Lung cancer is the most common malignancy worldwide
in terms of incidence and is also the leading cause of
cancer-related death (1), with
non-small cell lung cancer (NSCLC) accounting for ~89% of all lung
cancer cases (2). Although
chemoradiotherapy and targeted therapy have improved survival,
patients with NSCLC ultimately develop resistance to these
treatment regimens (3). Metastatic
lung cancer is considered incurable (4); therefore, understanding the signal
transduction pathways that drive lung cancer progression and
metastasis is essential for developing effective treatment
strategies. The Wnt/β-catenin pathway has a key role in embryonic
development and tissue homeostasis (5). Abnormal activation of this pathway,
which is essential for maintaining cellular homeostasis, has been
observed in breast, prostate, gastrointestinal and lung cancer
(6–9). The β-catenin protein is a central
component of the Wnt/β-catenin signaling pathway and plays a
critical role in maintaining cell-cell adhesion (10). Abnormal β-catenin expression
promotes the proliferation, metastasis and drug resistance of NSCLC
cells (11). The present review
examines the role of β-catenin protein in epithelial-mesenchymal
transition (EMT) and the tumor microenvironment (TME), discusses
the mechanisms regulating its nuclear translocation and its
contribution to the resistance against EGFR-tyrosine kinase
inhibitors (TKIs) and explores its potential (alongside that of its
coding gene) as a prognostic biomarker.
The Wnt signaling pathway is a highly conserved
pathway that is notably comparable in humans and insects; it plays
a crucial role in regulating cell growth, division and
carcinogenesis (12). The Wnt
protein family comprises cysteine-rich secreted glycoproteins that
are widely distributed in both vertebrates and invertebrates
(13). These proteins bind to the
extracellular domains of Frizzled receptors (14). Based on its dependence on β-catenin,
the Wnt signaling pathway is classified into two types: canonical
and non-canonical (15,16).
The canonical pathway plays a critical role in cell
division and proliferation, making it an important biological
target for cancer treatment (12).
The β-catenin protein, a key intracellular signaling molecule,
participates at every stage of this process (5). The adenomatous polyposis coli
(APC)-Axin-GSK-3β-casein kinase-1 (CK1) complex, which comprises
APC, Axin, CK1, protein phosphatase 2A and GSK-3β, facilitates
β-catenin degradation (17). This
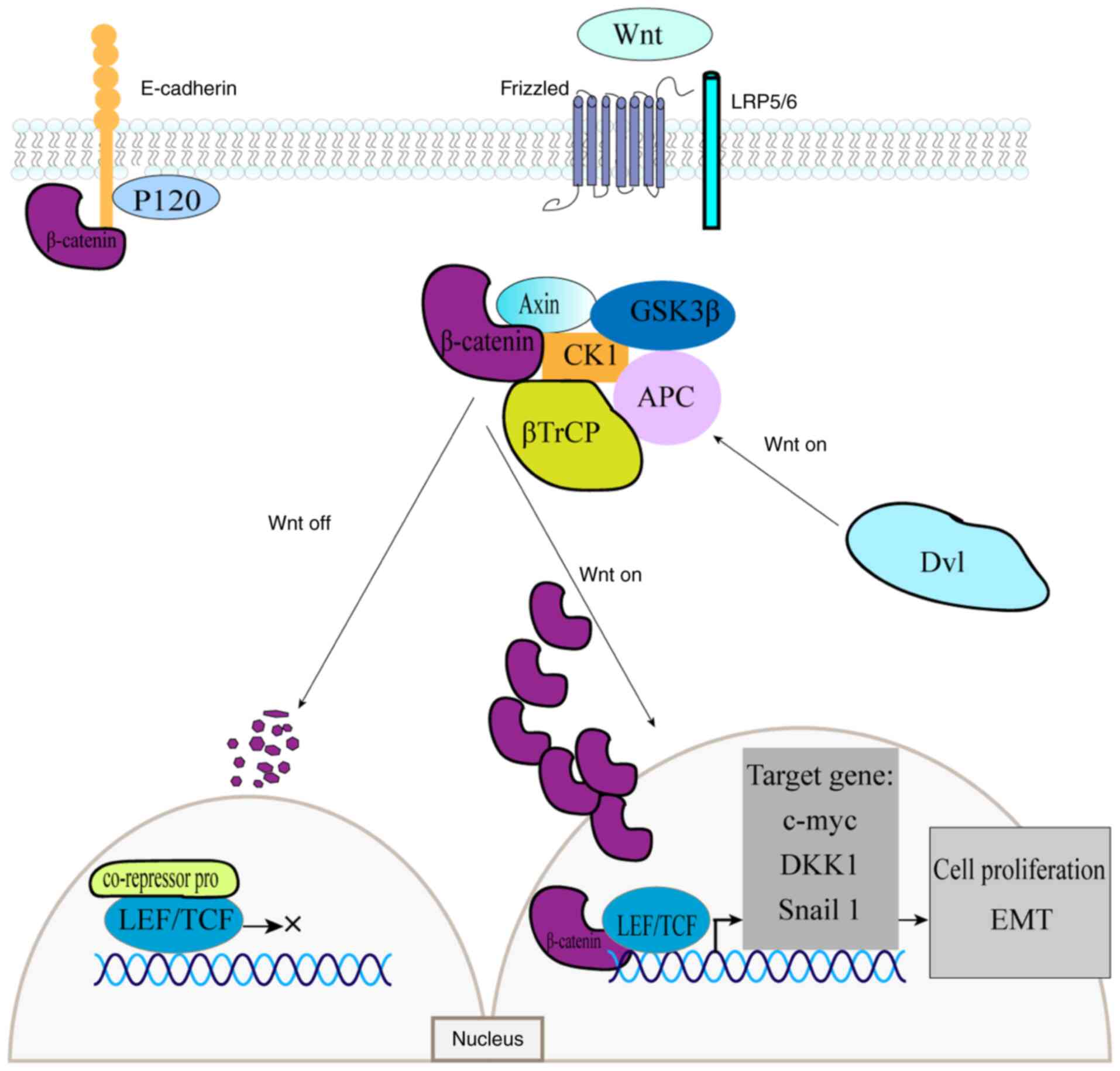
canonical pathway functions in two states depending on the presence
or absence of the Wnt ligand. In the absence of Wnt, β-catenin
binds to the destruction complex, where phosphorylation of its
N-terminal serine or threonine residues triggers ubiquitination and
subsequent proteasomal degradation (17). In the absence of nuclear β-catenin
protein accumulation, T-cell factor/lymphoid enhancer factor
(TCF/LEF) transcription factors remain bound to corepressor
proteins, thereby inhibiting transcription (18,19).
Wnt ligands can bind to Frizzled receptors and, with the
co-receptor assistance of low-density lipoprotein receptor-related
proteins 5/6 (LRP5/6), activate the cytoplasmic Dishevelled (Dvl)
protein (20,21). Ligand-receptor binding also
activates protein kinases (PKA, PKB and PKC), as well as the PI3K
and MAPK signaling pathways, which inhibit GSK-3β phosphorylation.
Concurrently, Dvl inactivates the destruction complex, thereby
preventing β-catenin phosphorylation and subsequent degradation
(22). As β-catenin accumulates in
the cytoplasm, it translocates into the nucleus, where it binds to
TCF/LEF transcription factors. This interaction activates the
transcription of Wnt target genes, including c-MYC, Cyclin
D1 gene (CCND1) and Dickkopf-1, which regulate cell growth
and development (23) (Fig. 1).
Non-canonical Wnt pathways regulate downstream
effects through alternative signaling molecules. Key pathways
include the Wnt/planar cell polarity (24), Wnt/Ras-related protein 1 signaling
(25), Wnt/PKA (26), Wnt/PKC (27) and Wnt/Ca2+ (28) pathways. These pathways exhibit
overlapping activities and are not entirely independent.
The Wnt signaling pathway plays a central role in
the initiation and progression of lung cancer; it regulates stem
cell renewal, migration, apoptosis and proliferation (29). Cancer development is associated with
aberrant Wnt signaling, particularly abnormal activation of the
canonical pathway driven by elevated β-catenin levels (30). Kren et al (31) reported that β-catenin protein
expression is correlated with tumor grade, stage and size, with 71%
of NSCLC cases showing upregulation of β-catenin in the cytoplasm
and plasma membrane.
