Introduction
Esophageal cancer is a prevalent upper digestive
tract tumor, currently ranking seventh in terms of incidence and
sixth in terms of mortality among all tumor types globally
(1). In Asia, esophageal squamous
cell carcinoma (ESCC) is the predominant pathological type of
esophageal cancer, accounting for ~90% of all malignant tumors of
the esophagus (2). Early esophageal
cancer typically lacks specific symptoms, resulting in most
patients seeking medical treatment at mid-to-late stages.
Currently, the main surgical methods for esophageal cancer include
the Sweet procedure with a single incision on the left thoracotomy,
the Ivor-Lewis procedure with two incisions on the right
thoracotomy and abdomen, and the McKeown procedure with three
incisions on the neck, right thoracotomy and abdomen. Those with
surgically resectable esophageal cancer are primarily diagnosed
with locally advanced disease (3).
According to the findings of the NEOCRTEC5010 and CROSS trials,
chemoradiotherapy is regarded as the standard treatment approach
for these patients (4,5).
The emergence of immunotherapy has led to numerous
clinical studies demonstrating its efficacy in the treatment of
various tumors, including esophageal cancer (6–8). The
KEYNOTE-590 study showed that immunotherapy combined with
chemotherapy could improve the overall survival (OS) of patients
with ESCC (9). The KEYNOTE-590
study demonstrated that the combination of immunotherapy and
chemotherapy improved OS in patients with ESCC (10,11).
Preoperative radiotherapy may increase perioperative complications
and complicate surgical procedures. By contrast, neoadjuvant
immunochemotherapy exhibits therapeutic efficacy comparable to that
of neoadjuvant immunotherapy combined with chemoradiotherapy
(12–14). Physicians have increasingly adopted
neoadjuvant immunochemotherapy as a treatment for locally advanced
ESCC, and the combination of platinum plus paclitaxel chemotherapy
with immunotherapy drugs such as nivolumab, pembrolizumab,
camrelizumab and sintilimab is currently widely used (15–17).
Pathological complete response (pCR) is
characterized by the absence of histological evidence of tumor in
the surgical resection specimen (18). Numerous studies have indicated that
patients achieving pCR exhibit improved OS and recurrence-free
survival (RFS) (19,20). Consequently, pCR has been utilized
as a key indicator to assess the efficacy of neoadjuvant therapy in
various clinical trials (19–21).
Theoretically, patients achieving pCR may be cured; however, ~30%
of these patients experience recurrence within 2 years post-surgery
(19,21,22).
Neoadjuvant immunochemotherapy has been gradually implemented as a
treatment for ESCC over the past decade (10–14).
Consequently, there is a lack of multicenter studies investigating
the relationship between pCR and prognosis, as well as recurrence
patterns in this patient population. The present study included
data from 300 patients across four esophageal cancer treatment
centers to investigate the impact of pCR on the prognosis and
recurrence patterns in patients with ESCC undergoing neoadjuvant
immunochemotherapy.
Materials and methods
Selection of patients
The present study included 300 patients diagnosed
with locally advanced esophageal cancer, all of whom underwent
surgical treatment across four medical centers between December
2019 and April 2024. The four medical centers were the Beijing
Chest Hospital (Beijing, China), National Cancer Center/National
Clinical Research Center for Cancer/Cancer Hospital & Shenzhen
Hospital (Shenzhen, China), Shaanxi Provincial People's Hospital
(Xi'an, China) and Anyang Tumor Hospital (Anyang, China). The
present study was approved by the Ethics Committee of National
Cancer Center/National Clinical Research Center for Cancer/Cancer
Hospital & Shenzhen Hospital (approval no. YW2024-3-1), Anyang
Tumor Hospital (approval no. 2023YP18H01), Shaanxi Provincial
People's Hospital (approval no. 2023K-S129) and Beijing Chest
Hospital (approval no. 2023-ky-59). The patients provided written
informed consent to participate.
The inclusion criteria were as follows: i)
Histological confirmation of ESCC through endoscopic biopsy before
treatment; ii) preoperative clinical stage of T1b-2N1-3M0 or
T3-4aN0-3M0 (patients with T1b-2N0M0 do not receive neoadjuvant
therapy due to the early stage of disease, while patients with
TxNxM1 do not undergo surgical treatment due to distant
metastasis); and iii) all patients underwent esophagectomy
following neoadjuvant immunochemotherapy. The criteria for
exclusion were: i) Patients who died within 30 days following
surgery; ii) patients who did not achieve radical resection
verified by postoperative pathology; iii) patients without
differentiation information in the postoperative pathology report;
and iv) history of radiotherapy before surgery. The case selection
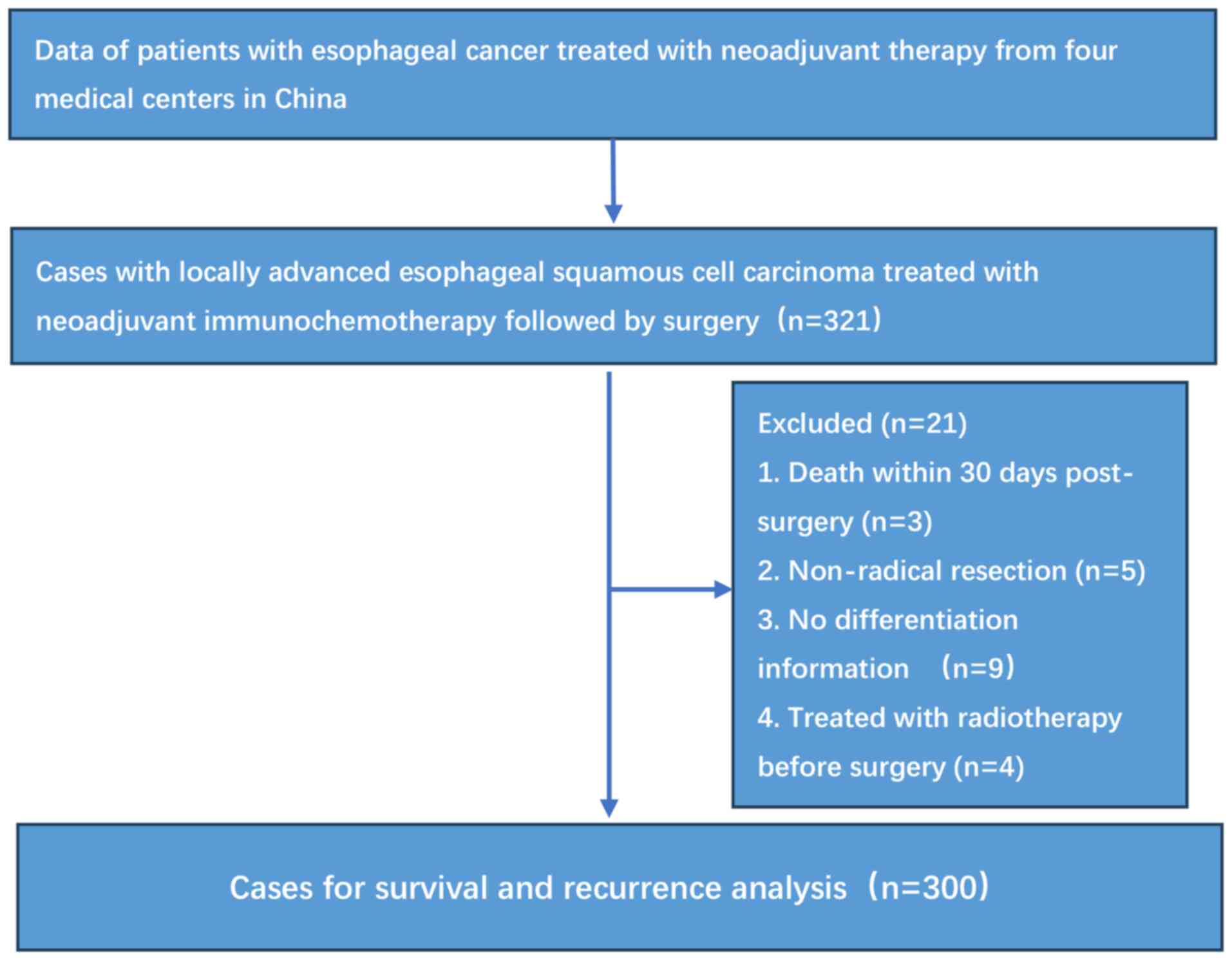
process is illustrated in Fig. 1.
