Introduction
Salivary adenoid cystic carcinoma (SACC) is a
relatively rare but aggressive malignancy of the salivary glands,
accounting for ~25% of all salivary gland tumors (1,2). The
tendency of SACC to invade locally and metastasize distantly makes
it difficult to manage clinically. Despite advancements in
multimodal therapies, including surgery in combination with
radiotherapy or chemotherapy, >30% of patients experience local
recurrence and face poor prognosis (3–5). Thus,
elucidating the molecular mechanisms underlying SACC invasion and
metastasis is key for improving patient outcomes.
Epithelial-mesenchymal transition (EMT), a key
biological process during tumor progression, endows cancer cells
with increased plasticity, promoting their migration and invasion
capabilities (6). EMT is marked by
the loss of epithelial characteristics, such as polarity and
intercellular adhesion, and the acquisition of mesenchymal markers
(7,8). Integrin-linked kinase (ILK) serves as
a serine/threonine kinase and scaffolding protein involved in
diverse signaling pathways, such as the PI3K/Akt and Wnt/β-catenin
pathways (9,10). Its N-terminal ankyrin repeat domain
binds particularly interesting new cysteine-histidine-rich protein
(PINCH), while the C-terminal kinase domain associates with Parvin
to form the ILK-PINCH-Parvin complex. This complex broadly
participates in the molecular regulation of malignant phenotypes,
including cell cycle progression, migration, invasion and apoptosis
resistance (10–14). ILK upregulation is observed in
various types of cancer, such as prostate and colorectal cancer,
and demonstrates significant associations with tumor progression
and poor prognosis (15,16). A previous study demonstrated that
ILK knockdown induces apoptosis of prostate cancer cells by
decreasing AKT activity (15). Our
previous research also revealed that overexpression of ILK is
associated with perineural invasion, local recurrence and distant
metastasis in SACC (17). Moreover,
ILK levels are associated with the expression of EMT markers,
suggesting its involvement in EMT regulation (16). However, the precise molecular
mechanism remains unclear.
S100 calcium-binding protein A4 (S100A4), a member
of the S100 protein family, exerts its functions in a
calcium-dependent manner and serves both intracellular and
extracellular roles. Within cells, S100A4 expression is associated
with regulation of cell migration, apoptosis and maintenance of
stemness (18,19). In the extracellular space, S100A4
primarily stimulates pro-inflammatory signaling pathways and
induces the expression of effector molecules, including growth
factors, extracellular matrix components and MMPs, thereby
activating diverse physiological processes (20). Recognized as a promoter of tumor
progression, metastasis and poor clinical outcomes (21,22),
S100A4 is frequently upregulated in tumor cells and the tumor
microenvironment. It contributes to the pathogenesis and
advancement of multiple malignancies, such as breast and lung
cancer (23–25). Although both ILK and S100A4 have
been independently linked to the regulation of EMT (26,27),
the mechanistic connection between them remains poorly understood.
The present study aimed to determine the contribution of ILK to the
malignant phenotypes of SACC cells and the regulatory association
between ILK and S100A4 in the context of EMT and tumor invasion.
The present results could offer new perspectives on SACC
pathogenesis and reveal promising targets for therapeutic
intervention.
Materials and methods
Patients and specimen collection
A total of 52 SACC tissue samples were obtained
following approval by the Ethics Committee of the Affiliated
Stomatological Hospital of Southwest Medical University (approval
no. 20211129004; Luzhou, China). The study enrolled 52 patients (21
male and 31 female), aged 23–84 years, who underwent radical
resection between January 2020 and December 2024 and had not
received any prior radiotherapy or chemotherapy. Patients who had
previously undergone radiotherapy or chemotherapy, or those with
ill-defined primary lesions accompanied by distant metastasis, were
excluded. Written informed consent was obtained from each
participant before tissue collection. Clinicopathological
characteristics and follow-up data were obtained from the database
of the Affiliated Stomatological Hospital of Southwest Medical
University. According to the World Health Organization
Classification of Head and Neck Tumors (28), the dominant histological pattern was
determined based on the pattern occupying the largest proportion of
the tumor, and those with a solid component >30% were classified
as the solid subtype. Tumor staging was performed according to the
8th edition of the Union for International Cancer Control/American
Joint Committee on Cancer (AJCC) TNM classification system
(29). Normal parotid gland tissue
samples (n=5) were collected from the disease-free surgical margins
of patients undergoing parotidectomy for benign pleomorphic adenoma
at the Affiliated Stomatological Hospital of Southwest Medical
University between January 2024 and December 2024. Exclusion
criteria included prior head and neck radiotherapy or chemotherapy.
The control cohort consisted of 2 males and 3 females, aged 35 to
56 years. All tissues were reviewed and confirmed to be
histologically normal by two independent pathologists.
Cell lines and culture
The human SACC cell lines SACC-83 and SACC-LM were
obtained from the State Key Laboratory of Oral Diseases, West China
Hospital of Stomatology. SACC-83 and SACC-LM cell lines are derived
from ductal epithelial cells (30).
Cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (Vazyme)
and 1% penicillin-streptomycin solution (Beyotime Institute of
Biotechnology), and maintained at 37°C in a humidified incubator
with 5% CO2.
Cell transfection
Lentiviruses targeting ILK and S100A4 were
constructed and synthesized by OBIO Technology. Short hairpin
(sh)RNA sequences targeting ILK were inserted into the
pSLenti-U6-shRNA-CMV-EGFP-F2A-Puro-WPRE silencing vector. S100A4
sequences were cloned into the
pSLenti-EF1-EGFP-P2A-Puro-CMV-S100A4-3XFLAG-WPRE overexpression
vector. The negative control lentivirus was obtained from OBIO
Technology. Lentiviral particles were harvested at 48 and 72 h
after transfecting the recombinant vectors into 293T cells (ATCC).
SACC-83 and SACC-LM cells were seeded in 12-well plates at a
density of 5×104 cells per well and cultured at 37°C for
24 h until 70% confluence. Cells were then transduced with
lentivirus at a multiplicity of infection of 60 in the presence of
5 µg/ml Polybrene Plus (OBIO Technology). Following 72 h
transfection at 37°C, 2 µg/ml puromycin (Beijing Solarbio Science
& Technology Co., Ltd.) was introduced for selection to
establish stably transfected cell lines. Subsequent experiments
utilized stable cells that were cultured for 5–7 days
post-selection and maintained within five passages of transfection.
The sequences of ILK-targeting shRNAs were as follows: shRNA-1,
5′-CCGTAGTGTAATGATTGATGA-3′; shRNA-2, 5′-CGACCCAAATTTGACATGATT-3′
and shRNA-3, 5′-GCAATGACATTGTCGTGAAGG-3′. Untreated cells were
defined as the control group (CON). Cells transfected with a
scramble non-targeting shRNA lentivirus served as the negative
control for the ILK knockdown experiment (NC-ILK), and cells
transfected with an empty vector lentivirus served as the negative
control for the S100A4 overexpression experiment (NC-S100A4).
Cell proliferation assay
SACC cells in the logarithmic growth phase were
trypsinized and seeded into 96-well plates at a density of
3×103 cells/well (n=5/group). After 24 h incubation at
37°C, Cell Counting Kit (CCK)-8 reagent (Dojindo Laboratories,
Inc.) was administered at 0, 24, 48, 72, 96 and 120 h. Optical
density at 450 nm following 1 h incubation at 37°C was measured to
quantify cell proliferation.
Colony formation assay
SACC cells were seeded in 6-well plates at a density
of 700 cells/well and cultured in complete RPMI-1640 medium at 37°C
under 5% CO2 for 14 days, with the medium (Gibco; Thermo
Fisher Scientific, Inc.) replaced every 48 h. Colonies (defined as
cell clusters containing ≥50 cells) were fixed with 4%
paraformaldehyde for 20 min at room temperature, stained with 0.1%
crystal violet for 15 min at room temperature, rinsed with PBS, and
then imaged using a light microscope. Colony counting was performed
automatically using ImageJ (v1.54, National Institutes of
Health).
Cell cycle analysis
SACC cells (5×105) were fixed in 75%
ethanol at −20°C overnight. Cells were treated with RNase A (50
µg/ml at 37°C for 30 min) and stained with PI (5 µl; 4°C for 30 min
in the dark). Cell cycle distribution was analyzed using a flow
cytometer (BD FACSCalibur), and data were processed with FlowJo
software (v10.8, FlowJo LLC).
Wound healing assay
SACC cells (2×106 cells/well) were
cultured at 37°C for 24 h in 6-well plates until >95%
confluency. The monolayers were scratched using a sterile pipette
tip and incubated at 37°C in serum-free medium. Images were
captured at 0, 12 and 24 h using an inverted light microscope
(Olympus GX53). The wound area was quantified using ImageJ software
(v1.54, National Institutes of Health) to evaluate migration.
