Introduction
Lymphoma is a malignant tumor caused by abnormal
clonal proliferation of lymphocytes (1). Based on pathological characteristics,
lymphomas can be divided into Hodgkin lymphoma (HL) and non-Hodgkin
lymphoma (NHL), with NHL accounting for ~90% of cases and HL only
10% (2). NHL can be further
classified based on cell origin into B-cell, T cell and natural
killer (NK)-cell NHL (3,4). Clinical practice indicates that
although lymphoid tissue is the common origin of most lymphomas,
different types of lymphomas exhibit notable differences in
clinical manifestations, molecular characteristics and case
structures. Therefore, therapeutic approaches vary substantially.
In recent years, emerging treatments such as programmed cell death
protein-1/programmed cell death ligand-1 inhibitors, chimeric
antigen receptor T (CAR-T) cell therapy and bispecific antibodies
have been increasingly applied for the management of NHL, offering
notable therapeutic potential compared with conventional
chemotherapy (5). With the
continuous advancement of modern medical knowledge, the
classification and treatment of lymphoma have been consistently
updated; while some lymphoma subtypes have achieved satisfactory
outcomes with standard treatment protocols, a subset of patients
still experience relapse or develop resistance (6).
For patients with NHL unsuitable for stem cell
transplantation, chemotherapy remains the preferred treatment
(7). Oxaliplatin, a
third-generation platinum-based anticancer drug, is a platinum
compound of diaminocyclohexane and it targets DNA, forming
cross-links with DNA strands, thereby inhibiting tumor DNA
replication and transcription. Multiple clinical studies have
reported the positive impact of oxaliplatin on improving the
prognosis of patients with NHL (8,9).
However, clinical practice has indicated that traditional
platinum-based chemotherapy drugs, such as oxaliplatin, have low
treatment response rates and high incidence of adverse reactions
(e.g., leukopenia, acute neurotoxicity, gastrointestinal reactions)
(10). In recent years, novel
anticancer drugs such as gemcitabine have entered clinical use
(11). Gemcitabine, a novel
cytidine analog, shares a similar mechanism with cytarabine,
primarily inhibiting ribonucleotide reductase to suppress
deoxycytidine deaminase, thereby reducing the degradation of
intracellular metabolites and enhancing self-potentiation (11). In vitro experiments have
confirmed its efficacy against various solid tumors, such as
pancreatic cancer and non-small cell lung cancer (12,13).
Currently, there is limited research on the combined
use of oxaliplatin and gemcitabine in NHL. However, existing
research has demonstrated the efficacy of this combination.
Previous clinical data on patients with NHL treated with the two
agents demonstrated that the majority of participants benefited
from the regimen, with markedly improved overall response and
disease control rates (DCRs) compared with monotherapy, while
maintaining a favorable safety profile (14). The present study aimed to analyze
the clinical value of the two-drug combination in patients with NHL
and to explore the safety of their combined use, which will
potentially provide more references for the clinical treatment of
patients with NHL in the future.
Materials and methods
Study design and patient
selection
The present study was a retrospective analysis,
approved by the Xi'an Central Hospital/Xi'an Institute of
Hematology Ethics Committee (approval no. 2024-JT-023; Xi'an,
China). All study participants provided written informed consent
prior to participation in the present study. The clinical data of
patients with NHL treated at Xi'an Central Hospital/Xi'an Institute
of Hematology from August 2018 to April 2022 were collected. Cases
were screened according to the following inclusion criteria: i) All
cases confirmed as NHL through pathological examination; ii)
patients treated with oxaliplatin or oxaliplatin combined with
gemcitabine in Xi'an Central Hospital/Xi'an Institute of
Hematology; iii) patients who received at least two cycles of
chemotherapy (with the option to include >2 cycles); iv)
patients with complete baseline data [sex, age, pathological type,
Ann Arbor stage and International Prognostic Index (IPI) score for
lymphoma (15)]; v) patients with
blood samples collected before and after chemotherapy and examined
for serum lactate dehydrogenase (LDH) and TGF-β1 levels; vi)
patients with Karnofsky Performance Status (KPS) score assessed
before and after chemotherapy (16); and vii) patients with definitive
follow-up results by the end of the follow-up period (case
inclusion ended in April 2022, with a follow-up duration of 2 years
ending in April 2024). The exclusion criteria were as follows: i)
Patients with other malignant tumors; ii) patients with incomplete
clinical data; and iii) patients with infectious diseases.
After screening the patients according to the
inclusion and exclusion criteria, a total of 106 patients were
included, comprising 68 men and 38 women, with an age range of
37–69 years and a median age of 48 years. Based on the differences
in treatment regimens, patients were divided into a control group
(CG; n=50), who received oxaliplatin treatment and an observation
group (OG; n=56), who received a combined treatment of oxaliplatin
and gemcitabine.
Observation indices
Differences in baseline clinical data between
patients in the OG and CG were collected and compared.
According to the Lugano classification criteria
published in 2014 for malignant lymphoma response assessment
(17), the clinical efficacy of
patients after chemotherapy was categorized as progressive disease
(PD), stable disease (SD), partial remission (PR) and complete
remission (CR). The complete remission rate (CRR) was calculated as
(PR + CR)/total cases ×100% and the DCR was calculated as (PR + CR
+ SD)/total cases ×100%. The clinical efficacy differences of
chemotherapy between the two groups were compared and their overall
survival (OS) and progression-free survival (PFS) were recorded
through follow-up. OS was defined as the duration from the first
administration to mortality or the follow-up endpoint, while PFS
was defined as the duration from the first administration to tumor
progression, mortality or follow-up endpoint.
