Introduction
Nasopharyngeal carcinoma (NPC) arises from
epithelial cells that cover the surface and line of the
nasopharynx. NPC shows marked variations between ethnic
populations. The incidence of NPC is higher in China, particularly
in some regions of Southern China with an incidence rate of up to
54.7 per 100,000 (1). Symptoms
related to the primary tumor include epistaxis, nasal obstruction,
otitis media and tinnitus. Because nasal and aural symptoms are
non-specific and full clinical examination of the nasopharynx is
not easily performed, the majority of NPC patients are diagnosed
after the tumor reaches advanced stages. Therefore, the treatment
effect of the majority of patients with NPC is not ideal.
The cancer stem cell (CSC) theory proposes that a
small subset of cells is responsible for the initiation,
proliferation and metastasis of cancer. CSCs generate a
heterogeneous population of cells that constitute the cancer
(2). The existence of CSCs and
their ability to self-renew, differentiate into multiple lineages,
resist apoptosis and extensively proliferate results in these cells
being particularly detrimental. Thus far, CSCs have been
distinguished from the bulk-tumor population by their expression
pattern of cell surface proteins (e.g., CD24, CD44 and CD133) and
cellular activities such as the efflux of Hoechst dye (3). The latter method, which we exploit in
our studies, is the so-called side population (SP) identification
and separation. The SP phenotype has been proven to be invaluable
for stem cell isolation in the absence of definitive cell surface
markers (4–6).
Targeting key signaling pathways that are active in
CSC self-renewal is a therapeutic approach to treating cancer
(7). Notch signaling is important
for the self-renewal and maintenance of stem cells and is receiving
increased attention as a target to eliminate CSCs (8). It has been found that the Notch
signaling pathway is highly activated in NPC (9). Another study found that Notch
signaling is mainly activated in human primary NPC cells that
express embryonic stem cell proteins (10). In our previous study, we
demonstrated that downregulation of Notch signaling by the Notch
inhibitor
N-[(3,5-difluorophenyl)acetyl]-L-alanyl-2-phenyl]glycine-1,1-dimethylethyl
ester (DAPT) could enhance radiosensitivity of NPC cells (11). On the other hand, CSCs contribute
toward radiation resistance (12–14).
Thus, we hypothesized that the Notch signaling pathway may be
crucial to the maintenance of CSCs in NPC, and Notch inhibition may
suppress NPC by depleted SP cells. To understand the underlying
mechanism, we investigated the expression and activation of Notch
signaling in SP and non-SP (NSP) cells from the NPC cell lines, and
examined the effect of DAPT on the proportional changes of SP cells
and on the inhibition of NPC cells.
Materials and methods
Cell culture
Human NPC cell lines (CNE1 and CNE2) were obtained
from the Xiangya Central Experiment Laboratory, Central South
University, China. Cells were cultured in RPMI-1640 (Hyclone, USA)
supplemented with 10% fetal bovine serum (FBS; Sijiqing, China),
100 U/ml penicillin G and 100 U/ml streptomycin (Gibco, Carlsbad,
CA, USA) under standard conditions.
Proliferation assay
The effect of Notch inhibition on cell proliferation
was determined by an MTT assay, as described in our previous
studies (15). In brief, CNE1 cells
were treated with various concentrations of DAPT or the same volume
of DMSO (control) for 1–3 days at 37°C. For the proliferation
assay, the MTT dye was added to each well and incubated for 2 h at
37°C according to the manufacturer’s instructions (GenMed, China).
Cell proliferation was expressed as the optical density (OD) of
each well.
SP cell analysis and isolation
SP cell analysis and isolation were performed by
fluorescence-activated cell sorting (FACS) (Beckman Coulter, Epic
Altra, New York, NY, USA) as described elsewhere (16). Before SP cell analysis, cells were
pretreated with 10 μmol/l DAPT or DMSO for 1–3 days. Cells at 80%
confluence were washed twice with calcium/magnesium free
phosphate-buffered saline (PBS), detached with 0.25% trypsin-EDTA
(Gibco) and suspended at 1×106 cells/ml in PBS
supplemented with 2% FBS. Then, cells were incubated with 5 μg/ml
Hoechst 33342 (Sigma, St. Louis, MO, USA) either alone or with 100
μg/ml verapamil (Sigma) at 37°C in the dark for 70 min. Cells were
washed, centrifuged and resuspended in cold PBS supplemented with
2% FBS, and then 1 μg/ml propidium iodide (PI; Sigma) was added.
All cells were kept at 4°C in the dark before FACS using dual
wavelength analysis. Verapamil is traditionally used as a guiding
parameter to determine the boundary between SP and NSP cells.
Isolated cells were analyzed for purity, which was typically
>99.5%.