Transwell invasion assay
SACC cells (5×104/insert) suspended in
serum-free RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
were seeded into the upper chamber of Matrigel-coated Transwell
inserts (Corning, Inc.). The lower chamber was filled with medium
containing 10% FBS (Vazyme) as a chemoattractant. Following 48 h
incubation at 37°C, non-invading cells were removed and invaded
cells were fixed with 4% paraformaldehyde for 20 min at room
temperature, stained with 0.1% crystal violet for 15 min at room
temperature, and then imaged using a light microscope. A total of
five randomly selected fields per membrane were analyzed using
ImageJ software (v1.54, National Institutes of Health).
Western blotting
Total protein was extracted from SACC cells using
RIPA buffer containing protease and phosphatase inhibitors (AbMole
Bioscience). Protein concentration was determined using a BCA assay
(Vazyme). Equal amounts of protein (30 µg/lane) were separated by
10% SDS-PAGE and transferred to PVDF membranes. Following blocking
with 5% skimmed milk for 2 h at room temperature, membranes were
incubated overnight at 4°C with primary antibodies against ILK
(1:1,000, Abcam; cat. no. ab76468), S100A4 (1:5,000, Proteintech
Group, Inc.; cat. no. 16105-1-AP), E-cadherin (cat. no. ET1607-75),
N-cadherin (cat. no. ET1607-37), Snail (cat. no. ER1706-22), GSK-3β
(cat. no. ET1607-71) and phosphorylated (p-)GSK-3β (all 1:1,000;
all HuaBio; cat. no. ET1607-60). Membranes were incubated with
HRP-conjugated secondary antibodies (1:20,000; HuaBio; cat. no.
HA1023) for 1 h at room temperature. Protein bands were visualized
using ECL reagents (Biosharp Life Sciences) and analyzed with
ImageJ, using GAPDH (1:5,000, HuaBio; cat. no. HA721136) as the
internal control.
RNA sequencing
Total RNA was extracted using TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.), and RNA integrity was confirmed
using an Agilent 2100 Bioanalyzer. RNA libraries were prepared
using the NEBNext Ultra RNA Library Prep kit (cat. no. #7530; New
England Biolabs) and subjected to paired-end sequencing on an
Illumina NovaSeq 6000 platform (Gene Denovo) with a 150 bp read
length and a 20 pM loading concentration. To ensure high-quality
data for assembly and analysis, raw reads were filtered using fastp
v0.18.0 (31) to remove adapters
and low-quality bases, yielding high-quality clean reads.
Bioinformatics analysis
Differentially expressed genes (DEGs) were analyzed
using the Omicsmart platform (omicsmart.com), applying false
discovery rate (FDR) <0.05 and absolute fold-change >2. Gene
Set Enrichment Analysis (GSEA) was performed to identify signaling
pathways and molecular functions significantly enriched in the gene
expression profile. EMT-associated gene sets were obtained from
EMTome (emtome.org). Functional associations were explored by
construction of protein-protein interaction (PPI) networks using
the STRING database (string-db.org).
Immunohistochemistry and HE
staining
Fresh tissue was fixed in 10% formalin for 24 h at
room temperature and paraffin-embedded. Sections (4 µm thickness)
were deparaffinized in xylene for 20 min and rehydrated through a
graded ethanol series. Heat-induced antigen retrieval was conducted
in citrate buffer (pH 6.0) at 92–98°C for 10 min. After endogenous
peroxidase activity was blocked with 3% hydrogen peroxide, sections
were incubated with 5% normal goat serum (ZSGB-BIO; cat. no.
ZLI-9056) for 30 min at 37°C to block non-specific binding.
Subsequently, the sections were incubated overnight at 4°C with
primary antibodies against ILK (1:100, Abcam; cat. no. ab76468),
S100A4 (1:100, Proteintech Group, Inc.; cat. no. 16105-1-AP),
E-cadherin (1:100; cat. no. ET1607-75), N-cadherin (1:10,000; cat.
no. ET1607-37) and Snail (1:100; cat. no. ER1706-22; all HuaBio).
After washing with PBS, the sections were incubated with a
ready-to-use, enhanced horseradish peroxidase (HRP)-labeled goat
anti-rabbit IgG polymer (ZSGB-BIO; cat. no. PV-9000) at 37°C for 40
min. Signal detection was carried out using DAB chromogen
(ZSGB-BIO; cat. no. ZLI-9017), and nuclei were counterstained with
hematoxylin for 3 min at room temperature. Slides were examined
under a light microscope. Image analysis was performed using ImageJ
software (v1.54, National Institutes of Health). For HE) staining,
serial sections were prepared from the same paraffin-embedded
tissue blocks. Briefly, after standard deparaffinization and
rehydration, sections were stained with hematoxylin for 5 min,
followed by eosin for 2 min. The slides were then dehydrated,
cleared in xylene for morphological evaluation. A total of two
independent pathologists, blinded to the clinical data, performed
the evaluation. A total of five randomly selected fields of view
were examined under high-power magnification, and the pathologists
recorded the percentage of cells at each intensity level (0,
negative; 1, weak; 2, moderate and 3, strong) for both ILK and
S100A4. The H-score was calculated as: [0 × (% of cells with score
0) + 1 × (% of cells with score 1) + 2 × (% of cells with score 2)
+ 3 × (% of cells with score 3)] (32).
Statistical analysis
All statistical analyses were conducted using SPSS
version 27.0 (IBM Corp.). Data are presented as the mean ± SD of at
least three independent biological replicates. The normality of
data distribution was assessed using the Shapiro-Wilk test.
Associations between S100A4 expression and clinicopathological
features were evaluated using the independent samples unpaired
t-test or one-way ANOVA followed by Tukey's post hoc test. The
correlation between ILK and S100A4 expression was validated using
Spearman correlation analysis. Graphs and data visualizations were
generated using GraphPad Prism version 10.0 (Dotmatics, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
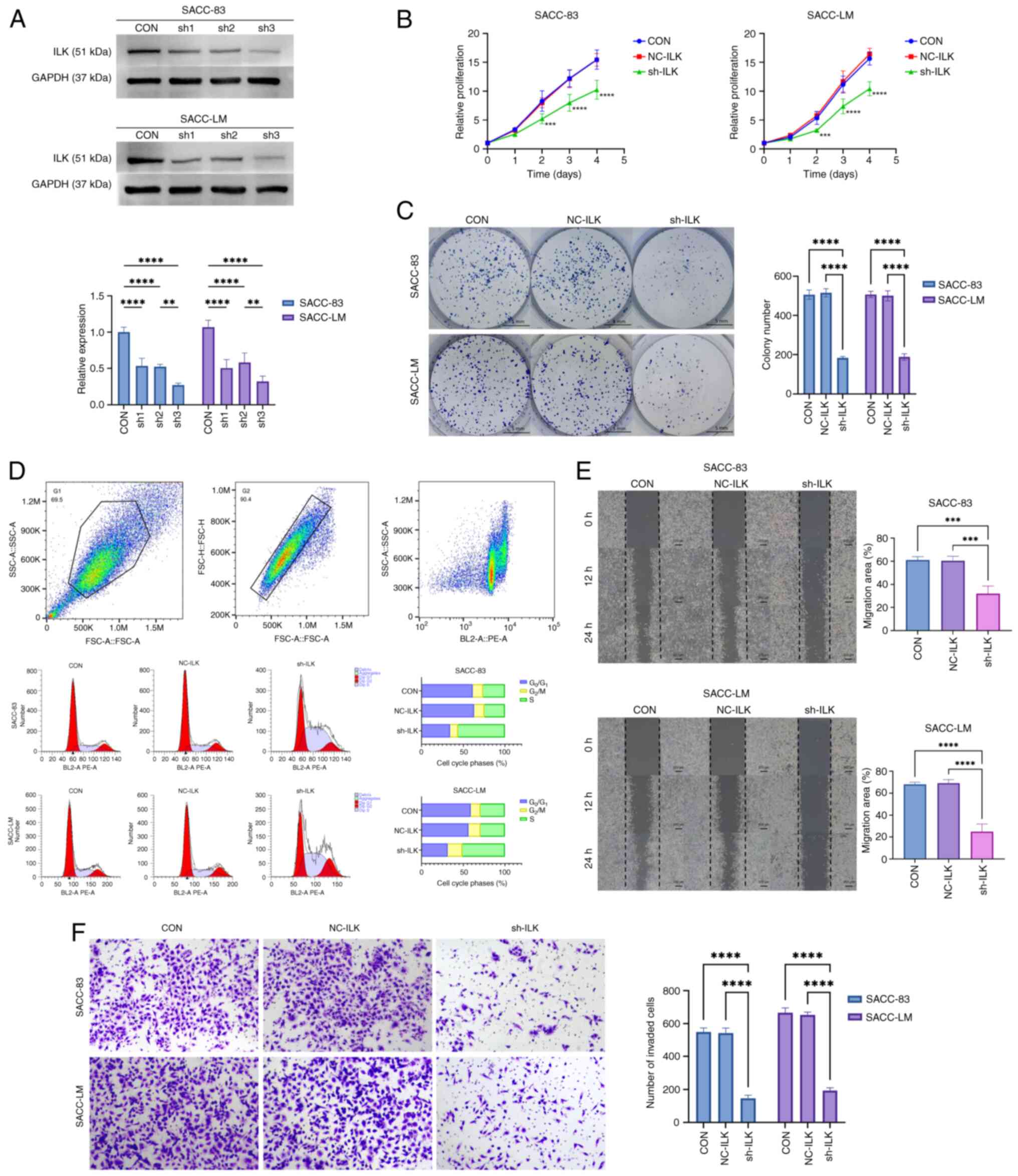
Knockdown ILK suppresses malignant
phenotypes in SACC cells
To investigate the biological function of ILK in
SACC cells, shRNA was used to stably knock down ILK expression. The
shRNA-3 sequence demonstrated the best knockdown efficiency, as
confirmed by western blotting, and was selected for subsequent
functional experiments (Fig. 1A).
