Introduction
Small cell lung cancer (SCLC) is an aggressive and
highly malignant subtype of lung cancer, accounting for ~15% of all
lung cancer cases (1,2). Its clinical features include rapid
tumor growth and a high propensity for distant metastasis. The fact
that most patients are diagnosed at the extensive stage
[extensive-stage SCLC (ES-SCLC)] (3) means that the prognosis of ES-SCLC is
poor, with the median overall survival (OS) typically <12
months, and survival time further shortens with the development of
treatment resistance (4,5). Currently, platinum-based doublet
chemotherapy [such as etoposide plus cisplatin (EP) or etoposide
plus carboplatin (EC)] remains the first-line standard treatment
for patients with ES-SCLC (6,7).
Although these chemotherapy regimens show high efficacy in the
early stages, patients often face a gradual decline in treatment
effectiveness due to the rapid progression of the tumor and the
development of resistance (8,9).
Moreover, chemotherapy-induced myelosuppression, which often causes
severe clinical adverse events such as anemia, neutropenia and
thrombocytopenia, significantly impacts the quality of life (QoL)
and survival prognosis of patients (10,11).
This myelosuppression, while a predictable consequence of
chemotherapy, poses a considerable challenge in maintaining
adequate treatment regimens and leads to complications such as
prolonged hospitalizations and additional supportive care needs.
Furthermore, these adverse effects often result in dose reductions
or interruptions, further reducing the overall efficacy of cancer
treatment. Traditional methods to address myelosuppression mainly
involve symptomatic supportive treatments, such as the use of
granulocyte colony-stimulating factor (G-CSF) or blood transfusions
(12,13). However, these methods are reactive
rather than preventive, often being administered after the onset of
myelosuppression. This delay in intervention fails to address the
underlying issue in a timely manner and may still carry certain
risks, such as infections from prolonged neutropenia or
transfusion-related complications.
Therefore, the development of novel strategies to
manage myelosuppression, especially preventive treatments, is of
utmost importance to improve patient outcomes and treatment
adherence. Trilaciclib, a short-acting and reversible
cyclin-dependent kinase 4/6 inhibitor, has garnered considerable
attention in clinical practice as a potential myeloprotective agent
for patients undergoing chemotherapy (14–16).
Trilaciclib functions by transiently blocking hematopoietic stem
cells in the G1 phase of the cell cycle, preventing
chemotherapy drugs from damaging bone marrow cells, thereby
effectively preventing chemotherapy-induced myelosuppression
(17). Several clinical studies
have demonstrated that trilaciclib has a significant
myeloprotective effect in patients with ES-SCLC. It not only
considerably reduces chemotherapy-related myelosuppression events
but also decreases related hospitalization rates, blood transfusion
needs and the use of supportive therapies, thereby improving the
QoL of patients without diminishing the antitumor efficacy of
chemotherapy (18–20). Given its promising efficacy,
trilaciclib has the potential to improve the treatment landscape
for ES-SCLC, particularly in managing the adverse effects of
chemotherapy while maintaining treatment efficacy. Despite the
promising results observed in these trials, there is a need for
more research focusing on the real-world application and safety of
trilaciclib in diverse patient populations, especially in China,
where clinical practices and patient demographics may differ.
Real-world data will offer more generalizable insights and
potentially highlight challenges or benefits that controlled trials
may not fully capture.
Although trilaciclib has shown positive results in
several international clinical trials and has been applied in some
countries and regions, research on the real-world application and
safety of trilaciclib in Chinese patients with ES-SCLC remains
relatively limited. In addition, cultural and healthcare system
differences may affect the accessibility and outcomes of treatment,
highlighting the importance of evaluating trilaciclib's role in
different settings. Therefore, the present study aims to
retrospectively assess the myeloprotective effect, safety and
impact on survival outcomes of trilaciclib in first-line
chemotherapy for patients with ES-SCLC in China. The findings from
this study may help bridge the gap in understanding the clinical
efficacy of trilaciclib in a Chinese population and contribute to
evidence-based support for its use in the treatment of ES-SCLC.
Moreover, the present study aims to offer stronger support for
clinical decision-making by providing insights into its practical
applications and potential benefits in improving the treatment
outcomes and overall care of patients with ES-SCLC.
Materials and methods
General clinical information
The present study is a single-center, retrospective
real-world research project conducted at Chengde Central Hospital.
A total of 180 patients diagnosed with ES-SCLC between January 2020
and January 2024 were included based on the following inclusion
criteria. The medical records of the patients were accessed
specifically for the present study between January 2024 and
February 2025, including all relevant treatment and follow-up data,
with patient mortality or the end of follow-up as the endpoint. The
inclusion criteria were as follows: i) Patients were histologically
or cytologically confirmed to have SCLC; ii) patients were
receiving platinum-based chemotherapy combined with immunotherapy;
iii) patients were aged ≥18 years; iv) patients with an Eastern
Cooperative Oncology Group (ECOG) performance status of 0–2
(21); v) completion of at least
two cycles of treatment with complete treatment data; vi)
first-time diagnosis of ES-SCLC, or recurrent or metastatic disease
meeting the criteria for extensive stage; and vii) patients had not
received prior systemic therapy for SCLC. The exclusion criteria
were as follows: i) Patients who had been diagnosed with other
malignant tumors; ii) patients with severe heart, liver or kidney
dysfunction, or other major complications; iii) patients with poor
treatment compliance or incomplete clinical data; iv) patients with
severe active infections or other major medical diseases; v)
patients with missing data; and vi) patients who received other
antitumor therapies or participated in other clinical trials during
the study period. After excluding patients with missing data, the
remaining 180 patients were included for final analysis. All
patients underwent a comprehensive evaluation before treatment,
including physical examinations, blood tests, biochemical
indicators (liver and kidney function, electrolytes, blood glucose
levels, lipid profile, myocardial enzymes, amylase, alkaline
phosphatase and lactate dehydrogenase), chest, abdominal and pelvic
computed tomography (CT) or magnetic resonance imaging (MRI) scans,
brain MRI or enhanced CT scans, and bone scans. Additionally,
baseline clinical data were recorded, including age, sex, smoking
history, ECOG performance status, programmed cell death 1 ligand 1
(PD-L1) expression status, tumor staging, number of metastatic
sites and brain metastasis status. Patients were divided into two
groups based on the treatment regimen: The trilaciclib group and
the control group. The trilaciclib group and the control group each
included 90 patients. The study was approved by the Ethics
Committee of Chengde Central Hospital (Chengde, China; approval no.
CDCHLL2023-407).
Treatment methods
Patients in the trilaciclib group received
trilaciclib in combination with chemotherapy and immunotherapy. The
chemotherapy regimen included EC (etoposide 100 mg/m2 on
days 1–3; carboplatin dosed according to an area under the curve of
5, corresponding to a dose of ~500 mg on day 1) or EP (etoposide
100 mg/m2 on days 1–3; cisplatin 75 mg/m2 on
day 1). The immunotherapy regimen included the following drugs: 71
Patients received surufilumab, 8 received tislelizumab (4.5
mg/m2 intravenously every 3 weeks), 6 received
toripalimab (240 mg intravenously every 3 weeks), 3 received
atezolizumab (1,200 mg intravenously every 3 weeks) and 2 received
durvalumab (1,500 mg intravenously every 3 weeks). Each treatment
cycle was 3 weeks, with a total of 4–6 cycles. After completing 4–6
cycles of chemotherapy, immunotherapy was continued as a
monotherapy until disease progression or intolerable toxicity
occurred. Trilaciclib was administered by intravenous infusion at a
dose of 240 mg/m2 on each day of chemotherapy (days 1–3
of the cycle), with administration completed 30 min prior to the
start of chemotherapy. For patients experiencing treatment delays
or dose adjustments, the dosage for subsequent chemotherapy cycles
was appropriately adjusted based on specific circumstances. After
disease progression, patients received second-line treatment,
primarily with topotecan or irinotecan, and some patients received
third-line treatment with paclitaxel or anlotinib.
Patients in the control group received the same
chemotherapy regimen as the trilaciclib group combined with
immunotherapy. The immunotherapy drugs included surufilumab (74
cases), tislelizumab (6 cases), toripalimab (6 cases), atezolizumab
(3 cases) and durvalumab (1 case), but without trilaciclib. The
second- and third-line treatment regimens after disease progression
were the same as those for the trilaciclib group, primarily
involving topotecan, irinotecan and in some cases, paclitaxel or
anlotinib.
Observation indicators
Adverse events were graded according to the Common
Terminology Criteria for Adverse Events version 5.0 by the National
Cancer Institute (22,23). Treatment efficacy was evaluated
using the Response Evaluation Criteria in Solid Tumors version 1.1
(24). The observation indicators
included myelosuppression-related indicators, chemotherapy dose
adjustment indicators, efficacy indicators, survival indicators and
safety indicators. The myelosuppression-related indicators
included: i) Incidence of grade 3 and 4 neutropenia (25); ii) duration of severe neutropenia
during the first cycle; iii) number of patients using G-CSF and the
average number of cycles of G-CSF use; iv) incidence of anemia
(≥grade 3) (26) and the lowest
hemoglobin level recorded for each patient during the study period;
v) number of patients requiring red blood cell transfusions and the
number of transfusion episodes; vi) incidence of thrombocytopenia
(≥grade 3) (27) and the lowest
platelet count recorded for each patient during the study period;
vii) number of patients requiring platelet transfusions and the
number of transfusion episodes; viii) incidence of febrile
neutropenia and the number of related hospitalizations; and ix)
number of patients hospitalized due to myelosuppression and the
number of hospitalization days. Chemotherapy dose adjustment
indicators included: i) Number of patients with chemotherapy cycle
delays, dose reductions and cycle interruptions; ii) number of
patients completing ≥4 chemotherapy cycles; and iii) the average
number of completed cycles. Efficacy indicators were as follows: i)
Objective response rate (ORR), including complete response (CR) and
partial response (PR); and ii) disease control rate, including CR,
PR and stable disease (SD). Survival indicators were
progression-free survival (PFS) and overall survival (OS). OS is
defined as the time from the initiation of treatment to death from
any cause or the time of last follow-up. PFS is defined as the time
from the initiation of treatment to disease progression or death
from any cause. The Cox proportional hazards model was used to
evaluate potential factors affecting PFS and OS, including age,
sex, ECOG score, immunotherapy regimen, number of metastatic sites,
baseline brain metastasis, brain radiotherapy, chest radiotherapy,
chemotherapy regimen and PD-L1 expression status. Safety indicators
consisted of the incidence of drug-related adverse events during
treatment.