Soft agar colony formation assay
Before the soft agar colony formation assay, CNE1
and CNE2 cells were pretreated with DAPT or DMSO for 2 days. The
soft agar colony formation assay was performed in 12-well plates
using a soft agar colony formation assay kit (GenMed) according to
the manufacturer’s instructions. DAPT- and DMSO-pretreated CNE1 and
CNE2 cells (2,500 per well) were plated in the soft agar gel with
10 μmol/l DAPT added to the nutrient solution of the continuously
treated cells. Every cell group (treated, continuously treated and
DMSO) were plated in triplicate wells. Cells were maintained at
37°C in a humidified incubator. On Day 14 after seeding, colonies
were washed twice with PBS and stained with MTT dye. After washing
out the dye, colonies >0.1 mm in diameter were counted under a
microscope in five selected fields (left upper, right upper, left
inferior, right inferior and central).
Apoptosis assay
The percentage of apoptotic cells was assessed using
the Annexin V-FITC/PI method with FACS as described in our previous
studies (15). CNE2 cells were
randomly assigned into three groups: DMSO, 48 and 72 h. Before the
apoptosis assay, CNE2 cells were incubated in 35-mm dishes with 10
μmol/l DAPT or the same volume of DMSO for 48 and 72 h. Then,
Annexin V-FITC/PI staining of the cells was performed according to
the manufacturer’s instructions (Biouniquer, China).
In vivo tumor formation assay
Ethics approval for this protocol was obtained from
The Institutional Animal Care and Use Committee at Fudan University
(SYXK20090082). All animal experiments were performed in accordance
with institutional guidelines for animal welfare. Five-week-old
female immune-deficient nude mice (BALB/C-nu) were purchased from
Shanghai Sino-British SIPPR/BK Lab Animal Co., Ltd., and housed in
microisolator individually ventilated cages with access to water
and food. Mice were randomly divided into DMSO- and DAPT-treated
groups (n=6, each group). CNE2 cells were pretreated with 10 μmol/l
DAPT or DMSO for 48 h, then 2×106 CNE2 cells were
subcutaneously injected into the right flank of the mice. After 4
weeks, xenografts were removed and processed into paraffin-embedded
sections.
Real-time PCR
Total RNA was extracted from SP and NSP cells
isolated from CNE2 cells using TRIzol reagent (Invitrogen,
Carlsbad, CA, USA) and converted to cDNA using MMLV reverse
transcriptase (Promega, Madison, WI, USA). The primers and the
methods of real-time PCR were described in our previous studies
(15).
Western blot analysis
Western blotting was performed as described
elsewhere (17). Briefly, the
concentration of protein extracted from SP and NSP cells isolated
from CNE2 cells was determined by the Lowry method. Equal amounts
of protein were separated by 10% SDS-PAGE and electrophoretically
transferred onto polyvinyldifluoridine membranes (Millipore,
Billerica, MA, USA). Rabbit anti-human Notch1 intracellular domain
(NICD) antibody (1:500; Cell Signaling Technology, Danvers, MA,
USA) and mouse anti-human Hes-1 antibody (1:500; Santa Cruz
Biotechnology, Santa Cruz, CA, USA) were used to detect the
expression of cleaved Notch1 and Hes-1. β-actin or Erk were used as
an internal control.
Statistical analysis
All experimental procedures were performed in
triplicate. Data from in vivo tumor formation assays were
analyzed for statistical significance using χ2 with SPSS
17.0 software (SPSS Inc., Chicago, IL, USA). Other data were
analyzed for statistical significance using analysis of variance
(ANOVA) or t-tests with the same software. A P-value <0.05 was
considered statistically significant.
