Introduction
The cancer with the highest mortality rate globally
is lung cancer, accounting for ~18.7% of all cancer-associated
deaths (1). Mutations in the
epidermal growth factor receptor (EGFR) are among the typical
driver gene alterations found in non-small cell lung carcinoma
(NSCLC) (2). For patients with
advanced NSCLC and sensitive EGFR mutations, tyrosine kinase
inhibitors (TKIs) are recommended as first-line therapy by
established guidelines (3–5). Targeted therapies can extend the
survival of patients with advanced NSCLC and driver gene mutations,
with life expectancy ranging from 12.9–21.9 months (6,7).
Despite the common use of first- and second-generation EGFR-TKIs in
treating patients with NSCLC, most patients eventually experience
acquired resistance, resulting in disease recurrence and
progression (8). The primary cause
of this resistance is the T790M mutation. The ASPIRATION study
demonstrated that adhering to the original targeted therapy after
disease progression whilst on erlotinib could slow down disease
progression, with a notable difference in progression-free survival
(PFS). Specifically, patients who continued with the original
therapy achieved a PFS of 14.1 months, whereas those who
discontinued the therapy experienced a shorter PFS of 11.0 months,
with corresponding median overall survival (OS) of 33.6 and 22.5
months, respectively (9). Moreover,
a study by Chaft et al (10)
reported that among 61 cases, 14 experienced disease recurrence
after discontinuing EGFR-TKIs, with a median recurrence time of 8
days. These findings underscore the possibility for the original
targeted therapy to control disease in patients who develop
resistance. Therefore, guidelines from Europe, America and China
recommend that these patients continue with the original EGFR-TKI
treatment regimen.
Certain patients exhibit oligometastasis, with
metastasis limited to a few organs or regions. Generally,
oligometastasis is categorized as inoperable stage III or IV;
however, it is a unique condition between locally advanced and
broadly disseminated stage IV. Guidelines recommend local treatment
for these patients (11), and
common local treatment methods include surgical resection,
radiation therapy, microwave ablation, radiofrequency ablation,
particle implantation and cryoablation (12,13).
Gamma Knife is a type of stereotactic body radiation
therapy (SBRT) radiotherapy technology. Through non-coplanar
rotational irradiation, the radiation converges at a single focal
point, creating an ellipsoidal high-dose region to cover the tumor
target area. The dose in the central area of the tumor is markedly
higher than that in the peripheral area. This design can more
effectively kill hypoxic cells inside the tumor that are resistant
to radiotherapy. The dose rapidly attenuates at the tumor
periphery, and the notable focusing capability results in a smaller
overall volume of normal lung tissue being irradiated and a lower
irradiation dose, making it more suitable for treating multiple
lesions. Gamma Knife is commonly used in Europe and the United
States to treat brain tumors or brain metastases. However, in
China, it is also used to treat primary and metastatic tumors in
the body (14), with several
studies indicating its notable efficacy (14–16).
In 2006, Xia et al (14)
reported marked results using Gamma Knife therapy for the treatment
of early-stage lung cancer. Furthermore, Zhang et al
(15) reported certain survival
benefits using whole-body Gamma Knife radiotherapy combined with
thermochemotherapy for locally advanced pancreatic cancer. For
patients with oligometastatic NSCLC, systemic therapy combined with
surgery or radiation treatment was reported to provide improved
treatment effect compared with systemic therapy alone (17–19).
Similarly, the adoption of first-generation EGFR-TKIs in
conjunction with local consolidation therapy (LCT) has also been
reported to demonstrate superior treatment effects compared with
the TKI-only treatment group (20–24).
However, most existing studies have focused on the combination of
EGFR-TKIs with surgical interventions or radiotherapy for
intracranial or other metastatic lesions (21,25,26).
There are few reports on the combined treatment of primary lung
lesions, with recent studies mostly concerning third-generation
EGFR-TKIs in combination therapies (27,28).
Therefore, the present retrospective study aimed to evaluate the
impact of Gamma Knife therapy on primary pulmonary lesions combined
with first-generation EGFR-TKIs treatment for advanced lung
adenocarcinoma on disease control and survival of patients.
Patients and methods
Study population
The present retrospective, single-arm study was
performed on a small cohort of 35 patients diagnosed with advanced
lung adenocarcinoma harboring EGFR-sensitive mutations. These
patients underwent both targeted therapy and radiation at the 901st
Hospital of the Joint Logistics Support Force of the People's
Liberation Army (Hefei, China) between October 2014 and November
2021. The study was approved by the Hospital Ethics Commission and
all patients signed informed consent prior to treatment.
Patient selection criteria
The inclusion criteria were as follows: i) Any sex
or age; ii) pathologically confirmed diagnosis of lung
adenocarcinoma; iii) history of post-surgical lung cancer
recurrence; iv) prior exposure to adjuvant or neoadjuvant
chemotherapy; v) genetic status indicating EGFR-sensitive
mutations, specifically exon 19 deletion or exon 21 L858R mutation;
vi) clinical staging of unresectable stage III or IV with
oligometastatic; vii) use of the first generation EGFR-TKIs as a
first-line treatment that achieved documented disease control;
viii) resistance after first-generation EGFR-TKIs as first-line
therapy, followed by Gamma Knife treatment for the primary lesions;
ix) presence of at least one measurable lesion as defined by the
Response Evaluation Criteria in Solid Tumors (RECIST) criteria
(29); and x) Eastern Cooperative
Oncology Group (ECOG) performance status (30) ranging from 0–2.
The exclusion criteria were as follows: i)
Co-existing malignancies; ii) severe underlying conditions such as
cardiovascular diseases, liver disease or kidney disease; iii)
presence of other genetic mutations, including EGFR 20 insertions,
anaplastic lymphoma kinase, hepatocyte growth factor receptor and
c-ROS oncogene 1; iv) concurrent use of other antitumor agents as
part of first-line treatment, including anti-angiogenic agents,
immunotherapy or chemotherapy; v) unmanageable adverse reactions to
first-generation EGFR-TKIs; vi) disease not controlled following
first-generation EGFR-TKI treatment due to primary resistance; and
vii) presence of widespread systemic metastases.
