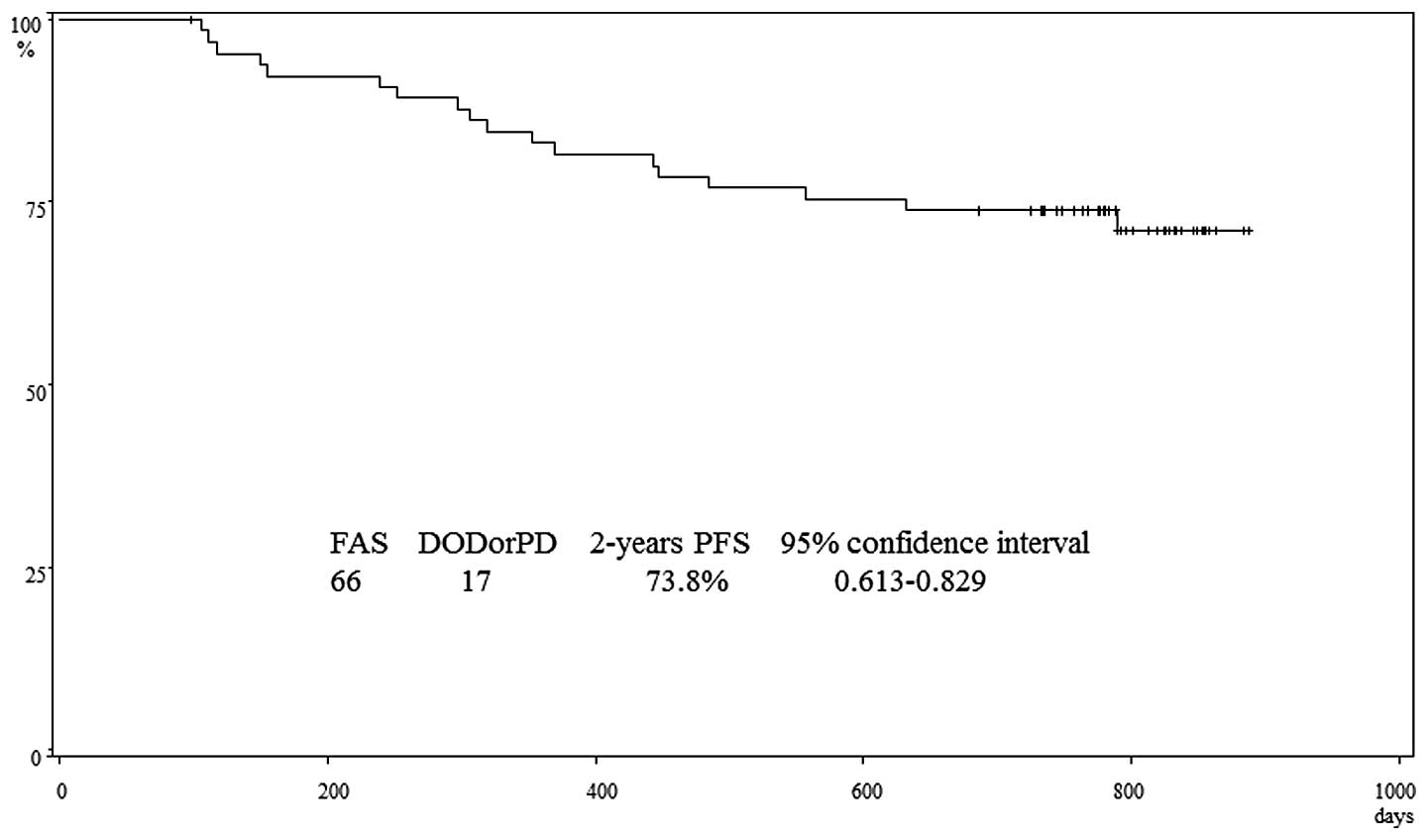
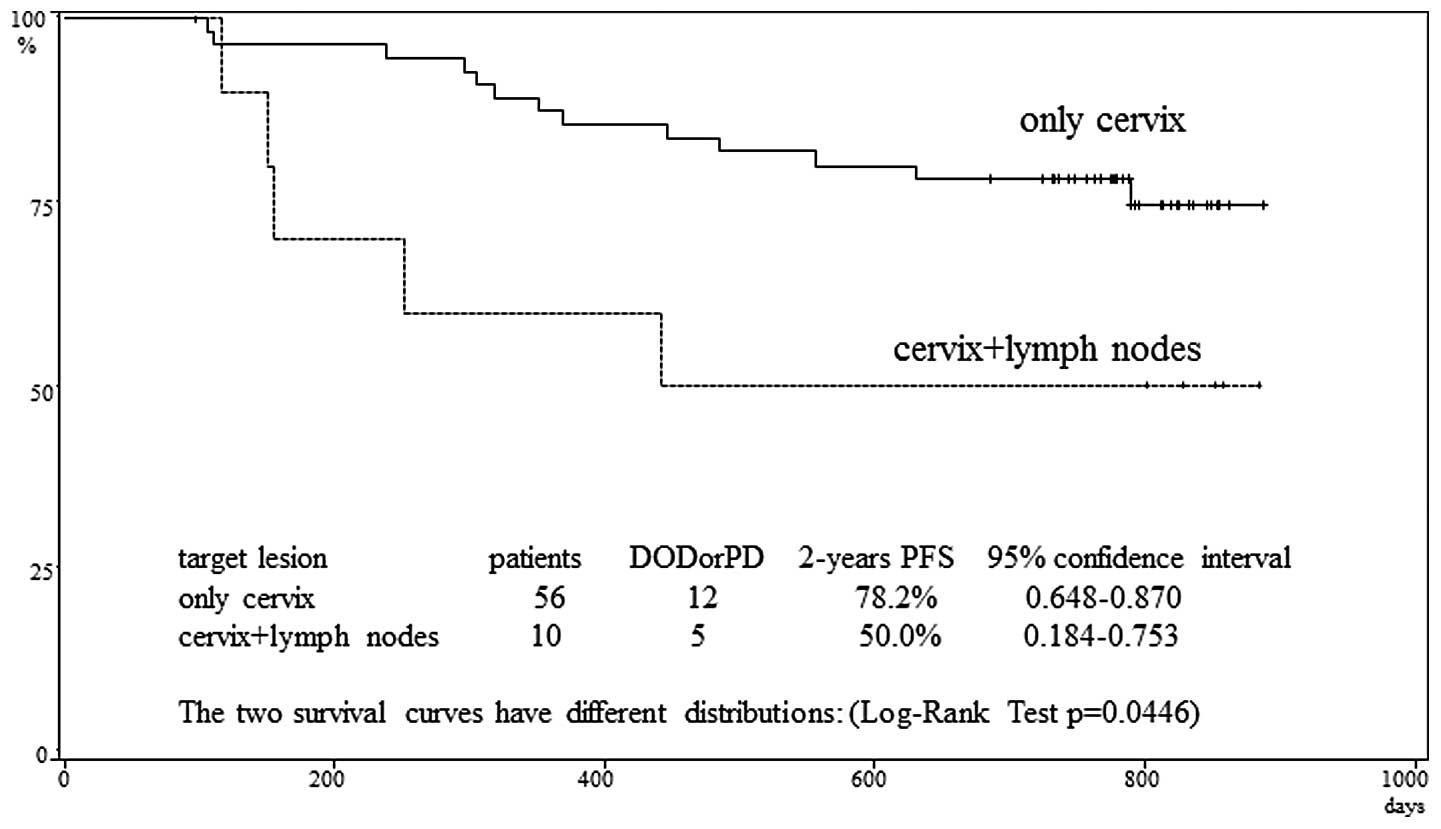
|
1
|
Yamamoto K, Kokawa K, Umesaki N, et al:
Phase I study of combination chemotherapy with irinotecan
hydrochloride and nedaplatin for cervical squamous cell carcinoma:
Japanese Gynecologic Oncology Group study. Oncol Rep. 21:1005–1009.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takeuchi S, Dobashi K, Fujimoto S, et al:
A late phase II study of CPT-11 on uterine cervical cancer and
ovarian cancer. Jpn J Cancer Chemother. 18:1681–1689.