According to the Common Terminology Criteria for
Adverse Events (CTCAE) version 4.03 criteria (18) for adverse reaction evaluation,
chemotherapy adverse reactions were classified into grades 0–5,
with 0 indicating no adverse reaction, 1 indicating mild adverse
reaction, 2 indicating moderate adverse reaction, 3 indicating
severe adverse reaction, 4 indicating life-threatening or disabling
adverse reaction and 5 indicating a fatal adverse reaction. The
present study analyzed the incidence of leukopenia, liver and
kidney dysfunction and toxic side effects of gastrointestinal
reactions in the OG and CG.
The serum LDH levels and TGF-β1 levels of two groups
of patients were measured before and after chemotherapy using
enzyme-linked immunosorbent assay (cat. nos. EH0287 and EH0198;
Wuhan Saipei Biotechnology Co., Ltd.). Although TGF-β1 is not a
standard biomarker in routine NHL treatment, previous studies have
demonstrated its involvement in tumorigenesis and progression
through modulation of the tumor microenvironment, which justifies
its inclusion in the present analysis (19,20).
The differences in KPS scores before and after
chemotherapy for the two groups were also collected. The KPS
scores, evaluated as a percentage, indicated the health status of
the individual, with higher scores representing improved functional
status of the subjects.
Based on the 2-year follow-up clinical outcomes of
106 patients with NHL, they were divided into a death subgroup
(n=15) and a survival subgroup (n=91). Multivariate logistic
regression analysis was used to identify the risk factors affecting
the prognosis of patients with NHL.
Statistical analysis
Data collection for the present study was performed
using Excel 2021 (version no. 16.0.14332.20255_x64; Microsoft
Corp.) and data analysis was performed with SPSS (version 26.0; IBM
Corp.). The measurement data were normally distributed according to
the Shapiro-Wilk test and are expressed as the mean ± SD, and
intergroup differences were assessed using an independent-samples
t-test. The count data were expressed as rates and intergroup
differences were analyzed using a χ2 or Fisher's exact
test. The risk factors were analyzed using univariate and
multivariate logistic regression analysis. P<0.05 was considered
to indicate a statistically significant difference.
Results
Comparison of general clinical data
between the OG and the CG
Based on the inclusion and exclusion criteria, a
total of 106 patients were selected for the present study, with 56
