Introduction
Gastric cancer is one of the most common cancers
worldwide, and was the fifth leading cause of cancer-related deaths
in 2022 (1,2). Although considerable recent advances
in chemotherapy, including immune checkpoint inhibitors, have
improved the prognosis of gastric cancer (3,4),
gastrectomy remains the most effective treatment strategy, even for
elderly patients (5). However,
surgical resection often leads to functional disorders and
postoperative infectious complications (PICs) (6–8).
Previous studies have reported that PICs result in prolonged
hospital stays, increased treatment costs, and a lack of effective
adjuvant therapy (9–11). Additionally, PICs are reportedly
associated with unfavorable outcomes after radical surgery for
gastrointestinal cancers (7,12–14).
Therefore, the ability to predict PICs preoperatively is crucial
for optimizing surgical procedures, tailoring perioperative
management, and refining treatment strategies to improve patient
prognosis.
The American Society of Anesthesiologists Physical
Status (ASA-PS) classification (15), frailty (16,17),
sarcopenia (18,19), nutritional status (20,21),
and comorbidities (22) are
reportedly useful physical predictors of PICs. However, the
mechanisms by which these indicators influence PICs remain unclear.
Furthermore, the relationship between preoperative
immunosuppression and PICs is not well understood.
The programmed cell death-1 (PD-1)/programmed cell
death ligand (PD-L) signaling pathway has recently been implicated
as a potential immune escape mechanism in several malignancies
(23), and several anti-PD-L1
antibodies have been applied as standard treatments for various
malignancies (24,25). Additionally, PD-1 is a critical
costimulatory molecule and a pivotal immune checkpoint receptor
that inhibits T-cell activation. Studies have shown that
PD-1+CD4+ cells increase with age and that
PD-1+CD4+ cells derived from aged mice have a
reduced capacity to respond to antigen stimulation, demonstrating
that PD-1+CD4+ cells are associated with
immunosenescence (26). However,
the relationship between PD-1+CD4+ cells and
PICs has not been reported.
In this study, we aimed to investigate the
predictive value of preoperative PD-1+CD4+
cells for the development of PICs and their association with
preoperative immune-inflammatory markers, nutritional indices, and
physical vulnerability.
Materials and methods
Patients' selection
We retrospectively analyzed the data of 85 patients
(67 men, 18 women; median age 72, range 46–92) who underwent
curative gastrectomy for gastric cancer at the National Defense
Medical College Hospital between January 2014 and December 2020.
Patients who had undergone preoperative chemotherapy, which may
have affected preoperative lymphocyte counts, were excluded.
Patients were divided into two groups; PD-1high (N=43)
and PD-1low (N=42) groups, based on the median value of
preoperative PD-1+CD4+/CD4+ cells
(cutoff value: 22.3%).
We collected the patients' clinical records at
admission for gastrectomy and pathological records. Pathological
findings of the specimens were recorded according to the third
English edition of the Japanese Classification of Gastric Carcinoma
published by the Japanese Gastric Cancer Association (27).
Definition of postoperative infectious
complications
PICs were defined as Clavien-Dindo Grade ≥2 within
30 days postoperatively (28). In
this study, PICs referred to pneumonia, pancreatic fistula,
anastomotic leakage, intraabdominal abscess, cholecystitis,
cholangitis, pneumonia, pyothorax, intestinal ischemia, and sepsis.
Superficial wound infections were excluded because they have
minimal impact on the systemic immune response. All surgeries were
performed by expert surgeons with more than 10 years of
experience.
Flow cytometric analysis of PD-1
expression on lymphocytes
Ethylenediaminetetraacetic acid-anticoagulated blood
samples were collected preoperatively (immediately before
gastrectomy) and stored at 4°C; the analysis was performed within
48 h of sample collection. Blood samples were incubated for 30 min.
at 4°C with fluorescent dye-conjugated monoclonal antibodies
against PD-1 (CD179)-PE (clone: eBioJ105, Thermo Fisher Scientific,
Inc.), CD4-PC7 (clone: SFCI12T4D11, Beckman Coulter), and CD3-FITC
(clone: UCTH1, Beckman Coulter). After incubation, samples were
washed twice with cold calcium and magnesium-free
phosphate-buffered saline (PBS) supplemented with 2% fetal bovine
serum (FBS) and 0.1% sodium azide (2% flow buffer). Red blood cells
were lysed using a lysis buffer, and the remaining cells were
suspended in IsoFlow (Beckman Coulter). Following two additional
washes with 2% flow buffer and centrifugation at 1200 rpm for 2
min, the cell pellets were resuspended in 500 µl of IsoFlow, kept
on ice, and analyzed within 6 h using a flow cytometry (Cytomics
FC500, Beckman Coulter). Appropriate isotype-matched negative
controls were used for each antibody. Flow cytometric data were
analyzed using FlowJo software (Tree Star Inc.).
