Introduction
Burkitt's lymphoma (BL) is a rapidly developing,
aggressive B-cell non-Hodgkin lymphoma that is more prevalent in
pediatric patients than in adults (1). It is estimated that there were 553,389
new cases and 250,679 deaths worldwide in 2022 (2). The 5-year net survival rate was
>80% in high-income countries, and <50% in certain regions of
Central and South America (3). The
current mainstay of treatment for BL is multidrug chemotherapy,
which employs doxorubicin alkylating agents, vincristine and
etoposide. However, prolonged high-dose and high-intensity
chemotherapy is less tolerated by patients, frequently leading to
clinical toxicity and treatment-related complications.
Concurrently, these regimens impede bone marrow function, suppress
the immune response and induce clinical infections during treatment
(4–6). Therefore, it is necessary to develop a
novel type of treatment that is less immunosuppressive and more
tolerable.
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL; also referred to as Apo2L) is a member of the TNF
superfamily and a promising antitumor medication due to its unique
ability to destroy cancer cells whilst maintaining normal cells
(7). TRAIL induces apoptosis by
binding to the receptors TRAIL-R1 [(death receptor 4 (DR4)] and
TRAIL-R2 [(death receptor 5 (DR5)], leading to the formation of the
death-inducing signaling complex and subsequent activation of
caspase 8. As the apical caspase in this extrinsic pathway,
activated caspase-8 directly cleaves and activates the effector
caspase 3/7, which serve as the ultimate executioners of the
apoptotic program and can be activated by both extrinsic and
intrinsic pathways (8).
Although TRAIL has the capacity to induce apoptosis
in certain tumor cells, certain malignant lymphomas are resistant
to TRAIL-induced apoptosis (9).
Clinical combination therapies to increase the sensitivity of
cancer cells to TRAIL include chemotherapy drugs, radiation therapy
or immunotherapy, as well as several signal transduction modulators
and small molecule inhibitors, providing the optimal combination
for the use of TRAIL in cancer treatment (10–14).
The use of proteasome inhibitors has also been reported to be a
promising strategy to enhance the sensitivity of cancer cells to
TRAIL (15).
Bortezomib (PS-341; Velcade) is the first selective
proteasome inhibitor approved by the US Food and Drug
Administration for the treatment of multiple myeloma and relapsed
mantle cell lymphoma (16).
Bortezomib has been reported to synergistically enhance
TRAIL-induced apoptosis in multiple drug-resistant cancer cells,
such as SNU-216 gastric cancer cells (17), B16F10 melanoma cells and CT26 colon
carcinoma cells (18). The
induction of apoptosis by bortezomib in primary chronic lymphocytic
leukemia cells and the BJAB BL cell line has been reported to be
associated with the upregulation of TRAIL and its death receptors
DR4 and DR5 (19). However, to the
best of our knowledge, the synergistic antitumor effects of
bortezomib and TRAIL in drug-resistant BL cells, and the underlying
mechanism, have not yet been elucidated. Our previous study
reported that several BL cell lines, including Raji and CA46 cells,
were insensitive to TRAIL, with Raji cells exhibiting the lowest
sensitivity to TRAIL (20).
Therefore, in the present study, Raji cells were selected to
explore the synergistic inhibitory effect of bortezomib and TRAIL,
and the underlying mechanism, in order to provide a novel
therapeutic strategy for TRAIL-insensitive BL cells.
Materials and methods
Materials
TRAIL (cat. no. HY-P77256, purity, ≥95%) and
bortezomib (cat. no. HY-10227, purity, ≥99%) were purchased from
MedChemExpress. RPMI 1640 medium and FBS were purchased from Gibco
(Thermo Fisher Scientific, Inc.). A Cell Counting Kit-8 (CCK-8) was
purchased from Biosharp Life Sciences. ProteinSafe™ phosphatase
inhibitor cocktail and protease inhibitor cocktail (EDTA-free) were
purchased from TransGen Biotech Co., Ltd. An Annexin V-FITC/PI
apoptosis detection kit, a mitochondrial membrane potential (MMP)
assay kit with JC-1, a reactive oxygen species (ROS) kit,
N-acetylcysteine (NAC) and RIPA lysis buffer were purchased from
Beyotime Biotechnology. BCA and supersensitive ECL kits were
purchased from Oriscience Biotechnology Co., Ltd. PVDF membranes
were purchased from Merck KGaA. The phycoerythrin (PE)-CD262 (DR5)
monoclonal antibodies (cat. no. 12-9908-42) and PE-CD261 (DR4)
monoclonal antibodies (cat. no. 12-6644-42) were purchased from
eBioscience (Thermo Fisher Scientific, Inc.). Antibodies against
PARP (cat. no. 9532), cleaved PARP (cat. no. 5625), caspase 8 (cat.
no. 4790), cleaved caspase 8 (cat. no. 9496), caspase 9 (cat. no.
9504), cleaved caspase 9 (cat. no. 7237), caspase 3 (cat. no.
9662), cleaved caspase 3 (cat. no. 9664), Bcl-xl (cat. no. 2764),
Bcl-2 (cat. no. 3498), phosphorylated (p-) p38 (cat. no. 4511), p-
stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK;
cat. no. 4668), p-c-Jun (cat. no. 3270), p-activating transcription
factor 2 (ATF2; cat. no. 27934), p-extracellular signal-regulated
kinase (ERK)1/2 (cat. no. 4695), C/EBP homologous protein (CHOP;
cat. no. 2895), GAPDH (cat. no. 5174) and HRP-conjugated goat
anti-rabbit antibodies (cat. no. 7074) were purchased from Cell
Signaling Technology Inc. with GAPDH as the internal reference
protein. GAPDH antibodies were diluted at a ratio of 1:10,000,
whilst all other antibodies were diluted at a ratio of 1:1,000. All
other chemicals used were of analytical grade.
Cell culture
Raji (cat. no. CCL-86; American Type Culture
Collection) and CA46 (cat. no. CRL-1648; American Type Culture
Collection) BL cell lines were cryopreserved in a liquid nitrogen
tank in the laboratory. Cells were cultured in RPMI 1640 medium
supplemented with 10% FBS under humidified conditions with 5%
CO2 at 37°C, according to the culture conditions
recommended by American Type Culture Collection. Cells in the
logarithmic growth phase were used in subsequent experiments.
Cell proliferation assay
Cell viability was determined using a CCK-8 assay.
The TRAIL stock solution was prepared by dissolving the powder via
sonication according to the manufacturer's protocol, which suggests
a stock solution of ≥100 µg/ml in ddH2O. Additionally,
there should be no crystals when determining the maximum working
concentration. Viable Raji and CA46 cells were seeded in a 96-well
plate, incubated with 1×105, 5×104,
2.5×104, 1.25×104, 6.25×103,
3.125×103 or 0 ng/ml TRAIL, and incubated with 200, 100,
50, 25, 12.5, 6.25 or 0 nM bortezomib and/or 100 ng/ml TRAIL,
followed by the addition of CCK-8 to each well and incubation for
another 2 h at 37°C with 5% CO2. The absorbance at 450
nm was measured using a Powerwave XS multiplate reader (BioTek;
Agilent Technologies, Inc.). Data acquisition and half-maximal
inhibitory concentration analysis were performed using GraphPad
Prism 9.0 software (Dotmatics). The following formula was used to
determine the cell inhibition rate: Cell inhibition rate
(%)=[1-optical density (OD) mean value of the experimental group/OD
mean value of the control group] ×100%.
Apoptosis analysis
Viable Raji cells treated with 100, 50, 25 or 0 nM
bortezomib and/or 100 ng/ml TRAIL for 24 h at 37°C. A total of
2×105 cells/well were harvested and washed with ice-cold
PBS. The cells were stained with 200 µl staining buffer containing
10 µl annexin V-FITC and 5 µl PI, followed by incubation at 25°C
for 20 min, after which the proportion of apoptotic cells was
detected and analyzed using flow cytometry (Quanteon; ACEA
Biosciences, Inc.). NovoExpress software (version 1.6.1, Agilent
Technologies) was used for data analysis. Morphological changes in
Raji cells were observed under an inverted microscope (Olympus
Corporation) after treatment with bortezomib and TRAIL.