patients receiving oxaliplatin combined with gemcitabine treatment
and 50 patients receiving oxaliplatin treatment alone. Baseline
clinical data such as sex, mean age, pathological type, Ann Arbor
stage and IPI score for lymphoma were collected in both groups.
Comparative analysis revealed no statistically significant
differences between the two groups (all P>0.05; Table I).
 | Table I.Comparison of general clinical data
between the OG and the CG. |
Table I.
Comparison of general clinical data
between the OG and the CG.
| General clinical
data | OG (n=56) | CG (n=50) |
t/χ2/Fisher | P-value |
|---|
| Sex |
|
| 0.001 | 0.976 |
| Male | 36 | 32 |
|
|
|
Female | 20 | 18 |
|
|
| Age, years | 49.63±7.83 | 50.26±6.39 | 0.450 | 0.653 |
| Pathological
type |
|
| 0.709 | 0.400 |
| B
cell | 38 | 30 |
|
|
| T
cell | 18 | 20 |
|
|
| Ann Arbor stage |
|
| 2.083 | 0.347 |
| II | 16 | 12 |
|
|
| III | 26 | 30 |
|
|
| IV | 14 | 8 |
|
|
| International
Prognostic Index score for lymphoma, points |
|
| 1.156 | 0.628 |
| 0-1 | 16 | 12 |
|
|
| 2-3 | 39 | 38 |
|
|
| 4-5 | 1 | 0 |
|
|
Comparison of clinical efficacy
between the two groups
All enrolled patients underwent a 24-month
follow-up, which concluded in April 2024. The assessment revealed
that the OG had a CRR of 10.71%, a PR rate of 62.50%, an SD rate of
17.85% and a PD rate of 8.93%, with a complete remission rate (CRR)
of 73.21% and a DCR of 91.07%. The CG had a CRR of 4.00%, a PR rate
of 48.00%, an SD rate of 28.00% and a PD rate of 20.00%, with a CRR
of 52.00% and a DCR of 80.00%. Comparisons between groups indicated
that the CRR and DCR of the OG were significantly higher compared
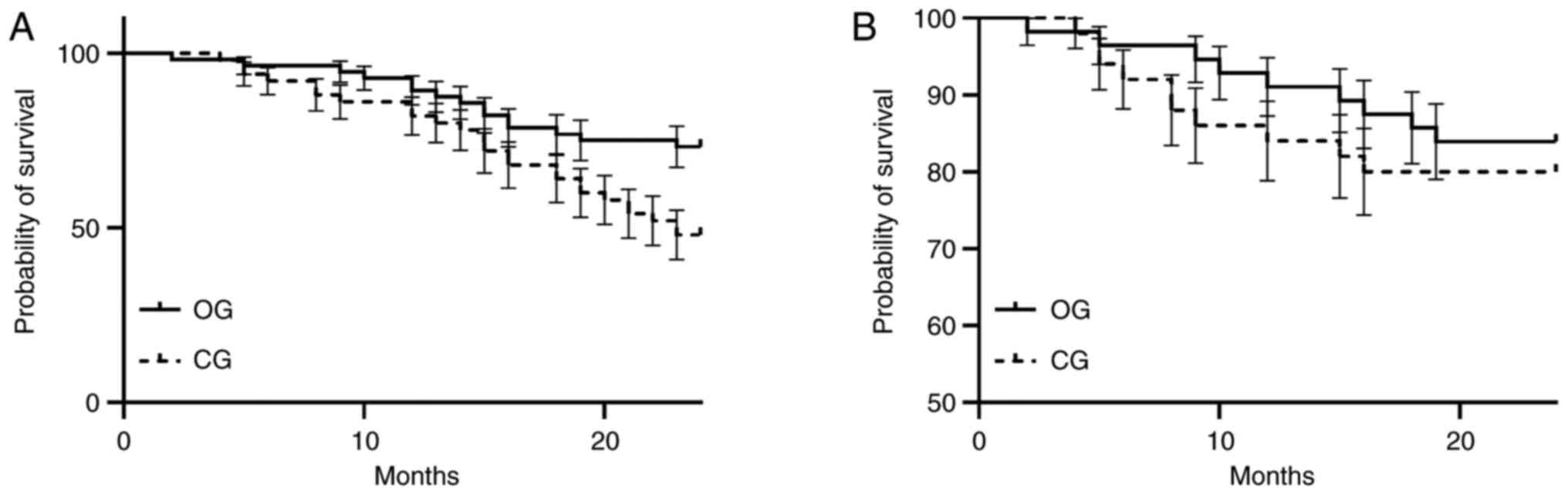
with those of the CG (P=0.024, P=0.031). The OG had an OS of 13.6
months and a PFS of 8.9 months and the CG had an OS of 11.3 months
and a PFS of 6.1 months (Fig.
1).
Comparison of toxic side effects
between the two groups
According to the CTCAE v4.03 criteria, two groups of
patients undergoing chemotherapy were assessed for the incidence of
various toxic side effects (leukopenia, gastrointestinal reactions,
and liver and kidney damage) and intergroup comparisons were
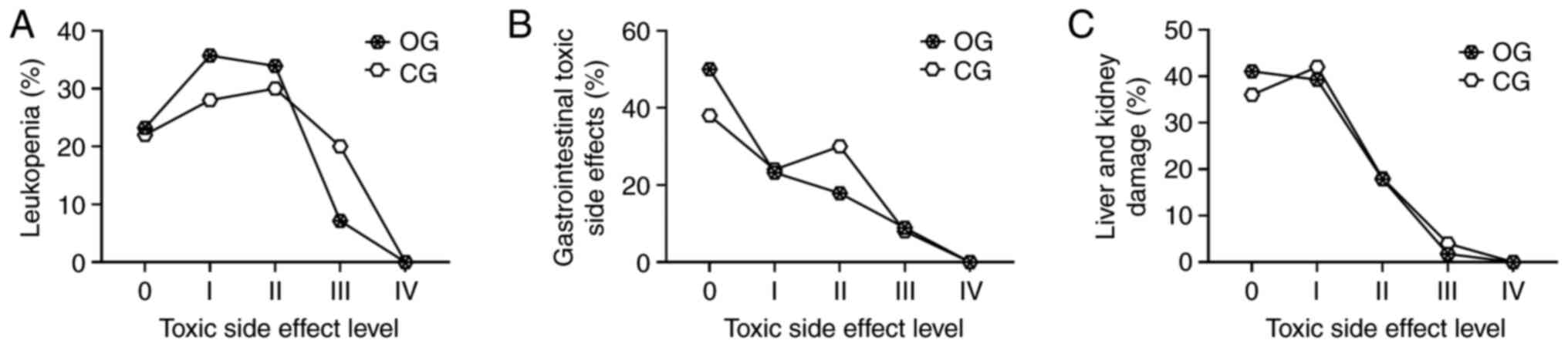
performed. The results indicated that there were no statistically
significant differences between the groups in terms of
leukopenia-related toxic side effects (Fig. 2A), gastrointestinal reactions
(nausea, vomiting, diarrhea, and loss of appetite) (Fig. 2B), and liver and kidney damage
(abnormal liver and kidney function) (all P>0.05) (Fig. 2C).
