Introduction
Neuroendocrine neoplasms (NENs) are tumors with
neuroendocrine differentiation and expression of neuroendocrine
markers, which can occur in various organs; however, they most
commonly in the gastrointestinal tract, accounting for 55–70% of
all NEN cases (1). Esophageal
neuroendocrine carcinoma (ENEC) is a rare subtype within digestive
tract NENs, comprising only 0.4–1% of gastroenteropancreatic NENs.
ENEC is highly aggressive, prone to widespread metastasis and has a
markedly worse prognosis compared with other common types of
esophageal cancer, such as squamous cell carcinoma and
adenocarcinoma (2).
Epidemiological studies have indicated that ENEC is
more common in middle-aged and elderly men, and that it shows
geographical clustering with higher incidence rates in certain
regions, such as East Asia (including in China and Japan) (2,3). The
risk factors for ENEC partially overlap with those of esophageal
squamous cell carcinoma (ESCC), such as smoking, alcohol
consumption, and a diet characterized by the frequent consumption
of foods and beverages at very high temperatures (>65°C has been
classified as probably carcinogenic to humans by the International
Agency for Research on Cancer) (4),
which may induce DNA damage and epigenetic changes in the
esophageal mucosa (5,6). Certain patients with ENEC also present
with Barrett's esophagus, which suggests that chronic inflammation
might drive neuroendocrine differentiation through abnormal
proliferative signaling pathways (7).
ENEC exhibits high biological heterogeneity. Early
studies have suggested that it originates from amine precursor
uptake and decarboxylation cells in the esophageal mucosa, derived
from the neuroectoderm (8,9). However, more recent research has
proposed that it likely originates from pluripotent basal
epithelial stem cells in the esophagus, which can differentiate
into squamous or glandular epithelium under normal conditions but
may aberrantly differentiate into NEC under epigenetic or
microenvironmental pressure, forming mixed tumors (10).
The present review particularly focuses on ENEC due
to its distinct clinicopathological features and highly aggressive
biological behavior. This focused approach is predicated on the
distinct clinicopathological and molecular features of ENEC, which
are justified by the fundamental differences between ENEC and other
gastrointestinal NECs, particularly gastric NEC (GNEC). First, ENEC
and GNEC possess unique molecular profiles; ENECs are characterized
by a high frequency of co-mutations in tumor protein 53 and
retinoblastoma gene 1, resembling small cell lung cancer, whereas
GNECs exhibit considerable heterogeneity with alterations in genes
such as low-density lipoprotein receptor-related protein 1B and
dysregulation of pathways such as Wnt/β-catenin (11). Second, they arise from different
epidemiological origins; ENEC shares strong associations with risk
factors for ESCC (for example, smoking and alcohol) (12), whereas GNEC is often associated with
chronic atrophic gastritis analogous to gastric adenocarcinoma
(13). Notably, ENEC is associated
with a markedly worse prognosis compared with GNEC, as confirmed by
large-scale database analyses (3).
Current evidence for the management of ENEC is often extrapolated
from small cell lung cancer or aggregated with other NECs,
underscoring the need for this particular synthesis of
ENEC-specific data to provide clinicians with a nuanced overview of
contemporary management and potential future directions.
While the seminal review by Ma et al
(1) provided a key foundation to
understand ENEC, the subsequent 8 years (2017–2025) have witnessed
a paradigm shift in its management, which forms the core
contribution of current research. The present review synthesizes
these recent advances, which include the successful application of
immune checkpoint inhibitors (for example, nivolumab and
camrelizumab) (14–16), the emergence of combination
immunotherapy strategies targeting cytotoxic
T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell
death protein-1 (PD-1) (17,18),
and novel regimens combining anti-angiogenic tyrosine kinase
inhibitors (TKIs) with immunotherapy (for example, anlotinib plus
camrelizumab) (19–21). The present review also provides a
key evaluation of refined chemotherapeutic approaches, such as the
validation of folinic acid + fluorouracil + irinotecan (FOLFIRI) in
second-line settings (22,23) and the exploration of modified
folinic acid + fluorouracil + irinotecan + oxaliplatin
(mFOLFIRINOX, which typically involves dose adjustments of the
constituent drugs to improve tolerability) (22), moving beyond the traditional
platinum-etoposide (EP) backbone. Furthermore, to translate
evidence into practice, the present review offers structured
guidance for clinicians, incorporating insights from recent trials
(24–26) and summaries of ongoing clinical
studies (for example, NCT04325425 and NCT04169672) (21,26).
This comprehensive and up-to-date review aimed to equip clinicians
with the knowledge to navigate the rapidly evolving therapeutic
landscape of this aggressive malignancy in the future.
Pathological features and diagnosis
Pathological classification
According to the 2019 World Health Organization
classification of digestive system NENs (27), NENs are classified into
well-differentiated neuroendocrine tumors (NETs), poorly
differentiated NECs and mixed neuroendocrine-non-NENs (MiNENs).
NETs are graded G1 to G3 based on mitotic count and Ki-67 index
(27). NECs are divided into small
cell NEC (SCNEC) and large cell NEC (LCNEC), with SCNEC accounting
for ~90% of all ENEC cases. Grossly, NECs often present as invasive
submucosal masses or ulcerative lesions with esophageal lumen
narrowing (28). Histologically,
SCNEC exhibits oat cell-like nests with necrosis, whereas LCNEC
features vesicular nuclei, prominent nucleoli and rosette
structures. MiNENs contain ≥30% neuroendocrine components and may
also exhibit adenocarcinoma or squamous differentiation (29). In China, MiNENs are usually
squamous-dominant, whereas in Western countries, they often coexist
with adenocarcinoma (30) (Table I).
 | Table I.Classification of NENs. |
Table I.
Classification of NENs.
| Type of NEN | Morphological and
molecular features |
|---|
| NET |
|
| G1 | Mitotic count,
<2/2 mm2 (or <2/10 HPF); Ki-67 index, ≤3% |
| G2 | Mitotic count,
2–10/2 mm2 (or 2–20/10 HPF); Ki-67, 3–20% |
| G3 | Mitotic count,
>10/2 mm2 (or >20/10 HPF); Ki-67, >20% |
| NEC |
|
| Small
cell NEC (accounts for | High mitotic count,
>10/2 mm2 (or >20/10 HPF); Ki-67, >20% (often
>55%) |
| ~90% of
esophageal NEC cases) |
|
| Large
cell NEC | High mitotic count,
>10/2 mm2; Ki-67, >20% (often >55%) |
| MiNEN | Biphasic tumor with
≥30% each of neuroendocrine (NET/NEC) and non-neuroendocrine
components |
Diagnosis
Pathological confirmation of NENs necessitates a
multimodal diagnostic approach. Immunohistochemically,
synaptophysin (Syn) exhibits high sensitivity (>95%) and is
expressed in virtually all NENs. Chromogranin A (CgA) is more
specific but less expressed in poorly differentiated NECs,
requiring additional markers such as CD56 and neuron-specific
enolase (31).
Insulinoma-associated protein 1, a sensitive and specific nuclear
marker for neuroendocrine differentiation, is increasingly used in
diagnostic panels, particularly when conventional markers are
equivocal (31–33). However, the interpretation of
immunohistochemical markers in NECs poses notable challenges. Key
interpretative pitfalls include: i) Heterogeneous or weak
expression levels of Syn and CgA in poorly differentiated tumors,
which may lead to false-negative diagnoses; ii) non-specific
staining of CD56 observed in a range of non-neuroendocrine
malignancies, such as small cell lung carcinoma, lymphoma, melanoma
and certain types of sarcoma; and iii) highly variable expression
patterns in MiNENs, underscoring the necessity of extensive tumor
sampling and the application of a comprehensive antibody panel to
prevent misclassification (32).