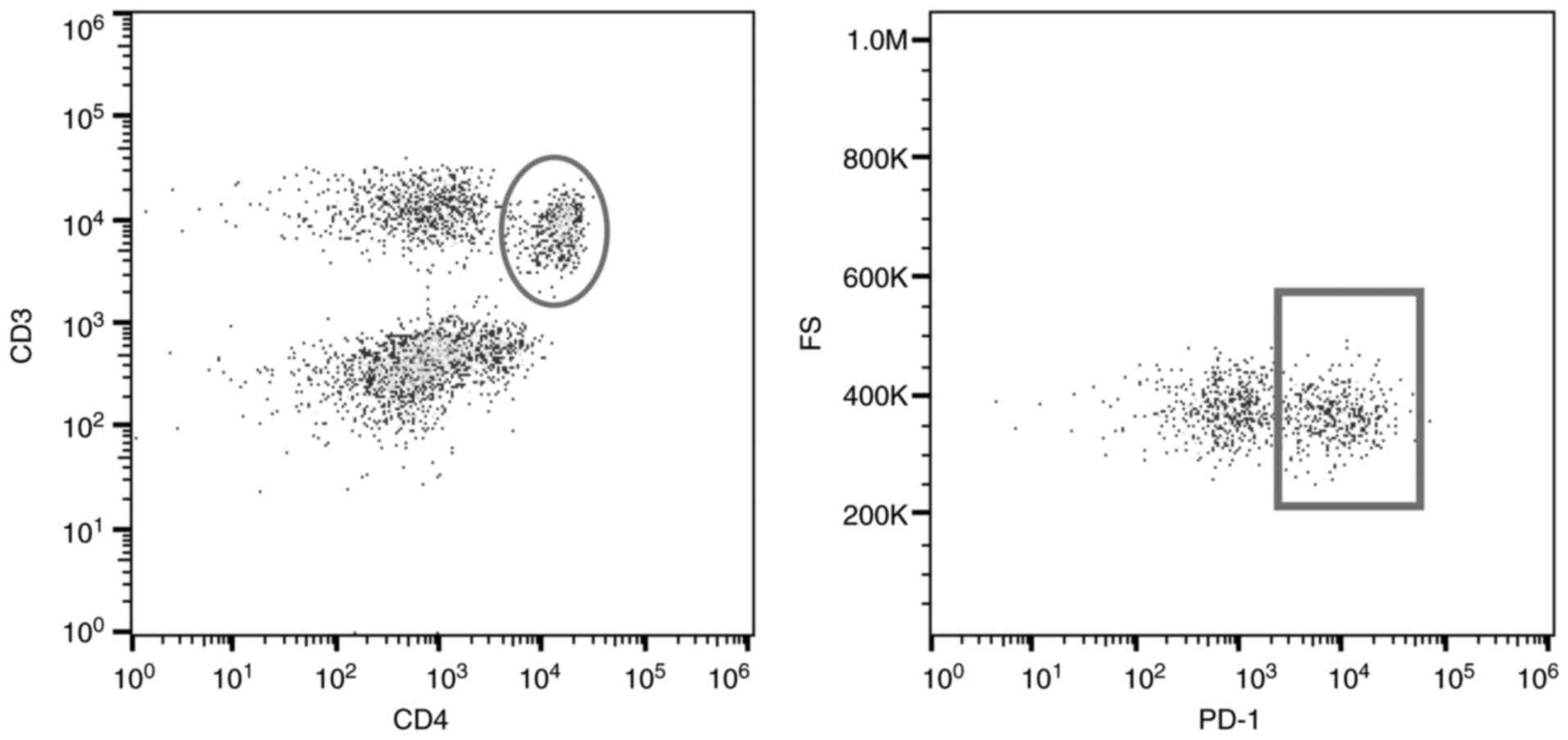
CD4+CD3+ cells were gated, and PD-1
expression on these cells was evaluated (Fig. 1) (29).
Immune-inflammatory markers,
nutritional indices, fall risk assessment score, and Charlson
comorbidity index
We assessed preoperative neutrophil-to-lymphocyte
ratio (NLR), C-reactive protein (CRP)-to-albumin ratio (CAR), and
platelet-to-lymphocyte ratio (PLR) as immune-inflammatory markers,
and prognostic nutrition index (PNI) and controlling nutritional
status (CONUT) as nutritional indexes (30–32).
ASA-PS was described from the anesthesia chart. Carlson comorbidity
index (CCI) (22) and the fall risk
assessment score (FRAS) (16) was
used to determine physical vulnerability. The FRAS was evaluated by
nursing staff upon admission (Table
SI), and was calculated as the sum of the scores for all items.
It includes seven categories: age, history of falls or syncope,
physical dysfunction, activity status, mental dysfunction,
medicines, and toileting needs. In total, these categories comprise
46 individual fall risk items. The CCI, developed by Charlson et
al (22), predicts the
mortality by accounting for a range of comorbid conditions, such as
renal, hepatic, and cardiac diseases, acquired immunodeficiency
syndrome, and cancer-spanning 17 categories in total.
Definition of immune-inflammatory
markers and nutritional indices
The markers and indices based on preoperative
laboratory data were calculated as follows using preoperative
laboratory data: NLR=neutrophil counts/lymphocyte counts,
CAR=C-reactive protein levels/albumin levels, PLR=platelet
counts/lymphocyte counts, PNI=10×serum albumin level (g/dl) +
0.005×total lymphocyte count (/µl) (31). The CONUT score was assessed using
serum albumin, total cholesterol, and total lymphocyte count levels
(32).
Ethics
All procedures followed were in accordance with the
ethical standards of the responsible committee on human
experimentation (institutional and national) and with the Helsinki
Declaration of 1964 and later versions. All protocols were approved
by the Institutional Review Board of the National Defense Medical
College (Approval number: 5070) and written informed consent was
obtained prior to the study.
Statistical analysis
Data are expressed as mean ± standard deviation,
unless otherwise stated. Welch's t-test, Mann-Whitney U-test, and
one-way analysis of variance were conducted, with post hoc Tukey
procedures employed to adjust for multiple comparisons, when
appropriate. To evaluate multicollinearity, we calculated the
Variance Inflation Factor (VIF) for all explanatory variables, with
VIF >5 indicating high collinearity. All statistical analyses
were performed using JMP Pro 17.0.0 (SAS Institute Inc.), and
statistical significance was set at P<0.05.
Results
Clinicopathologic characteristics of patients who
underwent gastrectomy are summarized in Table I. The PD-1high group was
significantly older (74.2±9.2 vs. 70.0±10.3) and had a higher
frequency of perioperative blood transfusion (32.6% vs. 14.3%)
compared to the PD-1low group. There were no significant
differences in sex, body mass index, comorbidities, postoperative
hospital stays, and pathological factors, such as tumor depth,
nodal metastasis, and pathological stage. The PD-1high
group demonstrated a significantly higher overall incidence of PICs
(41.9% vs. 19.0%), with notably increased rates of anastomotic
leakage (14.0% vs. 0%), pneumonia (11.6% vs. 0%), and sepsis (9.3%
vs. 0%) (all P<0.05). Fig. S1
shows the various types of complications and the ratio of
PD-1+CD4+/CD4+ cells.
 | Table I.Clinicopathological characteristics
according to the PD-1+CD4+/CD4+
cells. |
Table I.
