Introduction
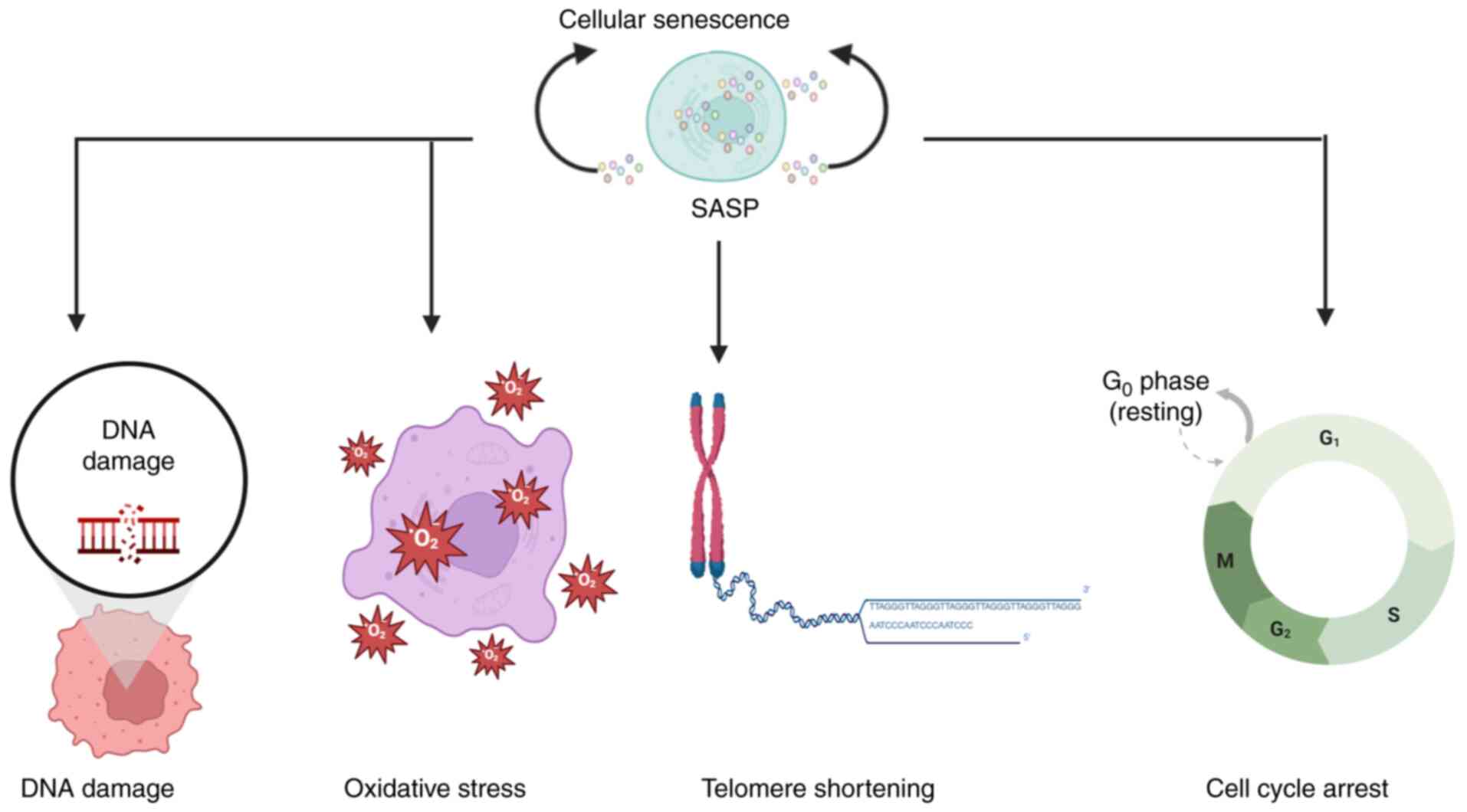
Cellular senescence exhibits a paradoxical dual role
in cancer biology, acting both as a barrier to tumorigenesis and as
a facilitator of tumor progression (Fig. 1) (1). In early tumor development, senescence
serves as a key tumor-suppressive mechanism by enforcing stable
cell cycle arrest through the p53/p21 and p16/ribosome (Rb)
pathways, thereby restricting the proliferation of damaged cells
(1,2). By contrast, increasing evidence
indicates that the senescence-associated secretory phenotype
(SASP), comprising inflammatory cytokines (such as IL-6 and IL-8),
growth factors and matrix-remodeling enzymes, can paradoxically
foster tumorigenesis through extensive remodeling of the tumor
microenvironment (TME) (2,3).
Despite these advances, several critical questions
remain unresolved: How do distinct components of the SASP
differentially influence tumor progression across cancer types? How
can we harness the cytoprotective properties of senescence while
avoiding its pro-tumorigenic effects? Recent findings underscore
the context-dependent nature of interactions between senescent
cells and the TME, which highlights the need for precision
strategies in senescence-targeted interventions (4–6). The
present review aims to address these gaps by dissecting SASP
heterogeneity and its therapeutic implications, and by evaluating
emerging modalities, including senolytics and SASP modulators, that
hold the potential to reshape cancer treatment paradigms.
Precise molecular targeting remains central to
therapeutic success, particularly in virus-associated malignancies.
For instance, hepatitis B virus X protein (HBx) drives
hepatocellular carcinoma (HCC) via regulating the expression of
microRNAs, underscoring the critical role of viral proteins in
promoting malignant transformation (5); additionally, distinct genotypes of
human T-cell lymphotropic virus type-1 (HTLV-1)-a virus closely
associated with adult T-cell leukemia/lymphoma-exhibit varying
prevalence among populations with 1.5% among blood donors in Iran)
and correlate with differential cancer susceptibility, which
supports genotype-based stratified screening for this hematological
malignancy (6–9). These observations underscore the
necessity for tailored screening and intervention strategies.
Within the TME, SASP-driven remodeling orchestrates
immune evasion, angiogenesis and therapeutic resistance,
compounding the complexity of cancer management (2,10,11).
In advanced disease, the persistent accumulation of senescent cells
and their secretome fosters an immunosuppressive niche through
recruitment of myeloid-derived suppressor cells and T-cell
dysfunction, while promoting metastatic dissemination (12,13).
This dynamic interplay between the protective and
deleterious facets of senescence has spurred the development of
targeted senotherapies (10,13).
Current approaches encompass the following: i) Senolytic agents,
which selectively ablate detrimental senescent cells (e.g.,
dasatinib-quercetin combinations); ii) senomorphic compounds, which
attenuate harmful SASP components without eliminating senescent
cells; and iii) novel candidates inspired by Traditional Chinese
Medicine (e.g., resveratrol), which may fine-tune senescence
responses (10,13,14).
Yet, notable challenges persist in clinical translation, including
the identification of biomarkers to distinguish protective from
pathogenic senescent subsets, mitigation of therapy-induced
SASP-mediated resistance and optimization of therapeutic timing to
maximize benefit while limiting harm (2,11).
Emerging evidence suggests that integrating
senescence-targeted agents with established treatments, such as
immunotherapy, may potentiate therapeutic efficacy (12). Advancing these strategies will
require robust preclinical models that accurately recapitulate the
complexity of the human TME (15,16).
Furthermore, incorporating single-cell profiling technologies and
systematically evaluating combination regimens, including those
pairing traditional Chinese and Western medicines, will be key to
fully exploit the therapeutic promise of senescence modulation in
oncology (14).
A nuanced understanding of cellular senescence in
cancer underscores its dualistic nature and positions it as an
attractive yet challenging therapeutic target. Future research can
delineate the molecular determinants that dictate whether
senescence exerts tumor-suppressive or tumor-promoting effects in
specific contexts, paving the way for precision interventions that
may potentially offer clinical benefit (1,2,11,12).
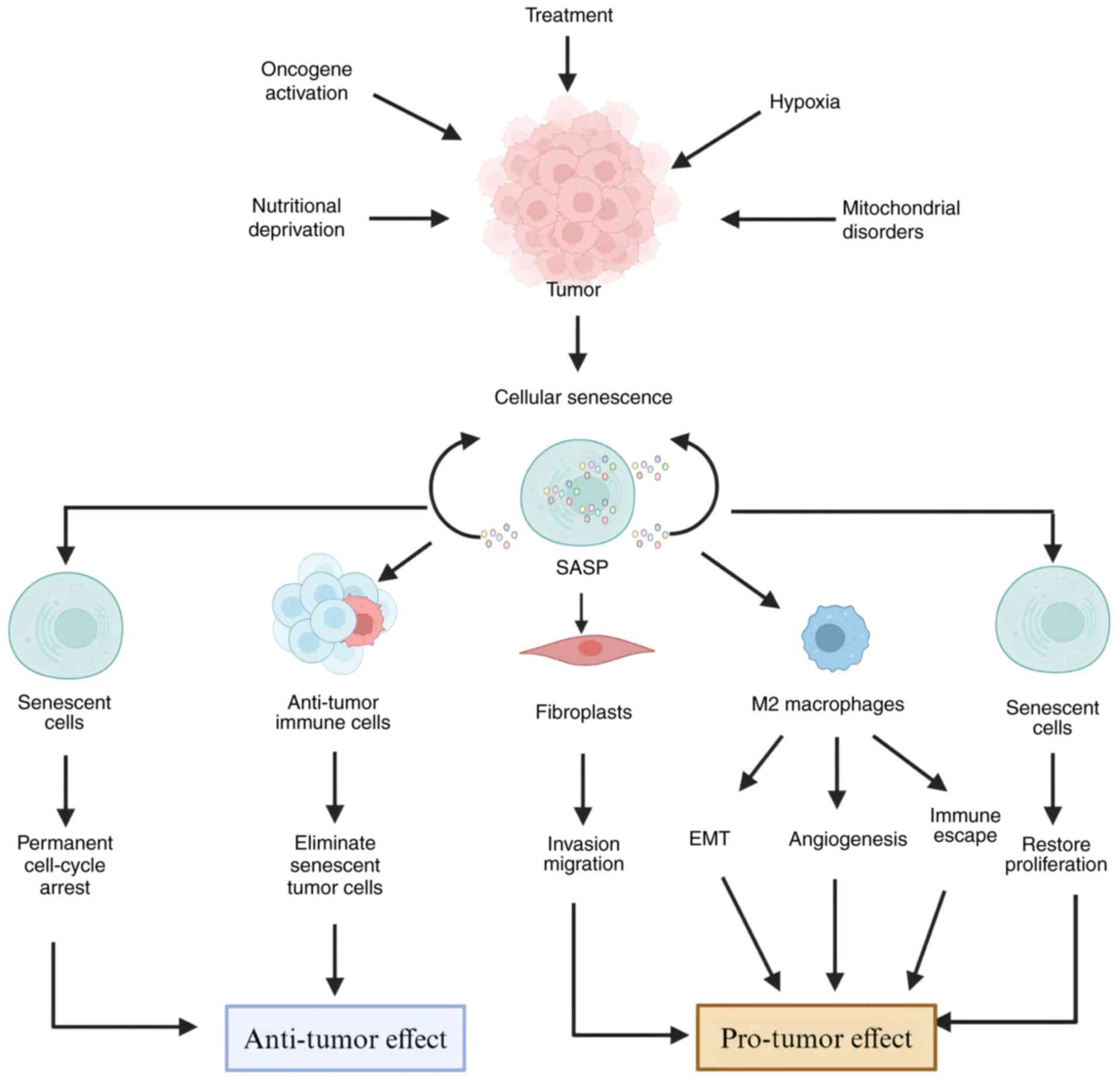
Dual-faced nature of cellular senescence in
cancer
Cellular senescence exerts a paradoxical influence
on cancer, functioning as both a tumor suppressor and promoter
(17) (Fig. 2). In the early stages of
tumorigenesis, senescence operates as a key defense mechanism,
arresting the proliferation of damaged cells via the p53/p21 and
p16/Rb pathways, thereby preventing malignant transformation
(2,18–21).