1991.PubMed/NCBI
|
|
3
|
Kanazawa F, Koizumi F, Koh Y, et al: In
vitro synergistic interactions between the cisplatin analogue
nedaplatin and the DNA topoisomerase I inhibitor irinotecan and the
mechanism of this interaction. Clin Cancer Res. 7:202–209.
2001.
|
|
4
|
Sugiyama T, Yakushiji M, Noda K, et al:
Phase II study of irinotecan and cisplatin as first-line
chemotherapy in advanced or recurrent cervical cancer. Oncology.
58:31–37. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sugiyama T, Nishida T, Kumagai S, et al:
Combination therapy with irinotecan and cisplatin as neoadjuvant
chemotherapy in locally advanced cervical cancer. Br J Cancer.
81:95–98. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raspagliesi F, Ditto A, Selvaggi L, et al:
A phase 2 multicenter study of irinotecan and cisplatinum as
neoadjuvant treatment in patients with locally advanced cervical
cancer. Int J Gynecol Cancer. 20:1569–1575. 2010.PubMed/NCBI
|
|
7
|
Matsumura M, Takeshima N, Ota T, et al:
Neoadjuvant chemotherapy followed by radical hysterectomy plus
postoperative chemotherapy but no radiotherapy for Stage IB2-IIB
cervical cancer - irinotecan and platinum chemotherapy. Gynecol
Oncol. 119:212–216. 2010. View Article : Google Scholar
|
|
8
|
Kato T, Nishimura H, Yakushiji M, et al:
Phase II study of 254-S (cis-diammine glycolate platinum) for
gynecological cancer. Jpn J Cancer Chemother. 19:695–701.
1992.PubMed/NCBI
|
|
9
|
Machida S, Ohwada M, Fujiwara H, et al:
Phase I study of combination chemotherapy using irinotecan
hydrochloride and nedaplatin for advanced or recurrent cervical
cancer. Oncology. 65:102–107. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsuda H, Hashiguchi Y, Nishimura S, et al:
Phase I-II study of irinotecan plus nedaplatin with recombinant
human granulocyte colony-stimulating factor support in patients
with advanced or recurrent cervical cancer. Br J Cancer.
91:1032–1037. 2004.
|
|
11
|
Ohwada M, Machida S, Fujiwara H, et al:
Phase II study of combination chemotherapy using irinotecan and
nedaplatin for patients with primary advanced or recurrent cervical
cancer. Proc ASCO: J Clin Oncol. 22(Suppl 14): abs. 50882004.
|
|
12
|
Zanetta G, Fei F, Mangioni C, et al:
Chemotherapy with paclitaxel, ifosmide, and cisplatin for the
treatment of squamous cell cervical cancer. Semin Oncol. 27:23–27.
2000.PubMed/NCBI
|
|
13
|
Haung HJ, Chang TC, Hong JH, et al:
Prognostic value of age and histologic type in neoadjuvant
chemotherapy plus radical surgery for bulky (≥4 cm) stage IB and
IIA cervical carcinoma. Int J Gynecol Cancer. 13:204–221.
2003.PubMed/NCBI
|
|
14
|
D’Agostino DG, Distefano M, Greggi S, et
al: Neoadjuvant treatment of locally advanced carcinoma of the
uterine cervix with epirubicin, paclitaxel and cisplatin. Cancer
Chemother Pharmacol. 49:256–260. 2002.PubMed/NCBI
|
|
15
|
Vagno GD, Cormio G, Pinata S, et al:
Cisplatin and vinorelbine as neoadjuvant chemotherapy in locally
advanced cervical cancer: a phase II study. Int J Gynecol Cancer.
13:308–312. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duenas-Gonzales A, Lopez-Graniel C,
Gonzalez-Enciso A, et al: A phase II study of multimodality
treatment for locally advanced cervical cancer: neoadjuvant
carboplatin and paclitaxel followed by radical hysterectomy and
adjyvant cisplatin chemoradiation. Ann Oncol. 14:1278–1284. 2003.
