|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
2
|
Morgan E, Soerjomataram I, Rumgay H,
Coleman HG, Thrift AP, Vignat J, Laversanne M, Ferlay J and Arnold
M: The global landscape of esophageal squamous cell carcinoma and
esophageal adenocarcinoma incidence and mortality in 2020 and
projections to 2040: New estimates from GLOBOCAN 2020.
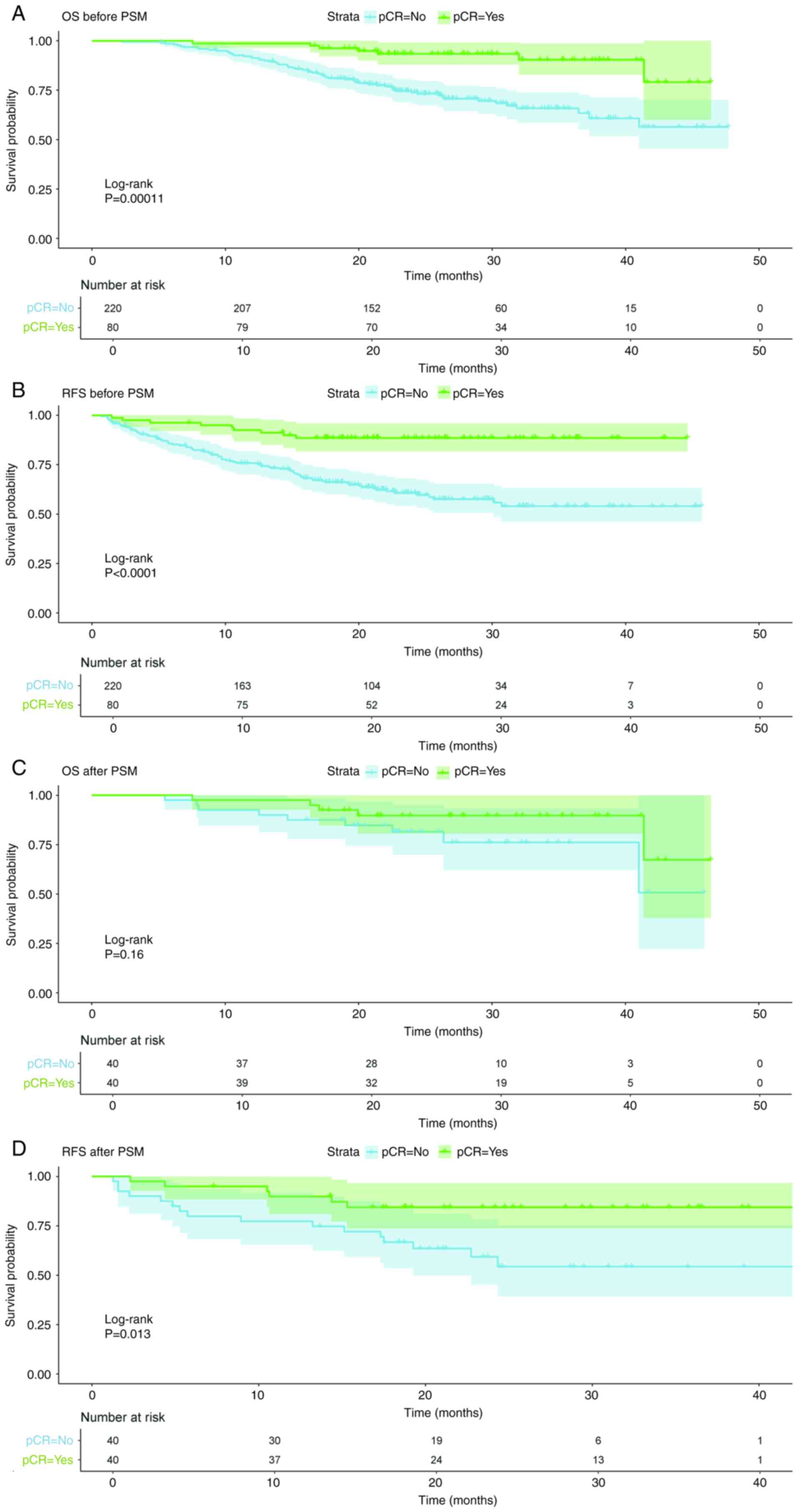
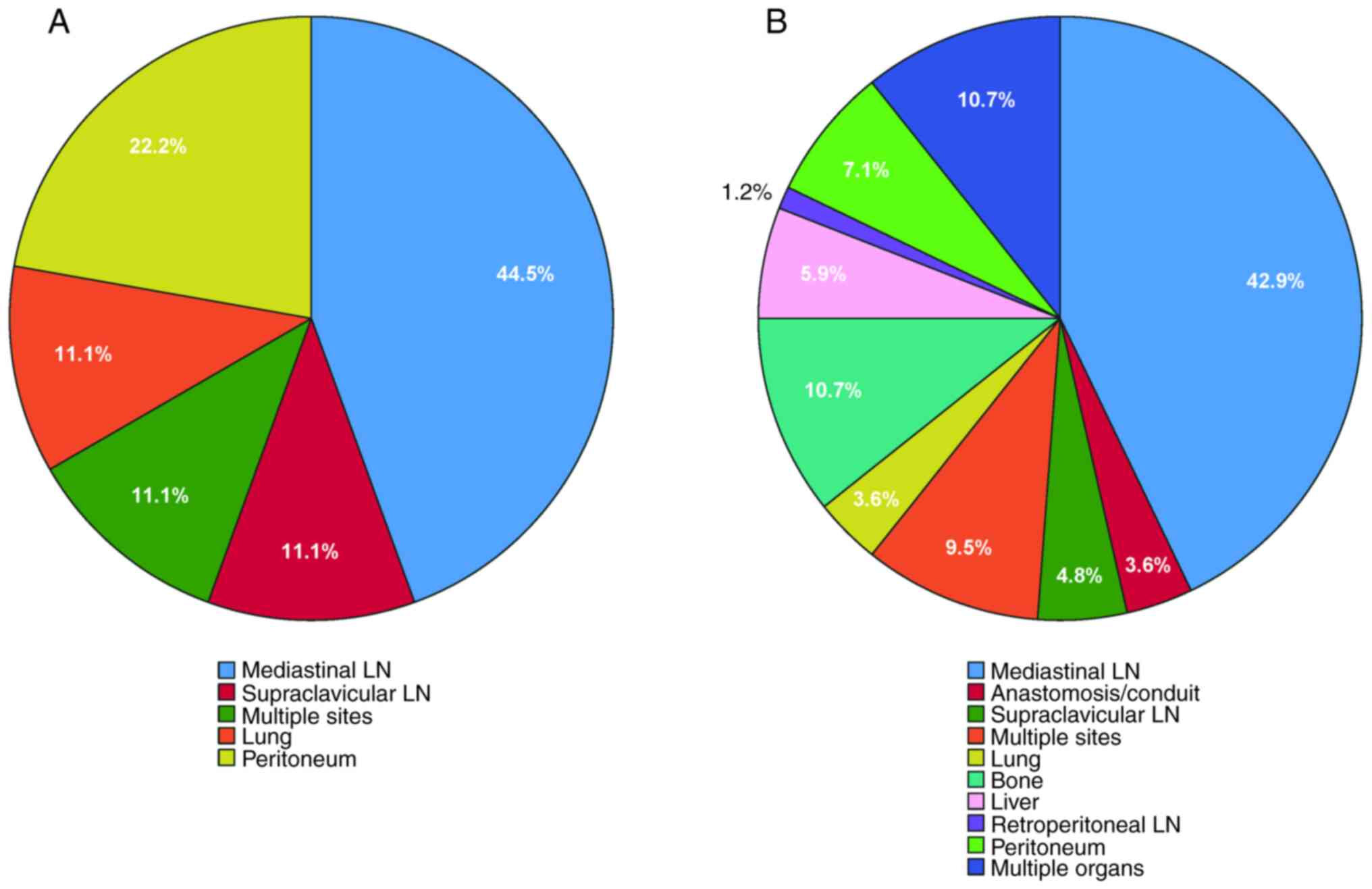
Gastroenterology. 163:649–658.e2. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu J, Zhu L, Huang X, Lu Z, Wang Y, Yang
Y, Ye J, Gu C, Lv W, Zhang C and Hu J: Does the time interval from
neoadjuvant camrelizumab combined with chemotherapy to surgery
affect outcomes for locally advanced esophageal squamous cell
carcinoma? J Cancer Res Clin Oncol. 150:1612024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eyck BM, van Lanschot JJB, Hulshof MCCM,
van der Wilk BJ, Shapiro J, van Hagen P, van Berge Henegouwen MI,
Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, et al:
Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for
esophageal cancer: The randomized controlled CROSS trial. J Clin
Oncol. 39:1995–2004. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu
Z, Mao W, Xiang J, Han Y, Chen Z, et al: Long-term efficacy of
neoadjuvant chemoradiotherapy plus surgery for the treatment of
locally advanced esophageal squamous cell carcinoma: The
NEOCRTEC5010 randomized clinical trial. JAMA Surg. 156:721–729.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Janjigian YY, Bendell J, Calvo E, Kim JW,