Comparison of LDH and TGF-β1 levels
between the two groups before and after chemotherapy
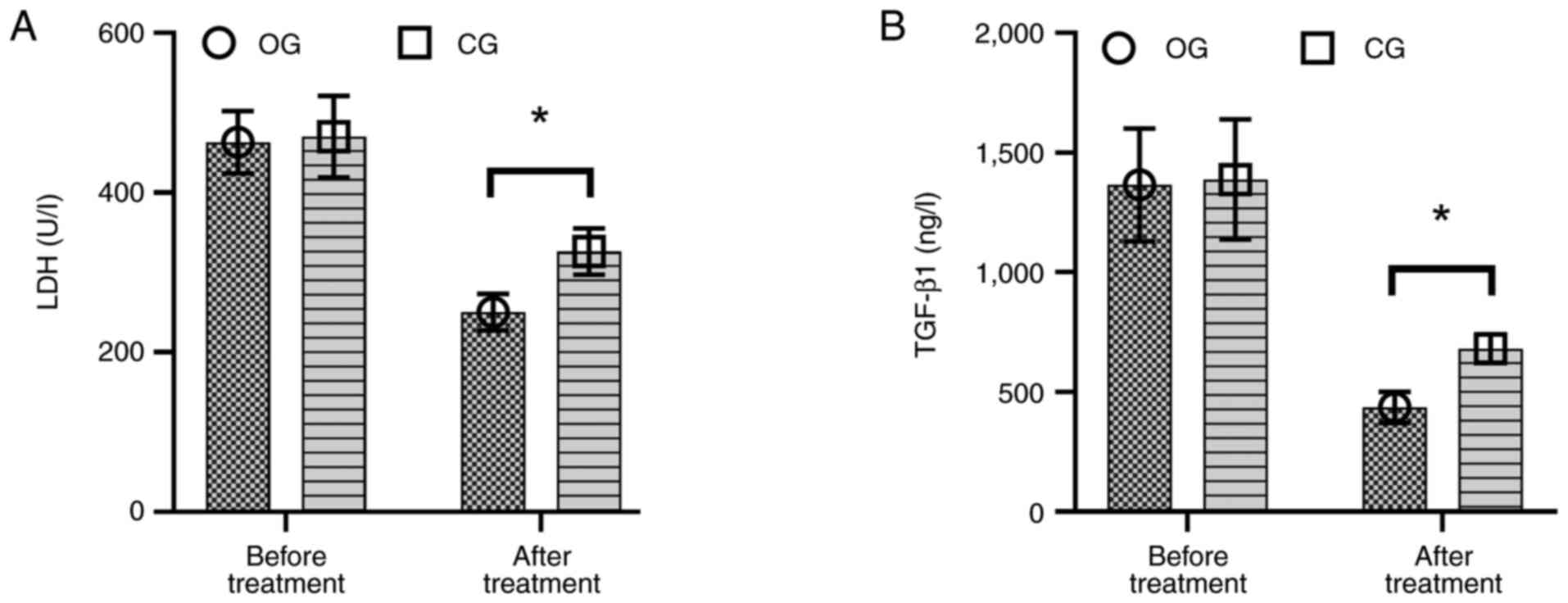
Before chemotherapy, there was no statistically
significant difference in serum LDH and TGF-β1 levels between the
two groups (P>0.05). However, upon discharge after chemotherapy,
patients in the OG exhibited significantly lower serum levels of
LDH (Fig. 3A) and TGF-β1 (Fig. 3B) compared with the CG (P=0.008,
P=0.003).
Comparison of KPS scores between the
two groups before and after treatment
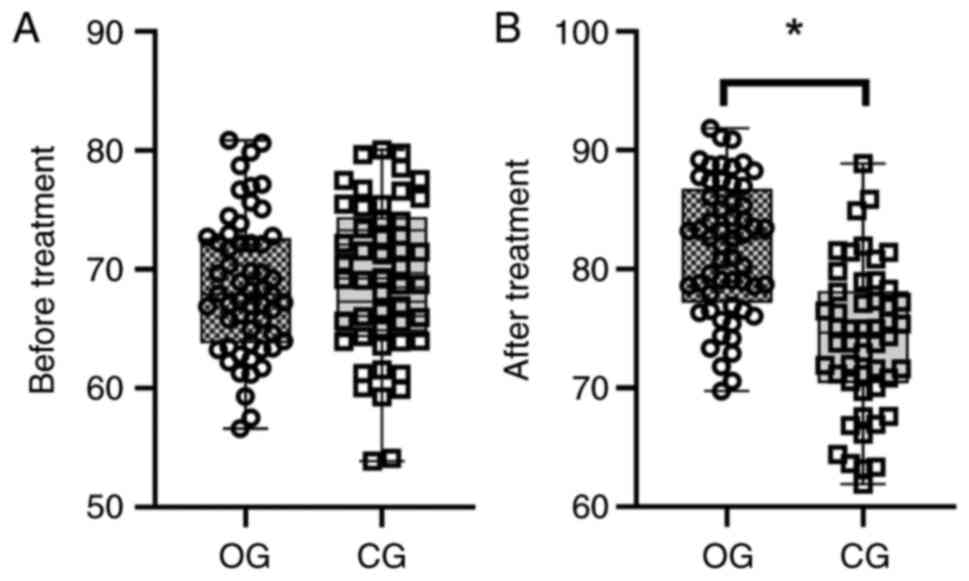
Before treatment, there was no statistically
significant difference in KPS scores between the two groups
(P>0.05; Fig. 4A). After
treatment, KPS scores in the OG were significantly higher compared
with those in the CG (P<0.001; Fig.
4B).