MMP assay
Changes in the MMP were detected by staining cells
with the fluorescent probe JC-1. Raji cells treated with 100, 50,
25 or 0 nM bortezomib and/or 100 ng/ml TRAIL for 24 h at 37°C with
2×105 cells/well were harvested, washed with ice-cold
PBS and stained with 5 mg/ml JC-1 at 37°C for 30 min in the dark.
Data acquisition and analysis of the MMP were performed using flow
cytometry. The analyte reporter was the fluorescent probe JC-1,
which exhibits a shift from red fluorescence (J-aggregates, ~590 nm
emission) to green fluorescence (monomers, ~527 nm emission) upon
mitochondrial membrane depolarization. Data acquisition was
performed using a NovoCyte Quanteon flow cytometer (ACEA
Biosciences, Inc.), and data analysis was conducted with
NovoExpress software (version 1.6.1, Agilent Technologies).
Intracellular ROS assay
NAC, a ROS scavenger and antioxidant, was used to
assess intracellular ROS generation (21). Raji cells were divided into two
groups: i) Pretreatment with NAC for 1 h at 37°C; and ii) untreated
(control) group. Subsequently, both groups were exposed to varying
concentrations of bortezomib (0, 25, 50 or 100 nM) and/or TRAIL
(100 ng/ml) for 24 h at 37°C, after which the cells were harvested,
washed with PBS, mixed with 10 µM 2′-7′-dichlorodihydrofluorescein
diacetate and incubated in the dark at 37°C for 30 min. Data
acquisition and analysis of ROS were performed using flow
cytometry. The analyte reporter was the fluorescent probe
2′,7′-dichlorodihydrofluorescein diacetate. Data acquisition was
performed using a NovoCyte Quanteon flow cytometer (ACEA
Biosciences, Inc.), and data analysis was conducted with
NovoExpress software (version 1.6.1, Agilent Technologies).
Analysis of DR5 expression on the cell
surface
To assess whether bortezomib enhances TRAIL
sensitivity via the ROS-dependent regulation of DR5 expression,
Raji cells were divided into two groups: i) Pretreatment with NAC
(10 mM) for 1 h at 37°C; and ii) untreated group. Both groups were
then exposed to increasing concentrations of bortezomib (0, 25, 50
or 100 nM) for 24 h at 37°C, and then stained with PE-CD262 (DR5)
monoclonal antibodies at 4°C for 30 min in the dark. Data
acquisition and analysis of DR5 expression in Raji cells were
performed using flow cytometry. The analyte reporter was a PE-CD262
(DR5) monoclonal antibody (cat. no. 12-9908-42) was directly
conjugated to PE. Data acquisition was performed using a NovoCyte
Quanteon flow cytometer (ACEA Biosciences, Inc.), and analysis was
conducted with NovoExpress software (version 1.6.1, Agilent
Technologies).
Western blot analysis
Raji cells treated with 100, 50, 25 or 0 nM
bortezomib and/or 100 ng/ml TRAIL for 24 h at 37°C with
5×106 cells were lysed with RIPA buffer containing
protease inhibitors or phosphatase inhibitors, then lysed on ice
for 30 min, mixed at 5-min intervals and centrifuged at 13,000 ×
g/min for 15 min at 4°C. The protein supernatants were collected,
and the protein concentration was determined using BCA kit.
Proteins (50 µg) were separated using 12% SDS-PAGE. The proteins
were subsequently transferred to PVDF membranes. The membranes were
blocked with 5% BSA for 1 h at 37°C, and incubated with primary
antibodies overnight at 4°C, and then with secondary antibodies
conjugated to HRP for another 2 h at 37°C. Subsequently, the PVDF
membranes were developed with ECL emitting solution and images were
captured using a gel imaging system (Bio-Rad Laboratories, Inc.).
The integrated optical density (IOD) values of the protein blots
were analyzed using ImageJ 1.54 analysis software (National
Institutes of Health). GAPDH served as a loading control to
normalize for equal protein loading across samples, and the IOD of
the target protein/IOD of GAPDH ratio reflected the relative
expression level of the target protein.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 10.0 software (Dotmatics). Each group of experiments was
independently repeated three times, and the experimental results
are presented as the mean ± SD. The differences between two groups
were compared using t-tests with Welch's correction, whilst the
differences between multiple groups were compared using one-way
ANOVA and Dunnett's post hoc test. Although all multiple
comparisons were formally assessed, only selected pairwise results
are displayed in the figures for clarity. P<0.05 was considered
to indicate a statistically significant difference.
Results
Bortezomib enhances TRAIL sensitivity
and synergistically inhibits proliferation in BL cells
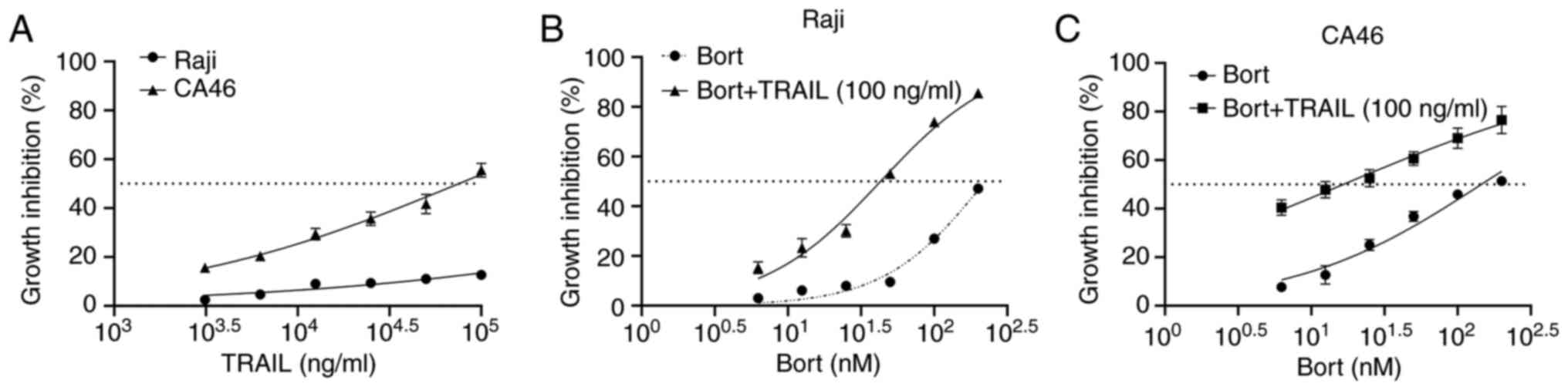
According to established criteria, cell lines
exhibiting <50% tumor growth inhibition following treatment with
100 ng/ml TRAIL are classified as TRAIL-insensitive (22). Cell viability assays revealed that
the BL cell lines (Raji and CA46) were insensitive to TRAIL. When
applied at a high concentration (1×105 ng/ml), TRAIL
achieved a proliferation inhibition rate of ~50% in CA46 cells,
whilst demonstrating notably less efficacy in Raji cells (<50%
inhibition; Fig. 1A). However, when
cells were treated with different concentrations of bortezomib
combined with 100 ng/ml TRAIL for 24 h, the inhibition of Raji
(Fig. 1B) and CA46 (Fig. 1C) cell proliferation noticeably
improved.
According to the Chou-Talalay combination index (CI)
method (23), synergistic, additive
and antagonistic effects between drugs are indicated when CI<1,
CI=1 and CI>1, respectively, whereas CI<0.9 indicates strong
synergistic effects. Bortezomib and TRAIL demonstrated strong
synergistic effects on Raji and CA46 cells (Table I). These results suggest that
bortezomib enhanced TRAIL sensitivity and synergistically inhibited
proliferation in BL cells. Consequently, the Raji cell line, the
least TRAIL-sensitive strain, was selected as the model for
subsequent experiments.
 | Table I.Combination index value of bortezomib
and tumor necrosis factor-related apoptosis-inducing ligand
combination therapy. |
Table I.