Ascierto PA, Sharma P, Ott PA, Peltola K, Jaeger D, Evans J, et al:
CheckMate-032 study: Efficacy and safety of nivolumab and nivolumab
plus ipilimumab in patients with metastatic esophagogastric Cancer.
J Clin Oncol. 36:2836–2844. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shah MA, Kojima T, Hochhauser D, Enzinger
P, Raimbourg J, Hollebecque A, Lordick F, Kim SB, Tajika M, Kim HT,
et al: Efficacy and safety of pembrolizumab for heavily pretreated
patients with advanced, metastatic adenocarcinoma or squamous cell
carcinoma of the esophagus: The phase 2 KEYNOTE-180 study. JAMA
Oncol. 5:546–550. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kojima T, Shah MA, Muro K, Francois E,
Adenis A, Hsu CH, Doi T, Moriwaki T, Kim SB, Lee SH, et al:
Randomized Phase III KEYNOTE-181 study of pembrolizumab versus
chemotherapy in advanced esophageal cancer. J Clin Oncol.
38:4138–4148. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun JM, Shen L, Shah MA, Enzinger P,
Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, et al:
Pembrolizumab plus chemotherapy versus chemotherapy alone for
first-line treatment of advanced oesophageal cancer (KEYNOTE-590):
A randomised, Placebo-controlled, phase 3 study. Lancet.
398:759–771. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li C, Zhao S, Zheng Y, Han Y, Chen X,
Cheng Z, Wu Y, Feng X, Qi W, Chen K, et al: Preoperative
pembrolizumab combined with chemoradiotherapy for oesophageal