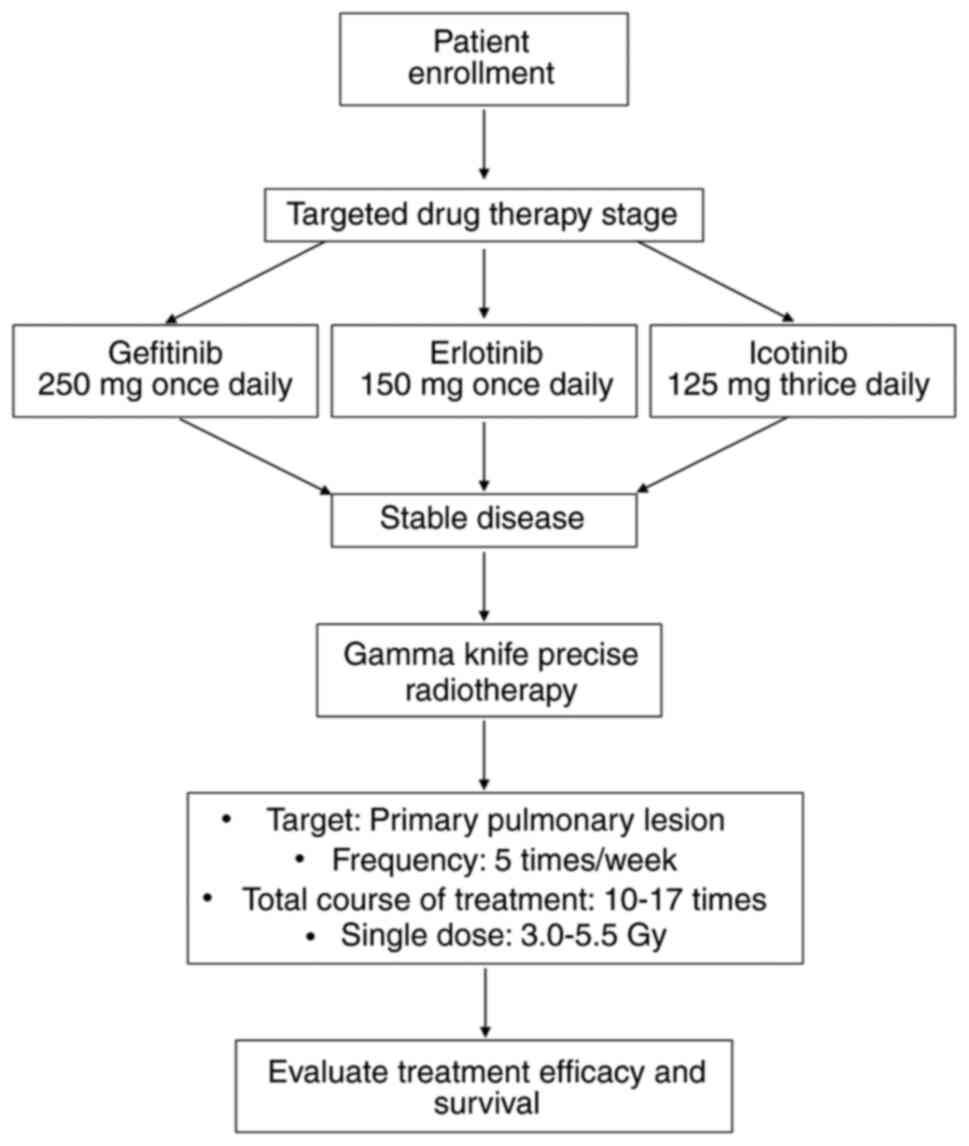
Treatment protocol
Following disease control achieved through
first-line treatment with first-generation EGFR-TKIs, lung lesions
exhibited stability or slight progression without reaching
progressive disease (PD). Consequently, patients underwent Gamma
Knife radiosurgery for lung cancer for 10–17 sessions administered
five times per week, with each session delivering a fractionated
dose ranging from 3.0–5.5 Gy. The specific treatment protocol is
detailed below.
EGFR-TKI targeted therapy
Patients received oral administration of either
gefitinib (250 mg, once daily), erlotinib (150 mg, once daily) or
icotinib (125 mg, thrice daily). Dose adjustments were permitted in
response to any adverse events experienced by the patients. Drugs
were preferentially selected according to the clinical features of
the patients. For example, for patients with mild abnormal liver
function, drugs mainly metabolized by the kidneys (such as
gefitinib) were preferred; for patients with concurrent chronic
lung diseases, the applicability of erlotinib was prioritized for
evaluation considering its potentially lower risk of pulmonary
adverse reactions. Additionally, drugs that were economically
affordable and easily accessible to patients were selected to
improve long-term treatment compliance. Furthermore, all drug
selections referred to the recommendations for first-generation
EGFR-TKIs in the latest version of lung cancer diagnosis and
treatment guidelines (such as National Comprehensive Cancer Network
Guidelines and Chinese Society of Clinical Oncology Guidelines)
(31,32). In scenarios where the guidelines did
not clearly recommend a priority, a multidisciplinary team
discussion model was adopted, and decisions were made jointly with
the wishes of the patients. Finally, the balance of the
distribution of baseline data on clinical characteristics was
assessed among the three groups of patients taking gefitinib,
erlotinib and icotinib. Table I
indicates that the samples of patients receiving targeted therapy
in the three groups were comparable in terms of sex, age, ECOG
score, disease stage, gene mutation type and smoking status, with
no significant differences demonstrated.
 | Table I.Analysis of baseline characteristics
of patients in three groups of targeted therapy. |
Table I.
Analysis of baseline characteristics
of patients in three groups of targeted therapy.
|
| Targeted drug |
|
|
|---|
|
|
|
|
|
|---|
| Characteristic | Erlotinib
(n=13) | Gefitinib
(n=10) | Icotinib
(n=12) | F | P-value |
|---|
| Sex |
|
|
| - | 0.817 |
|
Female | 9 (69.23) | 6 (60.00) | 9 (75.00) |
|
|
|
Male | 4 (30.77) | 4 (40.00) | 3 (25.00) |
|
|
| Age | 62.23±12.52 | 62.40±12.56 | 62.25±13.96 | 0.001 | 0.999 |
| ECOG performance
status |
|
|
| - | 0.821 |
| 0 | 1 (7.69) | 1 (10.00) | 2 (16.67) |
|
|
| 1 | 12 (92.31) | 9 (90.00) | 10 (83.33) |
|
|
| Stage |
|
|
| - | 0.821 |
|
III | 1 (7.69) | 1 (10.00) | 2 (16.67) |
|
|
| IV | 12 (92.31) | 9 (90.00) | 10 (83.33) |
|
|
| Mutation type |
|
|
| - | 0.228 |
| EGFR
19del | 10 (76.92) | 4 (40.00) | 8 (66.67) |
|
|
| EGFR
exon21 L858R | 3 (23.08) | 6 (60.00) | 4 (33.33) |
|
|
| Smoking status |
|
|
| - | 0.392 |
| No | 12 (92.31) | 7 (70.00) | 10 (83.33) |
|
|
|
Yes | 1 (7.69) | 3 (30.00) | 2 (16.67) |
|
|
Stereotactic radiation therapy
The fourth-generation LUNA-260 Gamma Knife system
(Shenzhen Yiti Medical Treatment Technology Co., Ltd.) was
employed. Patients were positioned supine and secured using a
vacuum bag. A helical CT scan (slice thickness, 3–5 mm) was
performed on the lesion area, including the entire lung, to obtain
localization images. The gross tumor volume (GTV) corresponded with
the visibility of the tumor in the lung window; the clinical target
volume (CTV) extended 5 mm beyond the GTV; and the planning target
volume (PTV) extended a further 5 mm from the CTV. Regarding the
determination of the radiation treatment target area, prior to
Gamma Knife treatment, the operator instructed patients to maintain
steady thoracic breathing (avoiding diaphragmatic breathing) to
minimize irregular breathing. Additionally, the hospital department
designed a set of devices to effectively reduce respiratory motion
and positioning errors. These included extra anchoring points for
positioning that were placed on the chest wall of the patient, and
a hydrolyzed plastic body mold to cover the external thorax.