Combination index value of bortezomib
and tumor necrosis factor-related apoptosis-inducing ligand
combination therapy.
| A, Raji cells |
|---|
|
|---|
| Bortezomib, nM | TRAIL, ng/ml | Effect | CIa |
|---|
| 200.00 | 100.0 | 0.85495 | 0.04971 |
| 100.00 | 100.0 | 0.73910 | 0.06212 |
| 50.00 | 100.0 | 0.53075 | 0.09787 |
| 25.00 | 100.0 | 0.30000 | 0.16464 |
| 12.50 | 100.0 | 0.23000 | 0.12932 |
| 6.25 | 100.0 | 0.15000 | 0.12527 |
|
| B, CA46
cells |
|
| Bortezomib,
nM | TRAIL,
ng/ml | Effect | CIa |
|
| 200.00 | 100.0 | 0.76543 | 0.24680 |
| 100.00 | 100.0 | 0.69000 | 0.22003 |
| 50.00 | 100.0 | 0.60597 | 0.19259 |
| 25.00 | 100.0 | 0.52500 | 0.15917 |
| 12.50 | 100.0 | 0.47773 | 0.10674 |
| 6.25 | 100.0 | 0.40460 | 0.08510 |
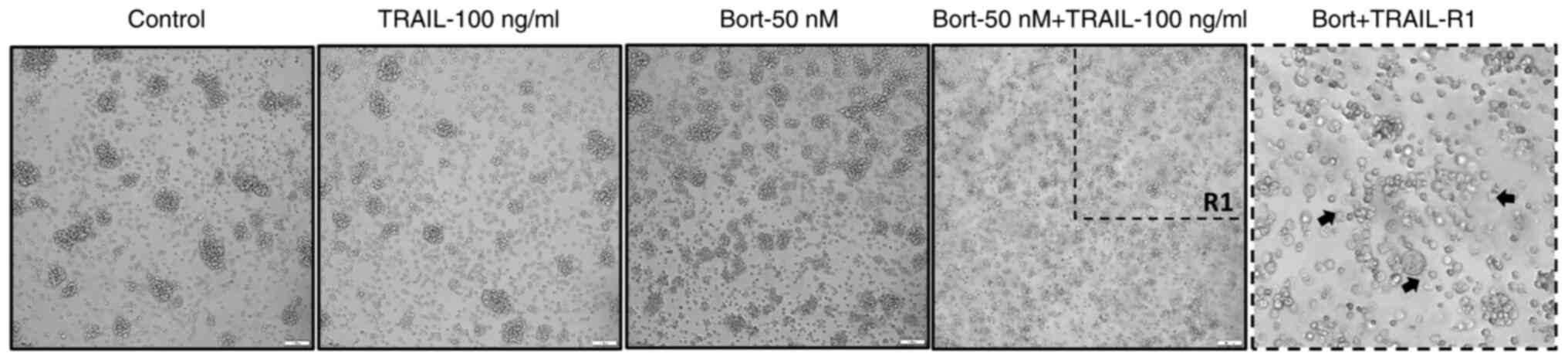
Combination of bortezomib and TRAIL
could induce morphological changes in Raji cells
Morphological analysis of Raji cells treated for 24
h with 50 nM bortezomib and/or 100 ng/ml TRAIL was performed using
inverted microscopy at a magnification of ×10. Compared with no
treatment, treatment with 100 ng/ml TRAIL alone did not induce
notable morphological changes. By contrast, 50 nM bortezomib alone
triggered characteristic apoptotic morphology, including cell
shrinkage. The combination of bortezomib and TRAIL produced
synergistic effects, as demonstrated by markedly reduced cell
density and widespread cell death. At a higher magnification of
×40, the R1 field displayed classical apoptotic hallmarks,
including marked cell shrinkage, nuclear condensation, membrane
blebbing and apoptotic body formation, confirming enhanced
cytotoxicity (Fig. 2).
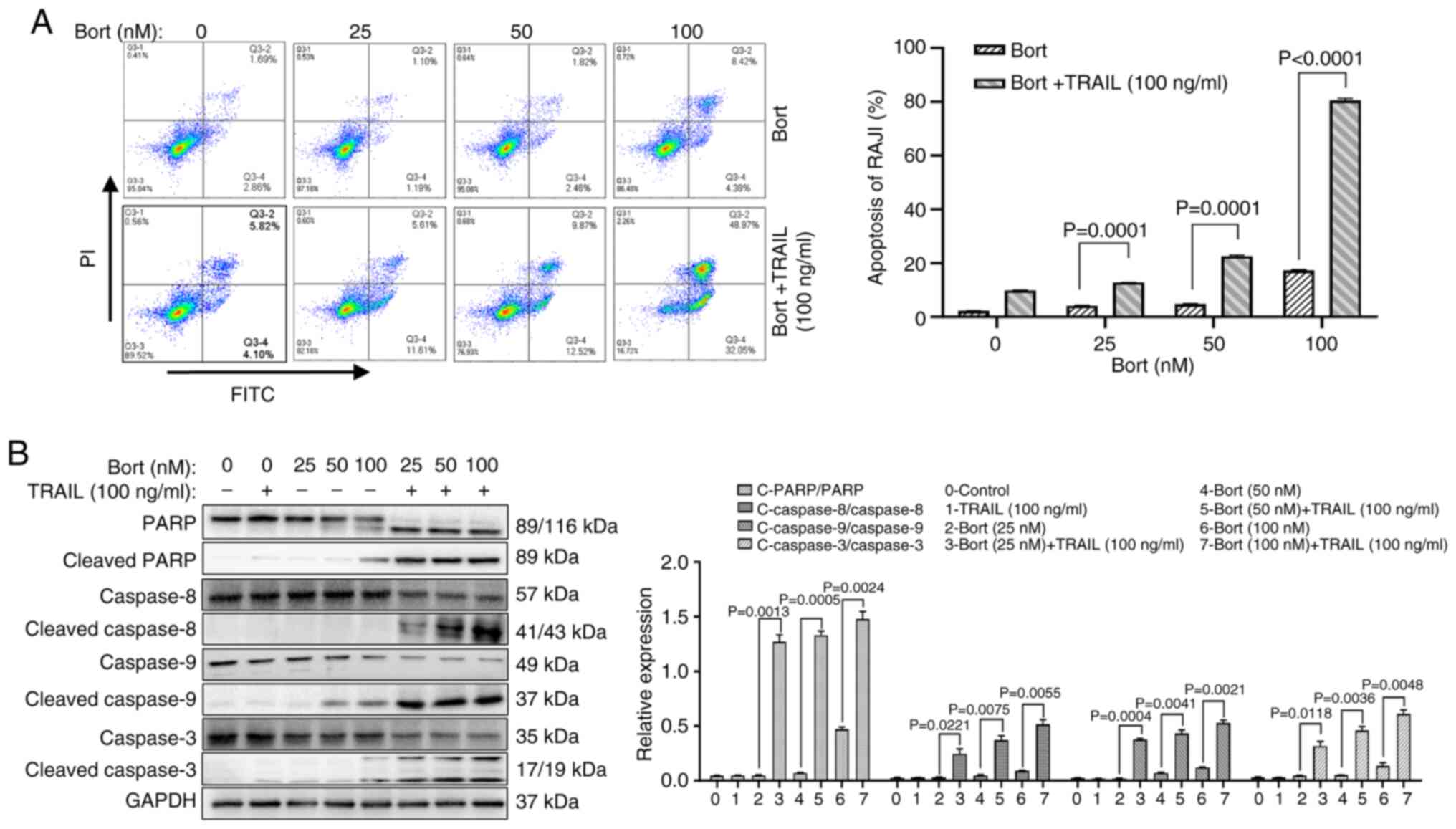
Bortezomib combined with TRAIL induces
the apoptosis of Raji cells
To further determine the pathway by which bortezomib
combined with TRAIL induced Raji cell death, annexin V-FITC/PI
double staining was used to detect the apoptosis of Raji cells.