Analysis of prognostic factors in
patients with NHL
Univariate analysis revealed that the Ann Arbor
stage and the IPI score for lymphoma were significantly associated
with the prognosis of patients with NHL (P<0.001, P=0.002;
Table II). Furthermore, using
patient prognosis as the dependent variable and the Ann Arbor stage
and IPI score for lymphoma as independent variables, a multivariate
logistic regression analysis was performed, which indicated that
these indicators were independent prognostic risk factors for
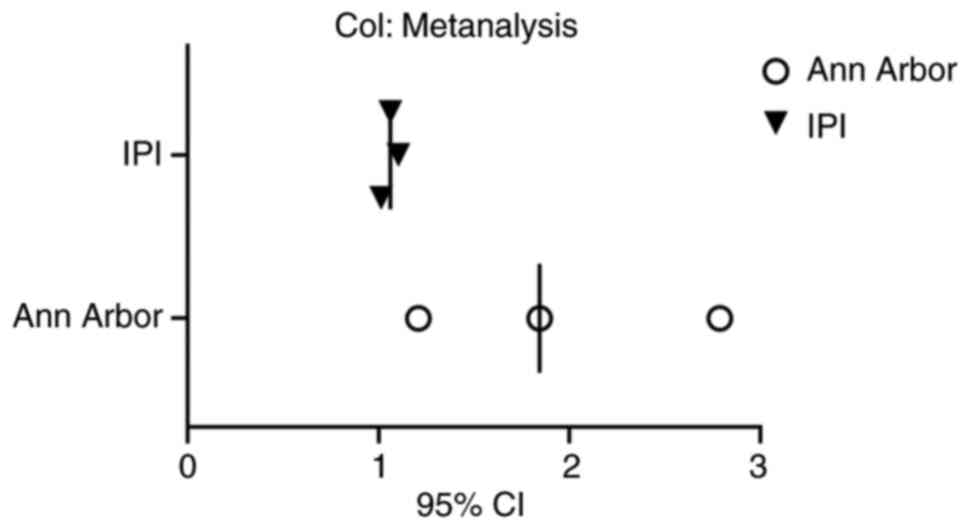
patients with NHL (P=0.005, P=0.008; Table III; Fig. 5).
 | Table II.Univariate analysis of prognostic
factors in patients with non-Hodgkin lymphoma. |
Table II.
Univariate analysis of prognostic
factors in patients with non-Hodgkin lymphoma.
| General clinical
data | Survival subgroup
(n=91) | Death subgroup
(n=15) |
t/χ2/Fisher | P-value |
|---|
| Sex |
|
| 2.323 | 0.128 |
|
Male | 61 | 7 |
|
|
|
Female | 30 | 8 |
|
|
| Age, years | 50.23±8.56 | 49.69±7.56 | 0.326 | 0.811 |
| Pathological
type |
|
| 0.889 | 0.346 |
| B
cell | 60 | 8 |
|
|
| T
cell | 31 | 7 |
|
|
| Ann Arbor
stage |
|
| 28.467 | <0.001 |
| II | 28 | 0 |
|
|
|
III | 52 | 4 |
|
|
| IV | 11 | 11 |
|
|
| International
Prognostic Index score for lymphoma, points |
|
| 10.892 | 0.002 |
|
0-1 | 28 | 0 |
|
|
|
2-3 | 63 | 14 |
|
|
|
4-5 | 0 | 1 |
|
|
| Therapeutic
measures |
|
| 2.665 | 0.103 |
|
Oxaliplatin combined with
gemcitabine | 51 | 5 |
|
|
|
Oxaliplatin | 40 | 10 |
|
|
 | Table III.Multivariate analysis of prognostic
factors in patients with non-Hodgkin lymphoma. |
Table III.
Multivariate analysis of prognostic
factors in patients with non-Hodgkin lymphoma.
| Risk factors | B | SE | Wald
χ2 | P-value | OR | 95% CI |
|---|
| Ann Arbor
stage | 0.617 | 0.203 | 8.135 | 0.005 | 1.844 | 1.209–2.787 |
| International
Prognostic Index score for lymphoma | 0.067 | 0.020 | 7.216 | 0.008 | 1.062 | 1.015–1.104 |
Discussion
Lymphoma is one of the most common types of
malignant tumors. Data have indicated that the incidence of
lymphoma has been increasing globally in recent years, which
accounts for 3–4% of all malignant tumor cases (21). Epidemiological studies have
indicated that lymphoma ranks 12th in terms of incidence in China,
with its mortality rate positioned 9th among malignant tumors in
men and 15th in women (22). NHL is
not a singular disease, rather a collective term for various
lymphomas with diverse biological behaviors, 85–90% of which
originate from B lymphocytes, with the remainder arising from T or
NK lymphocytes. Due to its high heterogeneity, there are notable
differences in treatment and prognosis (23). Currently, chemotherapy remains the
primary treatment for NHL. Although the advent of drugs such as
rituximab, alemtuzumab and oxaliplatin has improved the prognosis
of patients with NHL, clinical practice indicates that some
patients still experience poor efficacy and notable drug resistance
with oxaliplatin treatment or notable toxic side effects during
chemotherapy, adversely affecting their prognosis (24).
The present retrospective study compared the
intervention effects of oxaliplatin alone vs. oxaliplatin combined
with gemcitabine in patients with NHL. The results indicated that
the combination therapy significantly improves the overall
treatment efficacy and DCR compared with oxaliplatin monotherapy. A
multicenter study involving 196 patients with NHL indicated that
the combined use of oxaliplatin and gemcitabine is effective, with
63% of patients benefiting from the treatment, 33% achieving CR, a
PFS of 5 months and an OS of 10 months (25). Although oxaliplatin has a stronger
DNA inhibitory effect compared with traditional cisplatin
chemotherapy drugs (26),
oxaliplatin still belongs to the conventional platinum-based
chemotherapy category and carries a risk of resistance, thereby
reducing its efficacy. Gemcitabine is a novel nucleoside analogue
that primarily acts on S-phase cells, which inhibits DNA synthesis
and thereby induces apoptosis, and this mechanism endows
gemcitabine with broad-spectrum antitumor activity (27). Furthermore, the mechanisms of
gemcitabine and oxaliplatin are distinct, and their combination
produces a synergistic effect. Therefore, combination therapy is
more effective compared with monotherapy, as reflected in the
serological indicators and KPS scores of the two groups of patients
in the present study.