Clinicopathological characteristics
according to the PD-1+CD4+/CD4+
cells.
|
|
|
PD1+CD4+/CD4+
cells (%) |
|
|---|
|
|
|
|
|
|---|
| Factors | Groups | PD-1high
(N=43) | PD-1low
(N=42) | P-value |
|---|
| Clinical
factors |
|
|
|
|
| Mean age (range),
years |
| 74.2 (46–89) | 70.0 (47–88) | 0.04 |
| Sex, n (%) | Male | 33 (76.7) | 34 (81.0) | 0.63 |
|
| Female | 10 (23.3) | 8 (19.0) |
|
| BMI (range),
kg/m2 |
| 22.7
(15.7–31.1) | 22.6
(13.7–35.3) | 0.94 |
| Comorbidity, n
(%) | Yes | 38 (88.4) | 31 (73.8) | 0.08 |
|
| No | 5 (11.6) | 11 (26.2) |
|
| Cardiovascular, n
(%) | Yes | 27 (62.8) | 23 (54.8) | 0.45 |
|
| No | 16 (37.2) | 19 (45.2) |
|
| Diabetes, n
(%) | Yes | 9 (20.9) | 8 (19.0) | 0.82 |
|
| No | 34 (79.1) | 34 (81.0) |
|
| Respiratory, n
(%) | Yes | 4 (9.3) | 2 (4.8) | 0.41 |
|
| No | 39 (90.7) | 40 (95.2) |
|
| Previous
laparotomy, n (%) | Yes | 13 (30.2) | 15 (35.7) | 0.59 |
|
| No | 30 (69.8) | 27 (64.3) |
|
| Postoperative
hemoglobin ± SD, g/dl |
| 11.7±2.4 | 12.3±1.9 | 0.16 |
| Postoperative
hospital stays (range), days median |
| 11 (6–255) | 9 (6–138) | 0.46 |
| Surgical
factors |
|
|
|
|
| Time ± SD, min |
| 268±96 | 257±76 | 0.74 |
| Bleeding ± SD,
g |
| 481±601 | 319±348 | 0.23 |
| Surgical approach,
n (%) | Laparotomy | 19 (44.2) | 18 (42.9) | 0.90 |
|
| Laparoscopy | 24 (55.8) | 24 (57.1) |
|
| Blood transfusion,
n (%) | Yes | 14 (32.6) | 6 (14.3) | 0.04a |
|
| No | 29 (67.4) | 36 (85.7) |
|
| Pathological
factors |
|
|
|
|
| Localization, n
(%) | U | 13 (30.2) | 13 (31.0) | 0.92 |
|
| M | 12 (27.9) | 13 (31.0) |
|
|
| L | 18 (41.9) | 16 (38.0) |
|
| Diameter ± SD,
mm |
| 60±40 | 46±30 | 0.08 |
| Pathological type
(Lauren classification), n (%) | Diffuse | 22 (51.2) | 16 (38.1) | 0.20 |
|
| Intestinal | 21 (48.8) | 24 (57.1) |
|
|
| Othersa | 0 (0) | 2 (4.8) |
|
| Tumor depth, n
(%) | T1 | 13 (30.2) | 19 (45.2) | 0.42 |
|
| T2 | 7 (16.3) | 7 (16.7) |
|
|
| T3 | 11 (25.6) | 6 (14.3) |
|
|
| T4 | 12 (27.9) | 10 (23.8) |
|
| Nodal metastasis, n
(%) | N0 | 22 (51.2) | 24 (57.1) | 0.16 |
|
| N1 | 9 (20.9) | 6 (14.3) |
|
|
| N2 | 7 (16.3) | 2 (4.8) |
|
|
| N3 | 5 (11.6) | 10 (23.8) |
|
| Pathological stage,
n (%) | Stage I | 18 (41.9) | 23 (54.7) | 0.46 |
|
| Stage II | 8 (18.6) | 7 (16.7) |
|
|
| Stage III | 17 (39.5) | 12 (28.6) |
|
| Postoperative
infectious complicationsb, n (%) | Yes | 18 (41.9) | 8 (19.0) | 0.02a |
|
| No | 25 (58.1) | 34 (81.0) |
|
| Pancreatic fistula,
n (%) |
| 8 (18.6) | 4 (9.5) | 0.22 |
| Anastomotic
leakage, n (%) |
| 6 (14.0) | 0 (0.0) | 0.01a |
| Intraabdominal
abscess, n (%) |
| 5 (11.6) | 4 (9.5) | 0.75 |
| Sepsis, n (%) |
| 4 (9.3) | 0 (0.0) | 0.04a |
|
Cholecystitis/cholangitis, n (%) |
| 2 (4.6) | 1 (2.4) | 0.57 |
| Pneumonia, n
(%) |
| 5 (11.6) | 0 (0.0) | 0.02a |
| Intestinal
ischemia/necrosis, n (%) |
| 3 (7.0) | 0 (0.0) | 0.08 |
| Pyothorax, n
(%) |
| 2 (4.6) | 0 (0.0) | 0.15 |
| Catheter-related
bloodstream infection, n (%) |
| 1 (2.3) | 0 (0.0) | 0.32 |
There were no significant differences in
preoperative white blood cell, neutrophil, and platelet counts
between the two groups (Table II).
However, the PD-1high group had significantly lower
preoperative total lymphocyte counts (1476±534/µl vs. 1780±536/µl)
and serum albumin (3.5±0.5 g/dl vs. 3.9±0.6g/dl) and had a
significantly higher preoperative CRP level (1.0±1.4 mg/dl vs.
0.1±1.1mg/dl). In addition, the PD-1high group had
significantly higher preoperative NLR (3.3±1.6 vs. 2.3±0.8), CAR
(0.4±0.6 vs. 0.2±0.7), CONUT score (2.9±2.6 vs. 1.5±1.8), and FRAS
(5.5±3.6 vs. 3.9±3.1) as well as significantly lower preoperative
PNI (42.6±6.7 vs. 47.0±9.5) (all P<0.05).
 | Table II.Preoperative laboratory data,
immune-inflammatory markers, nutritional status and fall risk
assessment score according to the
PD-1+CD4+/CD4+ cells. |
Table II.