Compared with that of cells treated with bortezomib alone, the
apoptosis rate of Raji cells increased significantly in a
dose-dependent manner when this was combined with 100 ng/ml TRAIL.
Specifically, the apoptosis rate rose from 12.63±0.17% for 100 nM
bortezomib alone to 80.82±0.20% for the combination treatment with
TRAIL and bortezomib (Fig. 3A).
Moreover, the expression levels of apoptosis-related proteins were
detected using western blot, and the results revealed that compared
with the bortezomib group the expression levels of PARP were
significantly decreased when TRAIL was combined with bortezomib,
and the levels of cleaved PARP and cleaved caspase 8/9/3 were
significantly increased (P<0.05; Fig. 3B).
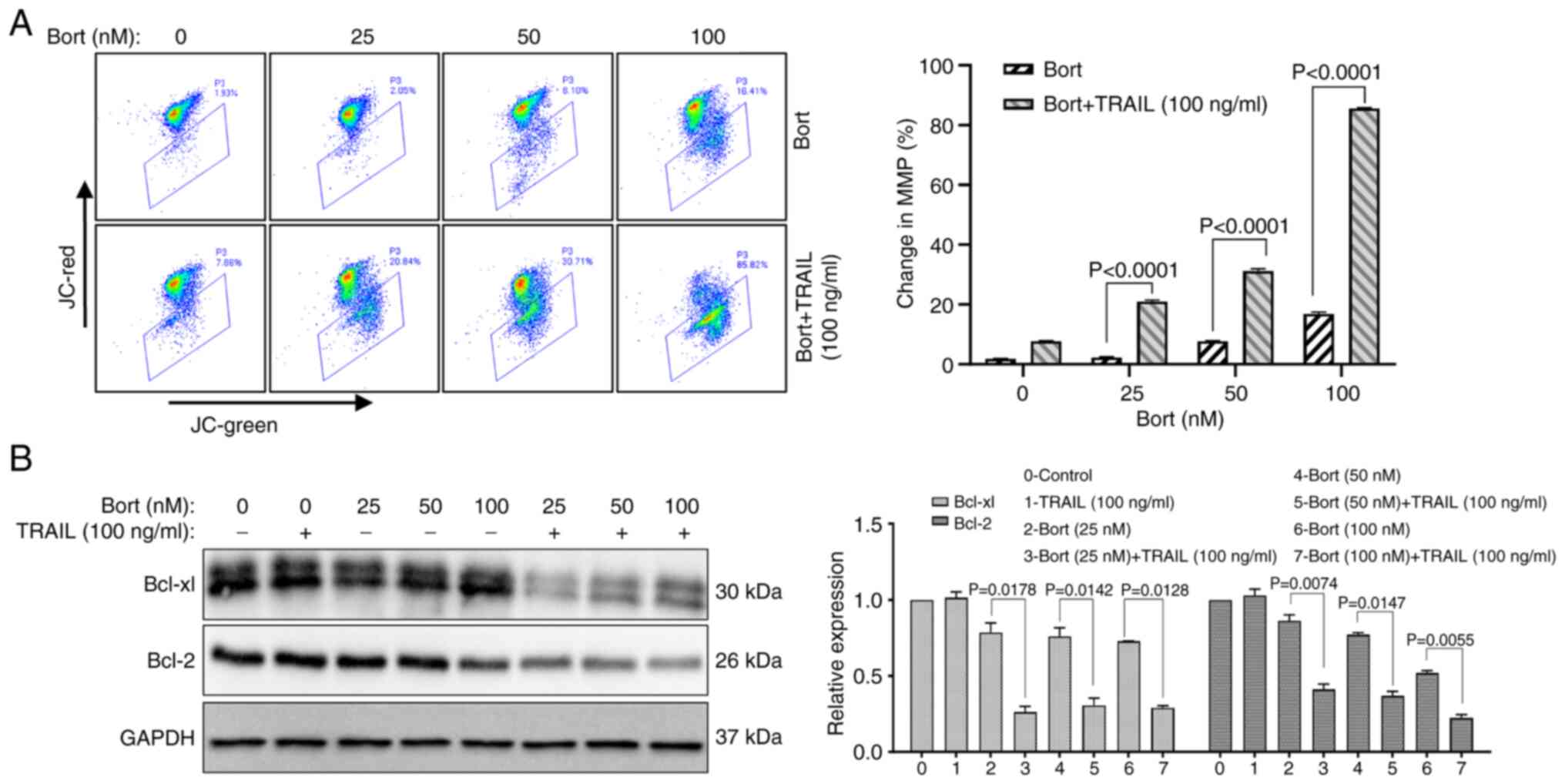
Bortezomib combined with TRAIL induces
the apoptosis of Raji cells via the mitochondrial apoptosis
pathway
The mitochondrial pathway is an important apoptotic
pathway. To further evaluate whether the mitochondrial apoptotic
pathway is involved in the increased sensitivity of Raji cells to
TRAIL caused by bortezomib, changes of the MMP were determined
using JC-1 staining. Compared with that of the TRAIL alone group,
the MMP changed significantly from 7.71±0.15 to 85.64±0.18% when
TRAIL was combined with 100 nM bortezomib (Fig. 4A). Furthermore, the western blot
analysis results revealed that compared with the bortezomib group
the expression levels of the antiapoptotic proteins Bcl-2 and
Bcl-xl were significantly decreased when TRAIL was combined with
bortezomib, (P<0.05; Fig. 4B).
These results suggest that bortezomib combined with TRAIL induces
apoptosis via the mitochondrial apoptotic pathway.
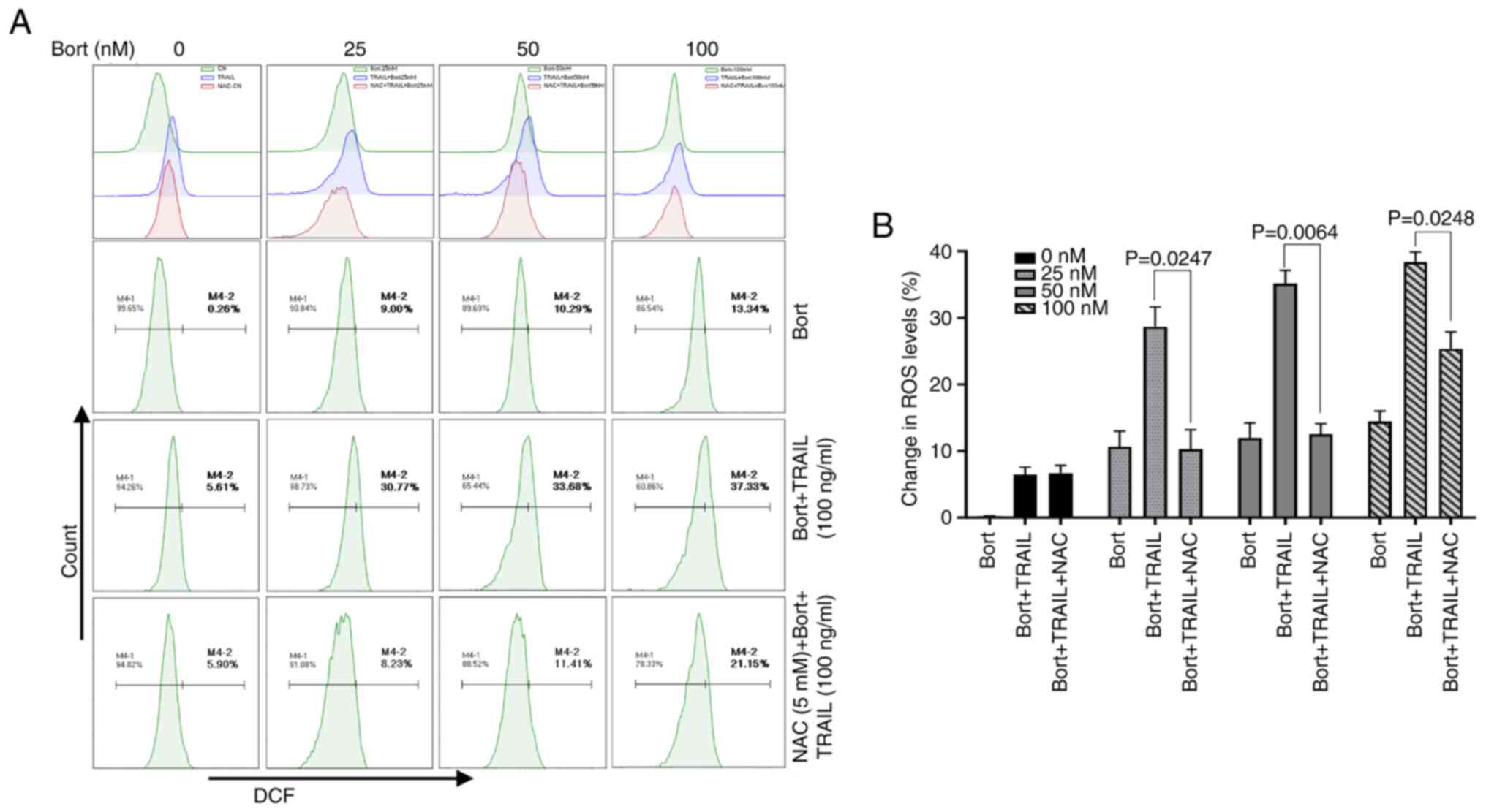
Bortezomib combined with TRAIL induces
ROS production
ROS are considered to be potential modulators of
apoptosis (24). The combination of
TRAIL and bortezomib markedly increased ROS levels in Raji cells
from 14.45±1.11% (100 nM bortezomib) to 36.56±0.77% (TRAIL combined
with 100 nM bortezomib; Fig. 5A).