The biopsy and postoperative pathological findings for all patients
were validated by two experienced pathologists. The TNM staging was
based on the 8th edition of the American Joint Committee on Cancer
staging manual for esophageal cancer (23).
Treatment and follow-up
Pre-treatment diagnosis and clinical staging were
conducted, encompassing endoscopy, enhanced computed tomography
(CT) scans and Doppler ultrasound of the neck. Positron emission
tomography (PET) scans were performed when deemed necessary. All
patients in the present study were evaluated by a multidisciplinary
team (MDT) consisting of specialists in surgery, oncology,
pathology, radiology and radiotherapy. Decisions concerning the
neoadjuvant therapy regimen, surgical timing and method, and
postoperative adjuvant therapy were determined after an MDT
discussion. The surgical techniques included the Ivor-Lewis or
McKeown procedures with thoracic and abdominal lymph node
dissection. In instances of suspected lymph node metastasis in the
neck, three-field lymphadenectomy was regarded as a viable option.
The Sweet surgery was indicated exclusively for patients with
esophagogastric junction cancer and those who were unsuitable for
right-sided thoracotomy.
Patients receiving neoadjuvant therapy should
undergo a re-evaluation of the neck, chest and abdomen using
enhanced CT 3–4 weeks after the second cycle of treatment. In the
first 2 years, patients were scheduled for follow-up visits at
3-month intervals, after which the frequency was reduced to once a
year. Subsequent evaluations included enhanced CT scans of the
head, neck and chest, in addition to hematological analyses.
Endoscopy, bone scans, ultrasound examinations and PET-CT were
performed as indicated when necessary.
Statistical analysis
The χ2 test and Fisher's exact test were
applied to compare characteristics among groups. The Kaplan-Meier
method was utilized to estimate OS and RFS, and the log-rank test
was used to compare survival outcomes between groups. The Cox
proportional hazards model was selected for regression analysis,
incorporating variables with a P-value <0.1 from the univariate
analysis into the multivariate analysis. Propensity score matching
(PSM) was employed to reduce data bias and confounding variables,
with a caliper value set at 0.05 for the analysis. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using R software (version
4.4.3; Posit Software, PBC), utilizing the ‘survminer’ (version
0.5.0; http://rpkgs.datanovia.com/survminer/index.html)
and ‘survival’ (version 3.8–3; http://github.com/therneau/survival) packages for
survival analysis and the visualization of survival curves and
forest plots. The ‘matchit’ (version 4.7.2; http://kosukeimai.github.io/MatchIt) package enabled
PSM. Pie charts were generated utilizing the ‘ggplot2’ (version
3.5.2; http://ggplot2.tidyverse.org)
package. The optimal cut-off value for age was established using
the X-tile software (version 3.6.1; http://medicine.yale.edu/lab/rimm/research/software).
Results
Baseline characteristics
Of the 300 patients included in the present study,
80 achieved pCR, whereas 220 did not (Table I). The median age was 67 and a
significant proportion (67.3%) of the participants were aged ≥63
years. Furthermore, ~68.3% of the patients were male. Most patients
(61.3%) had a BMI ranging between 18.5 and 23.9, which indicated
that they were primarily classified as normal weight. Patients with
a history of smoking, alcohol consumption and a family history of
cancer constituted 42.0, 33.7 and 27.7% of the study population,
respectively. The majority of patients were categorized as stage
III, with 55.0% presenting with moderately differentiated tumors.
Furthermore, ~84.7% of the cases exhibited tumors with a length of
≥4 cm. Additionally, ~86.0% of patients underwent 1–2 cycles of
neoadjuvant immunochemotherapy, whereas only 14.0% received ≥3
cycles of neoadjuvant treatment. Furthermore, ~87.7% of patients
underwent the McKeown procedure, 8.6% received the Ivor-Lewis
