Introduction
Papillary thyroid carcinoma (PTC) accounts for
80–85% of thyroid malignancies and typically carries an excellent
prognosis (>90% 10-year survival rate) (1). However, 10–15% of patients develop
aggressive disease with recurrence or distant metastases,
underscoring the need for precise risk stratification. Radioactive
iodine (RAI) therapy is essential for treating persistent or
metastatic PTC, effectively ablating residual thyroid tissue and
iodine-absorbing metastases (2).
Although there is strong evidence linking tumor biology to
suboptimal responses to RAI therapy, it is still challenging for
clinicians to identify specific patients with poor therapeutic
outcomes. Additionally, while several studies have explored
prognostic factors in PTC, including age, tumor size, lymph node
involvement and molecular markers [such as B-Raf proto-oncogene
serine/threonine kinase (BRAF) and telomerase reverse transcriptase
(TERT) mutations], an individualized nomogram to predict outcomes
after RAI therapy is still lacking (3–6).
Nomograms are powerful predictive models that have
recently been developed and widely used in oncology to provide
personalized risk estimates on the basis of multiple clinical and
pathological variables. In PTC, a nomogram could enhance risk
stratification by quantifying the likelihood of treatment failure,
recurrence or disease-specific survival following RAI therapy. For
instance, Valero et al (7)
developed a nomogram for survival outcomes in well-differentiated
thyroid cancer, while Wei et al (8) constructed a model based on clinical
features and gene mutations to predict lymph node metastasis.
However, these models do not incorporate dynamic post-treatment
biomarkers or focus on the critical post-RAI phase. Similarly, Wen
et al (9) proposed a
nomogram incorporating pre-ablation ratio for predicting
therapeutic response, yet its applicability is limited to
intermediate- and high-risk patients. In addition, existing
prognostic models often focus on preoperative or postoperative
factors, but not those after RAI injection (10,11).
Thyroglobulin (Tg) serves as a pivotal tumor marker
for PTC, with serum levels directly correlating with disease
prognosis. However, Tg-based predictive systems face significant
limitations, including requirements for long-time monitoring of
unreliable measurements and interference from non-malignant thyroid
tissue. A major drawback is the suppression of Tg levels in
patients with Tg antibodies (TgAb), which affects measurement
accuracy (12). While one study has
proposed TgAb levels as an alternative predictive factor in
TgAb-positive patients, their efficacy remains unconfirmed and
long-time monitoring proves equally unreliable (13). Given these challenges, developing a
predictive model that does not rely on Tg levels has substantial
clinical value. Therefore, the present study attempted to develop
and validate a nomogram based on readily available clinical
features to predict prognostic factors in patients with PTC after
RAI treatment. By incorporating demographic, pathological and
treatment-related variables, this model may provide clinicians with
information to individualize follow-up, treatment and counseling
after RAI treatment. The findings may aid in achieving more
personalized PTC treatment in the era of personalized medicine.
Materials and methods
Study population
The study protocol and data collection were approved
by the Ethical Board of the Second Hospital of Shandong University
(Jinan, China). All the subjects were informed about the
Declaration of Helsinki guidelines for research with human
subjects. This study was approved by the Institutional Ethics
Committee of the Second Hospital of Shandong University (approval
no. KYLL-2022LW077).
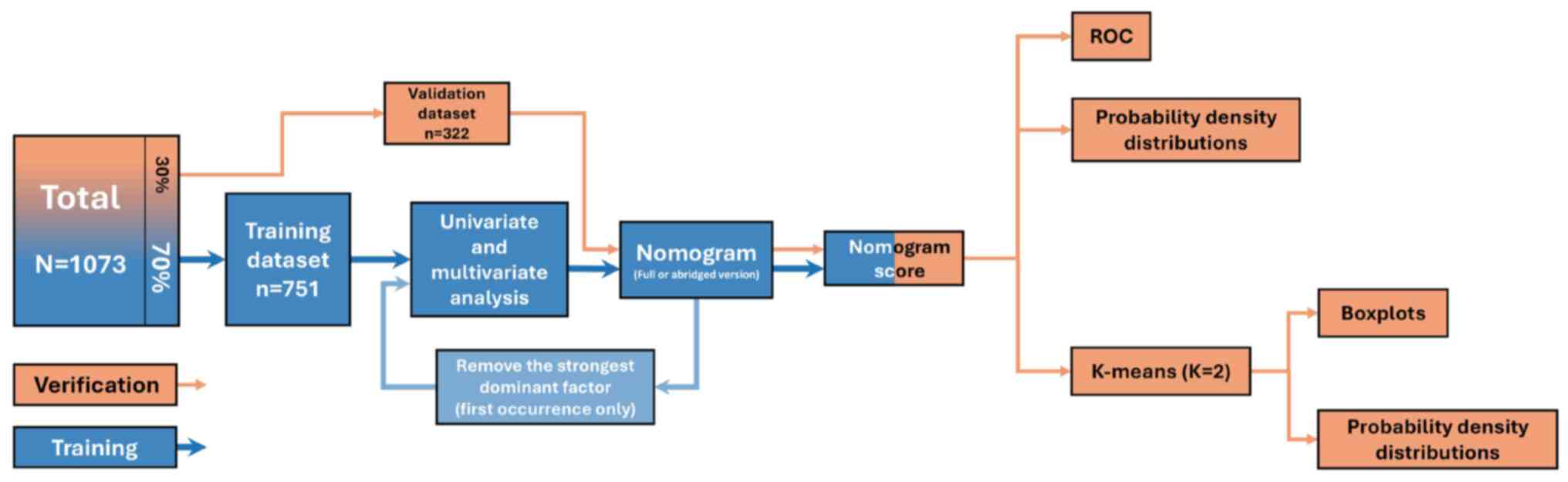
A total of 3,681 consecutive patients with
pathologically confirmed PTC were retrospectively reviewed, and all
these patients received RAI therapy at the Second Hospital of
Shandong University between May 2016 and April 2022. The inclusion
criteria to select the subjects were: i) Patients with PTC
confirmed by pathology, all of whom had undergone thyroidectomy;
and ii) patients with PTC scheduled for RAI therapy. A total of
2,608 patients with the following criteria were excluded: i) Peak
thyroid-stimulating hormone (TSH) level <30 µIU/ml (n=193); ii)
lack of complete clinical follow-up information on RAI therapy
(n=927); and iii) insufficient or unavailable mutation analysis
results (n=1,488). Finally, 1,073 patients were enrolled (319 men
and 754 women; mean age, 42.26±12.11 years; age range, 18–70
years), and the overall median follow-up time was 42 months
(interquartile range, 38–62 months) after initial RAI therapy.
Patients were managed according to American Thyroid
Association (ATA) guidelines (2015) (14). Clinical and pathological
characteristics were retrospectively analyzed from medical records,
including tumor subtype, multifocality, peripheral tissue invasion,
BRAFV600E mutation status, coexisting Hashimoto
thyroiditis (HT), Tumor-Node-Metastasis (TNM) stage (classified per
the AJCC 8th edition staging system), lymph node metastasis ratio
(LNR), Tg levels, TgAb levels and TSH levels (15). All pathological assessments were
conducted by board-certified pathologists. The flowchart of the
study cohort is presented in Fig.
1.