Moreover, NAC is a widely used thiol-containing antioxidant that
clears ROS from cells by interacting with OH and
H2O2, thereby affecting ROS-mediated
signaling pathways (25). The flow
cytometry results revealed that NAC pretreatment significantly
blocked the ROS production caused by bortezomib + TRAIL compared
with that in the group without NAC pretreatment (P<0.05;
Fig. 5B).
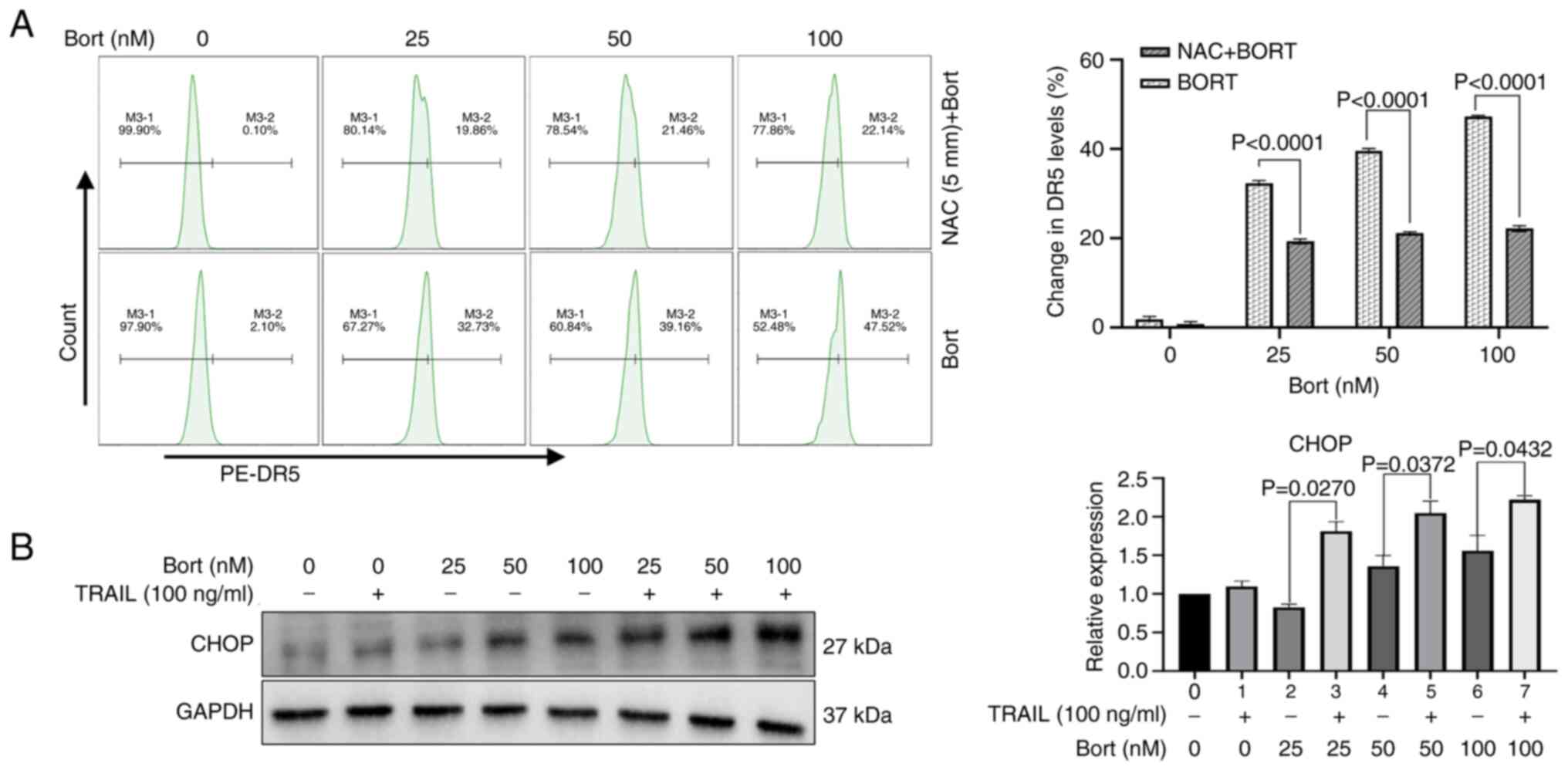
Bortezomib-induced DR5 upregulation
depends on ROS generation
DR5 is a receptor of TRAIL and serves a crucial role
in TRAIL-induced apoptosis (26).
Using staining with PE-CD262 (DR5) monoclonal antibodies, the
present study revealed that compared with control, bortezomib
significantly increased DR5 expression when in the presence of
bortezomib at 100 nM in Raji cells, from 1.60±0.50% (control group)
to 47.33±0.19% (in the presence of bortezomib at 100 nM). In
addition, NAC pretreatment significantly blocked the DR5
upregulation induced by bortezomib compared with that in the group
without NAC pretreatment (P<0.05; Fig. 6A). Furthermore, ROS generation is
associated with endoplasmic reticulum stress (ERS) and CHOP, and
the CHOP motif has been identified at the proximal region of the
DR5 gene promoter (27). Western
blot results identified that compared with that of the bortezomib
group the expression levels of CHOP were significantly increased
when TRAIL was combined with bortezomib (Fig. 6B). Based on these results, it was
hypothesize that bortezomib sensitizes Raji cells to TRAIL by
activating the ROS-CHOP-DR5 signaling axis, thereby promoting their
synergistic effect in inducing apoptosis and inhibiting cell
proliferation.