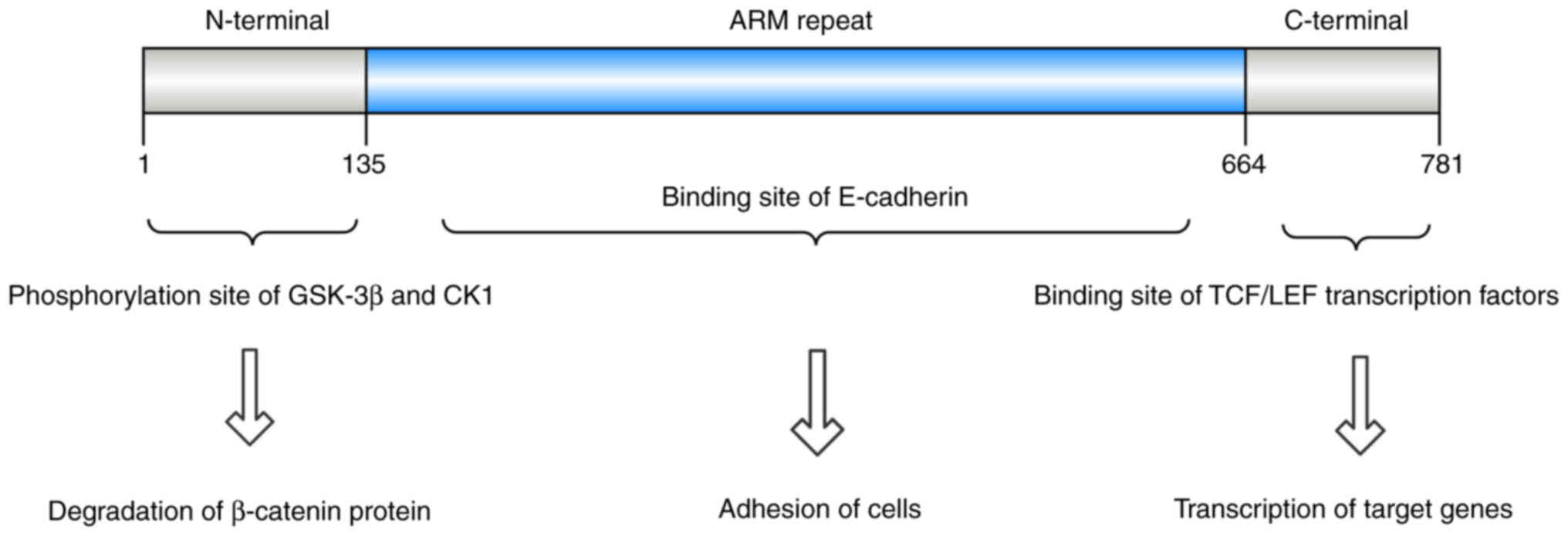
Structurally, β-catenin comprises three primary
domains: A central armadillo (ARM) repeat region of ~550 amino
acids, an N-terminal domain of 150 amino acids and a C-terminal
domain containing 100 amino acids (32,33).
The N-terminal domain is the site of phosphorylation by GSK-3β and
CK1, whereas the C-terminal domain interacts with nuclear TCF/LEF
transcription factors. On the cell membrane, β-catenin forms a
complex through ARM domain binding to E-cadherin (34–36)
(Fig. 2).
Nuclear translocation of β-catenin is essential for
initiating the expression of proliferation-related genes (49). Theoretically, sufficient production
of stable β-catenin protein enables its entry into the cell
nucleus, where it can activate proliferative programs (50). This nuclear transport is not a
process of simple free diffusion but is instead governed by precise
and complex regulatory mechanisms (50).
Cytoplasmic regulatory mechanisms play a crucial
role in determining the stability and accumulation of the β-catenin
protein, which is essential for its nuclear transport (23). In normal cells, β-catenin is
primarily localized at the cell membrane and is degraded in the
cytoplasm by the destruction complex (17). Mutations in the CTNNB1 gene
impair β-catenin phosphorylation and ubiquitination, resulting in
its cytoplasmic accumulation (51).
Additionally, various factors can increase cytoplasmic β-catenin
levels through upregulation of CTNNB1 transcription. For
example, FLVCR1-AS1 and LINC01006 are associated with high levels
of CTNNB1 mRNA in lung adenocarcinoma (LUAD) cells, thereby
promoting the activation of the canonical Wnt pathway (52–54).
The transcription of CTNNB1 is regulated by eIF3a and WD
repeat and SOCS box-containing protein 2 (53,55),
while cold-inducible RNA-binding protein binds to CTNNB1
mRNA to modulate its expression (56). In addition, cytoplasmic β-catenin
protein is regulated by the destruction complex, which, as
aforementioned, consists of GSK-3β, APC, Axin and CK1. Aquaporin-3
(57), microRNA (miR)-1246
(58) and long non-coding RNA
(IncRNA) PHLDA3 (59) downregulate
GSK-3β expression at the transcriptional, translational and protein
levels, respectively, thereby disrupting the function of the
destruction complex. Zbed3 (60)
and IncRNA DLX6-AS1 (61)
downregulate Axin expression; however, the expression of APC is
regulated by multiple miRNAs (62,63).
These regulatory abnormalities collectively promote the
accumulation of β-catenin in the cytoplasm, providing sufficient
substrate for its nuclear translocation.
The mechanism by which β-catenin is transported from
the cytoplasm to the nucleus remains a subject of debate. Early
studies suggested that β-catenin lacks a classical nuclear
localization signal, does not bind to the nuclear transport
receptor Importin-β1 (64) and is
independent of the Ran-mediated classical nuclear import pathway.
Instead, its ARM structure was thought to enable selective passage
through the nuclear pore complex (65). However, more recent studies indicate
that the nuclear translocation of β-catenin is Ras-related nuclear
protein-dependent, with nuclear transport proteins such as KPNA2
(66) and Importin-11 (67) facilitating its nuclear import.
Once inside the nucleus, β-catenin binds with the
cofactors BCL9 and Pygo to form a complex that anchors it within
the nuclear space. This BCL9/Pygo complex significantly enhances
the affinity of β-catenin for the TCF/LEF transcriptional complex,
thereby preventing its nuclear export (68).
Other regulatory mechanisms can also influence
pathway activity. Beyond the classical Wnt pathway, the nuclear
translocation of β-catenin is affected by additional pathways. For
instance, the amplification or mutation of the EGFR gene can
activate AKT, which in turn stabilizes β-catenin by inhibiting
GSK-3β or directly phosphorylating the Ser552 residue, thereby
facilitating its nuclear entry (69).
The invasion and metastasis processes of malignant
cells rely on EMT, a key driver of cancer-related mortality
(70,71). EMT refers to the transformation of
epithelial cells into mesenchymal-like phenotypes. This process has
an important role in various biological processes, including
embryonic development, tissue regeneration and tumor invasion and
metastasis (72). During EMT, cells
acquire mesenchymal characteristics, such as cytoskeletal
alterations, loss of polarity and reduced intercellular adhesion.
These changes enhance the invasiveness, resistance to apoptosis and
migration ability of cells (73).
EMT is regulated by the Wnt signaling pathway. When
extracellular Wnt proteins bind to Frizzled receptors and LRP5/6,
they form trimeric complexes that activate the Dvl protein
(20). This activation inhibits
GSK-3β phosphorylation, leading to the accumulation of cytoplasmic
and intranuclear β-catenin, thereby promoting the transcription of
Wnt target genes, such as Snail (78). This process accelerates EMT by
inhibiting the E-cadherin protein (5).
In addition to its role in cell adhesion, E-cadherin
inhibits β-catenin, thereby negatively regulating the classical Wnt
pathway (80). By forming a complex
with β-catenin, E-cadherin prevents the nuclear translocation of
β-catenin and helps maintain the cell-cell link. The nuclear
translocation of β-catenin, which stimulates transcriptional
activity and promotes EMT, is facilitated by both the stimulation
of the Wnt pathway and reduced E-cadherin protein expression
(81).
Additionally, Wnt regulators can modulate EMT by
affecting the transcriptional and translational levels of the
Wnt/β-catenin signal transduction pathway (82). Among these, miRNAs are key
regulators of Wnt signaling. These small molecules, typically 18–24
nucleotides in length, bind complementarily to the 3′ untranslated
region (UTR) of target mRNAs, leading to their degradation or
translational inhibition (83).
Through this mechanism, miRNAs play a notable regulatory role in
tumorigenesis (84). A number of
miRNAs have been implicated in tumorigenesis, acting either as
oncogenes or tumor suppressors. Specifically, miRNAs can directly
regulate EMT-promoting signaling pathways and major transcription
factors involved in E-cadherin expression (74). Notably, the miR-200 family, along
with miR-101, miR-506, miR-155, miR-31 and miR-214, serves critical
roles in controlling both EMT and mesenchymal-epithelial
transition, thereby enabling cellular flexibility between the
epithelial and mesenchymal states during transition (84,85).
For example, in meningiomas, miR-200 suppresses the Wnt pathway by
blocking the translation of CTNNB1 mRNA (86). Similarly, miR-34 inhibits both the
Wnt/β-catenin/TCF signaling cascade and EMT by targeting conserved
regions within the WNT1, WNT3, CTNNB1, AXIN2, LRP6 and
LEF1 genes (87).