Management and follow-up
assessment
Within 2–3 months postoperatively, patients
underwent RAI therapy based on their recurrence risk
stratification. The initial administered activity of iodine-131
ranged from 2.96 to 5.55 GBq (80–150 mCi). Prior to RAI therapy,
all patients prepared with 4 weeks of levothyroxine withdrawal and
a low-iodine diet, proceeding only when TSH levels were >30
µIU/ml. On the day of RAI administration, serological parameters,
including TSH, pre-ablation stimulated Tg and TgAb, were measured.
Post-therapeutic whole-body scintigraphy was performed at 72 h
post-RAI, supplemented by single-photon emission computed
tomography/computed tomography for equivocal thyroid bed or nodal
uptake. Patients were routinely followed up every 3–6 months with
neck ultrasonography and serum assays (Tg, TgAb and TSH).
Persistent disease prompted additional RAI therapies (6–12-month
intervals), guided by treatment response. Final outcomes were
determined based on the last documented clinical and biochemical
status during follow-up.
Evaluation of the response to RAI
therapy
On the basis of these data, the clinical outcomes of
RAI therapy were classified as excellent response (ER),
indeterminate response (IDR), biochemical incomplete response (BIR)
and structural incomplete response (SIR). ER was defined as
negative imaging with either suppressed Tg ≤0.2 ng/ml or
TSH-stimulated Tg ≤1 ng/ml and the absence of TgAb. IDR was defined
as negative imaging with suppressed Tg at 0.2–1 ng/ml, stimulated
Tg at 1–10 ng/ml or TgAb kept stable or declining. BIR was defined
as negative imaging with suppressed Tg >1 ng/ml, stimulated Tg
>10 ng/ml or rising TgAb levels. SIR was defined as patients
with structural or functional evidence of disease with any Tg and
TgAb levels (14). A non-ER (NER)
included IDR, BIR and SIR. In the present study, BIR or SIR was
defined as indicative of a poor clinical outcome.
Statistical analysis
Continuous variables are expressed as the mean ±
standard deviation or median (interquartile range). Normality was
assessed via the Shapiro-Wilk test. Parametric data were analyzed
with unpaired Student's t test, whereas non-parametric data were
evaluated via the Mann-Whitney U test. Categorical variables are
summarized as n (%), with associations analyzed via the
χ2 test or Fisher's exact test. Group allocation was
performed via simple random sampling. Univariate and multivariate
logistic regression models were used to screen for prognostic risk
factors. Adjusted odds ratios with 95% confidence intervals (CIs)
were calculated through multivariable logistic regression to
compare recurrence risk among independent predictors. A nomogram
model was constructed on the basis of multivariate regression
results to quantify and visualize risk factors. Model calibration
was assessed via calibration curves, and predictive accuracy was
quantified via the mean absolute error (MAE). Model validation was
performed through probability density distributions and receiver
operating characteristic curve analysis, with risk stratification
achieved via the K-means clustering algorithm. For quantitative
visualization, boxplots are used to demonstrate data dispersion,
and probability density distributions are used to assess central
tendency. All analyses adopted a two-sided significance threshold
of P<0.05 to indicate a statistically significant difference.
Statistical computations were performed in SPSS 26.0 (IBM Corp.),
while regression modeling, nomogram construction and visualization
were implemented in RStudio (version 4.3.1; http://www.R-project.org/). The rms (https://cran.r-project.org/package=rms),
ggplot2 (https://ggplot2.tidyverse.org), dplyr (https://cran.r-project.org/package=dplyr), haven
(https://cran.r-project.org/package=haven), tidyr
(https://cran.r-project.org/package=tidyr), tibble
(https://cran.r-project.org/package=tibble), patchwork
(https://cran.r-project.org/package=patchwork), scales
(https://cran.r-project.org/package=scales) and magick
(https://cran.r-project.org/package=magick) packages
were used.
Results
Baseline characteristics and
comparative analysis between the training and validation
datasets
The study analyzed 1,073 patients with PTC treated
with RAI therapy, divided into the training (n=751) and validation
(n=322) datasets. The overall cohort had a female predominance
(70.3%, n=754), with a high rate of BRAFV600E mutation
(66.9% positive) and metastasis (M) stage M1 (8.8%). No significant
differences were found in most baseline characteristics between
datasets, except TgAb positivity (P=0.023) and primary tumor (T)
stage (P=0.034). In the cohort, 132 patients (12.3%) were
TgAb-positive. Notably, the clinicopathological profiles, including
age, sex distribution, tumor subtype composition, multifocality,
tumor size, capsule invasion, extrathyroidal extension,
BRAFV600E mutation status, coexisting HT, regional lymph
node (N) stage, M stage, LNR, pre-ablation TSH levels, Tg levels,
and 6-month or 3-year responses were well-balanced across both
datasets (all P>0.05), supporting the robustness of the random
allocation and the validity of subsequent model training and
testing (Table I).
 | Table I.Baseline characteristics of patients
with papillary thyroid carcinoma in the training and validation
cohorts. |
Table I.
Baseline characteristics of patients
with papillary thyroid carcinoma in the training and validation
cohorts.
|
Characteristics | Total
(n=1,073) | Training dataset
(n=751) | Validation dataset
(n=322) | P-value |
|---|
| Mean age ± SD,
years | 42.26±12.11 | 42.14±12.10 | 42.56±12.15 | 0.773 |
| Sex, n (%) |
|
|
|
|
|
Male | 319 (29.7) | 226 (30.1) | 93 (28.9) | 0.691 |
|
Female | 754 (70.3) | 525 (69.9) | 229 (71.1) |
|
| Subtypes, n
(%) |
|
|
|
|
|
Classic | 901 (84.0) | 631 (84.0) | 270 (83.9) | 0.37 |
| FV | 131 (12.2) | 95 (12.6) | 36 (11.2) |
|
|
TCV | 41 (3.8) | 25 (3.3) | 16 (5.0) |
|
| Multifocality, n
(%) |
|
|
|
|
| No | 453 (42.2) | 319 (42.5) | 134 (41.6) | 0.793 |
|
Yes | 620 (57.8) | 432 (57.5) | 188 (58.4) |
|
| Tumor size,
cma | 1.1 (0.7–1.8) | 1.1 (0.7–1.8) | 1.1 (0.8–1.7) | 0.604 |
| Capsule invasion, n
(%) |
|
|
|
|
| No | 454 (42.3) | 318 (42.3) | 136 (42.2) | 0.974 |
|
Yes | 619 (57.7) | 433 (57.7) | 186 (57.8) |
|
| Extrathyroidal
invasion, n (%) |
|
|
|
|
| No | 508 (47.3) | 356 (47.4) | 152 (47.2) | 0.952 |
|
Yes | 565 (52.7) | 395 (52.6) | 170 (52.8) |
|
| BRAF, n (%) |
|
|
|
|
|
Negative | 355 (33.1) | 253 (33.7) | 102 (31.7) | 0.521 |
|
Positive | 718 (66.9) | 498 (66.3) | 220 (68.3) |
|
| HT, n (%) |
|
|
|
|
| No | 875 (81.5) | 609 (81.1) | 266 (82.6) | 0.557 |
|
Yes | 198 (18.5) | 142 (18.9) | 56 (17.4) |
|
| T stage, n (%) |
|
|
|
|
| Tx | 35 (3.3) | 21 (2.8) | 14 (4.3) | 0.034 |
| T1 | 616 (57.4) | 424 (56.5) | 192 (59.6) |
|
| T2 | 120 (11.2) | 76 (10.1) | 44 (13.7) |
|
| T3 | 235 (21.9) | 181 (24.1) | 54 (16.8) |
|
| T4 | 67 (6.2) | 49 (6.5) | 18 (5.6) |
|
| N stage, n (%) |
|
|
|
|
|
Nx/N0 | 72 (6.7) | 53 (7.1) | 19 (5.9) | 0.466 |
|
N1a | 487 (45.4) | 347 (46.2) | 140 (43.5) |
|
|
N1b | 514 (47.9) | 351 (46.7) | 163 (50.6) |
|
| LNR, n (%) |
|
|
|
|
|
<5 | 541 (50.4) | 384 (51.1) | 157 (48.8) | 0.476 |
| ≥5 | 532 (49.6) | 367 (48.9) | 165 (51.2) |
|
| M stage, n (%) |
|
|
|
|
| M0 | 979 (91.2) | 684 (91.1) | 295 (91.6) | 0.776 |
| M1 | 94 (8.8) | 67 (8.9) | 27 (8.4) |
|
| Mean TSH level ±
SD, mIU/l | 106.75±48.54 | 106.64±49.03 | 107.00±47.45 | 0.231 |
| TgAb level
[TgAb(+)], kIU/la | 26.85
(10.67–87.65) | 19.3
(8.1–62.2) | 38.2
(17.4–120.5) | 0.023 |
| Tg level [TgAb(−)],
µg/la | 3.08
(0.94–9.20) | 3.3 (0.9–10.0) | 2.8 (1.0–7.5) | 0.427 |
| 6-month response, n
(%) |
|
|
|
|
| ER +
IDR | 815 (76.0) | 567 (75.5) | 248 (77.0) | 0.594 |
| BIR +
SIR | 258 (24.0) | 184 (24.5) | 74 (23.0) | 3-year |
| 3-year response, n
(%) |
|
|
|
|
| ER +
IDR | 929 (86.6) | 646 (86.0) | 283 (87.9) | 0.41 |
| BIR +
SIR | 144 (13.4) | 105 (14.0) | 39 (12.1) |
|
A comprehensive review of the dataset was conducted.