Imaging features of ENEC markedly overlap with ESCC
and esophageal adenocarcinoma (EAC), making radiological
distinction challenging. Specifically, ENEC typically presents on
X-ray barium swallow as irregular mucosal destruction, strictures
or filling defects, similar to the appearances seen in ESCC and
EAC. On CT and enhanced CT, ENEC commonly manifests as focal or
circumferential wall thickening with heterogeneous enhancement,
patterns that are also frequently observed in advanced ESCC and
EAC. For ENEC, enhanced CT is crucial for assessing primary tumor
location, local invasion and distant metastasis (particularly to
the liver), with reported sensitivity for detecting liver
metastases reaching up to 79% (34,35).
Furthermore, endoscopic ultrasound accurately assesses tumor
origin, size and invasion depth, although it cannot reliably
differentiate ENEC from ESCC or EAC based solely on imaging
characteristics (34). PET-CT is
used for staging and recurrence detection (36). Somatostatin receptor (SSTR)
scintigraphy, using 111In-labeled octreotide, is more sensitive for
the detection of well-differentiated NETs and their metastases
(particularly in the liver and lungs). The sensitivity of SSTR
scintigraphy for poorly differentiated NECs is generally lower due
to reduced or absent SSTR expression (37).
The assessment of SSTR expression via functional
imaging also has potential therapeutic implications. Peptide
receptor radionuclide therapy (PRRT), such as
177Lu-oxodotreotide, is a well-established treatment
option for well-differentiated, SSTR+ NETs (38). However, to the best of our
knowledge, its role in poorly differentiated NECs, including ENEC,
remains limited and non-standard; this is primarily due to the
frequent absence or low density of SSTR expression in poorly
differentiated NECs, which precludes the use of SSTR-targeted
therapies (36). Therefore, patient
selection for PRRT in high-grade disease hinges on demonstrating
adequate SSTR expression on functional imaging, a finding that is
uncommon in NEC. Although anecdotal case reports exist (38,39),
robust clinical trial data supporting the efficacy of PRRT in ENEC
are currently lacking.
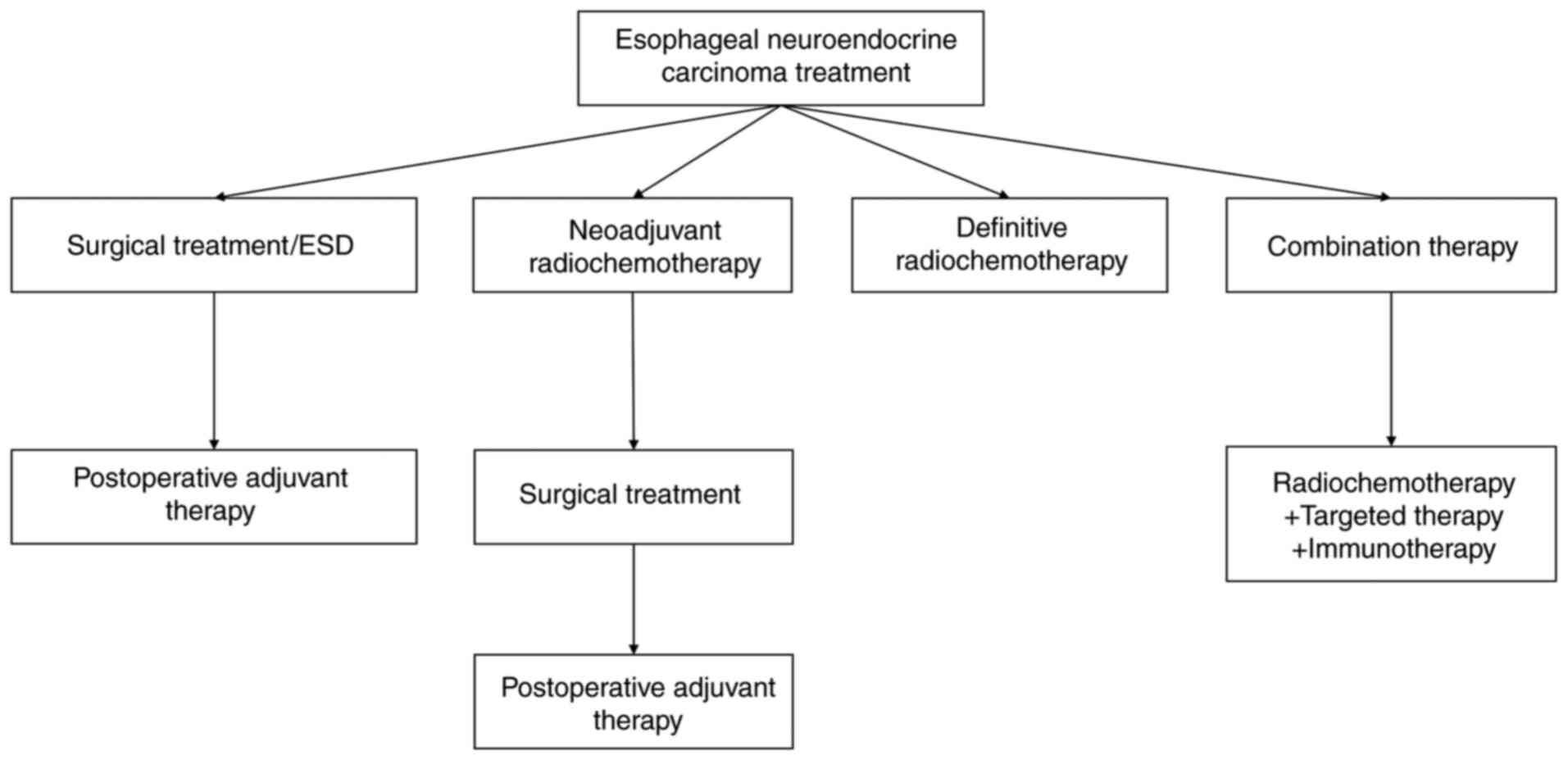
Treatment strategies
The management of ENEC involves a multimodal
approach, including surgery, platinum-based chemotherapy,
radiotherapy, targeted therapy and immunotherapy (Fig. 1).
Endoscopic treatment
Endoscopic submucosal dissection (ESD) can be
considered for highly selected cases of early ENEC confined to the
mucosa (Tis or T1a according to the American Joint Committee on
Cancer TNM staging system, 8th edition) (40) with a diameter of <1 cm (41). Although rare, such early cases have
been successfully treated with ESD. Case reports have described
patients achieving long-term disease-free survival following ESD
without adjuvant therapy (42,43).
For example, Fukui et al (42) documented a patient with pT1a ENEC
(muscularis mucosae invasion) who declined adjuvant therapy
post-ESD and remained recurrence-free during the 15-month
follow-up. Similarly, Cheng et al (43) reported a 3-year disease-free
survival in a case of NEC arising at the esophagogastric junction
without lymphovascular invasion, treated solely by ESD.
Neoadjuvant and/or adjuvant combined
surgery
Multimodal therapy combining neoadjuvant and/or
adjuvant therapy with surgery is standard for locally advanced
ENEC. Patients with resectable tumors and no lymph node metastasis
may undergo surgery first, followed by adjuvant chemoradiotherapy
if necessary (13). For initially
unresectable cases, neoadjuvant therapy may enable surgery
(1). Previous studies have
suggested that neoadjuvant chemoradiotherapy followed by surgery
markedly improves median overall survival (mOS) compared with
surgery or chemoradiotherapy alone (44–46).
In patients with initially unresectable locally
advanced ENEC, neoadjuvant therapy may facilitate surgical
conversion, followed by personalized adjuvant regimens (47). A multicenter trial by Shapiro et
al (48) demonstrated that
neoadjuvant chemoradiotherapy combined with surgery markedly
improved mOS in locally advanced ESCC (81.6 vs. 21.1 months) and
adenocarcinoma (43.2 vs. 27.1 months), establishing this approach
as the standard of care. Although ENEC was not included in this
previous study, extrapolation of these principles supports
guideline recommendations. The Clinical Practice Guidelines for
Gastrointestinal and Pancreatic Neuroendocrine Tumors conditionally
endorse neoadjuvant chemotherapy for borderline resectable ENEC
(49).