Results
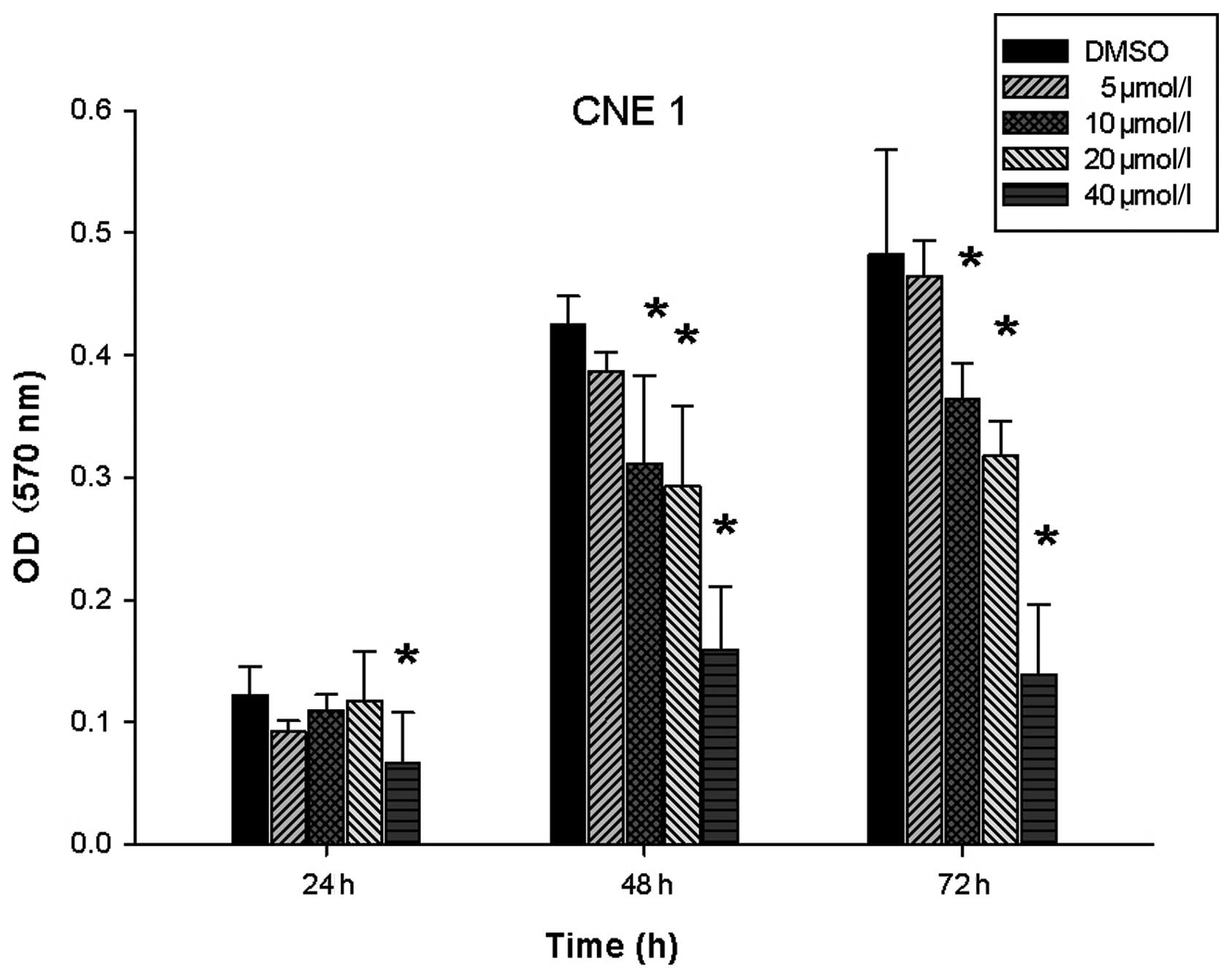
DAPT inhibits NPC cell proliferation
In our previous studies (15), we found that DAPT could inhibit CNE2
growth and the lowest applicable concentration was 10 μmol/l. CNE2
is a poorly differentiated squamous cell carcinoma line. To further
determine whether the Notch inhibitor (DAPT) could inhibit NPC cell
proliferation and the lowest applicable concentration, we examined
the effect of DAPT on the proliferation of CNE1 cells. CNE1 is
well-differentiated squamous cell carcinoma cell line. CNE1 cells
were also treated with various DAPT concentrations (0, 5, 10, 20 or
40 μmol/l). The MTT assay revealed that DAPT could inhibit CNE1
proliferation in a dose-dependent manner. For CNE1 cells, 40 μmol/l
DAPT inhibited proliferation after 24 h (P<0.05), and 10 μmol/l
DAPT inhibited proliferation after 48 h (P<0.05, Fig. 1). Similarly to the results in CNE2
cells, 10 μmol/l was the lowest concentration that inhibited CNE1
proliferation. Thus, DAPT inhibited proliferation in poorly as well
as in well-differentiated NPC cells.