The present study further compared the safety
differences in chemotherapy between two groups of patients, which
indicated that the addition of gemcitabine did not significantly
increase the incidence of various toxic side effects, including
leukopenia and abnormal liver and kidney function. This may be
attributed to the reduction in the dosage of oxaliplatin due to the
addition of gemcitabine. This finding has also been corroborated in
previous studies. Qu et al (28) administered a salvage chemotherapy
regimen of decitabine combined with oxaliplatin to 14 patients with
relapsed NHL and the results indicated a 2-year OS rate of 42.7%,
markedly higher compared with previously reported levels; the most
common toxic side effect during treatment was hematologic toxicity,
but all adverse effects were reversible, suggesting a favorable
safety profile for the combination therapy. The advantage of
gemcitabine over conventional chemotherapeutic agents lies in its
self-potentiation mechanism, which enhances the efficacy of
combination therapy, allowing for a reduced dosage of other
chemotherapeutic drugs and thereby ensuring the safety of the
combined treatment (29).
The results of the present study indicated that
there were no statistically significant differences in serum LDH
and TGF-β1 levels between the two groups before chemotherapy;
however, after chemotherapy, the OG exhibited significantly lower
levels of both serum LDH and TGF-β1 compared with the CG. LDH, a
key glycolytic enzyme widely distributed in tissues, is often
elevated in patients with lymphoma, reflecting increased tumor
burden and heightened proliferative activity of malignant cells
(30). In the present study, the
post-treatment LDH levels in the OG were significantly reduced
compared with the CG, which suggests that the combination therapy
of gemcitabine and oxaliplatin more effectively suppressed the
metabolic activity and proliferation of tumor cells, thereby
reducing tumor burden. This finding aligns with the higher CRR and
DCR observed in the OG, further substantiating the advantage of the
combined regimen (19). TGF-β1, a
multifunctional cytokine, serves a complex role in tumorigenesis
and progression (31). While TGF-β1
may inhibit cellular proliferation during early tumor development,
it promotes tumor progression and metastasis in advanced stages
through mechanisms such as angiogenesis, immunosuppression and
epithelial-mesenchymal transition (20). The significant post-treatment
reduction in TGF-β1 levels observed in the OG suggests that
gemcitabine combined with oxaliplatin may exert a synergistic
antitumor effect by modulating the tumor microenvironment and
inhibiting the TGF-β1 signaling pathway.
Lastly, multivariate logistic regression analysis
was performed to investigate the risk factors affecting the
prognosis of patients with NHL and the results indicate that both
the Ann Arbor stage and the IPI score for lymphoma are independent
prognostic risk factors for patients with NHL, inconsistent with
the expected outcomes. We hypothesized that differences in
treatment regimens would impact the prognosis of patients with NHL.
The observed phenomenon may be attributed to the small sample size
of enrolled patients and the relatively short follow-up period.
Furthermore, the follow-up outcomes of the present study were only
survival or mortality, whereas recurrence rate, which serves as
another key indicator for evaluating NHL prognosis, was not
explored due to limitations in follow-up data.
Although the present study offers notable insights
into the treatment and prognostic analysis of patients with NHL, it
has certain limitations: i) The present study is a retrospective
analysis with a relatively small sample size; however, the
inability to randomize patient grouping may introduce some bias in
the results; ii) due to the retrospective design in nature, the
selection of certain variables was limited. For instance,
prognostic factor analysis may be influenced by elements such as
molecular biomarkers and gene mutations; however, these factors
could not be assessed in the present study; iii) in assessing the
prognosis of patients with NHL, the present study only used OS as
the primary evaluation criterion; however, factors such as quality
of life and disease recurrence may also exert a considerable
influence on patient prognosis; and iv) due to the limited number
of cases, it was not possible to comprehensively classify all NHL
subtypes in accordance with the 2016 WHO classification. The
intrinsic heterogeneity of NHL may impact the findings.
Furthermore, even within the same NHL subtype, notable variations
may exist in genetic background, immune status and treatment
response. These differences warrant further elucidation of the
molecular heterogeneity of NHL through genomic,
immunohistochemistry and other advanced techniques, thereby
informing and guiding personalized therapeutic strategies.
Due to the aforementioned limitations of the present
study, future research directions may include the following. First,
the design of prospective randomized controlled trials is
warranted. Future studies should be performed as multicenter,
large-sample, randomized prospective studies to further validate
the efficacy and safety of the gemcitabine-oxaliplatin regimen in
the treatment of NHL, thereby enhancing the level of evidence.