A previous systematic review revealed notably
increased survival outcomes with perioperative chemotherapy
(neoadjuvant, 31 months; adjuvant, 25 months) versus surgery alone
(9 months) in stage I–III ENEC, although no notable difference
existed between the adjuvant and neoadjuvant groups (50). Notably, adding radiotherapy to
neoadjuvant chemotherapy enhances surgical resectability and
pathological complete response rates but does not improve survival
(13). Awada et al (51) documented a case of poorly
differentiated ENEC treated with neoadjuvant chemoradiotherapy and
surgery, achieving >5 years of survival.
Radiation therapy
For patients with locally advanced, inoperable ENEC
tumors, definitive chemoradiotherapy is recommended (52). Treatment plans should be based on
the extent of invasion and lymph node involvement. Combined
chemoradiotherapy yields a 3-year survival rate of ~31.6% (53). A Japanese retrospective study
further supported chemoradiotherapy as a viable option for locally
advanced ENEC (45). However,
unlike in limited-stage small cell lung cancer where it is a
standard of care, prophylactic brain irradiation is not routinely
recommended due to the low incidence of brain metastases in ENEC
(3).
Chemotherapy
First-line treatment
For advanced ENEC, systemic chemotherapy remains
essential (46). First-line
regimens are platinum-based doublets, either etoposide + platinum
(EP) or irinotecan + platinum (IP). Capecitabine with temozolomide
(CAPTEM), folinic acid + fluorouracil + oxaliplatin (FOLFOX),
FOLFIRI and FOLFIRINOX have also demonstrated activity (Table II) (54). Previous studies have reported
variable response rates [objective response rate (ORR), 14–75%] and
a median progression-free survival (PFS) time of 1.8–8.9 months
(25,54–56).
 | Table II.Comparison between first-line
chemotherapy regimens. |
Table II.
Comparison between first-line
chemotherapy regimens.
| Regimen | Key data | Toxicity
profile | Evidence level |
|---|
| EP/EC | mOS, 12.5 months
(25); ORR, 14–75% (56,57);
mPFS, 5.36 months (25) | Neutropenia (90%),
anemia and thrombocytopenia |
Guideline-recommended (69) |
| IP | mOS, 10.9 months;
no notable difference vs. EP (25) | Diarrhea (50%),
neutropenia (60%) | Phase III
trial |
| CAPTEM | mOS, 12.6 months;
mPFS, 2.43 months (markedly lower compared with EP) (24) | Grade 3/4 adverse
events, 29% (markedly lower compared with EP) | Phase II trial |
| FOLFOX | DCR, 91.3%
(first-line); mPFS, 10 months (66) | Mild toxicity
(neurotoxicity and diarrhea) | Retrospective
study |
| FOLFIRI | DCR, 91%
(metastatic GI NEC) (67) | Neutropenia and
diarrhea | Small-sample
study |
| FOLFIRINOX | ORR, 46%; mOS, 17.8
months (n=8) (62) | High toxicity
(hematological and gastrointestinal) | Early
exploratory |
| mFOLFIRINOX | ORR, 77%; mOS, 20.6
months (n=35) (22) | Markedly higher
severe toxicity risk in women | Modified regimen
study |
The EP or etoposide and carboplatin (EC) regimen is
widely used in NEC, while IP demonstrates comparable efficacy but
distinct toxicity profiles. Retrospective studies have indicated
variable response rates (ORR, 14–75%) and a median PFS time of
1.8–8.9 months (57). The phase III
JCOG1213 TOPIC-NEC trial identified no notable difference in mOS
between EP and IP regimens (EP group, 12.5 months vs. IP group,
10.9 months). The ENEC subgroup (15.5% in EP vs. 9.3% in IP)
demonstrated no clear advantage in overall survival compared with
the IP regimen. Toxicity profiles differed; for example,
neutropenia occurred in 90% of patients treated with EP, whereas
diarrhea affected ~50% of patients treated with IP, necessitating
regimen selection based on individual tolerance (25). For patients who are
cisplatin-intolerant, the EC regimen serves as an alternative,
indicating efficacy comparable to EP (58). Notably, patients with Ki-67 >55%
exhibit lower ORR to platinum-based chemotherapy but improved
survival, a paradoxical phenomenon requiring further mechanistic
study and context-specific clinical strategies (59).
Despite high response rates, the short PFS of
first-line platinum-based regimens has prompted exploration of
alternative therapies. It is important to distinguish high-grade
NENs here; while the CAPTEM regimen is an option for advanced
well-differentiated G3 NET (neoplasia), it is not a standard
first-line therapy for poorly differentiated NEC. The role of
CAPTEM has primarily been explored in later-line settings (24,60).
Trials such as NCT04325425 are ongoing to compare mFOLFIRINOX and
EP regimens (61). FOLFOX and
FOLFIRI are being evaluated as alternatives or second-line
regimens, with varying degrees of disease control (62–64).
These exhibit antitumor activity in NEC; however, to the best of
our knowledge, randomized prospective phase II studies remain
limited and no international consensus exists. In a previous study
by Merola et al (65)
involving 72 patients with advanced cases (44.5% NET; 55.5% NEC),
FOLFOX achieved a disease control rate (DCR) of 75% and mPFS of 8
months, with first-line DCR reaching 91.3% (mPFS, 10 months) and
manageable toxicity. Extended treatment cycles were recommended for
well-tolerated patients. Du et al (66) reported a DCR of 91% for FOLFIRI in
11 metastatic gastrointestinal NEC cases. An early small-sample
study (n=8) of FOLFIRINOX demonstrated an ORR of 46% and mOS of
17.8 months in first-line treatment (61). A mFOLFIRINOX regimen (n=35) achieved
an ORR of 77% (mOS, 20.6 months), although severe toxicity in
female patients warrants caution (22). Most of these aforementioned studies
were retrospective with small sample sizes (n, 8–72), which
warrants the validation of FOLFOX efficacy. The ongoing randomized
phase II trial NCT04325425 comparing mFOLFIRINOX and EP regimens
may provide higher-level evidence for first-line NEC treatment
(26).
Second and multiple lines of
treatment
Second-line treatments have demonstrated limited
effectiveness (ORR, ~18%) (56).
Platinum rechallenge is considered for patients with progression
>6 months after first-line therapy (67). Irinotecan-based (FOLFIRI) and
oxaliplatin-based (FOLFOX) regimens are used in platinum-resistant
cases (62,64). Temozolomide-based regimens have
exhibited modest activity, particularly in O6-methylguanine-DNA
methyltransferase (MGMT)-deficient tumors. Randomized trials are
ongoing to refine second-line therapy strategies. Table III compares the main second-line
chemotherapy regimens.
 | Table III.Second-line chemotherapy regimens
comparison. |
Table III.
Second-line chemotherapy regimens
comparison.
| Regimen | mOS, months | mPFS, months | ORR, % | DCR, % | Evidence level |
|---|
| Platinum
rechallenge | 11.7 (71) | 3.2 (71) | 17/31 (56,70) | 62 (71) | Retrospective
study |
| FOLFIRI | 5.9–18 (63,64,72) | 4.4–5.8 (63,64,72) | - | 44-80 (63,64,72) | Heterogeneous
evidence |
| FOLFOX | - | - | - | 64 (65) | Retrospective
study |
| CAPTEM | 12.1–22 (74,76) | 5.86 (76) | 26 (61) | - | Evidence from
studies with conflicting results |
| Topoisomerase I
inhibitors | 4.3 (78) | 1.8 (78) | - | 15 (78) | TLC388 trial |
Platinum rechallenge
Current consensus, as reflected in international
guidelines such as those from the National Comprehensive Cancer
Network (NCCN) and expert group recommendations, recommends regimen
selection based on the duration of response to first-line
chemotherapy (67,68). Patients with a time to progression
of >6 months may consider EP rechallenge, whereas those with a
time to progression of ≤6 months may benefit from regimens such as
FOLFIRI or CAPTEM (67).
Platinum-based rechallenge strategies have achieved ORRs of 17 and
31% in extrapulmonary NEC (55,69).