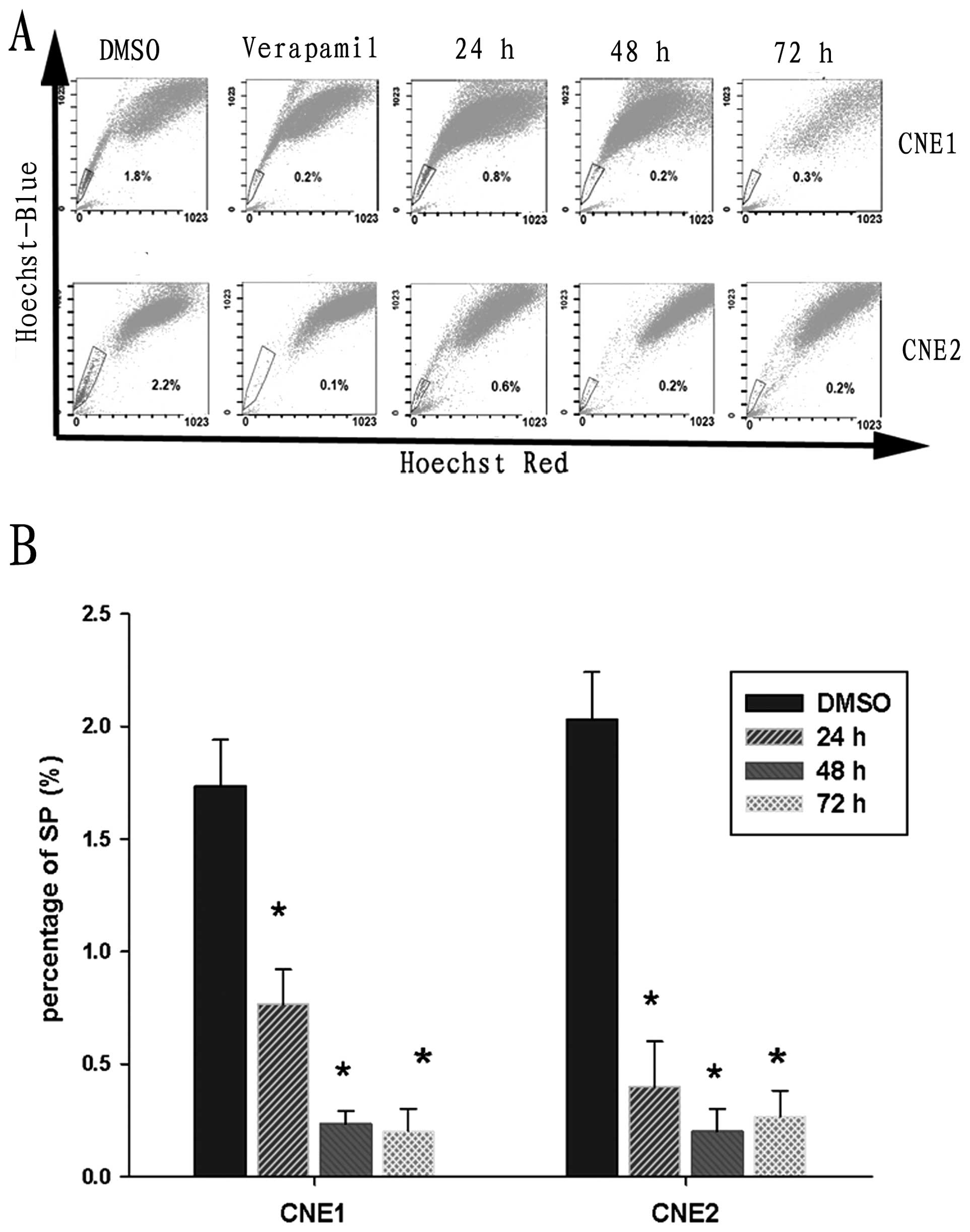
DAPT depletes SP cells in CNE1 and CNE2
cell lines
CSC theory states that CSCs are responsible for
cancer growth. We hypothesized that DAPT inhibition of NPC cell
proliferation may be mediated by decreasing the number of CSCs.
Therefore, we analyzed the percentage of SP cells after the two NPC
cell lines were pretreated with DAPT. For CNE1 cells, the
percentage of SP cells was 1.73±0.21%, while the percentage of SP
cells in the CNE2 cell line was 2.03±0.21%. After pretreatment with
10 μmol/l DAPT for 24 h, the percentage of SP cells was
significantly decreased in both cell lines based on FACS analysis.
Few SP cells were detected in CNE1 and CNE2 cell lines after
pretreatment with DAPT for 48 h (Fig.
2). Taken together, these data suggest that Notch inhibition
significantly depletes SP cells in the CNE1 and CNE2 cell line.