This protective effect is mediated by cell-autonomous processes
such as growth arrest and by non-cell-autonomous mechanisms through
SASP, which remodels the TME via cytokines, chemokines and
matrix-degrading enzymes (21–24).
For instance, in HCC, senescent cells suppress tumor initiation
through activation of p53-p21-Rb signaling, whereas SASP-derived
IL-24 recruits cytotoxic T cells to eliminate premalignant cells
(5,25).
By contrast, at advanced stages, the SASP
paradoxically fosters tumor progression by establishing an
inflammatory niche conducive to metastasis and therapeutic
resistance (15,26–33).
This duality is shaped by tumor-specific microenvironmental
contexts, resulting in divergent outcomes that can either restrain
or accelerate cancer progression (34,35).
In gastric cancer (GC), SASP-derived IL-6 and C-X-C motif chemokine
ligand 12 (CXCL12) promote immune evasion and extracellular matrix
(ECM) remodeling by recruiting myeloid-derived suppressor cells
(MDSCs) and cancer-associated fibroblasts (CAFs) (5,25).
Similarly, in lung adenocarcinoma, SASP-driven upregulation of
secreted phosphoprotein 1 (SPP1) and Tenascin enhances invasion and
immune escape through CD44 and integrin signaling (26,31).
Furthermore, senescence-associated epigenetic alterations, such as
the demethylation of histone 3 lysine 9 dimethylation (H3K9me2, a
key repressive histone modification), can reactivate oncogenes such
as yes-associated protein 1 (YAP1), driving chemoresistance in GC
(36).
The TME further modulates these outcomes, producing
cancer type-specific consequences (15,26-29,31,32). In melanoma,
the expression of senescence-associated genes, including BRCA2, is
associated with increased CD8+ T-cell infiltration and
favorable prognosis (37). By
contrast, in HCC, high senescence scores are associated with
elevated tumor mutational burden and poor survival, mediated by
immunosuppressive SASP components (38,39).
Long non-coding RNAs (lncRNAs) also influence this dynamic; for
instance, nuclear paraspeckle assembly transcript 1 (NEAT1)
suppresses senescence in HCC by stabilizing kinesin family member
11 (KIF11) and repressing CDK inhibitor 2A (CDKN2A) transcription
(5), which underscores the
context-dependent regulation of senescence across malignancies.
These opposing roles highlight the necessity of
nuanced therapeutic strategies. Recent advances in senotherapy aim
to exploit this complexity by selectively eliminating deleterious
senescent cells using senolytics (e.g., dasatinib plus quercetin),
attenuating SASP signaling via senomorphics or integrating these
approaches with standard treatments (40,41).
Key challenges include distinguishing beneficial from pathological
senescence and mitigating therapy-induced senescence in normal
tissues, which may drive recurrence (42). Future directions emphasize the
development of: i) Biomarkers for senescent subpopulation
profiling; ii) temporal control of senescence induction; and iii)
combinatorial regimens to optimize efficacy while minimizing
adverse effects. Successfully addressing these hurdles could
transform cancer therapy by harnessing the protective potential of
senescence while neutralizing its pro-tumorigenic properties
(39,41). However, notable barriers remain
before these strategies can be translated into safe and effective
clinical applications.
Underlying mechanisms of context-dependent
cellular senescence in cancer
Cellular senescence exhibits pronounced context
dependency in cancer, functioning as either a tumor-suppressive
barrier or a tumor-promoting factor depending on the specific
biological and microenvironmental context. This paradox stems from
the interplay between tumor-specific biology, SASP heterogeneity
and dynamic remodeling of the TME. The present review delineates
the core mechanisms underlying these contradictions, drawing on
evidence from specific cancer types highlighted below.
The SASP is highly heterogeneous; its composition,
cytokines, chemokines and matrix-remodeling enzymes show a marked
variation across cancer types, like breast cancer, gastric cancer,
lung adenocarcinoma, HCC, melanoma, colorectal cancer and glioma,
shaping the functional outcome of senescence. In melanoma,
senescent cells secrete factors that enhance antitumor immunity.
For instance, senescence-associated genes such as BRCA2 are
associated with increased infiltration of CD8+ T cells,
a hallmark of effective immune surveillance (37). Consistent with this, the SASP in
melanoma tends to favor pro-inflammatory cytokines that recruit
cytotoxic lymphocytes, which may potentially promote tumor
suppression (25).
By contrast, HCC exhibits a pro-tumor SASP profile.
High senescence scores in HCC are associated with elevated
immunosuppressive factors that attract MDSCs and CAFs (38,39).
These factors remodel the TME, promoting immune evasion and ECM
deposition, thereby converting senescence into a tumor-promoting
force. Such disparities underscore that SASP-driven outcomes depend
on its specific components: Pro-immune vs. immunosuppressive
factors determine whether senescence restrains or accelerates tumor
growth.
The immune architecture of the TME further amplifies
these contradictions, as senescent cells recruit distinct immune
subsets via SASP signaling. In melanoma, senescence-associated cues
bolster adaptive immunity; as noted, senescence-related genes are
associated with enhanced CD8+ T-cell infiltration,
suggesting a pro-inflammatory, antitumor milieu (37). By contrast, in other malignancies,
senescence dampens immune surveillance. In GC, senescent cells
foster immune suppression via SASP. SASP-derived IL-6 and CXCL12,
for instance, recruit MDSCs and regulatory T cells (Tregs),
blunting cytotoxic T-cell activity and enabling immune escape
(27,28). Similarly, in lung adenocarcinoma,
SASP-induced SPP1 and Tenascin interact with CD44 and integrins on
immune cells, impairing their cytotoxicity and promoting evasion
(26,31). These examples demonstrate that the
net effect of senescence hinges on the immune subsets it recruits,
cytotoxic vs. immunosuppressive.
Intrinsic genetic and epigenetic programs further
govern this context dependency by modulating senescence pathway
activity. In HCC, the lncRNA NEAT1 represses senescence by
stabilizing KIF11 and inhibiting CDKN2A transcription, a key
senescence inducer (5). This
epigenetic silencing diminishes the tumor-suppressive capacity of
senescence, which allows malignant cells to evade growth arrest. By
contrast, such repressive mechanisms appear less pronounced in
melanoma, enabling senescence to exert antitumor effects. In
glioma, TNF receptor-associated factor 7 knockdown induces
senescence and synergizes with lomustine to suppress tumor growth
(43). This contrasts with HCC,
where senescence pathways are often epigenetically silenced,
conferring resistance to similar strategies. Thus, the genetic and
epigenetic landscape, including lncRNA networks and senescence gene
expression, notably determines whether senescence serves as a
barrier or is co-opted for tumor progression.
Senescence also evolves with the tumor stage,
shifting from protective in early disease to deleterious in
advanced stages. Initially, senescence acts as a barrier to
transformation through p53/p21- and p16/Rb-mediated cell cycle
arrest (1,2). In premalignant lesions, SASP factors,
like IL-6, TNF-α, p21/p16, SPP1, MMPs and YAP1, may trigger
immune-mediated clearance of damaged cells (5,27). At
later stages, persistent senescence and chronic SASP reshape the
TME to foster metastasis and therapy resistance. In lung
adenocarcinoma, late-stage senescent cells upregulate SPP1, which
enhances invasiveness (31), while
in GC, senescence-driven H3K9me2 demethylation reactivates
oncogenes such as YAP1, which promotes chemoresistance (36). This stage-dependent shift
underscores the dynamic nature of senescence and its adaptation to
the evolving tumor ecosystem.
In summary, the opposing roles of cellular
senescence in cancer arise from SASP heterogeneity, immune
composition of the TME, cancer-specific regulatory networks and
disease stage. Deciphering these context-dependent mechanisms is
essential for precision strategies that exploit the
tumor-suppressive effects of senescence while mitigating its
pro-tumor consequences. These insights underpin the rationale for
SASP-targeted interventions, such as inhibiting the IL-6/STAT3
pathway in breast cancer or blocking the IL-8/CXCR2 axis in lung
cancer (33,34) (Table
I), to enable more effective senotherapy.
 | Table I.Therapeutic strategies harnessing
context-specific senescence mechanisms. |
Table I.
Therapeutic strategies harnessing
context-specific senescence mechanisms.
| Therapeutic
strategy | Mechanism of
action | Target cancer
types | Key findings | (Refs.) |
|---|
| Senolytics
(exisulind) | Induces apoptosis
in senescent cells; synergizes with palbociclib | GC | Reduces
SASP-mediated immunosuppression; enhances response to senescence
induction | (83) |
| Senescence
induction (FEN1-PBX1 inhibition) | Inhibits FEN1-PBX1
axis to trigger senescence via ROS accumulation | Breast cancer | Suppresses tumor
growth by activating p53/p21 pathways | (84) |
| LncRNA modulation
(NEAT1 knockdown) | Promotes KIF11
degradation, activating CDKN2A-mediated senescence | HCC | NEAT1 upregulation
is associated with senescence evasion; knockdown reduces tumor
progression | (85) |
| Senescence-related
gene targeting (EZH2 inhibition) | Reactivates CDKN2A
by reversing H3K27me3-mediated silencing | HCC | EZH2 inhibitors
reduce tumor growth and enhance immune infiltration | (86,87) |
| Combination therapy
(TRAF7 knockdown + lomustine) | TRAF7 knockdown
induces senescence; lomustine enhances senescent cell
clearance | Glioma | Reduces recurrence
by synergizing senescence induction with chemotherapy | (43) |
| Traditional Chinese
Medicine (Jianpi Huayu decoction) | Suppresses
senescence via p53-p21-Rb pathway | Colorectal
cancer | Inhibits tumor
growth by reducing SASP and stabilizing the TME | (83) |
Tumor-specific mechanisms underlying SASP
component heterogeneity
The heterogeneity of SASP components across cancer
types reflects tumor-specific signaling networks and interactions
within the TME, as exemplified by IL-6 and IL-8.
In breast cancer, IL-6 promotes metastasis through
context-dependent pathways. Preclinical evidence demonstrated that
IL-6 activates downstream cascades regulating
epithelial-mesenchymal transition (EMT) and matrix remodeling, both
key for metastatic dissemination. Consistently, IL-6-driven
signaling is associated with aggressive phenotypes in breast
cancer, where senescent cells secrete IL-6 to reinforce a
pro-metastatic niche.
In GC, IL-6 primarily reshapes immune landscapes
within the TME. Acting through chemokine networks such as chemokine
(C-C motif) ligand 2 (CCL2)/C-C chemokine receptor 2, it recruits
MDSCs and CAFs, fostering immunosuppression and enabling immune
evasion (31,32). This divergence from breast cancer
partly reflects distinct IL-6 receptor (IL-6R) distribution, with
GC enriched for IL-6R on stromal and immune cells compared with
tumor cells.
IL-8 exhibits similarly context-dependent functions.
In lung adenocarcinoma, SASP-derived IL-8 underpins therapy
resistance by sustaining drug-tolerant cell survival via CXC
receptor 1/2 (R1/2) signaling, thereby maintaining tumor
persistence despite treatment (26,29).
