Introduction
We previously reported a phase I study of
combination chemotherapy with irinotecan hydrochloride (CPT-11) and
nedaplatin (NED) for cervical squamous cell carcinoma (JGOG 1063)
(1). We conducted a phase II
clinical trial to evaluate the effectiveness and toxicity of
neoadjuvant chemotherapy (NAC) with CPT-11 and NED in women with
stage Ib2 or II cervical squamous cell carcinoma.
CPT-11 is a DNA topoisomerase I inhibitor developed
in Japan. In a phase II study in patients with cervical carcinoma,
it exhibited relatively high efficacy, with a response rate of 23%
(2).
NED is a second-generation platinum compound
developed in Japan. The response rate was 46% to patients with
cervical cancer in phase II clinical trials. Its antitumor activity
was suggested to be at least equivalent to that of cisplatin
(CDDP). Since NED is less nephrotoxic than CDDP, its indication
range was extended to patients with renal dysfunction. NED can be
administered on an outpatient basis, without rehydration therapy.
In vitro studies using gynecological cancer cell lines NDP
exerted stronger antitumor activity than CDDP (3).
Patients with cervical cancer have been reported to
have a high rate of response to CPT-11 plus CDDP (4–7). To
further enhance efficacy and safety, CPT-11 has been combined with
nedaplatin. Good outcomes have been reported (8–10).
Clinically, Ohwada et al reported a high response rate of
81% in patients with primary cervical cancer (11). Combined therapy with CPT-11 and NED
is thus expected to be useful for the management of advanced
cervical cancer.
The purpose of NAC in this study was to increase the
radicality of surgery by inducing tumor shrinkage, not to achieve a
histological complete response. Therefore, patients could receive
up to 3 courses of NAC, but if PR or CR was achieved during 1 or 2
courses, the physician could decide whether to proceed to surgery.
NAC regimens need a high local response rate of the primary tumor
and a prompt onset of effect.
Patients and methods
The study group comprised women in whom radical
hysterectomy was indicated, but surgery was considered difficult.
In clinical practice, this category includes patients with bulky
tumors and those with high-grade parametrial invasion. Clinical
stage Ib2 and IIa tumors were defined as measurable lesions >4
cm in diameter, and clinical stage IIb tumors were defined as
measurable lesions >2 cm in diameter. Patients were enrolled
from January 2007 through July 2007 at member hospitals of the
Japanese Gynecologic Oncology Group (JGOG). The study protocol was
approved by the institutional review board of each participating
hospital. All patients provided informed consent before
enrollment.
Eligibility criteria
Eligibility criteria were as follows: i) a
histopathologically confirmed diagnosis of cervical cancer
(squamous cell carcinoma); ii) any of the following clinical stages
according to the Federation of Gynecology and Obstetrics (FIGO)
staging system (1994 version): stage Ib2, stage IIa [measurable
lesions >4 cm in greatest diameter on direct measurement or
magnetic resonance imaging (MRI)], or stage IIb (measurable lesions
>2 cm in greatest diameter on direct measurement or MRI); iii)
the primary tumor can be directly measured or measured on MRI; iv)
no previous treatment; v) an age of 20–75 years at enrollment; vi)
a performance status (Eastern Cooperative Oncology Group) of 0 or
1; vii) extended hysterectomy is feasible; viii) preserved function
of major organs (bone marrow, heart, liver, kidney, etc.)
[neutrophil count ≥2000/μl, platelet count ≥100×103/μl,
hemoglobin level ≥9.0 g/dl (values after blood transfusion are
accepted), levels of aspartate aminotransferase and alanine
aminotransferase ≤100 IU/l, total bilirubin level ≤1.5 mg/dl, serum
creatinine level ≤1.2 mg/dl, creatinine clearance ≥60 ml/min,
normal electrocardiogram or electrocardiographic changes not
requiring treatment]; and ix) written informed consent for
voluntary participation in this study.
Exclusion criteria
The exclusion criteria were as follows: i) distinct
evidence of infectious disease; ii) serious concurrent disease
(cardiac disease, uncontrolled diabetes mellitus, malignant
hypertension, bleeding tendency, etc.); iii) active double cancer;
iv) interstitial pneumonia or pulmonary fibrosis; v) body fluid
retention requiring treatment; vi) unstable angina, a history of
myocardial infarction within 6 months before enrollment, or serious
arrhythmias requiring treatment; vii) contraindications for
irinotecan or nedaplatin; viii) diarrhea (watery stool); ix)
intestinal paralysis or ileus; x) pregnant women, breast-feeding
women, or women who want to become pregnant; xi) a history of
serious drug hypersensitivity or drug allergy; and xii) patients
considered unsuitable as subjects by their attending physicians for
study-related reasons.
Treatment
Patients received irinotecan (60 mg/m2)
on days 1 and 8 and nedaplatin (80 mg/m2) on day 1 of a
21-day cycle. After 1–3 courses of chemotherapy, extended
hysterectomy was performed. If a partial or better response was
obtained after 1 or 2 courses, or if a response was considered
unlikely, surgery could be performed.
Criteria for skipping treatment with
irinotecan on day 8
Laboratory tests were always performed 1 day before
or on the same day as treatment with irinotecan on day 8 to confirm
the severity of adverse drug reactions and the patients’ status.