Furthermore, miR-214 interacts with β-catenin as a
hetero-transcriptional complex and negatively regulates the
transcription of downstream target genes (88). In NSCLC cells, miR-214 inhibits the
transmission of the Wnt signaling pathway by directly targeting the
3′-UTR of CTNNB1 (84).
Overall, alterations in the E-cadherin protein
expression levels, certain miRNAs and the CTNNB1 gene can
each regulate β-catenin protein expression, modulate the canonical
Wnt pathway and drive the progression of EMT.
The widespread use of immune checkpoint inhibitors
(ICIs) has provided significant clinical benefits for patients with
NSCLC (89). However, the
combination of pembrolizumab and chemotherapy achieves only a
~45.7% 2-year overall survival rate in these patients (90). Resistance to ICIs is commonly
associated with three mechanisms: Deficiency of major
histocompatibility complex class I (91), β-2-microglobulin gene deletions
(92) and mutations in the IFN-γ
signaling pathway (93). Studies
have also indicated that activation of the Wnt/β-catenin pathway in
tumors contributes to ICI resistance in lung cancer. This pathway
promotes immune evasion, supports carcinogenesis, facilitates
cancer progression and plays a role in shaping the TME (94–96).
The Wnt/β-catenin pathway promotes the expression of
activating transcription factor 3 (ATF3), which in turn suppresses
the expression of C-C motif chemokine ligand 4 (CCL4) (97). Reduced CCL4 expression leads to
decreased infiltration of antigen-presenting cells, impairs
lymphocyte homing to tumors and enables cancer cells to evade the
immune surveillance more effectively (98). Beyond inhibiting CCL4 synthesis
through ATF3, the Wnt/β-catenin pathway also exerts multiple
additional effects that promote immune evasion. In NSCLC,
activation of this pathway is associated with a higher tumor
mutational burden, suggesting that these cancer cells are likely to
be highly immunogenic (94).
Consequently, immune editing may be required for their survival.
Immune editing driven by the Wnt/β-catenin pathway involves three
primary mechanisms. First, the Wnt/β-catenin pathway modulates
tumor-associated macrophages (TAMs), which are typically classified
as M1 and M2. The M2 subtype promotes tumor proliferation,
migration and immune escape (99).
IL-1β generated by TAMs can phosphorylate GSK-3β, stabilizing
β-catenin and Snail and activating the Wnt/β-catenin pathway. This
activation, in turn, stimulates macrophages to produce additional
IL-1β, thereby enhancing tumor survival and metastatic potential
(100). Second, tumor cells with
activated β-catenin produce elevated levels of IL-10, which impairs
dendritic cells (DCs) and inhibits their maturation (101). Third, the Wnt/β-catenin pathway
reshapes tumor metabolism, contributing to an immunosuppressive
TME. Activation of the Wnt/β-catenin signaling pathway can increase
the expression of pyruvate dehydrogenase kinase 1 and
monocarboxylate transporter 1 (102), shifting cancer cell metabolism
from oxidative phosphorylation to aerobic glycolysis and creating a
more acidic TME. Collectively, these mechanisms facilitate tumor
cell evasion of the immune response. In addition to preventing the
activation of T cells and DCs, the lactic acid-rich
microenvironment can also stimulate the polarization of TAMs toward
the M2 subtype, as well as the expression of VEGF and
hypoxia-inducible factor-1α (103). This microenvironment, shaped by
aerobic glycolysis, further suppresses T-cell activity, resulting
in resistance to ICIs (104).
In summary, tumor immune escape is closely
associated with hyperactivation of the Wnt/β-catenin pathway and
aberrant β-catenin production. Moreover, research has indicated
that patients with NSCLC who are β-catenin-positive generally
experience a poorer prognosis when treated with anti-programmed
cell death protein 1 (PD-1) monotherapy (95). These findings suggest that β-catenin
expression may serve as a prognostic marker for the efficacy of
anti-PD-1 therapy in patients with NSCLC.
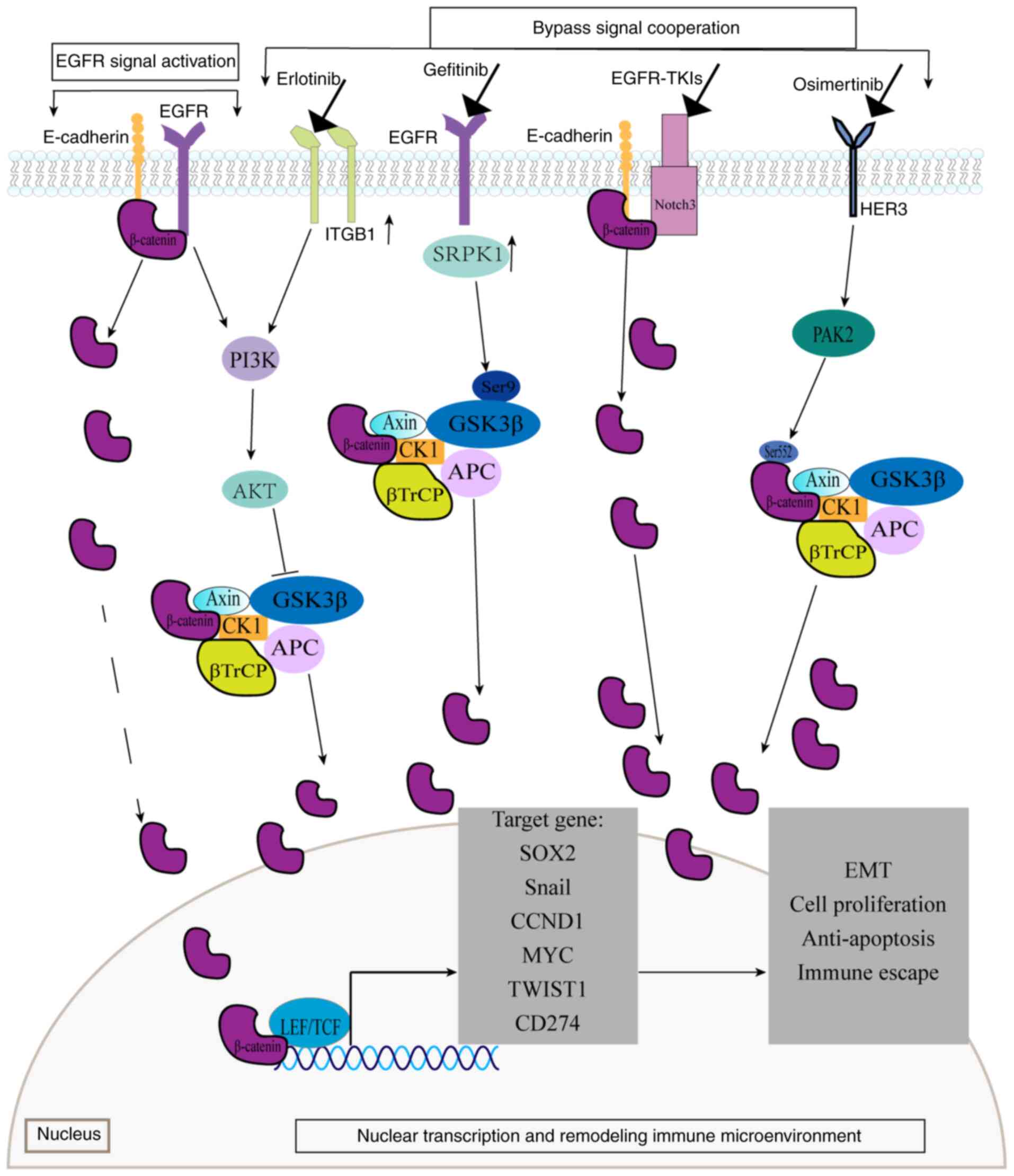
EGFR phosphorylates β-catenin at tyrosine residues
rather than at serine/threonine sites. This tyrosine
phosphorylation causes β-catenin to dissociate from the
α-catenin/E-cadherin complex, leading to increased intranuclear
β-catenin and activation of the Wnt/β-catenin signaling pathway
(106). EGFR can also bind
directly to β-catenin, transactivating the classical Wnt pathway
through PI3K signaling (107).
Activation of the EGFR/PI3K/AKT signaling pathway inhibits GSK-3β
activity, preventing β-catenin phosphorylation and degradation.
Consequently, the β-catenin protein accumulates in the cytoplasm
and initiates the classical Wnt pathway (108). These interactions demonstrate a
close connection between β-catenin and EGFR, with the Wnt and EGFR
signaling pathways functioning cooperatively. Therefore, β-catenin
can influence the effectiveness of EGFR-TKIs by activating its
signaling independently of direct EGFR interaction.