Follow-up
The follow-up methods in the present study included
regular outpatient visits, telephone follow-up and review of
medical records. The specific follow-up schedule was as follows:
Year 1, follow-up every 2 months; years 2–3, follow-up every 3–4
months; years 4–5, follow-up every 6 months; and >5 years,
annual follow-up. The follow-up content included medical history
inquiry, physical examination, laboratory tests and imaging
examinations (such as chest, abdominal and pelvic CT or MRI, brain
MRI or CT) to assess disease progression, treatment efficacy and
the occurrence of adverse events. During follow-up, the time of
disease progression, mortality and cause of death were recorded in
detail. The follow-up cut-off date was set as February 1, 2025,
with patient mortality or the end of follow-up as the endpoint.
Throughout the study, efforts were made to ensure the authenticity
and accuracy of follow-up data, with timely updates to the
follow-up database. Strict quality control and data verification
procedures were implemented, including regular audits of patient
data, cross-checking of medical records, validation of key clinical
outcomes, and verification of follow-up dates to ensure consistency
and accuracy.
Statistical analysis
Data analysis in the present study was performed
using Statistical Package for the Social Sciences 27.0 statistical
software (IBM Corp.). For normally distributed continuous data,
values were expressed as mean ± standard deviation, and inter-group
comparisons were performed using the unpaired independent sample
t-test. For non-normally distributed continuous data, normality was
assessed using the Shapiro-Wilk test, and values were expressed as
median (interquartile range), with inter-group comparisons
conducted using the Mann-Whitney U test. Categorical data were
expressed as frequency (percentage), and inter-group comparisons
were performed using the χ2 test or Fisher's exact
probability test. Survival data were analyzed using the
Kaplan-Meier method to estimate PFS and OS, and the median survival
time and its 95% confidence interval (CI) were calculated. The
log-rank test was used to compare survival curves between groups,
with a two-tailed P<0.05 considered statistically significant.
Additionally, Cox proportional hazards models were used for
multivariate analysis to identify factors influencing PFS and OS.
Cox regression analysis was performed to evaluate the independent
effects of clinical variables such as age, sex, ECOG performance
status and immunotherapy regimen on survival outcomes. The
corresponding hazard ratio (HR) and 95% CI were calculated.
Variables were selected based on clinical significance and
univariate analysis results. P<0.05 was considered to indicate a
statistically significant difference. Propensity score matching
(PSM) was used to match patients between the trilaciclib group and
the control group based on clinical variables such as age, sex,
ECOG performance status, PD-L1 expression and the number of
metastatic sites. After matching, the balance between the two
groups was assessed using standardized mean differences (SMD), with
all variables having SMD values of <0.1, indicating adequate
matching.
Results
Comparison of general clinical
data
There were no statistically significant differences
between the trilaciclib group and the control group in terms of
age, sex, smoking history, ECOG performance status, PD-L1
expression status, number of metastatic sites, baseline brain
metastasis and brain radiotherapy status (all P>0.05),
indicating that the baseline characteristics of the two groups were
well-matched and comparable, as shown in Table I. Brain metastasis is considered a
key controlled variable in this study due to its potential impact
on prognosis and treatment outcomes in patients with ES-SCLC. The
brain is a common site for metastasis in ES-SCLC, and its presence
may influence both the treatment approach and survival outcomes
(28).
 | Table I.Comparison of general clinical data
between the trilaciclib group and the control group. |
Table I.
Comparison of general clinical data
between the trilaciclib group and the control group.
| Characteristic | Trilaciclib group,
N (%) | Control group, N
(%) | χ2 | P-value |
|---|
| Age, years |
|
|
|
|
|
Median | 63.5 | 61.5 |
|
|
|
<65 | 52 (57.8) | 61 (67.8) |
|
|
|
≥65 | 38 (42.2) | 29 (32.2) | 1.926 | 0.165 |
| Sex |
|
|
|
|
|
Female | 43 (47.8) | 34 (37.8) |
|
|
|
Male | 47 (52.2) | 56 (62.2) | 1.838 | 0.175 |
| ECOG performance
status |
|
|
|
|
| 0 | 68 (75.6) | 74 (82.2) |
|
|
| 1 | 22 (24.4) | 16 (17.8) | 1.201 | 0.273 |
| Smoking
history |
|
|
|
|
| Never
smoked | 14 (15.6) | 8 (8.9) |
|
|
| Former
smoker | 72 (80.0) | 75 (83.3) |
|
|
| Current
smoker | 4 (4.4) | 7 (7.8) | 2.516 | 0.284 |
| PD-L1
expression |
|
|
|
|
|
Negative | 71 (78.9) | 62 (68.9) |
|
|
|
Positive | 19 (21.1) | 28 (31.1) | 2.332 | 0.127 |
| Number of
metastatic sites |
|
|
|
|
| 1 | 29 (32.2) | 18 (20.0) |
|
|
|
2-3 | 40 (44.4) | 53 (58.9) |
|
|
|
>3 | 21 (23.3) | 19 (21.1) | 0.639 | 0.726 |
| Baseline brain
metastasis |
|
|
|
|
| No | 74 (82.2) | 77 (85.6) |
|
|
|
Yes | 16 (17.8) | 13 (14.4) | 0.370 | 0.543 |
| Brain
radiotherapy |
|
|
|
|
| No | 75 (78.3) | 79 (85.0) |
|
|
|
Yes | 15 (21.7) | 9 (15.0) | 0.891 | 0.345 |
| Chest
radiotherapy |
|
|
|
|
| No | 63 (70.0) | 71 (78.9) |
|
|
|
Yes | 27 (30.0) | 19 (21.1) | 1.869 | 0.172 |
| Chemotherapy
regimen |
|
|
|
|
| EP | 47 (52.2) | 52 (57.8) |
|
|
| EC | 43 (47.8) | 38 (42.2) | 0.561 | 0.454 |
Comparison of myelosuppression
Compared with the control group, the trilaciclib
group had a significantly lower incidence of grade 3 (14.4 vs.
45.6%) and grade 4 neutropenia (3.3 vs. 20.0%)
(χ2=42.263; P<0.001). The duration of severe
neutropenia during the first cycle was significantly shorter in the
trilaciclib group [0 (0–1) days vs. 4 (2–6) days;
Z=−9.592; P<0.001]. The proportion of patients using G-CSF in
the trilaciclib group was also significantly lower (18.9 vs. 51.1%;
χ2=20.537), as was the average number of cycles of G-CSF
use (1.5±0.6 vs. 3.2±1.5; t=−10.681) (both P<0.001). In terms of
anemia, the incidence of grade ≥3 anemia in the trilaciclib group
was 15.6%, significantly lower than the control group at 30.0%
(χ2=5.338; P=0.021). The lowest hemoglobin value was
higher in the trilaciclib group (94±9 vs. 76±11 g/l; t=7.862;
P<0.001), and the number of patients requiring red blood cell
transfusions (18.9 vs. 43.3%; χ2=12.546; P=0.002) and
the number of transfusion episodes (1.0±0.6 vs. 2.0±0.8; t=−7.697;
P<0.001) were also significantly lower. For thrombocytopenia,
the incidence of grade ≥3 thrombocytopenia in the trilaciclib group
was 11.1%, significantly lower than the control group at 25.6%
(χ2=6.271; P=0.012). The lowest platelet count was
higher in the trilaciclib group (84±16×109 vs.
61±13×109/l; t=8.109; P<0.001), and the number of
patients requiring platelet transfusions (7.8 vs. 21.1%;
χ2=6.474; P=0.011) and the number of transfusion
episodes (1.0±0.4 vs. 1.9±0.6; t=−7.682; P<0.001) were
significantly reduced. Additionally, the incidence of febrile
neutropenia in the trilaciclib group was significantly lower than
in the control group (4.4 vs. 17.8%; χ2=8.100; P=0.004),
as was the number of hospitalizations due to febrile neutropenia
(3.3 vs. 15.6%; χ2=7.860; P=0.005). The number of
hospitalizations related to myelosuppression (7.8 vs. 21.1%;
χ2=6.474; P=0.011) and the number of hospitalization
days (4.2±0.9 vs. 8.2±2.7 days; t=−7.128; P<0.001) were also
significantly lower in the trilaciclib group. Regarding
chemotherapy dose adjustments, the trilaciclib group had
significantly lower rates of chemotherapy cycle delays (12.2 vs.
41.1%; χ2=19.205; P<0.001), dose reductions (15.6 vs.
37.8%; χ2=11.364; P<0.001) and cycle interruptions
(5.6 vs. 20.0%; χ2=8.424; P=0.004) compared with the
control group. The proportion of patients completing ≥4
chemotherapy cycles was higher in the trilaciclib group (91.1 vs.
75.6%; χ2=7.840; P=0.005), and the average number of
completed chemotherapy cycles was also higher (5.3±0.7 vs. 4.1±1.9;
t=6.274; P=0.037). Detailed data are shown in Table II.
 | Table II.Comparison of myelosuppression
between the trilaciclib group and the control group. |
Table II.
