Strum JC
and Roberts PJ: CDK4/6 inhibition enhances antitumor efficacy of
chemotherapy and immune checkpoint inhibitor combinations in
preclinical models and enhances T-cell activation in patients with
SCLC receiving chemotherapy. J Immunother Cancer. 8:e0008472020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goldschmidt J, Hart L, Scott J, Boykin K,
Bailey R, Heritage T, Lopez-Gonzalez L, Zhou ZY, Edwards ML,
Monnette A, et al: Real-world outcomes of trilaciclib among
patients with extensive-stage small cell lung cancer receiving
chemotherapy. Adv Ther. 40:4189–4215. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qiu J, Sheng D, Lin F, Jiang P and Shi N:
The efficacy and safety of Trilaciclib in preventing
chemotherapy-induced myelosuppression: A systematic review and
meta-analysis of randomized controlled trials. Front Pharmacol.
14:11572512023. View Article : Google Scholar
|
|
18
|
Weiss JM, Csoszi T, Maglakelidze M, Hoyer
RJ, Beck JT, Domine Gomez M, Lowczak A, Aljumaily R, Rocha Lima CM,
Boccia RV, et al: Myelopreservation with the CDK4/6 inhibitor
trilaciclib in patients with small-cell lung cancer receiving
first-line chemotherapy: A phase Ib/randomized phase II trial. Ann
Oncol. 30:1613–1621. 2019. View Article : Google Scholar
|
|
19
|
Cheng Y, Wu L, Huang D, Wang Q, Fan Y,
Zhang X, Fan H, Yao W, Liu B, Yu G, et al: Myeloprotection with
trilaciclib in Chinese patients with extensive-stage small cell
lung cancer receiving chemotherapy: Results from a randomized,
double-blind, placebo-controlled phase III study (TRACES). Lung
Cancer. 188:1074552024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ferrarotto R, Anderson I, Medgyasszay B,
García-Campelo MR, Edenfield W, Feinstein TM, Johnson JM, Kalmadi
S, Lammers PE, Sanchez-Hernandez A, et al: Trilaciclib prior to
chemotherapy reduces the usage of supportive care interventions for
chemotherapy-induced myelosuppression in patients with small cell
lung cancer: Pooled analysis of three randomized phase 2 trials.
Cancer Med. 10:5748–5756. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sok M, Zavrl M, Greif B and Srpčič M:
Objective assessment of WHO/ECOG performance status. Support Care
Cancer. 10:3793–3798. 2019.
|
|
22
|
Atkinson TM, Ryan SJ, Bennett AV, Stover
AM, Saracino RM, Rogak LJ, Jewell ST, Matsoukas K, Li Y and Basch
E: The association between clinician-based common terminology
criteria for adverse events (CTCAE) and patient-reported outcomes
(PRO): A systematic review. Support Care Cancer. 24:3669–3676.
2016.PubMed/NCBI
|
|
23
|
Trask PC, Dueck AC, Piault E and Campbell
A: Patient-reported outcomes version of the common terminology
criteria for adverse events: Methods for item selection in
industry-sponsored oncology clinical trials. Clin Trials.
15:616–623. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schwartz LH, Litière S, de Vries E, Ford
R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J,
et al: RECIST 1.1-update and clarification: From the RECIST
committee. Eur J Cancer. 62:132–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aapro MS, Bohlius J, Cameron DA, Dal Lago
L, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC,
Walewski J, et al: 2010 Update of EORTC guidelines for the use of
granulocyte-colony stimulating factor to reduce the incidence of
chemotherapy-induced febrile neutropenia in adult patients with
lymphoproliferative disorders and solid tumours. Eur J Cancer.
47:8–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang Y, Su C, Jiang H, Liu F, Yu Q and
Zhou S: The association between pretreatment anemia and overall
survival in advanced non-small cell lung cancer: A retrospective
cohort study using propensity score matching. J Cancer. 13:51–61.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie W, Hu N and Cao L: Immune
thrombocytopenia induced by immune checkpoint inhibitrs in lung
cancer: Case report and literature review. Front Immunol.
12:7900512021. View Article : Google Scholar
|
|
28
|
Shan Q, Shi J, Wang X, Guo J, Han X, Wang
Z and Wang H: A new nomogram and risk classification system for
predicting survival in small cell lung cancer patients diagnosed
with brain metastasis: A large population-based study. BMC Cancer.
21:6402021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reck M, Mok TSK, Mansfield A, De Boer R,
Losonczy G, Sugawara S, Dziadziuszko R, Krzakowski M, Smolin A,
Hochmair M, et al: Brief report: Exploratory analysis of
maintenance therapy in patients with extensive-stage SCLC treated
first line with atezolizumab plus carboplatin and etoposide. J
Thorac Oncol. 17:1122–1129. 2022. View Article : Google Scholar
|
|
30
|
Li Y, Bao Y, Zheng H, Qin Y and Hua B: A
nomogram for predicting severe myelosuppression in small cell lung
cancer patients following the first-line chemotherapy. Sci Rep.
13:174642023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dai HR, Yang Y, Wang CY, Chen YT, Cui YF,
Li PJ, Chen J, Yang C and Jiao Z: Trilaciclib dosage in Chinese
patients with extensive-stage small cell lung cancer: A pooled
pharmacometrics analysis. Acta Pharmacol Sin. 45:2212–2225. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Daniel D, Kuchava V, Bondarenko I,
Ivashchuk O, Reddy S, Jaal J, Kudaba I, Hart L, Matitashvili A,
Pritchett Y, et al: Trilaciclib prior to chemotherapy and
atezolizumab in patients with newly diagnosed extensive-stage small
cell lung cancer: A multicentre, randomised, double-blind,
placebo-controlled phase II trial. Int J Cancer. 148:2557–2570.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Y, Wu L, Huang D, Wang Q, Yang C, Zhou
L, Sun S, Jiang X and Cheng Y: Effect of trilaciclib administered
before chemotherapy in patients with extensive-stage small-cell
lung cancer: A pooled analysis of four randomized studies. Cancer
Treat Res Commun. 42:1008692024.PubMed/NCBI
|
|
34
|
Cheng Y, Chen J, Zhang W, Xie C, Hu Q,
Zhou N, Huang C, Wei S, Sun H, Li X, et al: Benmelstobart,
anlotinib and chemotherapy in extensive-stage small-cell lung
cancer: A randomized phase 3 trial. Nat Med. 30:2967–2976. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bianco A, Perrotta F, Barra G, Malapelle
U, Rocco D and De Palma R: Prognostic factors and biomarkers of
responses to immune checkpoint inhibitors in lung cancer. Int J Mol
Sci. 20:49312019. View Article : Google Scholar
|
|
36
|
Liu J, Cao Y, Shao T and Wang Y: Exploring
the prognostic impact of differences in treatment strategies for
SCLC with different histologies and prognostic factors for C-SCLC:
A SEER population-based study. Heliyon. 10:e329072024. View Article : Google Scholar : PubMed/NCBI
|