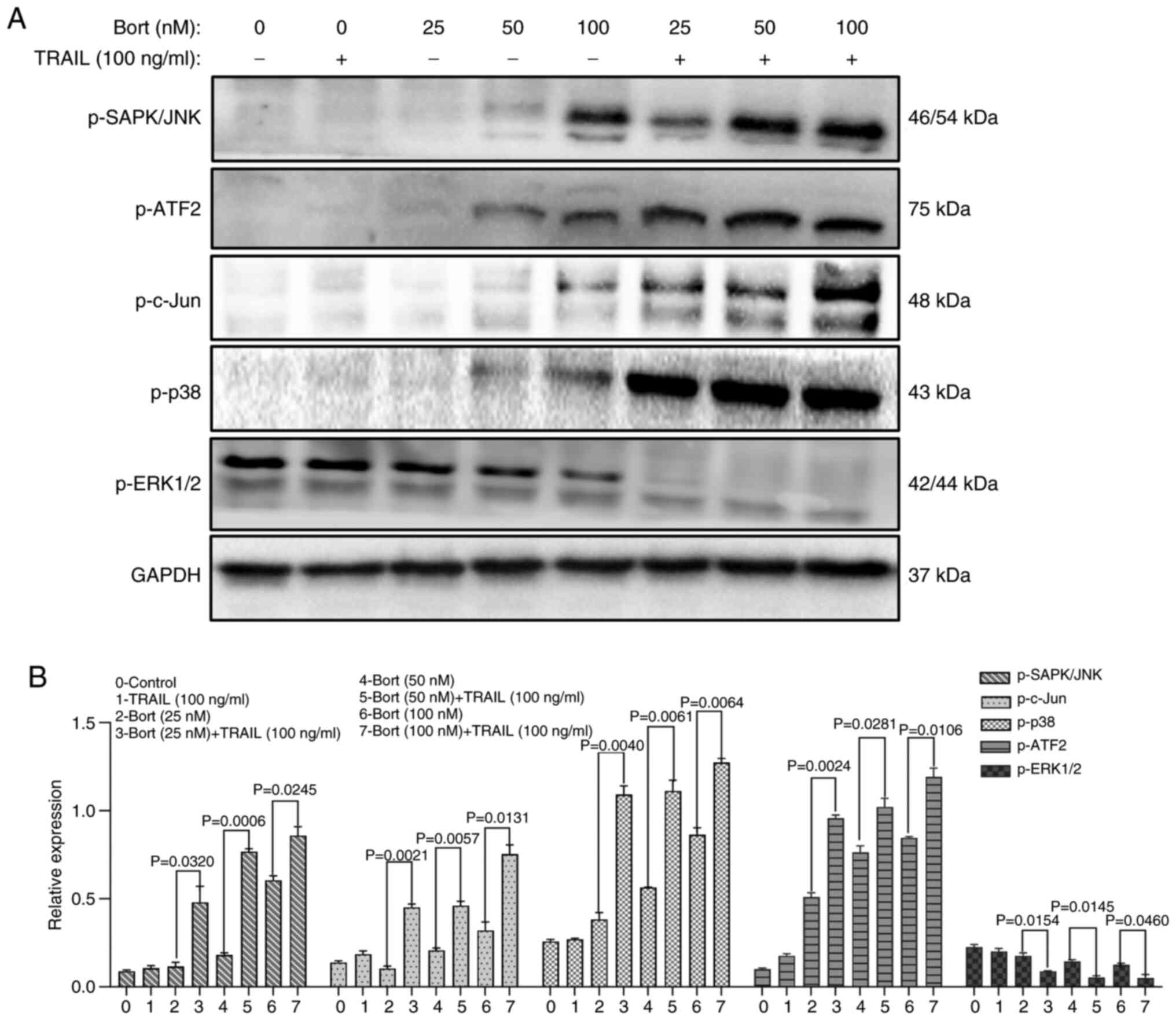
Bortezomib increases the sensitivity
of Raji cells to TRAIL through the MAPK signaling pathway
The MAPK signaling pathway can be activated by
several stimuli, regulating physiological processes such as cell
proliferation and death. It also serves an important role in the
process of apoptosis (28). To
assess the effect of bortezomib combined with TRAIL on the MAPK
signaling pathway in Raji cells, western blot was used to detect
the expression levels of MAPK signaling pathway-related proteins
(Fig. 7A). Compared with that of
the bortezomib group the levels of p-ERK1/2 in the MAPK/ERK
signaling pathway were significantly decreased, and the levels of
p-SAPK/JNK, p-c-Jun, p-ATF2 and p-p38 in the MAPK/p38 and MAPK/JNK
signaling pathways were significantly increased when TRAIL was
combined with bortezomib (P<0.05; Fig. 7B). These results suggest that
bortezomib may increase sensitivity and regulate the apoptosis of
Raji cells by activating the MAPK signaling pathway.
Discussion
TRAIL is a member of the TNF family that promotes
apoptosis, and can selectively kill tumor cells through specific
cell surface receptors, making it a promising antitumor drug.
However, in malignant lymphoma, the development of TRAIL resistance
is a major bottleneck limiting therapeutic effectiveness (9). Bortezomib, a proteasome inhibitor,
exhibits great therapeutic potential for sensitizing tumor cells to
TRAIL (16–18). Moreover, it has been reported that
A549 cells with acquired bortezomib resistance exhibit phenotypic
reversal of sensitivity to TRAIL (29).
Previous research has reported that the combination
of TRAIL and bortezomib was able to induce cell death in
TRAIL-resistant cancer cells via the intracellular TRAIL pathway
(17,18). Our previous study demonstrated that
BL cells have different sensitivities to TRAIL, and that the Raji
cell line is the strain with the most pronounced resistance to
TRAIL (20). Furthermore, the
present study revealed a potential synergistic effect of bortezomib
combined with TRAIL in promoting TRAIL-resistant BL cell apoptosis,
and explored its molecular mechanisms. The findings demonstrated
that bortezomib markedly increased the drug sensitivity of Raji and
CA46 cells to TRAIL. Moreover, when bortezomib was combined with
100 ng/ml TRAIL, it significantly inhibited the proliferation of BL
cells. Bortezomib also enhanced TRAIL-induced apoptosis in BL cells
in a concentration-dependent manner.
The TRAIL pathway consists of two caspase-level
pathways. After the initial activation of caspase 8 by TRAIL, the
signals differentiate in two directions: i) Direct activation of
caspase 3, without the involvement of mitochondria; and ii)
apoptotic bodies (mitochondrial proteins, dATP and Apaf-1) are
formed, resulting in the activation of caspase 9, followed by
activation of caspase 3 (30).
After bortezomib was combined with TRAIL in Raji cells, the levels
of cleaved caspase 8/9/3 were increased, and the changes of the
MMP, an important factor of mitochondrial dysfunction,
significantly decreased. It has been reported that downregulation
of Bcl-2 expression enhances the sensitivity of tumor cells to
TRAIL, and decreased intracellular Bcl-xl levels are responsible
for the acquisition of TRAIL resistance in tumor cells (31,32).
The western blot results in the present study revealed that the
expression levels of the antiapoptotic proteins Bcl-2 and Bcl-xl,
key members of the Bcl-2 family that target the mitochondrial
apoptotic pathway, were significantly decreased in Raji cells
(33). These findings suggest that
the mitochondrial apoptotic pathway serves a role in bortezomib
sensitization of Raji cells to TRAIL-induced apoptosis.
Mitochondria are the primary sites of oxygen
utilization in eukaryotic cells. Under stress (intracellular ROS
accumulation), mitochondrial outer membrane permeability increases
and the MMP decreases, leading to the release of proapoptotic
proteins into the cytoplasm, the activation of caspases and
ultimately apoptosis (34,35). Oxidative stress serves a crucial
role as a mediator of cell death. Studies have reported that cancer
chemopreventive drugs promote ROS generation, which upregulates
DR4/DR5 and potentiates TRAIL pathway, ultimately increasing cancer
cell sensitivity to TRAIL (36,37).
Furthermore, the death receptor DR5 has an important function in
the transduction of TRAIL activity and can activate signaling
pathways associated with cell death in cancer cells (38). However, the reduced or lost
expression of DR5 in cancer cells leads to TRAIL resistance
(39). The present study
demonstrated that bortezomib combined with TRAIL could
significantly increase intracellular ROS levels and upregulate DR5
expression, whilst DR4 expression revealed no significant change
(Fig S1). Furthermore, scavenging
ROS with the antioxidant NAC abolished bortezomib-induced DR5
expression, suggesting that ROS serve a major role in the
regulation of the TRAIL receptor DR5. In addition, the present
study revealed that bortezomib combined with TRAIL enhanced the
expression of the ERS-related marker protein CHOP. ROS, key
regulators of endoplasmic reticulum function and unfolded protein
response activation, can induce apoptosis by inducing ERS and
activating the downstream effector CHOP (40,41).