By random sampling, 1,073 cases not included in the analysis were
selected as the ‘Excluded group’, and the model-trained data was
the ‘Included group’. This revealed that no statistically
significant differences in baseline characteristics. A comparative
analysis of key baseline characteristics (for example, age, sex,
tumor size, T stage, N stage, AJCC Stage and response to therapy)
was conducted between the included cohort (n=1,073) and the
excluded cohort with missing BRAF and TSH levels data (n=1,315). No
statistically significant differences were found in the
distribution of age, sex, HT, subtypes, multifocality, tumor size,
capsule invasion, extrathyroidal invasion, T stage, N stage, M
stage, LNR, Tg level, TgAb level or initial treatment response
between the two groups. This suggests that the cohort with complete
BRAF data is representative of the larger patient population in
these fundamental clinical aspects (Table SI).
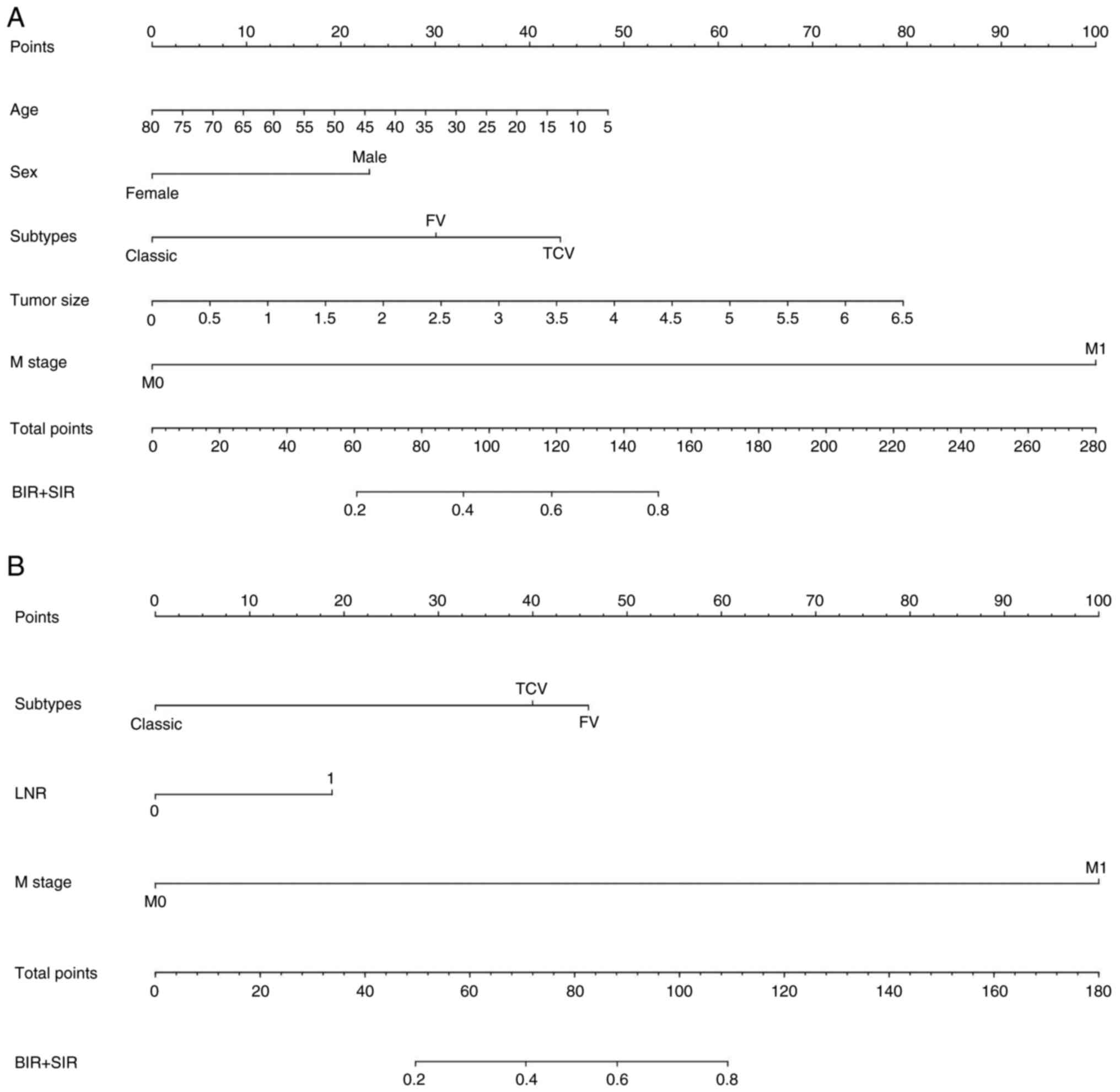
Univariate and multivariate analysis
results and nomogram excluding Tg
Univariate and multivariate analysis identified age,
female sex, follicular variant (FV) and tall cell variant (TCV)
subtypes, tumor size and M1 stage as independent risk factors for
adverse 6-month outcomes (all P<0.05; Fig. 2A; Tables SII and II), with excellent calibration (MAE,
0.009). For 3-year outcomes, risk factors included FV and TCV
subtypes, M1 stage and LNR (all P<0.05; Fig. 2B), showing strong calibration (MAE,
0.012). The 6-month nomogram highlighted M stage as dominant: M1
status contributed 100 points to total score, but only 40%
cumulative risk for BIR/SIR (Table
II).
 | Table II.Excluding thyroglobulin multivariate
logistic regression analysis of factors associated with treatment
response in patients with papillary thyroid carcinoma after
radioactive iodine therapy. |
Table II.