Treatment with irinotecan on day 8 was skipped if patients met any
of the following criteria: neutrophil count <1500/μl, platelet
count <100×103/μl, grade 1 or higher infection, a
fever of 38°C or higher, or grade 1 or higher diarrhea.
Criteria for starting the next
course
Before starting the second and subsequent course of
chemotherapy, laboratory tests were always performed within 24 h
before the time scheduled for treatment to adequately confirm the
severity of adverse drug reactions and the patients’ status. The
next course of treatment was postponed if patients did not meet the
following criteria: neutrophil count ≥1500/μl, platelet count
≥100×103/μl, serum creatinine level ≤1.5 mg/dl, grade 0
infection, grade 0 fever, and grade 0 diarrhea. However, the study
treatment was discontinued if these criteria were not met up to a
maximum of 5 weeks after the start of the previous course.
Furthermore, if a granulocyte colony-stimulating factor (G-CSF)
preparation was used to treat neutropenia, patients were observed
for at least 3 days after the completion of treatment to confirm
that the neutrophil count was ≥1500/μl.
The dose for the next course of treatment was
reduced according to the severity of the adverse drug reactions
that occurred during the previous course. If grade 4
non-hematologic toxicity developed, the protocol treatment was
discontinued.
Criteria for reduction of irinotecan
dose
The dose of irinotecan was decreased 10
mg/m2 for the next course of treatment in patients who
had any of the following conditions during the previous course:
grade 3 or higher febrile neutropenia; grade 4 neutropenia
persisting for ≥5 days; grade 3 or higher non-hematologic toxicity,
excluding hair loss, nausea, vomiting, and fatigue; or if dose
reduction was considered necessary by the patient’s attending
physician. Once the dose was reduced, treatment was continued at
the lower dose (Table II).
 | Table IIClinical response of neoadjuvant
chemotherapy with CPT-11+NED. |
Table II
Clinical response of neoadjuvant
chemotherapy with CPT-11+NED.
| Clinical
response | Patients | Response rate | | |
|---|
| CR | 2 | 3.0% |  | |
| PR | 48 | 72.7% | 75.8% |
| SD | 12 | 18.1% | | |
| PD | 0 | 0% | | |
| Evaluation
failure | 4 | 6.1% | | |
|
| No. of courses
required until response | Average | | |
|
| 1 course | | 30 |  | |
| 2 courses | | 19 | 1.42 courses |
| 3 courses | | 1 | |
Criteria for reduction of nedaplatin
dose
The criteria for reducing the dose of nedaplatin
were based on the platelet count. The dose of nedaplatin was
reduced 10 mg/m2 for the next course in patients who had
any of the following conditions during the previous course: grade 4
thrombocytopenia (platelet count, <25×103/μl); a
bleeding tendency caused by grade 3 thrombocytopenia (platelet
count, ≥25×103/μl to <50×103/μl); platelet
transfusion was received; or if dose reduction was considered
necessary by the patient’s attending physician. Once the dose was
reduced, treatment was continued at the lower dose.
Evaluation of adverse events
Adverse events were evaluated according to the JCOG
Japanese version of the Common Terminology Criteria for Adverse
Events (CTCAE), Version 3.0.
Surgery
Surgery was to be performed between 4 and 6 weeks
after the completion of preoperative chemotherapy. In principle,
radical hysterectomy was performed. However, if surgery was not
considered feasible after NAC or if surgery was precluded by
concurrent disease or other factors, non-surgical treatment such as
radiotherapy could be administered at the discretion of the
attending physician.
Postoperative treatment
In this clinical trial, postoperative therapy was
not specified. Patients were followed up and given radiography or
chemotherapy according to the criteria of each hospital.
Endpoints
The primary endpoint in this study was the response
(PR+CR) rate. Response was evaluated at a single timepoint,
referring to the World Health Organization Response Evaluation
Criteria in Solid Tumors (RECIST guidelines). Time to treatment
failure was not required. Secondary endpoints were: i) the number
of courses required for response; ii) incidence of adverse events;
iii) tumor marker levels (serum squamous cell carcinoma antigen);
iv) completeness of surgery (presence or absence of residual tumor
on intraoperative examination, rate of complete lymph node
dissection, and negative resection margin rate); v) results of
pathological examination of resected organs (histologic response,
parametrial invasion, resection margin status, stromal invasion,
vascular invasion, and lymphatic invasion); vi) relative ease or
difficulty of surgery (bleeding volume and operation time); vii)
proportion of patients with surgical complications; and viii)
recurrence-free survival rate at 2 years.
Evaluation criteria for target lesion
response
Tumor response was evaluated in accordance with
RECIST guidelines. Before treatment and at the completion of each
course, the longest diameter of target lesions was measured in a
single direction on MRI or by direct measurement. We used MRI
instead of computed tomography (CT) for two reasons. First, as
compared with CT, MRI can more clearly depict tumor borders,
facilitating the measurement of tumor diameter. Second, because
cervical cancer invasion often extends vertically, MRI is more
useful for measuring vertical extension on sagittal sections.
Response was defined as: complete response (CR), disappearance of
all target lesions including tumor-induced secondary changes;
partial response (PR), a ≥30% decrease in the sum of the longest
diameter of target lesions, as compared with the value before the
start of treatment; stable disease (SD): no evidence of tumor
shrinkage corresponding to PR or of tumor growth corresponding to
progressive disease; progressive disease (PD), a ≥20% increase in
the sum of the longest diameter of target lesions, as compared with
the smallest previous value; not evaluable (NE), examination cannot
be performed for some reason or response is not evaluated to be CR,
PR, PD, or SD.