By contrast, in colorectal cancer, IL-8 primarily drives
angiogenesis by stimulating vascular endothelial proliferation and
migration, which enhances tumor perfusion and growth.
These functional disparities arise from three
principal factors: i) Cell-type specificity of SASP receptor
expression (e.g., IL-6R on tumor vs. stromal cells); ii)
preferential activation of downstream pathways (e.g., EMT-related
cascades in breast cancer vs. immune-modulatory pathways in GC);
and iii) TME composition, which dictates whether SASP factors
engage tumor, immune or stromal compartments (15,33–36).
Collectively, these mechanisms highlight the need for
context-specific targeting of SASP components in cancer
therapy.
Targeting IL-6 illustrates the following principle:
Neutralizing IL-6 or blocking its downstream signaling (e.g.,
anti-IL-6 antibodies or STAT3 inhibitors) may suppress metastasis
in breast cancer, whereas in GC, such interventions may primarily
mitigate immunosuppression by reducing MDSC recruitment (37–43),
consistent with strategies outlined in Table I. Similarly, the divergent roles of
IL-8 underscore the therapeutic value of CXCR1/2 blockade in lung
adenocarcinoma to disrupt cancer stem cell maintenance and therapy
resistance, whereas in colorectal cancer, inhibiting IL-8-mediated
hypoxia inducible factor-1α upregulation could attenuate
angiogenesis. These approaches align with the SASP-modulating
therapies summarized in Table I,
emphasizing that therapeutic efficacy depends on matching
interventions to the dominant IL-8-driven pathway in each type of
cancer.
Relationship between cellular senescence and
the TME
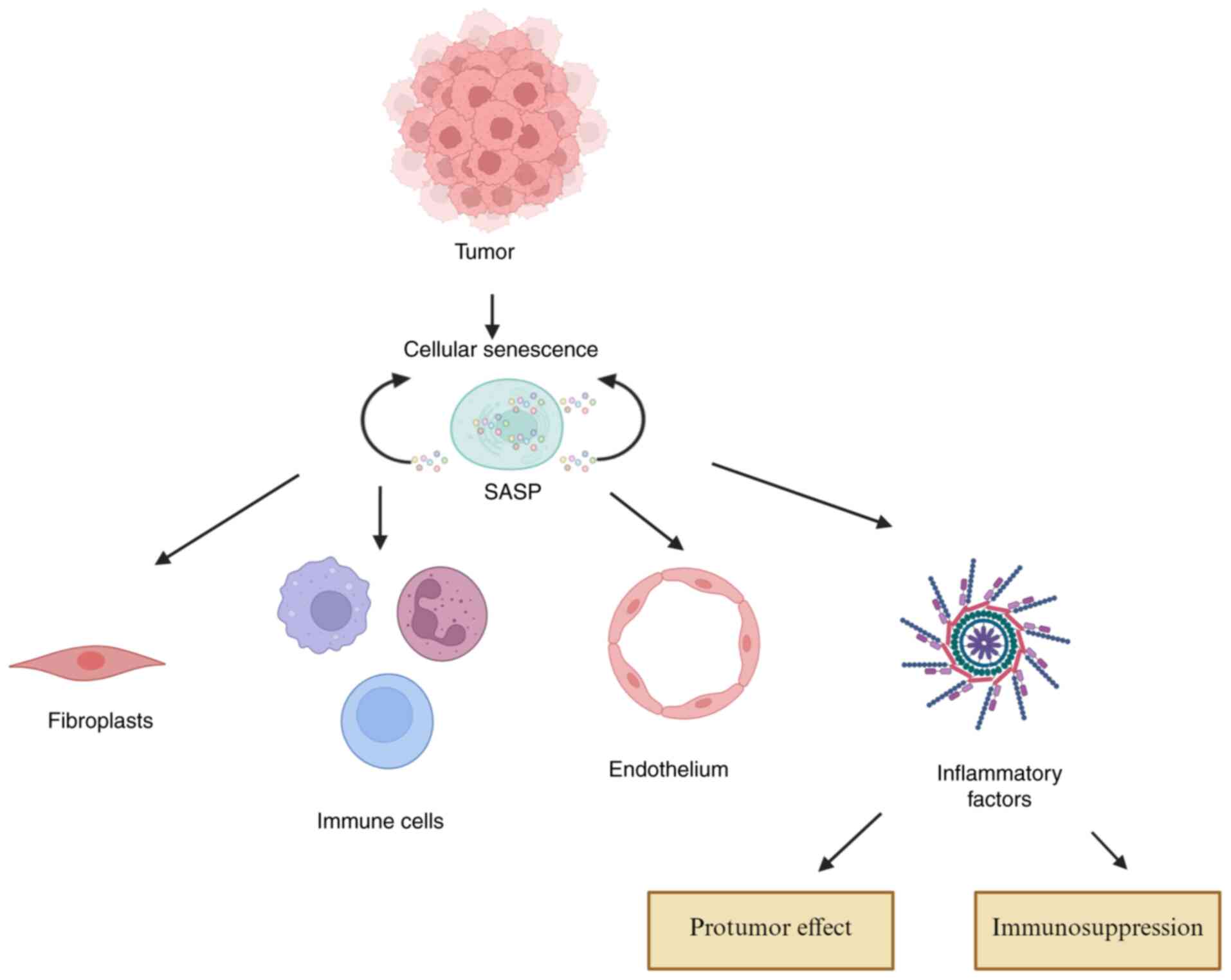
Cellular senescence exerts profound,
context-dependent effects on the TME, which shapes cancer
progression through complex interactions. The accumulation of
senescent cells within the TME promotes tumor development by
secreting pro-inflammatory and pro-tumorigenic factors collectively
known as the SASP (3,44,45).
SASP components remodel the ECM and establish a malignant niche by
driving cancer cell proliferation, invasion and immune evasion
(45,46). Furthermore, SASP factors recruit and
activate stromal cells, such as CAFs, further reinforcing a
tumor-permissive microenvironment (17,44,47).
Senescent immune cells also contribute to immune dysfunction by
disrupting metabolic balance (e.g., glucose competition) and
amplifying immunosuppressive signals (e.g., cAMP), thereby
facilitating immune escape (48).
Dual roles of senescent cells in the
TME
The influence of senescent cells within the TME is
highly dynamic. While SASP components can elicit antitumor
responses by enhancing immune surveillance and suppressing
angiogenesis in certain contexts (49,50),
specifically referring to four scenarios supported by preclinical
and clinical evidence: First, in premalignant lesions or
early-stage tumors, where senescent cells secrete immune-activating
SASP factors to trigger clearance of abnormal cells-for instance,
in HCC premalignancy, senescent hepatocytes release IL-24 to
recruit cytotoxic CD8+T cells for eliminating
premalignant cells (5,15,25–34).
Second, in cancer types with high immunogenicity, such as melanoma,
senescent cells express senescence-associated genes and secrete
pro-inflammatory SASP factors that promote CD8+ T-cell
infiltration, correlating with favorable patient prognosis
(37). Third, in the acute phase
after therapy-induced senescence, short-term SASP secretion from
acutely senescent tumor cells activates immunogenic cell death,
recruiting natural killer (NK) cells and dendritic cells to enhance
antitumor immunity (18,51). Fourth, in tissues rich in innate
immune cells, the tissue-specific TME amplifies the antitumor
effect of SASP-for example, senescent hepatic stellate cells in the
liver induce M1 macrophage polarization via SASP, inhibiting liver
fibrosis and early tumorigenesis (49). They more frequently drive tumor
progression through chronic inflammation, immunosuppression and
metastasis, as persistent senescence in advanced cancers leads to
accumulated immunosuppressive SASP components that recruit
myeloid-derived suppressor cells (MDSCs) and cancer-associated
fibroblasts (CAFs), fostering immune evasion and metastatic
dissemination (30,31,36).
They more frequently drive tumor progression through chronic
inflammation, immunosuppression and metastasis (51). This paradox extends beyond oncology;
in neurodegenerative models, such as MAPTP301SPS19 mice, clearance
of senescent cells improves cognitive function (51). These observations highlight the
broad pathological impact of senescence and its potential as a
therapeutic target in cancer and aging-related diseases.
Therapeutic strategies targeting senescence
in the TME
Addressing these complexities requires therapeutic
strategies that precisely modulate senescent cells to enhance
antitumor effects while minimizing pro-tumorigenic consequences.
Senolytics (e.g., dasatinib and quercetin) represent a promising
approach, selectively eliminating harmful senescent cells to reduce
SASP-driven inflammation and restore treatment sensitivity
(52–57). Another strategy integrates
senotherapies with immunotherapy to reverse immune suppression in
the TME, thereby improving tumor recognition while counteracting
SASP-mediated resistance (58–63).
These approaches underscore the need for precision medicine,
tailoring treatment regimens to tumor-specific senescence
profiles.
Future research can elucidate mechanisms governing
senescence plasticity within the TME, identify biomarkers to
distinguish protective from pathogenic senescence and optimize
combinatorial strategies that harness antitumor benefits without
promoting malignancy. Emerging insights into senescence
reprogramming hold the potential to reshape cancer therapy by
disrupting tumor ecosystems and improving patient outcomes
(64).
Biomarkers and therapeutic targeting of
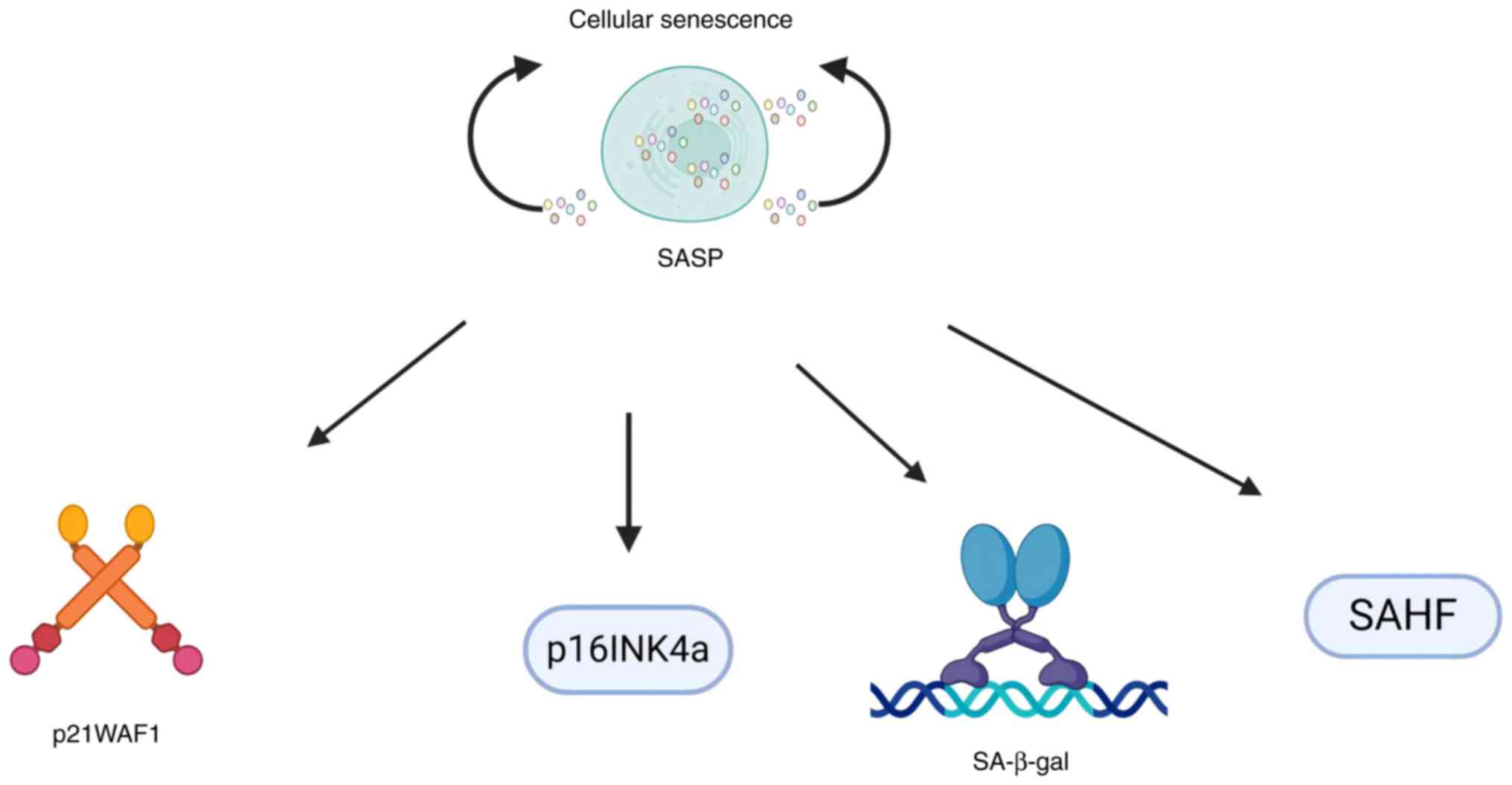
senescent cells in the TME
Biomarkers for identification and functional
analysis of senescent cells
The identification and functional characterization
of senescent cells within the TME rely on robust biomarkers,
including cell cycle regulators [e.g., CDKN2A inhibitor (p16INK4a,
where INK4a denotes the p16 protein subtype) and p21 wild-type
p53-activated fragment 1 (p21WAF1, where WAF1 is the functional
alias of the p21 protein)], senescence-associated β-galactosidase
activity (SA-β-gal) and specific chromatin modifications such as
senescence-associated heterochromatin foci (SAHF) (65,66).