Furthermore, β-catenin mediates EGFR-TKI resistance
through synergistic interactions with bypass signaling pathways. In
the NOTCH3-dependent β-catenin resistance pathway, EGFR-TKI
treatment rapidly activates NOTCH3, which physically binds to the
β-catenin protein. This interaction increases the stability and
activation of β-catenin, promoting EMT and cell stemness and
ultimately driving EGFR-TKI resistance (109). Another critical mechanism involves
the HER3/p21-activated kinase2 (PAK2)/β-catenin signaling pathway,
which plays a significant role in osimertinib resistance (110). This pathway is activated in
osimertinib-treated cells, where PAK2 increases the phosphorylation
of β-catenin at Ser552, preventing its ubiquitination and
proteasomal degradation. This allows β-catenin to translocate into
the nucleus, where it upregulates the expression of the target
gene, SOX2, enhancing tumor stem cell-like characteristics
and contributing to osimertinib resistance. The membrane β1
integrin/AKT/β-catenin signaling pathway is associated with
erlotinib resistance. In erlotinib-resistant cells, the Rab25
protein is highly expressed, mediating the recycling of ITGB to the
plasma cell membrane, activating AKT and phosphorylating GSK-3β.
This results in β-catenin accumulation and enhanced tumor cell
proliferation (111). Similarly,
the serine-arginine protein kinase 1 (SRPK1)/GSK-3β axis promotes
gefitinib resistance. In gefitinib-treated cells, highly expressed
SRPK1 binds to Ser9 of GSK-3β, promoting GSK-3β autophosphorylation
and activating the Wnt pathway. SRPK1 also promotes the binding of
LEF1, β-catenin protein and the EGFR promoter, leading to an
increase in EGFR expression and contributing to drug resistance
(112).
β-catenin protein mediates EGFR-TKI resistance
through nuclear transcriptional regulation. Upon entering the
nucleus, β-catenin binds to TCF4, forming a complex that activates
genes associated with multidrug resistance. This includes the
activation of Snail transcription, which downregulates
E-cadherin expression and promotes EMT and tumor metastasis
(113). This complex also triggers
the transcription of CCND1, relieving cell cycle arrest and
sustaining tumor cell proliferation under TKI stress (114). Additionally, β-catenin activates
MYC transcription, promoting tumor cell growth and helping
cells evade immune surveillance (115). Furthermore, this complex induces
Twist1 transcription, which directly binds to the intron and
promoter regions of BCL2L11 (BIM), inhibiting
BIM transcription and conferring resistance to TKI-induced
apoptosis (116).
NSCLC resistance to EGFR-TKIs is associated with the
upregulation of programmed death-ligand 1 (PD-L1) expression
(117,118). Recent research indicates that
β-catenin contributes to EGFR-TKI resistance by promoting the
upregulation of PD-L1 in NSCLC (119). As noted earlier, EGFR signaling
activates the AKT/β-catenin pathway, and nuclear β-catenin can
increase PD-L1 levels while reducing CD8+ T cell
recruitment in tumors, thereby inducing drug resistance (97). The mechanism by which β-catenin
upregulates PD-L1 involves two pathways: First, nuclear β-catenin
forms a complex with TCF/LEF, increasing the transcriptional
activity of the CD274 promoter and upregulating PD-L1
expression (120); and second, the
same complex activates the target gene MYC, which further
stimulates PD-L1 transcription (115). Together, these processes promote
tumor immune escape, ultimately contributing to EGFR-TKI
resistance.
Chemotherapy and radiation resistance are associated
with aberrant β-catenin levels. For instance, in lung carcinoma
cells, the transfer of exosomal targeting protein for Xenopus
kinesin-like protein 2 promotes docetaxel resistance and cell
migration by increasing β-catenin expression and activating
downstream Wnt signaling (121).
In vitro experiments indicate that elevated β-catenin levels
can reduce the effect of cisplatin in lung cancer cells,
potentially contributing to cisplatin resistance (122). Additionally, Yin et al
(123) demonstrated through
bioinformatic analyses that ubiquitin-conjugating enzyme E2T
promotes β-catenin upregulation by facilitating the
ubiquitin-dependent degradation of FOXO1, thereby driving EMT and
conferring radioresistance in NSCLC. Tumor cell resistance to
chemotherapy and radiation is associated with the characteristics
of cancer stem cells (CSCs) (124). Tumor stemness, a key biological
feature regulated by the CSC signaling network, describes the
ability of CSCs to self-renew and promote tumor formation (125). CSCs exhibit self-regeneration,
differentiation and dedifferentiation capacities, as well as
participate in the reprogramming of epithelium-mesenchymal,
immune-mediated, metabolic and epigenetic systems. These
adaptations enable them to survive the TME and resist host defenses
and therapeutic interventions (125). Furthermore, the expansion and
maintenance of CSCs are influenced, directly or indirectly, by the
classical Wnt pathway (126,127).
The intracellular localization and expression level
of β-catenin protein can be assessed using immunohistochemistry.
Tumor prognosis is negatively affected by both increased and
decreased membrane expression of β-catenin. In addition, increased
levels of cytoplasmic β-catenin protein or nuclear positivity are
correlated with reduced overall survival (43). This suggests that β-catenin levels
in the cytoplasm, nucleus and cell membrane can serve as important
prognostic indicators in patients with NSCLC (128). Elevated β-catenin levels are also
correlated with poorer immunotherapy results, suggesting its
potential as a predictor of the effectiveness of immunotherapy
(95). However, the commonly used
methods to determine β-catenin protein, such as western blotting,
immunohistochemistry and reporter gene plasmid assays, have
specific requirements regarding experimental conditions,
pathological tissues and cell states, which limits their clinical
applicability. By contrast, gene mutation data can be more readily
obtained through next-generation sequencing. For instance, ~6% of
LUADs harbor CTNNB1 mutations, which can be used in
predicting the postoperative recurrence of EGFR-mutated LUAD
(129). Moreover, CTNNB1
co-mutations may serve as potential predictors of the efficacy of
EGFR-TKIs in treating NSCLC, as patients with EGFR and
CTNNB1 co-mutations exhibit reduced responses to EGFR-TKIs
(130).
Despite extensive efforts to develop drugs targeting
the Wnt/β-catenin signaling pathway, to the best of our knowledge,
no effective treatments currently exist that directly target the
β-catenin protein. Recent clinical investigations indicate that 5
µM berberine liquid crystalline nanoparticles efficiently target
the Wnt signal by suppressing the production of CTNNB1 and
its corresponding protein, reducing both the gene and protein
levels in the human lung tumor cell line, A549 (131). The CTNNB1 gene can be
specifically targeted using RNA interference triggers incorporated
into the nanoparticle-based therapeutic known as Dicer-substrate
small interfering RNA targeting CTNNB1 (DCR-BCAT). DCR-BCAT
effectively suppresses β-catenin protein expression, significantly
increases T-cell infiltration and enhances tumor susceptibility to
ICIs, as demonstrated in a mouse tumor model (132). The nuclear localization inhibitor
of β-catenin, IMU-1003, significantly reduces colonies resistant to
osimertinib, suggesting that blocking intranuclear β-catenin can
overcome transgenerational EGFR-TKI resistance (133). Triptolide has also been reported
to reverse paclitaxel resistance and EMT in LUAD cells while
suppressing tumor progression by blocking the p70S6K/GSK3/β-catenin
pathway (134). Through its
interaction with β-catenin, ubiquitin-specific peptidase 5 (USP5)
helps to deubiquitinate, stabilize and activate the classical Wnt
pathway. When the small molecule WP1130 binds to USP5, it increases
β-catenin breakdown, which markedly lowers lung cancer cell
migration and infiltration (135).
Additionally, fucoxanthin exhibits antitumor potential in LUAD by
reversing EMT, limiting proliferation, inducing apoptosis and
downregulating the expression of TGF-β1, which can induce β-catenin
expression (136). Asiaticoside, a
triterpenoid saponin with antitumor properties, has been observed
to reduce intranuclear β-catenin levels and inhibit the
Wnt/β-catenin pathway by promoting GSK-3β phosphorylation,
increasing APC expression and lowering Axin levels. This blockade
of EMT suppresses NSCLC growth and metastasis (137). In conclusion, various experimental
approaches primarily focus on directly targeting the CTNNB1
gene or lowering β-catenin levels to modulate the TME, reverse EMT,
suppress treatment resistance and inhibit tumor growth.
NSCLC development and progression are driven by the
β-catenin protein. Elevated nuclear β-catenin levels activate the
Wnt/β-catenin pathway, leading to aberrant cell division and
promoting carcinogenesis. This process induces EMT, remodels the
TME and facilitates cancer migration and immune evasion.
Not applicable.
Funding: Not applicable.
Not applicable.
LP wrote the manuscript and prepared the figures. RG
and LH assisted with the literature search and manuscript revision.