Excluding thyroglobulin multivariate
logistic regression analysis of factors associated with treatment
response in patients with papillary thyroid carcinoma after
radioactive iodine therapy.
| A, 6-month
response |
|---|
|
|---|
|
|
|
| 95% CI |
|
|---|
|
|
|
|
|
|
|---|
| Variate | B | OR | Lower | Upper | P-value |
|---|
| Age | −0.020 | 0.980 | 0.964 | 0.997 | 0.020 |
| Sex (female) | −0.713 | 0.490 | 0.323 | 0.744 | <0.001 |
| Subtypes (FV) | 0.932 | 2.539 | 1.491 | 4.322 | <0.001 |
| Subtypes (TCV) | 1.340 | 3.819 | 1.295 | 11.266 | 0.015 |
| Tumor size | 0.379 | 1.461 | 1.202 | 1.776 | <0.001 |
| M stage (M1) | 3.097 | 22.137 | 9.843 | 49.788 | <0.001 |
| LNR |
|
|
|
|
|
|
| B, 3-year
response |
|
|
|
|
| 95% CI |
|
|
|
|
|
|
|
| Variate | B | OR | Lower | Upper | P-value |
|
| Age |
|
|
|
|
|
| Sex (female) |
|
|
|
|
|
| Subtypes (FV) | 2.136 | 8.467 | 4.343 | 16.509 | <0.001 |
| Subtypes (TCV) | 1.860 | 6.427 | 1.786 | 23.125 | 0.004 |
| Tumor size |
|
|
|
|
|
| M stage (M1) | 4.653 | 104.872 | 40.932 | 268.689 | <0.001 |
| INR | 0.871 | 2.389 | 1.276 | 4.472 | 0.006 |
Assessment of the nomogram excluding
Tg
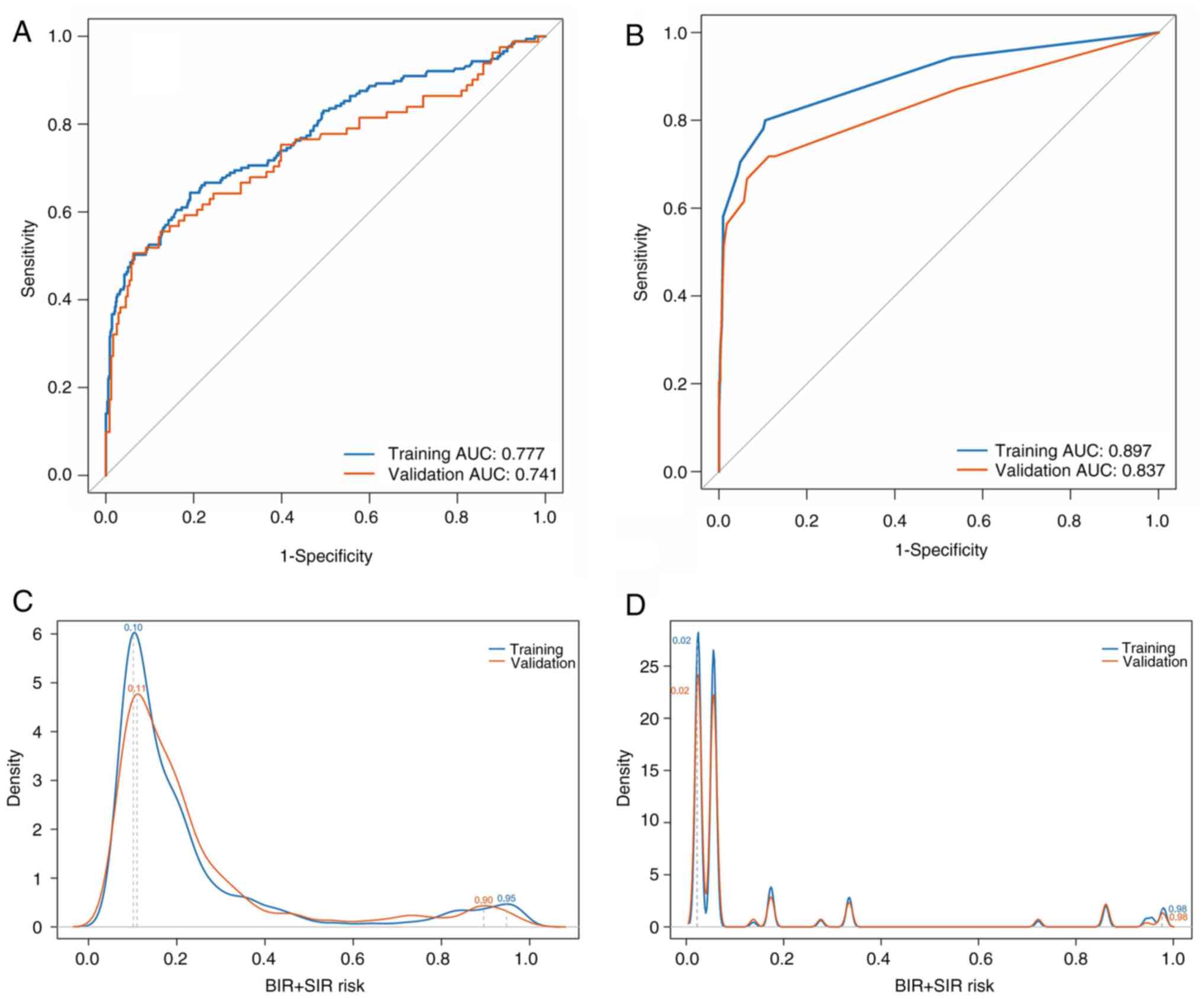
For the 6-month model, AUCs were 0.777 (training:
95% CI, 0.725–0.793) and 0.741 (validation: 95% CI, 0.721–0.790),
while for the 3-year model, AUCs were 0.897 (95% CI, 0.843–0.909)
and 0.837 (95% CI, 0.826–0.913). Probability density curves
revealed bimodal peaks: For the 6-month model, the training dataset
peaked at 0.10 and 0.95, and the validation dataset peaked at 0.11
and 0.90. For the 3-year model, both datasets peaked at 0.02 and
0.98. The dichotomous distribution supported K=2 for K-means
clustering, categorizing patients into low-risk and high-risk
groups. Case counts showed no significant differences for the
6-month (P=0.780) or 3-year (P=0.867) models between datasets,
indicating consistent distributions (Table III). Probability density curves
and boxplots confirmed distributional concordance in the validation
dataset (Figs. 3 and S1).
 | Table III.Distribution and comparison of
excluding thyroglobulin nomogram risk scores between the training
and validation cohorts after K-means clustering. |
Table III.