In a nationwide multicenter study by Hadoux et al (70), platinum rechallenge chemotherapy
demonstrated a DCR of 62% (mPFS, 3.2 months; mOS, 11.7 months).
Patients with relapse-free intervals ≥3 months after first-line EP
chemotherapy who received rechallenge therapy had markedly longer
mOS (12 vs. 5.9 months). However, ENEC-specific subgroup analyses
were lacking.
FOLFIRI program
Irinotecan-based regimens have been demonstrated to
have heterogeneous efficacy. Previous studies have reported DCRs of
62–80% with FOLFIRI after platinum resistance (mPFS, 4–5.8 months;
mOS, 11–18 months) (62,63). The PRODIGE 41-BEVANEC study
validated FOLFIRI as a second-line option but identified no added
benefit with bevacizumab (23). By
contrast, Bardasi et al (71) demonstrated a lower DCR (44.1%; mOS,
5.9 months; mPFS, 4.4 months), highlighting discrepancies possibly
due to study design or population characteristics. While FOLFIRI
remains feasible after EP failure, its efficacy requires
confirmation in prospective trials with controlled confounding
factors. The NET-02 trial (n=102) compared liposomal irinotecan +
5-fluorouracil versus docetaxel in extrapulmonary NEC. Although no
notable difference in mPFS or mOS was observed, the 6-month PFS
rate doubled (29.6 vs. 13.8%), suggesting subgroup benefits. The
trial failed its primary endpoint, Therefore, the potential
advantage of the liposomal irinotecan combination regimen over
docetaxel remains to be fully elucidated (72).
FOLFOX program
A retrospective study by Hadoux et al
(64) demonstrated antitumor
activity of FOLFOX as second- or third-line therapy in
EP-refractory patients, with a DCR of 64% and <30% incidence of
major hematological toxicity. These findings suggested that FOLFOX
may offer survival benefits with manageable toxicity for aggressive
ENEC lacking backline options.
CAPTEM program
Temozolomide efficacy remains debatable.
Retrospective studies have reported an ORR of 26% and mOS of 22
months with CAPTEM in high-grade gastroenteropancreatic NEC after
first-line failure (60,73). However, a prospective phase II study
of temozolomide monotherapy (B160101021) demonstrated a lower ORR
and mOS with an mPFS of 1.8 months, albeit with minimal toxicity
(74). Notably, MGMT-deficient
patients exhibited partial responses, suggesting that MGMT
deficiency may serve as a potential predictive biomarker for
sensitivity to temozolomide-based chemotherapy. The recent
NCT04122911 trial reported improved outcomes (mPFS, 5.86 months;
mOS, 12.1 months) for second-line temozolomide (75). These findings indicate modest
efficacy and manageable toxicity, particularly in combination or
MGMT-deficient subgroups.
The NCT03387592 trial compared CAPTEM and FOLFIRI in
metastatic NEC. At 12 weeks, DCRs were 39.1% (CAPTEM) and 28.0%
(FOLFIRI), with no notable difference in 12-month survival (28.4
vs. 32.4%). Both regimens demonstrated mild toxicity (<35%
incidence). High microRNA expression was associated with poor
prognosis, offering insights for stratification. Early termination
of the trial (n=53) limited conclusions, but safety and similar
antitumor activity were confirmed (76).
Topoisomerase I inhibitors
Topoisomerase I inhibitor monotherapy lacks notable
efficacy in multiple studies and is not recommended in in major
oncology guidelines, such as the NCCN Guidelines for Neuroendocrine
and Adrenal Tumors (26,68). The NCT02457273 trial evaluated the
novel camptothecin analog TLC388 in EP-refractory metastatic NEC,
demonstrating a DCR of 15% (mPFS, 1.8 months; mOS, 4.3 months). The
trial was halted due to unmet efficacy endpoints. However, MutS
homolog 6 mutations (40% of samples) in the studied cohort of
poorly differentiated NEC (including cases from various primary
sites) have been associated with tumor mutational burden, thus
suggesting potential for targeted therapies (77).
Targeted therapy
Targeted therapy inhibits tumor growth and
proliferation by interfering with specific molecular targets. While
molecular-targeted drugs are approved for various solid tumors,
such as non-small cell lung cancer, breast cancer and renal cell
carcinoma (78–80), their efficacy in NEC remains to be
elucidated. Current research focuses on mammalian target of
rapamycin (mTOR) inhibitors and anti-angiogenic agents. It is
considered that with the continuous development of research,
traditional treatment combined with targeted therapy may provide
hope for patients in the future.
mTOR inhibitors
mTOR, a key kinase regulating cell proliferation,
metabolism and angiogenesis, promotes tumor progression and
represents a potential therapeutic target. Everolimus, an mTOR
inhibitor, has demonstrated limited efficacy as monotherapy in
phase II trials (mPFS, 1.2–1.3 months), but has shown enhanced
antitumor activity in combination regimens (81,82).
The NCT02695459 study reported that everolimus combined with
cisplatin was effective as first-line therapy for advanced
extrapulmonary NEC, which improved quality of life by avoiding
etoposide-related side effects. Subgroup analysis identified three
patients with sustained remission >1 year, suggesting there may
be molecular predictors of sensitivity that remain to be
identified; however, their specific identities remain to be
elucidated due to a lack of correlative biomarker analysis in the
trial (83). Similarly, NCT01317615
reported that everolimus combined with carboplatin and paclitaxel
was effective and well-tolerated in metastatic lung LCNEC (84). In contrast to the potential benefits
of mTOR inhibitors, trials such as PRODIGE 41-BEVANEC did not
report notable survival benefits from the addition of bevacizumab
to FOLFIRI (85).
Antitumor neovascular drugs
Bevacizumab, an anti-angiogenic agent, exhibits
variable efficacy in NEN. Early retrospective studies have
suggested that bevacizumab combined with temozolomide may provide
benefits for patients with poorly differentiated NEC, although
incomplete data have limited conclusions (23,73).
The PRODIGE 41-BEVANEC trial (NCT02820857) identified no survival
difference between FOLFIRI with or without bevacizumab in
platinum-resistant gastrointestinal-pancreatic NEC (6-month OS, 53
vs. 60%) (23). This may reflect
the high Ki-67 index (>55%) and complex angiogenesis of NEC
compared with vascular-dependent NET (G1-G2), which may demonstrate
improved response to bevacizumab. Retrospective studies have
supported combining bevacizumab with FOLFIRI, FOLFOX, FOLFIRINOX or
temozolomide for NET (23,85).
Immunotherapy
Checkpoint inhibitors [PD-1, programmed cell
death-ligand 1 (PD-L1) and CTLA-4] offer potential but lack
large-scale ENEC-specific evidence (72). Case reports have described complete
remission (CR) when combining immunotherapy with radiotherapy or
targeted agents, such as anlotinib or apatinib (14,15,19,20).
Dual immunotherapy (for example, nivolumab + ipilimumab)
demonstrates promise in high-grade NENs (17,18).
Immunotherapy combined with
radiotherapy
Takagi et al (14) reported complete response in a
patient with metastatic ENEC treated with nivolumab and
radiotherapy, maintaining relapse-free survival for 42 months
despite ≤1% PD-L1 expression, which suggests radiotherapy may
modulate the immune microenvironment. Hanzawa et al
(15) described sustained disease
control in unresectable esophagogastric NEC with nivolumab and
radiotherapy, achieving >4.5-year survival.
Immunotherapy combined with
chemotherapy
Based on previous trials of extensive-stage small
cell lung cancer (86–89), immunotherapy combined with
platinum-based chemotherapy has been hypothesized to benefit
gastrointestinal NEC. Ongoing phase II trials (NCT03901378,
NCT03147404 and NCT03352934) are evaluating
pembrolizumab-chemotherapy and avelumab monotherapy. However, the
NET-001/002 trial reported a DCR of only 21% for avelumab in grade
2–3 NEN (90), which underscores
the need for optimized regimens.