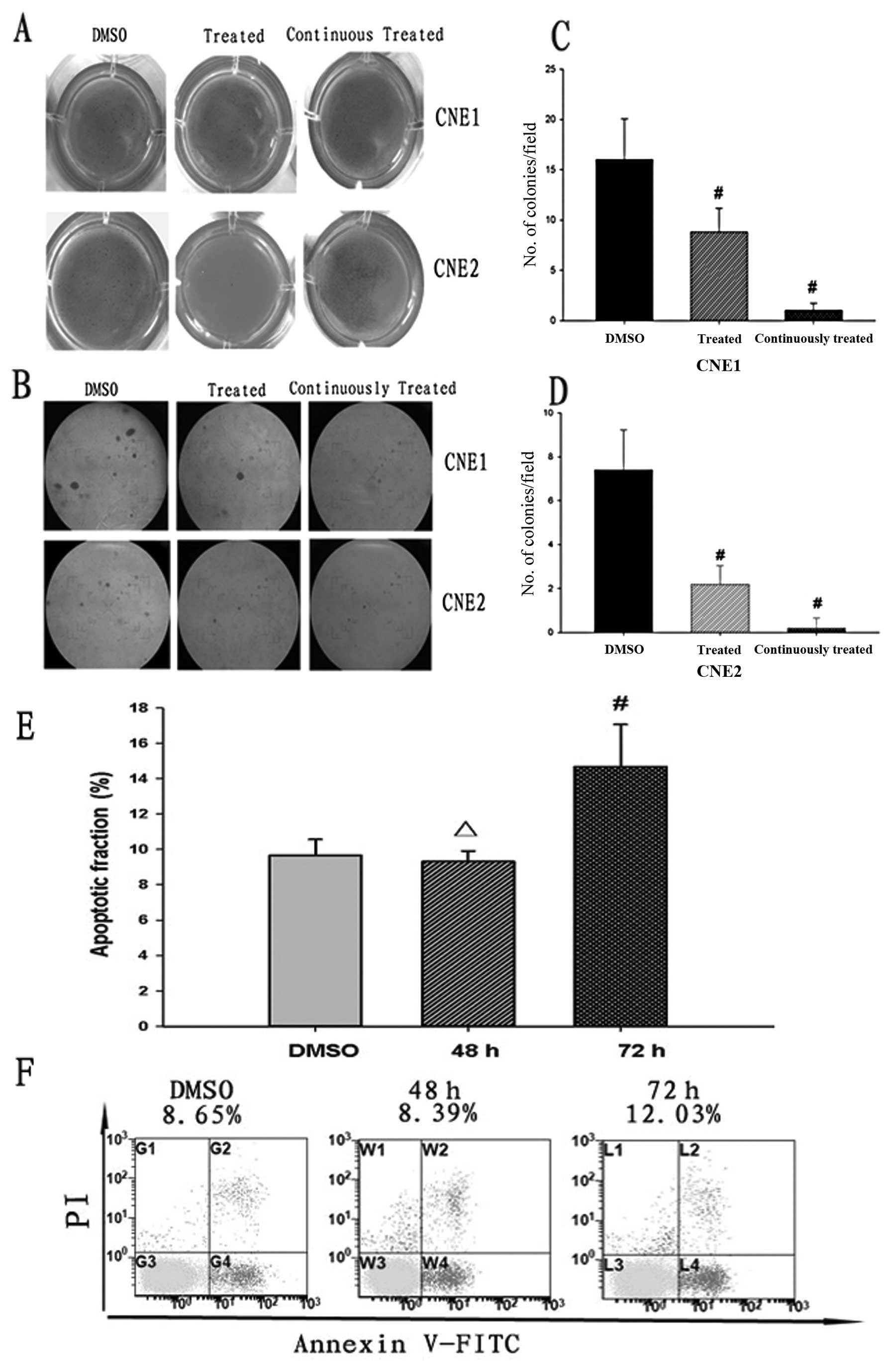
DAPT reduces colony formation and induces
apoptosis in NPC cells
It is well known that differentiation and the
anti-apoptotic ability of CSCs is higher than those of non-CSCs,
and SP cells of NPC demonstrate higher colony formation ability
(16). Thus, after the proportion
of SP to NSP cells decreases, the colony formation and
anti-apoptotic abilities of NPC cells may also decrease. After CNE1
and CNE2 cells were pretreated with DAPT (10 μmol/l) for 2 days, a
soft agar colony formation assay was performed. The colony
formation ability of DAPT-pretreated cells was significantly
decreased (P<0.05, Fig. 3C and
D). After the two cell lines were continuously treated with
DAPT, there were few colonies formed. The colony formation assay
results were easily observed with the naked-eye and under a
microscope (Fig. 3A and B). We
further examined whether reduced colony formation was due to
apoptosis induced by DAPT. In our previous studies (15), we found that DAPT had no obvious
impact on the apoptotic fraction when the cells were only treated
for 2 days. So, we investigated the apoptotic fraction in the
presence of DAPT for a longer time. The result demonstrated that
DAPT had an obvious impact on the percentage of apoptotic cells
after DAPT pretreatment for 72 h (P<0.05, Fig. 3E). Therefore, DAPT induces apoptosis
in NPC cells and decreases colony formation in continuously treated
groups, which may be associated with apoptosis induced by DAPT.