TF and CB provided guidance and assisted in revising and correcting
the manuscript. Data authentication is not applicable. All authors
have read and approved the final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
2
|
Luo G, Zhang Y, Rumgay H, Morgan E,
Langselius O, Vignat J, Colombet M and Bray F: Estimated worldwide
variation and trends in incidence of lung cancer by histological
subtype in 2022 and over time: A population-based study. Lancet
Respir Med. 13:348–363. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Georgakopoulos I, Kouloulias V, Ntoumas G,
Desse D, Koukourakis I, Kougioumtzopoulou A, Charpidou A, Syrigos
KN and Zygogianni A: Combined use of radiotherapy and tyrosine
kinase inhibitors in the management of metastatic non-small cell
lung cancer: A literature review. Crit Rev Oncol Hematol.
204:1045202024. View Article : Google Scholar
|
|
4
|
Mieras A, Pasman HRW, Onwuteaka-Philipsen
BD, Dingemans AMMC, Kok EV, Cornelissen R, Jacobs W, van den Berg
JW, Welling A, Bogaarts BAHA, et al: Is in-hospital mortality
higher in patients with metastatic lung cancer who received
treatment in the last month of life? A retrospective cohort study.
J Pain Symptom Manage. 58:805–811. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang
X, Zhou Z, Shu G and Yin G: Wnt/β-catenin signalling: Function,
biological mechanisms, and therapeutic opportunities. Signal
Transduct Target Ther. 7:32022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
White BD, Chien AJ and Dawson DW:
Dysregulation of Wnt/β-catenin signaling in gastrointestinal
cancers. Gastroenterology. 142:219–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yeh Y, Guo Q, Connelly Z, Cheng S, Yang S,
Prieto-Dominguez N and Yu X: Wnt/beta-catenin signaling and
prostate cancer therapy resistance. Adv Exp Med Biol. 1210:351–378.
2019. View Article : Google Scholar
|
|
8
|
Mukherjee N, Bhattacharya N, Alam N, Roy
A, Roychoudhury S and Panda CK: Subtype-specific alterations of the
Wnt signaling pathway in breast cancer: clinical and prognostic
significance. Cancer Sci. 103:210–220. 2012. View Article : Google Scholar
|
|
9
|
Skronska-Wasek W, Gosens R, Königshoff M
and Baarsma HA: WNT receptor signalling in lung physiology and
pathology. Pharmacol Ther. 187:150–166. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Z, Westover D, Tang Z, Liu Y, Sun J,
Sun Y, Zhang R, Wang X, Zhou S, Hesilaiti N, et al: Wnt/β-catenin
signaling in the development and therapeutic resistance of
non-small cell lung cancer. J Transl Med. 22:5652024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao C, Wang Y, Broaddus R, Sun L, Xue F
and Zhang W: Exon 3 mutations of CTNNB1 drive tumorigenesis: A
review. Oncotarget. 9:5492–5508. 2017. View Article : Google Scholar
|
|
12
|
Ma Q, Yu J, Zhang X, Wu X and Deng G:
Wnt/β-catenin signaling pathway-a versatile player in apoptosis and
autophagy. Biochimie. 211:57–67. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar
|
|
14
|
Vallée A, Lecarpentier Y and Vallée JN:
The key role of the WNT/β-catenin pathway in metabolic
reprogramming in cancers under normoxic conditions. Cancers
(Basel). 13:55572021. View Article : Google Scholar
|
|
15
|
Parsons MJ, Tammela T and Dow LE: WNT as a
driver and dependency in cancer. Cancer Discov. 11:2413–2429. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kleeman SO and Leedham SJ: Not all Wnt
activation is equal: ligand-dependent versus ligand-independent Wnt
activation in colorectal cancer. Cancers (Basel). 12:33552020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu F and Millar SE: Wnt/beta-catenin
signaling in oral tissue development and disease. J Dent Res.
89:318–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao DM, Yu S, Zhou X, Haring JS, Held W,
Badovinac VP, Harty JT and Xue HH: Constitutive activation of Wnt
signaling favors generation of memory CD8 T cells. J Immunol.
184:1191–1199. 2010. View Article : Google Scholar
|
|
19
|
Hurlstone A and Clevers H: T-cell factors:
Turn-ons and turn-offs. EMBO J. 21:2303–2311. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dale TC: Signal transduction by the Wnt
family of ligands. Biochem J. 329:209–223. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Howell BW and Herz J: The LDL receptor
gene family: Signaling functions during development. Curr Opin
Neurobiol. 11:74–81. 2001. View Article : Google Scholar
|
|
22
|
Moon RT, Bowerman B, Boutros M and
Perrimon N: The promise and perils of Wnt signaling through
beta-catenin. Science. 296:1644–1646. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharma A, Mir R and Galande S: Epigenetic
regulation of the Wnt/β-catenin signaling pathway in cancer. Front
Genet. 12:6810532021. View Article : Google Scholar
|
|
24
|
Yamamoto S, Nishimura O, Misaki K, Nishita
M, Minami Y, Yonemura S, Tarui H and Sasaki H: Cthrc1 selectively
activates the planar cell polarity pathway of Wnt signaling by
stabilizing the Wnt-receptor complex. Dev Cell. 15:23–36. 2008.
View Article : Google Scholar
|
|
25
|
Feng D, Wang J, Yang W, Li J, Lin X, Zha
F, Wang X, Ma L, Choi NT, Mii Y, et al: Regulation of Wnt/PCP
signaling through p97/VCP-KBTBD7-mediated Vangl ubiquitination and
endoplasmic reticulum-associated degradation. Sci Adv.
7:eabg20992021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cai Y, Cai T and Chen Y: Wnt pathway in
osteosarcoma, from oncogenic to therapeutic. J Cell Biochem.
115:625–631. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martineau X, Abed É, Martel-Pelletier J,
Pelletier JP and Lajeunesse D: Alteration of Wnt5a expression and
of the non-canonical Wnt/PCP and Wnt/PKC-Ca2+ pathways in human
osteoarthritis osteoblasts. PLoS One. 12:e01807112017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
De A: Wnt/Ca2+ signaling pathway: A brief
overview. Acta Biochim Biophys Sin (Shanghai). 43:745–756. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang X, Lou Y, Zheng X, Wang H, Sun J,
Dong Q and Han B: Wnt blockers inhibit the proliferation of lung
cancer stem cells. Drug Des Devel Ther. 9:2399–2407.
2015.PubMed/NCBI
|
|
31
|
Kren L, Hermanová M, Goncharuk VN, Kaur P,
Ross JS, Pavlovský Z and Dvorák K: Downregulation of plasma
membrane expression/cytoplasmic accumulation of beta-catenin
predicts shortened survival in non-small cell lung cancer. A
clinicopathologic study of 100 cases. Cesk Patol. 39:17–20.
2003.
|
|
32
|
Daniels DL, Eklof Spink K and Weis WI:
beta-catenin: Molecular plasticity and drug design. Trends Biochem
Sci. 26:672–678. 2001. View Article : Google Scholar
|
|
33
|
Städeli R, Hoffmans R and Basler K:
Transcription under the control of nuclear Arm/beta-catenin. Curr
Biol. 16:R378–R385. 2006. View Article : Google Scholar
|
|
34
|
Kishida S, Yamamoto H, Ikeda S, Kishida M,
Sakamoto I, Koyama S and Kikuchi A: Axin, a negative regulator of
the wnt signaling pathway, directly interacts with adenomatous
polyposis coli and regulates the stabilization of beta-catenin. J
Biol Chem. 273:10823–10826. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aberle H, Bauer A, Stappert J, Kispert A
and Kemler R: beta-catenin is a target for the ubiquitin-proteasome
pathway. EMBO J. 16:3797–3804. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Conacci-Sorrell M, Zhurinsky J and
Ben-Ze'ev A: The cadherin-catenin adhesion system in signaling and
cancer. J Clin Invest. 109:987–991. 2002. View Article : Google Scholar
|
|
37
|
Xing Y, Takemaru KI, Liu J, Berndt JD,
Zheng JJ, Moon RT and Xu W: Crystal structure of a full-length
beta-catenin. Structure. 16:478–487. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kase S, Sugio K, Yamazaki K, Okamoto T,
Yano T and Sugimachi K: Expression of E-cadherin and beta-catenin
in human non-small cell lung cancer and the clinical significance.
Clin Cancer Res. 6:4789–4796. 2000.PubMed/NCBI
|
|
39
|
Baum B and Georgiou M: Dynamics of
adherens junctions in epithelial establishment, maintenance, and
remodeling. J Cell Biol. 192:907–917. 2011. View Article : Google Scholar
|
|
40
|
Li LF, Wei ZJ, Sun H and Jiang B: Abnormal
β-catenin immunohistochemical expression as a prognostic factor in
gastric cancer: A meta-analysis. World J Gastroenterol.
20:12313–12321. 2014. View Article : Google Scholar
|
|
41
|
Klaus A and Birchmeier W: Wnt signalling
and its impact on development and cancer. Nat Rev Cancer.