Comparison of myelosuppression
between the trilaciclib group and the control group.
|
Myelosuppression-related indicators | Trilaciclib group,
N=90 | Control group,
N=90 |
χ2/T/Z | P-value |
|---|
| Neutropenia, n
(%) |
|
|
|
|
| Grade
3 | 13 (14.4) | 41 (45.6) |
|
|
| Grade
4 | 3 (3.3) | 18 (20.0) | 42.263 | <0.001 |
| Duration of severe
neutropenia, days (range) | 0 (0–1) | 4 (2–6) | −9.592 | <0.001 |
| G-CSF use |
|
|
|
|
| Number
of patients using G-CSF, n (%) | 17 (18.9) | 46 (51.1) | 20.537 | <0.001 |
| Average
number of cycles of G-CSF use | 1.5±0.6 | 3.2±1.5 | −10.681 | <0.001 |
| Anemia |
|
|
|
|
| Grade
≥3, n (%) | 14 (15.6) | 27 (30.0) | 5.338 | 0.021 |
| Lowest
hemoglobin value, g/l | 94±9 | 76±11 | 7.862 | <0.001 |
| Number
of patients requiring RBC transfusions, n (%) | 17 (18.9) | 39 (43.3) | 12.546 | 0.002 |
| Number
of RBC transfusion episodes (per person) | 1.0±0.6 | 2.0±0.8 | −7.697 | <0.001 |
|
Thrombocytopenia |
|
|
|
|
| Grade
≥3, n (%) | 10 (11.1) | 23 (25.6) | 6.271 | 0.012 |
| Lowest
platelet count, ×109/l | 84±16 | 61±13 | 8.109 | <0.001 |
| Number
of patients requiring platelet transfusions, n (%) | 7 (7.8) | 19 (21.1) | 6.474 | 0.011 |
| Number
of platelet transfusion episodes (per person) | 1.0±0.4 | 1.9±0.6 | −7.682 | <0.001 |
| FN |
|
|
|
|
|
Incidence of FN, n (%) | 4 (4.4) | 16 (17.8) | 8.100 | 0.004 |
| Number
of hospitalizations due to | 3 (3.3) | 14 (15.6) | 7.860 | 0.005 |
| FN, n
(%) |
|
|
|
|
|
Myelosuppression-related
hospitalizations |
|
|
|
|
| Number
of hospitalizations, n (%) | 7 (7.8) | 19 (21.1) | 6.474 | 0.011 |
|
Hospitalization days | 4.2±0.9 | 8.2±2.7 | −7.128 | <0.001 |
| Chemotherapy dose
adjustments |
|
|
|
|
| Number
of patients with chemotherapy cycle delays, n (%) | 11 (12.2) | 37 (41.1) | 19.205 | <0.001 |
| Number
of patients with dose reductions, n (%) | 14 (15.6) | 34 (37.8) | 11.364 | <0.001 |
| Number
of patients with chemotherapy cycle interruptions, n (%) | 5 (5.6) | 18 (20.0) | 8.424 | 0.004 |
| Number
of patients completing ≥4 chemotherapy cycles, n (%) | 82 (91.1) | 68 (75.6) | 7.840 | 0.005 |
| Average
number of chemotherapy cycles completed (cycles) | 5.3±0.7 | 4.1±1.9 | 6.274 | 0.037 |
Survival analysis comparison
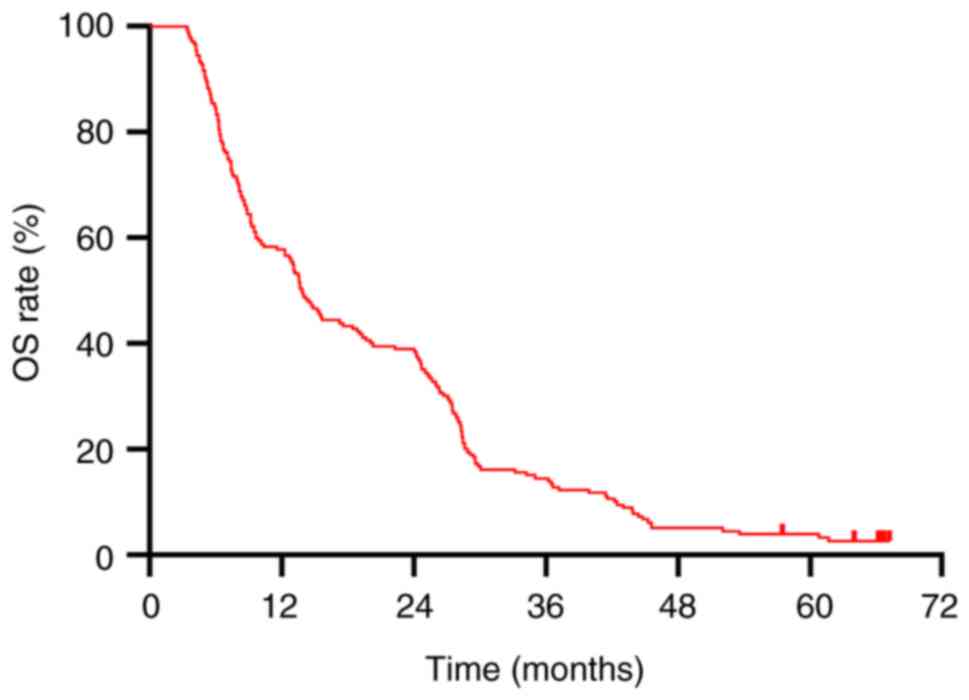
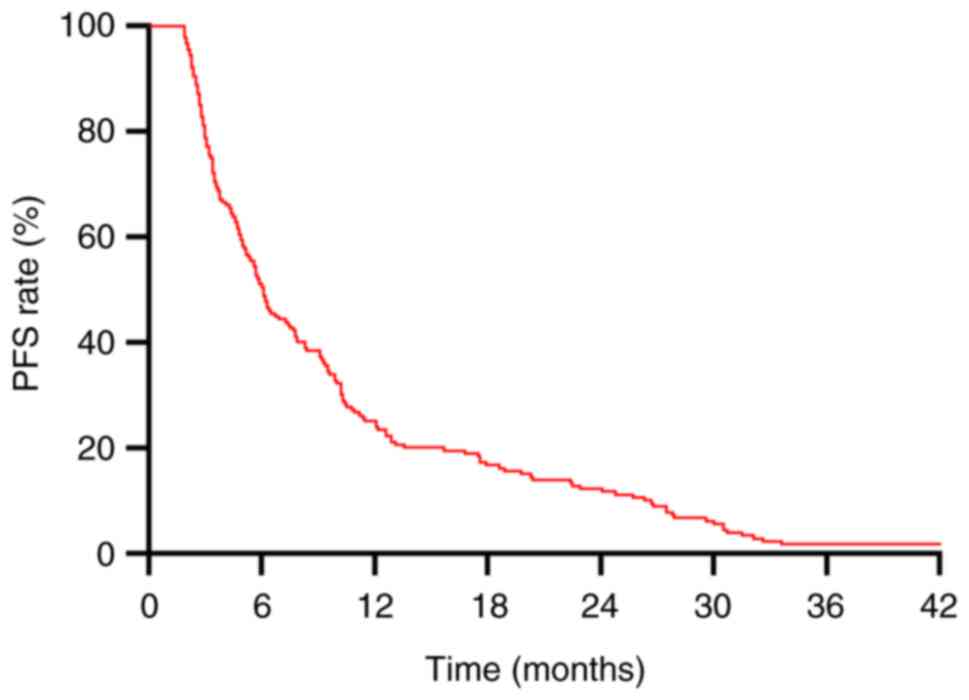
In the present study, the median OS for all patients
was 13.9 months (Fig. 1), and the
median PFS was 6.1 months (Fig. 2).
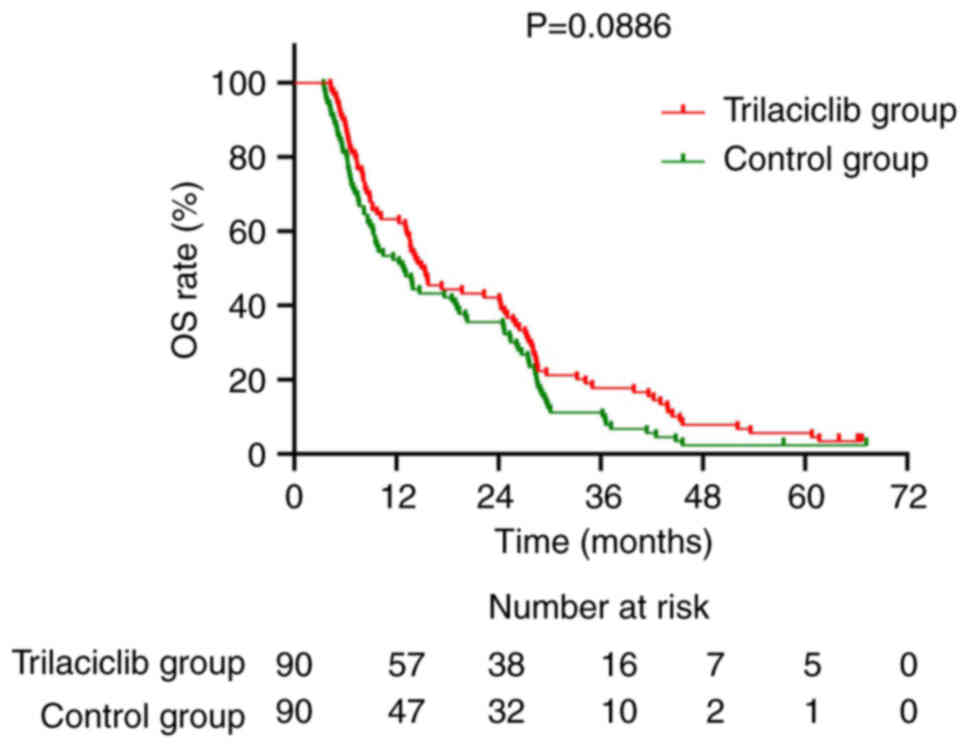
Group analysis showed that the median OS for the trilaciclib group
was 15.1 months, compared with 12.8 months for the control group.