Preoperative laboratory data,
immune-inflammatory markers, nutritional status and fall risk
assessment score according to the
PD-1+CD4+/CD4+ cells.
|
|
|
PD1+CD4+/CD4+
cells |
|
|---|
|
|
|
|
|
|---|
| Factors | Groups | PD-1high
(N=43) | PD-1low
(N=42) | P-value |
|---|
| ASA-PS, n (%) | I | 6 (13.9) | 8 (19.0) | 0.08 |
|
| II | 30 (69.8) | 33 (78.6) |
|
|
| III | 7 (16.3) | 1 (2.4) |
|
| FRAS, median
(range) |
| 4 (1–16) | 3 (0–14) | 0.02 |
|
| I (1–5), n
(%) | 25 (58.2) | 31 (73.8) | 0.23 |
|
| II (6–15), n
(%) | 17 (39.5) | 11 (26.2) |
|
|
| III (−16), n
(%) | 1 (2.3) | 0 (0.0) |
|
| CCI ± SD |
| 6.7±1.5 | 6.1±1.4 | 0.08 |
| CONUT score, median
(range) |
| 2 (0–11) | 1 (0–8) | <0.01 |
|
| Normal (0–1), n (%) | 16 (37.2) | 27 (64.3) | 0.07 |
|
| Mild (2–4), n
(%) | 18 (41.9) | 11 (26.2) |
|
|
| Moderate (5–8), n
(%) | 8 (18.6) | 4 (9.5) |
|
|
| Severe (9–12), n
(%) | 1 (2.3) | 0 (0.0) |
|
| White blood cell
counts ± SD, /µl |
| 6165±1810 | 6104±2043 | 0.77 |
| Neutrophil counts ±
SD, /µl |
| 4068±1487 | 3778±1658 | 0.28 |
| Lymphocyte counts ±
SD, /µl |
| 1476±534 | 1780±536 | 0.02 |
| Platelet counts ±
SD, ×103/µl |
| 246±100 | 254±108 | 0.81 |
| Hemoglobin ± SD,
g/dl |
| 11.7±2.4 | 12.3±1.9 | 0.16 |
| Albumin ± SD,
g/dl |
| 3.5±0.5 | 3.9±0.6 | <0.01 |
| CRP ± SD,
mg/dl |
| 1.0±1.4 | 0.5±1.1 | 0.01 |
| NLR ± SD |
| 3.32±1.56 | 2.33±0.84 | <0.01 |
| CAR ± SD |
| 0.37±0.56 | 0.24±0.66 | 0.03 |
| PLR ± SD |
| 185.6±101.1 | 148.3±62.1 | 0.15 |
| PNI ± SD |
| 42.6±6.7 | 47.0±9.5 | <0.01 |
| CD4+
cell counts ± SD, /µl |
| 544±439 | 733±574 | 0.06 |
|
PD1+CD4+ cell counts
± SD, /µl |
| 596±373 | 459±408 | 0.04 |
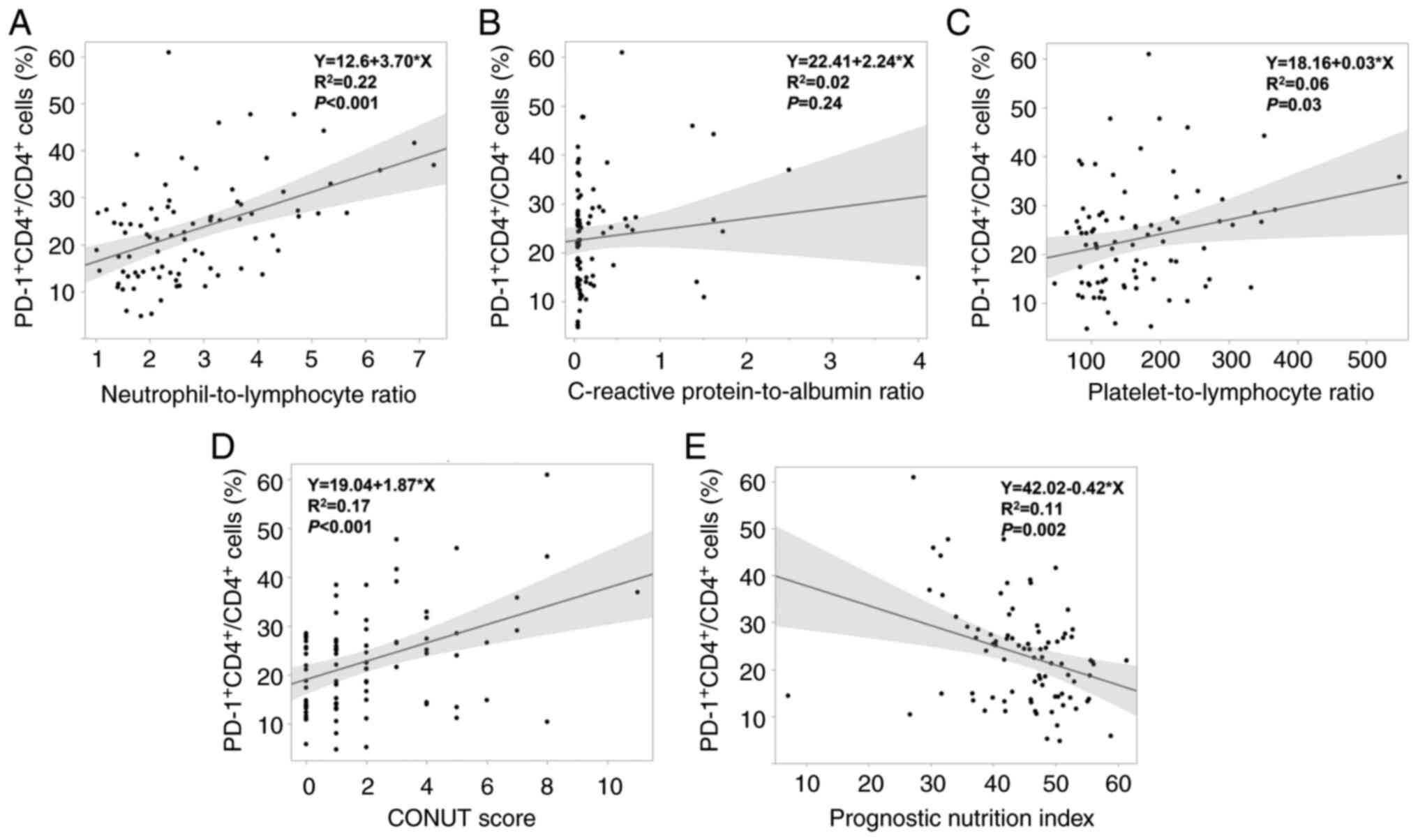
Regarding the association of preoperative
PD-1+CD4+/CD4+ cells and each
preoperative immune-inflammatory marker, there were significant
positive correlations between
PD-1+CD4+/CD4+ cells and NLR, PLR,
and CONUT score (all P<0.05) (Fig.