Distribution and comparison of
excluding thyroglobulin nomogram risk scores between the training
and validation cohorts after K-means clustering.
| A, 6-month
response |
|---|
|
|---|
| Group | Level | Mean | SD | Minimum | Maximum | Q1 | Median | Q3 | Frequency | P-value |
|---|
| Training | Low | 0.17 | 0.09 | 0.05 | 0.50 | 0.10 | 0.13 | 0.20 | 673 | 0.780 |
|
| High | 0.84 | 0.12 | 0.51 | 0.99 | 0.80 | 0.88 | 0.94 | 78 |
|
| Validation | Low | 0.17 | 0.09 | 0.06 | 0.47 | 0.10 | 0.15 | 0.21 | 286 |
|
|
| High | 0.79 | 0.14 | 0.49 | 0.99 | 0.71 | 0.83 | 0.90 | 36 |
|
|
| B, 3-year
response |
|
| Group | Level | Mean | SD | Minimum | Maximum | Q1 | Median | Q3 |
Frequency | P-value |
|
| Training | Low | 0.06 | 0.07 | 0.02 | 0.33 | 0.02 | 0.06 | 0.06 | 684 | 0.867 |
|
| High | 0.91 | 0.08 | 0.72 | 0.98 | 0.86 | 0.94 | 0.98 | 67 |
|
| Validation | Low | 0.06 | 0.07 | 0.02 | 0.33 | 0.02 | 0.06 | 0.06 | 295 |
|
|
| High | 0.89 | 0.09 | 0.72 | 0.98 | 0.86 | 0.86 | 0.98 | 27 |
|
Univariate and multivariate analysis
results and nomogram incorporating Tg
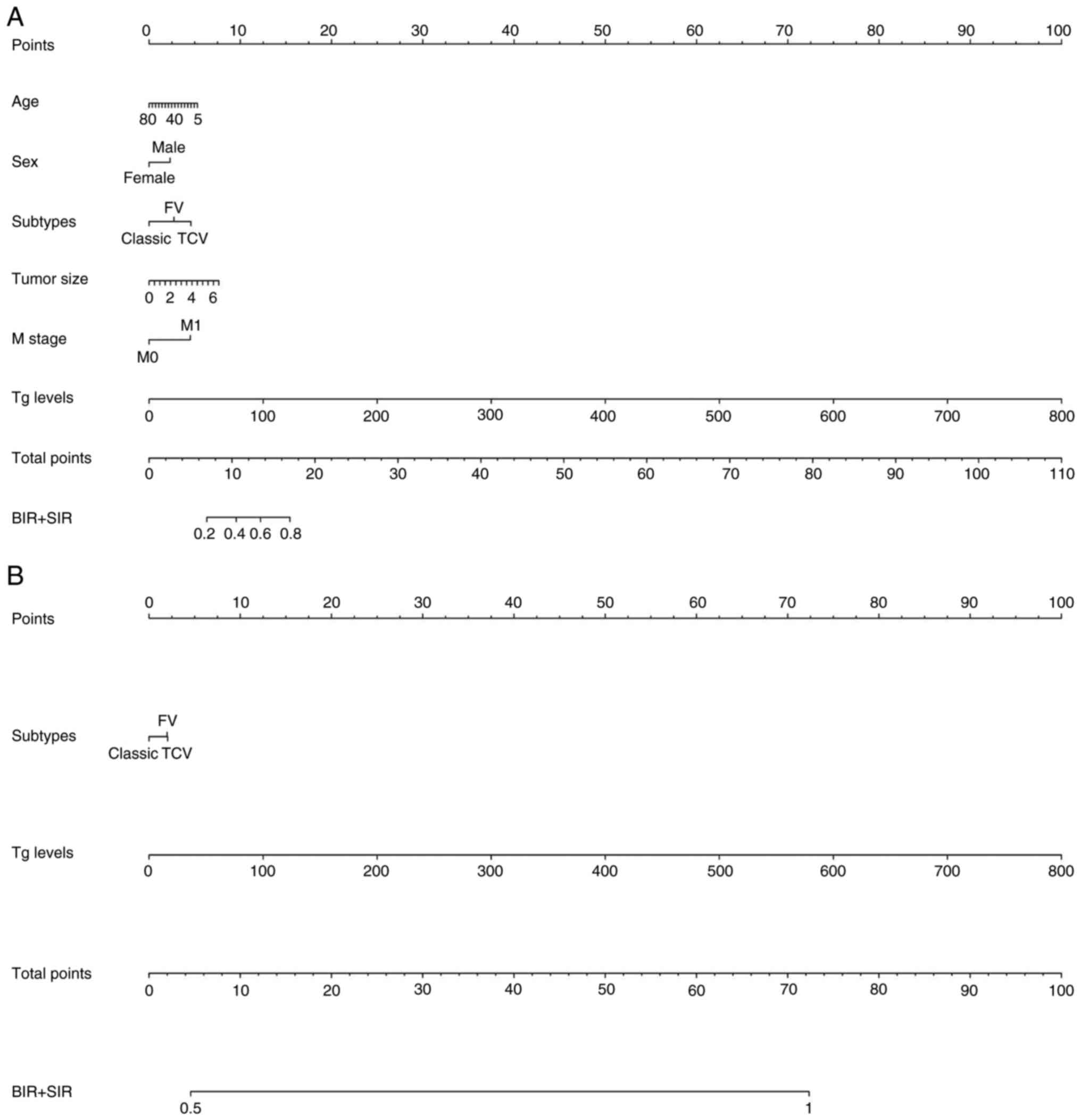
Univariate and multivariate analysis identified age,
female sex, FV and TCV subtype, tumor size, M1 stage and Tg levels
as risk factors for BIR/SIR at 6 months (P<0.05; Fig. 4A; Table
SII). Incorporating these variables yielded a predictive model
with reliable calibration (MAE, 0.018). For 3-year outcomes, risk
factors were FV and TCV subtypes, and Tg levels (P<0.05;
Fig. 4B), with excellent
calibration (MAE, 0.020) (Table
IV; Table SII). Tg level
dominated prediction for both time points. Specifically, 200 µg/l
Tg contributed 22.5 points to total score and accounted for 100%
risk for BIR/SIR.
 | Table IV.Incorporating Tg multivariate
logistic regression analysis of factors associated with treatment
response in patients with papillary thyroid carcinoma after
radioactive iodine therapy. |
Table IV.
Incorporating Tg multivariate
logistic regression analysis of factors associated with treatment
response in patients with papillary thyroid carcinoma after
radioactive iodine therapy.