These molecular signatures serve dual purposes: Enabling precise
tracking of senescent cell populations across spatial and temporal
scales and providing mechanistic insights into their roles in tumor
progression and therapeutic response (Fig. 3). Quantitative analysis of these
biomarkers has become essential to evaluate the efficacy of
senolytic interventions and understanding the dynamic processes of
cellular senescence during cancer development (67–69)
(Fig. 3).
SASP and its dual roles
Central to the paradoxical effects of senescent
cells is the SASP, a complex repertoire of secreted
factors-including pro-inflammatory cytokines, chemokines, and
ECM-remodeling enzymes whose composition dictates tumor suppression
or promotion (64). In
glioblastoma, for instance, senescent malignant cells secrete high
levels of IL-6 and IL-8, which activate STAT3 signaling in
neighboring tumor cells, while MMP-9 released through SASP mediates
ECM degradation to facilitate tumor invasion (65–70).
This dynamic secretion profile, conserved in patient glioblastomas,
underscores how SASP components contribute to the aggressive
phenotype of certain cancers, aligning with the notion that
senescent cells exert context-dependent influences through their
secretory phenotype. While the SASP can initiate tumor-suppressive
responses, such as immune activation and inhibition of
angiogenesis, it frequently promotes tumor progression by
facilitating immune evasion and remodeling the surrounding
microenvironment (71). Epigenetic
regulation further modulates SASP activity, where chromatin
modifications can diminish tumor-suppressive functions of
senescence and instead support malignant progression.
Therapeutic strategies targeting senescent
cells and future directions
Therapeutic strategies have evolved to address the
complex biology of senescent cells through two complementary
approaches: i) Senolytic compounds selectively eliminate senescent
cells, thereby mitigating the deleterious effects of the
SASP-navitoclax achieves this by targeting BCL-xL in senescent
cells to induce apoptosis (68),
while fisetin preferentially clears senescent populations via
p53-dependent pathways (72),
consistent with broader senolytic mechanisms reported previously
(24,38,73–76);
and ii) senomorphic agents, by contrast, modulate specific SASP
components to suppress harmful effects while preserving beneficial
secretory functions-for example, compounds that inhibit NF-.κB or
restore mitochondrial function reduce pro-inflammatory SASP without
eliminating senescent cells, maintaining factors critical for
tissue repair (77). Combining
senolytics with immunotherapy holds promise, enhancing antitumor
immune responses by modulating key checkpoints and cytokine
networks.
Current research priorities include the
identification of predictive biomarkers for patient stratification,
optimization of senolytic treatment timing, preservation of
physiologically beneficial senescent populations and integration
with existing therapeutic modalities (28,76–78).
These efforts aim to establish precision senotherapy, tailoring
interventions based on comprehensive profiling of tumor-specific
senescence landscapes.
Emerging technologies such as single-cell
multi-omics and advanced computational modeling are expected to
provide further insights into the spatiotemporal heterogeneity of
tumor-associated senescent cells, enabling refined and personalized
treatment strategies that consider both neoplastic and
microenvironmental factors (77–80).
From a clinical perspective, senescence biomarkers
may serve as predictors of treatment responsiveness, while SASP
profiling can guide selection of senomorphic interventions.
Temporal optimization of senolytic administration and careful
consideration of the immune context are key for therapeutic
success. Collectively, these strategies highlight the importance of
mechanistic studies to elucidate adaptive responses of senescent
cell populations, with the ultimate goal of developing targeted
interventions that may potentially promote tumor suppression.
Biomarkers of senescent cells in the
TME
Identification and characterization of
senescent cells
The identification and characterization of senescent
cells within the TME rely on established biomarkers, including
upregulation of cell cycle inhibitors p21WAF1 and p16INK4a,
increased SA-β-gal activity and the formation of SAHF (65,66).
These markers not only enable the detection and monitoring of
senescent cells but also provide insights into their roles in
cancer progression and therapeutic response (Fig. 4). Quantitative assessment of these
biomarkers allows evaluation of the prevalence, spatial
distribution and functional impact of senescent cells within the
TME, which supports detailed studies on their contributions to
tumor biology and treatment resistance (67–69).
 | Figure 4.Biomarkers of cellular senescence.
The schematic summarizes the key biomarkers used to identify and
monitor senescent cells, including cell cycle inhibitors (p16INK4a,
p21WAF1), SA-β-gal and SAHF. For consistency with the text, the
‘INK4a’ in p16INK4a and ‘WAF1’ in p21WAF1 in this figure correspond
to the gene subtype (INK4a) or protein alias (WAF1) described in
the main text, with the same functional meaning. The black arrows
in this figure visually connect senescent cells to their key
biomarkers, as detailed in the review: i) Arrows for intracellular
biomarkers point from senescent cells to cell cycle inhibitors,
SA-β-gal and SAHF. These markers reflect cell-autonomous senescence
features and are critical for ‘tracking senescent cell
populations’. ii) An arrow for the secretory biomarker SASP points
from senescent cells to SASP, highlighting its origin from
senescent cells and aligning with the document's definition of SASP
as a ‘dynamic secreted phenotype’ that also mediates TME
interactions. SA-β-gal, senescence-associated β-galactosidase
activity; SAHF, senescence-associated heterochromatin foci; SASP,
senescence-associated secretory phenotype. |
SASP and its dual roles
A hallmark of senescent cells is the SASP, a complex
and dynamic ensemble of secreted cytokines, chemokines and
matrix-remodeling proteases. SASP has complex, context-dependent
effects. Although SASP can activate antitumor immune responses, it
could promote tumorigenesis by altering the microenvironment and
impairing immune surveillance as well (70). Epigenetic regulation-such as histone
acetylation of SASP-related genes further modulates SASP activity,
which influences whether senescent cells function as tumor
suppressors or facilitators of malignancy. Epigenetic regulation
further modulates SASP activity, which influences whether senescent
cells function as tumor suppressors or facilitators of malignancy.
Dysregulation of tumor-suppressor pathways including p53-p21,
p16INK4a-Rb, PTEN-PI3K-AKT, p27Kip1 and TGF-β-Smad that often
undergo dysregulation, which disrupts their ability to regulate the
cell cycle and enforce senescence-induced growth arrest, ultimately
enabling cancer progression, which can bypass senescence-induced
growth arrest, enabling cancer progression (1,5,15,25,33,64).
Understanding these mechanisms is essential to develop therapies
that selectively mitigate pro-tumorigenic SASP functions while
preserving beneficial anticancer effects.
Therapeutic implications and future
directions
Recent advances in senotherapy have expanded
potential intervention strategies. Immune-based strategies
represent a breakthrough, with uPAR-targeted CAR-T cells
eliminating 67–90% of senescent cells in aged mouse tissues,
improving glucose tolerance and exercise capacity for over 12
months through specific recognition of the senescence marker uPAR
(80). For SASP modulation, JAK
inhibitor ruxolitinib and mTOR inhibitor rapamycin showed dual
efficacy in silencing pro-tumor inflammation while preserving
regenerative functions, validated in myelofibrosis trials and
preclinical studies on age-related pathologies (81). These advancements collectively
highlight the shift from single-agent senolysis to precision
strategies combining targeted delivery, immune engineering, and
SASP regulation, with clinical trials and guideline endorsements
solidifying their translational value. Senolytic drugs, such as
navitoclax and fisetin, selectively eliminate senescent cells,
thereby reducing SASP-mediated tumor promotion. Senomorphic agents,
by contrast, modulate SASP activity without inducing cell death,
providing refined control over downstream effects (19). The interplay between senescent cells
and immune regulation offers additional therapeutic opportunities.
The interplay between senescent cells and immune regulation offers
additional therapeutic opportunities specifically for enhancing
cancer treatment efficacy, such as reversing SASP-mediated
immunosuppression, developing senescence-targeted immunotherapies
and preventing therapy-induced recurrence by blocking
senescence-driven immune escape; these opportunities also extend to
alleviating age-related diseases by targeting their dysregulated
crosstalk. By shaping immune responses through secreted factors,
senescent cells can influence the efficacy of immunotherapies.
Combining senolytics with checkpoint inhibitors or adoptive cell
therapies may enhance antitumor immunity while counteracting
SASP-mediated immune evasion (13).
Future research aims to refine these strategies
using single-cell omics, spatial transcriptomics and artificial
intelligence (AI)-driven modeling to resolve the heterogeneity of
tumor-associated senescence. Identification of predictive
biomarkers will enable patient stratification, optimizing treatment
timing and combination regimens to maximize therapeutic benefit.
The ultimate goal is precision senotherapy, where interventions are
tailored to individual senescence profiles, harmonizing tumor
suppression with microenvironmental homeostasis. Such advances may
transform cancer care, moving from broadly cytotoxic treatments to
mechanism-driven, immune-compatible therapies that improve outcomes
while minimizing toxicity (82,83).
Emerging strategies exploit context-specific
senescence mechanisms, as summarized in Table I. These include targeting lncRNAs in
HCC or combining senescence induction with chemotherapy in glioma,
demonstrating how context-specific interventions can potentially
promote tumor suppression (36,84,85).
Clearance of senescent cells and tumor
treatment
Senescence as a tumor-suppressive mechanism
and pro-senescence therapy
Clinical evidence increasingly demonstrates that
chemotherapy-induced accumulation of senescent cells within tumors
is often associated with improved patient outcomes (86,87).
Preclinical studies corroborated these observations, which
demonstrates that defects in p53-dependent senescence pathways can
promote tumorigenesis and confer chemotherapy resistance (18,88,89).