procedure and 3.7% underwent the Sweet procedure, with ~71.0% of
cases treated with minimally invasive surgery.
 | Table I.Baseline characteristics of original
and matched datasets. |
Table I.
Baseline characteristics of original
and matched datasets.
|
| Original
dataset | Matched
dataset |
|---|
|
|
|
|
|---|
| Variable | Total (n=300) | Non-pCR
(n=220) | pCR (n=80) | P-value | Total (n=80) | Non-pCR (n=40) | pCR (n=40) | P-value |
|---|
| Age, years |
|
|
| 0.510 |
|
|
| 1.000 |
|
≤63 | 98 (32.7) | 69 (31.4) | 29 (36.2) |
| 31 (38.8) | 15 (37.5) | 16 (40.0) |
|
|
>63 | 202 (67.3) | 151 (68.6) | 51 (63.7) |
| 49 (61.3) | 25 (62.5) | 24 (60.0) |
|
| Sex |
|
|
| 0.147 |
|
|
| 0.811 |
|
Female | 95 (31.7) | 64 (29.1) | 31 (38.8) |
| 26 (32.5) | 14 (35.0) | 12 (30.0) |
|
|
Male | 205 (68.3) | 156 (70.9) | 49 (61.3) |
| 54 (67.5) | 26 (65.0) | 28 (70.0) |
|
| BMI |
|
|
| 0.880 |
|
|
| 0.451 |
|
<18.5 | 26 (8.67) | 18 (8.18) | 8 (10.0) |
| 12 (15.0) | 4 (10.0) | 8 (20.0) |
|
|
18.5–23.9 | 184 (61.3) | 136 (61.8) | 48 (60.0) |
| 44 (55.0) | 23 (57.5) | 21 (52.5) |
|
|
≥24 | 90 (30.0) | 66 (30.0) | 24 (30.0) |
| 24 (30.0) | 13 (32.5) | 11 (27.5) |
|
| Smoker |
|
|
| 0.412 |
|
|
| 1.000 |
| No | 174 (58.0) | 124 (56.4) | 50 (62.5) |
| 52 (65.0) | 26 (65.0) | 26 (65.0) |
|
|
Yes | 126 (42.0) | 96 (43.6) | 30 (37.5) |
| 28 (35.0) | 14 (35.0) | 14 (35.0) |
|
| Alcohol
consumption |
|
|
| 0.692 |
|
|
| 0.621 |
| No | 199 (66.3) | 144 (65.5) | 55 (68.8) |
| 57 (71.2) | 30 (75.0) | 27 (67.5) |
|
|
Yes | 101 (33.7) | 76 (34.5) | 25 (31.2) |
| 23 (28.7) | 10 (25.0) | 13 (32.5) |
|
| Family history |
|
|
| 1.000 |
|
|
| 0.274 |
| No | 217 (72.3) | 159 (72.3) | 58 (72.5) |
| 63 (78.8) | 34 (85.0) | 29 (72.5) |
|
|
Yes | 83 (27.7) | 61 (27.7) | 22 (27.5) |
| 17 (21.2) | 6 (15.0) | 11 (27.5) |
|
|
Differentiation |
|
|
| <0.001 |
|
|
| 0.848 |
|
Well | 91 (30.3) | 30 (13.6) | 61 (76.2) |
| 47 (58.8) | 23 (57.5) | 24 (60.0) |
|
|
Moderate | 165 (55.0) | 156 (70.9) | 9 (11.2) |
| 16 (20.0) | 9 (22.5) | 7 (17.5) |
|
|
Poor | 44 (14.7) | 34 (15.5) | 10 (12.5) |
| 17 (21.2) | 8 (20.0) | 9 (22.5) |
|
| Length, cm |
|
|
| 0.655 |
|
|
| 1.000 |
|
<4 | 46 (15.3) | 32 (14.5) | 14 (17.5) |
| 13 (16.2) | 6 (15.0) | 7 (17.5) |
|
| ≥4 | 254 (84.7) | 188 (85.5) | 66 (82.5) |
| 67 (83.8) | 34 (85.0) | 33 (82.5) |
|
| cTNM |
|
|
| 0.244 |
|
|
| 0.348 |
| II | 36 (12.0) | 23 (10.5) | 13 (16.2) |
| 12 (15.0) | 8 (20.0) | 4 (10.0) |
|
|
III | 264 (88.0) | 197 (89.5) | 67 (83.8) |
| 68 (85.0) | 32 (80.0) | 36 (90.0) |
|
| No. of cycles of
neoadjuvant therapy |
|
|
| 0.625 |
|
|
| 1.000 |
|
1-2 | 258 (86.0) | 191 (86.8) | 67 (83.8) |
| 65 (81.2) | 33 (82.5) | 32 (80.0) |
|
| ≥3 | 42 (14.0) | 29 (13.2) | 13 (16.2) |
| 15 (18.8) | 7 (17.5) | 8 (20.0) |
|
| Surgery |
|
|
| 0.041 |
|
|
| 0.755 |
|
McKeown | 263 (87.7) | 193 (87.7) | 70 (87.5) |
| 68 (85.0) | 33 (82.5) | 35 (87.5) |
|
| Ivor
Lewis | 26 (8.6) | 16 (7.3) | 10 (12.5) |
| 12 (15.0) | 7 (17.5) | 5 (12.5) |
|
|
Sweet | 11 (3.7) | 11 (5.0) | 0 (0.0) |
| 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| Approach |
|
|
| 0.287 |
|
|
| 0.439 |
|
Minimally invasive | 213 (71.0) | 152 (69.1) | 61 (76.2) |
| 60 (75.0) | 32 (80.0) | 28 (70.0) |
|
|
Open | 87 (29.0) | 68 (30.9) | 19 (23.8) |
| 20 (25.0) | 8 (20.0) | 12 (30.0) |
|
Prognostic factor analysis
Univariate analysis was performed on all 300
patients, employing the Cox proportional hazards regression model
(Table II). The P-values for two
variables, differentiation and pCR, were <0.1 for OS. The
P-values for five variables (age, sex, differentiation, cycles of
neoadjuvant therapy and pCR) were all <0.1 for RFS. The
aforementioned variables were integrated into the multivariate
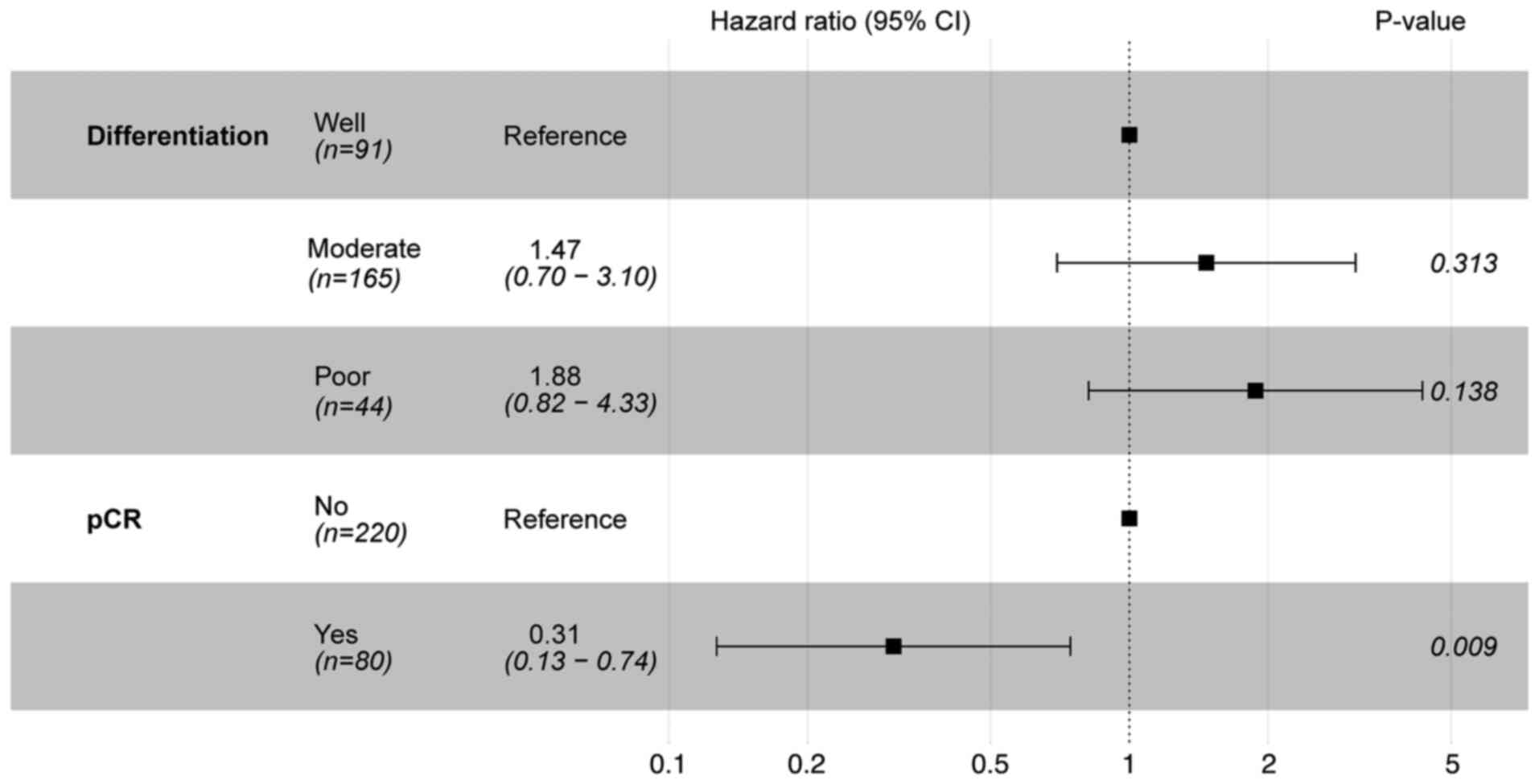
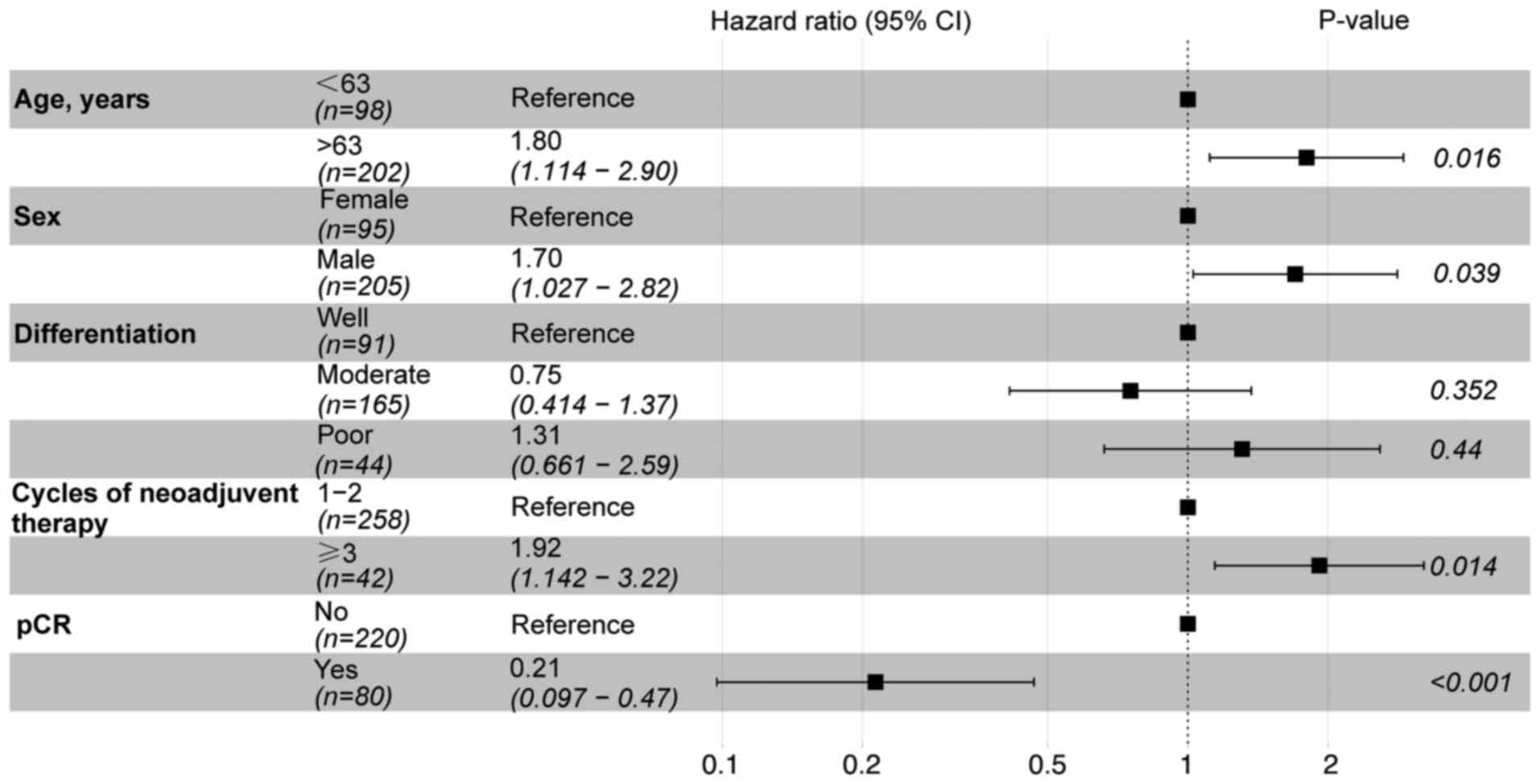
analysis (OS, Fig. 2; RFS, Fig. 3). The results of the multivariate
analysis for OS demonstrated that only pCR reached statistical
significance (P<0.05). In the context of RFS, age, sex, cycles
of neoadjuvant therapy and pCR were identified as statistically
significant prognostic factors (P<0.05).