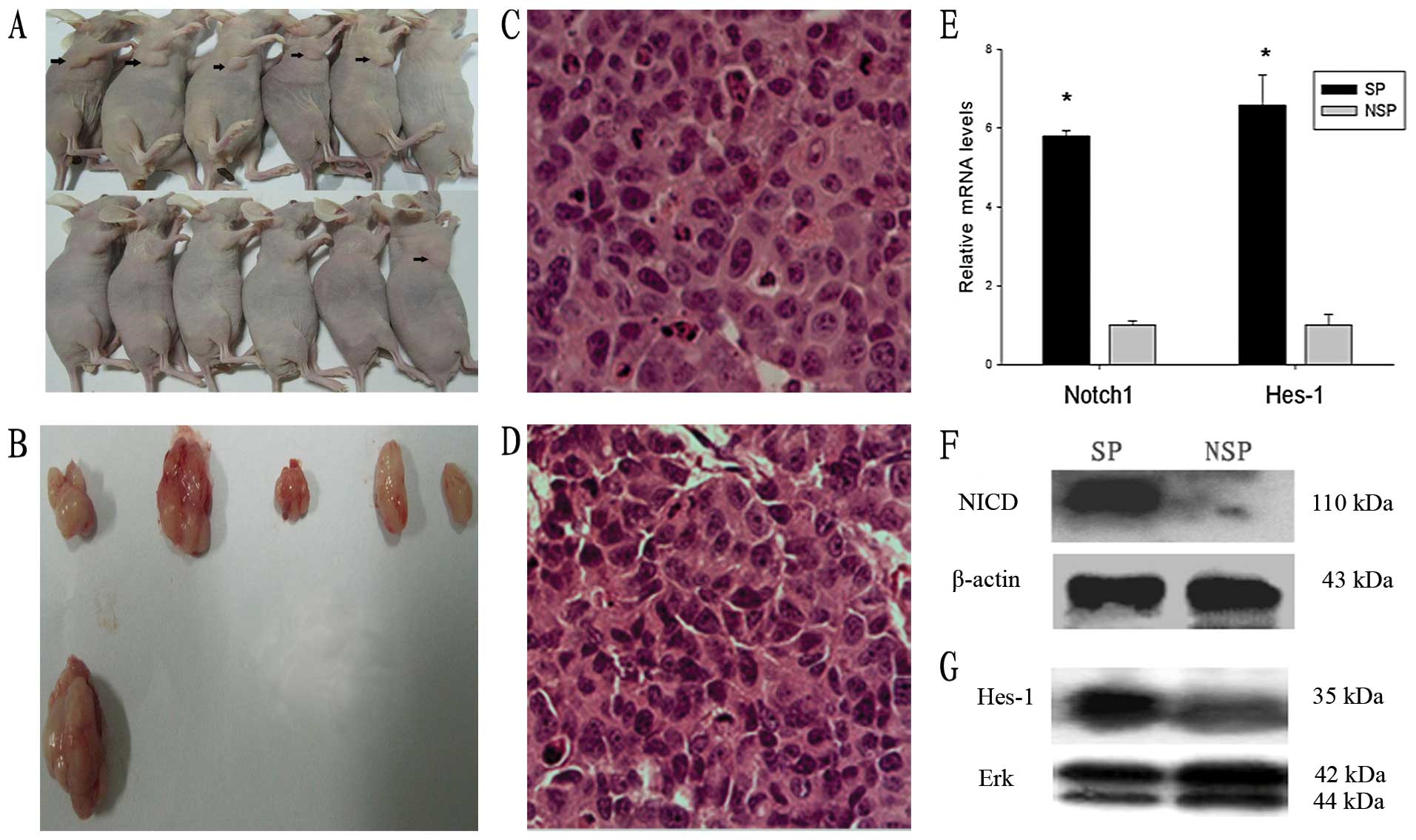
DAPT reduces tumor formation of NPC cells
in immune-deficient nude mice
Previous studies show that SP cells in NPC possess
higher tumorigenicity in vivo compared with that of NSP
cells (16). In the colony
formation assay, we showed that DAPT inhibited NPC cell
proliferation in vitro, but the cytotoxic effect of 10
μmol/l DAPT was rather limited after treatment for 2 days. In in
vivo tumor formation assays, only 1 out of 6 mice formed
xenografts in DAPT-treated groups. In contrast, 5 out of 6 mice
formed xenografts in DMSO-treated groups (P<0.05, Fig. 4A and B). Histological analysis
suggested that all xenografts showed the distinct pathological
features of poorly differentiated cells (Fig. 4C and D). These results demonstrate
that DAPT decreases tumor formation and reduces the tumorigenicity
of NPC cells.