Dual immunotherapy
Dual immunotherapy (CTLA-4 + PD-1 inhibition)
targets complementary immune pathways. The SWOG S1609 DART trial
reported a 26% ORR and 32% 6-month PFS with ipilimumab and
nivolumab in high-grade NEN, with manageable toxicity (grade 3/4
alanine aminotransferase elevation was most common) (17). The GETNE 1601 trial achieved a 36.1%
9-month survival rate with durvalumab and tremelimumab in
chemotherapy-refractory gastroenteropancreatic NEN (18). While promising, dual therapy
requires caution due to potential toxicity.
Immunotherapy combined with targeted
therapy
Combining checkpoint inhibitors with multi-target
TKIs such as surufatinib, anlotinib or apatinib demonstrate
promise. Previous studies have reported DCRs of >80% and durable
remission in certain cases (18,21).
The combination benefits from synergistic effects on the tumor
immune microenvironment (91).
Sulfatinib, a multi-target kinase inhibitor [VEGF
receptor VEGFR)1-3, fibroblast growth factor receptor 1 (FGFR1) and
colony stimulating factor 1 receptor], approved for non-pancreatic
NET in China, combined with toripalimab (PD-1 inhibitor) achieved
an 80% DCR (mPFS, 4.1 months; mOS, 13.7 months) in advanced NEC
with manageable toxicity in the NCT04169672 trial (21). No further subgroup analyses were
performed in this trial.
Camrelizumab (a PD-1 inhibitor) has demonstrated
efficacy in esophageal cancer, including ENEC. In a specific trial
including patients with esophageal cancer, ORRs ranging from 17.4
to 73.1% and a mOS of 8.3 months were reported, with efficacy
appearing to be influenced by PD-L1 expression and other
biomarkers, such as tumor mutational burden or microsatellite
instability (16). Liu et al
(16) reported camrelizumab
combined with apatinib (VEGFR-2 inhibitor) in third-line ENEC
recurrence, achieving >10-month PFS, which suggests that tumor
microenvironment modulation enhances efficacy.
Anlotinib, a multi-target TKI (VEGFR,
platelet-derived growth factor receptor, FGFR and c-Kit), improved
survival in the ALTER 1202 trial for small cell lung cancer
(92). Zhou et al (19) described a patient with metastatic
ENEC achieving 29-month PFS and >50-month OS with camrelizumab
and anlotinib after chemoradiation failure, reaching CR on
PET/CT.
Tislelizumab (PD-1 inhibitor), which has been
approved for the treatment of multiple malignancies such as
non-small cell lung cancer and hepatocellular carcinoma (93), combined with anlotinib in
second-line metastatic ENEC achieved CR (PFS, 16 months; OS, 21
months) with minimal toxicity in an elderly patient (20).
Targeted-immunotherapy combinations synergistically
regulate the tumor microenvironment, offering a novel strategy to
overcome traditional treatment limitations. While current evidence
derives from small studies or case reports (14–16,19,20),
preliminary data have suggested manageable toxicity and survival
benefits. Future high-quality trials and translational research are
warranted to validate efficacy and optimize personalized
treatment.
Immunotherapy summary
Immunotherapy exploration in ENEC highlights
multidimensional advances. Radiotherapy combinations may enhance
efficacy by modulating the immune microenvironment. Dual
immunotherapy (CTLA-4 + PD-1 inhibition) improves ORR and survival
in high-grade NEN but requires caution regarding toxicity.
Chemotherapy combinations lack randomized trial validation in
gastrointestinal NEC despite success in small cell lung cancer.
Targeted-immunotherapy regimens remodel the tumor microenvironment,
achieving survival benefits. To the best of our knowledge, current
evidence is limited to small studies or case reports with
heterogeneous populations and undefined biomarkers. Future efforts
should prioritize prospective trials, tumor microenvironment
dynamics and epigenetic analyses to establish precision treatment
models and address drug resistance.
Conclusion
ENEC is a rare, highly aggressive gastrointestinal
tumor with diagnosis dependent on pathology and treatment requiring
stratified management. Endoscopic or surgical resection is
preferred in early stages, although curable cases are rare. For
locally advanced disease, neoadjuvant/adjuvant therapy plus surgery
or chemoradiotherapy improves survival. Platinum-based chemotherapy
remains first-line in advanced stages, with individualized
second-line regimens. Emerging therapies, particularly
immunotherapy and targeted therapies, have achieved long-term
remission in individual cases. Future large-scale clinical trials
are warranted to optimize molecular subtyping, refine therapeutic
strategies and improve outcomes for this high-grade malignancy.
Finally, although the exclusive focus on ENEC in the present review
is justified by its distinct biology, the omission of direct
comparisons with other gastrointestinal NECs (such as GNEC)
represents a limitation. Future studies integrating multi-origin
NEC data may help refine both site-specific and common therapeutic
strategies.
Acknowledgements
The authors would like to thank Dr Fangyuan Kong
(Department of Oncology, The General Hospital of Western Theater
Command, Chengdu, China) for their perspectives on the
multidisciplinary management of esophageal neuroendocrine carcinoma
and Dr Yong Diao (Department of Oncology, The General Hospital of
Western Theater Command) for sharing their clinical experience
regarding immunotherapy in rare malignancies. Their specialized
knowledge enhanced the clinical viewpoints presented in the present
review.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
JS and BH conceptualized the present review, curated
the literature, devised the methodology and prepared the original
draft. HZ and CJ contributed to the study conception and data
acquisition, performed systematic literature retrieval, data
extraction and validation, conducted the comparative analysis and
interpretation of data from the included literature, and were
responsible for the design and creation of all tables and figures.
HZ and CJ also participated in drafting and critically reviewing
the manuscript. LZ made substantial contributions to the conception
of the work, participated in drafting the manuscript and provided
critical revision for important intellectual content. Data
authentication is not applicable. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ma Z, Cai H and Cui Y: Progress in the
treatment of esophageal neuroendocrine carcinoma. Tumour Biol.
39:10104283177113132017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fraenkel M, Kim MK, Faggiano A and Valk
GD: Epidemiology of gastroenteropancreatic neuroendocrine tumours.
Best Pract Res Clin Gastroenterol. 26:691–703. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dasari A, Mehta K, Byers LA, Sorbye H and
Yao JC: Comparative study of lung and extrapulmonary poorly
differentiated neuroendocrine carcinomas: A SEER database analysis
of 162,983 cases. Cancer. 124:807–815. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans, . Drinking Coffee, Mate, and Very Hot
Beverages. International Agency for Research on Cancer; Lyon: pp.
4882018
|
|
5
|
Hirabayashi K, Zamboni G, Nishi T, Tanaka
A, Kajiwara H and Nakamura N: Histopathology of gastrointestinal
neuroendocrine neoplasms. Front Oncol. 3:22013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nevárez A, Saftoiu A and Bhutani MS:
Primary small cell carcinoma of the esophagus: Clinico-
pathological features and therapeutic options. Curr Health Sci J.
37:31–34. 2011.PubMed/NCBI
|
|
7
|
Huang Q, Fang DC, Yu CG, Zhang J and Chen
MH: Barrett's esophagus-related diseases remain uncommon in China.
J Dig Dis. 12:420–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Al Mansoor S, Ziske C and Schmidt-Wolf
IGH: Primary small cell carcinoma of the esophagus: Patient data
metaanalysis and review of the literature. Ger Med Sci.
11:Doc122013.PubMed/NCBI
|
|
9
|
Imai T, Sannohe Y and Okano H: Oat cell
carcinoma (apudoma) of the esophagus: A case report. Cancer.
41:358–364. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Giannetta E, Guarnotta V, Rota F, de Cicco
F, Grillo F, Colao A and Faggiano A; NIKE: A rare rarity:
Neuroendocrine tumor of the esophagus. Crit Rev Oncol Hematol.