 | Table II.Univariate analysis of OS and RFS
before propensity score matching. |
Table II.
Univariate analysis of OS and RFS
before propensity score matching.
|
|
| OS | RFS |
|---|
|
|
|
|
|
|---|
| Variable | Total (n=300) | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years |
|
| 0.125 |
| 0.061 |
|
≤63 | 98 (32.7) | Reference |
| Reference |
|
|
>63 | 202 (67.3) | 1.50
(0.89–2.54) |
| 1.55
(0.98–2.47) |
|
| Sex |
|
| 0.208 |
| 0.011 |
|
Female | 95 (31.7) | Reference |
| Reference |
|
|
Male | 205 (68.3) | 1.40
(0.83–2.36) |
| 1.88
(1.14–3.08) |
|
| BMI |
|
| 0.812 |
| 0.141 |
|
<18.5 | 26 (8.67) | Reference |
| Reference |
|
|
18.5–23.9 | 184 (61.3) | 1.00
(0.43–2.34) |
| 1.27
(0.54–2.95) |
|
|
≥24 | 90 (30.0) | 0.84
(0.34–2.10) |
| 1.85
(0.78–4.40) |
|
| Smoker |
|
| 0.469 |
| 0.246 |
| No | 174 (58.0) | Reference |
| Reference |
|
|
Yes | 126 (42.0) | 1.19
(0.75–1.89) |
| 1.27
(0.85–1.91) |
|
| Alcohol
consumption |
|
| 0.126 |
| 0.144 |
| No | 199 (66.3) | Reference |
| Reference |
|
|
Yes | 101 (33.7) | 1.44
(0.90–2.30) |
| 1.36
(0.90–2.06) |
|
| Family history |
|
| 0.468 |
| 0.381 |
| No | 217 (72.3) | Reference |
| Reference |
|
|
Yes | 83 (27.7) | 1.20
(0.73–1.99) |
| 1.22
(0.78–1.89) |
|
|
Differentiation |
|
| 0.004 |
| 0.009 |
|
Well | 91 (30.3) | Reference |
| Reference |
|
|
Moderate | 165 (55.0) | 2.71
(1.40–5.24) |
| 1.75
(1.04–2.96) |
|
|
Poor | 44 (14.7) | 3.09
(1.42–6.72) |
| 2.59
(1.39–4.82) |
|
| Length, cm |
|
| 0.249 |
| 0.952 |
|
<4 | 46 (15.3) | Reference |
| Reference |
|
| ≥4 | 254 (84.7) | 1.54
(0.74–3.20) |
| 1.02
(0.58–1.80) |
|
| cTNM |
|
| 0.623 |
| 0.368 |
| II | 36 (12.0) | Reference |
| Reference |
|
|
III | 264 (88.0) | 1.20
(0.57–2.52) |
| 1.37
(0.69–2.73) |
|
| No. of cycles of
neoadjuvant therapy |
|
| 0.127 |
| 0.030 |
|
1-2 | 258 (86.0) | Reference |
| Reference |
|
| ≥3 | 42 (14.0) | 1.57
(0.88–2.81) |
| 1.74
(1.05–2.88) |
|
| Surgery |
|
| 0.571 |
| 0.138 |
|
McKeown | 263 (87.7) | Reference |
| Reference |
|
| Ivor
Lewis | 26 (8.67) | 1.36
(0.65–2.86) |
| 0.69
(0.30–1.59) |
|
|
Sweet | 11 (3.67) | 1.45
(0.53–4.01) |
| 2.04
(0.89–4.67) |
|
| Approach |
|
| 0.179 |
| 0.217 |
|
Minimally invasive | 213 (71.0) | Reference |
| Reference |
|
|
Open | 87 (29.0) | 1.39
(0.86–2.24) |
| 1.31
(0.85–2.02) |
|
| pCR |
|
| <0.001 |
| <0.001 |
| No | 220 (73.3) | Reference |
| Reference |