squamous cell carcinoma (PALACE-1). Eur J Cancer. 144:232–241.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van den Ende T, de Clercq NC, van Berge
Henegouwen MI, Gisbertz SS, Geijsen ED, Verhoeven RHA, Meijer SL,
Schokker S, Dings MPG, Bergman JJGHM, et al: Neoadjuvant
chemoradiotherapy combined with atezolizumab for resectable
esophageal adenocarcinoma: A Single-arm phase II feasibility trial
(PERFECT). Clin Cancer Res. 27:3351–3359. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamamoto S, Kato K, Daiko H, Kojima T,
Hara H, Abe T, Tsubosa Y, Nagashima K, Aoki K, Mizoguchi Y, et al:
Feasibility study of nivolumab as neoadjuvant chemotherapy for
locally esophageal carcinoma: FRONTiER (JCOG1804E). Future Oncol.
16:1351–1357. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patel MA, Kratz JD, Lubner SJ, Loconte NK
and Uboha NV: Esophagogastric cancers: Integrating immunotherapy
therapy into current practice. J Clin Oncol. 40:2751–2762. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen X, Xu X, Wang D, Liu J, Sun J, Lu M,
Wang R, Hui B, Li X, Zhou C, et al: Neoadjuvant sintilimab and
chemotherapy in patients with potentially resectable esophageal
squamous cell carcinoma (KEEP-G 03): An open-label, single-arm,
phase 2 trial. J Immunother Cancer. 11:e0058302023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hong ZN, Gao L, Weng K, Huang Z, Han W and
Kang M: Safety and feasibility of esophagectomy following combined
immunotherapy and chemotherapy for locally advanced esophageal
squamous cell carcinoma: A propensity score matching analysis.