Verification through 4-dimensional CT has shown that using a GTV +
10 mm margin can cover the risk of subclinical lesion extension
whilst reducing the radiation dose to normal tissues (33). Therefore, the internal target volume
region was no longer delineated separately. For lesions <3 cm,
the 50% isodose line encompassed 100% of the PTV, the 60% isodose
line encompassed 90% of the CTV and the 70% isodose line
encompassed 80% of the GTV. In cases where the lesion was >5 cm,
the 50–60% isodose line encompassed 100% of the GTV. The
fractionated dose delivered per session ranged from 3.0–5.5 Gy and
it was provided five times weekly for a total of 10–17 sessions,
with a cumulative peripheral dose of 45–55 Gy.
Treatment after progression
If disease progression occurred after the
combination of first-generation EGFR-TKI treatment and Gamma Knife
therapy, subsequent treatment options were determined according to
genetic testing results. These options included third-generation
EGFR-TKIs, chemotherapy, anti-angiogenic therapy, immunotherapy and
radiation therapy directed at metastatic lesions.
Efficacy evaluation and prognostic
analysis indicators
Clinical data, including patient demographics, tumor
characteristics, staging and radiological features, were recorded
prior to treatment. Patients were followed up to gather
radiological assessment results and details of any adverse events
following therapy: Following completion of Gamma Knife treatment,
patients underwent chest and abdominal CT scans after 1, 3, 6 and
12 months during the first year, and at 6-month intervals during
the second year. RECIST 1.1 criteria were employed to assess the
treatment efficacy. Short-term treatment outcomes were assessed
within 3–6 months post-therapy. Patients were classified into 1/4
categories based on their response: Complete response (CR), partial
response (PR), stable disease (SD) or PD. In the present study, the
objective response rate (ORR), which equals CR + PR, was considered
indicative of treatment effectiveness. For long-term outcomes,
survival data, specifically PFS and OS, were collected until the
follow-up cutoff date of December 31, 2023. Finally, any adverse
reactions were recorded: The primary side effects of EGFR-TKIs
included rash and diarrhea, whilst the main adverse reactions
observed following lung Gamma Knife treatment were radiation
pneumonitis.
Statistical methods
Primary study endpoints included PFS and OS, whilst
secondary endpoints included the ORR and safety. PFS was defined as
the time from the initiation of EGFR-TKIs to the occurrence of
disease progression, and OS was defined as the time from the
initiation of EGFR-TKIs to death or the end of follow-up. SPSS 25
software (IBM Corp.) was used for analysis, with statistical
methods including univariate linear regression (single-factor) and
multivariate linear regression (multi-factor). The Kaplan-Meier
method was adopted for assessing clinical treatment efficacy in
patients. Moreover, Fisher's Exact Test or analysis of variance was
used to assess the balanced distribution of baseline data for
clinical characteristics (including sex, age, EGFR mutation
subtype, tumor stage, ECOG performance status and smoking status)
among the three groups of patients treated with gefitinib,
erlotinib and icotinib. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
A cohort of 35 patients was included in the final
analysis. Fig. 1 illustrates the
treatment plan for these patients. Detailed clinical
characteristics of the patients are presented in Table II.
 | Table II.Characteristics of the 35
patients |
Table II.
Characteristics of the 35
patients
| Characteristic | n (%) |
|---|
| Sex |
|
|
Male | 11 (31) |
|
Female | 24 (69) |
| Age |
|
| ≥62
years | 20 (57) |
| <62
years | 15 (43) |
| Stage |
|
|
III | 4 (11) |
| IV | 31 (89) |
| Visceral
metastasis |
|
|
Present | 12 (34) |
|
Absent | 23 (66) |
| CEA |
|
| ≥10
ng/ml | 17 (49) |
| <10
ng/ml | 18 (51) |
| Smoking status |
|
|
Yes | 6 (17) |
| No | 29 (83) |
| ECOG performance
status |
|
| 0 | 4 (11) |
| 1 | 31 (89) |
| Mutation type |
|
| Exon 19
Del | 22 (63) |
| Exon 21
L858R | 13 (37) |
| Type of
EGFR-TKIs |
|
|
Gefitinib | 10 (29) |
|
Afatinib | 12 (34) |
|
Erlotinib | 13 (37) |
| Diameter of lung
lesions treated with gamma knife |
|
| ≥3
cm | 13 (37) |
| <3
cm | 22 (63) |
Efficacy and survival
Among the patients, 15 (43%) achieved CR, 12 (34%)
had a PR, 8 (23%) showed SD and no patients exhibited PD, leading
to an ORR of 77%. Moreover, according to the Radiation Therapy
Oncology Group classification, radiation pneumonitis was graded as
grade III in 1 patient (3%), grade II in 6 patients (17%) and grade
I in 17 patients (49%), whilst no adverse reactions were observed
in 11 patients (31%) (34).