|
|
Yes | 80 (26.7) | 0.24
(0.11–0.53) |
| 0.24
(0.12–0.47) |
|
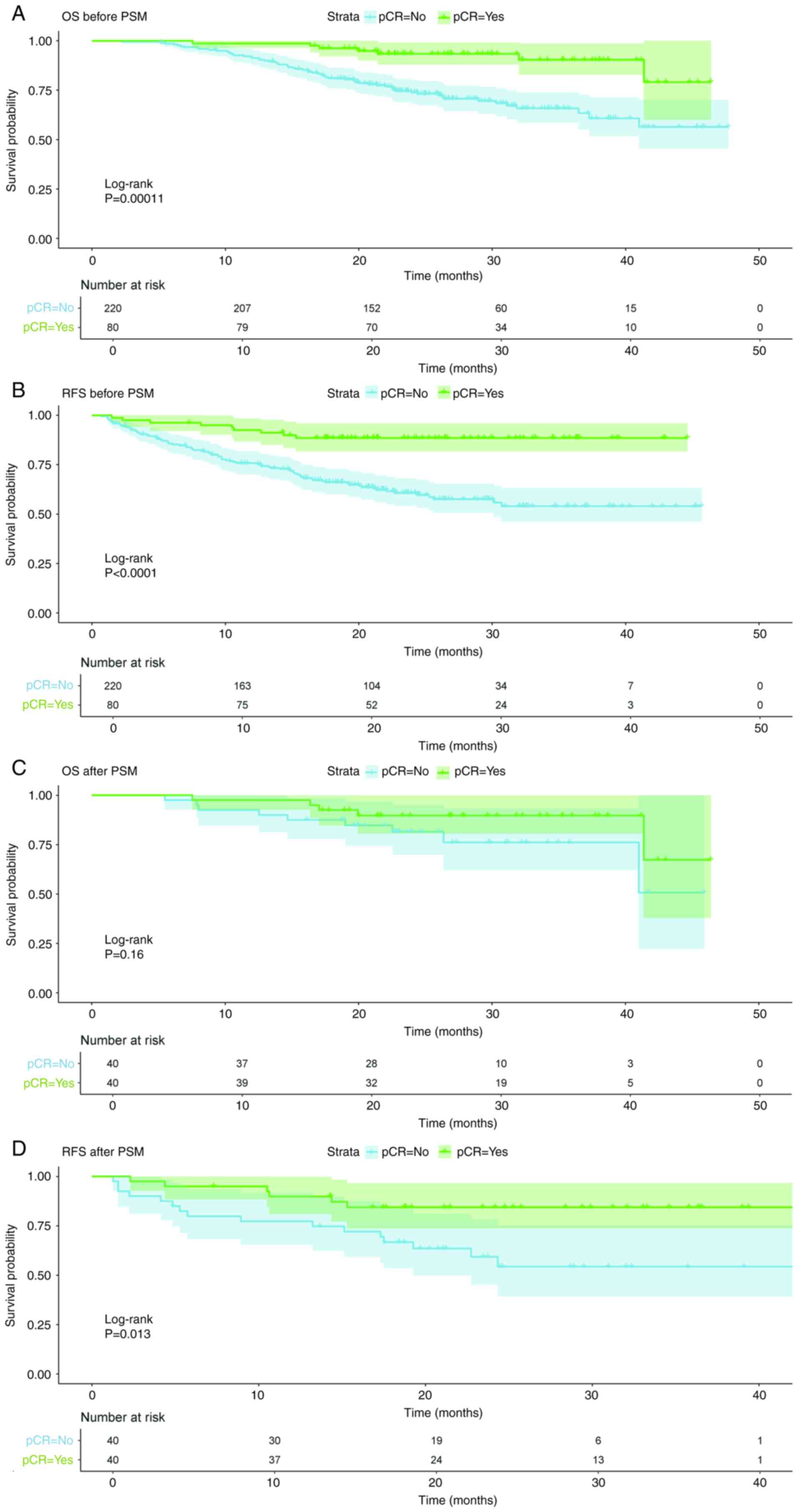
Survival analysis after PSM
To reduce bias, 1:1 PSM was conducted on all
included cases. All variables included in the present study, except
pCR, were used to calculate the propensity score. A total of 80
cases were included in the matched dataset, with no statistical
differences observed in the distribution of all variables between
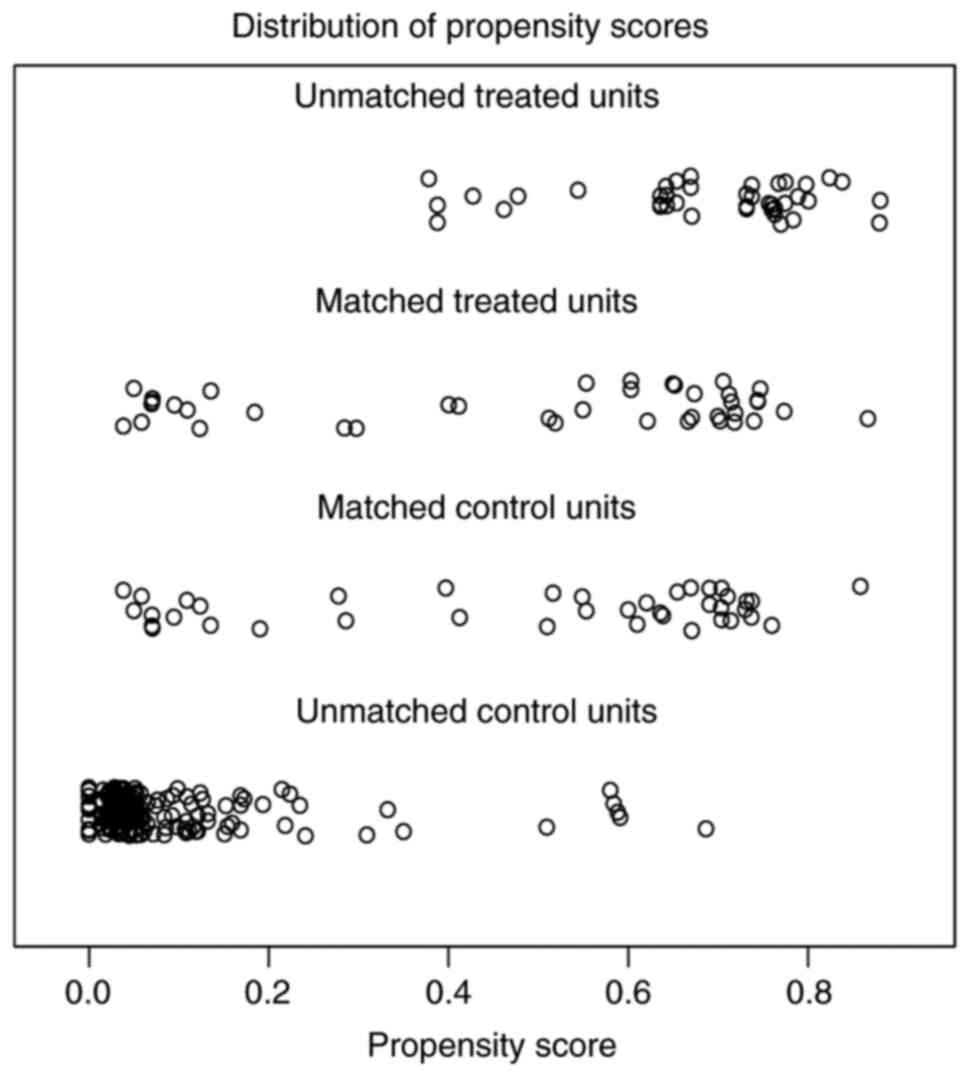
the pCR and non-pCR group after PSM. Before PSM, the propensity
scores of the unmatched treated group (pCR cases) were mostly
concentrated in the >0.6 range, while the propensity scores of
the unmatched control group (non-pCR cases) were mostly
concentrated in the <0.2 range. However, after PSM, the
propensity scores of the matched treated group (pCR cases) and the
matched control group (non-pCR cases) were distributed within the
same intervals; the propensity scores of most cases were in the
ranges of 0.0–0.2 and 0.6–0.8 (Fig.
4). The distribution of the propensity score was more
consistent between pCR and non-PCR groups after matching, which
indicated that a successful match was attained after PSM. Survival
curves were produced before and after PSM, and log-rank tests were
performed. Before PSM, OS and RFS in the pCR group were superior
compared with those in the non-pCR group (P<0.05; Fig. 5). After PSM, the survival curve
indicated that the pCR group exhibited superior survival compared
with the non-pCR group. However, this was only statistically
significant for RFS (P<0.05), while the P-value for OS was
0.16.
Patterns of recurrence
Among the 300 cases analyzed in the present study,
93 cases exhibited recurrence (Table
III). The recurrence rate in the pCR group was 11.2%, whereas
the rate in the non-pCR group was 38.2%. A statistically
significant difference was observed between these two groups
(P<0.05). Additionally, categorization of recurrence sites into
local-regional and distant recurrence demonstrated that the
local-regional recurrence rate (23.2%) and distant recurrence rate
(15.0%) in the non-pCR group were higher compared with those in the
pCR group (7.5 and 3.7%, respectively), with the differences being
statistically significant (P<0.05). The distribution of
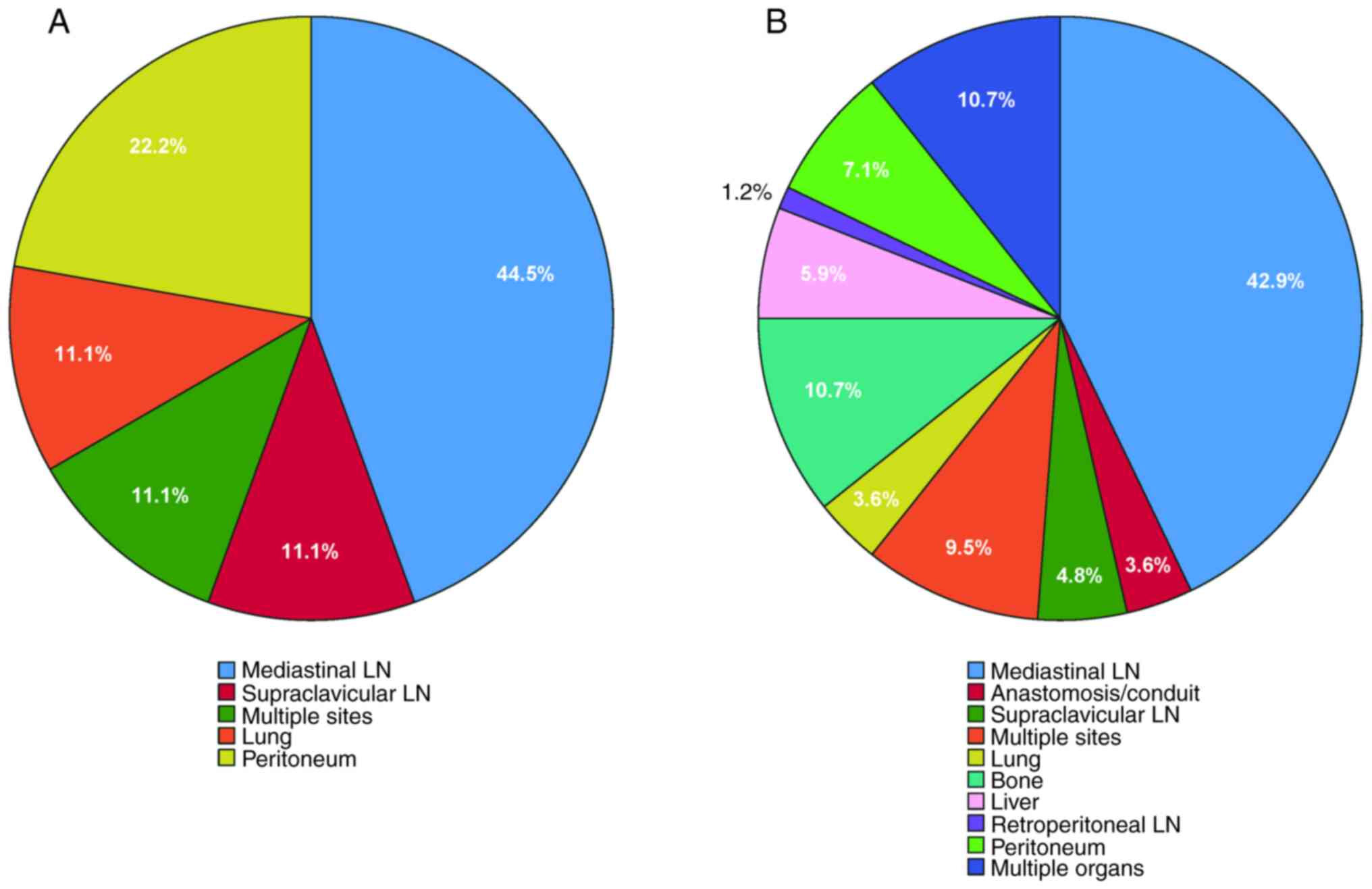
recurrence sites in the pCR group (Fig.