Front Immunol. 13:8363382022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hong Z, Xu J, Chen Z, Xu H, Huang Z, Weng
K, Cai J, Ke S, Chen S, Xie J, et al: Additional neoadjuvant
immunotherapy does not increase the risk of anastomotic leakage
after esophagectomy for esophageal squamous cell carcinoma: A
multicenter retrospective cohort study. Int J Surg. 109:2168–2178.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao Y, Huang B, Tang H, Dong D, Shen T,
Chen X, Feng X, Zhang J, Shi L, Li C, et al: Online tools to
predict individualised survival for primary oesophageal cancer
patients with and without pathological complete response after
neoadjuvant therapy followed by oesophagectomy: Development and
external validation of two independent nomograms. BMJ Open
Gastroenterol. 11:e0012532024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xi M, Yang Y, Zhang L, Yang H, Merrell KW,
Hallemeier CL, Shen RK, Haddock MG, Hofstetter WL, Maru DM, et al:
Multi-institutional analysis of recurrence and survival after
neoadjuvant chemoradiotherapy of esophageal cancer: Impact of
histology on recurrence patterns and outcomes. Ann Surg.
269:663–670. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meguid RA, Hooker CM, Taylor JT, Kleinberg
LR, Cattaneo SM II, Sussman MS, Yang SC, Heitmiller RF, Forastiere
AA and Brock MV: Recurrence after neoadjuvant chemoradiation and
surgery for esophageal cancer: Does the pattern of recurrence
differ for patients with complete response and those with partial
or no response? J Thorac Cardiovasc Surg. 138:1309–1317. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blum Murphy M, Xiao L, Patel VR, Maru DM,
Correa AMG, Amlashi F, Liao Z, Komaki R, Lin SH, Skinner HD, et al:
Pathological complete response in patients with esophageal cancer
after the trimodality approach: The association with baseline
variables and survival-The University of Texas MD Anderson cancer
center experience. Cancer. 123:4106–4113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jipping KM, Hulshoff JB, van Amerongen EA,
Bright TI, Watson DI and Plukker JTM: Influence of tumor response
and treatment schedule on the distribution of tumor recurrence in
esophageal cancer patients treated with neoadjuvant
chemoradiotherapy. J Surg Oncol. 116:1096–1102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vallböhmer D, Hölscher AH, DeMeester S,
DeMeester T, Salo J, Peters J, Lerut T, Swisher SG, Schröder W,
Bollschweiler E and Hofstetter W: A multicenter study of survival
after neoadjuvant radiotherapy/chemotherapy and esophagectomy for
ypT0N0M0R0 esophageal cancer. Ann Surg. 252:744–749. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rice TW, Ishwaran H, Hofstetter WL, Kelsen
DP, Apperson-Hansen C and Blackstone EH: Recommendations for
pathological staging (pTNM) of cancer of the esophagus and
esophagogastric junction for the 8th edition AJCC/UICC staging
manuals. Dis Esophagus. 29:897–905. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Corvera C, Das P, Denlinger CS, Enzinger PC, Fanta P, Farjah F, et
al: Esophageal and esophagogastric junction cancers, version
2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr
Canc Netw. 17:855–883. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Muro K, Lordick F, Tsushima T,
Pentheroudakis G, Baba E, Lu Z, Cho BC, Nor IM, Ng M, Chen LT, et
al: Pan-Asian adapted ESMO Clinical Practice Guidelines for the
management of patients with metastatic oesophageal cancer: A
JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann
Oncol. 30:34–43. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu
Z, Mao W, Xiang J, Han Y, Chen Z, et al: Neoadjuvant
chemoradiotherapy followed by surgery versus surgery alone for
locally advanced squamous cell carcinoma of the esophagus
(NEOCRTEC5010): A phase III multicenter, randomized, Open-label
clinical trial. J Clin Oncol. 36:2796–2803. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Warren S, Partridge M, Carrington R, Hurt
C, Crosby T and Hawkins MA: Radiobiological determination of dose
escalation and normal tissue toxicity in definitive chemoradiation
therapy for esophageal cancer. Int J Radiat Oncol Biol Phys.