It has been reported that CHOP can directly regulate the expression
of DR5 through the CHOP binding site on the 5′ side of the DR5
promoter (27). Therefore, we
hypothesize that the ROS-mediated CHOP-dependent DR5 pathway may be
involved in bortezomib-induced sensitization to TRAIL.
Additionally, it has been reported that ROS act as
upstream signaling messengers that trigger the MAPK cascade
(42), and that the MAPK signaling
pathway is involved in the regulation of cell proliferation,
differentiation, apoptosis, the inflammatory response and vascular
development in the human body. ERK, JNK and p38 kinase belong to
the most common mammalian MAPK subclass (43). Studies have reported that
dysregulation of the MAPK system alters normal physiological
processes and is frequently involved in tumorigenesis, development
and drug resistance, and thus, the MAPK system is considered to be
a viable target for cancer therapy (44,45).
It is well established that the activation of MAPK family members
(such as ERK, JNK and p38) requires dual phosphorylation, a process
in which both threonine and tyrosine residues within the activation
loop are phosphorylated by upstream kinases (MEK or MKK), resulting
in full kinase activity (46).
Accordingly, the phosphorylated forms of these kinases (such as
p-ERK, p-JNK and p-p38) serve as reliable indicators of pathway
activation. In the present study, following combination treatment
with bortezomib and TRAIL, western blot was used to assess the
expression levels of phosphorylated (activated) forms of key
proteins within the MAPK signaling pathway. However, the total
protein expression levels were not simultaneously detected due to
insufficient consideration in the experimental design. The results
revealed that the levels of p-ERK1/2 in the MAPK/ERK signaling
pathway were significantly decreased., and the levels of p-p38 in
the MAPK/p38 signaling pathway, and the levels of p-SAPK/JNK,
p-c-Jun and p-ATF2 in the MAPK/JNK signaling pathway, were
significantly increased. These findings suggest that bortezomib
enhanced the sensitivity of Raji cells to TRAIL by activating the
MAPK signaling pathway and synergistically inducing apoptosis.
In summary, the mechanism by which bortezomib
increased the sensitivity of Raji cells to TRAIL and
synergistically induced apoptosis is hypothesized to be as follows:
In Raji cells, bortezomib combined with TRAIL can increase the
level of oxidative stress, increase the level of ROS, decrease the
MMP, inhibit the expression of the antiapoptotic factor Bcl-2
family, activate the caspase cascades of caspase 8/9, caspase 3 and
PARP, and lead to the apoptosis of Raji cells through the
mitochondrial pathway. Furthermore, the increase in intracellular
ROS may trigger ERS, and then induce DR5 upregulation through the
transcription factor CHOP, thus increasing the sensitivity of Raji
cells to TRAIL. Finally, the increase in the sensitivity of Raji
cells to bortezomib may regulate the apoptosis of Raji cells by
activating the MAPK signaling pathway.
However, there are limitations of the present study:
Among TRAIL-resistant BL, the present study focused on two
representative cell lines, CA46 and Raji, which exhibited
differential sensitivity profiles. Furthermore, whilst the present
study identified the mechanism through which bortezomib sensitized
Raji cells, such as DR5 upregulation and caspase-8 activation, the
generalizability of this mechanism across other TRAIL-resistant BL
cell lines warrants further investigation. Future studies should
systematically evaluate this sensitization pathway in vivo
using xenograft mouse models to validate the therapeutic efficacy
of the bortezomib-TRAIL combination for BL treatment.
In conclusion, bortezomib appeared to increase the
sensitivity of Raji BL cells to TRAIL through ROS-dependent
upregulation of DR5, and to induce apoptosis via the MAPK signaling
pathway and the mitochondrial apoptosis pathway with synergistic
effects. This combined approach may provide a more effective and
less harmful option for the treatment of TRAIL-resistant BL.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Sichuan Science and
Technology Program of China (grant no. 2023NSFSC0725), the National
Undergraduates Innovating Experimentation Project of China (grant
nos. 202513705003, 202513705007 and 202513705022) and the Research
and Innovation Fund for Postgraduates of Chengdu Medical College of
China (grant nos. YCX2024-01-10 and YCX2023-02-04).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JC, WZ, PY and ML designed the research study. JC,
WZ, YY, YZ, SP and XB performed the experiments. JC, WZ, YY, ML and
PY analyzed the data. JC and YY confirm the authenticity of all the
raw data. All authors contributed to editorial changes in the
manuscript. All authors read and approved the final manuscript. All
authors participated sufficiently in the work and agreed to be
accountable for all aspects of the work.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Saleh K, Michot JM, Camara-Clayette V,
Vassetsky Y and Ribrag V: Burkitt and burkitt-like lymphomas: A
systematic review. Curr Oncol Rep. 22:332020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun K, Wu H, Zhu Q, Gu K, Wei H, Wang S,
Li L, Wu C, Chen R, Pang Y, et al: Global landscape and trends in
lifetime risks of haematologic malignancies in 185 countries:
Population-based estimates from GLOBOCAN 2022. EClinicalMedicine.
83:1031932025. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chu Y, Liu Y, Fang X, Jiang Y, Ding M, Ge
X, Yuan D, Lu K, Li P, Li Y, et al: The epidemiological patterns of
non-Hodgkin lymphoma: Global estimates of disease burden, risk
factors, and temporal trends. Front Oncol. 13:10599142023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Molyneux EM, Rochford R, Griffin B, Newton
R, Jackson G, Menon G, Harrison CJ, Israels T and Bailey S:
Burkitt's lymphoma. Lancet. 379:1234–1244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holmes M, Scott GB, Heaton S, Barr T,
Askar B, Müller LME, Jennings VA, Ralph C, Burton C, Melcher A, et
al: Efficacy of coxsackievirus A21 against drug-resistant
neoplastic B cells. Mol Ther Oncolytics. 29:17–29. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jacobson C and LaCasce A: How I treat
Burkitt lymphoma in adults. Blood. 124:2913–2920. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naimi A, Movassaghpour AA, Hagh MF, Talebi
M, Entezari A, Jadidi-Niaragh F and Solali S: TNF-related
apoptosis-inducing ligand (TRAIL) as the potential therapeutic
target in hematological malignancies. Biomed Pharmacother.
98:566–576. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lemke J, von Karstedt S, Zinngrebe J and
Walczak H: Getting TRAIL back on track for cancer therapy. Cell
Death Differ. 21:1350–1364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu FT, Agrawal SG, Gribben JG, Ye H, Du
MQ, Newland AC and Jia L: Bortezomib blocks bax degradation in
malignant B cells during treatment with TRAIL. Blood.
111:2797–2805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deng L, Zhai X, Liang P and Cui H:
Overcoming TRAIL resistance for glioblastoma treatment.
Biomolecules. 11:5722021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Quiroz-Reyes AG, Delgado-Gonzalez P, Islas
JF, Gallegos JLD, Garza JH and Garza-Trevino EN: Behind the
adaptive and resistance mechanisms of cancer stem cells to TRAIL.
Pharmaceutics. 13:10622021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yagolovich AV, Gasparian ME, Isakova AA,
Artykov AA, Dolgikh DA and Kirpichnikov MP: Cytokine TRAIL death
receptor agonists: Design strategies and clinical prospects.
Russian Chemical Reviews. 94:RCR51542025. View Article : Google Scholar
|
|
13
|
Kundu M, Greer YE, Dine JL and Lipkowitz
S: Targeting TRAIL death receptors in triple-negative breast
cancers: Challenges and strategies for cancer therapy. Cells.
