Introduction
Head and neck carcinoma accounts for 4.6% of all
cancers worldwide, and there has been an annual 1.4-fold increase
in the number of new cases per year during the last decade
(1,2). Squamous cell carcinomas (SCC) comprise
the most common type of malignancy in this wide anatomical region,
which includes the oral cavity, pharynx, and larynx (3). Although tobacco smoke and alcohol
consumption are traditionally considered major risk factors for
head and neck SCC (HNSCC), viruses are also accountable (4). Two types of viruses, namely, Human
Papilloma Virus (HPV) and Epstein-Barr Virus, are strongly
associated with oropharyngeal and nasopharyngeal SCC, respectively,
since virus-associated carcinomas exhibit a predilection for sites
with lymphoid-based mucosa (3,5). In
fact, according to the latest recommendations of The College of
American Pathologists, all newly diagnosed oropharyngeal SCC should
be examined by immunohistochemistry, using p16 as a surrogate
marker for potential HPV infection (6), while HPV-related oropharyngeal cancer
is currently considered as a unique disease entity in both the
pathological (7) and clinical
(8) context. Emerging molecular
drivers such as lncRNA BLACAT1 have been implicated in adjacent
sites like hypopharyngeal SCC (9),
underscoring the need to integrate morphologic and transcriptomic
data in future studies. Epigenetic dysregulation is a common
feature across cancers; chromatin-remodeling complex
vulnerabilities may provide novel therapeutic targets in HNSCC, as
recently suggested for a different type of tumors (10).
Several studies have reported the significance of
both tumor cells and tumor microenvironment histopathological
characteristics in HNSCC. Emerging data have highlighted the
importance of phenotypic heterogeneity in these carcinomas and its
role in the development and progression of the tumors as well as in
treatment efficacy (11). In this
context, the prognostic value of traditional grading is debatable,
whereas other histopathological features, i.e. worst pattern of
invasion and intensity of lymphocytic infiltration in the invasive
margin of HNSCC, seem directly associated with patient overall
survival (OS) (12,13).
In the present study, we assessed histopathological
parameters of tumor cells and their microenvironment in tumor
compartments (center and invasive margin) of HNSCC originating in
different anatomical sites. We evaluated these parameters against
each other and against clinicopathological characteristics of the
patients to identify potential risk factors with clinical
relevance.
Materials and methods
Tissue samples and patient data
Formalin-fixed paraffin-embedded tissue samples from
patients with histologically confirmed HNSCC and available
clinical, histopathological, treatment, and outcome data were
retrieved from the Hellenic Cooperative Oncology Group (HeCOG)
clinical database and biologic material repository. Since
nasopharyngeal carcinoma is generally considered a biologically
different disease compared to SCC of the rest of the head and neck
region, especially in Far East and Mediterranean basin, we explored
this neoplasm separately on genomic, histopathological and clinical
basis (14–16) and it was excluded from this study.
In the same line, p16 positive HNSCC considered as HPV-related,
which are biologically different from other HNSCC (7), were excluded, as well. Patients with
HNSCC were diagnosed and treated between 1999 and 2018. Written
informed consent was obtained from all patients for the use of
their data and biologic material for research purposes. The
protocol was approved by the Bioethics Committee of the Aristotle
University of Thessaloniki, School of Health Sciences, Faculty of
Medicine (approval no. 5/18.12.2019; Thessaloniki, Greece).
New Hematoxylin and Eosin (H&E) sections from
all tissue blocks were reviewed for histological evaluation and
tumor tissue adequacy (tumor cells and stroma). The tumor surface
area was separated into tumor center and invasive margin according
to the literature (17,18). As such, the invasive margin was
defined as the region around the border where normal tissue meets
malignant cells, extending by 1 millimeter. Samples from advanced
HNSCC that were not obtained from the primary site (e.g.,
metastatic lymph nodes) or where a primary site could not be
determined, were annotated as ‘local spread’. Based on the
availability of clinical data and adequate tumor tissue, 248 tumors
from the same number of patients were examined. Patient age at
first diagnosis ranged from 20.9 to 85.1 years, with a median of 62
years.
Histological evaluation
Parameters assessed on whole H&E sections were:
i) Keratinization (present/absent). Tumors exhibiting squamous
maturation features on less than 10% of their surface were not
classified as keratinizing (19);
ii) area occupied by keratin pearls as percentage of the entire
tumor area; iii) presence of specific spatial distribution of
neutrophils, that is, neutrophils in tumor necrotic areas and in
close proximity to dyskeratotic cells, as well as microabscesses in
keratinization; iv) histologic grading, according to the three-tier
grading system (G1, G2, G3) (20);
v) extent of tumor necrosis, as absent, focal (≤10% of the tumor
area), moderate (11–29% of the tumor area), or extensive (≥30% of
the tumor area) (21); and the
presence of necrosis in keratinization areas. However, since only
one case exhibited extensive necrosis, it was included in the
moderate group; vi) anaplastic cells defined as cells with lack of
differentiation, showing pleomorphism, nuclear abnormalities,
atypical mitoses and loss of polarity (present/absent) (22); vii) cell cannibalism, which
corresponds to cell-in-cell phenomenon, a process of non-apoptotic
cell death, where one cancer cell surrounds another cancer cell
(present/absent) (23); viii) tumor
stroma classification, as myxoid, when there was an amorphous
stromal substance comprising of slightly basophilic extracellular
matrix; as keloid-like, regulated by specific channels (24), when thick bundles of hypocellular
collagen with bright eosinophilic hyalinization were evident; or as
fibroblastic, when only fine mature collagen fibers were evident
(25); ix) tumor infiltrating
lymphocyte (TIL) density; according to international guidelines
(17,26), ten high-power fields of each section
excluding pre-existing lymphoid background areas (17,27),
were assessed, and the mean average was estimated; and x)
perineural and lymphovascular invasion (present/absent). The
presence of calcification and giant cell reaction was also
recorded.
Eosinophil number per mm2 was evaluated
similarly to CD8+ counts on Tissue MicroArray (TMA)
sections (28).
Histological parameters in the invasive margin were
examined separately, based on previous studies (5,12,13,29):
keratinization was assessed as absent (0.5% of the invasive margin
area), low (6–20%), moderate (21–50%), and high (<50%); cellular
atypia and pleomorphism were estimated as mild, moderate, and
severe; mature squamous cells with angular shape, eosinophilic
cytoplasm, and intercellular bridges were recorded in a four-tier
scale, based on their abundance (0–25%, 26–50%, 51–75%, and
<75%); lymphocytic host response along the invasive margin was
recorded as dense (dense lymphocytic response rimming the tumor
and/or lymphoid nodules at advancing edge in each 4× field),
intermediate (intermediate lymphocytic response and/or lymphoid
nodules in some but not all 4× fields), and weak (sparse
lymphocytic response without lymphoid nodules) (13); and perineural invasion was assessed
separately in this tumor compartment. The worst pattern of
invasion, defined as the least differentiated part of the invasive
margin of tumors, included five categories, namely, pushing border,
finger-like growth, large tumor islands (>15 cells/island),
small tumor islands (<15 cells/island), and tumor satellites ≥1
mm away from the main tumor.