6A) compared with the non-pCR group (Fig. 6B) was assessed. The mediastinal
lymph node emerged as the predominant site of recurrence in both
groups. In both groups, local-regional recurrence sites encompassed
mediastinal lymph nodes, supraclavicular lymph nodes and multiple
sites. Furthermore, ~3.6% of patients in the non-pCR group
experienced anastomotic recurrence, while no patients in the pCR
group exhibited such recurrence. Both groups exhibited metastases
to the lung and peritoneum as distant metastatic sites. In the pCR
group, 22.2% of cases exhibited peritoneal metastasis, while 11.1%
exhibited lung metastasis. No bone, liver, retroperitoneal lymph
node or multiple organ metastases were recorded. In the non-pCR
group, multiple organ and bone metastases were the most prevalent,
comprising ~10.7% of patients, whereas liver metastases and
peritoneal metastases were observed in 5.9 and 7.1% of patients,
respectively. Additionally, ~1.2% of patients exhibited
retroperitoneal lymph node metastases, representing the lowest
incidence among all metastatic sites in the non-pCR group.
 | Table III.Comparison of recurrence patterns
between the pCR group and the non-pCR group. |
Table III.
Comparison of recurrence patterns
between the pCR group and the non-pCR group.
| Recurrence
pattern | Total (n=300) | Non-pCR
(n=220) | pCR (n=80) | P-value |
|---|
| Recurrence |
|
|
| <0.001 |
| No | 207 (69.0) | 136 (61.8) | 71 (88.8) |
|
|
Yes | 93 (31.0) | 84 (38.2) | 9 (11.2) |
|
| Recurrence
site |
|
|
| <0.001 |
| No | 207 (69.0) | 136 (61.8) | 71 (88.8) |
|
|
Loco-regional | 57 (19.0) | 51 (23.2) | 6 (7.5) |
|
|
Distant | 36 (12.0) | 33 (15.0) | 3 (3.7) | <0.001 |
Discussion
Before 2021, the combination of chemotherapy and
radiotherapy was regarded as the standard adjuvant treatment for
locally advanced ESCC (24,25). The results of the NEOCRTEC5010 and
CROSS trials indicated that neoadjuvant chemoradiotherapy was a
superior treatment approach for resectable locally advanced
esophageal cancer (4,26). In 2021, the KEYNOTE-590 study became
the first clinical trial globally to demonstrate that immunotherapy
enhanced survival rates in advanced esophageal cancer while
maintaining a favorable safety profile, thereby transforming the
treatment paradigm for this type of cancer (9). The research findings indicate that
neoadjuvant immunochemotherapy represents an auspicious treatment
approach for ESCC. As radiotherapy can potentially increase the
difficulty and complications of esophagectomy, especially in Asia,
for patients with locally advanced ESCC, an increasing number of
physicians are selecting neoadjuvant immunochemotherapy as the
preferred treatment approach (27).
There are few clinical trials involving neoadjuvant
immunochemotherapy over the past decade (11,12,14), a
therapeutic approach that has only been widely employed in ESCC in
recent years; therefore, the present study primarily focused on
this patient population.
A number of previous studies on neoadjuvant therapy
for various tumor types have identified an association between pCR
and the prognosis of patients (28–30).
Due to this association, pCR is frequently utilized as a
significant endpoint in drug clinical trials, serving as a
surrogate for OS, which necessitates extended follow-up for
observation. The Food and Drug Administration of the United States
has approved pCR as a surrogate endpoint for OS in breast cancer
clinical trials (30). However, for
esophageal cancer, the relationship between pCR and survival
remains controversial. A systematic review of 40 clinical trials
involving 55,344 patients undergoing neoadjuvant therapy for
esophageal cancer found that pCR was not a valid surrogate endpoint
for survival in clinical trials (31). However, other clinical trials on
neoadjuvant therapy for esophageal cancer have demonstrated that
treatment patterns enhancing the pCR rate could improve the OS and
RFS of patients (20,32). Neoadjuvant immunochemotherapy for
esophageal cancer has recently emerged, resulting in limited
research on the prognostic value of pCR for the survival of
patients with ESCC. The present study found that 80 out of 300
patients achieved pCR, with a pCR rate of 26.7%. This rate aligns
with the previously reported pCR rates of 16.7–50% for neoadjuvant
immunochemotherapy in ESCC (33).
The present study utilized the Cox proportional hazards model to
investigate prognostic factors, and demonstrated that pCR was a
statistically significant prognostic factor for OS and RFS
(P<0.05). To validate this conclusion, the patients were
categorized into pCR and non-pCR groups, PSM was utilized to reduce
the potential bias, and survival analysis was conducted on the
matched dataset. The OS and RFS of the pCR group before PSM and the
RFS of the pCR group after PSM were significantly superior to those
of the non-pCR group (P<0.05). After PSM, the OS of the pCR
group remained superior to that of the non-pCR group as indicated
by the survival curve; however, this difference was not
statistically significant (P>0.05). This may be attributed to
the limited number of included cases, resulting in only 80 matched
cases after PSM. By contrast, a previous study confirmed that pCR
is weakly associated with OS in patients with ESCC with neoadjuvant
therapy (31). As for the reason
for this finding, a study has suggested that the measurement of
pathological response is primarily based on the primary tumor site
rather than on micrometastatic sites, but the reaction of
micrometastases beyond the primary site is likely the principal
factor influencing clinical outcomes (34). Another study has indicated that the
potential survival benefit derived from the favorable treatment
effect on local lesions may be counterbalanced by the adverse
effects associated with the toxicity of neoadjuvant drugs, thus
diminishing the association between pCR and survival (35). In summary, in conjunction with prior
studies on neoadjuvant therapy for ESCC, the present study
indicated that patients achieving pCR experienced improved survival
benefits; however, this association was not consistently
significant across all studies. Future clinical trials with
improved designs and larger sample sizes are necessary to validate
this conclusion.
To the best of our knowledge, no research exists
regarding the relationship between pCR and recurrence patterns in
patients with ESCC treated with neoadjuvant immunochemotherapy; the
present study represents the initial investigation concerning this
population. Presently, research has predominantly focused on
patients treated with neoadjuvant chemotherapy or neoadjuvant
chemoradiotherapy (4,5,18,19,21,22). A
study on esophageal cancer following neoadjuvant chemotherapy has
demonstrated that the RFS of patients in the pCR group was superior
to that of the patients in the non-pCR group (36). In the present study, the RFS of the
pCR group was also superior to that of the non-pCR group both
before and after PSM, which indicated that patients with a pCR may
have improved RFS among patients with ESCC treated with neoadjuvant
immunochemotherapy. According to previous research, patients who
achieved a pCR following neoadjuvant chemoradiotherapy had a
recurrence incidence of 23.3–38.0% (37–39).