Notch signaling is highly activated in SP
cells, but not in NSP cells in the CNE2 cell line
To determine the underlying mechanism of
DAPT-mediated depletion of SP cells, the activation status of Notch
signaling in the SP and NSP cells of the CNE2 cell line was
examined by real-time PCR and western blot analyses. NICD and Hes-1
are important signaling molecules in the Notch signaling pathway.
NICD is the activated form of Notch1 and is cleaved by γ-secretase
from the cell membrane. Hes-1 is a downstream target gene of Notch
signaling. As shown in Fig. 4E-G,
Notch1 and Hes-1 expression levels were higher in SP cells compared
with those of NSP cells at both mRNA and protein levels. Combined
with the above results, these data suggest that Notch signaling is
required for the maintenance of SP cells.
Discussion
With the development of novel therapeutic
technologies, the 5-year survival rate (SR) of NPC has improved,
but in some regional areas the 5-year SR is still <50% and the
10-year SR is <30% (18). Thus,
it is important to explore new strategies to decrease the mortality
rates of NPC. CSCs are thought to contribute toward therapeutic
resistance, recurrence and metastasis. If the proportion of CSCs
could be decreased, then the mortality rates of cancer may also
decrease. SP cells are a rare subset of cells enriched with CSCs.
In a previous study, SP cells were isolated from NPC cell lines and
found to possess high proliferative, self-renewal, differentiation
and tumorigenic abilities compared with those of NSP cells
(16). In the present study, we
found that Notch signaling is highly activated in SP cells compared
with that of NSP cells in NPC cells. Therefore, Notch inhibition
significantly reduces the proportion of SP cells in NPC cells.
Additionally, the abilities of high proliferation, anti-apoptosis
and tumorigenesis of NPC cells are inhibited with the decrease of
SP cells.
Notch signaling plays a pivotal role in the
regulation of numerous fundamental cellular processes, such as
proliferation, stem cell maintenance and differentiation during
embryonic and adult development (19,20).
Dysfunction of Notch signaling can result in a variety of
developmental defects and adult pathologies (21), including tumorigenesis. Notably, the
Notch signaling pathway is frequently activated in several human
malignancies such as glioma (22),
breast cancer (23), colon cancer
(24) and NPC (9,10).
Subsequent studies have found that Notch signaling contributes
toward the maintenance of CSCs (25–27).
In the present study, we found that Notch signaling is highly
activated in SP cells compared with that in NSP cells of NPC, which
is consistent with previous studies (28–31).
The Notch receptor is a transmembrane protein
expressed on the cell surface as a heterodimer with an EGF-like
repeat-rich extracellular domain and an intracellular domain with a
single pass transmembrane domain (19). Signaling is initiated by
ligand-receptor binding, thereby inducing a series of cleavages
named S2, S3 and S4. Among them, the S3/4 cleavage is an
intramembranous cleavage mediated by presenilin-dependent
γ-secretase, which results in translocation of the NICD into the
nucleus. Nuclear NICD then interacts with the transcriptional
factor CSL (known as CBF1/RBPJκ in mammals) to activate downstream
target genes such as Hes-1 (32,33).
γ-secretase is a multi-subunit enzyme with specificity for cleavage
of intramembrane substrates such as Notch (34). Therefore, γ-secretase inhibitors
(GSIs) can block Notch activation. The advantage of using GSIs is
that all Notch receptors require γ-secretase for processing and
signaling (35). siRNA can inhibit
Notch signaling, but the approach cannot be easily used in the
clinic. Therefore, in the present study, we used the GSI DAPT to
inhibit Notch signaling and investigate the effects.
Based on the CSC theory, CSCs possess a capacity to
self-renew and generate a certain number of CSCs, which maintains
the balance between CSCs and non-CSCs (36). During this process, Notch and other
signaling pathways, such as Hedgehog (Hh) and Wnt, are key factors
in the regulation of self-renewal (2). In the present study, we found that
Notch signaling is highly activated in CSCs. In our previous study,
we found that DAPT inhibits Notch signaling in NPC cells (11). After the Notch signaling pathway is
inhibited, CSC differentiation to non-CSCs increases and the
percentage of CSCs decreases, which is similar to the effect of
inhibiting the Wnt signaling pathway (37).
Notch inhibitors are not traditional cytotoxic
drugs. In the present study, the cytotoxic effects of DAPT at 10
mol/l were rather limited. However, 10 μmol/l DAPT significantly
inhibited the Notch signaling pathway and decreased the proportion
of CSCs. After CSCs have been depleted in cell lines, it is
plausible that proliferation, antiapoptosis and tumorigenesis of
the cell lines will be impaired. Furthermore, we found that the
volume of single tumors in DAPT-treated groups was similar to those
of DMSO-treated groups in the in vivo tumor formation
assays. These results demonstrate that Notch inhibition can only
decrease the tumor formation of NPC cells, but has no effect on
long-term tumor growth. Therefore, to maximize the efficacy of
Notch inhibition, it will likely be necessary to administer a Notch
inhibitor in combination with other treatments such as radiation
therapy and other chemotherapies (29). For NPC treatment, Notch inhibition
combined with radiation therapy may be a good therapeutic approach.