Immunohistochemistry
Immunohistochemistry was performed on 3-μm TMA
sections. To confirm HPV-negative status, p16INK4A mouse
monoclonal antibody (clone IHC116, GenomeMe, Lab Inc., Richmond,
BC, Canada) was applied at 1:50 dilution, on a Bond Max Autostainer
using BOND Polymer Detection kit. Antigen retrieval was performed
with EDTA (pH 9) for 40 min, followed by primary antibody
(p16INK4A) incubation at 37°C for 30 min. For immune
profiling, CD8 mouse monoclonal antibody (clone C8/144B, Dako,
Glostrup, Denmark) was used at 1:60 dilution. For antigen
retrieval, EDTA (pH 9) was used for 20 min, before primary antibody
incubation at 37°C for 30 min. Staining was performed on a Dako
autostainer using the EnVision™ FLEX+ (Agilent, Santa Clara, CA)
visualization system. PD-L1 was assessed with a mouse monoclonal
antibody (clone 22C3, Dako, Glostrup, Denmark) at 1:100 dilution on
a Dako autostainer by using the EnVision™ FLEX+ visualization
system (Agilent, Santa Clara, CA). For antigen retrieval, EDTA (pH
9) was performed for 30 min, followed by primary antibody
incubation at 37°C for 30 min. All slides were counterstained with
hematoxylin. Both positive and negative controls were
simultaneously stained.
In regard to immunohistochemical evaluation, PD-L1
expression was assessed in both tumor and immune cells, according
to the guidelines (30). For tumor
cells, PD-L1 positivity was defined as complete and/or partial
circumferential linear cellular membrane staining of any intensity,
as assessed by the tumor proportion score (TPS). Immune cells were
evaluated as the proportion of the tumor area occupied by any
discernible PD-L1 staining of any intensity and the combined
positive score (CPS) was estimated in each case (30). CPS ≥1 was considered positive.
Staining interpretation was performed by two experienced
pathologists (SET, TK). CD8+ cell counts were obtained
from the entire area of each 1.5 mm diameter core. The density of
positive cells was assessed as the ratio of CD8+ cells
per mm2 of core surface area. Average values were used
for tumors represented by multiple cores. Spatial distribution of
CD8+ T cells was assessed by comparing two tissue cores
of each tumor to determine whether cells were evenly distributed,
clustered or concentrated in specific regions or showed different
values between the cores (28). For
p16, strong and diffuse nuclear and/or cytoplasmic positivity in
≥70% of neoplastic cells was used as a cut-off according to
standard guidelines for HNSCC, with a positive result indicating
HPV-related HNSCC (31). As
aforementioned, p16 immunohistochemistry (Fig. S1) was applied to all available
HNSCC cases and HPV-related carcinomas were excluded from the
present study; accordingly, all tumors analyzed here were p16
negative.
Statistical analysis
Histological parameters, categorical and continuous,
were evaluated against clinicopathological patient and tumor
characteristics and against each other. In case of two categorical
variables χ2 and Fisher's tests were applied. In case of
comparing a continuous variable across different groups, the
Kruskal-Wallis(if more than two groups) and the Mann-Whitney (if
two groups) tests were used. In case of post hoc comparisons,
Kruskal Wallis test, followed by Mann-Whitney U test and Bonferroni
correction was applied.
For the assessment of risk factors for OS, the
log-rank test and Cox regression were applied. For multivariable
analyses, we selected risk factors with a P-value lower than 0.2,
which were further filtered by using Akaike's information criterion
for entry into the multivariable model.
The significance level for univariable analyses was
set at 5%, with P<0.05 considered to indicate a statistically
significant difference. All analyses were conducted in R (v. 4.3.1;
http://www.R.project.org/).
Results
Patient and tumor characteristics
Most patients were men, heavy smokers, and diagnosed
with stage III or IV disease. The majority underwent surgical
treatment, while 26.25% received various therapeutic modalities in
the first line setting. Based on tumor location as per
histopathology report, SCC were categorized into oral,
oropharyngeal, hypopharyngeal, and laryngeal, the two latter
categories being analyzed as one. Twenty-seven cases of local
spread specimens were incorporated in the study. Most tumor tissues
were obtained at disease diagnosis and were surgical specimens.
Seventy-nine of them included the invasive margin of the tumor. The
resection margins were free of carcinoma/high grade dysplasia. All
available clinicopathological data are presented in Table I.
 | Table I.Head and neck squamous cell carcinoma
patient demographics and clinicopathological data. |
Table I.
Head and neck squamous cell carcinoma
patient demographics and clinicopathological data.
| Characteristic | Value |
|---|
| Median age at first
diagnosis, years | 62
(54.23–70.1; |
| (IQR min-max;
range) | 20.9–85.1) |
| Sex (n=248) |
|
|
Female | 31 (12.5) |
|
Male | 217 (87.5) |
| Smoking packs
(n=225) |
|
| No | 25 (11.1) |
| 1-10
pack years | 9 (4.0) |
| 11-20
pack years | 27 (12.0) |
| >20
pack years | 164 (72.9) |
| Alcohol abuse
(n=226) |
|
| No | 68 (30.1) |
|
Mild | 53 (23.5) |
|
Moderate | 41 (18.1) |
|
Heavy | 64 (28.3) |
| Disease stage
(n=229)a |
|
| I | 25 (10.9) |
| II | 27 (11.8) |
|
III | 65 (28.4) |
| IV | 112 (48.9) |
| Adjuvant treatment
(n=242) |
|
| No | 210 (86.8) |
|
Chemo | 1 (0.4) |
| RT | 31 (12.8) |
| First line
treatment (n=240) |
|
| No | 177 (73.8) |
|
Chemo | 38 (15.8) |
|
CT-RT | 7 (2.9) |
| RT | 18 (7.5) |
| Death (n=246) |
|
| No | 59 (24.0) |
|
Yes | 187 (76.0) |
| Tumor site
(n=248) |
|
|
Oral | 35 (14.1) |
|
Oropharyngeal | 30 (12.1) |
|
Laryngohypopharyngeal | 156 (62.9) |
| Local
spreadb | 27 (10.9) |
| Sample status
(n=242) |
|
| First
diagnosis | 202 (83.5) |
|
Pretreatedc | 23 (9.5) |
|
Relapse, naïved | 17 (7.0) |
| Type of specimen
(n=248) |
|
|
Biopsy | 78 (31.5) |
|
Surgical | 170 (68.5) |
Phenotype characteristics
Detailed histopathological and immunophenotypic
characteristics of the examined HNSCC are shown in Table II.
 | Table II.Histopathological and
immunohistochemical characteristics of head and neck squamous cell
carcinoma cases. |
Table II.
Histopathological and
immunohistochemical characteristics of head and neck squamous cell
carcinoma cases.