Statistical analysis
Kaplan-Meier curves were generated for time to first
recurrence and progression-free survival. We compared curves for
the two groups with the log-rank test. All time estimates were done
with the date of first chemotherapy as the baseline.
Results
A total of 68 patients were enrolled. Two patients
did not meet the eligibility criteria and were excluded, and the
remaining 66 were included in the full analysis set. As for their
demographic characteristics, the median age was 47 years (range
22–71); the FIGO stage was Ib2 in 18 patients, IIa in 10, and IIb
in 38; performance status was 0 in 61 patients and 1 in 5; and
tumor diameter was ≤4 cm in 16 patients and >4 cm in 50
(Table I). Radical hysterectomy was
performed after NAC, thereby completing the protocol treatment, in
63 patients (95.5%). Three patients discontinued protocol
treatment: 1 directly refused treatment; 1 only received an
exploratory laparotomy; and 1 did not undergo surgery because her
attending physician preoperatively judged that operation was not
feasible. The number of administered courses of NAC was 1 in 13
patients, 2 in 43, and 3 in 10. The mean interval from the date of
staring course 1 of NAC to the date of starting the course 2 was
27.6 days (range 20–42). G-CSF was used in 10 of 53 patients. The
reasons for postponing treatment were neutropenia in 35 patients,
thrombocytopenia in 2, diarrhea in 1, and others in 5. The mean
interval from the date of staring the course 2 of NAC to the date
of starting course 3 was 27.1 days (range 21–34). G-CSF was used in
2 of 10 patients. The reasons for postponing treatment were
neutropenia in 7 patients and thrombocytopenia in 1. Treatment with
irinotecan was skipped on day 8 in 4 (6.1%) of 66 patients during
course 1, 10 (18.9%) of 53 patients during course 2, and 3 (30.0%)
of 10 patients during course 3. All of these patients skipped
treatment because of neutropenia. The dose of irinotecan in the
next course was decreased from 60–50 mg/m2 in 4 (6.1%)
of the 66 patients. The reason for this dose reduction was grade 4
neutropenia in 2 (50%) of the 4 patients. The dose of nedaplatin
was reduced in 2 (3%) of the 66 patients. In 1 patient the dose was
decreased from 80 to 70 mg/m2, and in the other the dose
was decreased to 60 mg/m2. The reasons for dose
reduction were grade 3 and 4 thrombocytopenia.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | Patients (n=66) |
|---|
| Age |
| Median | 47 |
| Range | 22–71 |
| PS |
| 0 | 61 |
| 1 | 5 |
| FIGO stage |
| Ib2 | 18 |
| IIa | 10 |
| IIb | 38 |
| Tumor size (cm) |
| ≤4 | 16 |
| >4 | 50 |
The response rate, the primary endpoint of this
study, was 75.8% (CR in 2 patients, PR in 48, SD in 12, PD in 0,
and NE in 4). The mean number of treatment courses required for a
response was 1.42 (1 course in 30 patients, 2 courses in 19, and 3
courses in 1) (Table II). The
combination of irinotecan and nedaplatin was considered an
effective regimen for cervical cancer (squamous cell
carcinoma).
The incidences of grade 3 or 4 hematological
toxicities were as follows (in descending order): neutropenia
72.2%, leukopenia 16.7%, anemia 13.6%, thrombocytopenia 7.6%,
febrile neutropenia 1.5%, and elevations of alanine
aminotransferase and aspartate aminotransferase 1.5% (Table III). The incidences of grade 3 or
4 non-hematologic toxicities were: diarrhea 6.1%, nausea 3%,
anorexia 1.5%, vomiting 1.5%, fever 1.5%, allergic reactions 1.5%,
ileus 1.5%, and vesicovaginal fistula 1.5% (Table IV). There were no deaths or other
serious adverse events.
 | Table IIIHematological toxicities of
chemothrapy with CPT-11+NED. |
Table III
Hematological toxicities of
chemothrapy with CPT-11+NED.
| Grade |
|---|
|
|
|---|
| Toxicities | 0 | 1 | 2 | 3 | 4 | G3/4 (%) |
|---|
| Leukopenia | 11 | 10 | 34 | 8 | 3 | 16.7 |
| Neutropenia | 7 | 4 | 8 | 37 | 10 | 71.2 |
| Febrile
neutoropenia | 65 | 0 | 0 | 1 | 0 | 1.5 |
| Anemia | 15 | 15 | 27 | 8 | 1 | 13.6 |
|
Thrombocytropenia | 34 | 20 | 7 | 2 | 3 | 7.6 |
| AST/GOT
evelation | 53 | 12 | 1 | 0 | 0 | 0 |
| ALT/GPT
evelation | 47 | 14 | 4 | 1 | 0 | 1.5 |
| Creatinine | 65 | 1 | 0 | 0 | 0 | 0 |
| Bilirubine | 60 | 4 | 2 | 0 | 0 | 0 |
|
Hypoalbuminemia | 61 | 3 | 2 | 0 | 0 | 0 |
 | Table IVNon-hematologic toxicities of
chemothrapy with CPT-11+NED. |
Table IV
Non-hematologic toxicities of
chemothrapy with CPT-11+NED.