Outcomes of first-generation EGFR-TKIs
combined with Gamma Knife therapy
As of the December 2023 follow-up, descriptive
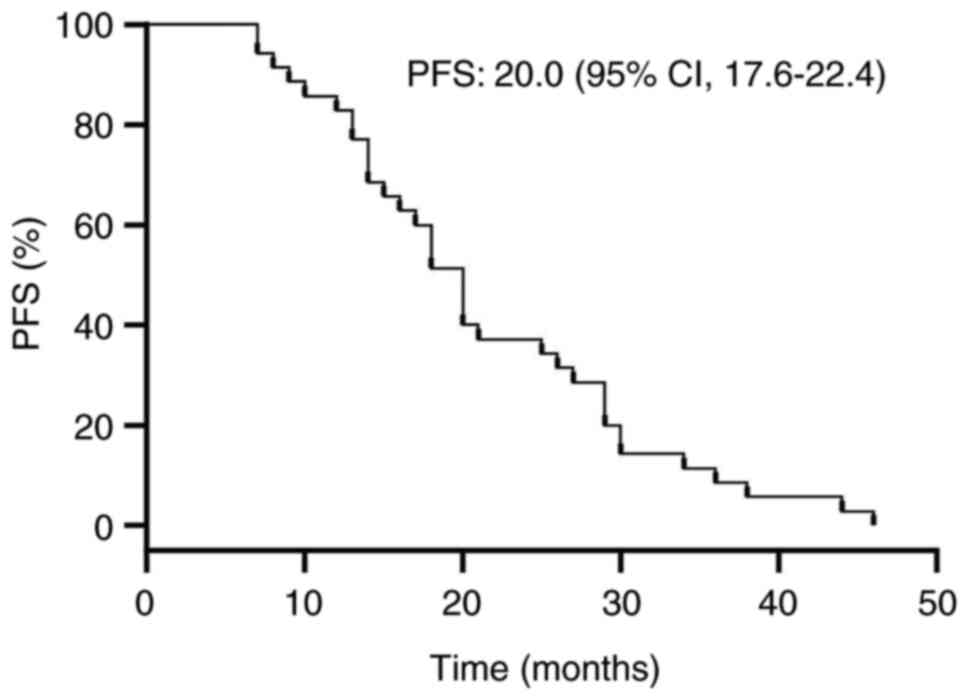
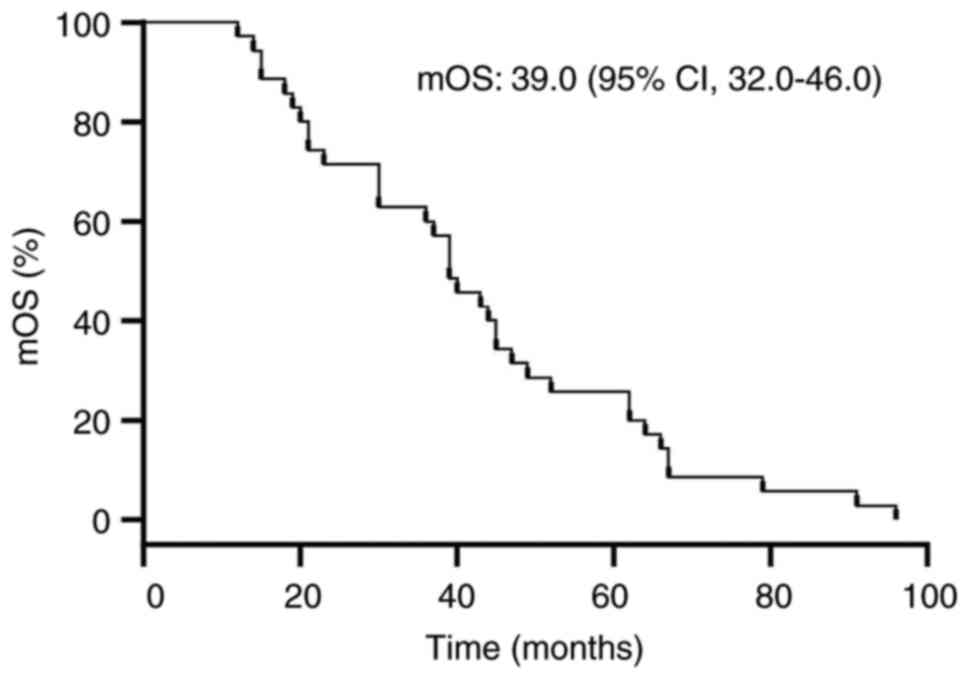
statistics indicated the PFS was 20 months (range, 17.6–22.4
months) and the median OS was 39 months (range, 32.0–46.0 months)
for the cohort of 35 patients. The corresponding PFS and median OS
survival curve functions are presented in Figs. 2 and 3.
Factors influencing the efficacy of
Gamma Knife treatment
Univariate and multivariate analyses were performed
to evaluate the association between different factors on PFS,
including sex, mutation type, cancer stage, visceral metastasis,
age, carcinoembryonic antigen (CEA) levels, types of targeted
therapies, ECOG performance status, size of lung lesions, smoking
status and severity of radiation pneumonitis (Table III). Univariate analysis revealed
a survival advantage among patients aged ≥62 years, with CEA <10
ng/ml and with grade I or no radiation pneumonitis; however, the
differences were not statistically significant. Multivariate
analysis demonstrated that non-smoking patients, CEA <10 ng/ml,
grade I or no radiation pneumonitis after treatment, and treated
with icotinib experienced significantly longer PFS (all
P<0.05).
 | Table III.Univariate and multivariate analyses
associated with progression-free survival. |
Table III.
Univariate and multivariate analyses
associated with progression-free survival.