| Characteristic | Value |
|---|
| Grade (n=248) |
|
| G1 | 42 (16.9) |
| G2 | 155 (62.5) |
| G3 | 51 (20.6) |
| Grade heterogeneity
(n=248) | 24 (9.7) |
| Tumor variant
(n=248) |
|
|
Keratinizing | 196 (79.0) |
| Non
keratinizing | 52 (21.0) |
| Anaplastic features
(n=248) | 138 (55.6) |
| Cell cannibalism
(n=248) | 139 (56.0) |
| Desmoplastic
stromal reaction (n=222) |
|
|
Fibroblastic | 134 (60.4) |
|
Keloid-like | 27 (12.1) |
|
Loose/myxoid | 61 (27.5) |
| Stromal reaction
heterogeneity (n=222) | 37 (16.7) |
|
Necrosisa (n=248) |
|
|
Absent | 220 (88.7) |
|
Focal | 21 (8.5) |
|
Moderate-extensive | 7 (2.8) |
| Microabscesses
(n=248) | 13 (5.2) |
| Necrosis in
keratinization (n=248) | 68 (27.4) |
| Neutrophils in
necrosis (n=248) | 14 (5.6) |
| Neutrophils in
dyskeratosis (n=248) | 126 (50.8) |
| Perineural
invasionb (n=248) | 12 (4.8) |
| Lymphovascular
invasion (n=248) | 4 (1.6) |
| CD8 heterogeneity
(n=159) | 41 (25.8) |
| PD-L1 status
(n=215) |
|
|
<1 | 190 (88.4) |
|
1-20 | 22 (10.2) |
|
≥20 | 3 (1.4) |
| PD-L1 heterogeneity
(n=215) | 12 (5.6) |
| Lymphocytic host
response in IM (n=79) |
|
|
Weak | 28 (35.4) |
|
Intermediate | 27 (34.2) |
|
Dense | 24 (30.4) |
| Perineural invasion
in IM (n=79) | 9 (11.4) |
| Multinucleated
macrophages (n=248) | 29 (11.7) |
| Median keratin
pearlsa (n=248)
[IQR] | 0 [0, 5] |
| Median
eosinophils/mm2 (n=214) [IQR] | 0.314 [0,
3.88] |
| Median TIL Salgado
density (n=242) | 20.75 |
| [IQR] | [11.15, 32.35] |
| Median
CD8+/mm2 (n=215) [IQR] | 198.05 |
|
| [98.45,
420.37] |
The most prevalent tumor variant was keratinizing of
intermediate grade (G2) with anaplastic features and cell
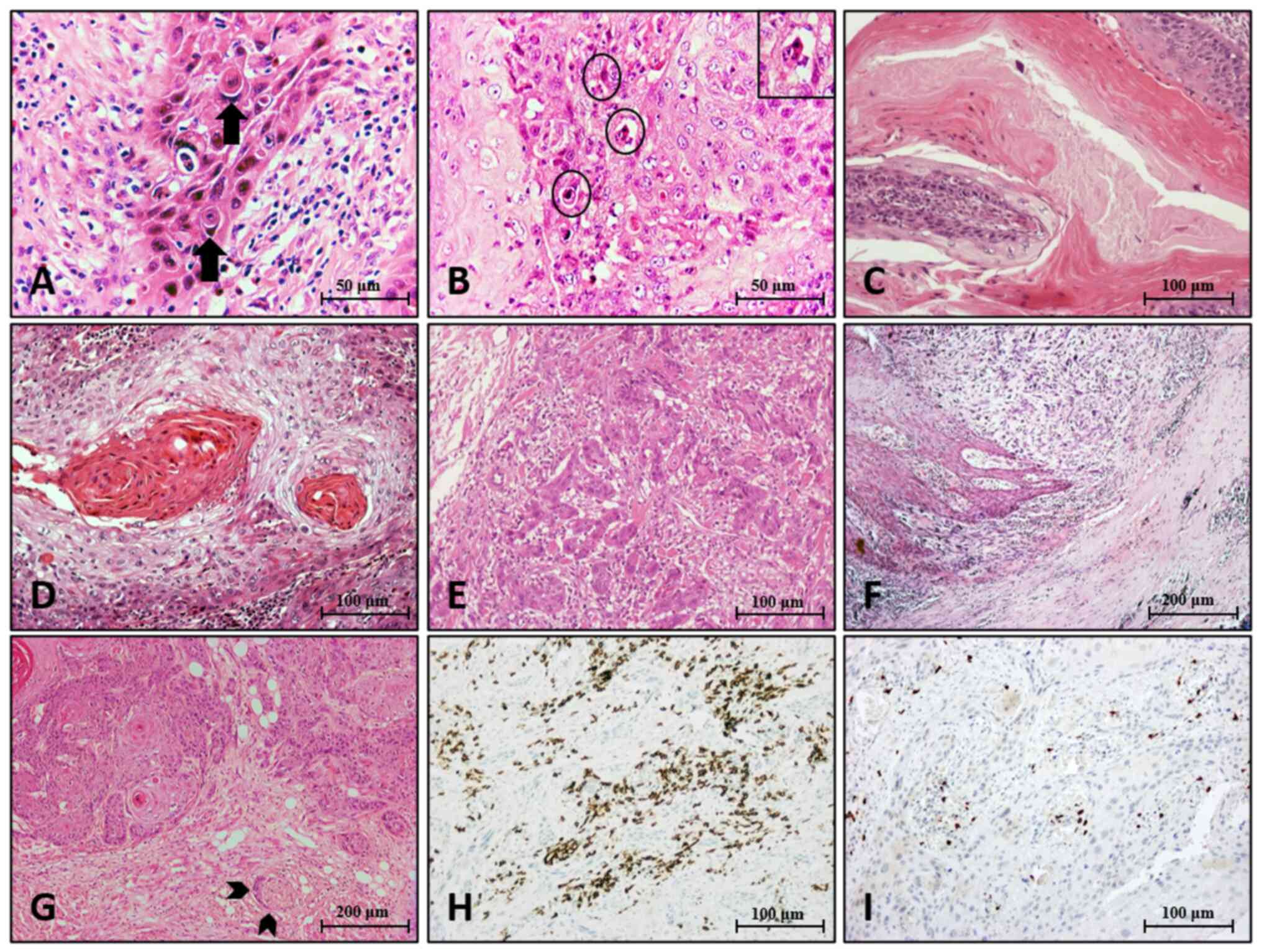
cannibalism (Fig. 1A). Among the
laryngeal SCC, two were designated as basaloid SCC variants. Two
additional basaloid SCC were found; one located in the oral cavity
and one in the oropharynx. Neutrophils in dyskeratosis (Fig. 1B) were quite common, whereas keratin
pearls in ≥5% of the tumor area, microabscesses, or necrosis in
keratinization (Fig. 1C) were
observed in a minority of tumors. Perineural and lymphovascular
invasion were rare. Grade (Fig. 1D and
E) heterogeneity was observed in a considerable number of
tumors. Specifically, while most of them presented with
intermediate grade (G2), ‘miniscule’ areas with features favoring
either low (G1) or high (G3) grade frequently coexisted. In all
cases with grading heterogeneity, the final grade was assigned
based on the worst component, if it comprised more than 5%.
Accordingly, stromal desmoplastic reaction presented with a variety
of features, from loose myxoid to dense collagenous, even within
the same tumor, in respect of stromal heterogeneity. In such cases,
stroma was characterized based on the predominant pattern.
The infiltrative margin in 63 (79.7%) out of 79
evaluable tumors was characterized by the absence of
keratinization. In the same tumor area, heterogeneous worst pattern
of invasion was identified in 32/79 cases (40.5%) (Fig. 1F); all degrees of lymphocytic host
response density were equally observed, while perineural invasion
(Fig. 1G) was noticed in 11.4% of
the tumors.
With respect to immune cell infiltrates, 10.3% of
tumors exhibited eosinophils >14.2/mm2, while 15% of
tumors had TIL density >50% and/or CD8+
>700/mm2. CD8+ infiltrates were
heterogeneously present in 25.8% of the tumors (Fig. 1H and I). Only 11.6% exhibited any
degree of PD-L1 positivity (Fig.
S1).
Site specificity of generic
histopathological characteristics
Histological characteristics significantly differed
among HNSCC from different anatomical areas, namely,
laryngohypopharyngeal, oropharyngeal, and oral carcinomas, as shown
in Table III. Regarding some of
these characteristics, the majority of laryngohypopharyngeal tumors
exhibited anaplastic features and cell cannibalism. Oropharyngeal
tumors featured higher grade and higher rates of tumor necrosis,
while being devoid of eosinophils. In contrast, eosinophils,
keratin pearls, and perineural invasion were more common in oral
carcinomas. In addition, keratinization was prevalent among
laryngohypopharyngeal tumors (P<0.001). Regarding the invasive
margin (Table III), perineural
invasion appeared more frequently in oral and oropharyngeal
tumors.
 | Table III.Associations between
clinicopathological variables and tumor site. |
Table III.