| Grade |
|---|
|
|
|---|
| Toxicities | 0 | 1 | 2 | 3 | 4 | G3/4 (%) |
|---|
| Anorexia | 14 | 38 | 13 | 1 | 0 | 1.5 |
| Nausea | 8 | 41 | 15 | 2 | 0 | 3.0 |
| Vomiting | 31 | 22 | 12 | 1 | 0 | 1.5 |
| Diarrhea | 26 | 24 | 12 | 4 | 0 | 6.1 |
| Fever | 46 | 14 | 5 | 1 | 0 | 1.5 |
| Hemorrhage | 61 | 5 | 0 | 0 | 0 | 0 |
| Hair loss | 11 | 37 | 18 | 0 | 0 | 0 |
| Allergic
reaction | 62 | 3 | 0 | 1 | 0 | 1.5 |
| Edema | 60 | 4 | 2 | 0 | 0 | 0 |
| Ileus | 63 | 0 | 2 | 1 | 0 | 1.5 |
| Constipation | 54 | 10 | 2 | 0 | 0 | 0 |
| Pyelonephritis | 63 | 1 | 2 | 0 | 0 | 0 |
| Fatigue | 64 | 2 | 0 | 0 | 0 | 0 |
| Weight loss | 43 | 19 | 4 | 0 | 0 | 0 |
| Fistula (GU-bladder
vagina) | 65 | 0 | 0 | 1 | 0 | 1.5 |
The serum level of squamous cell carcinoma antigen,
a tumor marker, was abnormal (≥1.5 ng/ml) before treatment in 52
(78.8%) of 66 patients. After chemotherapy, the level fell to the
normal range in 29 (55.8%) of these patients before surgery. Among
the 50 patients who responded to NAC (CR+PR), the squamous cell
carcinoma antigen level was abnormal in 41 (82.0%) before
treatment. In 25 (61.0%) of these patients, the level decreased to
normal after chemotherapy.
As for the radicality of surgery, 4 (6.4%) of 63
patients were judged by the operator to have residual tumor or to
have undergone incomplete lymph node resection, and 7 (11.1%) had
positive resection margins on histopathological examination of
their resected specimens.
Overall, 57 (90.5%) of 63 patients had a histologic
response on histopathological evaluation of their resected
specimens (grade 0 in 6 patients, grade 1a in 23, grade 1b in 8,
grade 2 in 22, and grade 3 in 4). Among the 63 patients,
parametrial invasion was present in 15 (23.8%), stromal invasion in
57 (90.5%; <50% in 21 and ≥50% in 36), vascular invasion in 31
(49.2%), and lymph node metastasis in 19 (30.2%) (Table V). Given that 38 (57.6%) of the 66
patients had stage IIb disease, the improvement in