ILK knockdown markedly suppressed cell proliferation, as
demonstrated by CCK-8 assay (Fig.
1B), and decreased the colony-forming ability of SACC cells
(Fig. 1C), compared with both the
CON and NC groups. Flow cytometric analysis of the cell cycle
indicated a decreased G1 and an increased S population in
ILK-knockdown cells (Fig. 1D),
suggesting an enhanced G1 to S transition and an S phase blockade,
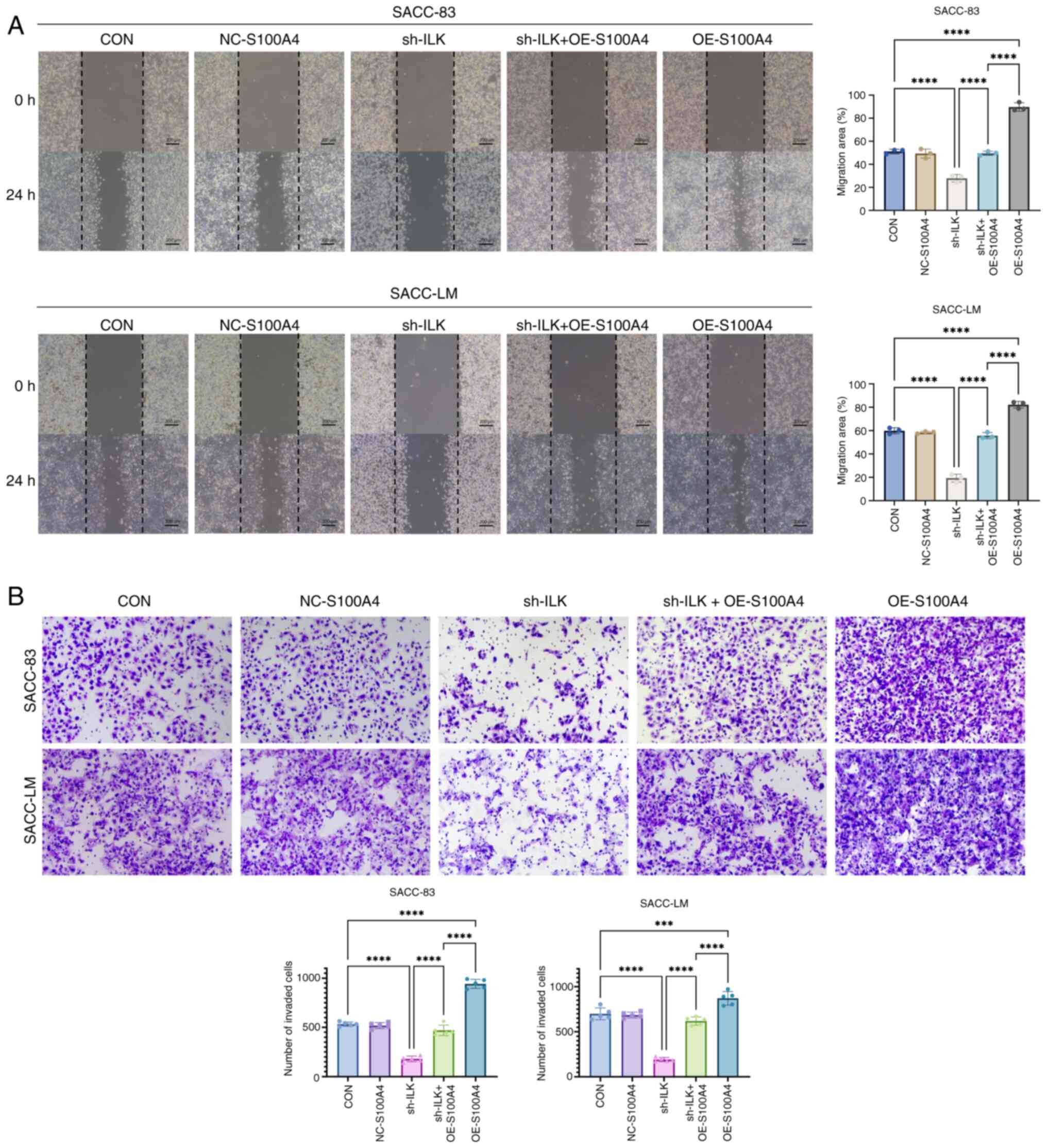
which hindered cellular DNA replication. In addition, wound healing
(Fig. 1E) and Transwell invasion
assays (Fig. 1F) showed that
ILK-knockdown cells exhibited significantly decreased migratory and
invasive capacities. Together, these findings indicated ILK was a
key regulator of SACC cell proliferation, colony formation,
migration and invasion, highlighting its potential role in
promoting tumor progression.
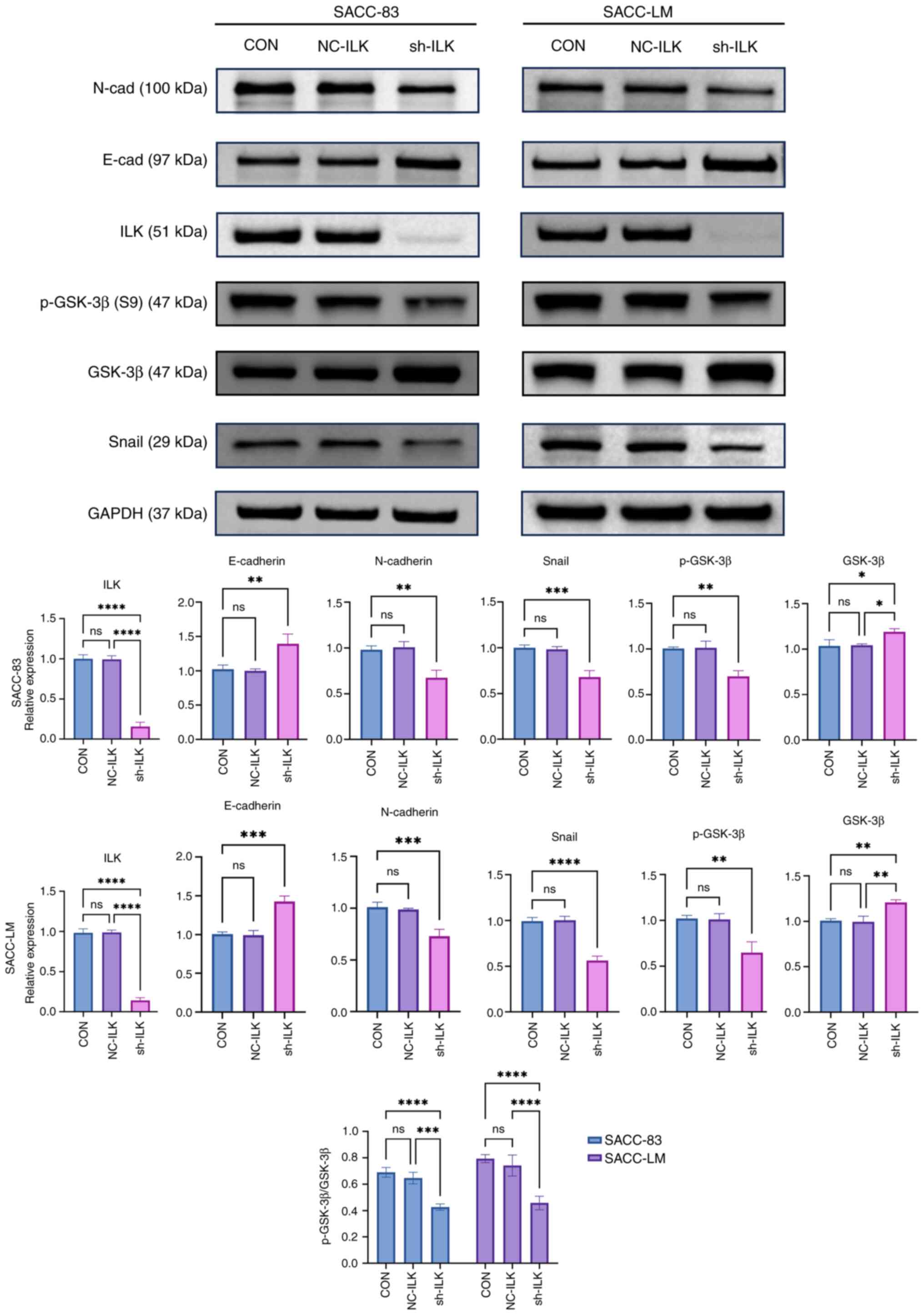
ILK regulates EMT progression in SACC
cells via the GSK-3β/Snail signaling pathway
EMT is characterized by the downregulation of the
epithelial marker E-cadherin and upregulation of the mesenchymal
marker N-cadherin; this is primarily controlled by transcription
factors (6). Building on previous
findings demonstrating a correlation between ILK and EMT markers in
SACC (14), the present study
examined the expression of key EMT markers following ILK knockdown.