View Article : Google Scholar
|
|
17
|
Umesaki N, Fujii T, Nishimura R, et al:
Combination chemotherapy with iirunotecan (irinotecan) and
mitomycin C (MMC) for advanced or recurrent squamous cell carcinoma
of the cervix: Japanese Gynecologic Oncology Group (JGOG) study.
Proc Am Soc Clin Oncol. 22:465abs. 18692003.PubMed/NCBI
|
|
18
|
Takeshima N, Umayahara K, Fujiwara K, et
al: Treatment results of adjuvant chemotherapy after radical
hysterectomy for intermediate-and high risk stage IB-IIA cervical
cancer. Gynecol Oncol. 103:618–622. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
NCCN Clinical Practice Guidelines in
Oncology-Cervical Cancer-v2. National Comprehensive Cancer Network;
2006
|
|
20
|
Cervical Cancer Treatment (PDQ®), Health
Professional Version. National Cancer Institute in the United
States (web-site). 2011
|
|
21
|
Sardi JE, Sananes CE, Giaroli AA, et al:
Long-term follow-up of the first randomized trial using neoadjuvant
chemotherapy in stage Ib squamous carcinoma of the cervix: the
final results. Gynecol Oncol. 67:61–69. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Benedetti-Panici P, Greggi S, Scambia G,
et al: Long-term survival following neoadjuvant chemotherapy and
radical surgery in locally advanced cervical cancer. Eur J Cancer.
34:341–346. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang TC, Lai CH, Hong JH, et al:
Randomized trial of neoadjuvant cisplatin, vincristine, bleomycin,
and radical hysterectomy versus radiation therapy for bulky stage
IB and IIA cervical cancer. J Clin Oncol. 18:1740–1747. 2000.
|
|
24
|
Buda A, Fossati R, Colombo N, et al:
Randomized trial of neoadjuvant chemotherapy comparing paclitaxel,
ifosfamide, and cisplatin with ifosfamide and cisplatin followed by
radical surgery in patients with locally advanced squamous cell
cervical carcinoma: the SNAP01 (studio neo-adjuvante portio)
Italian Collaborative Study. J Clin Oncol. 23:4137–4145. 2005.
|
|
25
|
Tzioras S, Pavlidis N, Paraskevaidis E, et
al: Effects of different chemotherapy regimens on survival for
advanced cervical cancer: systematic review and meta-analysis.
Cancer Treat Rev. 33:24–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Katsumata N, Yoshikawa H, Hirakawa T, et
al: Phase III randomized trial of neoadjuvant chemotherapy (NAC)
followed by radical hysterectomy (RH) versus RH for bulky stage
I/II in cervical cancer (JCOG0102). Proc Am Soc Clin Oncol.
24(S18): 1abs. 50132006.
|
|
27
|
Eddy GL, Bundy BN, Creasman WT, et al:
Treatment of (bulky) stage IB cervical cancer with or without
neoadjuvant vincristine and cisplatin prior to radical hysterectomy
and pelvic/para-aortic lymphadenectomy: a phase III trial of the
Gynecologic Oncology Group. Gynecol Oncol. 106:362–369. 2007.
View Article : Google Scholar
|
|
28
|
Mossa B, Mossa S, Corosu L, et al:
Follow-up in a long-term randomized trial with neoadjuvant
chemotherapy for squamous cell cervical carcinoma. Eur J Gynecol
Oncol. 31:497–503. 2010.PubMed/NCBI
|
|
29
|
Rydzewska L, Tierney J, Vale CL, et al:
Neoadjuvant chemotherapy plus surgery versus surgery for cervical
cancer. Cochrane Database Syst Rev. 20(1): CD0074062010.
|
|
30
|
Tierney J: Neoadjuvant chemotherapy for
locally advanced cervical cancer: a systematic review and
meta-analysis of individual patient data from 21 randomised trials.
Eur J Cancer. 39:2470–2086. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gonzalez-Martin A, Gonzalez-Cortijo L,
Carballo N, et al: The current role of neoadjuvant chemotherapy in
the management of cervical carcinoma. Gynecol Oncol. 110:S36–S40.
2008. View Article : Google Scholar : PubMed/NCBI
|