2A, C, D), whereas a significant negative correlation was
observed between PD-1+CD4+/CD4+
cells and PNI (P<0.01) (Fig.
2E). In addition,
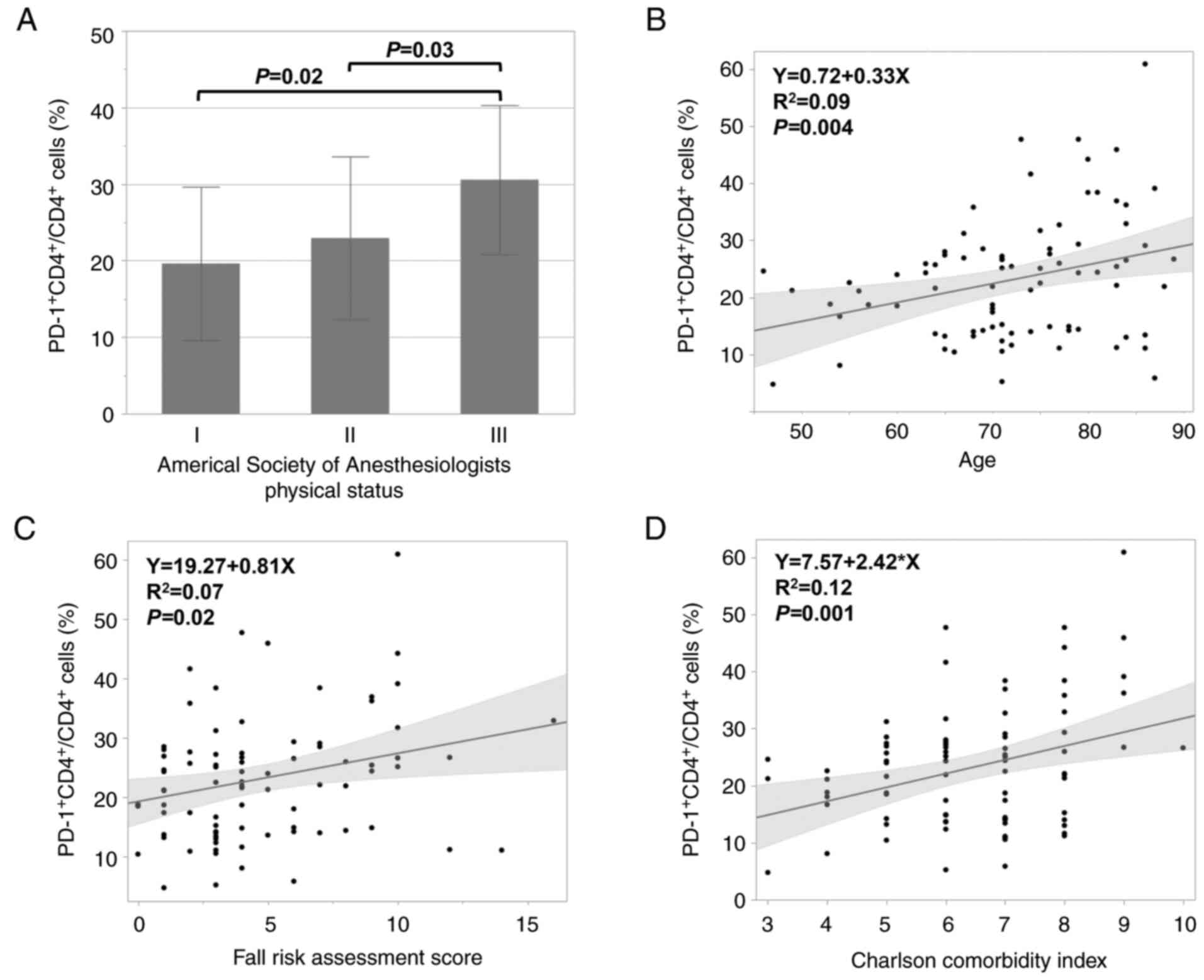
PD-1+CD4+/CD4+ cells significantly
increased as ASA-PS increased (P<0.05; Fig. 3A) and significantly positively
correlated with age, FRAS, and CCI (all P<0.05) (Fig. 3B-D).
The results of univariate and multivariate analyses
that may affect the incidence of PICs are shown in Table III and Fig. S2. Five variables (preoperative
FRAS, CAR, PNI, CCI, and
PD-1+CD4+/CD4+ cells) with
P<0.05 in the univariate analysis were selected as explanatory
variables for the multivariate analysis. Multivariate analysis
demonstrated that only CCI was identified as independent factors
for the development of PICs. No evidence of problematic
multicollinearity was found, as all VIF values were below 2.0.
These findings are in agreement with the results of the primary
multivariate analysis.
 | Table III.Univariate and multivariate analyses
that may affect the incidence of postoperative infectious
complications. |
Table III.