ILK knockdown significantly increased E-cadherin expression while
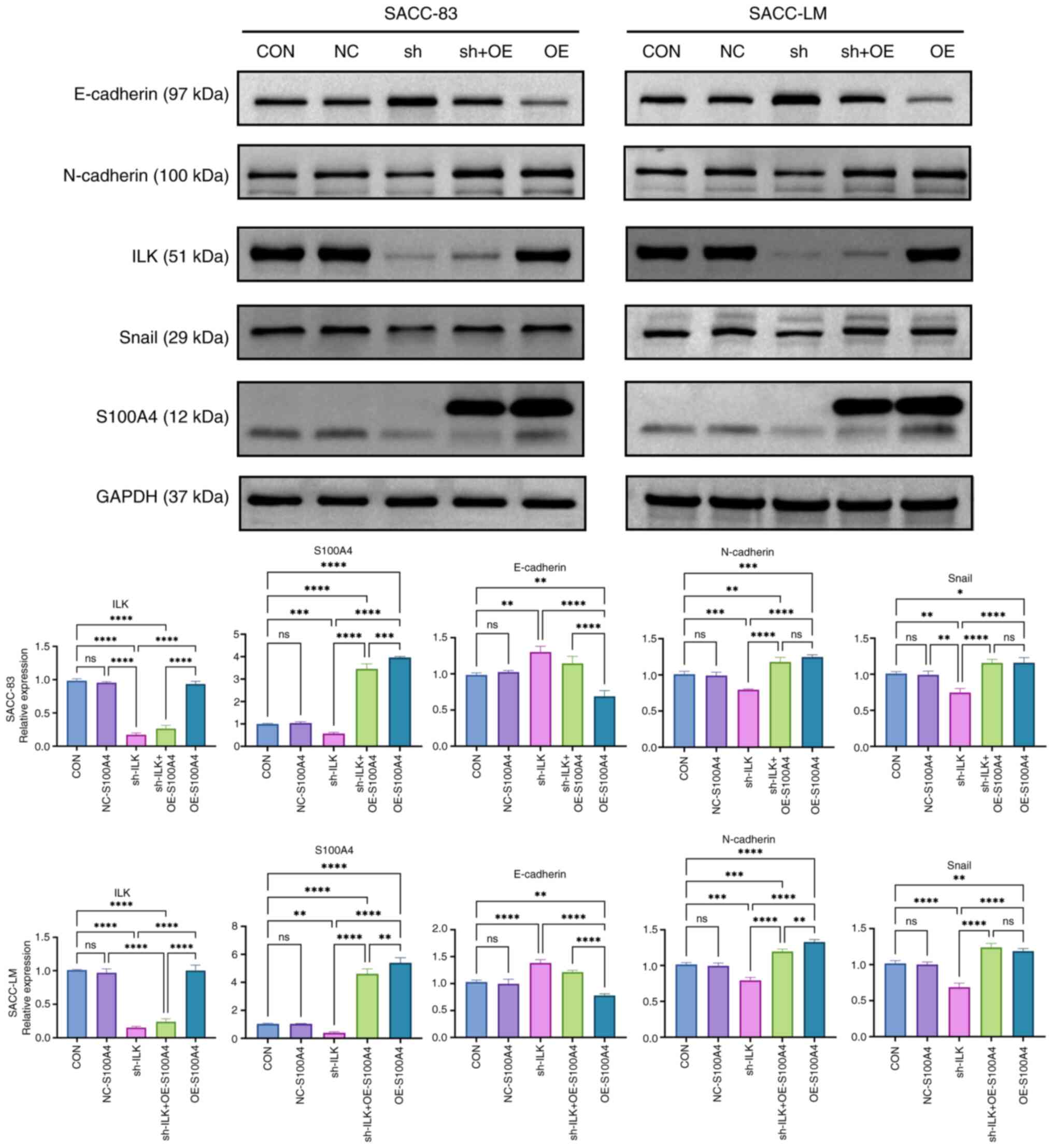
decreasing N-cadherin and the transcription factor Snail (Fig. 2). GSK-3β regulates the expression of
Snail protein via phosphorylation, thereby modulating EMT (33). ILK knockdown significantly decreased
the ratio of p-GSK-3β to total GSK-3β. These results suggest that
ILK may modulate EMT in SACC cells via the GSK-3β/Snail pathway,
and that its inhibition could effectively block this process.
ILK as a key regulator of EMT in
SACC
RNA sequencing was conducted on ILK-knockdown SACC
cells. Principal component analysis confirmed high reproducibility
among biological replicates (Fig.
S1A). Analysis of differential gene expression revealed 288
DEGs in SACC-83 cells and 134 in SACC-LM cells (Fig. S1B). The successful knockdown of ILK
was confirmed in both cell lines (Fig.
S1C). Additionally, a heatmap was generated to visualize the
hierarchical clustering of the top 50 DEGs, as ranked by FDR
(Fig. S1D).
GSEA revealed that ILK knockdown led to the
downregulation of cell cycle-associated pathways and enrichment of
pathways associated with epithelial phenotypes in both SACC-83-SH
and SACC-LM-SH cells (Fig. 3A).
Notably, the upregulated pathways showed negative correlations with
EMT signatures, providing direct evidence of EMT suppression. In
addition, pathways involving negative regulators of cell migration
and motility were enriched in SACC-LM-SH cells. These coordinated
transcriptomic changes suggested that ILK depletion enhanced
intercellular adhesion and suppressed cell motility, consistent
with in vitro findings.
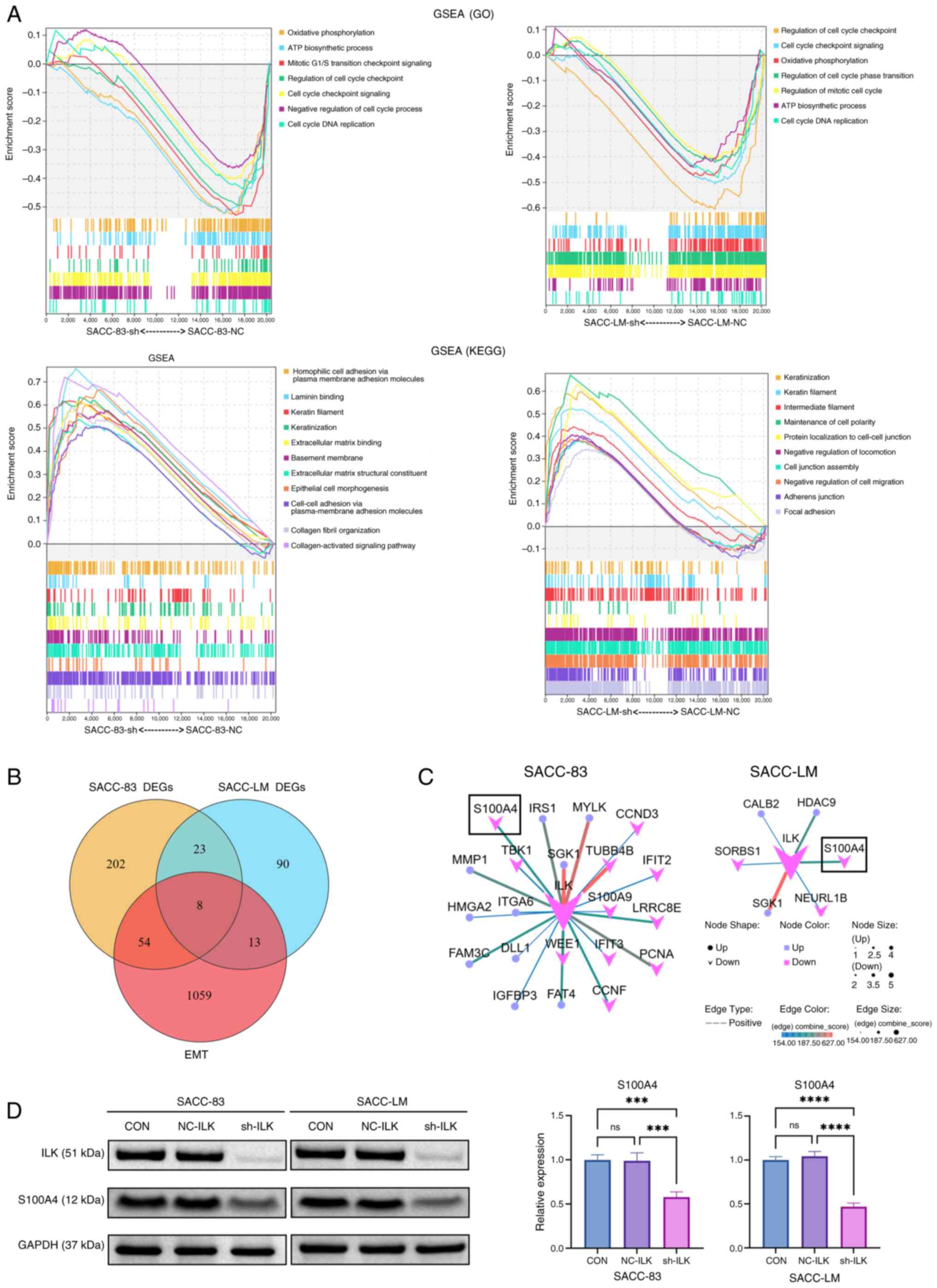 | Figure 3.Bioinformatics profiling and western
blotting identified S100A4 as a downstream effector of ILK. (A)
GSEA (P<0.05, false discovery rate <0.25) revealed that ILK
knockdown downregulated cell cycle pathways while upregulating
epithelial phenotype-associated pathways. (B) Venn diagram analysis
identified eight overlapping genes between DEGs and 1,153 EMT-core
genes from the EMTome database: CFH, CPA4, FST, ANGPTL2, LUM,
ADGRF1, ILK and S100A4. (C) Protein-protein interaction network
constructed via STRING database highlighted ILK as the central hub
node. (D) Western blot analysis validated that ILK knockdown
downregulated the protein expression of S100A4 in both cell lines.