8:387–398. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rim EY, Clevers H and Nusse R: The Wnt
pathway: From signaling mechanisms to synthetic modulators. Annu
Rev Biochem. 91:571–598. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yoo SB, Kim YJ, Kim H, Jin Y, Sun PL,
Jheon S, Lee JS and Chung JH: Alteration of the
E-cadherin/β-catenin complex predicts poor response to epidermal
growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI)
treatment. Ann Surg Oncol. 20 (Suppl 3):S545–S552. 2013. View Article : Google Scholar
|
|
44
|
Li XQ, Yang XL, Zhang G, Wu SP, Deng XB,
Xiao SJ, Liu QZ, Yao KT and Xiao GH: Nuclear β-catenin accumulation
is associated with increased expression of Nanog protein and
predicts poor prognosis of non-small cell lung cancer. J Transl
Med. 11:1142013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Polakis P: Wnt signaling and cancer. Genes
Dev. 14:1837–1851. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kikuchi A: Tumor formation by genetic
mutations in the components of the Wnt signaling pathway. Cancer
Sci. 94:225–229. 2003. View Article : Google Scholar
|
|
47
|
Amit S, Hatzubai A, Birman Y, Andersen JS,
Ben-Shushan E, Mann M, Ben-Neriah Y and Alkalay I: Axin-mediated
CKI phosphorylation of beta-catenin at Ser 45: A molecular switch
for the Wnt pathway. Genes Dev. 16:1066–1076. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou C, Jin H, Li W, Zhao R and Chen C:
CTNNB1 S37C mutation causing cells proliferation and migration
coupled with molecular mechanisms in lung adenocarcinoma. Ann
Transl Med. 9:6812021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hu S, Chang J, Ruan H, Zhi W, Wang X, Zhao
F, Ma X, Sun X, Liang Q, Xu H, et al: Cantharidin inhibits
osteosarcoma proliferation and metastasis by directly targeting
miR-214-3p/DKK3 axis to inactivate β-catenin nuclear translocation
and LEF1 translation. Int J Biol Sci. 17:2504–2522. 2021.
View Article : Google Scholar
|
|
50
|
Anthony CC, Robbins DJ, Ahmed Y and Lee E:
Nuclear regulation of Wnt/β-catenin signaling: It's a complex
situation. Genes (Basel). 11:8862020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kim W, Kim M and Jho E: Wnt/β-catenin
signalling: From plasma membrane to nucleus. Biochem J. 450:9–21.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang Y, Liu H, Zhang Q and Zhang Z: Long
noncoding RNA LINC01006 facilitates cell proliferation, migration,
and epithelial-mesenchymal transition in lung adenocarcinoma via
targeting the MicroRNA 129-2-3p/CTNNB1 axis and activating
Wnt/β-catenin signaling pathway. Mol Cell Biol. 41:e00380202021.
View Article : Google Scholar
|
|
53
|
Zheng JY, Zhu T, Zhuo W, Mao XY, Yin JY,
Li X, He YJ, Zhang W, Liu C and Liu ZQ: eIF3a sustains non-small
cell lung cancer stem cell-like properties by promoting
YY1-mediated transcriptional activation of β-catenin. Biochem
Pharmacol. 213:1156162023. View Article : Google Scholar
|
|
54
|
Liu S, Yang N, Wang L, Wei B, Chen J and
Gao Y: lncRNA SNHG11 promotes lung cancer cell proliferation and
migration via activation of Wnt/β-catenin signaling pathway. J Cell
Physiol. 235:7541–7553. 2020. View Article : Google Scholar
|
|
55
|
Wei X, Liao J, Lei Y, Li M, Zhao G, Zhou
Y, Ye L and Huang Y: Retraction: WSB2 as a target of Hedgehog
signaling promoted the malignantbiological behavior of Xuanwei lung
cancer through regulating Wnt/β-catenin signaling. Transl Cancer
Res. 13:51612024. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liao Y, Feng J, Sun W, Wu C, Li J, Jing T,
Liang Y, Qian Y, Liu W and Wang H: CIRP promotes the progression of
non-small cell lung cancer through activation of Wnt/β-catenin
signaling via CTNNB1. J Exp Clin Cancer Res. 40:2752021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu C, Liu L, Zhang Y and Jing H:
Molecular mechanism of AQP3 in regulating differentiation and
apoptosis of lung cancer stem cells through Wnt/GSK-3β/β-catenin
pathway. J BUON. 25:1714–1720. 2020.PubMed/NCBI
|
|
58
|
Yang F, Xiong H, Duan L, Li Q, Li X and
Zhou Y: MiR-1246 promotes metastasis and invasion of A549 cells by
targeting GSK-3β-mediated Wnt/β-catenin pathway. Cancer Res Treat.
51:1420–1429. 2019. View Article : Google Scholar
|
|
59
|
Lei L, Wang Y, Li ZH, Fei LR, Huang WJ,
Zheng YW, Liu CC, Yang MQ, Wang Z, Zou ZF and Xu HT: PHLDA3
promotes lung adenocarcinoma cell proliferation and invasion via
activation of the Wnt signaling pathway. Lab Invest. 101:1130–1141.
2021. View Article : Google Scholar
|
|
60
|
Shi X, Zhao Y and Fan C: Zbed3 promotes
proliferation and invasion of lung cancer partly through regulating
the function of Axin-Gsk3β complex. J Cell Mol Med. 23:1014–1021.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Xu X, Zhang Y, Wang M, Zhang X, Jiang W,
Wu S and Ti X: A peptide encoded by a long non-coding RNA DLX6-AS1
facilitates cell proliferation, migration, and invasion by
activating the wnt/β-catenin signaling pathway in non-small-cell
lung cancer cell. Crit Rev Eukaryot Gene Expr. 32:43–53. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Xu G, Zhang Z, Zhang L, Chen Y, Li N, Lv
Y, Li Y and Xu X: miR-4326 promotes lung cancer cell proliferation
through targeting tumor suppressor APC2. Mol Cell Biochem.
443:151–157. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Cen W, Yan Q, Zhou W, Mao M, Huang Q, Lin
Y and Jiang N: miR-4739 promotes epithelial-mesenchymal transition
and angiogenesis in ‘driver gene-negative’ non-small cell lung
cancer via activating the Wnt/β-catenin signaling. Cell Oncol
(Dordr). 46:1821–1835. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yokoya F, Imamoto N, Tachibana T and
Yoneda Y: beta-catenin can be transported into the nucleus in a
Ran-unassisted manner. Mol Biol Cell. 10:1119–1131. 1999.
View Article : Google Scholar
|
|
65
|
Andrade MA, Petosa C, O'Donoghue SI,
Müller CW and Bork P: Comparison of ARM and HEAT protein repeats. J
Mol Biol. 309:1–18. 2001. View Article : Google Scholar
|
|
66
|
Altan B, Yokobori T, Mochiki E, Ohno T,
Ogata K, Ogawa A, Yanai M, Kobayashi T, Luvsandagva B, Asao T and
Kuwano H: Nuclear karyopherin-α2 expression in primary lesions and
metastatic lymph nodes was associated with poor prognosis and
progression in gastric cancer. Carcinogenesis. 34:2314–2321. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Mis M, O'Brien S, Steinhart Z, Lin S, Hart
T, Moffat J and Angers S: IPO11 mediates βcatenin nuclear import in
a subset of colorectal cancers. J Cell Biol. 219:e2019030172020.
View Article : Google Scholar
|
|
68
|
Krieghoff E, Behrens J and Mayr B:
Nucleo-cytoplasmic distribution of beta-catenin is regulated by
retention. J Cell Sci. 119:1453–1463. 2006. View Article : Google Scholar
|
|
69
|
Fang D, Hawke D, Zheng Y, Xia Y,
Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T and Lu Z:
Phosphorylation of beta-catenin by AKT promotes beta-catenin
transcriptional activity. J Biol Chem. 282:11221–11229. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lee GA, Hwang KA and Choi KC: Roles of
dietary phytoestrogens on the regulation of epithelial-mesenchymal
transition in diverse cancer metastasis. Toxins (Basel). 8:1622016.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Dongre A and Weinberg RA: New insights
into the mechanisms of epithelial-mesenchymal transition and
implications for cancer. Nat Rev Mol Cell Biol. 20:69–84. 2019.
View Article : Google Scholar
|
|
73
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar
|
|
74
|
Guo F, Parker Kerrigan BC, Yang D, Hu L,
Shmulevich I, Sood AK, Xue F and Zhang W: Post-transcriptional
regulatory network of epithelial-to-mesenchymal and
mesenchymal-to-epithelial transitions. J Hematol Oncol. 7:192014.