Associations between
clinicopathological variables and tumor site.
| Parameter | Oral | Oropharyngeal |
Laryngohypopharyngeal | P-value |
|---|
| Smoking packs, n
(%) | | | | 0.009 |
| No | 6 (24%) | 2 (8.3%) | 17 (11.1%) |
|
|
1-10 | 4 (16%) | 2 (8.3%) | 3 (2%) |
|
|
11-20 | 4 (16%) | 2 (8.3%) | 20 (13.1%) |
|
|
>20 | 11 (44%) | 18 (75.1%) | 113 (73.8%) |
|
| Disease stage |
|
|
| 0.009 |
| I | 8 (28.6%) | 0 (0%) | 15 (9.9%) |
|
| II | 4 (14.3%) | 4 (16%) | 19 (12.5%) |
|
|
III | 3 (10.7%) | 6 (24%) | 53 (34.9%) |
|
| IV | 13 (46.4%) | 15 (60%) | 65 (42.7%) |
|
| Grade |
|
|
| 0.002 |
| G1 | 12 (34.3%) | 4 (13.3%) | 23 (14.7%) |
|
| G2 | 21 (60%) | 15 (50%) | 106 (68%) |
|
| G3 | 2 (5.7%) | 11 (36.7%) | 27 (17.3%) |
|
| Necrosis in
keratinization |
|
|
| <0.001 |
|
Absent | 34 (97.1%) | 27 (90%) | 97 (62.2%) |
|
|
Present | 1 (2.9%) | 3 (10%) | 59 (37.8%) |
|
| Anaplastic
features |
|
|
| 0.006 |
|
Absent | 21 (60%) | 17 (56.7%) | 55 (35.3%) |
|
|
Present | 14 (40%) | 13 (43.3%) | 101 (64.7%) |
|
| Cell
cannibalism |
|
|
| <0.001 |
|
Absent | 25 (71.4%) | 18 (60%) | 52 (33.3%) |
|
|
Present | 10 (28.6%) | 12 (40%) | 104 (66.7%) |
|
| Perineural invasion
(entire tumor area) |
|
|
| 0.004 |
|
Absent | 29 (82.9%) | 28 (93.3%) | 152 (97.4%) |
|
|
Present | 6 (17.1%) | 2 (6.7%) | 4 (2.6%) |
|
| Perineural invasion
(invasive margin) |
|
|
| 0.004 |
|
Absent | 11 (64.7%) | 0 (0%) | 59 (96.7%) |
|
|
Present | 6 (35.3%) | 1 (100%) | 2 (3.3%) |
|
| Tumor variant and
cell cannibalism status |
|
|
| <0.001 |
|
Keratinizing without cell
cannibalism | 20 (26.3%) | 13 (17.1%) | 43 (56.6%) |
|
| Non
keratinizing without cell cannibalism | 5 (26.3%) | 5 (26.3%) | 9 (47.4%) |
|
|
Keratinizing with cell
cannibalism | 9 (8.8%) | 7 (6.9%) | 86 (84.3%) |
|
| Non
keratinizing with cell cannibalism | 1 (4.2%) | 5 (20.8%) | 18 (75%) |
|
| Median keratin
pearls, % of tumor surface (IQR) | 6.31 (8.64) | 3.13 (6.33) | 5.81 (12.92) | 0.034 |
| Median TIL density,
% (IQR) | 22.53 (12.39) | 30.26 (20.71) | 23 (16.3) | 0.265 |
| Median
CD8+/mm2 (IQR) | 395.63
(365.59) | 305.74
(269.88) | 273.58
(281.18) | 0.123 |
| Median
eosinophils/mm2 (IQR) | 15.99 (29.16) | 2.57 (5.09) | 13.51 (74.71) | 0.003 |
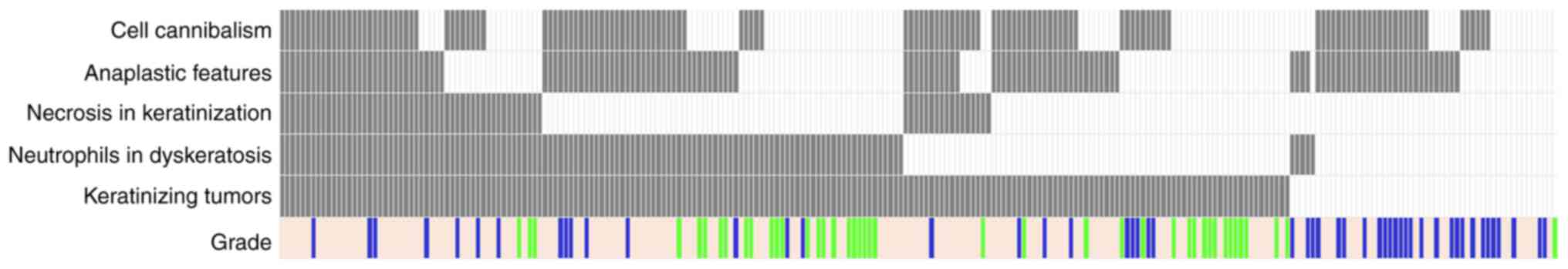
Next, we profiled histological parameters for all
tumors (Fig. 2). By definition,
parameters related to keratinization were identified at
significantly higher rates in keratinizing tumors (grade and
necrosis in keratinization, neutrophils in dyskeratosis; all
P-values <0.001). In comparison, anaplastic features, cell
cannibalism and perineural invasion were almost equally distributed
among tumors with and without keratinization. Modeling parameters
related and not related to keratinization revealed a site-specific
association; that is, concomitant keratinization and cell
cannibalism were rare among oral and oropharyngeal tumors
(P<0.001) (Table III). With
respect to clinical parameters, laryngohypopharyngeal and
oropharyngeal carcinomas were more frequent in heavy smokers
(P=0.009) and in patients with stage III and IV disease (P=0.009).
No further clinicopathological associations were observed.
No associations with tumor site were found for worst
pattern of invasion and lymphocytic host response in the invasive
margin. Similarly, there were no associations for tumor variant,
tumor necrosis, neutrophils in necrosis or in dyskeratosis,
microabscesses, stromal reaction, lymphovascular invasion,
multinucleated macrophages, CD8 and PD-L1 status.
Associations between
clinicopathological parameters and survival
All parameters were examined for their association
with OS at a follow-up period of 26 years (24/4/1997-30/11/2023).
The results of a univariable analysis are presented in Table IV.
 | Table IV.Univariable analysis with respect to
OS for the parameters studied in the entire cohort and in the
invasive margin. |
Table IV.
Univariable analysis with respect to
OS for the parameters studied in the entire cohort and in the
invasive margin.