| A, 6-month
response |
|---|
|
|---|
|
|
|
| 95% CI |
|
|---|
|
|
|
|
|
|
|---|
| Variate | B | OR | Lower | Upper | P-value |
|---|
| Age | −0.020 | 0.981 | 0.964 | 0.998 | 0.025 |
| Sex (female) | −0.635 | 0.530 | 0.345 | 0.813 | 0.004 |
| Subtypes (FV) | 0.755 | 2.127 | 1.217 | 3.717 | 0.008 |
| Subtypes (TCV) | 1.267 | 3.549 | 1.229 | 10.244 | 0.019 |
| Tumor size | 0.325 | 1.384 | 1.128 | 1.697 | 0.002 |
| M stage (M1) | 1.250 | 3.490 | 1.069 | 11.393 | 0.038 |
| Tg level | 0.035 | 1.035 | 1.014 | 1.057 | <0.001 |
|
| B, 3-year
response |
|
|
|
|
| 95% CI |
|
|
|
|
|
|
|
| Variate | B | OR | Lower | Upper | P-value |
|
| Age |
|
|
|
|
|
| Sex (female) |
|
|
|
|
|
| Subtypes (FV) | 1.705 | 5.500 | 2.662 | 11.365 | <0.001 |
| Subtypes (TCV) | 1.755 | 5.783 | 1.761 | 18.992 | 0.004 |
| Tumor size |
|
|
|
|
|
| M stage (M1) |
|
|
|
|
|
| Tg level | 0.108 | 1.114 | 1.087 | 1.141 | <0.001 |
Assessment of the nomogram
incorporating Tg
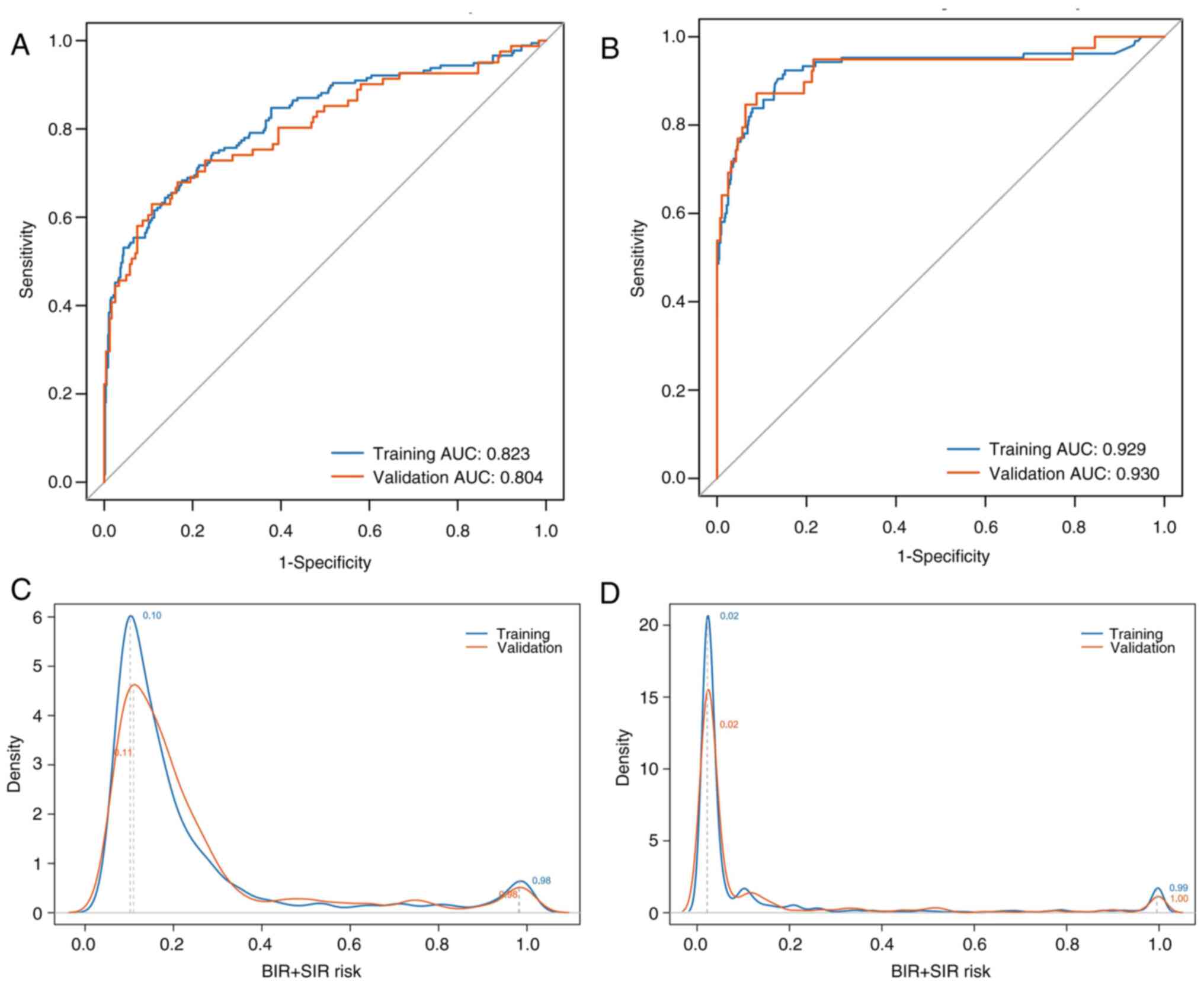
For 6-month outcomes, AUCs were 0.823 (training: 95%
CI, 0.801–0.845) and 0.804 (validation: 95% CI, 0.793–0.820),
indicating robust performance, while for 3-year outcomes, AUCs were
0.929 (training: 95% CI, 0.915–0.943) and 0.930 (validation: 95%
CI, 0.910–0.946), also confirming robustness. Probability density
distributions showed bimodal peaks: For 6-month outcomes, training
clusters were at risk values of 0.1 and 0.98, with validation
clusters at 0.11 and 0.98. For 3-year outcomes, training clusters
were at 0.02 and 0.99, with validation clusters at 0.02 and 1.0.
The distinct low-risk vs. high-risk separation indicated K=2 as
optimal for K-means clustering. Post-clustering, frequency
distributions showed no significant differences for 6-month
(P=0.714) or 3-year (P=0.767) outcomes between cohorts (Table V). Frequency density and box plots
revealed intracluster variations: At 6 months, validation low-risk
clusters agreed closely with training data, while high-risk
clusters exhibited slight dispersion. This pattern persisted for
the 3-year outcomes (Figs. 5 and
S2).
 | Table V.Distribution and comparison of
incorporating Tg nomogram risk scores between the training and
validation cohorts after K-means clustering. |
Table V.
Distribution and comparison of
incorporating Tg nomogram risk scores between the training and
validation cohorts after K-means clustering.
| A, 6-month
response |
|---|
|
|---|
| Group | Level | Mean | SD | Minimum | Maximum | Q1 | Median | Q3 | Frequency | P-value |
|---|
| Training | Low | 0.16 | 0.08 | 0.05 | 0.48 | 0.10 | 0.13 | 0.19 | 663 | 0.714 |
|
| High | 0.83 | 0.17 | 0.49 | 1.00 | 0.70 | 0.89 | 0.98 | 88 |
|
| Validation | Low | 0.17 | 0.08 | 0.06 | 0.47 | 0.10 | 0.15 | 0.21 | 281 |
|
|
| High | 0.78 | 0.18 | 0.48 | 1.00 | 0.63 | 0.78 | 0.97 | 41 |
|
|
| B, 3-year
response |
|
| Group | Level | Mean | SD | Minimum | Maximum | Q1 | Median | Q3 |
Frequency | P-value |
|
| Training | Low | 0.05 | 0.07 | 0.02 | 0.45 | 0.02 | 0.02 | 0.04 | 671 | 0.767 |
|
| High | 0.88 | 0.17 | 0.48 | 1.00 | 0.79 | 0.98 | 1.00 | 80 |
|
| Validation | Low | 0.05 | 0.07 | 0.02 | 0.39 | 0.02 | 0.02 | 0.04 | 285 |
|
|
| High | 0.81 | 0.22 | 0.43 | 1.00 | 0.54 | 0.90 | 1.00 | 37 |
|
Discussion
While conventional staging systems such as the AJCC
TNM classification and ATA risk stratification provide valuable
prognostic frameworks, they lack the precision required for
individualized outcome prediction following RAI treatment (16,17).
The present study developed and validated a nomogram incorporating
readily available clinical and pathological features to predict
prognostic outcomes in patients with PTC following RAI therapy.
Unlike previous PTC prognostic models that focused mainly on
preoperative or surgical outcomes (18–20),
the present study specifically addresses the post-RAI treatment
phase, a critical yet understudied period in disease management.
The present model advances current risk stratification approaches
by incorporating dynamic biomarkers, such as Tg levels, TgAb status
and early (6-month) treatment response, thereby achieving superior
predictive accuracy compared with static staging systems such as
AJCC TNM or ATA risk classifications.