|
|
|
| Univariate
regression | Multivariate
regression |
|---|
|
|
|
|
|
|
|---|
| Factor | n | Mean PFS,
months | Ba (95% CI) | P-value | Ba (95%CI) | P-value |
|---|
| Age |
|
|
|
|
|
|
| <62
years | 15 | 18.47 | - | - | - | - |
| ≥62
years | 20 | 23.50 | 5.033
(−1.698–11.765) | 0.152 | 5.047
(−1.561–11.655) | 0.149 |
| Sex |
|
|
|
|
|
|
|
Male | 11 | 21.91 | - | - | - | - |
|
Female | 24 | 21.08 | −0.826
(−8.226–6.575) | 0.828 | −6.390
(−14.740–1.960) | 0.148 |
| ECOG performance
status |
|
|
|
|
|
|
| 0 | 4 | 26.75 | - | - | - | - |
| 1 | 31 | 20.65 | −6.105
(−16.708–4.499) | 0.267 | −7.704
(−18.403–2.995) | 0.172 |
| Smoking status |
|
|
|
|
|
|
| No | 29 | 21.79 | - | - | - | - |
|
Yes | 6 | 19.17 | −2.626
(−11.705–6.452) | 0.575 | −14.113
(−24.541–3.685) | 0.015b |
| Mutation type |
|
|
|
|
|
|
| Exon 19
Del | 22 | 22.45 | - | - | - | - |
| Exon 21
L858R | 13 | 19.46 | −2.993
(−10.035–4.049) | 0.411 | −1.608
(−8.518–5.303) | 0.653 |
| Stage |
|
|
|
|
|
|
|
III | 4 | 20.75 | - | - | - | - |
| IV | 31 | 21.42 | 0.669
(−10.134–11.473) | 0.904 | 12.548
(0.566–24.529) | 0.052 |
| Visceral
metastasis |
|
|
|
|
|
|
| No | 23 | 21.52 | - | - | - | - |
|
Yes | 12 | 21.00 | −0.522
(−7.763–6.719) | 0.889 | −3.050
(−11.218–5.118) | 0.472 |
| CEA |
|
|
|
|
|
|
| <10
ng/ml | 18 | 24.17 | - | - | - | - |
| >10
ng/ml | 17 | 18.35 | −5.814
(−12.400–0.773) | 0.093 | −13.172
(−20.989–5.354) | 0.003c |
| Types of targeted
drugs |
|
|
|
|
|
|
|
Icotinib | 12 | 23.08 | - | - | - | - |
|
Erlotinib | 13 | 19.66 | −3.468
(−11.648–4.712) | 0.412 | −9.219
(−16.598–1.840) | 0.023b |
|
Gefitinib | 10 | 20.50 | −1.583
(−10.333–7.166) | 0.725 | −7.505
(−17.397–2.387) | 0.151 |
| Size of lung
lesions |
|
|
|
|
|
|
| <3
cm | 22 | 20.45 | - | - | - | - |
| ≥3
cm | 13 | 22.85 | 2.392
(−4.677–9.460) | 0.512 | 6.673
(−0.701–14.046) | 0.090 |
| Radiation
pneumonitis |
|
|
|
|
|
|
| Grade I
+ None | 28 | 22.46 | - | - | - | - |
| Grade
II + III | 7 | 16.86 | −5.607
(−13.987–2.772) | 0.199 | −8.168
(−15.524–0.812) | 0.041b |
Discussion
In the present study, Gamma Knife treatment extended
both PFS and OS in patients with advanced EGFR-mutant lung
adenocarcinoma receiving first-line EGFR-TKIs. Notably, enhanced
survival benefits were observed in specific subgroups, such as
non-smokers, patients with a CEA level of <10 ng/ml, grade I or
no radiation pneumonitis after treatment, and those receiving
icotinib. Previous studies have reported that integrating LCT with
systemic treatments can boost survival rates in patients with
oligometastatic NSCLC (18,35). Additionally, LCT has also been
reported to extend survival for patients undergoing EGFR-TKIs or
chemotherapy (12,18–20,36).
Moreover, previous studies have reported that
radiation therapy or primary lung tumor resection combined with
systemic treatment can yield survival benefits in patients with
metastatic NSCLC (21,22,37,38).
Takenaka et al (39)
reported that salvage surgery can extend the median OS of patients
treated with EGFR-TKI to ~66 months. Tseng et al (38) demonstrated that primary tumor
resection (PTR) could increase the PFS and OS of patients to 25.1
and 56.8 months, respectively. Kuo et al (21) reported that the median PFS of
patients with stage IV EGFR-mutant NSCLC receiving PRT and
first-line TKI treatment increased from 13.0 to 29.6 months,
compared with patients not receiving PTR treatment. Furthermore, in
the study by Hsu et al (22), compared with not receiving radiation
therapy, first-generation EGFR-TKI treatment followed by radiation
therapy for primary lung cancer increased PFS from 10.9 to 27.5
months and OS from 38.0 to not reached. Elamin et al
(40) reported that oligometastatic
disease (≤3 metastatic sites) occurred in 8/12 patients treated
with TKIs and LCT. Of the 12 cases, 11 underwent radiation therapy
and 1 underwent surgical resection. Furthermore, in comparison with
TKIs alone, LCT following TKI treatment notably prolonged the PFS
(14 vs. 36 months). Deng et al (41) assessed how synchronous radiation
therapy (CPRT) impacts the primary tumor during first-line
treatment with icotinib, and reported that the CPRT group achieved
a median PFS of 13.6 months, compared with only 10.6 months in the
non-CPRT group. Moreover, in a study by Peng et al (23), 13 patients with stage IV lung cancer
who received EGFR-TKIs and SBRT to primary lung lesions had an
median PFS of 27.3 months and an median OS of 49.1 months.
Additionally, radiation therapy to the primary site alone offered
improved benefits compared with radiation therapy targeted solely
at metastatic sites or a combination of both approaches. Notably,
osimertinib was not employed as the first-line treatment plan in
any of the aforementioned studies.
Reducing the tumor burden in the primary site can
enhance the efficacy of systemic therapy (42,43).
Lin et al (44) demonstrated
that salvage surgery performed before the disease advances can
remove TKI-resistant subclones, thereby improving survival results.
Furthermore. according to Al-Halabi et al (45), a notable number of patients
receiving EGFR-TKIs initially experienced failure at the primary
site, with a recurrence rate of 47.0% for primary tumors. The study
by Hsu et al (22) also
reported that the radiotherapy group had a reduced rate of primary
tumor progression compared with the group not receiving radiation
therapy. These findings indicate that integrating primary tumor
control therapy (PTCT), whether through surgical resection or
radiation therapy, enhances overall treatment outcomes and prolongs
survival.
Additionally, research has demonstrated that
combining EGFR-TKIs with radiotherapy can lead to improved survival
in patients. Radiotherapy employs high-energy rays to target tumor
cells and disrupt their genetic material. It can induce EGFR
phosphorylation, which affects the efficacy of radiotherapy and may
lead to resistance. Conversely, EGFR-TKIs can inhibit EGFR
phosphorylation, thereby enhancing the effectiveness of
radiotherapy (46). This
synergistic mechanism provides a theoretical foundation for
subsequent research. Furthermore, PTCT can restore immune function,
reduce subclones of tumor stem cells and decrease tumor
heterogeneity, thereby enhancing the effect of EGFR-TKIs (22,38,47).
In early studies, the values of PFS were smaller for patients with
the L858R mutation than for those with the 19 deletions (19del)
(48–50). In the present study, the PFS for the
19-exon deletion subgroup was 22.5 months, whereas for the L858R
mutation subgroup it was 19.5 months, with no statistically
significant difference observed. Factors such as sex, smoking
status and cancer stage did not significantly affect PFS,
indicating that all subgroups could benefit from Gamma Knife
therapy.
The FLAURA trial reported that osimertinib achieved
a PFS of 18.9 months and an OS of 38.6 months (51,52).