| A, Entire
cohort |
|---|
|
|---|
| Parameter | Cases | Events | HR | 95% CI | P-value |
|---|
| Sex |
|
|
|
| 0.102 |
|
Female | 30 | 17 | 1 | - |
|
|
Male | 216 | 164 | 1.51 | (0.92, 2.49) |
|
| Smoking |
|
|
|
| 0.264 |
| No | 25 | 20 | 1 | - |
|
|
Yes | 200 | 147 | 0.76 | (0.47, 1.23) |
|
| Smoking pack years;
only smokers |
|
| 1.03 | (0.88, 1.22) | 0.077 |
| Alcohol abuse |
|
|
|
| <0.001 |
| No | 68 | 44 | 1 | - |
|
|
Mild | 53 | 34 | 0.93 | (0.59, 1.46) |
|
|
Moderate | 41 | 31 | 1.44 | (0.91, 2.28) |
|
|
Heavy | 64 | 58 | 2.60 | (1.73, 3.89) |
|
| Disease stage |
|
|
|
| <0.001 |
| I | 25 | 13 | 1 | - |
|
| II | 25 | 17 | 1.01 | (0.49, 2.11) |
|
|
III | 65 | 38 | 0.83 | (0.44, 1.56) |
|
| IV | 112 | 99 | 2.46 | (1.38, 4.4) |
|
| Tumor site |
|
|
|
| <0.001 |
|
Oral | 33 | 25 | 1.57 | (1.01, 2.43) |
|
|
Oropharyngeal | 30 | 21 | 1.39 | (0.87, 2.23) |
|
|
Laryngohypopharyngeal | 156 | 112 | 1 | - |
|
| Local
spread | 27 | 23 | 2.38 | (1.51, 3.75) |
|
| Tumor variant |
|
|
|
| 0.616 |
| Non
keratinizing | 52 | 35 | 1 | - |
|
|
Keratinizing | 194 | 146 | 1.1 | (0.76, 1.59) |
|
| Microabscesses |
|
|
|
| 0.433 |
|
Absent | 233 | 173 | 1 | - |
|
|
Present | 13 | 8 | 0.75 | (0.37, 1.53) |
|
| Grade |
|
|
|
| 0.567 |
| G1 | 41 | 27 | 1 | - |
|
| G2 | 154 | 119 | 1.22 | (0.8, 1.85) |
|
| G3 | 51 | 35 | 1.3 | (0.78, 2.15) |
|
| Necrosis in
keratinization |
|
|
|
| 0.838 |
|
Absent | 178 | 128 | 1 | - |
|
|
Present | 68 | 53 | 1.03 | (0.75, 1.43) |
|
| Neutrophils in
dyskeratosis |
|
|
|
| 0.507 |
|
Absent | 120 | 85 | 1 | - |
|
|
Present | 126 | 96 | 1.10 | (0.82, 1.48) |
|
| Tumor necrosis (%
of tumor surface) |
|
|
|
| 0.147 |
|
Absent | 218 | 158 | 1 | - |
|
|
Focal | 21 | 16 | 1.09 | (0.65, 1.83) |
|
|
Moderate-extensive | 7 | 7 | 2.11 | (0.98, 4.55) |
|
| Neutrophils in
necrosis |
|
|
|
| 0.992 |
|
Absent | 232 | 170 | 1 | - |
|
|
Present | 14 | 11 | 1.00 | (0.54, 1.85) |
|
| Anaplastic
features |
|
|
|
| 0.804 |
|
Absent | 109 | 81 | 1 | - |
|
|
Present | 137 | 100 | 0.96 | (0.72, 1.29) |
|
| Cell
cannibalism |
|
|
|
| 0.495 |
|
Absent | 108 | 79 | 1 | - |
|
|
Present | 138 | 102 | 0.90 | (0.67, 1.21) |
|
| Perineural
invasion |
|
|
|
| 0.095 |
|
Absent | 70 | 47 | 1 | - |
|
|
Present | 8 | 7 | 1.71 | (0.9, 3.25) |
|
| Multinucleated
macrophages |
|
|
|
| 0.062 |
|
Absent | 217 | 164 | 1 | - |
|
|
Present | 29 | 17 | 0.62 | (0.37, 1.03) |
|
| Age at 1st
diagnosis | 246 | 181 | 1.01 | (1, 1.02) | 0.127 |
| Keratin pearls (%
of tumor surface) | 246 | 181 | 1.00 | (0.99, 1.02) | 0.459 |
| TIL density
(%) | 240 | 177 | 0.99 | (0.99, 1) | 0.279 |
|
Eosinophils/mm2 average | 212 | 154 | 1.00 | (1, 1) | 0.323 |
| CD8+/
mm2 average | 214 | 157 | 1.00 | (1, 1) | 0.184 |
| CD8
heterogeneity |
|
|
|
| 0.030 |
|
Homogeneous | 118 | 90 | 1 | - |
|
|
Heterogeneous | 40 | 25 | 0.61 | (0.39, 0.96) |
|
|
| B, Invasive
margin |
|
|
Parameter | Cases | Events | HR | 95% CI | P-value |
|
| Lymphocytic host
response | 78 | 54 | 0.56 | (0.39, 0.81) | 0.002 |
| Perineural invasion
present | 8 | 7 | 2.52 | (1.12, 5.66) | 0.025 |
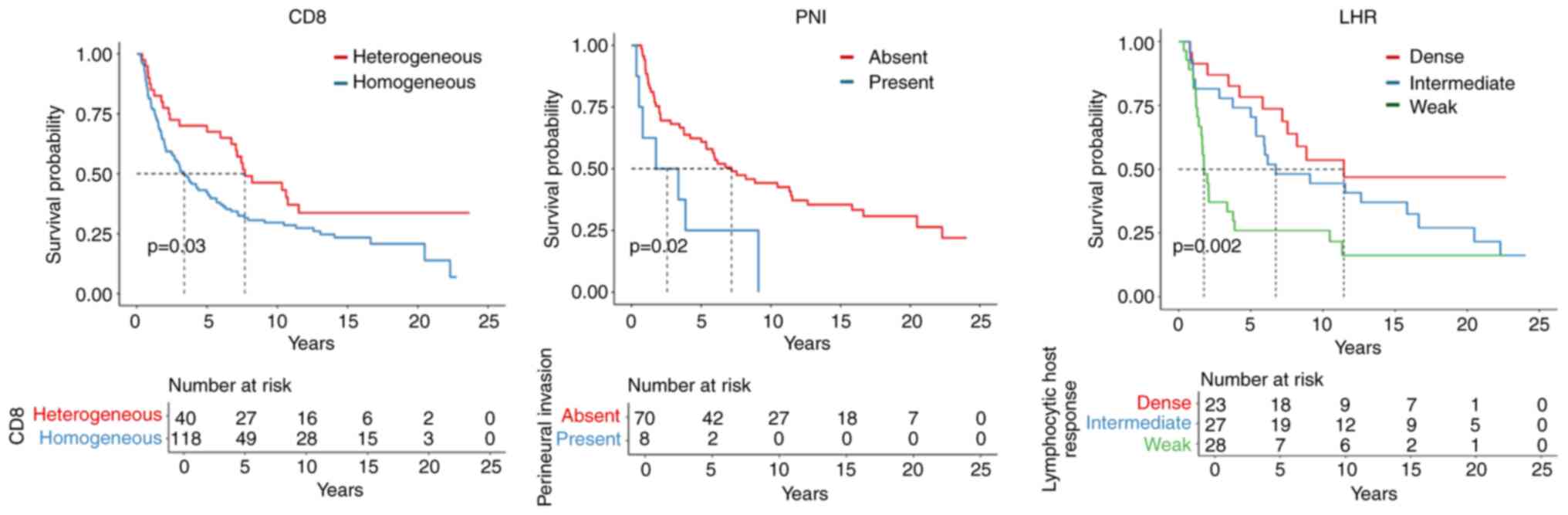
Alcohol consumption and disease stage had
significant impacts on patient OS. CD8+ heterogeneity
(P=0.038) was associated with a favorable prognosis, whereas
perineural invasion in the invasive margin (P=0.025) appeared to be
an adverse prognosticator. In addition, weak lymphocytic
infiltrates in the invasive margin had a negative impact on OS
(Fig. 3).
A subgroup analysis was conducted by separating
samples into three distinct groups based on the primary tumor site:
oral cavity, oropharynx, and laryngohypopharynx. This additional
analysis showed that heavy alcohol abuse and advanced disease stage
were significantly associated with worse OS in
laryngohypopharyngeal tumors (P<0.001). Furthermore, within the
same tumor site, perineural invasion (entire cohort and invasive
margin, P=0.007) and weak lymphocytic host response in the invasive
margin (P=0.028) were also significantly linked to poorer OS. In
oral cavity SCC, weak lymphocytic host response in the invasive
margin (P=0.052) was associated with an adverse prognosis. Detailed
results of these findings are presented in Table SI, Table SII, Table SIII and prognostic histological
features by site are illustrated in Fig. S2.