histopathological findings after NAC was regarded to be
considerable.
 | Table VPathological examination. |
Table V
Pathological examination.
| Pathological
findings | Yes | No |
|---|
| Parametrial
involvement | 15 | 48 |
| Histologic
margin | 7 | 56 |
| Depth of stromal
invasion | 21 (<50%) | 6 |
| 36 (>50%) | |
| Vascular-lymphatic
involvement | 31 | 32 |
| Lymph node
metastasis | 19 | 44 |
The mean bleeding volume was 998 ml (range
158–3362), and the mean operation time was 294 min (range 136–566).
As for surgical complications, grade 3 or 4 intraoperative bleeding
occurred in 3.2% of the patients, which did not differ from the
incidence associated with conventional extended hysterectomy.
Dysuria was grade 0 in 33.9% of the patients, grade 1 in 25.8%,
grade 2 in 19.4%, and grade 3 in 21.0%. The incidence of grade 1 or
2 lymphatic cyst was somewhat high (27.4%).
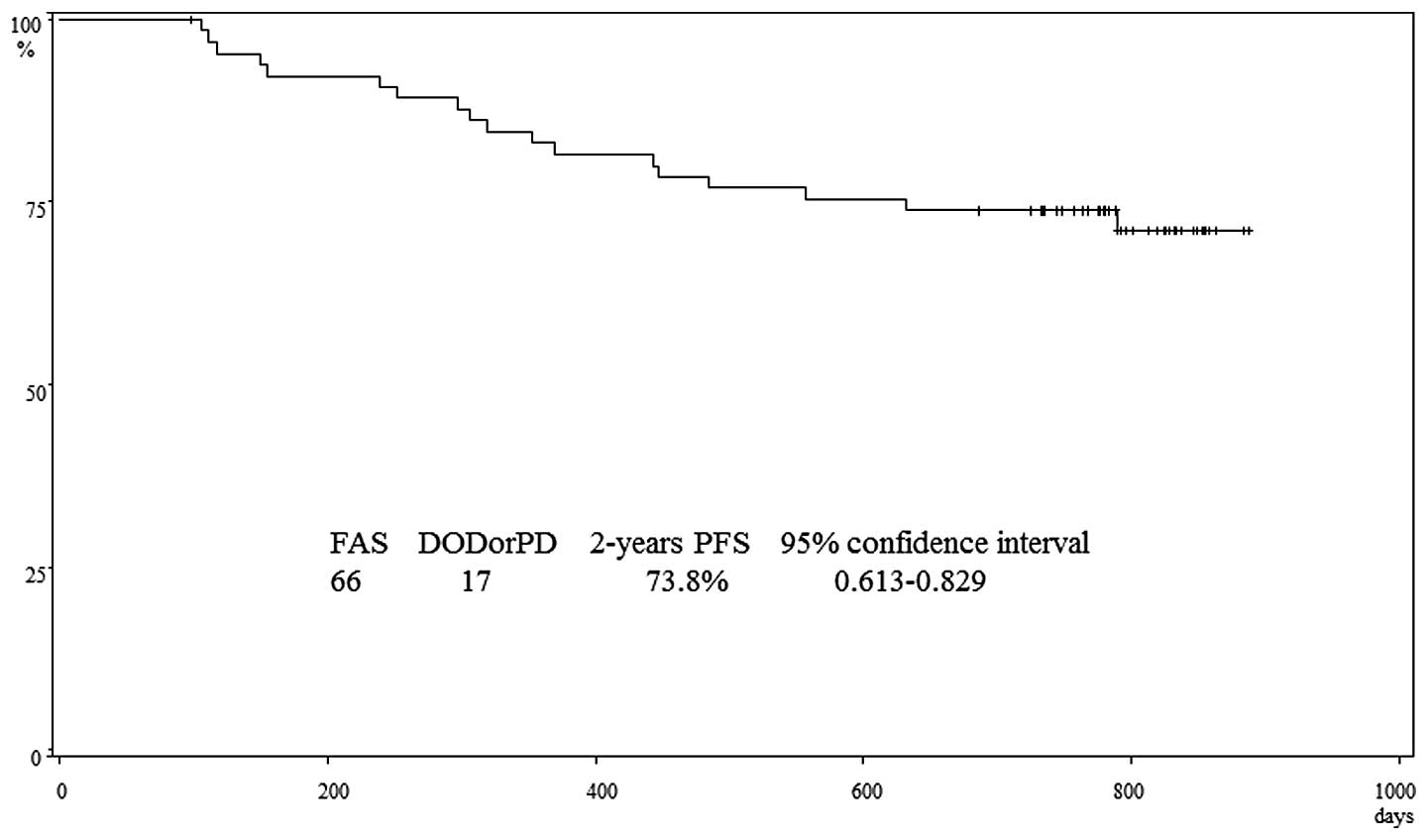
Two years after surgery, 17 patients had recurrence,
including 9 who died of cancer. The progression-free survival rate
at 2 years was 73.8% (95% confidence interval, 0.613–0.829)
(Fig. 1). In this study, additional
postoperative treatment was not specified and was left to the
treatment policy of each hospital and the discretion of the
attending physician. The breakdown of postoperative therapy was: no
additional treatment in 30 patients (recurrence in 8), radiotherapy
in 5 (recurrence in 1), concurrent chemoradiotherapy in 15
(recurrence in 7), and chemotherapy in 15 (recurrence in 1). In
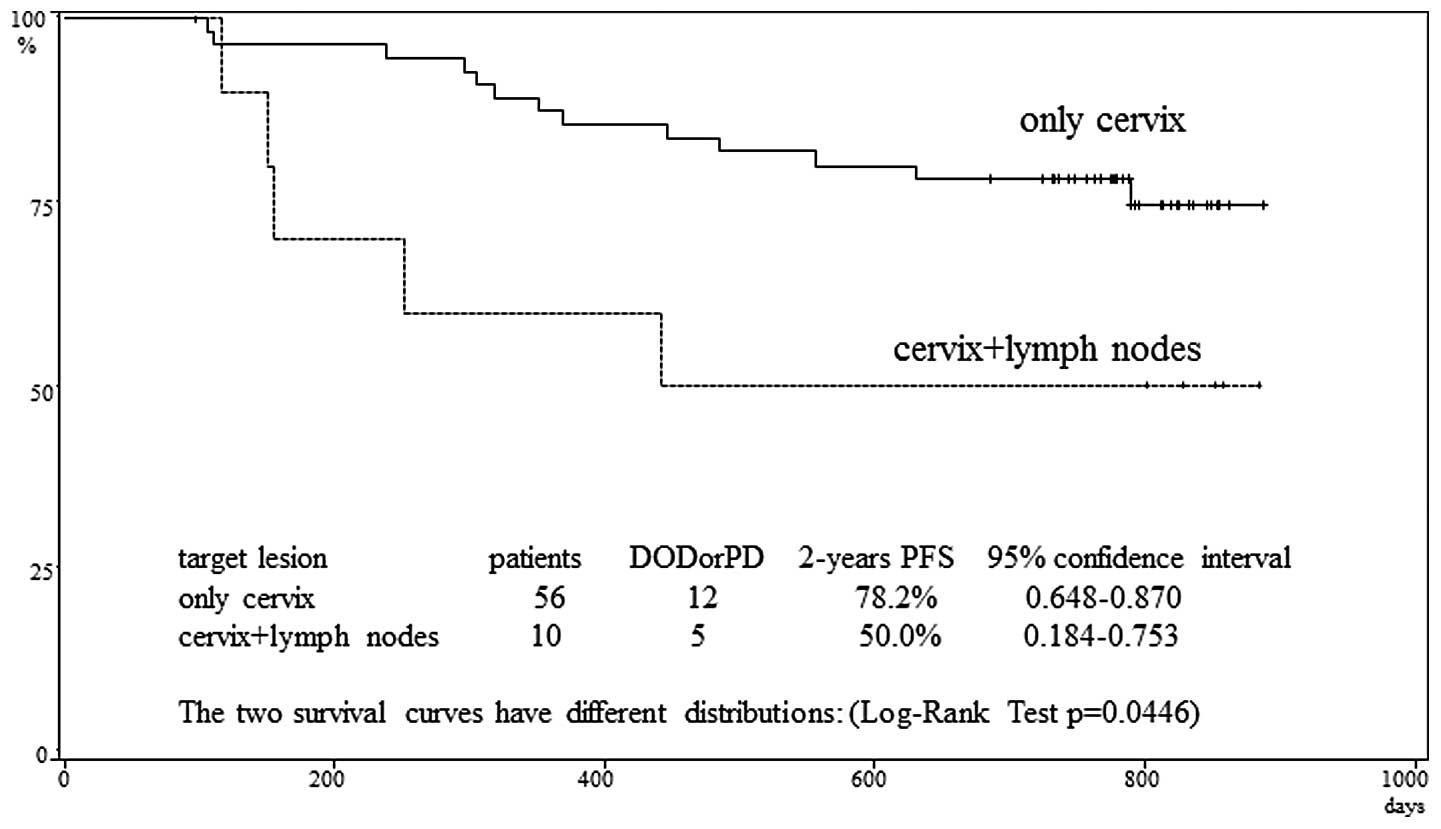
patients with target lesions only in the cervix and those with
target lesions in both the cervix and lymph nodes, the
progression-free survival rate at 2 years was 78.2 and 50.0%,
respectively. This difference was significant (p=0.0446) (Fig. 2). The progression-free survival rate
at 2 years according to disease stage was 94.1% for Ib2, 60.0% for
IIa, and 68.4% for IIb. These differences were not significant.