The recurrence rate for the pCR group in the present study was
11.2%, which was significantly lower compared with that of the
non-pCR group (P<0.05) and lower compared with the rates
reported in prior literature (36,37).
Some studies on neoadjuvant chemotherapy or chemoradiotherapy have
shown that patients achieving pCR may experience both local and
distant recurrences. However, the proportions of these recurrences
differ across various studies (36–39).
In the present study, the loco-regional and distant recurrence
rates in the pCR group were significantly lower compared with those
in the non-pCR group; however, a substantial proportion in each
group exhibited local recurrence. The mediastinal lymph node was
the most prevalent recurrence site in both groups, with mediastinal
lymph node metastasis being observed in <50% of cases. In the
pCR group, there were no instances of anastomotic recurrence, bone,
liver, retroperitoneal lymph node or multiple organ metastasis. By
contrast, metastasis was present at these sites in the non-pCR
group. The limited number of cases achieving pCR may have led to
some metastatic sites not being observed in this group. Future
studies with larger sample sizes are anticipated to investigate the
relationship between pCR and recurrence sites.
The present study had specific limitations. Firstly,
the present study had a limited sample size, especially after PSM,
leading to fewer matched cases, which may affect the research
conclusions. Secondly, the present study was retrospective.
Although the Cox proportional hazards model and PSM were employed
to reduce bias, some bias remains inevitable. Finally, the
practices of different centers influence the immunochemotherapy
regimen and the surgical approach. The variability in treatment
plans may affect the conclusions. In summary, the growing
acceptance of neoadjuvant immunochemotherapy for locally advanced
ESCC requires additional prospective randomized controlled studies
with larger sample sizes to clarify the association between pCR and
prognosis, along with recurrence patterns. This research would
establish a stronger basis for the treatment of patients with
esophageal cancer. Additionally, there are numerous other factors,
such as minimal residual disease or circulating tumor DNA, that may
influence the prognosis of patients after neoadjuvant
immunochemotherapy followed by surgery. In future studies, more
potential prognostic indicators such as minimal residual disease or
circulating tumor DNA could be added to construct a multivariate
prediction model to improve the predictive ability.
In conclusion, in patients with ESCC undergoing
neoadjuvant immunochemotherapy, pCR indicated improved OS and RFS;
however, the association between pCR and OS was not statistically
significant. Concurrently, pCR was associated with reduced local
and distant recurrence rates, and the recurrence pattern of
patients in the pCR group differed from that of patients in the
non-pCR group. Future research should involve larger sample sizes
to elucidate the relationship between pCR and survival, as well as
its influence on recurrence patterns.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Beijing Municipal
Administration of Hospitals Incubating Program (grant no.
PX2024057).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
FW and XY drafted the manuscript. JZ and YH
participated in the design of the study plan. YH and RY
participated in the revision and language editing of the
manuscript. FW, XY, JZ and RY participated in the collection of
data. LY and DY participated in the statistical analysis. FW and XY
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The studies involving human participants were
reviewed and approved by the Ethics Committee of National Cancer
Center/National Clinical Research Center for Cancer/Cancer Hospital
& Shenzhen Hospital (approval no. YW2024-3-1), Anyang Tumor
Hospital (approval no. 2023YP18H01), Shaanxi Provincial People's
Hospital (approval no. 2023K-S129) and Beijing Chest Hospital
(approval no. 2023-ky-59). The patients/participants provided
written informed consent to participate in the present study. The
study was conducted in strict compliance with the Declaration of
Helsinki (2013) and the Good Clinical Practice guidelines.
Patient consent for publication
The patients/participants provided written informed
consent for the publication of any data and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ESCC
|
esophageal squamous cell carcinoma
|
|
pCR
|
pathological complete response
|
|
PSM
|
propensity score matching
|
|
OS
|
overall survival
|
|
RFS
|
recurrence-free survival
|
|
CT
|
computed tomography
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
2
|
Morgan E, Soerjomataram I, Rumgay H,
Coleman HG, Thrift AP, Vignat J, Laversanne M, Ferlay J and Arnold
M: The global landscape of esophageal squamous cell carcinoma and
esophageal adenocarcinoma incidence and mortality in 2020 and
projections to 2040: New estimates from GLOBOCAN 2020.
Gastroenterology. 163:649–658.e2. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu J, Zhu L, Huang X, Lu Z, Wang Y, Yang
Y, Ye J, Gu C, Lv W, Zhang C and Hu J: Does the time interval from
neoadjuvant camrelizumab combined with chemotherapy to surgery
affect outcomes for locally advanced esophageal squamous cell
carcinoma? J Cancer Res Clin Oncol. 150:1612024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eyck BM, van Lanschot JJB, Hulshof MCCM,
van der Wilk BJ, Shapiro J, van Hagen P, van Berge Henegouwen MI,
Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, et al:
Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for
esophageal cancer: The randomized controlled CROSS trial. J Clin
Oncol. 39:1995–2004. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu
Z, Mao W, Xiang J, Han Y, Chen Z, et al: Long-term efficacy of
neoadjuvant chemoradiotherapy plus surgery for the treatment of
locally advanced esophageal squamous cell carcinoma: The
NEOCRTEC5010 randomized clinical trial. JAMA Surg. 156:721–729.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Janjigian YY, Bendell J, Calvo E, Kim JW,
Ascierto PA, Sharma P, Ott PA, Peltola K, Jaeger D, Evans J, et al:
CheckMate-032 study: Efficacy and safety of nivolumab and nivolumab
plus ipilimumab in patients with metastatic esophagogastric Cancer.
J Clin Oncol. 36:2836–2844. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shah MA, Kojima T, Hochhauser D, Enzinger
P, Raimbourg J, Hollebecque A, Lordick F, Kim SB, Tajika M, Kim HT,
et al: Efficacy and safety of pembrolizumab for heavily pretreated
patients with advanced, metastatic adenocarcinoma or squamous cell
carcinoma of the esophagus: The phase 2 KEYNOTE-180 study. JAMA
Oncol. 5:546–550. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kojima T, Shah MA, Muro K, Francois E,
Adenis A, Hsu CH, Doi T, Moriwaki T, Kim SB, Lee SH, et al:
Randomized Phase III KEYNOTE-181 study of pembrolizumab versus
chemotherapy in advanced esophageal cancer. J Clin Oncol.
38:4138–4148. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun JM, Shen L, Shah MA, Enzinger P,
Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, et al:
Pembrolizumab plus chemotherapy versus chemotherapy alone for
first-line treatment of advanced oesophageal cancer (KEYNOTE-590):
A randomised, Placebo-controlled, phase 3 study. Lancet.
398:759–771. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li C, Zhao S, Zheng Y, Han Y, Chen X,
Cheng Z, Wu Y, Feng X, Qi W, Chen K, et al: Preoperative
pembrolizumab combined with chemoradiotherapy for oesophageal
squamous cell carcinoma (PALACE-1). Eur J Cancer. 144:232–241.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van den Ende T, de Clercq NC, van Berge
Henegouwen MI, Gisbertz SS, Geijsen ED, Verhoeven RHA, Meijer SL,
Schokker S, Dings MPG, Bergman JJGHM, et al: Neoadjuvant
chemoradiotherapy combined with atezolizumab for resectable
esophageal adenocarcinoma: A Single-arm phase II feasibility trial
(PERFECT). Clin Cancer Res. 27:3351–3359. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamamoto S, Kato K, Daiko H, Kojima T,
Hara H, Abe T, Tsubosa Y, Nagashima K, Aoki K, Mizoguchi Y, et al:
Feasibility study of nivolumab as neoadjuvant chemotherapy for
locally esophageal carcinoma: FRONTiER (JCOG1804E). Future Oncol.