***P<0.001, ****P<0.0001. ns not significant; ILK,
integrin-linked kinase; GSEA, Gene Set Enrichment Analysis; DEG,
differentially expressed gene; CFH, Complement Factor H; CPA,
Carboxypeptidase A; FST, Follistatin; ANGPTL, Angiopoietin-like
Protein; LUM, Lumican; ADGRF, Adhesion G protein-coupled receptor
F; NC, negative control; CON, blank control; sh, short hairpin;
EMT, epithelial-mesenchymal transition; GO, Gene Ontology; KEGG,
Kyoto Encyclopedia of Genes and Genomes; SACC, salivary adenoid
cystic carcinoma. |
S100A4 is a key downstream effector of
ILK in EMT regulation
To elucidate the molecular mechanisms underlying
ILK-mediated EMT in SACC, the present study integrated DEGs from
two distinct SACC cell lines with 1,153 experimentally validated
EMT core genes from the EMTome database (34). Venn diagram analysis revealed eight
overlapping candidate genes: Complement Factor H), CPA4
(Carboxypeptidase A4), FST (Follistatin), ANGPTL2
(Angiopoietin-like Protein 2), LUM (Lumican), ADGRF1 (Adhesion G
protein-coupled receptor F1), ILK and S100A4 (Fig. 3B). PPI network analysis using the
STRING database highlighted S100A4 as a significantly
co-downregulated gene in ILK-knockdown cells (Fig. 3C). These findings were validated by
western blot analysis, which confirmed that S100A4 expression
markedly decreased following ILK knockdown in both SACC cell lines
(Fig. 3D).
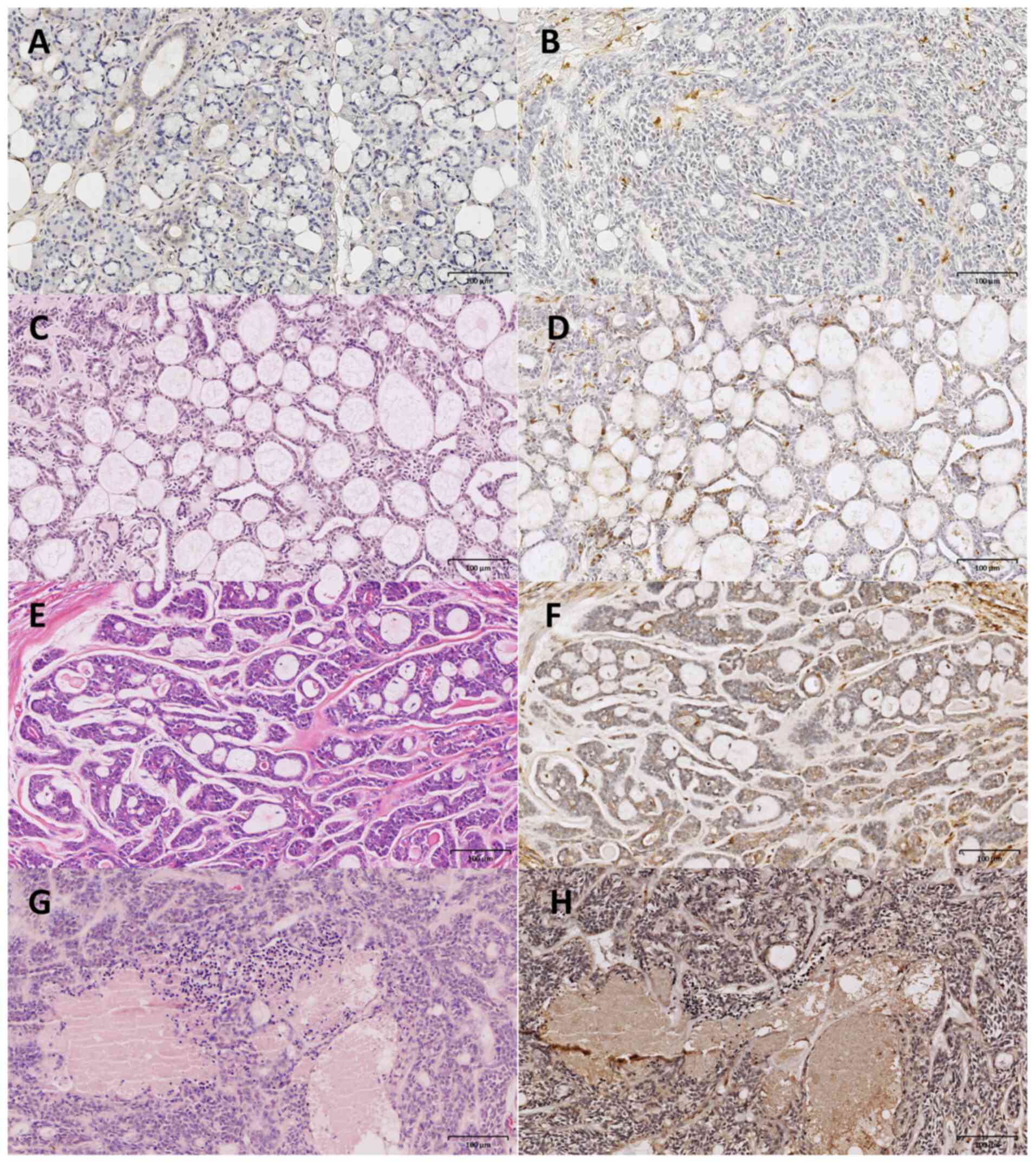
S100A4 expression is associated with
aggressive clinicopathological features and ILK expression in
SACC
S100A4 expression was nearly undetectable in the
acinar cells of normal salivary glands (Fig. 4A). Among 52 SACC specimens, 39 cases
exhibited S100A4 positivity, while 13 cases demonstrated no
observable S100A4 expression (Fig.
4B). By contrast, S100A4 expression was observed in both
tumor-derived myoepithelial (Fig. 4C
and D) and ductal epithelial cells (Fig. 4E and F), particularly in the solid
subtype of SACC, which is associated with the worse prognosis
(Fig. 4G and H). S100A4 expression
showed no significant association with patient age, sex, primary
tumor location or maximal tumor diameter (Table I). By contrast, S100A4 levels were
significantly associated with clinicopathological markers of
aggressive disease. Tumors classified as advanced stage (III/IV)
demonstrated significantly higher S100A4 expression compared with
early-stage (I/II) cases. Similarly, the solid histological subtype
exhibited stronger S100A4 positivity than either tubular or
cribriform subtypes. Furthermore, cases with perineural invasion
presented more intense staining of S100A4. These findings
demonstrated an association between S100A4 expression and adverse
clinicopathological features, underscoring its potential value as a
prognostic biomarker for assessing tumor aggressiveness in
SACC.
 | Table I.Association between S100A4 expression
and clinicopathological features of patients with salivary adenoid
cystic carcinoma. |
Table I.