Second, considering the potential impact of heterogeneity of NHL on
treatment outcomes, future studies should strictly classify NHL
subtypes according to the WHO 2016 classification criteria and
evaluate the differential responses of various subtypes to
combination therapy, thereby providing a foundation for precision
treatment. Third, prognostic analyses should incorporate a broader
range of molecular biomarkers and genetic mutations, such as Myc,
Bcl-2 and Bcl-6 rearrangements or expression, p53 mutations and
CD20 expression levels, combined with clinical staging and the IPI
to construct a more accurate prognostic evaluation model. Fourth,
in recent years, the therapeutic landscape of NHL has undergone
profound transformation with the emergence of various
immunotherapeutic strategies, including immune checkpoint
inhibitors, CAR-T cells and bispecific antibodies. These novel
therapies hold notable potential, particularly in certain NHL
subtypes, particularly among patients with relapsed or refractory
NHL. Future studies may compare the efficacy of the
gemcitabine-oxaliplatin regimen with these emerging immunotherapies
or explore the optimal sequencing or combination strategies
integrating chemotherapy with immunotherapy.
In conclusion, the combination therapy of
gemcitabine and oxaliplatin was confirmed to be safe for patients
with NHL. This treatment demonstrated notable overall efficacy and
improved functional improvement compared with oxaliplatin
monotherapy. The Ann Arbor stage and the IPI score for lymphoma are
independent prognostic factors for patients with NHL.
Acknowledgements
Not applicable.
Funding
The present study was supported by the 2022 National Health
Commission Clinical Specialist Talent Professional Ability
Innovation and Application Research Project (grant no.
RCLX2315009).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XZ and FL designed the present study. XZ, GL, FL,
RS, JW, WW and JD performed the research and analyzed the data. XZ
and GL contributed towards novel methods in the present study and
wrote the manuscript. WW and JD confirm the authenticity of the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Xi'an Central
Hospital/Xi'an Institute of Hematology ethics committee (approval
no. 2024-JT-023; Xi'an, China). All study participants provided
written informed consent before participating in the present
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Thandra KC, Barsouk A, Saginala K, Padala
SA, Barsouk A and Rawla P: Epidemiology of non-Hodgkin's lymphoma.
Med Sci (Basel). 9:52021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zanoni L, Bezzi D, Nanni C, Paccagnella A,
Farina A, Broccoli A, Casadei B, Zinzani PL and Fanti S: PET/CT in
non-Hodgkin lymphoma: An Update. Semin Nucl Med. 53:320–351. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Laurent C, Cook JR, Yoshino T,
Quintanilla-Martinez L and Jaffe ES: Follicular lymphoma and
marginal zone lymphoma: how many diseases? Virchows Arch.
482:149–162. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alderuccio JP and Kahl BS: Current
treatments in marginal zone lymphoma. Oncology (Williston Park).
36:206–215. 2022.PubMed/NCBI
|
|
5
|
Iwamuro M, Tanaka T and Okada H: Review of
lymphoma in the duodenum: An update of diagnosis and management.
Virchows Arch. 29:1852–186. 2023.
|
|
6
|
Goodlad JR, Cerroni L and Swerdlow SH:
Recent advances in cutaneous lymphoma-implications for current and
future classifications. Virchows Arch. 482:281–298. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Olsen EA, Whittaker S, Willemze R,
Pinter-Brown L, Foss F, Geskin L, Schwartz L, Horwitz S, Guitart J,
Zic J, et al: Primary cutaneous lymphoma: recommendations for
clinical trial design and staging update from the ISCL, USCLC, and
EORTC. Blood. 140:419–437. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bender JD, Rubinstein JD, Mizukawa B,
Perentesis JP and Pommert L: Use of gemcitabine, oxaliplatin, and
anti-CD20 therapy in children and adolescents with non-Hodgkin
lymphoma unfit for intensive therapy. Pediatr Blood Cancer.
70:e302142023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tian XP, Cai J, Xia Y, Zhang YC, Wang L,
Liu PP, Huang HQ, Li YJ, Zhou H, Li ZM, et al: First-line
sintilimab with pegaspargase, gemcitabine, and oxaliplatin in
advanced extranodal natural killer/T cell lymphoma (SPIRIT): A
multicentre, single-arm, phase 2 trial. Lancet Haematol.
11:e336–e344. 2024. View Article : Google Scholar
|
|
10
|
St-Pierre F and Gordon LI: CAR T-cell
therapy for relapsed/refractory non-Hodgkin's lymphoma: A
comprehensive review. Clin Adv Hematol Oncol. 20:309–318. 2022.
|
|
11
|
Alnasser SM, Alharbi KS, Almutairy AF,
Almutairi SM and Alolayan AM: Autologous stem cell transplant in
Hodgkin's and Non-Hodgkin's lymphoma, multiple myeloma, and AL
amyloidosis. Cells. 12:28552023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kelley RK, Ueno M, Yoo C, Finn RS, Furuse
J, Ren Z, Yau T, Klümpen HJ, Chan SL, Ozaka M, et al: Pembrolizumab
in combination with gemcitabine and cisplatin compared with
gemcitabine and cisplatin alone for patients with advanced biliary
tract cancer (KEYNOTE-966): A randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet. 401:1853–1865. 2023.