The developed nomogram effectively integrated
clinical and histological variables (age, BRAFV600E
mutation status, extrathyroidal extension and TNM stage), all
established predictors of PTC aggressiveness. Notably,
BRAFV600E-positive tumors and advanced T/N stages were
strongly associated with NER, which aligns with the literature
(21). Specifically, the present
data showed that patients with BRAFV600E mutations had a
2.5-fold increased risk of NER at 6 months, while those with M1
stage disease demonstrated a 22.1-fold increased risk of a poor
outcome. These findings underscore the importance of combining
molecular and clinicopathological factors for prognostication.
Molecular investigations by Fagin and Wells (22) revealed that impaired RAI efficacy is
strongly correlated with BRAF/RAS mutations and RET/PTC
rearrangements, which promote tumor progression through MAPK
pathway-mediated alterations in cell biological behavior. The
present results further substantiate this mechanism, showing that
BRAFV600E mutation was not only prevalent (66.9% in the
cohort), but also independently predictive of a poor treatment
response. Additionally, growing evidence suggests that adverse
pathological features, such as TCV, lymph node metastasis and gross
extrathyroidal extension, further compromise RAI avidity and are
associated with higher rates of treatment failure (23). In the present study, TCV was
associated with a significantly increased risk of poor outcomes at
both 6 months and 3 years, while a high LNR emerged as a strong
predictor of 3-year NER.
However, the differential impact of predictors over
time, with demographic factors such as age and sex diminishing in
significance, while tumor subtype, M stage and LNR persist as
long-term determinants, likely reflects the shifting balance
between initial therapeutic response and inherent tumor biology. In
the short term (6 months), the response to RAI therapy is a
function of both the patient's physiological context (for example,
hormonal milieu or immune status potentially influenced by age and
sex) and tumor characteristics. However, over the longer term (3
years), the influence of transient physiological factors wanes, and
the aggressive, innate biological drive of the tumor itself becomes
the dominant prognostic force. Factors such as TCV subtype and the
presence of M1 stage are well-established markers of
de-differentiation, reduced iodine avidity and increased metastatic
potential; their persistent significance underscores that they
represent a more aggressive disease phenotype that is harder to
eradicate with initial therapy and is more likely to progress or
recur despite an initial response (24,25).
This temporal shift in predictor weight underscores the dynamic
nature of PTC progression and highlights the need for time-specific
prognostic models (26).
The study cohort exhibited notable variations in
TgAb positivity rates (10.8% in the training dataset vs. 15.5% in
the validation dataset; P=0.023), aligning with reported TgAb
prevalence ranges of 10–25% in PTC populations (27). The 12.3% overall TgAb-positive rate
(132/1,073) highlights the clinical imperative for Tg-independent
predictive tools, particularly given that TgAb interference affects
~20% of Tg measurements in clinical practice. Although Tg is not
incorporated in the initial risk stratification under current ATA
guidelines, an associated has been suggested between undetectable
post-operative Tg and a low possibility of biochemical or
structural recurrence in a patient with low ATA risk (28). A notable finding was the
disproportionate influence of Tg in the initial model, where a
solitary Tg level of 200 µg/l could theoretically confer a 100%
predicted risk of poor prognosis; a clinically unrealistic scenario
that highlights the limitations of over-reliance on single
biomarkers. To address this, the present study developed an
alternative 3-year prognostic model that replaces Tg with more
balanced multivariable predictors, including LNR and M stage. This
refined version demonstrates three key advantages: i) It eliminates
the disproportionate weighting of any single variable; ii)
maintains clinically acceptable performance; and iii) provides
reliable risk stratification even when Tg measurements are
unavailable or unreliable, which is a common clinical challenge
given that 20–25% of patients with PTC have TgAb interference.
Furthermore, unlike models that rely heavily on
pre-ablation Tg levels or fixed-dose RAI protocols, the present
nomogram integrates both dynamic variables (for example, Tg levels
and 6-month treatment response) and static clinicopathological
features (for example, tumor subtype and M stage), enhancing its
predictive power for both short- and long-term outcomes (29). A particularly innovative aspect of
the present study is the demonstration that the model remains
clinically useful even when Tg is excluded, addressing a major
limitation in current practice where Tg measurements can be
unreliable due to antibody interference or other factors.
Additionally, the nomogram provides a more balanced risk assessment
by incorporating multifactorial interactions (for example, M stage
and LNR) that mitigate the over-reliance on any single predictor,
such as Tg. This contrasts with some existing tools that may
disproportionately weight individual variables. Rigorous validation
across independent cohorts further strengthens the reliability and
generalizability of the present model, as evidenced by robust AUC
values (>0.8) and excellent calibration (MAE <0.02). Garo
et al (30) noted that
currently available nomograms exhibit considerable heterogeneity,
and their predictive performance appears highly dependent on the
particular patient cohorts from which they were derived.
While the present study presents a clinically useful
nomogram for predicting RAI therapy outcomes in patients with PTC,
several limitations should be acknowledged. First, the
retrospective design may introduce selection bias, particularly due
to the exclusion of patients with incomplete data (for example,
missing BRAF mutation status), which could limit the
generalizability of the findings. Second, the model was developed
and validated at a single institution, and its performance should
be confirmed in multicenter, prospective cohorts to ensure broader
applicability across diverse populations and healthcare settings.
Third, although the nomogram incorporates key clinicopathological
variables, it does not account for emerging molecular markers, such
as TERT promoter mutations or programmed death-ligand 1 expression,
which have been associated with aggressive tumor behavior and
treatment resistance. Future iterations of the model could benefit
from integrating these biomarkers to enhance predictive
accuracy.
In conclusion, this study developed a clinical
nomogram that effectively predicts outcomes in patients with PTC
after RAI therapy by integrating key risk factors, such as tumor
subtype, M stage and Tg levels. The model showed strong predictive
accuracy and reliably stratified patients into low- and high-risk
groups. Even without Tg data, it maintained utility using
alternative markers. This tool supports personalized treatment
planning, though further external validation is needed to confirm
its broader applicability.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Author contributions
JC and HL wrote the main manuscript text. XL, HL and
HX prepared the figures and tables. JC, XG and HX conceived and
designed the study. JC, HL, XL and XG collected and analyzed the
data. HL, XL and XG confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study complies with the Declaration of
Helsinki. All of the study procedures were approved by The Second
Hospital of Shandong University institutional review board
(approval no. KYLL-2022LW077; Jinan, China). Written informed
consent was obtained from all individual participants included in
the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HT
|
Hashimoto's thyroiditis
|
|
T
|
primary tumor
|
|
N
|
regional lymph nodes
|
|
M
|
metastasis
|
|
TSH
|
thyroid-stimulating hormone
|
|
TgAb
|
thyroglobulin antibody
|
|
ER
|
excellent response
|
|
IDR
|
indeterminate response
|
|
BIR
|
biochemical incomplete response
|
|
SIR
|
structural incomplete response
|
References
|
1
|
Megwalu UC and Moon PK: Thyroid cancer
incidence and mortality trends in the United States: 2000–2018.
Thyroid. 32:560–570. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tuttle RM, Ahuja S, Avram AM, Bernet VJ,
Bourguet P, Daniels GH, Dillehay G, Draganescu C, Flux G, Führer D,
et al: controversies, consensus, and collaboration in the use of
131I therapy in differentiated thyroid cancer: A joint
statement from the American thyroid association, the European
association of nuclear medicine, the society of nuclear medicine
and molecular imaging, and the European thyroid association.