These findings position cellular senescence as a natural
tumor-suppressive mechanism, notably restraining cancer
progression. The recent Food and Drug Administration approvals of
CDK4/6 inhibitors, including abemaciclib and palbociclib, which
possess senolytic properties, further support ‘pro-senescence
therapy’ as a viable anticancer strategy, particularly in breast
cancer and non-small cell lung cancer (90).
Senolytic therapy: Targeting senescent cells
to remodel the TME
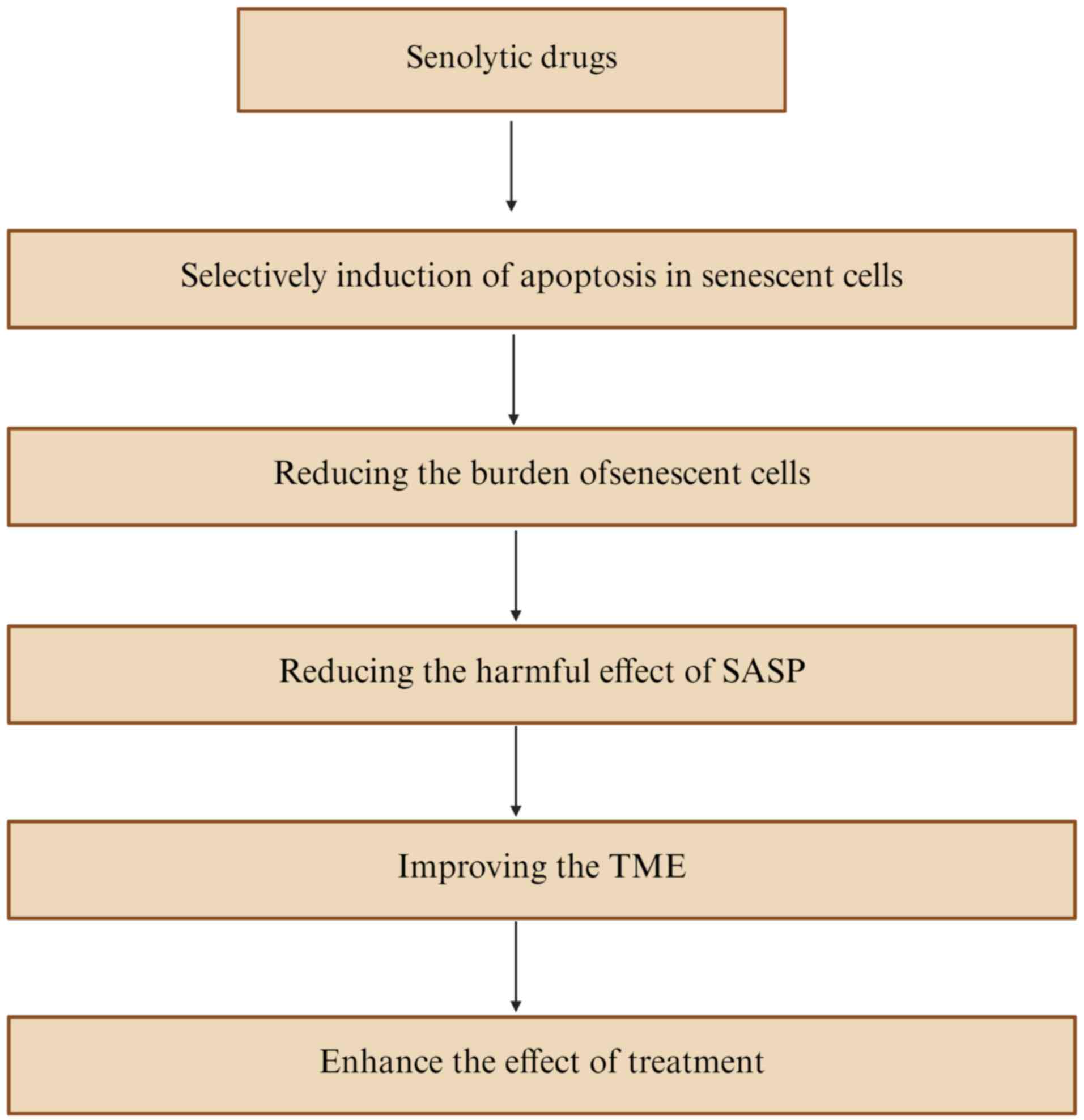
Senolytic therapy, which selectively eliminates
senescent cells, is emerging as a promising strategy to remodel the
TME and enhance therapeutic efficacy (91,92).
By inducing apoptosis in senescent cells, senolytics such as
navitoclax and fisetin reduce their abundance, thereby mitigating
deleterious SASP effects including angiogenesis, invasion and
metastasis (93). This approach
addresses the accumulation of age-associated senescent cells, which
contributes to cancer susceptibility and represents a notable
advance in oncology (94,95). Depletion of senescent cells
suppresses pro-inflammatory and tumorigenic SASP factors, creating
a TME more conducive to effective anticancer therapies (96). Numerous preclinical compounds
including BH3 mimetics, PROTAC degraders, dual-mechanism small
molecules, and epigenetic modulators which exhibit potential by
selectively eliminating senescent cells, reversing SASP-mediated
immunosuppression, or synergizing with other therapies; as
mechanistic understanding of their targeting of
senescence-associated pathways increases, senescence-targeted
interventions are poised to become a central component of cancer
treatment.
However, the dual role of senescent cells
complicates therapeutic implementation. While their clearance can
counteract pro-tumorigenic effects, residual SASP factors may
persist and influence neighboring cells, potentially maintaining a
tumor-permissive environment (97).
Premature removal of senescent cells during specific treatment
phases such as the early stage after chemotherapy or radiotherapy,
when SASP still secretes antitumor factors to recruit cytotoxic
CD8+T cells could also disrupt SASP-mediated immune
responses that support antitumor activity (98); therefore, optimizing the timing and
context of senolytic interventions is key. Future studies can
refine senolytic mechanisms and elucidate dynamic crosstalk among
senescent cells, immune populations and stromal components within
the TME. Balancing senescence induction with selective elimination
will be essential to maximize therapeutic benefit while minimizing
tumor-promoting risks (99).
Addressing senescence-driven therapeutic resistance remains a key
challenge, which requires strategies to counteract immune evasion
and treatment failure (100)
(Fig. 5).
Combining senolytics with immunotherapy and
emerging therapeutic strategies
SASP-mediated immunosuppression enables tumor cells
to evade immune surveillance and resist therapy (101), highlighting the potential of
combining senolytics with immunotherapy, particularly immune
checkpoint inhibitors, such as PD-1 blockers, PD-L1 inhibitors,
CTLA-4 antibodies and novel LAG-3 inhibitors. Such combinations can
concurrently eliminate immunosuppressive senescent cells and
enhance antitumor immunity, potentially overcoming SASP-mediated
therapy resistance (102).
Targeting specific SASP factors, including IL-6 and TGF-β, may
further reprogram the TME from immunosuppressive to immune-active,
opening opportunities for synergy with adoptive cell therapies or
cancer vaccines (103).
Implementing these strategies requires a precision
medicine framework, incorporating biomarker-driven patient
stratification to identify optimal candidates for
senolytic-immunotherapy combinations. Advanced approaches, such as
single-cell profiling and AI-based predictive modeling, can help
resolve tumor-associated senescence heterogeneity and guide
personalized regimens (104).
Translating these innovations will necessitate multidisciplinary
collaboration among oncologists, immunologists and computational
biologists to ensure safe and effective application across diverse
patient populations.
The dualistic nature of senescent cells, both
restraining and promoting cancer, presents a complex yet promising
therapeutic frontier. While pro-senescence therapies can inhibit
tumor growth, senolytic strategies offer a means to counterbalance
their detrimental effects. Optimizing combination approaches that
integrate senescence modulation with immunotherapy, chemotherapy
and targeted agents will be pivotal specifically for balancing the
tumor-suppressive and pro-tumorigenic effects of senescence,
overcoming therapy-induced senescence-related resistance,
remodeling the immunosuppressive TME which can reduce MDSC
recruitment via IL-6 inhibition and reinvigorating CD8+T cells with
PD-1 blockers in HCC, enabling precision senotherapy tailored to
cancer heterogeneity and minimizing treatment toxicity. By
systematically dissecting the role of senescence in cancer,
next-generation therapies can exploit its protective mechanisms
while neutralizing pro-tumorigenic potential, ultimately improving
survival and quality of life for patients.
Beyond established senolytics such as dasatinib and
quercetin, emerging agents such as exisulind have demonstrated
efficacy in GC by inducing apoptosis in senescent cells and
synergizing with palbociclib to enhance senescence clearance
(83). In breast cancer, inhibition
of the flap endonuclease 1-pre-B cell leukemia factor 1 axis
triggers senescence via reactive oxygen species accumulation and
activation of p53/p21 pathways, which suppresses tumor growth
(84). These findings support the
expansion of senolytic and senescence-inducing strategies beyond
conventional regimens.
Dual nature of senescent cells in tumor
immune evasion and therapeutic opportunities
Paradoxical roles of senescence and SASP in
tumor immunity
Cellular senescence exerts a key yet paradoxical
influence in cancer, simultaneously constraining tumor initiation
while promoting immune evasion and therapy resistance. The SASP is
central to this duality, comprising a complex mixture of
inflammatory cytokines (e.g., IL-6 and TNF-α), chemokines (e.g.,
CCL2 and CXCL12) and proteases that notably shape the TME (105,106). Initially, SASP factors recruit
immune effector cells; however, chronic SASP activity drives
immunosuppression through multiple mechanisms: Polarizing
macrophages toward tumor-promoting M2 phenotypes (107), recruiting Tregs (107,108) and inducing T-cell dysfunction via
p16INK4a-mediated senescence (108). Therefore, a permissive niche is
established, enabling malignant cells to evade immune surveillance
despite the tumor-suppressive growth arrest in senescent cells.
Oncogene-induced senescence and
context-dependent tumorigenesis
The interplay between senescence and tumorigenesis
is particularly evident in oncogene-induced senescence. Activation
of oncogenes such as human Ras oncogene G12V, HER2, EGFR and PI3K
triggers senescence as an intrinsic tumor-suppressive response
(109), yet concurrent SASP
secretion undermines this protection by promoting immunosuppression
and remodeling tissue architecture. Conversely, oncogene
inactivation (e.g., Myc) can induce senescence and tumor regression
across various cancer types (110), like lymphoma, HCC, triple-negative
breast cancer, highlighting the context-dependent nature of these
processes. Senescent cells disrupt normal tissue structure and
suppress antitumor immunity via their secretory profile, thereby
facilitating immune evasion and disease progression (28).
Therapeutic strategies targeting senescence
and SASP in cancer
This biological paradox presents both challenges and
opportunities for intervention. While senescence induction halts
tumor growth, SASP activity may drive therapeutic resistance and
immune escape (111). Current
strategies focus on three complementary approaches: i) Senolytic
agents that selectively eliminate senescent cells to abrogate
pro-tumorigenic effects; ii) SASP-modulating therapies that inhibit
deleterious factors while preserving beneficial functions; and iii)
rational combinations with immunotherapy to overcome SASP-mediated
immunosuppression (112). Research
suggests that precisely timed senolytic interventions can enhance
immune checkpoint blockades by remodeling the TME and
reinvigorating antitumor T-cell responses (3,113).
These findings align with the document's emphasis on temporal
precision in senolytic-immunotherapy combinations to avoid
late-stage immunosuppressive SASP while preserving early IFN-driven
antitumor immunity (5,93) (Table
II).
 | Table II.Therapeutic approaches and
challenges. |
Table II.