In the present study, the PFS and OS data of Gamma Knife treatment
combined with first-generation EGFR-TKI were broadly consistent
with those of third-generation TKI monotherapy (such as
osimertinib) in a similar population reported in the FLAURA trial,
and the side effects were controllable; therefore, it is a viable
option from a pharmacoeconomic perspective. However, it should be
noted that the present study did not set up a third-generation TKI
treatment group as a control. For patients eligible for Gamma
Knife, the use of TKIs alone without local treatment (such as
Stereotactic Radiosurgery/Gamma Knife) was not a routine or
recommended practice in the 901st Hospital of the Joint Logistics
Support Force of the People's Liberation Army. Therefore, among the
concurrent, same-center patient population eligible for Gamma
Knife, it was difficult for the present study to identify a
sufficient number of suitable patients with comparable baseline
characteristics who only received TKIs without any local treatment
as the control group. Forcibly incorporating a poorly qualified
control group would undermine the overall credibility of the
research. Therefore, the current conclusion is based on a
comparative analysis of indirect efficacy indicators among similar
patient populations in different trials, rather than direct
comparisons through head-to-head trials. Due to the differences in
the treatment scenarios and patient screening criteria between the
two, the statistical significance of the comparisons must be
carefully interpreted. The main contribution of the present study
lies in the description and preliminarily evaluation of a promising
sequential treatment strategy, whose results need to be validated
in future prospective controlled studies.
The focus of the present study was on consolidation
therapy for the primary lesion of lung cancer (excluding metastatic
lesions), which has not been systematically explored in existing
studies, to the best of our knowledge. Current similar studies
mainly focus on local treatment of metastatic lesions (12,26),
lacking a specific analysis of the combination therapy of
first-generation TKIs and Gamma Knife for the primary lesion.
Through long-term follow-up data (PFS of 20 months and median OS of
39 months), the present efficacy and safety profile provide
patients with one more treatment option.
Case inclusion in the present study preceded the
subsequent regulatory approval of osimertinib for this indication
(51). The treatment regimen of the
present study may serve as an alternative option for situations in
which third-generation TKIs cannot be used for treatment due to
limited drug supply in certain developing countries or regions,
insufficient financial affordability of patients, adverse reactions
or contraindications. In the future, head-to-head research is
needed to further compare the cost-effectiveness of
first-generation TKIs combined with Gamma Knife, and of osimertinib
monotherapy, in specific populations, providing more basis for
individualized treatment.
Moreover, it should be noted that the present study
has certain limitations. First, as it is a retrospective study
without a control group, there may be selection bias. Second, a
small sample size can lead to limited statistical power, which may
affect the statistical significance of certain subgroup
comparisons. Third, the follow-up time may have not been sufficient
to obtain mature OS results from subgroup analyses and follow-up
studies should extend the follow-up time to enhance the credibility
of long-term interpretations. Large-scale prospective experiments
are still needed to verify the findings proposed in the present
study in the future.
In conclusion, combining first-generation EGFR-TKIs
with Gamma Knife therapy can delay EGFR resistance and extend PFS
and OS with a low incidence of toxicity and side effects. The
results of the present study indicate that the combination of
first-generation EGFR-TKIs and Gamma Knife therapy for the
treatment of primary lung lesions is a viable therapeutic option
for patients with advanced EGFR-sensitive mutations. Nevertheless,
these results should be further verified in future prospective
research.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Anhui Province Health
Research Project Key Project (grant no. AHWJ2023BAc10023).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
DLv was responsible for the study's conception and
research protocol design. BX, DLu and ZL participated in study data
acquisition, including data collection and patient management. DW,
HM, LZ and ML analyzed the collected data and interpreted the
results to derive key findings. DLv, BX, and DW collaborated on
writing the manuscript's initial draft. All authors actively
participated in the subsequent review and critical revisions, and
all authors have read and approved the final manuscript. DLv and BX
confirm the authenticity of all the raw data. All authors are
accountable for the intellectual content of the work and agree to
be responsible for all aspects of it.
Ethics approval and consent to
participate
The present study was approved by the review board
ethics committee of the 901st Hospital of the Joint Logistics
Support Force of the People's Liberation Army (approval no.
LYAHW2023BAc1003). The requirement for written informed consent was
waived due to the retrospective nature of the study. All data were
anonymized prior to analysis and handled according to institutional
and ethical standards.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Thai AA, Solomon BJ, Sequist LV, Gainor JF
and Heist RS: Lung cancer. Lancet. 398:535–554. 2021. View Article : Google Scholar
|
|
2
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular profiling of lung adenocarcinoma. Nature.
511:543–550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar
|
|
5
|
Shen YW, Zhang XM, Li ST, Lv M and Yang J,
Wang F, Chen ZL, Wang BY, Li P, Chen L and Yang J: Efficacy and
safety of icotinib as first-line therapy in patients with advanced
non-small-cell lung cancer. Onco Targets Ther. 9:929–935. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu YL, Zhou C, Cheng Y, Lu S, Chen GY,
Huang C, Huang YS, Yan HH, Ren S, Liu Y and Yang JJ: Erlotinib as
second-line treatment in patients with advanced non-small-cell lung
cancer and asymptomatic brain metastases: A phase II study
(CTONG-0803). Ann Oncol. 24:993–999. 2013. View Article : Google Scholar
|
|
7
|
Iuchi T, Shingyoji M, Sakaida T, Hatano K,
Nagano O, Itakura M, Kageyama H, Yokoi S, Hasegawa Y, Kawasaki K
and Iizasa T: Phase II trial of gefitinib alone without radiation
therapy for Japanese patients with brain metastases from
EGFR-mutant lung adenocarcinoma. Lung Cancer. 82:282–287. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roper N, Brown AL, Wei JS, Pack S,
Trindade C, Kim C, Restifo O, Gao S, Sindiri S, Mehrabadi F, et al:
Clonal evolution and heterogeneity of osimertinib acquired
resistance mechanisms in EGFR mutant lung cancer. Cell Rep Med.
1:1000072020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park K, Yu CJ, Kim SW, Lin MC, Sriuranpong
V, Tsai CM, Lee JS, Kang JH, Chan KC, Perez-Moreno P, et al:
First-line erlotinib therapy until and beyond response evaluation
criteria in solid tumors progression in asian patients with
epidermal growth factor receptor Mutation-positive Non-Small-Cell
lung cancer: The ASPIRATION study. JAMA Oncol. 2:305–312. 2016.