16:1351–1357. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patel MA, Kratz JD, Lubner SJ, Loconte NK
and Uboha NV: Esophagogastric cancers: Integrating immunotherapy
therapy into current practice. J Clin Oncol. 40:2751–2762. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen X, Xu X, Wang D, Liu J, Sun J, Lu M,
Wang R, Hui B, Li X, Zhou C, et al: Neoadjuvant sintilimab and
chemotherapy in patients with potentially resectable esophageal
squamous cell carcinoma (KEEP-G 03): An open-label, single-arm,
phase 2 trial. J Immunother Cancer. 11:e0058302023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hong ZN, Gao L, Weng K, Huang Z, Han W and
Kang M: Safety and feasibility of esophagectomy following combined
immunotherapy and chemotherapy for locally advanced esophageal
squamous cell carcinoma: A propensity score matching analysis.
Front Immunol. 13:8363382022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hong Z, Xu J, Chen Z, Xu H, Huang Z, Weng
K, Cai J, Ke S, Chen S, Xie J, et al: Additional neoadjuvant
immunotherapy does not increase the risk of anastomotic leakage
after esophagectomy for esophageal squamous cell carcinoma: A
multicenter retrospective cohort study. Int J Surg. 109:2168–2178.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao Y, Huang B, Tang H, Dong D, Shen T,
Chen X, Feng X, Zhang J, Shi L, Li C, et al: Online tools to
predict individualised survival for primary oesophageal cancer
patients with and without pathological complete response after
neoadjuvant therapy followed by oesophagectomy: Development and
external validation of two independent nomograms. BMJ Open
Gastroenterol. 11:e0012532024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xi M, Yang Y, Zhang L, Yang H, Merrell KW,
Hallemeier CL, Shen RK, Haddock MG, Hofstetter WL, Maru DM, et al:
Multi-institutional analysis of recurrence and survival after
neoadjuvant chemoradiotherapy of esophageal cancer: Impact of
histology on recurrence patterns and outcomes. Ann Surg.
269:663–670. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meguid RA, Hooker CM, Taylor JT, Kleinberg
LR, Cattaneo SM II, Sussman MS, Yang SC, Heitmiller RF, Forastiere
AA and Brock MV: Recurrence after neoadjuvant chemoradiation and
surgery for esophageal cancer: Does the pattern of recurrence
differ for patients with complete response and those with partial
or no response? J Thorac Cardiovasc Surg. 138:1309–1317. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blum Murphy M, Xiao L, Patel VR, Maru DM,
Correa AMG, Amlashi F, Liao Z, Komaki R, Lin SH, Skinner HD, et al:
Pathological complete response in patients with esophageal cancer
after the trimodality approach: The association with baseline
variables and survival-The University of Texas MD Anderson cancer
center experience. Cancer. 123:4106–4113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jipping KM, Hulshoff JB, van Amerongen EA,
Bright TI, Watson DI and Plukker JTM: Influence of tumor response
and treatment schedule on the distribution of tumor recurrence in
esophageal cancer patients treated with neoadjuvant
chemoradiotherapy. J Surg Oncol. 116:1096–1102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vallböhmer D, Hölscher AH, DeMeester S,
DeMeester T, Salo J, Peters J, Lerut T, Swisher SG, Schröder W,
Bollschweiler E and Hofstetter W: A multicenter study of survival
after neoadjuvant radiotherapy/chemotherapy and esophagectomy for
ypT0N0M0R0 esophageal cancer. Ann Surg. 252:744–749. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rice TW, Ishwaran H, Hofstetter WL, Kelsen
DP, Apperson-Hansen C and Blackstone EH: Recommendations for
pathological staging (pTNM) of cancer of the esophagus and
esophagogastric junction for the 8th edition AJCC/UICC staging
manuals. Dis Esophagus. 29:897–905. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Corvera C, Das P, Denlinger CS, Enzinger PC, Fanta P, Farjah F, et
al: Esophageal and esophagogastric junction cancers, version
2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr
Canc Netw. 17:855–883. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Muro K, Lordick F, Tsushima T,
Pentheroudakis G, Baba E, Lu Z, Cho BC, Nor IM, Ng M, Chen LT, et
al: Pan-Asian adapted ESMO Clinical Practice Guidelines for the
management of patients with metastatic oesophageal cancer: A
JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann
Oncol. 30:34–43. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu
Z, Mao W, Xiang J, Han Y, Chen Z, et al: Neoadjuvant
chemoradiotherapy followed by surgery versus surgery alone for
locally advanced squamous cell carcinoma of the esophagus
(NEOCRTEC5010): A phase III multicenter, randomized, Open-label
clinical trial. J Clin Oncol. 36:2796–2803. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Warren S, Partridge M, Carrington R, Hurt
C, Crosby T and Hawkins MA: Radiobiological determination of dose
escalation and normal tissue toxicity in definitive chemoradiation
therapy for esophageal cancer. Int J Radiat Oncol Biol Phys.
90:423–429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Passot G, You B, Boschetti G, Fontaine J,
Isaac S, Decullier E, Maurice C, Vaudoyer D, Gilly FN, Cotte E and
Glehen O: Pathological response to neoadjuvant chemotherapy: A new
prognosis tool for the curative management of peritoneal colorectal
carcinomatosis. Ann Surg Oncol. 21:2608–2614. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Corsini EM, Weissferdt A, Pataer A, Zhou
N, Antonoff MB, Hofstetter WL, Mehran RJ, Rajaram R, Rice DC, Roth
JA, et al: Pathological nodal disease defines survival outcomes in
patients with lung cancer with tumour major pathological response
following neoadjuvant chemotherapy. Eur J Cardiothorac Surg.
59:100–108. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Conforti F, Pala L, Sala I, Oriecuia C, De
Pas T, Specchia C, Graffeo R, Pagan E, Queirolo P, Pennacchioli E,
et al: Evaluation of pathological complete response as surrogate
endpoint in neoadjuvant randomised clinical trials of early stage
breast cancer: Systematic review and Meta-analysis. BMJ.
375:e0663812021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Su F, Yang X, Yin J, Shen Y and Tan L:
Validity of using pathological response as a surrogate for overall
survival in neoadjuvant studies for esophageal cancer: A systematic
review and Meta-analysis. Ann Surg Oncol. 30:7461–7471. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chao YK, Chen HS, Wang BY, Hsu PK, Liu CC
and Wu SC: Factors associated with survival in patients with
oesophageal cancer who achieve pathological complete response after
chemoradiotherapy: A nationwide population-based study. Eur J
Cardiothorac Surg. 51:155–159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu L, Wei XF, Li CJ, Yang ZY, Yu YK, Li
HM, Xie HN, Yang YF, Jing WW, Wang Z, et al: Pathological responses
and surgical outcomes after neoadjuvant immunochemotherapy versus
neoadjuvant chemoradiotherapy in patients with locally advanced
esophageal squamous cell carcinoma. Front Immunol. 13:10525422022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rose BS, Winer EP and Mamon HJ: Perils of
the pathological complete response. J Clin Oncol. 34:3959–3962.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Colori A and Hiley C: The interaction of
preexisting cardiac dysfunction and heart dose from radical
radiotherapy on All-cause mortality in locally advanced NSCLC. J
Thorac Oncol. 18:14–16. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu YY, Dai L, Yang YB, Yan WP, Cheng H,
Fan MY, Gao YM and Chen KN: Long-term survival and recurrence
patterns in locally advanced esophageal squamous cell carcinoma
patients with pathological complete response after neoadjuvant
chemotherapy followed by surgery. Ann Surg Oncol. 31:5047–5054.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
van Hagen P, Wijnhoven BP, Nafteux P,
Moons J, Haustermans K, De Hertogh G, van Lanschot JJ and Lerut T:
Recurrence pattern in patients with a pathologically complete
response after neoadjuvant chemoradiotherapy and surgery for
oesophageal cancer. Br J Surg. 100:267–273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Agoston AT, Zheng Y, Bueno R, Lauwers GY,
Odze RD and Srivastava A: Predictors of disease recurrence and
survival in esophageal adenocarcinomas with complete response to
neoadjuvant therapy. Am J Surg Pathol. 39:1085–1092. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xi M, Hallemeier CL, Merrell KW, Liao Z,
Murphy MAB, Ho L, Hofstetter WL, Mehran R, Lee JH, Bhutani MS, et
al: Recurrence risk stratification after preoperative
chemoradiation of esophageal adenocarcinoma. Ann Surg. 268:289–295.
2018. View Article : Google Scholar : PubMed/NCBI
|