137:92–107. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu H, Yu Z, Liu Y, Guo L, Teng L, Guo L,
Liang L, Wang J, Gao J, Li R, et al: Genomic characterization
reveals distinct mutation landscapes and therapeutic implications
in neuroendocrine carcinomas of the gastrointestinal tract. Cancer
Commun (Lond). 42:1367–1386. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ji A, Jin R, Zhang R and Li H: Primary
small cell carcinoma of the esophagus: Progression in the last
decade. Ann Transl Med. 8:5022020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gonzalez RS: Diagnosis and management of
gastrointestinal neuroendocrine neoplasms. Surg Pathol Clin.
13:377–397. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takagi K, Kamada T, Fuse Y, Kai W,
Takahashi J, Nakashima K, Nakaseko Y, Suzuki N, Yoshida M, Okada S,
et al: Nivolumab in combination with radiotherapy for metastatic
esophageal neuroendocrine carcinoma after esophagectomy: A case
report. Surg Case Rep. 7:2212021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hanzawa S, Asami S, Kanazawa T, Oono S and
Takakura N: Multimodal treatment with nivolumab contributes to
long-term survival in a case of unresectable esophagogastric
junction neuroendocrine carcinoma. Cureus. 16:e659812024.PubMed/NCBI
|
|
16
|
Liu L, Liu Y, Gong L, Zhang M and Wu W:
Salvage camrelizumab plus apatinib for relapsed esophageal
neuroendocrine carcinoma after esophagectomy: A case report and
review of the literature. Cancer Biol Ther. 21:983–989. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Patel SP, Othus M, Chae YK, Giles FJ,
Hansel DE, Singh PP, Fontaine A, Shah MH, Kasi A, Baghdadi TA, et
al: A phase II basket trial of dual anti-CTLA-4 and Anti-PD-1
blockade in rare tumors (DART SWOG 1609) in patients with
nonpancreatic neuroendocrine tumors. Clin Cancer Res. 26:2290–2296.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Capdevila J, Teule A, López C,
García-Carbonero R, Benavent M, Custodio A, Cubillo A, Alonso V,
Gordoa TA, Carmona-Bayonas A, et al: 1157O A multi-cohort phase II
study of durvalumab plus tremelimumab for the treatment of patients
(pts) with advanced neuroendocrine neoplasms (NENs) of
gastroenteropancreatic or lung origin: The DUNE trial (GETNE 1601).
Ann Oncol. 31:S770–S771. 2020. View Article : Google Scholar
|
|
19
|
Zhou L, Xu G, Chen T, Wang Q, Zhao J,
Zhang T, Duan R and Xia Y: Anlotinib plus camrelizumab achieved
long-term survival in a patient with metastatic esophageal
neuroendocrine carcinoma. Cancer Rep (Hoboken).
6:e18552023.PubMed/NCBI
|
|
20
|
Zhang Y, Liu X, Liang H, Liu W, Wang H and
Li T: Late-stage esophageal neuroendocrine carcinoma in a patient
treated with tislelizumab combined with anlotinib: A case report. J
Int Med Res. 51:30006052311879422023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang P, Shi S, Xu J, Chen Z, Song L,
Zhang X, Cheng Y, Zhang Y, Ye F, Li Z, et al: Surufatinib plus
toripalimab in patients with advanced neuroendocrine tumours and
neuroendocrine carcinomas: An open-label, single-arm, multi-cohort
phase II trial. Eur J Cancer. 199:1135392024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Borghesani M, Reni A, Lauricella E, Rossi
A, Moscarda V, Trevisani E, Torresan I, Al-Toubah T, Filoni E,
Luchini C, et al: Efficacy and toxicity analysis of mFOLFIRINOX in
high-grade gastroenteropancreatic neuroendocrine neoplasms. J Natl
Compr Canc Netw. 22:e2470052024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Walter T, Lievre A, Coriat R, Malka D,
Elhajbi F, Di Fiore F, Hentic O, Smith D, Hautefeuille V, Roquin G,
et al: Bevacizumab plus FOLFIRI after failure of platinum-etoposide
first-line chemotherapy in patients with advanced neuroendocrine
carcinoma (PRODIGE 41-BEVANEC): A randomised, multicentre,
non-comparative, open-label, phase 2 trial. Lancet Oncol.
24:297–306. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eads JR, Catalano PJ, Fisher GA, Rubin D,
Iagaru A, Klimstra DS, Konda B, Kwong MS, Chan JA, de Jesus-Acosta
A, et al: Randomized phase II study of platinum and etoposide (EP)
versus temozolomide and capecitabine (CAPTEM) in patients (pts)
with advanced G3 non-small cell gastroenteropancreatic
neuroendocrine neoplasms (GEPNENs): ECOG-ACRIN EA2142. J Clin
Oncol. 40 (16_suppl):S40202022. View Article : Google Scholar
|
|
25
|
Morizane C, Machida N, Honma Y, Okusaka T,
Boku N, Kato K, Nomura S, Hiraoka N, Sekine S, Taniguchi H, et al:
Effectiveness of etoposide and cisplatin vs irinotecan and
cisplatin therapy for patients with advanced neuroendocrine
carcinoma of the digestive system: The TOPIC-nec phase 3 randomized
clinical trial. JAMA Oncol. 8:1447–1455. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weaver JMJ, Hubner RA, Valle JW and
McNamara MG: Selection of chemotherapy in advanced poorly
differentiated extra-pulmonary neuroendocrine carcinoma. Cancers
(Basel). 15:49512023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nagtegaal ID, Odze RD, Klimstra D, Paradis
V, Rugge M, Schirmacher P, Washington KM, Carneiro F and Cree IA;
WHO Classification of Tumours Editorial Board, : The 2019 WHO
classification of tumours of the digestive system. Histopathology.
76:182–188. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang L, Sun X, Zou Y and Meng X: Small
cell type neuroendocrine carcinoma colliding with squamous cell
carcinoma at esophagus. Int J Clin Exp Pathol. 7:1792–1795.
2014.PubMed/NCBI
|
|
29
|
Wilson CI, Summerall J, Willis I, Lubin J
and Inchausti BC: Esophageal collision tumor (Large cell
neuroendocrine carcinoma and papillary carcinoma) arising in a
Barrett esophagus. Arch Pathol Lab Med. 124:411–415. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maru DM, Khurana H, Rashid A, Correa AM,
Anandasabapathy S, Krishnan S, Komaki R, Ajani JA, Swisher SG and
Hofstetter WL: Retrospective study of clinicopathologic features
and prognosis of high-grade neuroendocrine carcinoma of the
esophagus. Am J Surg Pathol. 32:1404–1411. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Klöppel G: Classification and pathology of
gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat
Cancer. 18 (Suppl 1):S1–S16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Capelli P, Fassan M and Scarpa A:
Pathology-grading and staging of GEP-NETs. Best Pract Res Clin
Gastroenterol. 26:705–717. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rooper LM, Bishop JA and Westra WH: INSM1
is a sensitive and specific marker of neuroendocrine
differentiation in head and neck tumors. Am J Surg Pathol.
42:665–671. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang D, Zhang GB, Yan L, Wei XE, Zhang YZ
and Li WB: CT and enhanced CT in diagnosis of gastrointestinal
neuroendocrine carcinomas. Abdom Imaging. 37:738–745. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schott M, Klöppel G, Raffel A, Saleh A,
Knoefel WT and Scherbaum WA: Neuroendocrine neoplasms of the
gastrointestinal tract. Dtsch Arztebl Int. 108:305–312.
2011.PubMed/NCBI
|
|
36
|
Islam O, Sarti K, Verbruggen L,
Vandersmissen V, Bulcke KV, Annys L, Verslype C, Van Laethem JL,
Kalantari HR, Janssens J, et al: Management of high-grade
neuroendocrine neoplasms: Impact of functional imaging. Endocr
Relat Cancer. 32:e2402312025. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sahani DV, Bonaffini PA, Fernández-Del
Castillo C and Blake MA: Gastroenteropancreatic neuroendocrine
tumors: Role of imaging in diagnosis and management. Radiology.