View Article : Google Scholar
|
|
10
|
Chaft JE, Oxnard GR, Sima CS, Kris MG,
Miller VA and Riely GJ: Disease flare after tyrosine kinase
inhibitor discontinuation in patients with EGFR-mutant lung cancer
and acquired resistance to erlotinib or gefitinib: Implications for
clinical trial design. Clin Cancer Res. 17:6298–6303. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heitmann J and Guckenberger M:
Perspectives on oligometastasis: Challenges and opportunities. J
Thorac Dis. 10:113–117. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu F, Xu J, Zhang B, Li C, Nie W, Gu P, Hu
P, Wang H, Zhang Y, Shen Y, et al: Efficacy of local consolidative
therapy for oligometastatic lung adenocarcinoma patients harboring
epidermal growth factor receptor mutations. Clin Lung Cancer.
20:e81–e90. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seong H, Kim SH, Kim MH, Kim J and Eom JS:
Additional local therapy before disease progression for
EGFR-mutated advanced lung cancer: A systematic review and
meta-analysis. Transl Lung Cancer Res. 13:491–502. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xia T, Li H, Sun Q, Wang Y, Fan N, Yu Y,
Li P and Chang JY: Promising clinical outcome of stereotactic body
radiation therapy for patients with inoperable Stage I/II
non-small-cell lung cancer. Int J Radiat Oncol Biol Phys.
66:117–125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang LP, Nie Q, Kang JB, Wang B, Cai CL,
Li JG and Qi WJ: Efficacy of whole body gamma-knife radiotherapy
combined with thermochemotherapy on locally advanced pancreatic
cancer. Chin J Cancer. 27:1204–1207. 2008.(In Chinese).
|
|
16
|
Yu W, Tang L, Lin F, Li D, Wang J, Yang Y
and Shen Z: Stereotactic radiosurgery, a potential alternative
treatment for pulmonary metastases from osteosarcoma. Int J Oncol.
44:1091–1098. 2014. View Article : Google Scholar
|
|
17
|
Liu K, Zheng D, Xu G, Du Z and Wu S: Local
thoracic therapy improve prognosis for stage IV non-small cell lung
cancer patients combined with chemotherapy: A Surveillance,
Epidemiology, and End Results database analysis. PLoS One.
12:e01873502017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gomez DR, Tang C, Zhang J, Blumenschein GR
Jr, Hernandez M, Lee JJ, Ye R, Palma DA, Louie AV, Camidge DR, et
al: Local consolidative therapy vs. maintenance therapy or
observation for patients with oligometastatic Non-Small-Cell lung
cancer: Long-Term results of a Multi-Institutional, Phase II,
randomized study. J Clin Oncol. 37:1558–1565. 2019. View Article : Google Scholar
|
|
19
|
Palma DA, Olson R, Harrow S, Gaede S,
Louie AV, Haasbeek C, Mulroy L, Lock M, Rodrigues GB, Yaremko BP,
et al: Stereotactic ablative radiotherapy for the comprehensive
treatment of oligometastatic cancers: Long-term results of the
SABR-COMET phase II randomized trial. J Clin Oncol. 38:2830–2838.
2020. View Article : Google Scholar
|
|
20
|
Wang XS, Bai YF, Verma V, Yu RL, Tian W,
Ao R, Deng Y, Zhu XQ, Liu H, Pan HX, et al: Randomized trial of
First-line tyrosine kinase inhibitor with or without radiotherapy
for synchronous oligometastatic EGFR-Mutated Non-Small cell lung
cancer. J Natl Cancer Inst. 115:742–748. 2023. View Article : Google Scholar
|
|
21
|
Kuo SW, Chen PH, Lu TP, Chen KC, Liao HC,
Tsou KC, Tsai TM, Lin MW, Hsu HH and Chen JS: Primary tumor
resection for Stage IV Non-small-cell lung cancer without
progression After First-Line epidermal growth factor
receptor-tyrosine kinase inhibitor treatment: A retrospective
Case-Control study. Ann Surg Oncol. 29:4873–4884. 2022. View Article : Google Scholar
|
|
22
|
Hsu KH, Huang JW, Tseng JS, Chen KW, Weng
YC, Yu SL, Yang TY, Huang YH, Chen JJW, Chen KC, et al: Primary
tumor radiotherapy during EGFR-TKI disease control improves
survival of treatment naïve advanced EGFR-Mutant lung
adenocarcinoma patients. Onco Targets Ther. 14:2139–2148. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peng P, Gong J, Zhang Y, Zhou S, Li Y, Han
G, Meng R, Chen Y, Yang M, Shen Q, et al: EGFR-TKIs plus
stereotactic body radiation therapy (SBRT) for stage IV Non-small
cell lung cancer (NSCLC): A prospective, multicenter, randomized,
controlled phase II study. Radiother Oncol. 184:1096812023.
View Article : Google Scholar
|
|
24
|
Xu Q, Zhou F, Liu H, Jiang T, Li X, Xu Y
and Zhou C: Consolidative local ablative therapy improves the
survival of patients with synchronous oligometastatic NSCLC
harboring EGFR Activating mutation treated with first-line
EGFR-TKIs. J Thorac Oncol. 13:1383–1392. 2018. View Article : Google Scholar
|
|
25
|
Wang Y, Li Y, Xia L, Niu K, Chen X, Lu D,
Kong R, Chen Z and Sun J: Continued EGFR-TKI with concurrent
radiotherapy to improve time to progression (TTP) in patients with
locally progressive non-small cell lung cancer (NSCLC) after
front-line EGFR-TKI treatment. Clin Transl Oncol. 20:366–373. 2018.
View Article : Google Scholar
|
|
26
|
Chiou GY, Chiang CL, Yang HC, Shen CI, Wu
HM, Chen YW, Chen CJ, Luo YH, Hu YS, Lin CJ, et al: Combined
stereotactic radiosurgery and tyrosine kinase inhibitor therapy
versus tyrosine kinase inhibitor therapy alone for the treatment of
non-small cell lung cancer patients with brain metastases. J
Neurosurg. 137:563–570. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu JJ, Tseng JS, Zheng ZR, Chu CH, Chen
KC, Lin MW, Huang YH, Hsu KH, Yang TY, Yu SL, et al: Primary tumor
consolidative therapy improves the outcomes of patients with
advanced EGFR-mutant lung adenocarcinoma treated with first-line
osimertinib. Ther Adv Med Oncol. 16:175883592312206062024.
View Article : Google Scholar
|
|
28
|
Guo T, Ni J, Yang X, Li Y, Li Y, Zou L,
Wang S, Liu Q, Chu L, Chu X, et al: Pattern of Recurrence analysis
in metastatic EGFR-Mutant NSCLC treated with osimertinib:
Implications for consolidative stereotactic body radiation therapy.
Int J Radiat Oncol Biol Phys. 107:62–71. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ando M, Ando Y, Hasegawa Y, Shimokata K,
Minami H, Wakai K, Ohno Y and Sakai S: Prognostic value of
performance status assessed by patients themselves, nurses, and
oncologists in advanced non-small cell lung cancer. Br J Cancer.