Association between S100A4 expression
and clinicopathological features of patients with salivary adenoid
cystic carcinoma.
| Clinicopathological
feature | Cases (n=52) | Mean S100A4
expression | P-value |
|---|
| Age, years |
|
|
|
|
≤60 | 29 | 148.0±48.4 | 0.463 |
|
>60 | 23 | 158.8±56.3 |
|
| Sex |
|
|
|
|
Male | 21 | 151.2±54.0 | 0.861 |
|
Female | 31 | 153.8±51.1 |
|
| Tumor site |
|
|
|
| Minor
salivary gland | 25 | 151.5±52.5 | 0.863 |
| Major
salivary gland | 27 | 154.0±52.1 |
|
| Tumor diameter,
cm |
|
|
|
| ≤2 | 18 | 161.4±37.3 | 0.207 |
| >2,
≤4 | 23 | 140.1±42.4 |
|
|
>4 | 11 | 165.2±61.5 |
|
| Clinical stage |
|
|
|
|
I+II | 27 | 134.1±42.9 | 0.002 |
|
III+IV | 25 | 173.0±41.1 |
|
| Histological
subtype |
|
|
|
|
Tubulara,b | 17 | 140.7±37.7 | 0.023 |
|
Cribriformc | 21 | 143.7±45.8 |
|
|
Solid | 14 | 181.1±46.1 |
|
| Perineural
invasion |
|
|
|
|
Positive | 24 | 185.4±37.6 | <0.001 |
|
Negative | 28 | 124.8±32.1 |
|
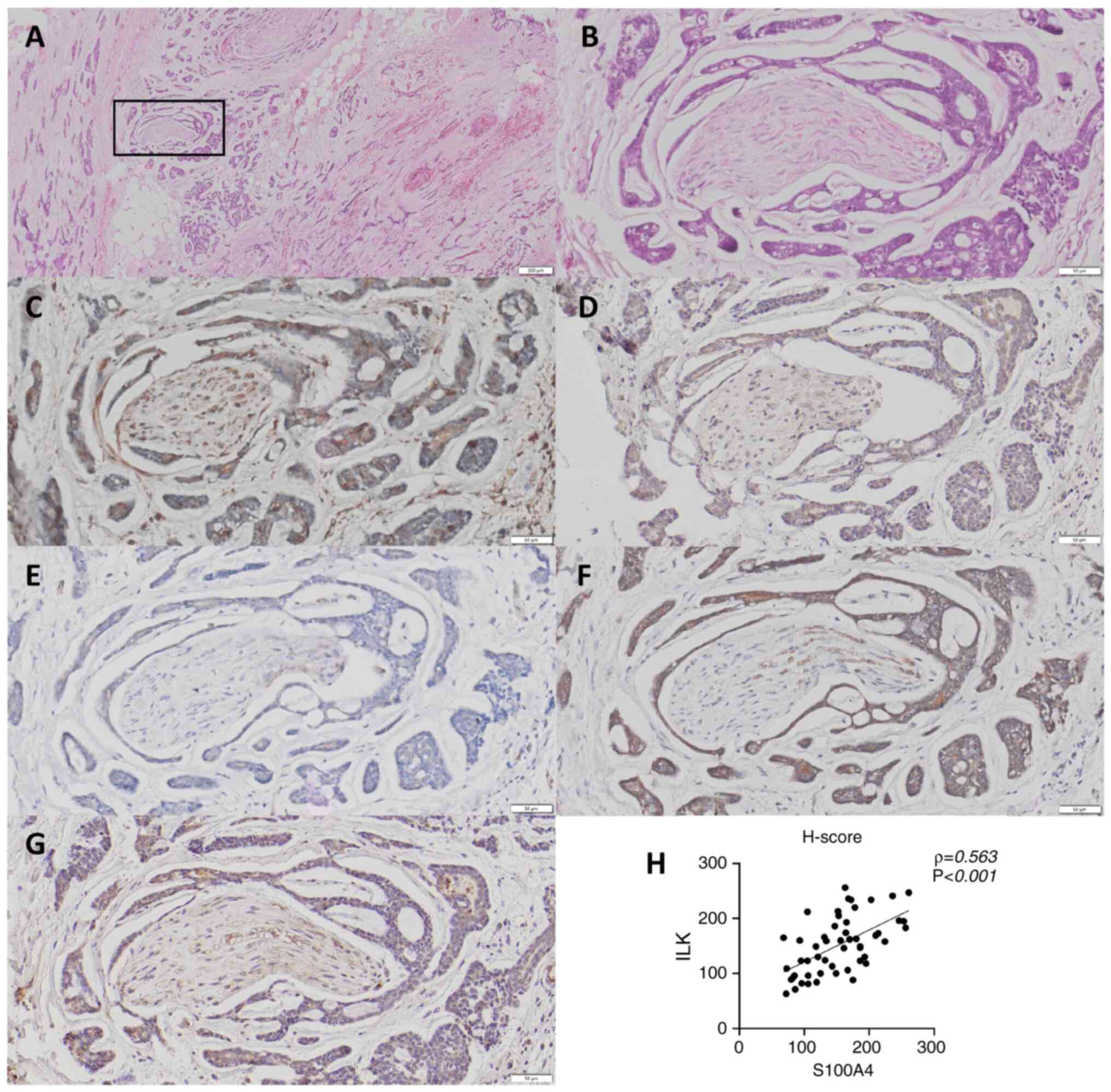
ILK expression was undetectable in normal salivary
gland tissue, with the exception of partial immunoreactivity in
ductal epithelial cells (17). By
contrast, S100A4 and ILK expression was observed in the cytoplasm
of tumor cells as well as in surrounding stromal cells of SACC
tissue. Immunohistochemical analysis revealed that tumor cells at
the invasive front surrounding nerves frequently exhibited a
spindle-shaped or fusiform morphology (Fig. 5A and B), accompanied by enhanced
expression of S100A4 (Fig. 5C), ILK
(Fig. 5D), N-cadherin (Fig. 5F) and Snail (Fig. 5G), along with decreased expression
of E-cadherin (Fig. 5E).
Immunohistochemical evaluation using H-score assessment
demonstrated a significant positive correlation between S100A4 and
ILK expression in tumor tissue (ρ=0.563; Fig. 5H).
S100A4 mediates ILK-regulated EMT
progression via Snail restoration
To elucidate the regulatory association between ILK
and S100A4, the present study established cell models with combined
ILK knockdown and S100A4 overexpression. Western blot analysis of
EMT markers demonstrated that S100A4 overexpression led to
decreased E-cadherin and elevated N-cadherin levels, effectively
reversing the suppression of EMT caused by ILK knockdown (Fig. 6). Notably, the expression of Snail
protein was restored, suggesting S100A4 may regulate Snail
expression. Functional assays demonstrated that S100A4
overexpression enhanced both the migratory and invasive
capabilities of SACC cells and effectively reversed the inhibitory
effects caused by ILK depletion (Fig.
7A and B). In summary, ILK knockdown may inhibit the
phosphorylation of GSK-3β, leading to downregulation of S100A4 and
Snail expression, which decreases N-cadherin and increases
E-cadherin levels. Overexpression of S100A4 restored Snail
expression and re-induced the EMT phenotype, thereby attenuating
the inhibitory effect of ILK knockdown on EMT progression.
Discussion
Salivary adenoid cystic carcinoma accounts for ~1%
of all head and neck malignancies (35,36).
Although it typically exhibits an indolent growth pattern, the
long-term prognosis for SACC remains poor (37). Once metastasis occurs, the 10-year
survival rate is 10%. EMT in SACC is associated with solid subtype
(38), tumor progression,
perineural invasion, recurrence and distant metastasis (39). Although it is challenging to define
EMT features in SACC using HE staining alone, the present study
demonstrated that tumor cells at the perineural invasion front
frequently exhibited a spindle-shaped morphology, accompanied by
decreased E-cadherin expression and increased expression of
N-cadherin and Snail. The present study further demonstrated that
ILK knockdown significantly attenuated the migratory and invasive
capabilities of SACC cells, concurrent with upregulated E-cadherin
expression and downregulated N-cadherin and Snail levels. These
changes were consistent with the results of our previous
clinicopathological study (17).
These findings suggested that ILK may serve a key role in promoting
EMT in SACC.
To identify the mechanism by which ILK participates
in EMT, the present study conducted transcriptomic screening and
identified S100A4 as a key target. In vitro results were
supported by GSEA. Although previous studies have demonstrated the
involvement of S100A4 in the invasion and metastasis of numerous
types of cancer (40,41), to the best of our knowledge, its
specific role in SACC has not been reported. The present
clinicopathological analysis revealed that high S100A4 expression
was significantly associated with advanced clinical stage, solid
histological subtype and perineural invasion, suggesting its
potential as a molecular marker of aggressive tumor behavior. While
both ILK and S100A4 were nearly undetectable in normal salivary
gland tissue, consistent with previous reports (17,42),
their expression in SACC tumors was implicated in the promotion of
local and perineural invasion.
To clarify the role of S100A4 in ILK-driven EMT, the
present study examined the expression of S100A4 in ILK-knockdown
cell lines. S100A4 expression was significantly downregulated in
ILK-knockdown cells. When S100A4 expression was induced in
ILK-knockdown cells, the expression of Snail and N-cadherin was
markedly enhanced, while E-cadherin expression was downregulated.