11:37172022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thorburn A, Behbakht K and Ford H: TRAIL
receptor-targeted therapeutics: Resistance mechanisms and
strategies to avoid them. Drug Resist Updat. 11:17–24. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cingoz A, Ozyerli-Goknar E, Morova T,
Seker-Polat F, Selvan ME, Gümüş ZH, Bhere D, Shah K, Solaroglu I
and Bagci-Onder T: Generation of TRAIL-resistant cell line models
reveals distinct adaptive mechanisms for acquired resistance and
re-sensitization. Oncogene. 40:3201–3216. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Robak P and Robak T: Bortezomib for the
treatment of hematologic malignancies: 15 years later. Drugs R D.
19:73–92. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bui HTT, Le NH, Le QA, Kim SE, Lee S and
Kang D: Synergistic apoptosis of human gastric cancer cells by
bortezomib and TRAIL. Int J Med Sci. 16:1412–1423. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ryu S, Ahn YJ, Yoon C, Chang JH, Park Y,
Kim TH, Howland AR, Armstrong CA, Song PI and Moon AR: The
regulation of combined treatment-induced cell death with
recombinant TRAIL and bortezomib through TRAIL signaling in
TRAIL-resistant cells. BMC Cancer. 18:4322018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kabore AF, Sun J, Hu X, McCrea K, Johnston
JB and Gibson SB: The TRAIL apoptotic pathway mediates proteasome
inhibitor induced apoptosis in primary chronic lymphocytic leukemia
cells. Apoptosis. 11:1175–1193. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qin X, Chen Z and Chen Y: Sensitivity of
tumor necrosis factor-related apoptosis-inducing ligands in B
lymphoma cell lines and mechanisms of apoptosis induction. J
Chengdu Med Coll. 11:413–442. 2016.
|
|
21
|
Pedre B, Barayeu U, Ezeriņa D and Dick TP:
The mechanism of action of N-acetylcysteine (NAC): The emerging
role of H(2)S and sulfane sulfur species. Pharmacol Ther.
228:1079162021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Qiao X, Shang Y, Zhang S, Li Y, He
H and Chen SZ: RGD and NGR modified TRAIL protein exhibited potent
anti-metastasis effects on TRAIL-insensitive cancer cells in vitro
and in vivo. Amino Acids. 49:931–941. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu M, Wu X, Cui Y, Liu P, Xiao B, Zhang
X, Zhang J, Sun Z, Song M, Shao B and Li Y: Mitophagy and apoptosis
mediated by ROS participate in AlCl(3)-induced MC3T3-E1 cell
dysfunction. Food Chem Toxicol. 155:1123882021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim BR, Park SH, Jeong YA, Na YJ, Kim JL,
Jo MJ, Jeong S, Yun HK, Oh SC and Lee DH: RUNX3 enhances
TRAIL-induced apoptosis by upregulating DR5 in colorectal cancer.
Oncogene. 38:3903–3918. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamaguchi H and Wang HG: CHOP is involved
in endoplasmic reticulum stress-induced apoptosis by enhancing DR5
expression in human carcinoma cells. J Biol Chem. 279:45495–45502.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han SH, Lee JH, Woo JS, Jung GH, Jung SH,
Han EJ, Park YS, Kim BS, Kim SK, Park BK, et al: Myricetin induces
apoptosis through the MAPK pathway and regulates JNK-mediated
autophagy in SK-BR-3 cells. Int J Mol Med. 49:542022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
De Wilt L, Sobocki BK, Jansen G, Tabeian
H, de Jong S, Peters GJ and Kruyt F: Mechanisms underlying reversed
TRAIL sensitivity in acquired bortezomib-resistant non-small cell
lung cancer cells. Cancer Drug Resist. 7:122024.PubMed/NCBI
|
|
30
|
Xi H, Wang S, Wang B, Hong X, Liu X, Li M,
Shen R and Dong Q: The role of interaction between autophagy and
apoptosis in tumorigenesis (Review). Oncol Rep. 48:2082022.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim HJ, Kang S, Kim DY, You S, Park D, Oh
SC and Lee DH: Diallyl disulfide (DADS) boosts TRAIL-Mediated
apoptosis in colorectal cancer cells by inhibiting Bcl-2. Food Chem
Toxicol. 125:354–360. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fresquet V, Rieger M, Carolis C,
Garcia-Barchino MJ and Martinez-Climent JA: Acquired mutations in
BCL2 family proteins conferring resistance to the BH3 mimetic
ABT-199 in lymphoma. Blood. 123:4111–4119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim R: Unknotting the roles of Bcl-2 and
Bcl-xL in cell death. Biochem Biophys Res Commun. 333:336–343.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Waltz F, Salinas-Giege T, Englmeier R,
Meichel H, Soufari H, Kuhn L, Pfeffer S, Förster F, Engel BD, Giegé
P, et al: How to build a ribosome from RNA fragments in
Chlamydomonas mitochondria. Nat Commun. 12:71762021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lan Q, Lim U, Liu CS, Weinstein SJ,
Chanock S, Bonner MR, Virtamo J, Albanes D and Rothman N: A
prospective study of mitochondrial DNA copy number and risk of
non-Hodgkin lymphoma. Blood. 112:4247–4249. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jin CY, Molagoda IMN, Karunarathne W, Kang
SH, Park C, Kim GY and Choi YH: TRAIL attenuates
sulforaphane-mediated Nrf2 and sustains ROS generation, leading to
apoptosis of TRAIL-resistant human bladder cancer cells. Toxicol
Appl Pharmacol. 352:132–141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jeong S, Farag AK, Yun HK, Jeong YA, Kim
DY, Jo MJ, Park SH, Kim BR, Kim JL, Kim BG, et al: AF8c, a
multi-kinase inhibitor induces apoptosis by activating DR5/Nrf2 via
ROS in colorectal cancer cells. Cancers (Basel). 14:30432022.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lv Z, Hu J, Su H, Yu Q, Lang Y, Yang M,
Fan X, Liu Y, Liu B, Zhao Y, et al: TRAIL induces podocyte
PANoptosis via death receptor 5 in diabetic kidney disease. Kidney
Int. 107:317–331. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim HH, Lee SY and Lee DH: Apoptosis of
pancreatic cancer cells after co-treatment with eugenol and tumor
necrosis factor-related apoptosis-inducing ligand. Cancers (Basel).
16:30922024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liao H, Li X, Zhang H, Yin S, Hong Y, Chen
R, Gui F, Yang L, Yang J and Zhang J: The ototoxicity of
chlorinated paraffins via inducing apoptosis, oxidative stress and
endoplasmic reticulum stress in cochlea hair cells. Ecotoxicol
Environ Saf. 284:1169362024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu M, Yao Y, Chen R, Fu B, Sun Y, Yu Y,
Liu Y, Feng H, Guo S, Yang Y and Zhang C: Effects of melatonin and
3,5,3′-Triiodothyronine on the development of rat granulosa cells.
Nutrients. 16:30852024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rezatabar S, Karimian A, Rameshknia V,
Parsian H, Majidinia M, Kopi TA, Bishayee A, Sadeghinia A, Yousefi
M, Monirialamdari M and Yousefi B: RAS/MAPK signaling functions in
oxidative stress, DNA damage response and cancer progression. J
Cell Physiol. 234:14951–14965. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lewis TS, Shapiro PS and Ahn NG: Signal
transduction through MAP kinase cascades. Adv Cancer Res.
74:49–139. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li HC, Li JY, Wang XC, Zeng M, Wu YK, Chen
YL, Kong CH, Chen KL, Wu JR, Mo ZX, et al: Network pharmacology,
experimental validation and pharmacokinetics integrated strategy to
reveal pharmacological mechanism of goutengsan on methamphetamine
dependence. Front Pharmacol. 15:14805622024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ju Z, Bi Y, Gao M, Yin Y, Xu T and Xu S:
Emamectin benzoate and nanoplastics induce PANoptosis of common
carp (Cyprinus carpio) gill through MAPK pathway. Pestic Biochem
Physiol. 206:1062022024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kciuk M, Gielecinska A, Budzinska A,
Mojzych M and Kontek R: Metastasis and MAPK pathways. Int J Mol
Sci. 23:38472022. View Article : Google Scholar : PubMed/NCBI
|