Discussion
The response rate, the primary endpoint, was 75.8%
(CR in 2 patients, PR in 48, SD in 12, PD in 0, and NE in 4). The
tumor shrinkage rate was <30% in 11 of the 12 patients with SD.
The response rate was non-inferior to those previously reported for
NAC in patients with cervical cancer. It is noteworthy that only
1.42 courses of NAC on average were required to produce a response
(1 course in 30 patients, 2 courses in 19, and 3 courses in 1).
Fifty-six of 66 patients (84.8%) received 1 or 2 courses of NAC.
Moreover, no patient had PD. CPT-11 and NED had a high response
rate against primary lesions, as well as a prompt onset of effect,
making it an optimal regimen for NAC.
Although a number of studies of NAC using a variety
chemotherapeutic agents have been evaluated in patients with
locally advanced cervical cancer and high response rates ranging
from 76 to 95% have been demonstrated (12–17).
The rate of progression-free survival at 2 years was
73.8% (95% confidence interval, 0.613–0.829) in initially treated
patients with stage Ib2 to IIb cervical squamous cell carcinoma who
received NAC with irinotecan plus nedaplatin followed by radical
hysterectomy. Because 57.6% of the study group had stage IIb
disease and 75.8% had tumors exceeding 4 cm in diameter, we
consider our results to be satisfactory. In addition, the study
protocol did not specify additional treatment after surgery, and
such treatment was left to the treatment policies of each hospital
and the discretion of the attending physician. Postoperative
treatment was performed as follows: no additional treatment in 30
patients (recurrence in 8), radiotherapy in 5 (recurrence in 1),
concurrent chemoradiotherapy in 15 (recurrence in 7), and
chemotherapy in 15 (recurrence in 1). Because chemotherapy was
associated with good outcomes, future studies should assess whether
multidisciplinary treatment combining surgery with preoperative and
postoperative chemotherapy can improve outcomes (7,18).
Patients with lymph nodes as target lesions had significantly
poorer outcomes. More aggressive, individualized treatment
strategies may be necessary in this subgroup of patients.
In Japan, stage Ib2 to IIb cervical cancer is
generally treated by radical hysterectomy. In the United States,
the National Comprehensive Cancer Network (NCCN) and National
Cancer Institute (NCI) guidelines recommend concurrent
chemoradiotherapy for patients with stage Ib2 to IIb disease, and
surgery is not included as a treatment option (19,20).
Since the 1980s, however, many attempts have been made to improve
survival by performing surgery after preoperative chemotherapy in
patients with stage Ib2 to IIb cervical cancer (21–28).
Preoperative chemotherapy may eliminate micrometastases, facilitate
complete tumor resection, and enable resection of previously
unresectable tumors.
Some studies have shown that NAC is therapeutically
useful, whereas others have not. The Meta-analysis Group in the UK
conducted a meta-analysis of 6 randomized controlled trials
comparing NAC plus surgery with surgery alone. NAC was found to
significantly improve progression-free survival (hazard ratio,
0.76; 95% confidence interval 0.62–0.94; p=0.01), but not overall
survival (hazard ratio, 0.85; 95% confidence interval 0.67–1.07;
p=0.17). In the NAC group, histopathological findings such as lymph
node metastasis and parametrial invasion improved significantly.
The NAC group also showed slight trends toward better outcomes in
terms of local recurrence, distant recurrence, and resection rate.
However, the meta-analysis concluded that it was unclear whether
NAC improves long-term outcomes (29).
In 2003, the results of a meta-analysis of 5
clinical studies comparing NAC plus surgery with surgery alone in
872 patients with stage I or II (some with stage III) cervical
cancer were reported. As compared with surgery alone, NAC plus
surgery was found to significantly improve overall survival (hazard
ratio, 0.65) and disease-free survival (hazard ratio, 0.68) at 5
years (30). However, this
meta-analysis had several limitations, such as the inclusion of
patients with various stages of cervical cancer and the lack of a
comparison with chemoradiotherapy. Preoperative chemotherapy has
thus not been accepted as standard treatment.