View Article : Google Scholar
|
|
75
|
Pertz O, Bozic D, Koch AW, Fauser C,
Brancaccio A and Engel J: A new crystal structure, Ca2+ dependence
and mutational analysis reveal molecular details of E-cadherin
homoassociation. EMBO J. 18:1738–1747. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kim YS, Yi BR, Kim NH and Choi KC: Role of
the epithelial-mesenchymal transition and its effects on embryonic
stem cells. Exp Mol Med. 46:e1082014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Heuberger J and Birchmeier W: Interplay of
cadherin-mediated cell adhesion and canonical Wnt signaling. Cold
Spring Harb Perspect Biol. 2:a0029152010. View Article : Google Scholar
|
|
78
|
Eijkelenboom A and Burgering BMT: FOXOs:
Signalling integrators for homeostasis maintenance. Nat Rev Mol
Cell Biol. 14:83–97. 2013. View Article : Google Scholar
|
|
79
|
Bustamante A, Baritaki S, Zaravinos A and
Bonavida B: Relationship of signaling pathways between RKIP
expression and the inhibition of EMT-inducing transcription factors
SNAIL1/2, TWIST1/2 and ZEB1/2. Cancers (Basel). 16:31802024.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Schmalhofer O, Brabletz S and Brabletz T:
E-cadherin, beta-catenin, and ZEB1 in malignant progression of
cancer. Cancer Metastasis Rev. 28:151–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ghahhari NM and Babashah S: Interplay
between microRNAs and WNT/β-catenin signalling pathway regulates
epithelial-mesenchymal transition in cancer. Eur J Cancer.
51:1638–1649. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Stewart DJ: Wnt signaling pathway in
non-small cell lung cancer. J Natl Cancer Inst. 106:djt3562014.
View Article : Google Scholar
|
|
83
|
Mármol-Sánchez E, Luigi-Sierra MG,
Castelló A, Guan D, Quintanilla R, Tonda R and Amills M:
Variability in porcine microRNA genes and its association with mRNA
expression and lipid phenotypes. Genet Sel Evol. 53:432021.
View Article : Google Scholar
|
|
84
|
Zhao H, Wang Z, Wu G, Lu Y, Zheng J, Zhao
Y, Han Y, Wang J, Yang L, Du J and Wang E: Role of MicroRNA-214 in
dishevelled1-modulated β-catenin signalling in non-small cell lung
cancer progression. J Cancer. 14:239–249. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Tian Y, Pan Q, Shang Y, Zhu R, Ye J, Liu
Y, Zhong X, Li S, He Y, Chen L, et al: MicroRNA-200 (miR-200)
cluster regulation by achaete scute-like 2 (Ascl2): Impact on the
epithelial-mesenchymal transition in colon cancer cells. J Biol
Chem. 289:36101–36115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Saydam O, Shen Y, Würdinger T, Senol O,
Boke E, James MF, Tannous BA, Stemmer-Rachamimov AO, Yi M, Stephens
RM, et al: Downregulated microRNA-200a in meningiomas promotes
tumor growth by reducing E-cadherin and activating the
Wnt/beta-catenin signaling pathway. Mol Cell Biol. 29:5923–5940.
2009. View Article : Google Scholar
|
|
87
|
Cha YH, Kim NH, Park C, Lee I, Kim HS and
Yook JI: MiRNA-34 intrinsically links p53 tumor suppressor and Wnt
signaling. Cell Cycle. 11:1273–1281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yi B, Wang S, Wang X, Liu Z, Zhang C, Li
M, Gao S, Wei S, Bae S, Stringer-Reasor E, et al: CRISPR
interference and activation of the microRNA-3662-HBP1 axis control
progression of triple-negative breast cancer. Oncogene. 41:268–279.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Friedlaender A, Naidoo J, Banna GL, Metro
G, Forde P and Addeo A: Role and impact of immune checkpoint
inhibitors in neoadjuvant treatment for NSCLC. Cancer Treat Rev.
104:1023502022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Rodríguez-Abreu D, Powell SF, Hochmair MJ,
Gadgeel S, Esteban E, Felip E, Speranza G, De Angelis F, Dómine M,
Cheng SY, et al: Pemetrexed plus platinum with or without
pembrolizumab in patients with previously untreated metastatic
nonsquamous NSCLC: Protocol-specified final analysis from
KEYNOTE-189. Ann Oncol. 32:881–895. 2021. View Article : Google Scholar
|
|
91
|
Montesion M, Murugesan K, Jin DX, Sharaf
R, Sanchez N, Guria A, Minker M, Li G, Fisher V, Sokol ES, et al:
Somatic HLA class I loss is a widespread mechanism of immune
evasion which refines the use of tumor mutational burden as a
biomarker of checkpoint inhibitor response. Cancer Discov.
11:282–292. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zaretsky JM, Garcia-Diaz A, Shin DS,
Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY,
Abril-Rodriguez G, Sandoval S, Barthly L, et al: Mutations
associated with acquired resistance to PD-1 blockade in melanoma. N
Engl J Med. 375:819–829. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Han P, Dai Q, Fan L, Lin H, Zhang X, Li F
and Yang X: Genome-wide CRISPR screening identifies JAK1 deficiency
as a mechanism of T-cell resistance. Front Immunol. 10:2512019.
View Article : Google Scholar
|
|
94
|
Takeuchi Y, Tanegashima T, Sato E, Irie T,
Sai A, Itahashi K, Kumagai S, Tada Y, Togashi Y, Koyama S, et al:
Highly immunogenic cancer cells require activation of the WNT
pathway for immunological escape. Sci Immunol. 6:eabc64242021.
View Article : Google Scholar
|
|
95
|
Muto S, Ozaki Y, Yamaguchi H, Watanabe M,
Okabe N, Matsumura Y, Hamada K and Suzuki H: Tumor β-catenin
expression associated with poor prognosis to anti-PD-1 antibody
monotherapy in non-small cell lung cancer. Cancer Diagn Progn.
5:32–41. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Galluzzi L, Spranger S, Fuchs E and
López-Soto A: WNT signaling in cancer immunosurveillance. Trends
Cell Biol. 29:44–65. 2019. View Article : Google Scholar
|
|
97
|
Spranger S, Bao R and Gajewski TF:
Melanoma-intrinsic β-catenin signalling prevents anti-tumour
immunity. Nature. 523:231–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Muto S, Inomata S, Yamaguchi H, Mine H,
Takagi H, Watanabe M, Ozaki Y, Inoue T, Yamaura T, Fukuhara M, et
al: β-catenin expression in non-small cell lung cancer and
therapeutic effect of immune checkpoint inhibitors. Gan To Kagaku
Ryoho. 49:947–949. 2022.PubMed/NCBI
|
|
99
|
DeNardo DG and Ruffell B: Macrophages as
regulators of tumour immunity and immunotherapy. Nat Rev Immunol.
19:369–382. 2019. View Article : Google Scholar
|
|
100
|
Kaler P, Augenlicht L and Klampfer L:
Activating mutations in β-catenin in colon cancer cells alter their
interaction with macrophages; the role of snail. PLoS One.
7:e454622012. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Yaguchi T, Goto Y, Kido K, Mochimaru H,
Sakurai T, Tsukamoto N, Kudo-Saito C, Fujita T, Sumimoto H and
Kawakami Y: Immune suppression and resistance mediated by
constitutive activation of Wnt/β-catenin signaling in human
melanoma cells. J Immunol. 189:2110–2117. 2012. View Article : Google Scholar
|
|
102
|
Pate KT, Stringari C, Sprowl-Tanio S, Wang
K, TeSlaa T, Hoverter NP, McQuade MM, Garner C, Digman MA, Teitell
MA, et al: Wnt signaling directs a metabolic program of glycolysis
and angiogenesis in colon cancer. EMBO J. 33:1454–1473. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Pavlova NN and Thompson CB: The emerging
hallmarks of cancer metabolism. Cell Metab. 23:27–47. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Lim AR, Rathmell WK and Rathmell JC: The
tumor microenvironment as a metabolic barrier to effector T cells
and immunotherapy. Elife. 9:e551852020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Nakayama S, Sng N, Carretero J, Welner R,
Hayashi Y, Yamamoto M, Tan AJ, Yamaguchi N, Yasuda H, Li D, et al:
β-catenin contributes to lung tumor development induced by EGFR
mutations. Cancer Res. 74:5891–5902. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Lilien J and Balsamo J: The regulation of
cadherin-mediated adhesion by tyrosine
phosphorylation/dephosphorylation of beta-catenin. Curr Opin Cell
Biol. 17:459–465. 2005. View Article : Google Scholar
|
|
107
|
Yang W, Xia Y, Ji H, Zheng Y, Liang J,
Huang W, Gao X, Aldape K and Lu Z: Nuclear PKM2 regulates β-catenin
transactivation upon EGFR activation. Nature. 480:118–122. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Hu T and Li C: Convergence between
Wnt-β-catenin and EGFR signaling in cancer. Mol Cancer. 9:2362010.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Arasada RR, Shilo K, Yamada T, Zhang J,
Yano S, Ghanem R, Wang W, Takeuchi S, Fukuda K, Katakami N, et al:
Notch3-dependent β-catenin signaling mediates EGFR TKI drug
persistence in EGFR mutant NSCLC. Nat Commun. 9:31982018.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Yi Y, Li P, Huang Y, Chen D, Fan S, Wang
J, Yang M, Zeng S, Deng J, Lv X, et al: P21-activated kinase
2-mediated β-catenin signaling promotes cancer stemness and
osimertinib resistance in EGFR-mutant non-small-cell lung cancer.