Although the median OS in the trilaciclib group was 15.1 months
compared to 12.8 months in the control group, the difference was
not statistically significant (P=0.0886). The 1-, 2- and 3-year OS
rates for the trilaciclib group were 63.3, 42.2 and 17.8%,
respectively, while the rates for the control group were 52.2, 35.6
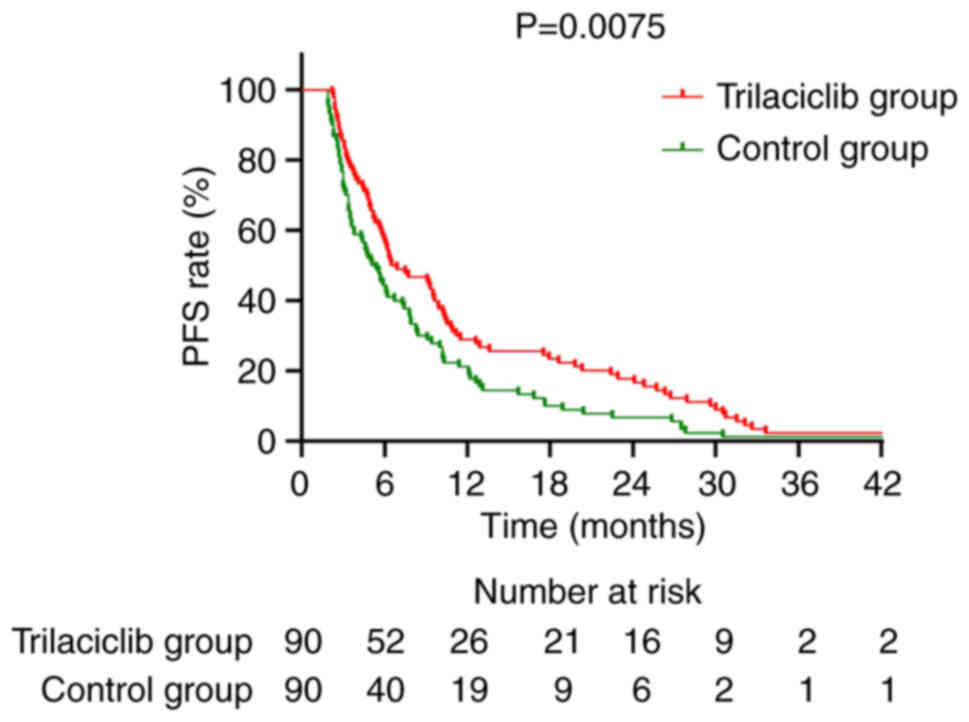
and 11.1% (Fig. 3). For PFS, the
median PFS for the trilaciclib group was 6.7 months, significantly
longer than the 5.3 months of the control group (HR=0.677; 95%
CI=0.502–0.912; P=0.0075). The 1-year PFS rate for the trilaciclib
group was 28.9% compared with 21.1% for the control group (Fig. 4).
Subgroup analyses were performed to evaluate the
impact of different immunotherapeutic agents and chemotherapy
regimens on survival outcomes. Among patients receiving
immunotherapy, those treated with Surufilumab had a median OS of
14.8 months, compared with 13.6 months in those receiving other
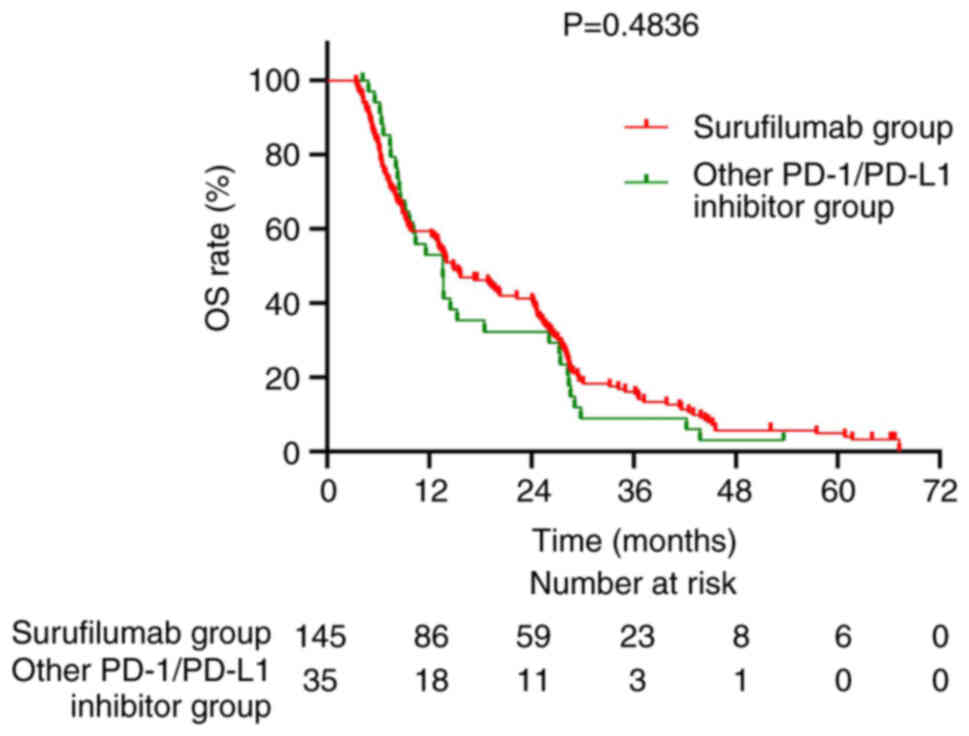
programmed cell death protein 1 (PD-1)/PD-L1 inhibitors (P=0.4836;
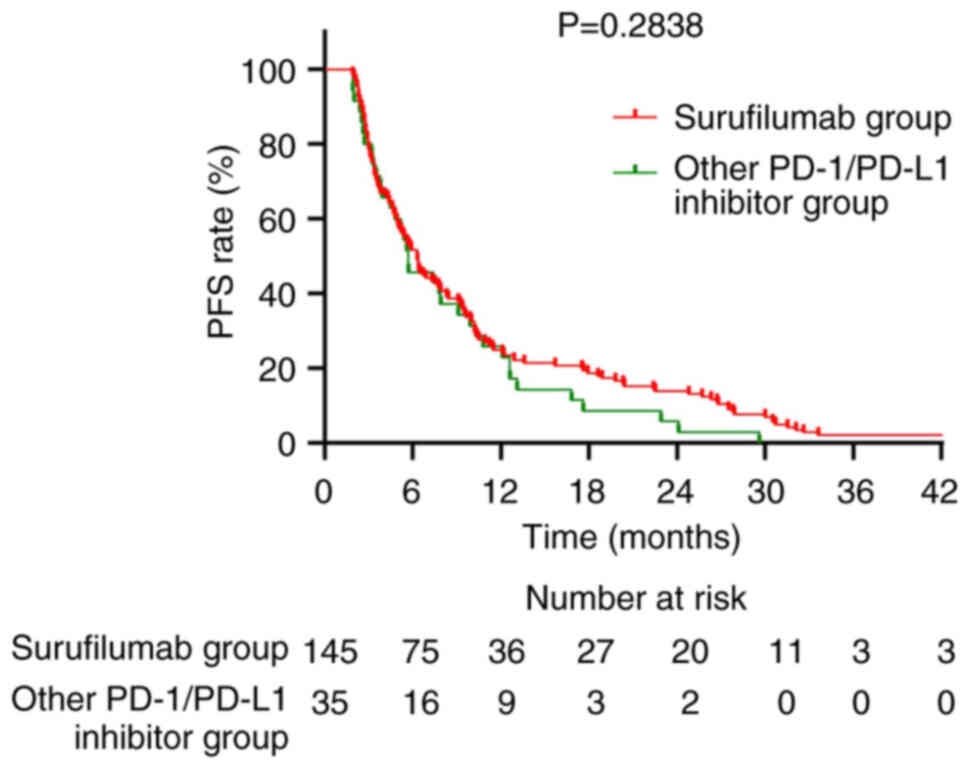
Fig. 5). The median PFS was 6.3
months in the Surufilumab group and 5.7 months in the PD-1/PD-L1
inhibitors group (P=0.2838; Fig.