The impaired invasion and migration phenotypes of SACC cells were
restored. These findings suggested that S100A4 serves a key role in
ILK-driven EMT. Studies have indicated that ILK promotes the
stabilization and nuclear translocation of β-catenin via the GSK-3β
pathway (43,44). β-catenin that enters the nucleus
forms a complex with T cell factor 4; this complex specifically
binds the promoter region of the S100A4 gene, thereby enhancing its
expression (45). Other studies
have revealed that the expression of S100A4 in cancer is
significantly associated with Snail family proteins (46) and regulates their expression
(47). ILK may regulate the
expression of S100A4 and Snail by modulating the phosphorylation of
GSK-3β, thereby influencing EMT.
The most prominent molecular characteristic of SACC
is the aberrant activation of the MYB proto-oncogene. A total of
~86% of cases harbor the characteristic MYB-nuclear factor IB gene
fusion (48). Research has shown
that MYB upregulation contributes to EMT, thereby promoting
invasion and metastasis in SACC (49). Although the association between MYB
and ILK remains unclear, MYB Proto-Oncogene Like 1) carried by
small extracellular vesicles upregulates the expression of integrin
proteins ITGB3 (Integrin Subunit Beta 3) and ITGAV (Integrin
Subunit Alpha V) (50). As a key
molecule in the integrin signaling pathway, ILK may also be
regulated by MYB. MYB and ILK modulate the expression of the
adhesion molecule ICAM-1 (Intercellular Adhesion Molecule 1)
(50,51). ICAM-1 serves a crucial role in
mediating EMT, highlighting its role in tumor metastasis (52). In summary, the integrin signaling
pathway may serve as a key bridge connecting the functions of MYB
and S100A4, and the synergistic mechanism between MYB and the
ILK-S100A4 axis requires further exploration.
Through clinicopathological correlation analysis and
in vitro experiments, the present study demonstrated the
functional role of the ILK-S100A4-Snail signaling axis in promoting
EMT in SACC. However, the specific molecular mechanisms by which
ILK regulates S100A4/Snail require further validation through in
vivo models and pharmacological approaches. Furthermore, the
prognostic value of ILK and S100A4 for survival in patients with
SACC remains to be elucidated.
In conclusion, S100A4 represents a potential
biomarker for predicting aggressive behavior in SACC. ILK may
facilitate tumor invasion and metastasis by modulating S100A4/Snail
expression via GSK-3β, leading to EMT. These findings suggest dual
targeting of ILK and S100A4 may offer synergistic therapeutic
benefits and represent a promising strategy to inhibit tumor
invasion in SACC.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank the Oral &
Maxillofacial Reconstruction and Regeneration of Luzhou Key
Laboratory (Luzhou, China) for providing the experimental site.
Funding
The present study was supported by The Scientific Research
Project of Southwest Medical University (grant no. 2021ZKMS017),
the Scientific Research Foundation of the Affiliated Stomatological
Hospital of Southwest Medical University (grant no. 2021BS01) and
Southwest Medical University Stomatology Special Program (grant
nos. 2024KQZX18 and 2024KQZX20).
Availability of data and materials
The data generated in the present study may be found
in the NCBI Sequence Read Archive (SRA) under BioProject accession
number PRJNA1320937 or at the following URL: www.ncbi.nlm.nih.gov/sra/PRJNA1320937.
Authors' contributions
YY conceived the study, designed and performed
experiments and wrote the manuscript. JL designed the experiments.
LY constructed figures and performed experiments. YL analyzed data.
KG performed the experiments. DZ conceived the study and edited the
manuscript. YY and DZ confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board and Ethics Committee of The Affiliated Stomatological
Hospital of Southwest Medical University (approval no. 20211129004;
Luzhou, China). Informed consent was obtained in writing from all
patients before the tissue collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
van der Wal JE, Becking AG, Snow GB and
van der Waal I: Distant metastases of adenoid cystic carcinoma of
the salivary glands and the value of diagnostic examinations during
follow-up. Head Neck. 24:779–783. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
da Cruz Perez DE, de Abreu Alves F, Nobuko
Nishimoto I, de Almeida OP and Kowalski LP: Prognostic factors in
head and neck adenoid cystic carcinoma. Oral Oncol. 42:139–146.
2006. View Article : Google Scholar
|
|
3
|
Gao M, Hao Y, Huang MX, Ma DQ, Luo HY, Gao
Y, Peng X and Yu GY: Clinicopathological study of distant
metastases of salivary adenoid cystic carcinoma. Int J Oral
Maxillofac Surg. 42:923–928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kokemueller H, Eckardt A, Brachvogel P and
Hausamen JE: Adenoid cystic carcinoma of the head and neck-a 20
year experience. Int J Oral Maxillofac Surg. 33:25–31. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Avery CM, Moody AB, McKinna FE, Taylor J,
Henk JM and Langdon JD: Combined treatment of adenoid cystic
carcinoma of the salivary glands. Int J Oral Maxillofac Surg.
29:277–279. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brabletz T, Kalluri R, Nieto MA and
Weinberg RA: EMT in cancer. Nat Rev Cancer. 18:128–134. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y, Chen L, Jiang D, Luan L, Huang J,
Hou Y and Xu C: HER2 promotes epithelial-mesenchymal transition
through regulating osteopontin in gastric cancer. Pathol Res Pract.
227:1536432021. View Article : Google Scholar
|
|
8
|
Dongre A and Weinberg RA: New insights
into the mechanisms of epithelial-mesenchymal transition and
implications for cancer. Nat Rev Mol Cell Biol. 20:69–84. 2019.
View Article : Google Scholar
|
|
9
|
Naves MA, Requião-Moura LR, Soares MF,
Silva-Júnior JA, Mastroianni-Kirsztajn G and Teixeira VPC: Podocyte
Wnt/ß-catenin pathway is activated by integrin-linked kinase in
clinical and experimental focal segmental glomerulosclerosis. J
Nephrol. 25:401–409. 2012. View Article : Google Scholar
|
|
10
|
Legate KR, Montañez E, Kudlacek O and
Fässler R: ILK, PINCH and parvin: The tIPP of integrin signalling.
Nat Rev Mol Cell Biol. 7:20–31. 2006. View
Article : Google Scholar
|
|
11
|
Jia YY, Yu Y and Li HJ: POSTN promotes
proliferation and epithelial-mesenchymal transition in renal cell
carcinoma through ILK/AKT/mTOR pathway. J Cancer. 12:4183–4195.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsirtsaki K and Gkretsi V: The focal
adhesion protein integrin-linked kinase (ILK) as an important
player in breast cancer pathogenesis. Cell Adh Migr. 14:204–213.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu S, Jin Z, Cao M, Hao D, Li C, Li D and
Zhou W: Periostin regulates osteogenesis of mesenchymal stem cells
from ovariectomized rats through actions on the ILK/Akt/GSK-3β
axis. Genet Mol Biol. 44:e202004612021. View Article : Google Scholar
|
|
14
|
Akrida I, Nikou S, Gyftopoulos K, Argentou
M, Kounelis S, Zolota V, Bravou V and Papadaki H: Expression of EMT
inducers integrin-linked kinase (ILK) and ZEB1 in phyllodes breast
tumors is associated with aggressive phenotype. Histol Histopathol.
33:937–949. 2018.
|
|
15
|
Yuan Y, Xiao Y, Li Q, Liu Z, Zhang X, Qin
C, Xie J, Wang X and Xu T: In vitro and in vivo effects of short
hairpin RNA targeting integrin-linked kinase in prostate cancer
cells. Mol Med Rep. 8:419–424. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsoumas D, Nikou S, Giannopoulou E,
Champeris Tsaniras S, Sirinian C, Maroulis I, Taraviras S, Zolota
V, Kalofonos HP and Bravou V: ILK expression in colorectal cancer
is associated with EMT, cancer stem cell markers and
chemoresistance. Cancer Genomics Proteomics. 15:127–141.
2018.PubMed/NCBI
|
|
17
|
Zhao D, Yang K, Tang XF, Lin NN and Liu
JY: Expression of integrin-linked kinase in adenoid cystic
carcinoma of salivary glands correlates with epithelial-mesenchymal
transition markers and tumor progression. Med Oncol. 30:6192013.
View Article : Google Scholar
|
|
18
|
Dahlmann M, Kobelt D, Walther W, Mudduluru
G and Stein U: S100A4 in cancer metastasis: Wnt signaling-driven
interventions for metastasis restriction. Cancers (Basel).
8:592016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chow KH, Park HJ, George J, Yamamoto K,
Gallup AD, Graber JH, Chen Y, Jiang W, Steindler DA, Neilson EG, et
al: S100A4 is a biomarker and regulator of glioma stem cells that
is critical for mesenchymal transition in glioblastoma. Cancer Res.