A multivariable analysis (Table V) revealed that alcohol abuse,
disease stage, and perineural invasion were independently
associated with unfavorable OS. In the invasive margin, weak
lymphocytic infiltrates and perineural invasion remained
independent predictors for unfavorable OS. No additional
associations between the clinicopathological parameters studied and
OS were observed. Based on these results, we recommend
histopathological parameters that should be included, among others,
in the histological reports, in each case of HNSCC (Table VI).
 | Table V.Multivariable analysis with respect
to OS for the parameters studied in the entire cohort and in the
invasive margin. |
Table V.
Multivariable analysis with respect
to OS for the parameters studied in the entire cohort and in the
invasive margin.
| A, Entire
cohort |
|---|
|
|---|
| Parameter | Cases | Events | HR | 95% CI | P-value |
|---|
| Alcohol abuse | 226 | 167 | 1.27 | (0.95, 1.7) | 0.105 |
| Disease stage | 227 | 168 | 1.67 | (1.11, 2.51) | 0.013 |
| Perineural invasion
present | 8 | 7 | 2.81 | (1.05, 7.49) | 0.039 |
|
| B, Invasive
margin |
|
|
Parameter | Cases | Events | HR | 95% CI | P-value |
|
| Lymphocytic host
response | 80 | 56 | 0.58 | (0.4, 0.83) | 0.003 |
| Perineural invasion
present | 8 | 7 | 2.2 | (0.97, 4.98) | 0.060 |
 | Table VI.Key histopathological parameters
recommended for routine reporting regardless of head and neck
squamous cell carcinoma anatomic site. |
Table VI.
Key histopathological parameters
recommended for routine reporting regardless of head and neck
squamous cell carcinoma anatomic site.
| Category | Parameter |
Assessment/reporting |
|---|
| Entire tumour | Histological
typea | Conventional
SCC/other (specify) |
|
| Perineural
invasiona | Present/absent |
|
| TIL density | % of all stromal
mononuclear cells (including lymphocytes and plasma cells) |
| Invasive front | Lymphocytic host
response |
Weak/moderate/dense |
|
| Perineural
invasion | Present/absent |
|
Immunohistochemistry | p16
immunohistochemistrya |
Positive/negative |
|
| PD-L1
immunohistochemistry | CPS |
Discussion
Grading is one of the primary prognostic factors in
malignant neoplasms; however, it does not seem to be an accurate
prognosticator for HNSCC, possibly due to the heterogeneity of
cytomorphological and architectural features in a pattern
previously described as ‘hybrid SCC variant’ (19). Accordingly, we identified limited
areas of keratinization in non-keratinizing tumors and anaplastic
cells in otherwise well-differentiated carcinomas. Our observation
of heterogeneity beyond neoplastic cells in microenvironmental
components, such as stroma and TILs/CD8+ cells, supports
the view of HNSCC as heterogeneous ecosystems.
The assessment of routinely reported histological
features may assist in developing an optimal prognostic tool in
line with the ongoing effort to establish multifactorial grading
systems (5,32). However, we first need to identify
which of the histological parameters are risk factors and whether
they are site-specific. In this study, as expected, keratinizing
tumors exhibited features related to keratinization, and
non-keratinizing tumors were mainly characterized as high-grade.
Anaplastic features, cell cannibalism, and perineural invasion were
observed independent of tumor variant. Comparisons between the SCC
of different anatomic locations revealed site-specific
characteristics. In oral SCC, high grade was not prevalent, and a
common feature was perineural invasion, as previously reported
(33). Eosinophil density was
higher, as well (34). Necrosis in
keratinization, anaplastic cells, and cell cannibalism were mainly
present in laryngohypopharyngeal SCC. Interestingly, cell
cannibalism and anaplastic features were relatively common in
laryngohypopharyngeal SCC, even in low-grade tumors, which is in
agreement with previous reports (23). Of note, the literature contains no
similar comparative morphological studies based on the HNSCC
anatomical site and the observed differences could be used in
developing site-specific prognostic profiling tools.
In addition, along the invasive margin, perineural
invasion and weak lymphocytic host response were adverse
prognosticators independently of the anatomical site. Hence, both
parameters should be included in all HNSCC histopathological
reporting guidelines, not only in oral carcinomas. Perineural
invasion proved to be an independent prognosticator in this study,
regardless of nerve diameter and intratumoral localization, in line
with previous publications (35,36).
There are ongoing studies focusing on the spatial
heterogeneity of the immune cells found in the tumor
microenvironment with potential therapeutic implications (37–40).
The observed heterogeneity of CD8+ T-cell distribution
has been previously mentioned in HNSCC (41,42)
without any reference to its prognostic relevance. The association
of heterogeneous CD8+ infiltrates with OS described in
this paper seems worth further investigation in terms of its
therapeutic implications, particularly with respect to immune
checkpoint inhibitors. In our series, PD-L1 evaluation showed no
significant association with OS, a finding in agreement with
previously published data (43). It
is emphasized, however, that only a minority of our cases showed
PD-L1 positivity, in contrast to former studies (44), raising concerns about its accurate
evaluation on TMAs or small biopsy specimens (45).
Recent AI pipelines that fuse histology with genomic
data have already achieved prognostic accuracy in other solid
tumors (46) and illustrate the
potential for similar multimodal models in HNSCC. Digital pathology
systems leverage image analysis algorithms and machine learning to
identify and quantify microscopic features with greater precision
than the standard evaluation (47).
Algorithms can be trained to recognize characteristic patterns of
LHR-IM and PNI by analyzing H&E-stained slides and even to
identify subtle changes in histology, possibly missed by
pathologists (48) or to determine
the clinical significance of spatial distribution of TILs (49). Machine learning models can be
trained on annotated datasets of HNSCC, regarding perineural
invasion, the number of nerves, the size of nerves or the different
patterns of LHR-IM (48,49). In addition, by using these tools,
the interobserver variability is reduced on morphological and
immunohistochemical grounds (50),
which is crucial with predictive biomarker evaluation, such as
PD-L1 CPS. In this context, the performance of these models may
exceed that of experienced pathologists.
One of the limitations of this study is the
inclusion of both biopsy and surgical material, as well as the
accurate representation of histologic characteristics in small
biopsy samples. Biopsy material was more common in oropharyngeal
tumors. In addition, histological parameters were mainly examined
in whole sections, whereas immunohistochemical parameters were
examined only on TMA sections. Further, site-specific subgroup
analysis for single or profiled parameters in terms of OS was not
possible due to the imbalanced respective group sizes. Male
predominance may affect the conclusions of this study due to
sex-related biological differences. Gender-specific
histopathological parameters with potential clinical value weren't
investigated and future studies examining sex as a potential
modifier of prognostic outcomes in HNSCC are needed. Finally, the
lack of HPV-positive tumors should be regarded as an additional
limitation.
In conclusion, based on our findings, it seems that
the malignant potential of HNSCC is reflected in several tumor
biology-related parameters, such as CD8+ spatial
heterogeneity, tumor center and/or invasive margin perineural
invasion and LHR-IM. Furthermore, perineural invasion and LHR-IM
are confirmed as independent histological risk factors in HNSCC.
Integrating the above parameters in pathology reports may provide
more accurate prognostic information for patients with HNSCC than
the routinely assessed histologic grade. The herein presented
site-specific and tumor compartment histological parameters may be
included in larger studies with deep learning approaches, in order
to enable revising histologic grade in HNSCC in a clinically
meaningful way, as required in the context of personalized
diagnostics.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Mrs. Maria Moschoni
(data coordinator of HeCOG) for administrative support.