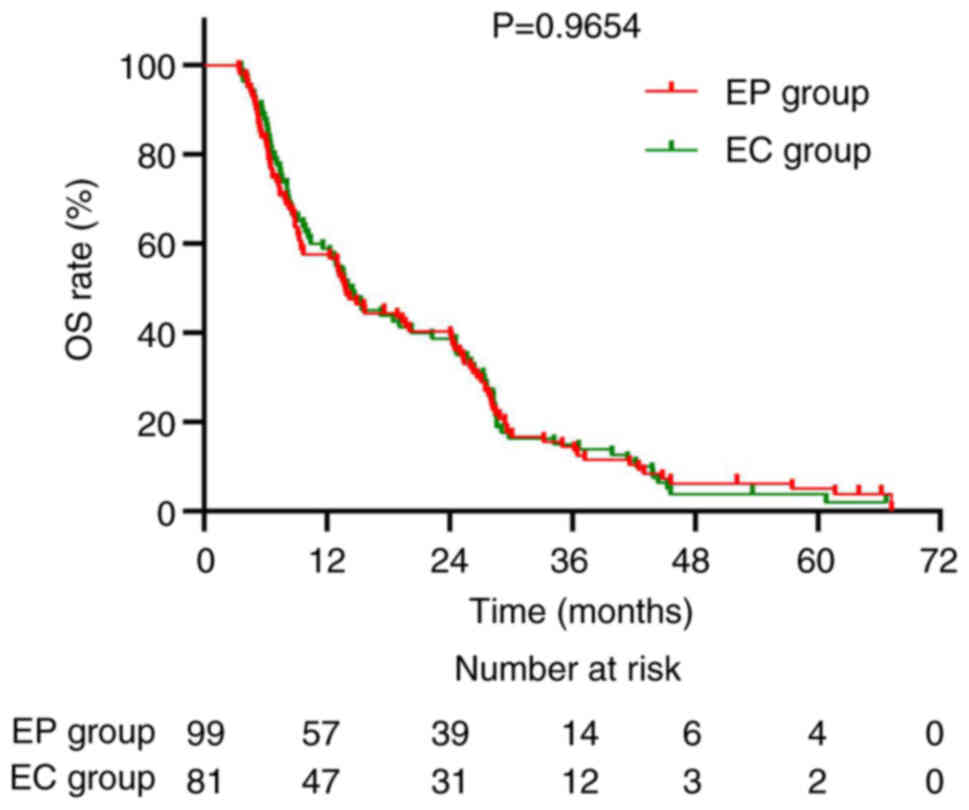
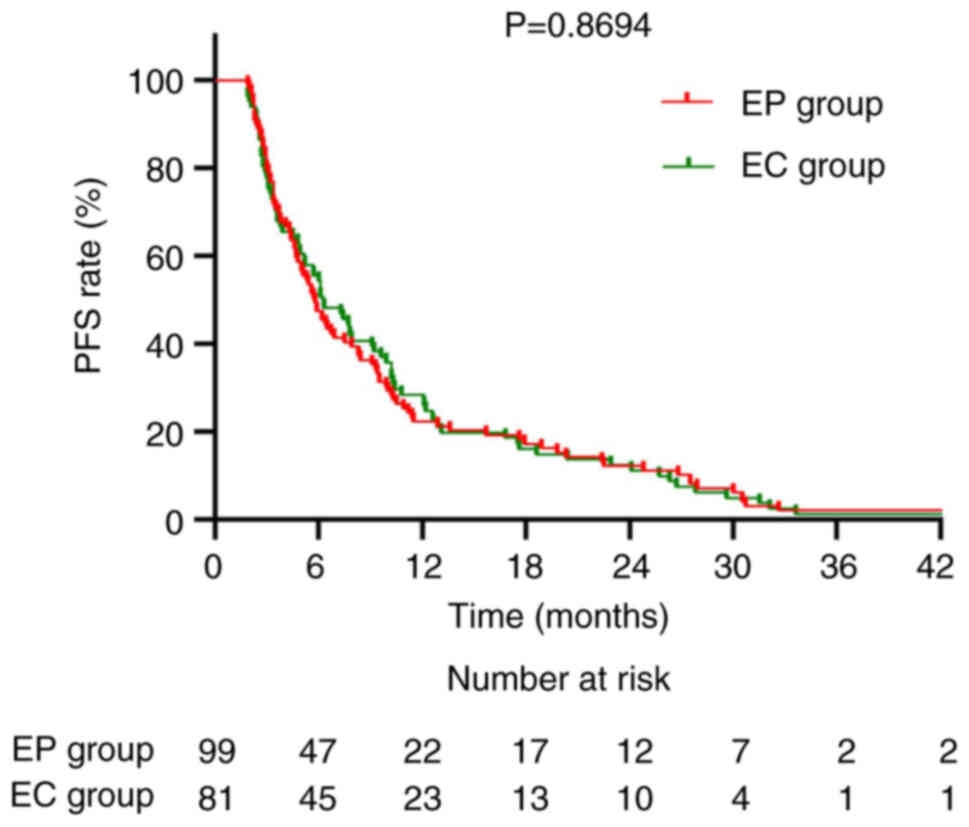
6). Regarding chemotherapy regimens, the median OS for the EP
group was 14.1 and 14.5 months for the EC group (P=0.9654; Fig. 7). The corresponding median PFS was
5.8 and 6.3 months, respectively (P=0.8694; Fig. 8).
PSM analysis
After performing PSM to balance key baseline
characteristics between the trilaciclib and control groups, each
group was reduced to 63 patients, resulting in 63 patients in the
trilaciclib group and 63 patients in the control group. Table III presents the baseline clinical
characteristics of the two groups after PSM, showing that the
groups were well-matched in terms of key clinical variables such as
age, ECOG performance status and number of metastatic sites. The
results of the survival analysis after PSM showed that the median
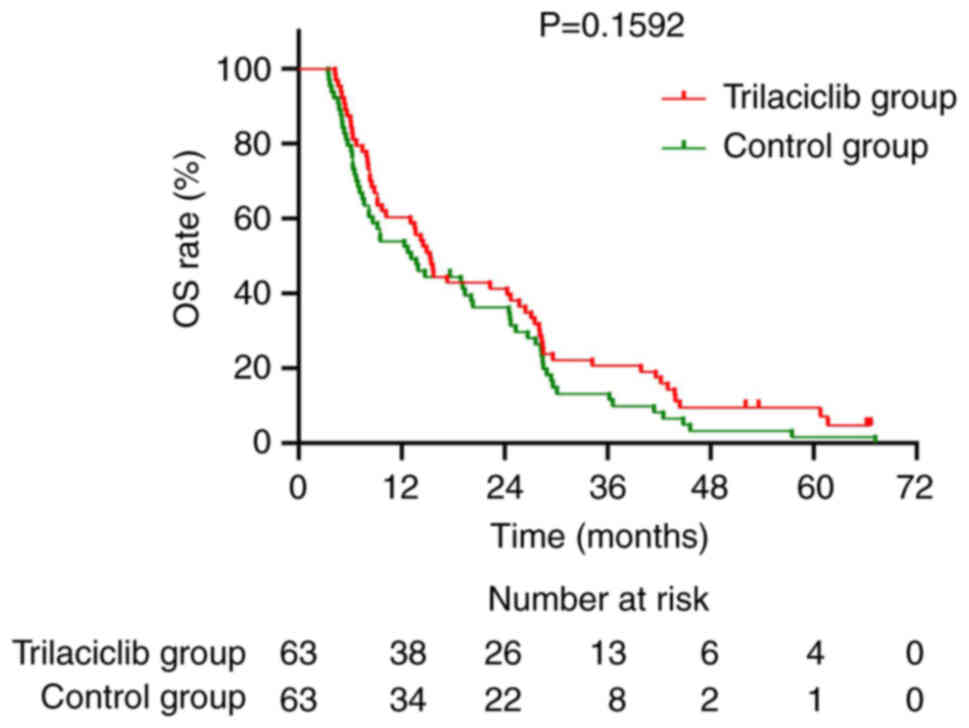
OS for the trilaciclib group was 15.3 months compared with 13.1
months for the control group, with no statistically significant
difference (P=0.1592; Fig. 9). By
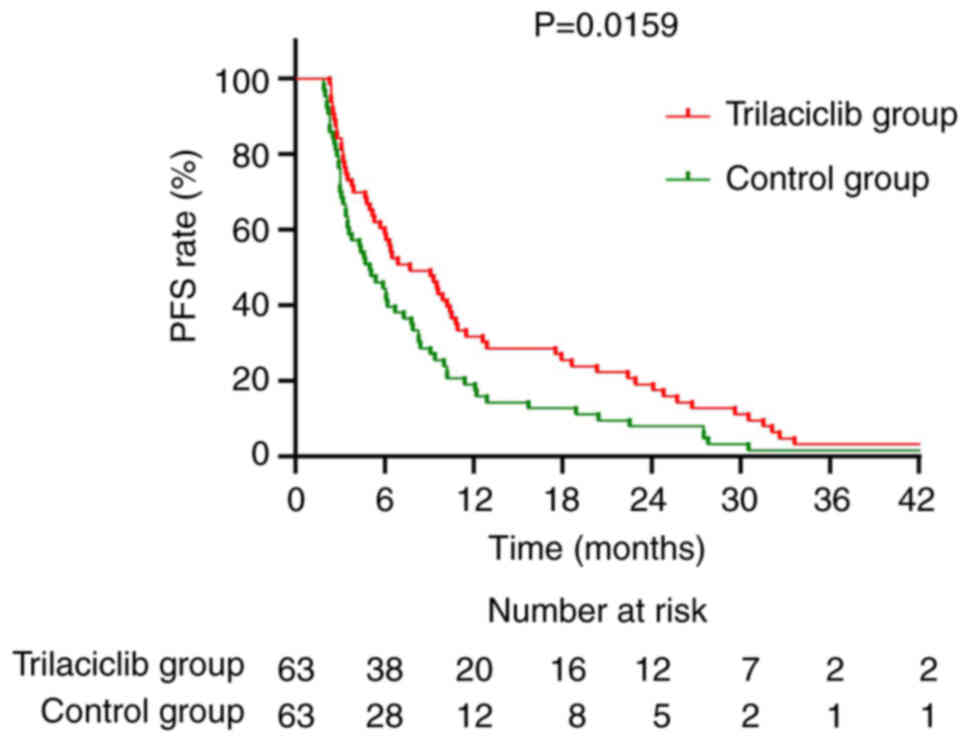
contrast, the median PFS for the trilaciclib group was 7.7 months,
significantly longer than the 5.0 months of the control group
(P=0.0159), with a HR of 0.6558 (95% CI=0.4581–0.9388; Fig. 10).
 | Table III.Baseline clinical characteristics of
the trilaciclib and control groups after propensity score
matching. |
Table III.