Therapeutic approaches and
challenges.
| Therapeutic
approach | Mechanism | Potential
benefit | Challenges |
|---|
| Senolytic
therapy | Selective
elimination of senescent cells | Reduces
SASP-mediated immunosuppression | May disrupt
tumor-suppressive senescence |
| SASP
modulation | Targeted cytokine
inhibition (e.g., IL-6 blockade) | Preserves
senescence arrest while blocking harmful secretions | Complex cytokine
network redundancy |
| Combination
immunotherapy | Senolytics +
checkpoint inhibitors | Enhances T cell
reinvigoration | Timing/dosing
optimization required |
Future cancer therapies are likely to integrate
advanced approaches leveraging the current growing understanding of
senescence biology. These include biomarker-driven patient
stratification, temporally controlled senolytic delivery and
senomorphic agents designed to reprogram rather than eliminate
senescent cells (113). By
simultaneously targeting malignant cells and reshaping the
immunosuppressive microenvironment, such strategies have the
potential to overcome treatment limitations, reduce recurrence and
improve long-term outcomes.
Tumor-specific immune modulation by SASP illustrates
the need for personalized approaches. In melanoma,
senescence-associated gene expression is associated with increased
CD8+ T-cell infiltration (36), whereas in HCC, high senescence
scores are associated with immunosuppressive SASP and poor
prognosis (38,39). Targeted interventions, such as
enhancer of zeste homolog 2 inhibition to reactivate CDKN2A and
enhance immune infiltration (85,86) or
lncRNA NEAT1 knockdown to restore senescence (84), exemplify strategies to exploit
senescence biology for precision oncology.
Interplay between senescent cells and
telomerase pathways in cancer
Telomerase reactivation and senescence
escape in tumor cells
Cellular senescence and telomerase activity are
closely intertwined in cancer development and progression.
Telomerase, which preserves telomere length, is largely inactive in
normal somatic cells but reactivated in ~85–90% of human cancer
types, enabling tumor cells to bypass replicative senescence and
sustain ‘immortal’ proliferation (114). This observation supports the
telomerase theory of cancer, in which telomerase activation is
regarded as a near-universal hallmark of malignancy (115). Nevertheless, notable molecular
distinctions exist between replicative senescence in normal cells,
mediated by p53/p16-dependent G0/G1 arrest
and telomere shortening, and senescence-like arrest in cancer
cells, which frequently occurs independently of telomere attrition
(116–118).
Therapeutic targeting of telomerase and
challenges of alternative lengthening of telomeres (ALT)
pathway
Targeting telomerase represents a promising
anticancer strategy. Natural compounds, including icaritin (from
Epimedium) and wogonin (from Scutellaria
baicalensis), have demonstrated potent telomerase inhibition in
leukemia (HL-60) and ovarian cancer (SKOV3) models, respectively
(119–121). These agents selectively induce
cytotoxicity in telomerase-positive tumors while sparing normal
tissues. Clinical translation, however, requires optimization of
pharmacokinetics and development of effective combination regimens
(Table III).
 | Table III.Natural compounds targeting
telomerase. |
Table III.
Natural compounds targeting
telomerase.
| Compound | Source | Target cancer (cell
line) | Key findings | (Refs.) |
|---|
| Icaritin | Epimedium
extract | Leukemia
(HL-60) | Notable telomerase
suppression | (120) |
| Wogonin | Scutellaria
baicalensis | Ovarian
(SKOV3) | Dose-dependent
inhibition in vitro/in vivo | (121) |
Certain cancer types (10–15%) evade telomerase
dependency by employing the ALT pathway, which maintains telomere
length via homologous recombination rather than telomerase. ALT is
particularly prevalent in osteosarcomas (~60%) and glioblastomas
(~40%), which poses a notable therapeutic challenge due to
resistance to conventional telomerase-targeted drugs, e.g.
olaparib, RAD51 inhibitor, telomere homologous recombination
inhibitor with oxadiazole scaffold, tazemetostat (122,123). Emerging strategies to overcome
ALT-mediated resistance include ataxia telangiectasia and
Rad3-related inhibitors, CRISPR-based disruption of ALT machinery
and synthetic lethality approaches that exploit ALT-specific
vulnerabilities (124).
Future perspectives: Integrating telomere
biology with senescence-targeted therapies
Future directions emphasize patient stratification
according to telomere maintenance mechanisms and the development of
combination therapies that integrate telomerase inhibitors with
immunotherapy or epigenetic modulators. The creation of novel
diagnostic tools will be key for real-time monitoring of telomerase
activity. The crosstalk between senescence and telomere dynamics
constitutes a pivotal frontier in cancer therapy, offering
opportunities to enhance treatment efficacy, prevent relapse and
overcome resistance in both telomerase-dependent and ALT-driven
malignancies. By combining telomerase inhibition with senolytic
strategies, it may be possible to simultaneously disrupt tumor cell
immortality and remodel the immunosuppressive TME, establishing
innovative therapeutic paradigms.
Conclusions
The present review highlights the central role of
cellular senescence in cancer, emphasizing its paradoxical function
as both a tumor-suppressive mechanism and a promoter of malignancy.
The intricate interplay between senescent cells and the TME,
largely mediated through SASP, presents novel avenues for
therapeutic intervention. Future research can prioritize the
elucidation of tumor-specific variations in SASP composition and
their distinct contributions to cancer progression. The development
of next-generation senotherapeutics, including innovative
senolytics and senomorphics, holds notable promise in selectively
targeting deleterious senescent cells while preserving their
protective functions.
Integrating Traditional Chinese Medicine with
contemporary therapeutic strategies offers additional opportunities
to enhance senescence modulation, advancing the field towards
precision oncology. Cutting-edge technologies, such as single-cell
omics, spatial transcriptomics and AI-driven modeling, are
essential to dissect the spatiotemporal dynamics of senescent cells
within the TME. Furthermore, novel approaches, including
SASP-specific inhibitors and senescent cell vaccines, may provide
targeted solutions to the complex interactions between senescence
and tumorigenesis.
Clinical translation will require robust biomarkers
for patient stratification and carefully designed trials,
particularly for aging populations and refractory types of cancer.
Future research can focus on balancing the protective functions of
senescence, such as clearance of damaged cells, against potential
detrimental effects, including chronic inflammation and immune
suppression. Optimizing the timing and context of senolytic
interventions is key to maximizing therapeutic benefit while
minimizing adverse effects. Strategically targeting pathological
senescence thus represents a transformative approach in oncology,
which offers personalized, effective therapies that simultaneously
disrupt tumor cell immortality and remodel the immunosuppressive
TME.
Acknowledgements
Not applicable.
Funding
The present review was funded by Shanghai Putuo District Health
System Science and Technology Innovation Project (grant no.
ptkwws202507).
Availability of data and materials
Not applicable.
Authors' contributions
YL conceptualized the present review, validated the
data and conducted the formal analysis, wrote the original draft,
and edited and reviewed the manuscript. JG collected, screened and
systematically organized key data from relevant literature and
existing studies, wrote the original draft, and edited and reviewed
the manuscript. PY conceptualized the present review, validated the
data, obtained funding, and edited and reviewed the manuscript.
Data authentication is not applicable. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Campisi J and d'Adda di Fagagna F:
Cellular senescence: When bad things happen to good cells. Nat Rev
Mol Cell Biol. 8:729–740. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schosserer M, Grillari J and Breitenbach
M: The dual role of cellular senescence in developing tumors and
their response to cancer therapy. Front Oncol. 7:2782017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nacarelli T, Liu P and Zhang R: Epigenetic
basis of cellular senescence and its implications in aging. Genes
(Basel). 8:3432017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lai P, Liu L, Bancaro N, Troiani M, Calì
B, Li Y, Chen J, Singh PK, Arzola RA, Attanasio G, et al:
Mitochondrial DNA released by senescent tumor cells enhances
PMN-MDSC-driven immunosuppression through the cGAS-STING pathway.
Immunity. 58:811–825.e7. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen D, Wang J, Li Y, Xu C, Fanzheng M,
Zhang P and Liu L: LncRNA NEAT1 suppresses cellular senescence in
hepatocellular carcinoma via KIF11-dependent repression of CDKN2A.
Clin Transl Med. 13:e14182023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu N, Wu J, Deng E, Zhong J, Wei B, Cai
T, Xie Z, Duan X, Fu S, Osei-Hwedieh DO, et al: Immunotherapy and
senolytics in head and neck squamous cell carcinoma: Phase 2 trial
results. Nat Med. 31:3047–3061. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zandi M, Behboudi E, Shojaei MR, Soltani S
and Karami H: Letter to the editor regarding ‘An overview on
serology and molecular tests for COVID-19: An important challenge
of the current century (doi: 10.22034/iji.2021.88660.1894.)’. Iran
J Immunol. 19:3372022.PubMed/NCBI
|
|
8
|
Khosravi M, Behboudi E, Razavi-Nikoo H and
Tabarraei A: Hepatitis B virus X protein induces expression changes
of miR-21, miR-22, miR-122, miR-132, and miR-222 in Huh-7 cell
line. Arch Razi Inst. 79:111–119. 2024.PubMed/NCBI
|
|
9
|
Edalat F, Gholamzad A, Ghoreshi ZA,
Dalfardi M, Golkar A, Behboudi E and Arefinia N: Prevalence and
genetic diversity of HTLV-1 among blood donors in Jiroft, Iran: A
comprehensive study. Virus Genes. 61:424–431. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hao X, Billings SD, Wu F, Stultz TW,
Procop GW, Mirkin G and Vidimos AT: Dermatofibrosarcoma
protuberans: Update on the diagnosis and treatment. J Clin Med.
9:17522020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Molina-Peña R, Tudon-Martinez JC and
Aquines-Gutiérrez O: A mathematical model of average dynamics in a
stem cell hierarchy suggests the combinatorial targeting of cancer
stem cells and progenitor cells as a potential strategy against
tumor growth. Cancers (Basel). 12:25902020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu L, Wang Y, Wang J, Zhai J, Ren L and
Zhu G: Radiation-induced osteocyte senescence alters bone marrow
mesenchymal stem cell differentiation potential via paracrine
signaling. Int J Mol Sci. 22:93232021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Childs BG, Baker DJ, Kirkland JL, Campisi
J and van Deursen JM: Senescence and apoptosis: Dueling or
complementary cell fates? EMBO Rep. 15:1139–1153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rhinn M, Ritschka B and Keyes WM: Cellular
senescence in development, regeneration and disease. Development.
146:dev1518372019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gorgoulis V, Adams PD, Alimonti A, Bennett
DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K,
Ferbeyre G, et al: Cellular senescence: Defining a path forward.
Cell. 179:813–827. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Olivieri F, Prattichizzo F, Grillari J and
Balistreri CR: Cellular senescence and inflammaging in Age-related
diseases. Mediators Inflamm. 2018:90764852018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Micco R, Krizhanovsky V, Baker D and
d'Adda di Fagagna F: Cellular senescence in ageing: From mechanisms
to therapeutic opportunities. Nat Rev Mol Cell Biol. 22:75–95.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mikuła-Pietrasik J, Niklas A, Uruski P,
Tykarski A and Książek K: Mechanisms and significance of
therapy-induced and spontaneous senescence of cancer cells. Cell
Mol Life Sci. 77:213–229. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ou HL, Hoffmann R, González-López C,
Doherty GJ, Korkola JE and Muñoz-Espín D: Cellular senescence in
cancer: From mechanisms to detection. Mol Oncol. 15:2634–2671.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Di Mitri D and Alimonti A:
Non-Cell-Autonomous regulation of cellular senescence in cancer.