Oncogene. 41:4318–4329. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wang J, Zhou P, Wang X, Yu Y, Zhu G, Zheng
L, Xu Z, Li F, You Q, Yang Q, et al: Rab25 promotes erlotinib
resistance by activating the β1 integrin/AKT/β-catenin pathway in
NSCLC. Cell Prolif. 52:e125922019. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Huang JQ, Duan LX, Liu QY, Li HF, Hu AP,
Song JW, Lin C, Huang B, Yao D, Peng B, et al: Serine-arginine
protein kinase 1 (SRPK1) promotes EGFR-TKI resistance by enhancing
GSK3β Ser9 autophosphorylation independent of its kinase activity
in non-small-cell lung cancer. Oncogene. 42:1233–1246. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Tripathi SK and Biswal BK: SOX9 promotes
epidermal growth factor receptor-tyrosine kinase inhibitor
resistance via targeting β-catenin and epithelial to mesenchymal
transition in lung cancer. Life Sci. 277:1196082021. View Article : Google Scholar
|
|
114
|
Liu B, Chen D, Chen S, Saber A and Haisma
H: Transcriptional activation of cyclin D1 via HER2/HER3
contributes to EGFR-TKI resistance in lung cancer. Biochem
Pharmacol. 178:1140952020. View Article : Google Scholar
|
|
115
|
Wang G, Li T, Wan Y and Li Q: MYC
expression and fatty acid oxidation in EGFR-TKI acquired
resistance. Drug Resist Updat. 72:1010192024. View Article : Google Scholar
|
|
116
|
Yochum ZA, Cades J, Wang H, Chatterjee S,
Simons BW, O'Brien JP, Khetarpal SK, Lemtiri-Chlieh G, Myers KV,
Huang EHB, et al: Targeting the EMT transcription factor TWIST1
overcomes resistance to EGFR inhibitors in EGFR-mutant
non-small-cell lung cancer. Oncogene. 38:656–670. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Ding W, Yang P, Zhao X and Wang X, Liu H,
Su Q and Wang X, Li J, Gong Z, Zhang D and Wang X: Unraveling
EGFR-TKI resistance in lung cancer with high PD-L1 or TMB in
EGFR-sensitive mutations. Respir Res. 25:402024. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Peng S, Wang R, Zhang X, Ma Y, Zhong L, Li
K, Nishiyama A, Arai S, Yano S and Wang W: EGFR-TKI resistance
promotes immune escape in lung cancer via increased PD-L1
expression. Mol Cancer. 18:1652019. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Huang Z, Wang J, Xia Z, Lv Q, Ruan Z and
Dai Y: Wnt/β-catenin pathway-mediated PD-L1 overexpression
facilitates the resistance of non-small cell lung cancer cells to
epidermal growth factor receptor tyrosine kinase inhibitors. Discov
Med. 36:2300–2308. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Du L, Lee JH, Jiang H, Wang C, Wang S,
Zheng Z, Shao F, Xu D, Xia Y, Li J, et al: β-Catenin induces
transcriptional expression of PD-L1 to promote glioblastoma immune
evasion. J Exp Med. 217:e201911152020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Hu J, He Q, Tian T, Chang N and Qian L:
Transmission of exosomal TPX2 promotes metastasis and resistance of
NSCLC cells to docetaxel. Onco Targets Ther. 16:197–210. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Jiang Y, Hu X, Pang M, Huang Y, Ren B, He
L and Jiang L: RRM2-mediated Wnt/β-catenin signaling pathway
activation in lung adenocarcinoma: A potential prognostic
biomarker. Oncol Lett. 26:4172023. View Article : Google Scholar
|
|
123
|
Yin H, Wang X, Zhang X, Zeng Y, Xu Q, Wang
W, Zhou F and Zhou Y: UBE2T promotes radiation resistance in
non-small cell lung cancer via inducing epithelial-mesenchymal
transition and the ubiquitination-mediated FOXO1 degradation.
Cancer Lett. 494:121–131. 2020. View Article : Google Scholar
|
|
124
|
Katoh M and Katoh M: WNT signaling and
cancer stemness. Essays Biochem. 66:319–331. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Katoh M: Canonical and non-canonical WNT
signaling in cancer stem cells and their niches: Cellular
heterogeneity, omics reprogramming, targeted therapy and tumor
plasticity (Review). Int J Oncol. 51:1357–1369. 2017. View Article : Google Scholar
|
|
126
|
Katoh M and Katoh M: WNT signaling pathway
and stem cell signaling network. Clin Cancer Res. 13:4042–4045.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Katoh M and Katoh M: Molecular genetics
and targeted therapy of WNT-related human diseases (Review). Int J
Mol Med. 40:587–606. 2017.PubMed/NCBI
|
|
128
|
Jin J, Zhan P, Katoh M, Kobayashi SS, Phan
K, Qian H, Li H, Wang X, Wang X and Song Y; written on behalf of
the AME Lung Cancer Collaborative Group, : Prognostic significance
of β-catenin expression in patients with non-small cell lung
cancer: A meta-analysis. Transl Lung Cancer Res. 6:97–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Kim Y, Ahn B, Yoon S, Lee G, Kim D, Chun
SM, Kim HR, Jang SJ and Hwang HS: An oncogenic CTNNB1 mutation is
predictive of post-operative recurrence-free survival in an
EGFR-mutant lung adenocarcinoma. PLoS One. 18:e02872562023.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Taniguchi Y, Tamiya A, Osuga M, Harada D,
Isa SI, Nakamura K, Mizumori Y, Shinohara T, Yanai H, Nakatomi K,
et al: Baseline genetic abnormalities and effectiveness of
osimertinib treatment in patients with chemotherapy-naïve
EGFR-mutated NSCLC based on performance status. BMC Pulm Med.
24:4072024. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Malyla V, De Rubis G, Paudel KR,
Chellappan DK, Hansbro NG, Hansbro PM and Dua K: Berberine
nanostructures attenuate ß-catenin, a key component of epithelial
mesenchymal transition in lung adenocarcinoma. Naunyn Schmiedebergs
Arch Pharmacol. 396:3595–3603. 2023. View Article : Google Scholar
|
|
132
|
Ganesh S, Shui X, Craig KP, Park J, Wang
W, Brown BD and Abrams MT: RNAi-mediated β-catenin inhibition
promotes T cell infiltration and antitumor activity in combination
with immune checkpoint blockade. Mol Ther. 26:2567–2579. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Katagiri H, Yonezawa H, Shitamura S,
Sugawara A, Kawano T, Maemondo M and Nishiya N: A Wnt/β-catenin
signaling inhibitor, IMU1003, suppresses the emergence of
osimertinib-resistant colonies from gefitinib-resistant non-small
cell lung cancer cells. Biochem Biophys Res Commun. 645:24–29.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Tian Y, Li P, Xiao Z, Zhou J, Xue X, Jiang
N, Peng C, Wu L, Tian H, Popper H, et al: Triptolide inhibits
epithelial-mesenchymal transition phenotype through the
p70S6k/GSK3/β-catenin signaling pathway in taxol-resistant human
lung adenocarcinoma. Transl Lung Cancer Res. 10:1007–1019. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Tung CH, Wu JE, Huang MF, Wang WL, Wu YY,
Tsai YT, Hsu XR, Lin SH, Chen YL and Hong TM: Ubiquitin-specific
peptidase 5 facilitates cancer stem cell-like properties in lung
cancer by deubiquitinating β-catenin. Cancer Cell Int. 23:2072023.
View Article : Google Scholar
|
|
136
|
Luan H, Yan L, Zhao Y, Ding X and Cao L:
Fucoxanthin induces apoptosis and reverses epithelial-mesenchymal
transition via inhibiting Wnt/β-catenin pathway in lung
adenocarcinoma. Discov Oncol. 13:982022. View Article : Google Scholar
|
|
137
|
Zhang Y, Liu J, Yang G, Zou J, Tan Y, Xi
E, Geng Q and Wang Z: Asiaticoside inhibits growth and metastasis
in non-small cell lung cancer by disrupting EMT via Wnt/β-catenin
pathway. Environ Toxicol. 39:4859–4870. 2024. View Article : Google Scholar
|