Baseline clinical characteristics of
the trilaciclib and control groups after propensity score
matching.
| Characteristic | Trilaciclib group,
n (%) | Control group, n
(%) | χ2 | P-value |
|---|
| Age, years |
|
|
|
|
|
Median | 63.2 | 63.0 |
|
|
|
<65 | 36 (57.1) | 35 (55.6) |
|
|
|
≥65 | 27 (42.9) | 28 (44.4) | 0.032 | 0.857 |
| Sex |
|
|
|
|
|
Female | 29 (46.1) | 31 (49.2) |
|
|
|
Male | 34 (53.9) | 32 (50.8) | 0.127 | 0.721 |
| ECOG performance
status |
|
|
|
|
| 0 | 48 (76.2) | 47 (74.6) |
|
|
| 1 | 15 (23.8) | 16 (25.4) | 0.043 | 0.836 |
| PD-L1
expression |
|
|
|
|
|
Negative | 51 (81.0) | 49 (77.8) |
|
|
|
Positive | 12 (19.0) | 14 (22.2) | 0.194 | 0.660 |
| Number of
metastatic sites |
|
|
|
|
| 1 | 20 (31.7) | 19 (30.2) |
|
|
|
2-3 | 28 (44.4) | 27 (42.9) |
|
|
|
>3 | 15 (23.8) | 17 (27.0) | 0.169 | 0.919 |
| Baseline brain
metastasis |
|
|
|
|
| No | 54 (85.7) | 52 (82.5) |
|
|
|
Yes | 9 (14.3) | 11 (17.5) | 0.238 | 0.626 |
Analysis of factors affecting OS and
PFS
To further explore the independent predictive
factors influencing survival outcomes, Cox proportional hazards
model analyses were performed for both OS and PFS, with results
presented in Tables IV and
V. In the multivariate analysis for
OS, the ECOG performance status, number of metastatic sites and
baseline brain metastasis were identified as independent risk
factors. Compared with patients with an ECOG score of 0, those with
a score of 1 had a significantly increased risk of mortality
(HR=1.876; 95% CI=1.231–2.858; P=0.003). Compared with patients
with only one metastatic site, those with 2–3 metastatic sites had
a significantly higher risk of mortality (HR=2.194; 95%
CI=1.485–3.241; P<0.001), and the risk further increased for
patients with >3 metastatic sites (HR=7.066; 95%
CI=4.114–12.139; P<0.001). Patients with baseline brain
metastasis also had a significantly higher risk of death compared
with those without brain metastasis (HR=2.183; 95% CI=1.394–3.417;
P<0.001). For overall survival (OS), no statistically
significant difference was observed between the trilaciclib and
control groups (HR=1.306; 95% CI=0.965–1.766; P=0.083). Patients in
the control group had a 30.6% higher risk of mortality compared
with those receiving trilaciclib, based on the hazard ratio
(HR=1.306). This calculation was derived from comparing the hazard
ratio for OS between the two groups, but the difference did not
reach statistical significance (P=0.083). Treatment modality
(trilaciclib vs. control), type of immunotherapeutic agent
(surufilumab vs. others) and chemotherapy regimen (EP vs. EC) were
not independently associated with OS in the multivariate model (all
P>0.05) (Table IV).
 | Table IV.Multivariate and univariate analysis
of factors affecting overall survival. |
Table IV.
Multivariate and univariate analysis
of factors affecting overall survival.
|
| Univariate
analysis | Multifactorial
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
<65 | 1.000 |
|
|
|
|
|
|
≥65 | 1.090 | 0.800–1.486 | 0.584 |
|
|
|
| Sex |
|
|
|
|
|
|
|
Male | 1.000 |
|
|
|
|
|
|
Female | 1.102 | 0.814–1.491 | 0.529 |
|
|
|
| ECOG performance
status |
|
|
|
|
|
|
| 0 | 1.000 |
|
| 1.000 |
|
|
| 1 | 2.751 | 1.892–4.000 | <0.001 | 1.876 | 1.231–2.858 | 0.003 |
| PD-L1
expression |
|
|
|
|
|
|
|
Negative | 1.000 |
|
|
|
|
|
|
Positive | 1.061 | 0.755–1.491 | 0.733 |
|
|
|
| Number of
metastatic sites |
|
|
|
|
|
|
| 1 | 1.000 |
| <0.001 | 1.000 |
| <0.001 |
|
2-3 | 2.473 | 1.689–3.620 | <0.001 | 2.194 | 1.485–3.241 | <0.001 |
|
>3 | 9.670 | 5.774–16.194 | <0.001 | 7.066 | 4.114–12.139 | <0.001 |
| Baseline brain
metastasis |
|
|
|
|
|
|
| No | 1.000 |
|
| 1.000 |
|
|
|
Yes | 2.629 | 1.728–3.999 | <0.001 | 2.183 | 1.394–3.417 | <0.001 |
| Brain
radiotherapy |
|
|
|
|
|
|
|
Yes | 1.000 |
|
| 1.000 |
|
|
| No | 1.696 | 1.110–2.590 | 0.015 | 1.173 | 0.902–2.148 | 0.129 |
| Chest
radiotherapy |
|
|
|
|
|
|
|
Yes | 1.000 |
|
|
|
|
|
| No | 1.267 | 0.898–1.788 | 0.177 |
|
|
|
| Chemotherapy
regimen |
|
|
|
|
|
|
| EC | 1.000 |
|
| 1.000 |
|
|
| EP | 1.047 | 0.774–1.416 | 0.765 | 1.101 | 0.809–1.500 | 0.540 |
| Immunotherapy
agent |
|
|
|
|
|
|
|
Surufilumab | 1.000 |
|
| 1.000 |
|
|
|
Others | 1.165 | 0.823–1.534 | 0.498 | 1.141 | 0.837–1.598 | 0.452 |
| Treatment
modality |
|
|
|
|
|
|
|
Trilaciclib group | 1.000 |
|
| 1.000 |
|
|
| Control
group | 1.306 | 0.965–1.766 | 0.083 | 1.168 | 0.853–1.600 | 0.333 |
 | Table V.Univariate and multivariate analysis
of factors affecting progression-free survival. |
Table V.
Univariate and multivariate analysis
of factors affecting progression-free survival.
|
| Univariate
analysis | Multifactorial
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
<65 | 1.000 |
|
|
|
|
|
|
≥65 | 1.197 | 0.882–1.624 | 0.248 |
|
|
|
| Sex |
|
|
|
|
|
|
|
Male | 1.000 |
|
|
|
|
|
|
Female | 1.068 | 0.792–1.438 | 0.667 |
|
|
|
| ECOG performance
status |
|
|
|
|
|
|
| 0 | 1.000 |
|
| 1.000 |
|
|
| 1 | 2.025 | 1.402–2.925 | <0.001 | 1.397 | 0.936–2.084 | 0.101 |
| PD-L1
expression |
|
|
|
|
|
|
|
Negative | 1.000 |
|
|
|
|
|
|
Positive | 1.038 | 0.741–1.455 | 0.828 |
|
|
|
| Number of
metastatic sites |
|
|
|
|
|
|
| 1 | 1.000 |
| <0.001 | 1.000 |
| <0.001 |
|
2-3 | 1.633 | 1.140–2.339 | 0.007 | 1.508 | 1.045–2.176 | 0.028 |
|
>3 | 4.081 | 2.590–6.430 | <0.001 | 3.150 | 1.922–5.164 | <0.001 |
| Baseline brain
metastasis |
|
|
|
|
|
|
| No | 1.000 |
|
| 1.000 |
|
|
|
Yes | 1.748 | 1.163–2.626 | 0.007 | 1.484 | 0.983–2.242 | 0.061 |
| Brain
radiotherapy |
|
|
|
|
|
|
|
Yes | 1.000 |
|
|
|
|
|
| No | 1.404 | 0.924–2.134 | 0.112 |
|
|
|
| Chest
radiotherapy |
|
|
|
|
|
|
|
Yes | 1.000 |
|
|
|
|
|
| No | 1.267 | 0.901–1.781 | 0.173 |
|
|
|
| Chemotherapy
regimen |
|
|
|
|
|
|
| EC | 1.000 |
|
|
|
|
|
| EP | 1.085 | 0.807–1.458 | 0.589 |
|
|
|
| Treatment
modality |
|
|
|
|
|
|
|
Trilaciclib group | 1.000 |
|
| 1.000 |
|
|
| Control
group | 2.387 | 1.319–3.745 | <0.001 | 1.495 | 1.109–2.014 | 0.008 |
In the multivariate analysis for PFS, the treatment
modality and number of metastatic sites were identified as
independent factors affecting PFS. Compared with patients with only
one metastatic site, those with 2–3 metastatic sites had a
significantly increased risk of disease progression (HR=1.508; 95%
CI=1.045–2.176; P=0.028), and the risk further increased for
patients with >3 metastatic sites (HR=3.150; 95% CI=1.922–5.164;
P<0.001). Compared with the trilaciclib group, patients in the
control group had a significantly increased risk of disease
progression (HR=1.495; 95% CI=1.109–2.014; P=0.008), indicating
that trilaciclib independently reduced the risk of progression.
Although ECOG performance status and baseline brain metastasis
showed some trend of impact in the multivariate analysis for PFS,
they did not reach statistical significance (Table V).
Efficacy comparison
The efficacy assessment results after treatment
showed that in the trilaciclib group, the CR rate was 3.3%, the PR
rate was 83.3%, the SD rate was 11.1% and the PD rate was 2.2%. In
the control group, the CR rate was 2.2%, the PR rate was 73.3%, the
SD rate was 18.9% and the PD rate was 5.6%. The differences between
the two groups in terms of each efficacy category (CR, PR, SD and
PD) did not reach statistical significance (all P>0.05; Table VI). In terms of ORR (CR + PR), the
trilaciclib group had an ORR of 86.6%, higher than the 75.5%
observed in the control group. However, the difference between the
groups did not reach statistical significance (χ2=3.626;
P=0.057; data not shown).
 | Table VI.Comparison of efficacy between the
trilaciclib group and the control group. |
Table VI.