90:423–429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Passot G, You B, Boschetti G, Fontaine J,
Isaac S, Decullier E, Maurice C, Vaudoyer D, Gilly FN, Cotte E and
Glehen O: Pathological response to neoadjuvant chemotherapy: A new
prognosis tool for the curative management of peritoneal colorectal
carcinomatosis. Ann Surg Oncol. 21:2608–2614. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Corsini EM, Weissferdt A, Pataer A, Zhou
N, Antonoff MB, Hofstetter WL, Mehran RJ, Rajaram R, Rice DC, Roth
JA, et al: Pathological nodal disease defines survival outcomes in
patients with lung cancer with tumour major pathological response
following neoadjuvant chemotherapy. Eur J Cardiothorac Surg.
59:100–108. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Conforti F, Pala L, Sala I, Oriecuia C, De
Pas T, Specchia C, Graffeo R, Pagan E, Queirolo P, Pennacchioli E,
et al: Evaluation of pathological complete response as surrogate
endpoint in neoadjuvant randomised clinical trials of early stage
breast cancer: Systematic review and Meta-analysis. BMJ.
375:e0663812021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Su F, Yang X, Yin J, Shen Y and Tan L:
Validity of using pathological response as a surrogate for overall
survival in neoadjuvant studies for esophageal cancer: A systematic
review and Meta-analysis. Ann Surg Oncol. 30:7461–7471. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chao YK, Chen HS, Wang BY, Hsu PK, Liu CC
and Wu SC: Factors associated with survival in patients with
oesophageal cancer who achieve pathological complete response after
chemoradiotherapy: A nationwide population-based study. Eur J
Cardiothorac Surg. 51:155–159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu L, Wei XF, Li CJ, Yang ZY, Yu YK, Li
HM, Xie HN, Yang YF, Jing WW, Wang Z, et al: Pathological responses
and surgical outcomes after neoadjuvant immunochemotherapy versus
neoadjuvant chemoradiotherapy in patients with locally advanced
esophageal squamous cell carcinoma. Front Immunol. 13:10525422022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rose BS, Winer EP and Mamon HJ: Perils of
the pathological complete response. J Clin Oncol. 34:3959–3962.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Colori A and Hiley C: The interaction of
preexisting cardiac dysfunction and heart dose from radical
radiotherapy on All-cause mortality in locally advanced NSCLC. J
Thorac Oncol. 18:14–16. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu YY, Dai L, Yang YB, Yan WP, Cheng H,
Fan MY, Gao YM and Chen KN: Long-term survival and recurrence
patterns in locally advanced esophageal squamous cell carcinoma
patients with pathological complete response after neoadjuvant
chemotherapy followed by surgery. Ann Surg Oncol. 31:5047–5054.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
van Hagen P, Wijnhoven BP, Nafteux P,
Moons J, Haustermans K, De Hertogh G, van Lanschot JJ and Lerut T:
Recurrence pattern in patients with a pathologically complete
response after neoadjuvant chemoradiotherapy and surgery for
oesophageal cancer. Br J Surg. 100:267–273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Agoston AT, Zheng Y, Bueno R, Lauwers GY,
Odze RD and Srivastava A: Predictors of disease recurrence and
survival in esophageal adenocarcinomas with complete response to
neoadjuvant therapy. Am J Surg Pathol. 39:1085–1092. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xi M, Hallemeier CL, Merrell KW, Liao Z,
Murphy MAB, Ho L, Hofstetter WL, Mehran R, Lee JH, Bhutani MS, et
al: Recurrence risk stratification after preoperative
chemoradiation of esophageal adenocarcinoma. Ann Surg. 268:289–295.
2018. View Article : Google Scholar : PubMed/NCBI
|