View Article : Google Scholar
|
|
13
|
Gravett AM, Dalgleish AG and Copier J: In
vitro culture with gemcitabine augments death receptor and NKG2D
ligand expression on tumour cells. Sci Rep. 9:15442019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van der Heijden MS, Sonpavde G, Powles T,
Necchi A, Burotto M, Schenker M, Sade JP, Bamias A, Beuzeboc P,
Bedke J, et al: Nivolumab plus gemcitabine-cisplatin in advanced
urothelial carcinoma. N Engl J Med. 389:1778–1789. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu J, Zhou Y, Li Q, Zhang J and Mao Y:
Primary biliary non-Hodgkin's lymphoma: A case report. Medicine
(Baltimore). 100:e261102021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gante J, Georg S, Robez JG, Dubuc A and
Lauwers F: Primary extra-nodal non-Hodgkin's lymphoma affecting
mandibular bone: A case report. Pan Afr Med J. 41:2312022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheson BD, Fisher RI, Barrington SF,
Cavalli F, Schwartz LH, Zucca E, Lister TA; Alliance, Australasian
Leukaemia; Lymphoma Group and Eastern Cooperative Oncology Group;
European Mantle Cell Lymphoma Consortium;, ; et al: Recommendations
for initial evaluation, staging, and response assessment of Hodgkin
and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol.
32:3059–3068. 2014. View Article : Google Scholar
|
|
18
|
Argumánez Tello V, Sánchez-Montes C,
Mínguez Sabater A, Bauza M and Bustamante-Balén M: Diagnosis of
non-Hodgkin's lymphoma due to a tiny polyp in the cecum. Rev Esp
Enferm Dig. 115:732–733. 2023.
|
|
19
|
Zheng Q, Xie Y, Xu L, Chen D, Wu J, Liu S,
Wu L, Fang P and Xie F: LDHA as a predictive biomarker and its
association with the infiltration of immune cells in pancreatic
adenocarcinoma. J Gastrointest Oncol. 15:1746–1759. 2024.
View Article : Google Scholar
|
|
20
|
Tas F, Yasasever CT, Karabulut S, Tastekin
D and Duranyildiz D: Serum transforming growth factor-beta1 levels
may have predictive and prognostic roles in patients with gastric
cancer. Tumour Biol. 36:2097–2103. 2015. View Article : Google Scholar
|
|
21
|
Lin WH and Wu MN: Non-Hodgkin's lymphoma
with intraspinal involvement mimics bilateral thoracolumbar
plexopath. Acta Neurol Taiwan. 32:122–126. 2023.
|
|
22
|
Kassar O, Kahla AB, Koubaa A, Kallel F,
Amor IB and Elloumi M: Non-Hodgkin's lymphoma developed during
imatinib mesylate treatment of chronic myeloid leukemia. J Oncol
Pharm Pract. 29:996–998. 2023. View Article : Google Scholar
|
|
23
|
Jariwal R, Raza N, Bhandohal J and Cobos
E: Non-Hodgkin's plasmablastic lymphoma as initial presentation of
human immunodeficiency virus. J Investig Med High Impact Case Rep.
9:232470962110146892021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Liu X, Yan P, Bi Y, Liu Y and
Zhang ZJ: Association between type 1 and type 2 diabetes and risk
of non-Hodgkin's lymphoma: A meta-analysis of cohort studies.
Diabetes Metab. 46:8–19. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cazelles C, Belhadj K, Vellemans H, Camus
V, Poullot E, Gaulard P, Veresezan L, Itti E, Becker S, Carvalho M,
et al: Rituximab plus gemcitabine and oxaliplatin (R-GemOx) in
refractory/relapsed diffuse large B-cell lymphoma: A real-life
study in patients ineligible for autologous stem-cell
transplantation. Leuk Lymphoma. 62:2161–2168. 2021. View Article : Google Scholar
|
|
26
|
Cui S, Huang J, Wei D, Yu L and Jiang S:
Oxaliplatin inhibits the EGFR-MAPK pathway to promote apoptosis in
HCT116 cells. Chin Pharmacol Bull. 38:1279–1280. 2022.
|
|
27
|
Zhang Z, Yu H, Yao W, Zhu N, Miao R, Liu
Z, Song X, Xue C, Cai C, Cheng M, et al: RRP9 promotes gemcitabine
resistance in pancreatic cancer via activating AKT signaling
pathway. Cell Commun Signal. 20:1882022. View Article : Google Scholar
|
|
28
|
Qu C, Ping N, Kong D, Liu A, Liu H, Xu T,
Xia F, Wu D and Jin Z: Dual epigenetic agents plus
rituximab-gemcitabine-oxaliplatin as salvage treatment in
relapsed/refractory diffuse large B-cell lymphoma patients failure
of salvage chemotherapy. Hematol Oncol. 40:914–921. 2022.
View Article : Google Scholar
|
|
29
|
Roider T, Wang X, Hüttl K, Müller-Tidow C,
Klapper W, Rosenwald A, Stewart JP, de Castro DG, Dreger P, Hermine
O, et al: The impact of SAMHD1 expression and mutation status in
mantle cell lymphoma: An analysis of the MCL Younger and Elderly
trial. Int J Cancer. 148:150–160. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Galleze A, Raache R, Cherif N, Eddaikra A,
Belhani M, Bensenouci A, Touil-Boukoffa C and Abbadi MC: Increased
level of lactate dehydrogenase correlates with disease growth in
Algerian children with lymphoma. J Hematol Oncol Res. 2:7–15. 2017.
View Article : Google Scholar
|
|
31
|
Xue VW, Chung JY, Córdoba CAG, Cheung AH,
Kang W, Lam EW, Leung KT, To KF, Lan HY and Tang PM: Transforming
growth factor-β: A multifunctional regulator of cancer immunity.
Cancers (Basel). 12:30992020. View Article : Google Scholar : PubMed/NCBI
|