Funding
The study is part of the NCR-17-12885 project funded by Astra
Zeneca and conducted by HeCOG. It was also partially supported by a
Hellenic Society of Medical Oncology (HeSMO) grant (grant no.
HE_TR5/25). The funders had no role in study design, data
collection and analysis, decision to publish or preparation of the
manuscript.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SET, VK, GK, PH, GF and TK conceptualized and
designed the study, performed the formal analysis and wrote the
original draft; KM, SP, KV, SC, AP, PH and GF collected and
reviewed clinical and histopathological data. GF and SC confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients for the use of their data and biological material for
research purposes. The protocol was approved by the Bioethics
Committee of the Aristotle University of Thessaloniki, School of
Health Sciences, Faculty of Medicine (approval no.
5/18.12.2019).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HeCOG
|
Hellenic Cooperative Oncology
Group
|
|
H&E
|
hematoxylin & eosin
|
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
HPV
|
human papilloma virus
|
|
IM
|
invasive margin
|
|
IQR
|
interquartile range
|
|
LHR
|
lymphocytic host response
|
|
OS
|
overall survival
|
|
PNI
|
perineural invasion
|
|
SCC
|
squamous cell carcinoma
|
|
TIL
|
tumor infiltrating lymphocyte
|
|
TMA
|
tissue microarray
|
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Ervik M, Lam F, Colombet M, Mery
L, Pineros M, Znaor A, Soerjomataram I and Bray F: Global cancer
observatory: Cancer today. International Agency for Research on
Cancer. (Lyon, France). 2020.
|
|
3
|
Johnson DE, Burtness B, Leemans CR, Lui
VWY, Bauman JE and Grandis JR: Head and neck squamous cell
carcinoma. Nat Rev Dis Primers. 6:922020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aupérin A: Epidemiology of head and neck
cancers: An update. Curr Opin Oncol. 32:178–186. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El-Naggar AK, Chan JKC, Grandis JR, Takata
T and Slootweg PJ: WHO classification of head and neck tumours.
International Agency for Research on Cancer; 2017
|
|
6
|
Lewis JS Jr, Beadle B, Bishop JA, Chernock
RD, Colasacco C, Lacchetti C, Moncur JT, Rocco JW, Schwartz MR,
Seethala RR, et al: Human papillomavirus testing in head and neck
carcinomas: Guideline from the college of american pathologists.
Arch Pathol Lab Med. 142:559–597. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
World Health Organization (WHO), . WHO
Classification of Head and Neck Tumors. 2023.
|
|
8
|
National Comprehensive Cancer Network
(NCCN), . NCCN Clinical Practice Guidelines in Oncology: Head and
Neck Cancers. 2025.
|
|
9
|
Liu FL, Zhang ZC, Zhou SL, Liu XL and Xu
W: Unlocking the therapeutic potential of LncRNA BLACAT1 in
hypopharynx squamous cell carcinoma. Discov Med. 36:546–558. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Z, Zhao Z, Xie L, Xiao Z, Li M, Li Y
and Luo T: Proteomic analysis reveals chromatin remodeling as a
potential therapeutical target in neuroblastoma. J Transl Med.
23:2342025. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Elmusrati A, Wang J and Wang CY: Tumor
microenvironment and immune evasion in head and neck squamous cell
carcinoma. Int J Oral Sci. 13:242021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bryne M, Jenssen N and Boysen M:
Histological grading in the deep invasive front of T1 and T2
glottic squamous cell carcinomas has high prognostic value. Virch
Arch. 427:277–281. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brandwein-Gensler M, Smith RV, Wang B,
Penner C, Theilken A, Broughel D, Schiff B, Owen RP, Smith J, Sarta
C, et al: Validation of the histologic risk model in a new cohort
of patients with head and neck squamous cell carcinoma. Am J Surg
Pathol. 34:676–688. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fountzilas G, Ciuleanu E, Bobos M,
Kalogera-Fountzila A, Eleftheraki AG, Karayannopoulou G,
Zaramboukas T, Nikolaou A, Markou K, Resiga L, et al: Induction
chemotherapy followed by concomitant radiotherapy and weekly
cisplatin versus the same concomitant chemoradiotherapy in patients
with nasopharyngeal carcinoma: A randomized phase II study
conducted by the hellenic cooperative oncology group (HeCOG) with
biomarker evaluation. Ann Oncol. 23:427–435. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krikelis D, Bobos M, Karayannopoulou G,
Resiga L, Chrysafi S, Samantas E, Andreopoulos D, Vassiliou V,
Ciuleanu E and Fountzilas G: Expression profiling of 21
biomolecules in locally advanced nasopharyngeal carcinomas of
Caucasian patients. BMC Clin Pathol. 13:12013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fountzilas G, Psyrri A, Giannoulatou E,
Tikas I, Manousou K, Rontogianni D, Ciuleanu E, Ciuleanu T, Resiga
L, Zaramboukas T, et al: Prevalent somatic BRCA1 mutations shape
clinically relevant genomic patterns of nasopharyngeal carcinoma in
Southeast Europe. Int J Cancer. 142:66–80. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hendry S, Salgado R, Gevaert T, Russell
PA, John T, Thapa B, Christie M, van de Vijver K, Estrada MV,
Gonzalez-Ericsson PI, et al: Assessing tumor-infiltrating
lymphocytes in solid tumors: A practical review for pathologists
and proposal for a standardized method from the international
immunooncology biomarkers working group: Part 1: Assessing the host
immune response, TILs in invasive breast carcinoma and ductal
carcinoma in situ, metastatic tumor deposits and areas for further
research. Adv Anat Pathol. 24:235–251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang D, He W, Wu C, Tan Y, He Y, Xu B,
Chen L, Li Q and Jiang J: Scoring system for tumor-infiltrating
lymphocytes and its prognostic value for gastric cancer. Front
Immunol. 10:712019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chernock RD: Morphologic features of
conventional squamous cell carcinoma of the oropharynx:
‘Keratinizing’ and ‘nonkeratinizing’ histologic types as the basis
for a consistent classification system. Head Neck Pathol. 6 (Suppl
1):S41–S47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amin MB, Edge SB, Greene FL, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC Cancer Staging Manual. 8th Edition.
Springer International Publishing; Cham, Switzerland: pp. 91–156.
2018
|
|
21
|
Pollheimer MJ, Kornprat P, Lindtner RA,
Harbaum L, Schlemmer A, Rehak P and Langner C: Tumor necrosis is a
new promising prognostic factor in colorectal cancer. Human Pathol.
41:1749–1757. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kumar V, Abbas AK, Aster JC and Perkins
JA: Robbins basic pathology. 10th Edition. Elsevier; Amsterdam,
Netherlands: pp. 1932017
|
|
23
|
Almangush A, Mäkitie AA, Hagström J,
Haglund C, Kowalski LP, Nieminen P, Coletta RD, Salo T and Leivo I:
Cell-in-cell phenomenon associates with aggressive characteristics
and cancer-related mortality in early oral tongue cancer. BMC
Cancer. 20:8432020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang Z, Chen Z, Xu Y, Li H, Li Y, Peng L,
Shan H, Liu X, Wu H, Wu L, et al: Low-frequency ultrasound
sensitive piezo1 channels regulate keloid-related characteristics
of fibroblasts. Adv Sci. 11:e23054892024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ueno H, Kanemitsu Y, Sekine S, Ishiguro M,
Ito E, Hashiguchi Y, Kondo F, Shimazaki H, Mochizuki S, Kajiwara Y,
et al: Desmoplastic pattern at the tumor front defines
poor-prognosis subtypes of colorectal cancer. Am J Surg Pathol.