Since 2002, the European Organization for Research
and Treatment of Cancer (EORTC) has been conducting a randomized
controlled trial (EORTC 55994) comparing concurrent
chemoradiotherapy, currently standard treatment, with preoperative
chemotherapy followed by surgery in patients with stage Ib2-IIb
cervical cancer. This study is ongoing and conclusions have yet to
be reached (30). At present,
evidence demonstrating that NAC plus surgery is superior to surgery
alone or concurrent chemoradiotherapy is still not available
(31).
Acknowledgements
We are indebted to the following participating
hospitals for cooperating in patient enrollment: Hyogo Cancer
Center, Tohoku University Hospital, St. Marianna University School
of Medicine Hospital, Iwate University School of Medicine Hospital,
Tottori University Hospital, Kitasato University, Osaka City
General Hospital, Cancer Institute Hospital Ariake, National
Hospital Organization Kure Medical Center-Chugoku Cancer Center,
Jikei University Kashiwa Hospital, Keio University Hospital,
Nagasaki University Hospital of Medicine and Dentistry, Hamamatsu
Medical Center, Shinshu University Hospital, Hiroshima University
Hospital, Dokkyo Medical University Hospital, National Hospital
Organization Shikoku Cancer Center, Kochi Health Sciences Center,
Yamaguchi University Hospital, Kinki University Hospital, Nara
Medical University Hospital, Jikei University Hospital, Tokyo
Dental College Ichikawa General Hospital, Hirosaki University
Hospital, National Hospital Organization Fukuyama Medical Center,
Kansai Rosai Hospital, Fukuoka University Hospital, Sapporo Railway
Hospital, Wakayama Medical University Hospital, Tokyo Medical
University Hospital, Kumamoto University Hospital, Japanese Red
Cross Ashikaga Hospital, and Nagasaki Municipal Hospital. We thank
all members of JGOG.
References
|
1
|
Yamamoto K, Kokawa K, Umesaki N, et al:
Phase I study of combination chemotherapy with irinotecan
hydrochloride and nedaplatin for cervical squamous cell carcinoma:
Japanese Gynecologic Oncology Group study. Oncol Rep. 21:1005–1009.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takeuchi S, Dobashi K, Fujimoto S, et al:
A late phase II study of CPT-11 on uterine cervical cancer and
ovarian cancer. Jpn J Cancer Chemother. 18:1681–1689.
1991.PubMed/NCBI
|
|
3
|
Kanazawa F, Koizumi F, Koh Y, et al: In
vitro synergistic interactions between the cisplatin analogue
nedaplatin and the DNA topoisomerase I inhibitor irinotecan and the
mechanism of this interaction. Clin Cancer Res. 7:202–209.
2001.
|
|
4
|
Sugiyama T, Yakushiji M, Noda K, et al:
Phase II study of irinotecan and cisplatin as first-line
chemotherapy in advanced or recurrent cervical cancer. Oncology.
58:31–37. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sugiyama T, Nishida T, Kumagai S, et al:
Combination therapy with irinotecan and cisplatin as neoadjuvant
chemotherapy in locally advanced cervical cancer. Br J Cancer.
81:95–98. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raspagliesi F, Ditto A, Selvaggi L, et al:
A phase 2 multicenter study of irinotecan and cisplatinum as
neoadjuvant treatment in patients with locally advanced cervical
cancer. Int J Gynecol Cancer. 20:1569–1575. 2010.PubMed/NCBI
|
|
7
|
Matsumura M, Takeshima N, Ota T, et al:
Neoadjuvant chemotherapy followed by radical hysterectomy plus
postoperative chemotherapy but no radiotherapy for Stage IB2-IIB
cervical cancer - irinotecan and platinum chemotherapy. Gynecol
Oncol. 119:212–216. 2010. View Article : Google Scholar
|
|
8
|
Kato T, Nishimura H, Yakushiji M, et al:
Phase II study of 254-S (cis-diammine glycolate platinum) for
gynecological cancer. Jpn J Cancer Chemother. 19:695–701.
1992.PubMed/NCBI
|
|
9
|
Machida S, Ohwada M, Fujiwara H, et al:
Phase I study of combination chemotherapy using irinotecan
hydrochloride and nedaplatin for advanced or recurrent cervical
cancer. Oncology. 65:102–107. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsuda H, Hashiguchi Y, Nishimura S, et al:
Phase I-II study of irinotecan plus nedaplatin with recombinant
human granulocyte colony-stimulating factor support in patients
with advanced or recurrent cervical cancer. Br J Cancer.
91:1032–1037. 2004.
|
|
11
|
Ohwada M, Machida S, Fujiwara H, et al:
Phase II study of combination chemotherapy using irinotecan and
nedaplatin for patients with primary advanced or recurrent cervical
cancer. Proc ASCO: J Clin Oncol. 22(Suppl 14): abs. 50882004.
|
|
12
|
Zanetta G, Fei F, Mangioni C, et al:
Chemotherapy with paclitaxel, ifosmide, and cisplatin for the
treatment of squamous cell cervical cancer. Semin Oncol. 27:23–27.