77:5360–5373. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bresnick AR, Weber DJ and Zimmer DB: S100
proteins in cancer. Nat Rev Cancer. 15:96–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grigorian M, Andresen S, Tulchinsky E,
Kriajevska M, Carlberg C, Kruse C, Cohn M, Ambartsumian N,
Christensen A, Selivanova G and Lukanidin E: Tumor suppressor p53
protein is a new target for the metastasis-associated Mts1/S100A4
protein: Functional consequences of their interaction. J Biol Chem.
276:22699–22708. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Semov A, Moreno MJ, Onichtchenko A,
Abulrob A, Ball M, Ekiel I, Pietrzynski G, Stanimirovic D and
Alakhov V: Metastasis-associated protein S100A4 induces
angiogenesis through interaction with Annexin II and accelerated
plasmin formation. J Biol Chem. 280:20833–20841. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pedersen KB, Nesland JM, Fodstad Ø and
Maelandsmo GM: Expression of S100A4, E-cadherin, alpha- and
beta-catenin in breast cancer biopsies. Br J Cancer. 87:1281–1286.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang J, Gu Y, Liu X, Rao X, Huang G and
Ouyang Y: Clinicopathological and prognostic value of S100A4
expression in non-small cell lung cancer: A meta-analysis. Biosci
Rep. 40:BSR202017102020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fei F, Qu J, Zhang M, Li Y and Zhang S:
S100A4 in cancer progression and metastasis: A systematic review.
Oncotarget. 8:73219–73239. 2017. View Article : Google Scholar
|
|
26
|
Zhou W, Peng Z, Zhang C, Liu S and Zhang
Y: ILK-induced epithelial-mesenchymal transition promotes the
invasive phenotype in adenomyosis. Biochem Biophys Res Commun.
497:950–956. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hua T, Liu S, Xin X, Cai L, Shi R, Chi S,
Feng D and Wang H: S100A4 promotes endometrial cancer progress
through epithelial-mesenchymal transition regulation. Oncol Rep.
35:3419–3426. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Szanto PA, Luna MA, Tortoledo ME and White
RA: Histologic grading of adenoid cystic carcinoma of the salivary
glands. Cancer. 54:1062–1069. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang SH and O'Sullivan B: Overview of the
8th edition TNM classification for head and neck cancer. Curr Treat
Options Oncol. 18:402017. View Article : Google Scholar
|
|
30
|
Wang L, Wang Y, Bian H, Pu Y and Guo C:
Molecular characteristics of homologous salivary adenoid cystic
carcinoma cell lines with different lung metastasis ability. Oncol
Rep. 30:207–212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen S, Zhou Y, Chen Y and Gu J: fastp: An
ultra-fast all-in-one FASTQ preprocessor. Bioinformatics.
34:i884–i890. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hirsch FR, Varella-Garcia M, Bunn PA Jr,
Di Maria MV, Veve R, Bremmes RM, Barón AE, Zeng C and Franklin WA:
Epidermal growth factor receptor in non-small-cell lung carcinomas:
Correlation between gene copy number and protein expression and
impact on prognosis. J Clin Oncol. 21:3798–3807. 2003. View Article : Google Scholar
|
|
33
|
Zhou BP, Deng J, Xia W, Xu J, Li YM,
Gunduz M and Hung MC: Dual regulation of Snail by
GSK-3beta-mediated phosphorylation in control of
epithelial-mesenchymal transition. Nat Cell Biol. 6:931–940. 2004.
View Article : Google Scholar
|
|
34
|
Vasaikar SV, Deshmukh AP, den Hollander P,
Addanki S, Kuburich NA, Kudaravalli S, Joseph R, Chang JT,
Soundararajan R and Mani SA: EMTome: A resource for pan-cancer
analysis of epithelial-mesenchymal transition genes and signatures.
Br J Cancer. 124:259–269. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
de Sousa LG, Jovanovic K and Ferrarotto R:
Metastatic adenoid cystic carcinoma: Genomic landscape and emerging
treatments. Curr Treat Options Oncol. 23:1135–1150. 2022.
View Article : Google Scholar
|
|
36
|
Ayoub N, Nozhy A, Shawki A, Hassouna A,
Ibraheem D, Elmahdy M and Amin A: Management of adenoid cystic
carcinoma of the head and neck: Experience of the national cancer
institute, Egypt. Gulf J Oncolog. 1:63–69. 2022.PubMed/NCBI
|
|
37
|
Laurie SA, Ho AL, Fury MG, Sherman E and
Pfister DG: Systemic therapy in the management of metastatic or
locally recurrent adenoid cystic carcinoma of the salivary glands:
A systematic review. Lancet Oncol. 12:815–824. 2011. View Article : Google Scholar
|
|
38
|
Sangala BN, Raghunath V, Kavle P, Gupta A,
Gotmare SS and Andey VS: Evaluation of immunohistochemical
expression of E-cadherin in pleomorphic adenoma and adenoid cystic
carcinoma. J Oral Maxillofac Pathol. 26:65–71. 2022. View Article : Google Scholar
|
|
39
|
Hoch CC, Stögbauer F and Wollenberg B:
Unraveling the role of epithelial-mesenchymal transition in adenoid
cystic carcinoma of the salivary glands: A comprehensive review.
Cancers (Basel). 15:28862023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ambartsumian N, Klingelhöfer J and
Grigorian M: The multifaceted S100A4 protein in cancer and
inflammation. Methods Mol Biol. 1929:339–365. 2019. View Article : Google Scholar
|
|
41
|
Mishra SK, Siddique HR and Saleem M:
S100A4 calcium-binding protein is key player in tumor progression
and metastasis: Preclinical and clinical evidence. Cancer
Metastasis Rev. 31:163–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shan C, Wei J, Hou R, Wu B, Yang Z, Wang
L, Lei D and Yang X: Schwann cells promote EMT and the Schwann-like
differentiation of salivary adenoid cystic carcinoma cells via the
BDNF/TrkB axis. Oncol Rep. 35:427–435. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Luo L, Liu H, Dong Z, Sun L, Peng Y and
Liu F: Small interfering RNA targeting ILK inhibits EMT in human
peritoneal mesothelial cells through phosphorylation of GSK-3β. Mol
Med Rep. 10:137–144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tan C, Costello P, Sanghera J, Dominguez
D, Baulida J, de Herreros AG and Dedhar S: Inhibition of integrin
linked kinase (ILK) suppresses beta-catenin-Lef/Tcf-dependent
transcription and expression of the E-cadherin repressor, snail, in
APC-/-human colon carcinoma cells. Oncogene. 20:133–140. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gong N, Shi L, Bing X, Li H, Hu H, Zhang
P, Yang H, Guo N, Du H, Xia M and Liu C: S100A4/TCF complex
transcription regulation drives epithelial-mesenchymal transition
in chronic sinusitis through Wnt/GSK-3β/β-catenin signaling. Front
Immunol. 13:8358882022. View Article : Google Scholar
|
|
46
|
Jian L, Zhihong W, Liuxing W and Qingxia
F: Role of S100A4 in the epithelial-mesenchymal transition of
esophageal squamous cell carcinoma and its molecular mechanism.
Zhonghua Zhong Liu Za Zhi. 37:258–265. 2015.(In Chinese).
|
|
47
|
Liang DP, Huang TQ, Li SJ and Chen ZJ:
Knockdown of S100A4 chemosensitizes human laryngeal carcinoma cells
in vitro through inhibition of Slug. Eur Rev Med Pharmacol Sci.
18:3484–3490. 2014.
|
|
48
|
Persson M, Andrén Y, Moskaluk CA, Frierson
HF Jr, Cooke SL, Futreal PA, Kling T, Nelander S, Nordkvist A,
Persson F and Stenman G: Clinically significant copy number
alterations and complex rearrangements of MYB and NFIB in head and
neck adenoid cystic carcinoma. Genes Chromosomes Cancer.
51:805–817. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xu LH, Zhao F, Yang WW, Chen CW, Du ZH, Fu
M, Ge XY and Li SL: MYB promotes the growth and metastasis of
salivary adenoid cystic carcinoma. Int J Oncol. 54:1579–1590.
2019.
|
|
50
|
Fu M, Gao Q, Xiao M, Li RF, Sun XY, Li SL,
Peng X and Ge XY: Extracellular vesicles containing circMYBL1
induce CD44 in adenoid cystic carcinoma cells and pulmonary
endothelial cells to promote lung metastasis. Cancer Res.
84:2484–2500. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang W, Matsukura M, Fujii I, Ito K, Zhao
JE, Shinohara M, Wang YQ and Zhang XM: Inhibition of high
glucose-induced VEGF and ICAM-1 expression in human retinal pigment
epithelium cells by targeting ILK with small interference RNA. Mol
Biol Rep. 39:613–620. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Qian WJ, Yan JS, Gang XY, Xu L, Shi S, Li
X, Na FJ, Cai LT, Li HM and Zhao MF: Intercellular adhesion
molecule-1 (ICAM-1): From molecular functions to clinical
applications in cancer investigation. Biochim Biophys Acta Rev
Cancer. 1879:1891872024. View Article : Google Scholar : PubMed/NCBI
|