Trends Cell Biol. 26:215–226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park SS, Choi YW, Kim JH, Kim HS and Park
TJ: Senescent tumor cells: An overlooked adversary in the battle
against cancer. Exp Mol Med. 53:1834–1841. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saleh T, Tyutynuk-Massey L, Cudjoe EK Jr,
Idowu MO, Landry JW and Gewirtz DA: Non-cell autonomous effects of
the Senescence-associated secretory phenotype in cancer therapy.
Front Oncol. 8:1642018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Herranz N and Gil J: Mechanisms and
functions of cellular senescence. J Clin Invest. 128:1238–1246.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rao SG and Jackson JG: SASP: Tumor
suppressor or promoter? Yes! Trends Cancer. 2:676–687. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He Y, Long K, Du B, Liao W, Zou R, Su J,
Luo J, Shi Z and Wang L: The cellular senescence score (CSS) is a
comprehensive biomarker to predict prognosis and assess senescence
and immune characteristics in hepatocellular carcinoma (HCC).
Biochem Biophys Res Commun. 739:1505762024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ru K, Cui L, Wu C, Tan XX, An WT, Wu Q, Ma
YT, Hao Y, Xiao X, Bai J, et al: Exploring the molecular and immune
landscape of cellular senescence in lung adenocarcinoma. Front
Immunol. 15:13477702024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Geng H, Huang C, Xu L, Zhou Y, Dong Z,
Zhong Y, Li Q, Yang C, Huang S, Liao W, et al: Targeting cellular
senescence as a therapeutic vulnerability in gastric cancer. Life
Sci. 346:1226320241
|
|
28
|
Liu H, Zhao H and Sun Y: Tumor
microenvironment and cellular senescence: Understanding therapeutic
resistance and harnessing strategies. Semin Cancer Biol.
86:769–781. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Higashiguchi M, Murakami H, Akita H,
Kobayashi S, Takahama S, Iwagami Y, Yamada D, Tomimaru Y, Noda T,
Gotoh K, et al: The impact of cellular senescence and
senescence-associated secretory phenotype in cancer-associated
fibroblasts on the malignancy of pancreatic cancer. Oncol Rep.
49:982023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu H, Lv R, Song F, Yang Y, Zhang F, Xin
L, Zhang P, Zhang Q and Ding C: A near-IR ratiometric fluorescent
probe for the precise tracking of senescence: A multidimensional
sensing assay of biomarkers in cell senescence pathways. Chem Sci.
15:5681–5693. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin W, Wang X, Wang Z, Shao F, Yang Y, Cao
Z, Feng X, Gao Y and He J: Comprehensive analysis uncovers
prognostic and immunogenic characteristics of cellular senescence
for lung adenocarcinoma. Front Cell Dev Biol. 9:7804612021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang W, Li Y, Lyu J, Shi F, Kong Y, Sheng
C, Wang S and Wang Q: An aging-related signature predicts favorable
outcome and immunogenicity in lung adenocarcinoma. Cancer Sci.
113:891–903. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pérez-Mancera PA, Young AR and Narita M:
Inside and out: The activities of senescence in cancer. Nat Rev
Cancer. 14:547–558. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang Y, Cai Q, Zhu M, Rong J, Feng X and
Wang K: Exploring the Double-edged role of cellular senescence in
chronic liver disease for new treatment approaches. Life Sci.
373:1236782025. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wyld L, Bellantuono I, Tchkonia T, Morgan
J, Turner O, Foss F, George J, Danson S and Kirkland JL: Senescence
and cancer: A review of clinical implications of senescence and
senotherapies. Cancers (Basel). 12:21342020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gu Y, Xu T, Fang Y, Shao J, Hu T, Wu X,
Shen H, Xu Y, Zhang J, Song Y, et al: CBX4 counteracts cellular
senescence to desensitize gastric cancer cells to chemotherapy by
inducing YAP1 SUMOylation. Drug Resist Updat. 77:1011362024.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liang X, Lin X, Lin Z, Lin W, Peng Z and
Wei S: Genes associated with cellular senescence favor melanoma
prognosis by stimulating immune responses in tumor
microenvironment. Comput Biol Med. 158:1068502023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao Q, Hu W, Xu J, Zeng S, Xi X, Chen J,
Wu X, Hu S and Zhong T: Comprehensive Pan-cancer analysis of
senescence with cancer prognosis and immunotherapy. Front Mol
Biosci. 9:9192742022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fan Y, Gao Z, Li X, Wei S and Yuan K: Gene
expression and prognosis of x-ray repair cross-complementing family
members in non-small cell lung cancer. Bioengineered. 12:6210–6228.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mavrogonatou E, Pratsinis H and Kletsas D:
The role of senescence in cancer development. Semin Cancer Biol.
62:182–191. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu H, Xu Q, Wufuer H, Li Z, Sun R, Jiang
Z, Dou X, Fu Q, Campisi J and Sun Y: Rutin is a potent senomorphic
agent to target senescent cells and can improve chemotherapeutic
efficacy. Aging Cell. 23:e139212024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sun Y, Coppé JP and Lam EW: Cellular
senescence: The sought or the unwanted? Trends Mol Med. 24:871–885.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen Y, Zhou T, Zhou R, Sun W, Li Y, Zhou
Q, Xu D, Zhao Y, Hu P, Liang J, et al: TRAF7 knockdown induces
cellular senescence and synergizes with lomustine to inhibit glioma
progression and recurrence. J Exp Clin Cancer Res. 44:1122025.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Calcinotto A, Kohli J, Zagato E,
Pellegrini L, Demaria M and Alimonti A: Cellular senescence: Aging,
cancer, and injury. Physiol Rev. 99:1047–1078. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kamal M, Shanmuganathan M, Kroezen Z,
Joanisse S, Britz-McKibbin P and Parise G: Senescent myoblasts
exhibit an altered exometabolome that is linked to
senescence-associated secretory phenotype signaling. Am J Physiol
Cell Physiol. 328:C440–C451. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lecot P, Alimirah F, Desprez PY, Campisi J
and Wiley C: Context-dependent effects of cellular senescence in
cancer development. Br J Cancer. 114:1180–1184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lian J, Yue Y, Yu W and Zhang Y:
Immunosenescence: A key player in cancer development. J Hematol
Oncol. 13:1512020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ruhland MK and Alspach E: Senescence and
immunoregulation in the tumor microenvironment. Front Cell Dev
Biol. 9:7540692021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lau L and David G: Pro- and
anti-tumorigenic functions of the senescence-associated secretory
phenotype. Expert Opin Ther Targets. 23:1041–1051. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gonzalez-Meljem JM, Apps JR, Fraser HC and
Martinez-Barbera JP: Paracrine roles of cellular senescence in
promoting tumourigenesis. Br J Cancer. 118:1283–1288. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang G, Cheng X, Zhang J, Liao Y, Jia Y
and Qing C: Possibility of inducing tumor cell senescence during
therapy. Oncol Lett. 22:4962021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Maggiorani D, Le O, Lisi V, Landais S,
Moquin-Beaudry G, Lavallée VP, Decaluwe H and Beauséjour C:
Senescence drives immunotherapy resistance by inducing an
immunosuppressive tumor microenvironment. Nat Commun. 15:24352024.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lasry A and Ben-Neriah Y:
Senescence-associated inflammatory responses: Aging and cancer
perspectives. Trends Immunol. 36:217–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Murray KO, Mahoney SA, Ludwig KR,
Miyamoto-Ditmon JH, VanDongen NS, Banskota N, Herman AB, Seals DR,
Mankowski RT, Rossman MJ and Clayton ZS: Intermittent
supplementation with fisetin improves physical function and
decreases cellular senescence in skeletal muscle with aging: A
comparison to genetic clearance of senescent cells and synthetic
senolytic approaches. Aging Cell. 24:e701142025. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ayoub M, Abou Jaoude C, Ayoub M, Hamade A
and Rima M: The immune system and cellular senescence: A complex
interplay in aging and disease. Immunology. Sep 12–2025.doi:
10.1111/imm.70036 (Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang W, Zhang K, Shi J, Qiu H, Kan C, Ma
Y, Hou N, Han F and Sun X: The impact of the senescent
microenvironment on tumorigenesis: Insights for cancer therapy.
Aging Cell. 23:e141822024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kirkland JL: Tumor dormancy and disease
recurrence. Cancer Metastasis Rev. 42:9–12. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
de Paula B, Kieran R, Koh SSY, Crocamo S,
Abdelhay E and Muñoz-Espín D: Targeting senescence as a therapeutic
opportunity for Triple-negative breast cancer. Mol Cancer Ther.
22:583–598. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lee S and Lee JS: Cellular senescence: A
promising strategy for cancer therapy. BMB Rep. 52:35–41. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang C, Hao X and Zhang R: Targeting
cellular senescence to combat cancer and ageing. Mol Oncol.
16:3319–3332. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Battram AM, Bachiller M and Martín-Antonio
B: Senescence in the development and response to cancer with
immunotherapy: A Double-edged sword. Int J Mol Sci. 21:43462020.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fan DN and Schmitt CA: Detecting markers
of Therapy-induced senescence in cancer cells. Methods Mol Biol.
1534:41–52. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ruhland MK, Coussens LM and Stewart SA:
Senescence and cancer: An evolving inflammatory paradox. Biochim
Biophys Acta. 1865:14–22. 2016.PubMed/NCBI
|
|
64
|
Song S, Lam EW, Tchkonia T, Kirkland JL
and Sun Y: Senescent cells: Emerging targets for human aging and
Age-related diseases. Trends Biochem Sci. 45:578–592. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Paleari L: Personalized assessment for
cancer prevention, detection, and treatment. Int J Mol Sci.
25:81402024. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ohtani N: The roles and mechanisms of
senescence-associated secretory phenotype (SASP): Can it be
controlled by senolysis? Inflamm Regen. 42:112022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kaur J and Farr JN: Cellular senescence in
Age-related disorders. Transl Res. 226:96–104. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hughes BK, Davis A, Milligan D, Wallis R,
Mossa F, Philpott MP, Wainwright LJ, Gunn DA and Bishop CL:
SenPred: A single-cell RNA sequencing-based machine learning
pipeline to classify deeply senescent dermal fibroblast cells for
the detection of an in vivo senescent cell burden. Genome Med.
17:22025. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Uyar B, Palmer D, Kowald A, Murua Escobar
H, Barrantes I, Möller S, Akalin A and Fuellen G: Single-cell
analyses of aging, inflammation and senescence. Ageing Res Rev.
64:1011562020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sprenger HG, MacVicar T, Bahat A, Fiedler
KU, Hermans S, Ehrentraut D, Ried K, Milenkovic D, Bonekamp N,
Larsson NG, et al: Cellular pyrimidine imbalance triggers
mitochondrial DNA-dependent innate immunity. Nat Metab. 3:636–650.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Liang A, Kong Y, Chen Z, Qiu Y, Wu Y, Zhu
X and Li Z: Advancements and applications of single-cell
multi-omics techniques in cancer research: Unveiling heterogeneity
and paving the way for precision therapeutics. Biochem Biophys Rep.