Comparison of efficacy between the
trilaciclib group and the control group.
| Efficacy | Trilaciclib group,
N (%) | Control group, N
(%) | χ2 | P-value |
|---|
| CR | 3 (3.3) | 2 (2.2) |
|
|
| PR | 75 (83.3) | 66 (73.3) |
|
|
| SD | 10 (11.1) | 17 (18.9) |
|
|
| PD | 2 (2.2) | 5 (5.6) | 3.875 | 0.275 |
Discussion
ES-SCLC is an aggressive subtype of lung cancer with
an poor prognosis. At present, the standard of care primarily
comprises platinum-based doublet chemotherapy in combination with
immunotherapy (29). However, these
regimens are frequently associated with severe myelosuppression,
which significantly affects patients' treatment tolerance and QoL
(30). As a novel myeloprotective
agent, trilaciclib has shown promise in mitigating this issue in
clinical practice (31). The
present study is, to the best of our knowledge, the first
real-world investigation conducted in a Chinese population to
comprehensively assess the myeloprotective effects, safety profile
and prognostic impact of trilaciclib in the first-line treatment of
ES-SCLC, offering important clinical insights and application
value.
The findings of the present study demonstrated that
trilaciclib, when combined with chemotherapy and immunotherapy,
significantly improved PFS, which aligns with previous clinical
trials involving trilaciclib. Notably, in a phase II randomized
trial conducted by Weiss et al (18), trilaciclib combined with EP
(etoposide + carboplatin) achieved a median PFS of 6.2 months in
ES-SCLC patients, consistent with the results of the present study
and reinforcing the beneficial effect of trilaciclib on PFS.
Another study confirmed that trilaciclib, in combination with
chemotherapy and atezolizumab, significantly reduced the incidence
of myelosuppression and improved QoL, with a notable improvement in
PFS but no significant advantage in OS (32). These findings are consistent with
the present study, in which PFS was significantly prolonged while
OS did not reach statistical significance.
In terms of response, the ORR in the trilaciclib
group was slightly higher than in the control group; however,
differences in CR, PR, SD and PD rates between the groups were not
statistically significant, in line with previous clinical studies
(17,33). Weiss et al (18) also reported that although
trilaciclib improved chemotherapy tolerability, it did not
significantly increase ORRs. The present study also found clear
myeloprotective effects of trilaciclib, including significant
reductions in severe neutropenia, anemia, thrombocytopenia, G-CSF
use and transfusion requirements. These findings are consistent
with those reported by Daniel et al (32), who demonstrated that trilaciclib
significantly reduced the incidence of chemotherapy-induced
myelosuppression in clinical settings. By enabling patients to
maintain scheduled chemotherapy doses and cycles, trilaciclib may
further translate into improved treatment outcomes and QoL, which
is one of its key clinical advantages.
Although the trilaciclib group showed a modest
improvement in median OS compared with the control group, the
difference was not statistically significant. This is similar to
the findings by Daniel et al (32), which showed that trilaciclib could
mitigate myelosuppression and maintain antitumor efficacy without
significantly prolonging OS. Possible reasons for this include
limited follow-up duration, relatively small sample size and
differences in post-progression treatment strategies. In fact,
second- and third-line treatment choices can substantially
influence overall survival outcomes. A recent study involving
benmelstobart, anlotinib and chemotherapy further demonstrated that
combining multi-targeted anti-angiogenic agents, such as anlotinib
and bevacizumab, with immunotherapy significantly improved OS (up
to 19.3 months), clearly outperforming the current standard
chemoimmunotherapy alone (34).
These findings suggest that trilaciclib could potentially be
explored in combination with other antitumor agents to further
enhance survival benefits. However, the potential influence of
heterogeneity in immunotherapeutic agents and chemotherapy regimens
on survival outcomes should be considered.
In the present subgroup analyses, surufilumab
demonstrated longer OS and PFS compared with other PD-1/PD-L1
inhibitors, although the differences were not statistically
significant. Similarly, no significant differences in OS or PFS
were observed between patients receiving EP vs. EC regimens. These
findings indicate that the observed clinical benefit of trilaciclib
is unlikely to be confounded by the type of immunotherapy or
chemotherapy regimen used, thereby supporting the robustness of the
primary results. To elucidate the prognostic value of trilaciclib,
multivariate Cox regression analysis was conducted. The results
showed that, compared with patients treated with trilaciclib, those
in the control group had a significantly increased risk of disease
progression (HR=1.495; 95% CI=1.109–2.014; P=0.008), indicating an
independent protective effect of trilaciclib on PFS. No
statistically significant difference in OS was observed between the
trilaciclib and control groups (HR=1.306; 95% CI=0.965–1.766;
P=0.083). Further analysis showed that ECOG performance status,
number of metastatic sites and baseline brain metastasis were
independent poor prognostic factors for OS, which aligns with the
results of other studies (35,36).
This highlights the importance of considering these variables in
treatment planning. The impact of immunotherapy type and
chemotherapy regimen on survival did not show statistically
significant differences in the present model, emphasizing the
robust effect of trilaciclib. This suggests that even after
controlling for confounding variables such as ECOG performance
status, number of metastatic sites and presence of brain
metastases, trilaciclib independently contributed to delayed
disease progression. The novelty of the present study lies in its
being, to the best of our knowledge, the first real-world clinical
investigation of trilaciclib in a Chinese ES-SCLC population. It
provides an objective and comprehensive evaluation of the
myeloprotective efficacy, antitumor performance and safety profile
of trilaciclib. Unlike prior randomized controlled trials, the
present real-world study reflects the complexity of routine
clinical practice, involves a broader patient population and yields
findings that are more generalizable to actual treatment settings.
The present results offer important evidence-based support for
clinicians and demonstrate strong clinical applicability and value
for broader implementation.
Nonetheless, the present study has several
limitations. First, as a retrospective, non-randomized study,
selection bias could not be fully eliminated. To mitigate this
limitation, PSM was employed to match patients in the trilaciclib
and control groups based on baseline characteristics such as age,
ECOG performance status and number of metastatic sites. While PSM
helped reduce baseline imbalances between the groups, it is
important to note that residual confounding variables may still
exist. Second, the relatively small sample size limited the
statistical power for subgroup analyses, potentially affecting the
robustness and generalizability of certain findings. Third, the
follow-up duration was limited and long-term survival data for some
patients are still being collected, which may underestimate or
overestimate the true OS difference. Fourth, the lack of blinding
in the study could have introduced subjectivity in efficacy and
safety assessments. Future prospective, randomized, controlled
trials are required to further validate the efficacy and safety of
trilaciclib across different patient populations.
In summary, the present real-world study supported
the myeloprotective role of trilaciclib in first-line treatment of
ES-SCLC. Trilaciclib effectively reduced chemotherapy-induced
myelosuppression, improved treatment compliance and prolonged PFS,
thereby enhancing overall treatment safety without compromising
antitumor efficacy. Although a statistically significant OS benefit
was not demonstrated, trilaciclib exhibited promising clinical
potential, particularly in preserving QoL by reducing
chemotherapy-induced myelosuppression. The reduction in severe
neutropenia, anemia and thrombocytopenia in the trilaciclib group
not only improved treatment tolerance but also contributed to
improved patient outcomes in terms of QoL. While OS did not show
significant improvement, these myeloprotective effects are
clinically meaningful, as they may lead to fewer treatment delays,
reduced hospitalization and improved overall treatment compliance.
Further large-scale, long-term prospective studies are warranted to
clarify role of trilaciclib in SCLC management and identify the
populations most likely to benefit.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and Technology
Program of Chengde (grant no. 202301A016).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YC performed the data analysis and wrote the paper.
LW was responsible for the research design and guided the revision
of the paper. HZ provided clinical cases, participated in data
analysis and interpretation, and revised the manuscript critically
for important intellectual content. XL contributed to the research
design, provided data collection support and assisted in drafting
the manuscript. YC and LW confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The current study was performed in accordance with
the Declaration of Helsinki and approved by the local Ethics
Committee of Chengde Central Hospital (Chengde, P.R. China;
approval no. CDCHLL2023-407). All patients and/or their families
signed informed consent forms for study participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ES-SCLC
|
extensive-stage small cell lung
cancer
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
ORR
|
objective response rate
|
|
CR
|
complete response
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
|
PD
|
progressive disease
|
|
G-CSF
|
granulocyte colony-stimulating
factor
|
|
ECOG
|
Eastern Cooperative Oncology Group
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
EP
|
etoposide plus cisplatin
|
|
EC
|
etoposide plus carboplatin
|
References
|
1
|
Megyesfalvi Z, Gay CM, Popper H, Pirker R,
Ostoros G, Heeke S, Lang C, Hoetzenecker K, Schwendenwein A,
Boettiger K, et al: Clinical insights into small cell lung cancer:
Tumor heterogeneity, diagnosis, therapy, and future directions. CA
Cancer J Clin. 73:620–652. 2023.PubMed/NCBI
|
|
2
|
Wang Q, Gümüş ZH, Colarossi C, Memeo L,
Wang X, Kong CY and Boffetta P: SCLC: Epidemiology, risk factors,
genetic susceptibility, molecular pathology, screening, and early
detection. J Thorac Oncol. 18:31–46. 2023. View Article : Google Scholar
|
|
3
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33.
2022.PubMed/NCBI
|
|
4
|
Schwendenwein A, Megyesfalvi Z, Barany N,
Valko Z, Bugyik E, Lang C, Ferencz B, Paku S, Lantos A, Fillinger
J, et al: Molecular profiles of small cell lung cancer subtypes:
Therapeutic implications. Mol Ther Oncolytics. 20:470–483. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang S, Tang J, Sun T, Zheng X, Li J, Sun
H, Zhou X, Zhou C, Zhang H, Cheng Z, et al: Survival changes in
patients with small cell lung cancer and disparities between
different sexes, socioeconomic statuses and ages. Sci Rep.