41:1506–1512. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Salgado R, Denkert C, Demaria S, Sirtaine
N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL,
Penault-Llorca F, et al: The evaluation of tumor-infiltrating
lymphocytes (TILs) in breast cancer: Recommendations by an
international TILs working group 2014. Ann Oncol. 26:259–271. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hendry S, Salgado R, Gevaert T, Russell
PA, John T, Thapa B, Christie M, van de Vijver K, Estrada MV,
Gonzalez-Ericsson PI, et al: Assessing tumor-infiltrating
lymphocytes in solid tumors: A practical review for pathologists
and proposal for a standardized method from the international
immuno-oncology biomarkers working group: Part 2: TILs in melanoma,
gastrointestinal tract carcinomas, non-small cell lung carcinoma
and mesothelioma, endometrial and ovarian carcinomas, squamous cell
carcinoma of the head and neck, genitourinary carcinomas, and
primary brain tumors. Adv Anat Pathol. 24:311–335. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koletsa T, Kotoula V, Koliou GA, Manousou
K, Chrisafi S, Zagouri F, Sotiropoulou M, Pentheroudakis G,
Papoudou-Bai A, Christodoulou C, et al: Prognostic impact of
stromal and intratumoral CD3, CD8 and FOXP3 in adjuvantly treated
breast cancer: Do they add information over stromal
tumor-infiltrating lymphocyte density? Cancer Immunol Immunother.
69:1549–1564. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brandwein-Gensler M, Teixeira MS, Lewis
CM, Lee B, Rolnitzky L, Hille JJ, Genden E, Urken ML and Wang BY:
Oral squamous cell carcinoma: Histologic risk assessment, but not
margin status, is strongly predictive of local disease-free and
overall survival. Am J Surg Pathol. 29:167–178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Agilent Technologies Inc, . PD-L1 IHC 22C3
pharmDx Interpretation Manual-Head and Neck Squamous Cell Carcinoma
(HNSCC). Santa Clara; CA, USA: pp. 1–72. https://www.agilent.com/cs/library/usermanuals/public/29314_22c3_pharmDx_hnscc_interpretation_manual_us.pdfApril
2–2024
|
|
31
|
Seethala RR, Baras A, Baskovich BW,
Birdsong GG, Fitzgibbons PL, Khoury JD and Schneider F: Head and
Neck Biomarker Reporting Template. Version 2.0.0.0. College of
American Patologists. 1–6. 2021.
|
|
32
|
Almangush A, Mäkitie AA, Triantafyllou A,
de Bree R, Strojan P, Rinaldo A, Hernandez-Prera JC, Suárez C,
Kowalski LP, Ferlito A and Leivo I: Staging and grading of oral
squamous cell carcinoma: An update. Oral Oncol. 107:1047992020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schmitd LB, Scanlon CS and D'Silva NJ:
Perineural invasion in head and neck cancer. J Dental Res.
97:742–750. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sharma HD, Mahadesh J, Monalisa W,
Gopinathan PA, Laxmidevi BL and Sanjenbam N: Quantitative
assessment of tumor-associated tissue eosinophilia and nuclear
organizing region activity to validate the significance of the
pattern of invasion in oral squamous cell carcinoma: A
retrospective study. J Oral Maxillofac Pathol. 25:258–265. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Binmadi N, Alsharif M, Almazrooa S,
Aljohani S, Akeel S, Osailan S, Shahzad M, Elias W and Mair Y:
Perineural invasion is a significant prognostic factor in oral
squamous cell carcinoma: A systematic review and meta-analysis.
Diagnostics (Basel). 13:33392023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang Q, Huang Y, Chen C, Zhang Y, Zhou J,
Xie C, Lu M, Xiong Y, Fang D, Yang Y, et al: Prognostic impact of
lymphovascular and perineural invasion in squamous cell carcinoma
of the tongue. Sci Rep. 13:38282023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yuan Y: Spatial heterogeneity in the tumor
microenvironment. Cold Spring Harb Perspect Med. 6:a0265832016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Galon J, Mlecnik B, Bindea G, Angell HK,
Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, et
al: Towards the introduction of the ‘Immunoscore’ in the
classification of malignant tumours. J Pathol. 232:199–209. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang XM, Song LJ, Shen J, Yue H, Han YQ,
Yang CL, Liu SY, Deng JW, Jiang Y, Fu GH, et al: Prognostic and
predictive values of immune infiltrate in patients with head and
neck squamous cell carcinoma. Hum Pathol. 82:104–112. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Almangush A, De Keukeleire S, Rottey S,
Ferdinande L, Vermassen T, Leivo I and Mäkitie AA:
Tumor-infiltrating lymphocytes in head and neck cancer: Ready for
prime time? Cancers (Basel). 14:15582022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Balermpas P, Michel Y, Wagenblast J, Seitz
O, Weiss C, Rödel F, Rödel C and Fokas E: Tumour-infiltrating
lymphocytes predict response to definitive chemoradiotherapy in
head and neck cancer. Br J Cancer. 110:501–509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chatzopoulos K, Kotoula V, Manoussou K,
Markou K, Vlachtsis K, Angouridakis N, Nikolaou A, Vassilakopoulou
M, Psyrri A and Fountzilas G: Tumor infiltrating lymphocytes and
CD8+ T cell subsets as prognostic markers in patients with
surgically treated laryngeal squamous cell carcinoma. Head Neck
Pathol. 14:689–700. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang WF, Wong MCM, Thomson PJ, Li KY and
Su YX: The prognostic role of PD-L1 expression for survival in head
and neck squamous cell carcinoma: A systematic review and
meta-analysis. Oral Oncol. 86:81–90. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Crosta S, Boldorini R, Bono F, Brambilla
V, Dainese E, Fusco N, Gianatti A, L'Imperio V, Morbini P and Pagni
F: PD-L1 testing and squamous cell carcinoma of the head and neck:
A multicenter study on the diagnostic reproducibility of different
protocols. Cancers (Basel). 13:2922021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
De Keukeleire SJ, Vermassen T, Deron P,
Huvenne W, Duprez F, Creytens D, Van Dorpe J, Ferdinande L and
Rottey S: Concordance, correlation, and clinical impact of
standardized PD-L1 and TIL scoring in SCCHN. Cancers (Basel).
14:24312022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
He B, Wang L, Zhou W, Liu H, Wang Y, Lv K
and He K: A fusion model to predict the survival of colorectal
cancer based on histopathological image and gene mutation. Sci Rep.
15:96772025. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Baxi V, Edwards R, Montalto M and Saha S:
Digital pathology and artificial intelligence in translational
medicine and clinical practice. Mod Pathol. 35:23–32. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee LY, Yang CH, Lin YC, Hsieh YH, Chen
YA, Chang MD, Lin YY and Liao CT: A domain knowledge enhanced yield
based deep learning classifier identifies perineural invasion in
oral cavity squamous cell carcinoma. Front Oncol. 12:9515602022.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Abousamra S, Gupta R, Hou L, Batiste R,
Zhao T, Shankar A, Rao A, Chen C, Samaras D, Kurc T and Saltz J:
Deep learning-based mapping of tumor infiltrating lymphocytes in
whole slide images of 23 types of cancer. Front Oncol.
11:8066032021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Badve S, Kumar GL, Lang T, Peigin E, Pratt
J, Anders R, Chatterjee D, Gonzalez RS, Graham RP, Krasinskas AM,
et al: Augmented reality microscopy to bridge trust between AI and
pathologists. NPJ Precis Oncol. 9:1392025. View Article : Google Scholar : PubMed/NCBI
|