266:38–61. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Strosberg J, El-Haddad G, Wolin E,
Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H,
et al: Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine
tumors. N Engl J Med. 376:125–135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chan DS, Kanagaratnam AL, Pavlakis N and
Chan DL: Peptide receptor chemoradionuclide therapy for
neuroendocrine neoplasms: A systematic review. J Neuroendocrinol.
37:e133552025. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017.PubMed/NCBI
|
|
41
|
Lee CG, Lim YJ, Park SJ, Jang BI, Choi SR,
Kim JK, Kim YT, Cho JY, Yang CH, Chun HJ, et al: The clinical
features and treatment modality of esophageal neuroendocrine
tumors: A multicenter study in Korea. BMC Cancer. 14:5692014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fukui H, Dohi O, Miyazaki H, Yasuda T,
Yoshida T, Ishida T, Doi T, Hirose R, Inoue K, Harusato A, et al: A
case of endoscopic submucosal dissection for neuroendocrine
carcinoma of the esophagus with invasion to the muscularis mucosae.
Clin J Gastroenterol. 15:339–344. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cheng YQ, Wang GF, Zhou XL, Lin M, Zhang
XW and Huang Q: Early adenocarcinoma mixed with a neuroendocrine
carcinoma component arising in the gastroesophageal junction: A
case report. World J Gastrointest Oncol. 16:563–570. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vest M, Shah D, Nassar M and Niknam N:
Neuroendocrine carcinoma of the esophagus with liver metastasis: A
case report. Cureus. 14:e288422022.PubMed/NCBI
|
|
45
|
Honma Y, Nagashima K, Hirano H, Shoji H,
Iwasa S, Takashima A, Okita N, Kato K, Boku N, Murakami N, et al:
Clinical outcomes of locally advanced esophageal neuroendocrine
carcinoma treated with chemoradiotherapy. Cancer Med. 9:595–604.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Alese OB, Jiang R, Shaib W, Wu C, Akce M,
Behera M and El-Rayes BF: High-grade gastrointestinal
neuroendocrine carcinoma management and outcomes: A national cancer
database study. Oncologist. 24:911–920. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Enjoji T, Kobayashi S, Hayashi K, Tetsuo
H, Matsumoto R, Maruya Y, Araki T, Honda T, Akazawa Y, Kanetaka K,
et al: Long-term survival after conversion surgery for an
esophageal neuroendocrine carcinoma: A case report. Gen Thorac
Cardiovasc Surg Cases. 3:282024.PubMed/NCBI
|
|
48
|
Shapiro J, van Lanschot JJB, Hulshof MCCM,
van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven
HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, et al:
Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for
oesophageal or junctional cancer (CROSS): Long-term results of a
randomised controlled trial. Lancet Oncol. 16:1090–1098. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ito T, Masui T, Komoto I, Doi R, Osamura
RY, Sakurai A, Ikeda M, Takano K, Igarashi H, Shimatsu A, et al:
JNETS clinical practice guidelines for gastroenteropancreatic
neuroendocrine neoplasms: diagnosis, treatment, and follow-up: A
synopsis. J Gastroenterol. 56:1033–1044. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kikuchi Y, Shimada H, Yamaguchi K and
Igarashi Y: Systematic review of case reports of Japanese
esophageal neuroendocrine cell carcinoma in the Japanese
literature. Int Cancer Conf J. 8:47–57. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Awada H, Ali AH, Bakhshwin A and Daw H:
High-grade large cell neuroendocrine carcinoma of the esophagus: A
case report and review of the literature. J Med Case Rep.
17:1442023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shah MH, Goldner WS, Benson AB, Bergsland
E, Blaszkowsky LS, Brock P, Chan J, Das S, Dickson PV, Fanta P, et
al: Neuroendocrine and adrenal tumors, version 2.2021, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
19:839–868. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wong AT, Shao M, Rineer J, Osborn V,
Schwartz D and Schreiber D: Treatment and survival outcomes of
small cell carcinoma of the esophagus: An analysis of the National
cancer data base. Dis Esophagus. 30:1–5. 2017.
|
|
54
|
Moertel CG, Kvols LK, O'Connell MJ and
Rubin J: Treatment of neuroendocrine carcinomas with combined
etoposide and cisplatin. Evidence of major therapeutic activity in
the anaplastic variants of these neoplasms. Cancer. 68:227–232.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yamaguchi T, Machida N, Morizane C, Kasuga
A, Takahashi H, Sudo K, Nishina T, Tobimatsu K, Ishido K, Furuse J,
et al: Multicenter retrospective analysis of systemic chemotherapy
for advanced neuroendocrine carcinoma of the digestive system.
Cancer Sci. 105:1176–1181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
McNamara MG, Frizziero M, Jacobs T,
Lamarca A, Hubner RA, Valle JW and Amir E: Second-line treatment in
patients with advanced extra-pulmonary poorly differentiated
neuroendocrine carcinoma: A systematic review and meta-analysis.
Ther Adv Med Oncol. 12:17588359209152992020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ooki A, Osumi H, Fukuda K and Yamaguchi K:
Potent molecular-targeted therapies for gastro-entero-pancreatic
neuroendocrine carcinoma. Cancer Metastasis Rev. 42:1021–1054.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Imai H, Shirota H, Okita A, Komine K,
Saijo K, Takahashi M, Takahashi S, Takahashi M, Shimodaira H and
Ishioka C: Efficacy and safety of carboplatin and etoposide
combination chemotherapy for extrapulmonary neuroendocrine
carcinoma: A retrospective case series. Chemotherapy. 61:111–116.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sorbye H, Welin S, Langer SW, Vestermark
LW, Holt N, Osterlund P, Dueland S, Hofsli E, Guren MG, Ohrling K,
et al: Predictive and prognostic factors for treatment and survival
in 305 patients with advanced gastrointestinal neuroendocrine
carcinoma (WHO G3): The NORDIC NEC study. Ann Oncol. 24:152–160.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chan DL, Bergsland EK, Chan JA, Gadgil R,
Halfdanarson TR, Hornbacker K, Kelly V, Kunz PL, McGarrah PW, Raj
NP, et al: Temozolomide in grade 3 gastroenteropancreatic
neuroendocrine neoplasms: A multicenter retrospective review.
Oncologist. 26:950–955. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Butt BP, Stokmo HL, Ladekarl M, Tabaksblat
EM, Sorbye H, Revheim ME and Hjortland GO: 1108P Folfirinox in the
treatment of advanced gastroenteropancreatic neuroendocrine
carsinomas. ESMO. 32:S9152021.
|
|
62
|
Hentic O, Hammel P, Couvelard A, Rebours
V, Zappa M, Palazzo M, Maire F, Goujon G, Gillet A, Lévy P, et al:
FOLFIRI regimen: An effective second-line chemotherapy after
failure of etoposide-platinum combination in patients with
neuroendocrine carcinomas grade 3. Endocr Relat Cancer. 19:751–757.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sugiyama K, Shiraishi K, Sato M, Nishibori
R, Nozawa K and Kitagawa C: Salvage chemotherapy by folfiri regimen
for poorly differentiated gastrointestinal neuroendocrine
carcinoma. J Gastrointest Cancer. 52:947–951. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hadoux J, Malka D, Planchard D, Scoazec
JY, Caramella C, Guigay J, Boige V, Leboulleux S, Burtin P,
Berdelou A, et al: Post-first-line FOLFOX chemotherapy for grade 3
neuroendocrine carcinoma. Endocr Relat Cancer. 22:289–298. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Merola E, Dal Buono A, Denecke T, Arsenic
R, Pape UF, Jann H, Wiedenmann B and Pavel ME: Efficacy and
toxicity of 5-Fluorouracil-Oxaliplatin in gastroenteropancreatic
neuroendocrine neoplasms. Pancreas. 49:912–917. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Du Z, Wang Y, Zhou Y, Wen F and Li Q:
First-line irinotecan combined with 5-fluorouracil and leucovorin
for high-grade metastatic gastrointestinal neuroendocrine
carcinoma. Tumori. 99:57–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lamarca A, Frizziero M, Barriuso J,
McNamara MG, Hubner RA and Valle JW: Urgent need for consensus:
International survey of clinical practice exploring use of
platinum-etoposide chemotherapy for advanced extra-pulmonary high
grade neuroendocrine carcinoma (EP-G3-NEC). Clin Transl Oncol.