85:1634–1639. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D'Amico
TA, et al: NCCN guidelines insights: Non-Small cell lung cancer,
version 2022. J Natl Compr Canc Netw. 20:497–530. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar
|
|
33
|
Ju X, Li M, Zhou Z, Zhang K, Han W, Fu G,
Cao Y and Wang L: 4D-CT-based plan target volume (PTV) definition
compared with conventional PTV definition using general margin in
radiotherapy for lung cancer. Zhonghua Zhong Liu Za Zhi. 36:34–38.
2014.(In Chinese).
|
|
34
|
Cox JD, Stetz J and Pajak TF: Toxicity
criteria of the radiation therapy oncology group (RTOG) and the
european organization for research and treatment of cancer (EORTC).
Int J Radiat Oncol Biol Phys. 31:1341–1346. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang S, Sun Q, Cai F, Li H and Zhou Y:
Local therapy treatment conditions for oligometastatic non-small
cell lung cancer. Front Oncol. 12:10281322022. View Article : Google Scholar
|
|
36
|
Iyengar P, Wardak Z, Gerber DE, Tumati V,
Ahn C, Hughes RS, Dowell JE, Cheedella N, Nedzi L, Westover KD, et
al: Consolidative radiotherapy for limited metastatic
Non-Small-Cell lung cancer: A Phase 2 randomized clinical trial.
JAMA Oncol. 4:e1735012018. View Article : Google Scholar
|
|
37
|
Ohtaki Y, Shimizu K, Suzuki H, Suzuki K,
Tsuboi M, Mitsudomi T, Takao M, Murakawa T, Ito H, Yoshimura K, et
al: Salvage surgery for non-small cell lung cancer after tyrosine
kinase inhibitor treatment. Lung Cancer. 153:108–116. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tseng JS, Hsu KH, Zheng ZR, Yang TY, Chen
KC, Huang YH, Su KY, Yu SL and Chang GC: Primary tumor resection is
associated with a better outcome among advanced EGFR-Mutant lung
adenocarcinoma patients receiving EGFR-TKI treatment. Oncology.
99:32–40. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takenaka M, Tanaka F, Kajiyama K, Manabe
T, Yoshimatsu K, Mori M, Kanayama M, Taira A, Kuwata T, Nawata A
and Kuroda K: Outcomes and pathologic response of primary lung
cancer treated with tyrosine kinase inhibitor/immune checkpoint
inhibitor before salvage surgery. Surg Today. 54:1146–1153. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Elamin YY, Gomez DR, Antonoff MB,
Robichaux JP, Tran H, Shorter MK, Bohac JM, Negrao MV, Le X,
Rinsurogkawong W, et al: Local consolidation therapy (LCT) after
first line tyrosine kinase inhibitor (TKI) for patients with EGFR
mutant metastatic Non-small-cell lung cancer (NSCLC). Clin Lung
Cancer. 20:43–47. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Deng R, Liu J, Song T, Xu T, Li Y, Duo L,
Xiang L, Yu X, Lei J and Cao F: Primary lesion radiotherapy during
first-line icotinib treatment in EGFR-mutated NSCLC patients with
multiple metastases and no brain metastases: A single-center
retrospective study. Strahlenther Onkol. 198:1082–1093. 2022.
View Article : Google Scholar
|
|
42
|
Chang SJ and Bristow RE: Evolution of
surgical treatment paradigms for advanced-stage ovarian cancer:
Redefining ‘optimal’ residual disease. Gynecol Oncol. 125:483–492.
2012. View Article : Google Scholar
|
|
43
|
Koole S, van Stein R, Sikorska K, Barton
D, Perrin L, Brennan D, Zivanovic O, Mosgaard BJ, Fagotti A and
Colombo PE: Primary cytoreductive surgery with or without
hyperthermic intraperitoneal chemotherapy (HIPEC) for FIGO stage
III epithelial ovarian cancer: OVHIPEC-2, a phase III randomized
clinical trial. Int J Gynecol Cancer. 30:888–892. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin MW, Yu SL, Hsu YC, Chen YM, Lee YH,
Hsiao YJ, Lin JW, Su TJ, Jeffrey Yang CF, Chiang XH, et al: Salvage
surgery for advanced lung adenocarcinoma after epidermal growth
factor receptor tyrosine kinase inhibitor treatment. Ann Thorac
Surg. 116:111–119. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Al-Halabi H, Sayegh K, Digamurthy SR,
Niemierko A, Piotrowska Z, Willers H and Sequist LV: Pattern of
failure analysis in metastatic EGFR-Mutant lung cancer treated with
tyrosine kinase inhibitors to identify candidates for consolidation
stereotactic body radiation therapy. J Thorac Oncol. 10:1601–1607.
2015. View Article : Google Scholar
|
|
46
|
Wee P and Wang Z: Epidermal growth factor
receptor cell proliferation signaling pathways. Cancers (Basel).
9:522017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Byrne NM, Tambe P and Coulter JA:
Radiation response in the tumour microenvironment: Predictive
biomarkers and future perspectives. J Pers Med. 11:532021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Won YW, Han JY, Lee GK, Park SY, Lim KY,
Yoon KA, Yun T, Kim HT and Lee JS: Comparison of clinical outcome
of patients with non-small-cell lung cancer harbouring epidermal
growth factor receptor exon 19 or exon 21 mutations. J Clin Pathol.
64:947–952. 2011. View Article : Google Scholar
|
|
49
|
Hong W, Wu Q, Zhang J and Zhou Y:
Prognostic value of EGFR 19-del and 21-L858R mutations in patients
with non-small cell lung cancer. Oncol Lett. 18:3887–3895.
2019.
|
|
50
|
Chen Y, Wang S, Zhang B, Zhao Y, Zhang L,
Hu M, Zhang W and Han B: Clinical factors affecting the response to
osimertinib in Non-Small cell lung cancer patients with an acquired
epidermal growth factor receptor T790M mutation: A Long-Term
survival analysis. Target Oncol. 15:337–345. 2020. View Article : Google Scholar
|
|
51
|
Soria JC, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura
F, Nogami N, Kurata T, et al: Osimertinib in untreated EGFR-Mutated
advanced Non-Small-Cell Lung cancer. N Engl J Med. 378:113–125.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ramalingam SS, Vansteenkiste J, Planchard
D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y,
Chewaskulyong B, et al: Overall survival with osimertinib in
untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 382:41–50.
2020. View Article : Google Scholar : PubMed/NCBI
|