Univariate and multivariate analyses
that may affect the incidence of postoperative infectious
complications.
|
|
| Univariate
analysis | Multivariate
analysis |
|
|---|
|
|
|
|
|
|
|---|
| Factor | Groups | OR (95% CI) | P-value | OR (95% CI) | P-value | VIF |
|---|
| ASA-PS | I–II | 1 | 0.053 |
|
| 1.35 |
|
| III | 4.44
(0.97–20.2) |
|
|
|
|
| FRAS | I | 1 | 0.04 | 1 | 0.8 | 1.43 |
|
| II–III | 2.68
(1.02–7.01) |
| 0.85
(0.24–3.00) |
|
|
| CONUT score | Normal, mild | 1 | 0.98 |
|
| 1.55 |
|
| Median, severe | 1.01
(0.28–3.63) |
|
|
|
|
| NLR (median) | <2.44 | 1 | 0.09 |
|
| 1.38 |
|
| ≥2.44 | 2.23
(0.86–5.82) |
|
|
|
|
| CAR (median) | <0.06 | 1 | 0.01 | 1 | 0.08 | 1.24 |
|
| ≥0.06 | 3.69
(1.29–10.5) |
| 2.83
(0.87–9.19) |
|
|
| PLR (median) | <145.5 | 1 | 0.31 |
|
| 1.42 |
|
| ≥145.5 | 1.61
(0.63–4.10) |
|
|
|
|
| PNI (median) | <46.1 | 1 | <0.01 | 1 | 0.36 | 1.82 |
|
| ≥46.1 | 0.21
(0.07–0.60) |
| 0.54
(0.15–2.03) |
|
|
| CCI (median) | <7 | 1 | <0.01 | 1 | <0.01 | 1.37 |
|
| ≥7 | 7.06
(2.33–21.4) |
| 6.05
(1.71–21.3) |
|
|
| PD-1+CD4+/CD4+
cells (%, median) | <22.3 | 1 | 0.02 | 1 | 0.15 | 1.23 |
|
| ≥22.3 | 3.15
(1.14–8.15) |
| 2.41
(0.73–7.95) |
|
|
Discussion
In this study, we investigated the predictive value
of preoperative PD-1+CD4+ cell counts for the
development of PICs and their association with preoperative
immune-inflammatory markers, nutritional indices, and physical
vulnerability. The results showed that patients who underwent
gastrectomy and developed PICs had significantly higher
preoperative PD-1+CD4+/CD4+ cells.
We also found significant associations between preoperative
PD-1+CD4+/CD4+ cells,
immune-inflammatory markers, nutritional indices, CCI, and the FRAS
on admission.
Although there are increasing reports regarding the
association of unfavorable long-term outcomes with PICs in several
malignancies (7,12–14,33),
the precise mechanism underlying this association remains unclear
(34). Various factors can lead to
the development of PICs after gastrectomy, including the patients'
physical condition, cancer stage, and surgical technique (35,36).
In their systematic review, Joharatnam-Hogan et
al (37) demonstrated that
elderly patients can benefit from curative treatment to a similar
extent as younger patients. However, they emphasized that improving
outcomes in physically frail populations requires an individualized
approach to treatment approach, with greater reliance on indicators
of functional age and frailty rather than chronological age when
determining gastric cancer treatment (37). In this study, we focused on
PD-1+CD4+ cells to evaluate their
immunological vulnerability to PICs. T-cell function, which is
essential for defense against infection, is regulated not only by
the T-cell receptor but also by costimulatory molecules, such as
PD-1 and cytotoxic T-lymphocyte-associated antigen-4 (38). Notably,
PD-1+CD4+ cells are characterized by
proliferative hyporesponsiveness and are incapable of responding to
antigenic stimulation (26). These
findings prompted us to investigate preoperative
PD-1+CD4+ cells in patients with PICs.
We demonstrated that
PD-1+CD4+/CD4+ cells were
significantly associated with age, which supports and extends
previous reports in mice (39).
Foldi et al demonstrated that human immunodeficiency virus
(HIV)-infected children not receiving antiretroviral therapy (ART)
had a higher proportion of
PD-1+CD4+/CD4+cells than healthy
and HIV-infected children on ART. They also indicated that
PD-1+CD4+ cells preferentially produce Th1
(interferon-γ) and Th17 cytokines, despite weak proliferative
potential (40). In humans, the
process of aging upregulates PD-1 in natural killer cells and
enhances pro-inflammatory cytokines, similar to that of
HIV-infected children. Thus, patients who develop PICs may already
be immunosuppressed, similar to older and HIV-infected individuals
(41,42). Our previous research has reported
that patients who developed PIC after surgery for gastric or
esophageal cancer had a high preoperative level of MDSCs, which
have a strong immunosuppressive effect (29). In addition, there was no correlation
between the disease stage and
PD-1+CD4+/CD4+ cells, suggesting
that the elevated PD-1+CD4+/CD4+
cells in patients with PICs were not affected by tumor
progression.
This study also revealed that preoperative
PD-1+CD4+/CD4+ cells are
associated with the FRAS and CCI, which reflects the patients'
activity, comorbidity, and frailty. Several FRAS have been widely
used in hospital-specific formats, including age, medical history,
physical dysfunction, activity status, mental dysfunction,
medications, and assistance required for toileting, all of which
may reflect patient physical frailty. We previously reported that
patients with higher FRAS had longer hospital stays and poorer
overall and recurrence-free survival than those with lower FRAS
(16). Our findings regarding the
association between preoperative
PD-1+CD4+/CD4+ cells, FRAS and CCI
implied an association between immunological frailty and physical
frailty. In a previous study, Wang et al (43) demonstrated that a decline in
CD4+ cells is associated with worse outcomes in older
and frail patients with severe community-acquired pneumonia.
Notably, although frailty is thought to be one of the causes of
PICs immunity, to our knowledge, no studies on the association
between PD-1+CD4+ cells and susceptibility to
PICs have been conducted.
This study has some limitations. This retrospective
and single-institutional study included a relatively small number
of patients, which is associated with the potential limitations
arising from heterogeneity in our retrospective cohort, including
patient background, tumor stage, and perioperative management. We
used the median value of
PD-1+CD4+/CD4+ cells as an
exploratory, hypothesis-generating cutoff value because no
clinically validated biologically relevant threshold exists;
however, the median split may not represent the optimal
biologically relevant threshold, and future studies should aim to
determine validated clinical cutoffs, ideally from larger
prospective cohorts. Furthermore, CD4+T cells,
particularly the Th17 subset, are known to secrete pro-inflammatory
cytokines such as IL-17. We are currently planning prospective
study based on this research, and we hope to publish further
findings on the relationship between preoperative
PD-1+CD4+/CD4+ cells, the Th17
subset, and IL-17. In this study, physical frailty was evaluated
using only ASA-PS, FRAS, and CCI; thus, it is necessary to assess
physical frailty as sarcopenia and kinesiological evaluations in
the future.