21:950–953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Shah MH, Goldner WS, Benson AB, Bergsland
E, Blaszkowsky LS, Brock P, Chan J, Das S, Dickson PV, Fanta P, et
al: Neuroendocrine and adrenal tumors, version 2.2021, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
19:839–868. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Frizziero M, Spada F, Lamarca A, Kordatou
Z, Barriuso J, Nuttall C, McNamara MG, Hubner RA, Mansoor W,
Manoharan P, et al: Carboplatin in combination with oral or
intravenous etoposide for extra-pulmonary, poorly-differentiated
neuroendocrine carcinomas. Neuroendocrinology. 109:100–112. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hadoux J, Walter T, Kanaan C, Hescot S,
Hautefeuille V, Perrier M, Tauveron I, Laboureau S, Do Cao C,
Petorin C, et al: Second-line treatment and prognostic factors in
neuroendocrine carcinoma: the RBNEC study. Endocr Relat Cancer.
29:569–580. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Bardasi C, Spallanzani A, Benatti S, Spada
F, Laffi A, Antonuzzo L, Lavacchi D, Marconcini R, Ferrari M,
Rimini M, et al: Irinotecan-based chemotherapy in extrapulmonary
neuroendocrine carcinomas: Survival and safety data from a
multicentric Italian experience. Endocrine. 74:707–713. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
McNamara MG, Swain J, Craig Z, Sharma R,
Faluyi O, Wadsley J, Morgan C, Wall LR, Chau I, Reed N, et al:
NET-02: A randomised, non-comparative, phase II trial of
nal-IRI/5-FU or docetaxel as second-line therapy in patients with
progressive poorly differentiated extra-pulmonary neuroendocrine
carcinoma. EClinicalMedicine. 60:1020152023. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Welin S, Sorbye H, Sebjornsen S, Knappskog
S, Busch C and Oberg K: Clinical effect of temozolomide-based
chemotherapy in poorly differentiated endocrine carcinoma after
progression on first-line chemotherapy. Cancer. 117:4617–4622.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kobayashi N, Takeda Y, Okubo N, Suzuki A,
Tokuhisa M, Hiroshima Y and Ichikawa Y: Phase II study of
temozolomide monotherapy in patients with extrapulmonary
neuroendocrine carcinoma. Cancer Sci. 112:1936–1942. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
von Arx C, Della Vittoria Scarpati G,
Cannella L, Clemente O, Marretta AL, Bracigliano A, Picozzi F,
Iervolino D, Granata V, Modica R, et al: A new schedule of one week
on/one week off temozolomide as second-line treatment of advanced
neuroendocrine carcinomas (TENEC-TRIAL): A multicenter, open-label,
single-arm, phase II trial. ESMO Open. 9:1030032024. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bongiovanni A, Liverani C, Foca F, Bergamo
F, Leo S, Pusceddu S, Gelsomino F, Brizzi MP, Di Meglio G, Spada F,
et al: A randomized phase II trial of Captem or Folfiri as
second-line therapy in neuroendocrine carcinomas. Eur J Cancer.
208:1141292024. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chen MH, Chou WC, Hsiao CF, Jiang SS, Tsai
HJ, Liu YC, Hsu C, Shan YS, Hung YP, Hsich CH, et al: An
open-label, single-arm, two-stage, multicenter, phase II study to
evaluate the efficacy of TLC388 and genomic analysis for poorly
differentiated neuroendocrine carcinomas. Oncologist. 25:e782–e788.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik
C, Kim ST, et al: Sunitinib versus interferon alfa in metastatic
renal-cell carcinoma. N Engl J Med. 356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Okuyama H, Ikeda M, Okusaka T, Furukawa M,
Ohkawa S, Hosokawa A, Kojima Y, Hara H, Murohisa G, Shioji K, et
al: A phase II trial of everolimus in patients with advanced
pancreatic neuroendocrine carcinoma refractory or intolerant to
platinum-containing chemotherapy (NECTOR Trial).
Neuroendocrinology. 110:988–993. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Tarhini A, Kotsakis A, Gooding W, Shuai Y,
Petro D, Friedland D, Belani CP, Dacic S and Argiris A: Phase II
study of everolimus (RAD001) in previously treated small cell lung
cancer. Clin Cancer Res. 16:5900–5907. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Levy S, Verbeek WHM, Eskens FALM, van den
Berg JG, de Groot DJA, van Leerdam ME and Tesselaar MET: First-line
everolimus and cisplatin in patients with advanced extrapulmonary
neuroendocrine carcinoma: A nationwide phase 2 single-arm clinical
trial. Ther Adv Med Oncol. 14:175883592210770882022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Christopoulos P, Engel-Riedel W, Grohé C,
Kropf-Sanchen C, von Pawel J, Gütz S, Kollmeier J, Eberhardt W,
Ukena D, Baum V, et al: Everolimus with paclitaxel and carboplatin
as first-line treatment for metastatic large-cell neuroendocrine
lung carcinoma: a multicenter phase II trial. Ann Oncol.
28:1898–1902. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ünal Ç and Sağlam S: Metronomic
Temozolomide (mTMZ) and Bevacizumab-The Safe and Effective Frontier
for Treating Metastatic Neuroendocrine Tumors (NETs): A
Single-Center Experience. Cancers (Basel). 23:56882023. View Article : Google Scholar
|
|
86
|
Horn L, Mansfield AS, Szczęsna A, Havel L,
Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio
M, et al: First-line atezolizumab plus chemotherapy in
extensive-stage small-cell lung cancer. N Engl J Med.
379:2220–2229. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liu SV, Reck M, Mansfield AS, Mok T,
Scherpereel A, Reinmuth N, Garassino MC, De Castro Carpeno J,
Califano R, Nishio M, et al: Updated overall survival and PD-L1
subgroup analysis of patients with extensive-stage small-cell lung
cancer treated with atezolizumab, carboplatin, and etoposide
(IMpower133). J Clin Oncol. 39:619–630. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N,
Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH,
et al: Durvalumab plus platinum-etoposide versus platinum-etoposide
in first-line treatment of extensive-stage small-cell lung cancer
(CASPIAN): A randomised, controlled, open-label, phase 3 trial.
Lancet. 394:1929–1939. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Goldman JW, Dvorkin M, Chen Y, Reinmuth N,
Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH,
et al: Durvalumab, with or without tremelimumab, plus
platinum-etoposide versus platinum-etoposide alone in first-line
treatment of extensive-stage small-cell lung cancer (CASPIAN):
Updated results from a randomised, controlled, open-label, phase 3
trial. Lancet Oncol. 22:51–65. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Chan DL, Rodriguez-Freixinos V, Doherty M,
Wasson K, Iscoe N, Raskin W, Hallet J, Myrehaug S, Law C, Thawer A,
et al: Avelumab in unresectable/metastatic, progressive, grade 2–3
neuroendocrine neoplasms (NENs): Combined results from NET-001 and
NET-002 trials. Eur J Cancer. 169:74–81. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Reddy SM, Reuben A and Wargo JA:
Influences of BRAF inhibitors on the immune microenvironment and
the rationale for combined molecular and immune targeted therapy.
Curr Oncol Rep. 18:422016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Cheng Y, Wang Q, Li K, Shi J, Liu Y, Wu L,
Han B, Chen G, He J, Wang J, et al: Anlotinib vs placebo as third-
or further-line treatment for patients with small cell lung cancer:
A randomised, double-blind, placebo-controlled Phase 2 study. Br J
Cancer. 125:366–371. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z,
Zhao J, Yu Y, Hu C, Yang K, et al: Tislelizumab plus chemotherapy
vs chemotherapy alone as first-line treatment for advanced squamous
non-small-cell lung cancer: A phase 3 randomized clinical trial.
JAMA Oncol. 7:709–717. 2021. View Article : Google Scholar : PubMed/NCBI
|