2000.PubMed/NCBI
|
|
13
|
Haung HJ, Chang TC, Hong JH, et al:
Prognostic value of age and histologic type in neoadjuvant
chemotherapy plus radical surgery for bulky (≥4 cm) stage IB and
IIA cervical carcinoma. Int J Gynecol Cancer. 13:204–221.
2003.PubMed/NCBI
|
|
14
|
D’Agostino DG, Distefano M, Greggi S, et
al: Neoadjuvant treatment of locally advanced carcinoma of the
uterine cervix with epirubicin, paclitaxel and cisplatin. Cancer
Chemother Pharmacol. 49:256–260. 2002.PubMed/NCBI
|
|
15
|
Vagno GD, Cormio G, Pinata S, et al:
Cisplatin and vinorelbine as neoadjuvant chemotherapy in locally
advanced cervical cancer: a phase II study. Int J Gynecol Cancer.
13:308–312. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duenas-Gonzales A, Lopez-Graniel C,
Gonzalez-Enciso A, et al: A phase II study of multimodality
treatment for locally advanced cervical cancer: neoadjuvant
carboplatin and paclitaxel followed by radical hysterectomy and
adjyvant cisplatin chemoradiation. Ann Oncol. 14:1278–1284. 2003.
View Article : Google Scholar
|
|
17
|
Umesaki N, Fujii T, Nishimura R, et al:
Combination chemotherapy with iirunotecan (irinotecan) and
mitomycin C (MMC) for advanced or recurrent squamous cell carcinoma
of the cervix: Japanese Gynecologic Oncology Group (JGOG) study.
Proc Am Soc Clin Oncol. 22:465abs. 18692003.PubMed/NCBI
|
|
18
|
Takeshima N, Umayahara K, Fujiwara K, et
al: Treatment results of adjuvant chemotherapy after radical
hysterectomy for intermediate-and high risk stage IB-IIA cervical
cancer. Gynecol Oncol. 103:618–622. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
NCCN Clinical Practice Guidelines in
Oncology-Cervical Cancer-v2. National Comprehensive Cancer Network;
2006
|
|
20
|
Cervical Cancer Treatment (PDQ®), Health
Professional Version. National Cancer Institute in the United
States (web-site). 2011
|
|
21
|
Sardi JE, Sananes CE, Giaroli AA, et al:
Long-term follow-up of the first randomized trial using neoadjuvant
chemotherapy in stage Ib squamous carcinoma of the cervix: the
final results. Gynecol Oncol. 67:61–69. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Benedetti-Panici P, Greggi S, Scambia G,
et al: Long-term survival following neoadjuvant chemotherapy and
radical surgery in locally advanced cervical cancer. Eur J Cancer.
34:341–346. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang TC, Lai CH, Hong JH, et al:
Randomized trial of neoadjuvant cisplatin, vincristine, bleomycin,
and radical hysterectomy versus radiation therapy for bulky stage
IB and IIA cervical cancer. J Clin Oncol. 18:1740–1747. 2000.
|
|
24
|
Buda A, Fossati R, Colombo N, et al:
Randomized trial of neoadjuvant chemotherapy comparing paclitaxel,
ifosfamide, and cisplatin with ifosfamide and cisplatin followed by
radical surgery in patients with locally advanced squamous cell
cervical carcinoma: the SNAP01 (studio neo-adjuvante portio)
Italian Collaborative Study. J Clin Oncol. 23:4137–4145. 2005.
|
|
25
|
Tzioras S, Pavlidis N, Paraskevaidis E, et
al: Effects of different chemotherapy regimens on survival for
advanced cervical cancer: systematic review and meta-analysis.
Cancer Treat Rev. 33:24–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Katsumata N, Yoshikawa H, Hirakawa T, et
al: Phase III randomized trial of neoadjuvant chemotherapy (NAC)
followed by radical hysterectomy (RH) versus RH for bulky stage
I/II in cervical cancer (JCOG0102). Proc Am Soc Clin Oncol.
24(S18): 1abs. 50132006.
|
|
27
|
Eddy GL, Bundy BN, Creasman WT, et al:
Treatment of (bulky) stage IB cervical cancer with or without
neoadjuvant vincristine and cisplatin prior to radical hysterectomy
and pelvic/para-aortic lymphadenectomy: a phase III trial of the
Gynecologic Oncology Group. Gynecol Oncol. 106:362–369. 2007.
View Article : Google Scholar
|
|
28
|
Mossa B, Mossa S, Corosu L, et al:
Follow-up in a long-term randomized trial with neoadjuvant
chemotherapy for squamous cell cervical carcinoma. Eur J Gynecol
Oncol. 31:497–503. 2010.PubMed/NCBI
|
|
29
|
Rydzewska L, Tierney J, Vale CL, et al:
Neoadjuvant chemotherapy plus surgery versus surgery for cervical
cancer. Cochrane Database Syst Rev. 20(1): CD0074062010.
|
|
30
|
Tierney J: Neoadjuvant chemotherapy for
locally advanced cervical cancer: a systematic review and
meta-analysis of individual patient data from 21 randomised trials.
Eur J Cancer. 39:2470–2086. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gonzalez-Martin A, Gonzalez-Cortijo L,
Carballo N, et al: The current role of neoadjuvant chemotherapy in
the management of cervical carcinoma. Gynecol Oncol. 110:S36–S40.
2008. View Article : Google Scholar : PubMed/NCBI
|