In conclusion, elevated preoperative
PD-1+CD4+/CD4+ cells were
associated with the development of PICs after gastrectomy, although
multivariate analysis did not identify them as an independent
predictor; rather, our findings highlight their associative value
with immunological and physical frailty, suggesting that
preoperative PD-1+CD4+/CD4+ cell
measurement may have potential clinical relevance as part of a
screening tool or multiparameter predictive model. Importantly,
although our results did not demonstrate superiority over
conventional clinical parameters such as immuno-inflammatory,
nutritional, and frailty indices,
PD-1+CD4+/CD4+ cell evaluation
offers unique mechanistic insight into host immune competence by
reflecting T-cell functional exhaustion mediated through immune
checkpoint pathways-an aspect not directly captured by standard
measures. Such immune profiling may provide complementary
information to conventional indices and could, when integrated into
composite prediction models, improve perioperative risk assessment
and individualized management strategies. Future prospective
studies are warranted to validate this potential additive
value.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Mrs. Takami Saiki
(National Defense Medical College Research Institute, Saitama,
Japan) for their assistance with the experiments.
Funding
This work was partially supported by JSPS KAKENHI (grant no.
24K11880).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
NU, HT, NI, TK, HM, and HU conceived and designed
the study. NU, NI, TK and HM conducted the experiments. NU, HH, KK,
SF, TS, YY, RK, AI and HT interpreted the data. NU, HT, and HU
prepared the manuscript. HT and HU supervised the study. NU and HT
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
All procedures followed were in accordance with the
ethical standards of the Institutional Review Board of the National
Defense Medical College on human experimentation and with the
Helsinki Declaration of 1964 and later versions. The Institutional
Review Board of the National Defense Medical College approved the
study (permission no. 5070), and written informed consent was
obtained prior to its commencement.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Hironori Tsujimoto, ORCID: 0000-0002-2808-4723.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
2
|
Higashi T and Kurokawa Y: Incidence,
mortality, survival, and treatment statistics of cancers in
digestive organs-Japanese cancer statistics 2024. Ann Gastroenterol
Surg. 8:958–965. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Janjigian YY, Shitara K, Moehler M,
Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T,
Campos Bragagnoli A, et al: First-line nivolumab plus chemotherapy
versus chemotherapy alone for advanced gastric, gastro-oesophageal
junction, and oesophageal adenocarcinoma (CheckMate 649): A
randomised, open-label, phase 3 trial. Lancet. 389:27–40. 2021.
View Article : Google Scholar
|
|
4
|
Rha SY, Oh DY, Yañez P, Bai Y, Ryu MH, Lee
J, Rivera F, Alves GV, Garrido M, Shiu KK, et al: Pembrolizumab
plus chemotherapy versus placebo plus chemotherapy for
HER2-negative advanced gastric cancer (KEYNOTE-859): A multicentre,
randomised, double-blind, phase 3 trial. Lancet Oncol.
24:1181–1195. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosa F, Schena CA, Laterza V, Quero G,
Fiorillo C, Strippoli A, Pozzo C, Papa V and Alfieri S: The role of
surgery in the management of gastric cancer: State of the art.
Cancers (Basel). 14:55422022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shimada H, Fukagawa T, Haga Y and Oba K:
Does postoperative morbidity worsen the oncological outcome after
radical surgery for gastrointestinal cancers? A systematic review
of the literature. Ann Gastroenterol Surg. 1:11–23. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsujimoto H, Ichikura T, Ono S, Sugasawa
H, Hiraki S, Sakamoto N, Yaguchi Y, Yoshida K, Matsumoto Y and Hase
K: Impact of postoperative infection on long-term survival after
potentially curative resection for gastric cancer. Ann Surg Oncol.
16:311–318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yaguchi Y, Tsujimoto H, Kumano I, Takahata
R, Matsumoto Y, Yoshida K, Horiguchi H, Ono S, Ichikura T, Yamamoto
J and Hase K: Sentinel node navigation surgery attenuates the
functional disorders in early gastric cancer. Oncol Rep.
27:643–649. 2012.PubMed/NCBI
|
|
9
|
Badia JM, Casey AL, Petrosillo N, Hudson
PM, Mitchell SA and Crosby C: Impact of surgical site infection on
healthcare costs and patient outcomes: A systematic review in six
European countries. J Hosp Infect. 96:1–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kusachi S, Kashimura N, Konishi T, Shimizu
J, Kusunoki M, Oka M, Wakatsuki T, Kobayashi J, Sawa Y, Imoto H, et
al: Length of stay and cost for surgical site infection after
abdominal and cardiac surgery in Japanese hospitals: Multi-center
surveillance. Surg Infect (Larchmt). 13:257–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsujimoto H, Kouzu K, Sugasawa H, Nomura
S, Ito N, Harada M, Sugihara T, Ishibashi Y, Kishi Y and Ueno H:
Impact of postoperative infectious complications on adjuvant
chemotherapy administration after gastrectomy for advanced gastric
cancer. Jpn J Clin Oncol. 51:379–386. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsuda A, Maruyama H, Akagi S, Inoue T,
Uemura K, Kobayashi M, Shiomi H, Watanabe M, Fujita T, Takahata R,
et al: Survival impact of surgical site infection in esophageal
cancer surgery: A multicenter retrospective cohort study. Ann
Gastroenterol Surg. 7:603–614. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsujimoto H, Ueno H, Hashiguchi Y, Ono S,
Ichikura T and Hase K: Postoperative infections are associated with
adverse outcome after resection with curative intent for colorectal
cancer. Oncol Lett. 1:119–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ueno H and Tsujimoto H: Postoperative
complications and impaired Long-term survival-Is this causation or
association? Ann Gastroenterol Surg. 7:5–6. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saklad M: Grading of patients for surgical
procedures. Anesthesiology. 2:281–284. 1941. View Article : Google Scholar
|
|
16
|
Kouzu K, Tsujimoto H, Nagata H, Sugasawa
H, Ishibashi Y, Hase K, Kishi Y and Ueno H: Preoperative fall risk
assessment score as a prognostic factor in gastric cancer patients
after gastrectomy. Jpn J Clin Oncol. 51:569–576. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Makary MA, Segev DL, Pronovost PJ, Syin D,
Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG,
Tian J and Fried LP: Frailty as a predictor of surgical outcomes in
older patients. J Am Coll Surg. 210:901–908. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fukuda Y, Yamamoto K, Hirao M, Nishikawa
K, Nagatsuma Y, Nakayama T, Tanikawa S, Maeda S, Uemura M, Miyake
M, et al: Sarcopenia is associated with severe postoperative
complications in elderly gastric cancer patients undergoing
gastrectomy. Gastric Cancer. 19:986–993. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nagata K, Tsujimoto H, Nagata H, Harada M,
Ito N, Kanematsu K, Nomura S, Horiguchi H, Hiraki S, Hase K, et al:
Impact of reduced skeletal muscle volume on clinical outcome after
esophagectomy for esophageal cancer: A retrospective study.