7:13392017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spigel DR, Townley PM, Waterhouse DM, Fang
L, Adiguzel I, Huang JE, Karlin DA, Faoro L, Scappaticci FA and
Socinski MA: Randomized phase II study of bevacizumab in
combination with chemotherapy in previously untreated
extensive-stage small-cell lung cancer: Results from the SALUTE
trial. J Clin Onco. 29:2215–2222. 2011. View Article : Google Scholar
|
|
7
|
Gomez-Randulfe I, Leporati R, Gupta B, Liu
S and Califano R: Recent advances and future strategies in
first-line treatment of ES-SCLC. Eur J Cancer. 200:1135812024.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang W, Wu G, Luo W, Lin L, Zhou C, Yao G,
Chen M, Wu X, Chen Z, Ye J, et al: Anlotinib plus oral
fluoropyrimidine S-1 in refractory or relapsed small-cell lung
cancer (SALTER TRIAL): A multicenter, single-arm, phase II trial.
BMC Cancer. 24:11822024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herzog BH, Devarakonda S and Govindan R:
Overcoming chemotherapy resistance in SCLC. J Thorac Oncol.
16:2002–2015. 2021. View Article : Google Scholar
|
|
10
|
Gajra A, Reeves P, O'Brien S, Carey A,
Moore M and Huang H: HSR25-156: Myelosuppression and healthcare
resource utilization in community oncology patients with
limited-stage small cell lung cancer (LS-SCLC) receiving first-line
therapy with or without trilaciclib. J Natl Compr Canc Netw.
23:HSR25–HSR156. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Povsic M, Enstone A, Wyn R, Kornalska K,
Penrod JR and Yuan Y: Real-world effectiveness and tolerability of
small-cell lung cancer (SCLC) treatments: A systematic literature
review (SLR). PLoS One. 14:e02196222019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Epstein RS, Aapro MS, Basu Roy UK, Salimi
T, Krenitsky J, Leone-Perkins ML, Girman C, Schlusser C and
Crawford J: Patient burden and real-world management of
chemotherapy-induced myelosuppression: Results from an online
survey of patients with solid tumors. Adv Ther. 37:3606–3618. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Adams JR, Lyman GH, Djubegovic B,
Feinglass J and Bennett CL: G-CSF as prophylaxis of febrile
neutropenia in SCLC. Expert Opin Pharmacother. 3:1273–1281. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dhillon S: Trilaciclib: First approval.
Drugs. 81:867–874. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lai AY, Sorrentino JA, Dragnev KH, Weiss
JM, Owonikoko TK, Rytlewski JA, Hood J, Yang Z, Malik RK, Strum JC
and Roberts PJ: CDK4/6 inhibition enhances antitumor efficacy of
chemotherapy and immune checkpoint inhibitor combinations in
preclinical models and enhances T-cell activation in patients with
SCLC receiving chemotherapy. J Immunother Cancer. 8:e0008472020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goldschmidt J, Hart L, Scott J, Boykin K,
Bailey R, Heritage T, Lopez-Gonzalez L, Zhou ZY, Edwards ML,
Monnette A, et al: Real-world outcomes of trilaciclib among
patients with extensive-stage small cell lung cancer receiving
chemotherapy. Adv Ther. 40:4189–4215. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qiu J, Sheng D, Lin F, Jiang P and Shi N:
The efficacy and safety of Trilaciclib in preventing
chemotherapy-induced myelosuppression: A systematic review and
meta-analysis of randomized controlled trials. Front Pharmacol.
14:11572512023. View Article : Google Scholar
|
|
18
|
Weiss JM, Csoszi T, Maglakelidze M, Hoyer
RJ, Beck JT, Domine Gomez M, Lowczak A, Aljumaily R, Rocha Lima CM,
Boccia RV, et al: Myelopreservation with the CDK4/6 inhibitor
trilaciclib in patients with small-cell lung cancer receiving
first-line chemotherapy: A phase Ib/randomized phase II trial. Ann
Oncol. 30:1613–1621. 2019. View Article : Google Scholar
|
|
19
|
Cheng Y, Wu L, Huang D, Wang Q, Fan Y,
Zhang X, Fan H, Yao W, Liu B, Yu G, et al: Myeloprotection with
trilaciclib in Chinese patients with extensive-stage small cell
lung cancer receiving chemotherapy: Results from a randomized,
double-blind, placebo-controlled phase III study (TRACES). Lung
Cancer. 188:1074552024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ferrarotto R, Anderson I, Medgyasszay B,
García-Campelo MR, Edenfield W, Feinstein TM, Johnson JM, Kalmadi
S, Lammers PE, Sanchez-Hernandez A, et al: Trilaciclib prior to
chemotherapy reduces the usage of supportive care interventions for
chemotherapy-induced myelosuppression in patients with small cell
lung cancer: Pooled analysis of three randomized phase 2 trials.
Cancer Med. 10:5748–5756. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sok M, Zavrl M, Greif B and Srpčič M:
Objective assessment of WHO/ECOG performance status. Support Care
Cancer. 10:3793–3798. 2019.
|
|
22
|
Atkinson TM, Ryan SJ, Bennett AV, Stover
AM, Saracino RM, Rogak LJ, Jewell ST, Matsoukas K, Li Y and Basch
E: The association between clinician-based common terminology
criteria for adverse events (CTCAE) and patient-reported outcomes
(PRO): A systematic review. Support Care Cancer. 24:3669–3676.
2016.PubMed/NCBI
|
|
23
|
Trask PC, Dueck AC, Piault E and Campbell
A: Patient-reported outcomes version of the common terminology
criteria for adverse events: Methods for item selection in
industry-sponsored oncology clinical trials. Clin Trials.
15:616–623. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schwartz LH, Litière S, de Vries E, Ford
R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J,
et al: RECIST 1.1-update and clarification: From the RECIST
committee. Eur J Cancer. 62:132–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aapro MS, Bohlius J, Cameron DA, Dal Lago
L, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC,
Walewski J, et al: 2010 Update of EORTC guidelines for the use of
granulocyte-colony stimulating factor to reduce the incidence of
chemotherapy-induced febrile neutropenia in adult patients with
lymphoproliferative disorders and solid tumours. Eur J Cancer.
47:8–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang Y, Su C, Jiang H, Liu F, Yu Q and
Zhou S: The association between pretreatment anemia and overall
survival in advanced non-small cell lung cancer: A retrospective
cohort study using propensity score matching. J Cancer. 13:51–61.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie W, Hu N and Cao L: Immune
thrombocytopenia induced by immune checkpoint inhibitrs in lung
cancer: Case report and literature review. Front Immunol.
12:7900512021. View Article : Google Scholar
|
|
28
|
Shan Q, Shi J, Wang X, Guo J, Han X, Wang
Z and Wang H: A new nomogram and risk classification system for
predicting survival in small cell lung cancer patients diagnosed
with brain metastasis: A large population-based study. BMC Cancer.
21:6402021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reck M, Mok TSK, Mansfield A, De Boer R,
Losonczy G, Sugawara S, Dziadziuszko R, Krzakowski M, Smolin A,
Hochmair M, et al: Brief report: Exploratory analysis of
maintenance therapy in patients with extensive-stage SCLC treated
first line with atezolizumab plus carboplatin and etoposide. J
Thorac Oncol. 17:1122–1129. 2022. View Article : Google Scholar
|
|
30
|
Li Y, Bao Y, Zheng H, Qin Y and Hua B: A
nomogram for predicting severe myelosuppression in small cell lung
cancer patients following the first-line chemotherapy. Sci Rep.
13:174642023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dai HR, Yang Y, Wang CY, Chen YT, Cui YF,
Li PJ, Chen J, Yang C and Jiao Z: Trilaciclib dosage in Chinese
patients with extensive-stage small cell lung cancer: A pooled
pharmacometrics analysis. Acta Pharmacol Sin. 45:2212–2225. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Daniel D, Kuchava V, Bondarenko I,
Ivashchuk O, Reddy S, Jaal J, Kudaba I, Hart L, Matitashvili A,
Pritchett Y, et al: Trilaciclib prior to chemotherapy and
atezolizumab in patients with newly diagnosed extensive-stage small
cell lung cancer: A multicentre, randomised, double-blind,
placebo-controlled phase II trial. Int J Cancer. 148:2557–2570.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Y, Wu L, Huang D, Wang Q, Yang C, Zhou
L, Sun S, Jiang X and Cheng Y: Effect of trilaciclib administered
before chemotherapy in patients with extensive-stage small-cell
lung cancer: A pooled analysis of four randomized studies. Cancer
Treat Res Commun. 42:1008692024.PubMed/NCBI
|
|
34
|
Cheng Y, Chen J, Zhang W, Xie C, Hu Q,
Zhou N, Huang C, Wei S, Sun H, Li X, et al: Benmelstobart,
anlotinib and chemotherapy in extensive-stage small-cell lung
cancer: A randomized phase 3 trial. Nat Med. 30:2967–2976. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bianco A, Perrotta F, Barra G, Malapelle
U, Rocco D and De Palma R: Prognostic factors and biomarkers of
responses to immune checkpoint inhibitors in lung cancer. Int J Mol
Sci. 20:49312019. View Article : Google Scholar
|
|
36
|
Liu J, Cao Y, Shao T and Wang Y: Exploring
the prognostic impact of differences in treatment strategies for
SCLC with different histologies and prognostic factors for C-SCLC:
A SEER population-based study. Heliyon. 10:e329072024. View Article : Google Scholar : PubMed/NCBI
|