37:1015892023.PubMed/NCBI
|
|
72
|
Avelar RA, Ortega JG, Tacutu R, Tyler EJ,
Bennett D, Binetti P, Budovsky A, Chatsirisupachai K, Johnson E,
Murray A, et al: A multidimensional systems biology analysis of
cellular senescence in aging and disease. Genome Biol. 21:912020.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wu L, Xie X, Liang T, Ma J, Yang L, Yang
J, Li L, Xi Y, Li H, Zhang J, et al: Integrated Multi-omics for
novel aging biomarkers and antiaging targets. Biomolecules.
12:392021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Aird KM and Zhang R: Nucleotide
metabolism, oncogene-induced senescence and cancer. Cancer Lett.
356:204–210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yang K, Li X and Xie K: Senescence program
and its reprogramming in pancreatic premalignancy. Cell Death Dis.
14:5282023. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hwang HJ, Jung SH, Lee HC, Han NK, Bae IH,
Lee M, Han YH, Kang YS, Lee SJ, Park HJ, et al: Identification of
novel therapeutic targets in the secretome of ionizing
radiation-induced senescent tumor cells. Oncol Rep. 35:841–850.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Matjusaitis M, Chin G, Sarnoski EA and
Stolzing A: Biomarkers to identify and isolate senescent cells.
Ageing Res Rev. 29:1–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ziglari T, Calistri NL, Finan JM, Derrick
DS, Nakayasu ES, Burnet MC, Kyle JE, Hoare M, Heiser LM and Pucci
F: Senescent Cell-derived extracellular vesicles inhibit cancer
recurrence by coordinating immune surveillance. Cancer Res.
85:859–874. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Davalli P, Mitic T, Caporali A, Lauriola A
and D'Arca D: ROS, cell senescence, and novel molecular mechanisms
in aging and Age-related diseases. Oxid Med Cell Longev.
2016:35651272016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Amor C, Fernández-Maestre I, Chowdhury S,
Ho YJ, Nadella S, Graham C, Carrasco SE, Nnuji-John E, Feucht J,
Hinterleitner C, et al: Prophylactic and Long-lasting efficacy of
senolytic CAR T cells against Age-related metabolic dysfunction.
Nat Aging. 4:336–349. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Alqahtani S, Alqahtani T, Venkatesan K,
Sivadasan D, Ahmed R, Sirag N, Elfadil H, Abdullah Mohamed H, T A
H, Elsayed Ahmed R, et al: SASP modulation for cellular
rejuvenation and tissue homeostasis: Therapeutic strategies and
molecular insights. Cells. 14:6082025. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Schmitt CA, Fridman JS, Yang M, Lee S,
Baranov E, Hoffman RM and Lowe SW: A senescence program controlled
by p53 and p16INK4a contributes to the outcome of cancer therapy.
Cell. 109:335–346. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wang S, Xing Y, Wang R and Jin Z: Jianpi
Huayu Decoction suppresses cellular senescence in colorectal cancer
via p53-p21-Rb pathway: Network pharmacology and in vivo
validation. J Ethnopharmacol. 319:1173472024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wu M, Wu B, Huang X, Wang Z, Zhu M, Zhu Y,
Yu L and Liu J: Inhibition of the FEN1-PBX1 axis elicits cellular
senescence in breast cancer via the increased intracellular
reactive oxygen species levels. J Transl Med. 23:2482025.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wang K, Jiang X, Jiang Y, Liu J, Du Y,
Zhang Z, Li Y, Zhao X, Li J and Zhang R: EZH2-H3K27me3-mediated
silencing of mir-139-5p inhibits cellular senescence in
hepatocellular carcinoma by activating TOP2A. J Exp Clin Cancer
Res. 42:3202023. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yuan W, Xu Y, Wu Z, Huang Y, Meng L, Dai
S, Ying S, Chen Z and Xu A: Cellular senescence-related genes:
Predicting prognosis in hepatocellular carcinoma. BMC Cancer.
23:10012023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhang C, Wang X and Zhang C: Icaritin
inhibits CDK2 expression and activity to interfere with tumor
progression. iScience. 25:1049912022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Sieben CJ, Sturmlechner I, van de Sluis B
and van Deursen JM: Two-Step senescence-focused cancer therapies.
Trends Cell Biol. 28:723–737. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sikora E, Bielak-Zmijewska A and Mosieniak
G: Targeting normal and cancer senescent cells as a strategy of
senotherapy. Ageing Res Rev. 55:1009412019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Short S, Fielder E, Miwa S and von
Zglinicki T: Senolytics and senostatics as adjuvant tumour therapy.
EBioMedicine. 41:683–692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yang N and Sen P: The senescent cell
epigenome. Aging (Albany NY). 10:3590–3609. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Choi YW, Kim YH, Oh SY, Suh KW, Kim YS,
Lee GY, Yoon JE, Park SS, Lee YK, Park YJ, et al: Senescent tumor
cells build a cytokine shield in colorectal cancer. Adv Sci
(Weinh). 8:20024972021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Vilgelm AE, Johnson CA, Prasad N, Yang J,
Chen SC, Ayers GD, Pawlikowski JS, Raman D, Sosman JA, Kelley M, et
al: Connecting the dots: Therapy-induced senescence and a
Tumor-suppressive immune microenvironment. J Natl Cancer Inst.
108:djv4062015.PubMed/NCBI
|
|
94
|
Aimono Y, Endo K and Sekiya I: Cellular
senescence contributes to spontaneous repair of the rat meniscus.
Aging Cell. 24:e143852025. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ohtani N: The role of SASP in tumor
microenvironment. Clin Calcium. 27:835–843. 2017.(In Japanese).
PubMed/NCBI
|
|
96
|
Hatzikirou H, Alfonso JC, Mühle S, Stern
C, Weiss S and Meyer-Hermann M: Cancer therapeutic potential of
combinatorial immuno- and vasomodulatory interventions. J R Soc
Interface. 12:201504392015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Rebbaa A: Targeting senescence pathways to
reverse drug resistance in cancer. Cancer Lett. 219:1–13. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Cuollo L, Antonangeli F, Santoni A and
Soriani A: The Senescence-associated secretory phenotype (SASP) in
the challenging future of cancer therapy and Age-related diseases.
Biology (Basel). 9:4852020.PubMed/NCBI
|
|
99
|
Chen J, Wang J, Lucas M, Liu H, Wheeler C,
Johnson K, Woodard K, Chang C, Frey G, Boyle WJ and Short JM:
Abstract B022: Targeting senescence cells in cancer and aging by
conditionally active biologic therapeutics In: Proceedings of the
AACR Special Conference: Aging and Cancer; 2022 Nov 17–20, San
Diego, CA, AACR, Philadelphia, PA. Cancer Res. 83 (2
Suppl_1):Abstract nr B022. 2022.
|
|
100
|
Butt AQ and Mills KH: Immunosuppressive
networks and checkpoints controlling antitumor immunity and their
blockade in the development of cancer immunotherapeutics and
vaccines. Oncogene. 33:4623–4631. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Ivey JW, Bonakdar M, Kanitkar A, Davalos
RV and Verbridge SS: Improving cancer therapies by targeting the
physical and chemical hallmarks of the tumor microenvironment.
Cancer Lett. 380:330–339. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Papismadov N, Gal H and Krizhanovsky V:
The anti-aging promise of p21. Cell Cycle. 16:1997–1998. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Gonzalez-Meljem JM and Martinez-Barbera
JP: Adamantinomatous craniopharyngioma as a model to understand
paracrine and Senescence-induced tumourigenesis. Cell Mol Life Sci.
78:4521–4544. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
He S and Sharpless NE: Senescence in
health and disease. Cell. 169:1000–1011. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Janelle V, Neault M, Lebel MÈ, De Sousa
DM, Boulet S, Durrieu L, Carli C, Muzac C, Lemieux S, Labrecque N,
et al: p16INK4a regulates cellular senescence in PD-1-expressing
human T cells. Front Immunol. 12:6985652021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Park SS, Lee YK, Kim YH, Park SH, Kang HY,
Kim JC, Kim DJ, Lim SB, Yoon G, Kim JH, et al: Distribution and
impact of p16INK4A+ senescent cells in elderly tissues: A focus on
senescent immune cell and epithelial dysfunction. Exp Mol Med.
56:2631–2641. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Baker DJ, Childs BG, Durik M, Wijers ME,
Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC,
Pezeshki A, et al: Naturally occurring p16(Ink4a)-positive cells
shorten healthy lifespan. Nature. 530:184–189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Courtois-Cox S, Jones SL and Cichowski K:
Many roads lead to Oncogene-induced senescence. Oncogene.
27:2801–2809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Angelini PD, Zacarias Fluck MF, Pedersen
K, Parra-Palau JL, Guiu M, Bernadó Morales C, Vicario R,
Luque-García A, Navalpotro NP, Giralt J, et al: Constitutive HER2
signaling promotes breast cancer metastasis through cellular
senescence. Cancer Res. 73:450–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhang B, Lam EW and Sun Y: Senescent
cells: A new Achilles' heel to exploit for cancer medicine? Aging
Cell. 18:e128752019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Du PY, Gandhi A, Bawa M and Gromala J: The
ageing immune system as a potential target of senolytics. Oxf Open
Immunol. 4:iqad0042023. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Saleh T, Tyutyunyk-Massey L and Gewirtz
DA: Tumor cell escape from Therapy-induced senescence as a model of
disease recurrence after dormancy. Cancer Res. 79:1044–1046. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Salam R, Saliou A, Bielle F, Bertrand M,
Antoniewski C, Carpentier C, Alentorn A, Capelle L, Sanson M,
Huillard E, et al: Cellular senescence in malignant cells promotes
tumor progression in mouse and patient Glioblastoma. Nat Commun.
14:4412023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zheng J, Liu Y, Lau YL and Tu W: γδ-T
cells: An unpolished sword in human Anti-infection immunity. Cell
Mol Immunol. 10:50–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Wang W, Luo HS and Yu BP: Expression of
NF-kappaB and human telomerase reverse transcriptase in gastric
cancer and precancerous lesions. World J Gastroenterol. 10:177–181.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Meyerson M, Counter CM, Eaton EN, Ellisen
LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ,
Liu Q, et al: hEST2, the putative human telomerase catalytic
subunit gene, is up-regulated in tumor cells and during
immortalization. Cell. 90:785–795. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Saleh S, Lam AK and Ho YH: Real-time PCR
quantification of human telomerase reverse transcriptase (hTERT) in
colorectal cancer. Pathology. 40:25–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Eastley N, Ottolini B, Garrido C, Shaw JA,
McCulloch TA, Ashford RU and Royle NJ: Telomere maintenance in soft
tissue sarcomas. J Clin Pathol. 70:371–377. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Bojko A, Czarnecka-Herok J, Charzynska A,
Dabrowski M and Sikora E: Diversity of the senescence phenotype of
cancer cells treated with chemotherapeutic agents. Cells.
8:15012019. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Ganesan K and Xu B: Telomerase inhibitors
from natural products and their anticancer potential. Int J Mol
Sci. 19:132017. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Banik K, Khatoon E, Harsha C, Rana V,
Parama D, Thakur KK, Bishayee A and Kunnumakkara AB: Wogonin and
its analogs for the prevention and treatment of cancer: A
systematic review. Phytother Res. 36:1854–1883. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Shay JW and Wright WE: Telomeres and
telomerase: Three decades of progress. Nat Rev Genet. 20:299–309.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Saretzki G: Role of telomeres and
telomerase in cancer and aging. Int J Mol Sci. 24:99322023.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Bollmann FM: Targeting ALT: The role of
alternative lengthening of telomeres in pathogenesis and prevention
of cancer. Cancer Treat Rev. 33:704–709. 2007. View Article : Google Scholar : PubMed/NCBI
|