Medicine (Baltimore). 97:e114502018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takano Y, Haruki K, Kai W, Tsukihara S,
Kobayashi Y, Ito D, Kanno H, Son K, Hanyu N and Eto K: The
influence of serum cholinesterase levels and sarcopenia on
postoperative infectious complications in colorectal cancer
surgery. Surg Today. 53:816–823. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu H and Kong F: Malnutrition-related
factors increased the risk of anastomotic leak for rectal cancer
patients undergoing surgery. Biomed Res Int. 2020:50596702020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Charlson ME, Pompei P, Ales KL and
MacKenzie CR: A new method of classifying prognostic comorbidity in
longitudinal studies: Development and validation. J Chronic Dis.
40:373–383. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bilgin B, Sendur MA, Bulent Akinci M,
Sener Dede D and Yalcin B: Targeting the PD-1 pathway: A new hope
for gastrointestinal cancers. Curr Med Res Opin. 33:749–759. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y,
Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in
patients with advanced gastric or gastro-oesophageal junction
cancer refractory to, or intolerant of, at least two previous
chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet.
390:2461–2471. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Muro K, Chung HC, Shankaran V, Geva R,
Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, et al:
Pembrolizumab for patients with PD-L1-positive advanced gastric
cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial.
Lancet Oncol. 17:717–726. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shimada Y, Hayashi M, Nagasaka Y,
Ohno-Iwashita Y and Inomata M: Age-associated up-regulation of a
negative co-stimulatory receptor PD-1 in mouse CD4+ T cells. Exp
Gerontol. 44:517–522. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Association JGC: Japanese classification
of gastric carcinoma: 3rd English edition. Gastric Cancer.
14:101–112. 2011. View Article : Google Scholar
|
|
28
|
Dindo D, Demartines N and Clavien PA:
Classification of surgical complications: A new proposal with
evaluation in a cohort of 6336 patients and results of a survey.
Ann Surg. 240:205–213. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ito N, Tsujimoto H, Miyazaki H, Takahata R
and Ueno H: Pivotal role of myeloid-derived suppressor cells in
infection-related tumor growth. Cancer Med. 13:e69172024.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ishibashi Y, Tsujimoto H, Hiraki S, Kumano
I, Yaguchi Y, Horiguchi H, Nomura S, Ito N, Shinto E, Aosasa S, et
al: Prognostic value of preoperative systemic immunoinflammatory
measures in patients with esophageal cancer. Ann Surg Oncol.
25:3288–3299. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Onodera T, Goseki N and Kosaki G:
Prognostic nutritional index in gastrointestinal surgery of
malnourished cancer patients. Nihon Geka Gakkai Zasshi.
85:1001–1005. 1984.(In Japanese). PubMed/NCBI
|
|
32
|
Ignacio de Ulibarri J, Gonzalez-Madrono A,
de Villar NG, González P, González B, Mancha A, Rodríguez F and
Fernández G: CONUT: A tool for controlling nutritional status.
First validation in a hospital population. Nutr Hosp. 20:38–45.
2005.PubMed/NCBI
|
|
33
|
Murthy BL, Thomson CS, Dodwell D, Shenoy
H, Mikeljevic JS, Forman D and Horgan K: Postoperative wound
complications and systemic recurrence in breast cancer. Br J
Cancer. 97:1211–1217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsujimoto H, Kobayashi M, Sugasawa H, Ono
S, Kishi Y and Ueno H: Potential mechanisms of tumor progression
associated with postoperative infectious complications. Cancer
Metastasis Rev. 40:285–296. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tan ZK, Tang WZ, Jia K, Li DN, Qiu LY,
Chen X and Yang L: Relation between frailty and adverse outcomes in
elderly patients with gastric cancer: A scoping review. Ann Med
Surg (Lond). 86:1590–1600. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Matsui R, Inaki N and Tsuji T: Impact of
preoperative muscle quality on postoperative severe complications
after radical gastrectomy for gastric cancer patients. Ann
Gastroenterol Surg. 5:510–518. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Joharatnam-Hogan N, Shiu KK and Khan K:
Challenges in the treatment of gastric cancer in the older patient.
Cancer Treat Rev. 85:1019802020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Khoury SJ and Sayegh MH: The roles of the
new negative T cell costimulatory pathways in regulating
autoimmunity. Immunity. 20:529–538. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Channappanavar R, Twardy BS, Krishna P and
Suvas S: Advancing age leads to predominance of inhibitory receptor
expressing CD4 T cells. Mech Ageing Dev. 130:709–712. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Foldi J, Kozhaya L, McCarty B, Mwamzuka M,
Marshed F, Ilmet T, Kilberg M, Kravietz A, Ahmed A, Borkowsky W, et
al: HIV-infected children have elevated levels of PD-1+ memory CD4
T cells with low proliferative capacity and high inflammatory
cytokine effector functions. J Infect Dis. 216:641–650. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Deng M, Zeng Y, Liu Y, Wang X, Chen N,
Zhang M, Jiang M, Zhao H and Du J: Increased PD-1+ NK cell subset
in the older population. Int J Gen Med. 17:651–661. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ono S, Aosasa S, Tsujimoto H, Ueno C and
Mochizuki H: Increased monocyte activation in elderly patients
after surgical stress. Eur Surg Res. 33:33–38. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang J, Pei L, Zhao T, Liu X, Wang Q,
Zhang S, Li J, Wu H and Niu D: CD4+ T cells related to
disease severity in elderly and frailty community-acquired
pneumonia patients: A retrospective cohort study. Immun Inflamm
Dis. 11:e10092023. View Article : Google Scholar : PubMed/NCBI
|