Thyroid. 29:461–470. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xing M, Alzahrani AS, Carson KA, Viola D,
Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM, et al:
Association between BRAF V600E mutation and mortality in patients
with papillary thyroid cancer. JAMA. 309:1493–1501. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moon S, Song YS, Kim YA, Lim JA, Cho SW,
Moon JH, Hahn S, Park DJ and Park YJ: Effects of coexistent
BRAFV600E and TERT promoter mutations on poor clinical
outcomes in papillary thyroid cancer: A meta-analysis. Thyroid.
27:651–660. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bournaud C, Descotes F, Decaussin-Petrucci
M, Berthiller J, de la Fouchardière C, Giraudet AL,
Bertholon-Gregoire M, Robinson P, Lifante JC, Lopez J and
Borson-Chazot F: TERT promoter mutations identify a high-risk group
in metastasis-free advanced thyroid carcinoma. Eur J Cancer.
108:41–49. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Valero C, Eagan A, Adilbay D, Matsuura D,
Harries V, Shaha AR, Shah JP, Tuttle RM, Akhmedin D, Pinheiro RA,
et al: A clinical nomogram to predict survival outcomes in patients
with well-differentiated thyroid cancer. Thyroid. 35:397–405. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei M, Wang R, Zhang W, Zhang J, Fang Q,
Fang Z, Liu B and Li Y: Landscape of gene mutation in Chinese
thyroid cancer patients: Construction and validation of lymph node
metastasis prediction model based on clinical features and gene
mutation marker. Cancer Med. 12:12929–12942. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wen R, Zhao M, Chen C, Yang Y and Zhang B:
A novel nomogram integrated with preablation stimulated
thyroglobulin and thyroglobulin/thyroid-stimulating hormone ratio
to predict the therapeutic response of intermediate- and high-risk
differentiated thyroid cancer patients: A bi-center retrospective
study. Endocrine. 84:989–998. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li S, Yun M, Hong G, Tian L, Yang A and
Liu L: Development and validation of a nomogram for preoperative
prediction of level VII nodal spread in papillary thyroid cancer:
Radiologic-pathologic correlation. Surg Oncol. 37:1015202021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Wang J, Hui J, Hou G, He W, Sun T,
Gao L, Wei Y, Zhang W, Zheng L, et al: Postoperative nomogram for
predicting involved lymph node in differentiated thyroid carcinoma:
An observational study. Future Oncol. 21:2785–2793. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Persoon ACM, Links TP, Wilde J, Sluiter
WJ, Wolffenbuttel BHR and van den Ouweland JMW: Thyroglobulin (Tg)
recovery testing with quantitative Tg antibody measurement for
determining interference in serum Tg assays in differentiated
thyroid carcinoma. Clin Chem. 52:1196–1199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Neshandar Asli I, Siahkali AS, Shafie B,
Javadi H and Assadi M: Prognostic value of basal serum
thyroglobulin levels, but not basal antithyroglobulin antibody
(TgAb) levels, in patients with differentiated thyroid cancer. Mol
Imaging Radionucl Ther. 23:54–59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American thyroid association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American thyroid association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Duda OR, Slipetsky RR and Bojko NI:
Principles of medullary thyroid cancer staging according to AJCC
TNM 8th edition. AML. 27:101–116. 2021. View Article : Google Scholar
|
|
16
|
Suh S, Kim YH, Goh TS, Lee J, Jeong DC, Oh
SO, Hong JC, Kim SJ, Kim IJ and Pak K: Outcome prediction with the
revised American joint committee on cancer staging system and
American thyroid association guidelines for thyroid cancer.
Endocrine. 58:495–502. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghaznavi SA, Ganly I, Shaha AR, English C,
Wills J and Tuttle RM: Using the American thyroid association
risk-stratification system to refine and individualize the American
joint committee on cancer eighth edition disease-specific survival
estimates in differentiated thyroid cancer. Thyroid. 28:1293–1300.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Balachandran VP, Gonen M, Smith JJ and
DeMatteo RP: Nomograms in oncology: More than meets the eye. Lancet
Oncol. 16:e173–e180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun J, Jiang Q and Wang X, Liu W and Wang
X: Nomogram for preoperative estimation of cervical lymph node
metastasis risk in papillary thyroid microcarcinoma. Front
Endocrinol (Lausanne). 12:6139742021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang R, Tang Z, Wu Z, Xiao Y, Li J, Zhu J,
Zhang X and Ming J: Construction and validation of nomograms to
reduce completion thyroidectomy by predicting lymph node metastasis
in low-risk papillary thyroid carcinoma. Eur J Surg Oncol.
49:1395–1404. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Q, Yu B, Zhang S, Wang D, Xiao Z,
Meng H, Dong L, Zhang Y, Wu J, Hou Z, et al: Papillary thyroid
carcinoma: correlation between molecular and clinical features. Mol
Diagn Ther. 28:601–609. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fagin JA and Wells SA Jr: Biologic and
clinical perspectives on thyroid cancer. N Engl J Med.
375:1054–1067. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nappi C, Megna R, Zampella E, Volpe F,
Piscopo L, Falzarano M, Vallone C, Pace L, Petretta M, Cuocolo A
and Klain M: External validation of a predictive model for
post-treatment persistent disease by 131I whole-body
scintigraphy in patients with differentiated thyroid cancer. Eur J
Nucl Med Mol Imaging. 52:2867–2874. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang X, Zheng X, Zhu J, Li Z and Wei T: A
nomogram and risk classification system for predicting
cancer-specific survival in tall cell variant of papillary thyroid
cancer: A SEER-based study. J Endocrinol Invest. 46:893–901. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim DH, Jung JH, Son SH, Kim CY, Hong CM,
Jeong SY, Lee SW, Lee J and Ahn BC: Difference of clinical and
radiological characteristics according to radioiodine avidity in
pulmonary metastases of differentiated thyroid cancer. Nucl Med Mol
Imaging. 48:55–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lamartina L, Durante C, Lucisano G, Grani
G, Bellantone R, Lombardi CP, Pontecorvi A, Arvat E, Felicetti F,
Zatelli MC, et al: Are evidence-based guidelines reflected in
clinical practice? An analysis of prospectively collected data of
the Italian thyroid cancer observatory. Thyroid. 27:1490–1497.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Anderson H, Lim KH, Gull S, Oprean R,
Spence K and Cvasciuc T: Predicting clinical outcomes of patients
with serum thyroglobulin antibodies after thyroidectomy for
differentiated thyroid cancer: A retrospective study from a UK
regional center. Minerva Endocrinol (Torino). 49:60–68.
2024.PubMed/NCBI
|
|
28
|
Zhang Y, Hua W, Zhang X, Peng J, Liang J
and Gao Z: The predictive value for excellent response to initial
therapy in differentiated thyroid cancer: Preablation-stimulated
thyroglobulin better than the TNM stage. Nucl Med Commun.
39:405–410. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu L, Li Q, Ge Z, Lu Y, Lin C, Lv J, Huang
J, Mu X and Fu W: Development of a predictive nomogram for
intermediate-risk differentiated thyroid cancer patients after
fixed 3.7GBq (100mCi) radioiodine remnant ablation. Front
Endocrinol (Lausanne). 15:13616832024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Garo ML, Deandreis D, Campennì A,
Vrachimis A, Petranovic Ovcaricek P and Giovanella L: Accuracy of
papillary thyroid cancer prognostic nomograms: A systematic review.
Endocr Connect. 12:e2204572023.
|