In our previous study, we found that Notch inhibition enhances the
radiosensitivity of NPC cells (11).
In conclusion, our data indicate that Notch
signaling is highly activated in SP cells compared with that of NSP
cells in NPC. Thus, Notch inhibition significantly depletes SP
cells. With the decrease of SP cells, proliferation, anti-apoptosis
and tumorigenesis are also decreased. These results indicate that
Notch inhibition may be a promising preclinical application in
CSC-targeting therapy for NPC. Certainly, the application of Notch
inhibition is not limited to NPC because the Notch signaling
pathway is not only critical for the regulation of CSCs in NPC, but
also of CSCs in other cancers.
Acknowledgements
This study was supported by the Shanghai Health
Bureau Scientific Research Found (no. 2007002). The authors thank
Guoping Zhang for technical support in flow cytometry.
References
|
1
|
Tao Q and Chan AT: Nasopharyngeal
carcinoma: molecular pathogenesis and therapeutic developments.
Expert Rev Mol Med. 9:1–24. 2007.PubMed/NCBI
|
|
2
|
Kawasaki BT, Hurt EM, Mistree T and Farrar
WL: Targeting cancer stem cells with phytochemicals. Mol Interv.
8:174–184. 2008. View
Article : Google Scholar
|
|
3
|
Keysar SB and Jimeno A: More than markers:
biological significance of cancer stem cell-defining molecules. Mol
Cancer Ther. 9:2450–2457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patrawala L, Calhoun T,
Schneider-Broussard R, Zhou J, Claypool K and Tang DG: Side
population is enriched in tumorigenic, stem-like cancer cells,
whereas ABCG2+ and ABCG2− cancer cells are
similarly tumorigenic. Cancer Res. 65:6207–6219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ho MM, Ng AV, Lam S and Hung JY: Side
population in human lung cancer cell lines and tumors is enriched
with stem-like cancer cells. Cancer Res. 67:4827–4833. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J, Wei X, Ling J, Huang Y, Huo Y and
Zhou Y: The presence of a side population and its marker ABCG2 in
human deciduous dental pulp cells. Biochem Biophys Res Commun.
400:334–339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alison MR, Lim SM and Nicholson LJ: Cancer
stem cells: problems for therapy. J Pathol. 223:147–161. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pannuti A, Foreman K, Rizzo P, et al:
Targeting Notch to target cancer stem cells. Clin Cancer Res.
16:3141–3152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang M, Li JT, Zeng YX, Hou JH and Lin QQ:
Expression and significance of Notch1, p21WAF1 and involucrin in
nasopharyngeal carcinoma. Ai Zheng. 24:1230–1234. 2005.(In
Chinese).
|
|
10
|
Zhang Y, Peng J, Zhang H, et al: Notch1
signaling is activated in cells expressing embryonic stem cell
proteins in human primary nasopharyngeal carcinoma. J Otolaryngol
Head Neck Surg. 39:157–166. 2010.PubMed/NCBI
|
|
11
|
Yu S, Zhang R, Liu F, Hu H, Yu S and Wang
H: Down-regulation of Notch signaling by a γ-secretase inhibitor
enhances the radiosensitivity of nasopharyngeal carcinoma cells.
Oncol Rep. 26:1323–1328. 2011.
|
|
12
|
Bao S, Wu Q, McLendon RE, et al: Glioma
stem cells promote radioresistance by preferential activation of
the DNA damage response. Nature. 756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Phillips TM, McBride WH and Pajonk F: The
response of CD24(-/low)/CD44+ breast cancer-initiating
cells to radiation. J Natl Cancer Inst. 98:1777–1785.
2006.PubMed/NCBI
|
|
14
|
Woodward WA, Chen MS, Behbod F, Alfaro MP,
Buchholz TA and Rosen JM: WNT/beta-catenin mediates radiation
resistance of mouse mammary progenitor cells. Proc Natl Acad Sci
USA. 104:618–623. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu S, Zhang R, Liu F, Hu H, Yu S and Wang
H: Down-regulation of Notch signaling by a γ-secretase inhibitor
enhances the radiosensitivity of nasopharyngeal carcinoma cells.
Oncol Rep. 26:1323–1328. 2011.
|
|
16
|
Wang J, Guo LP, Chen LZ, Zeng YX and Lu
SH: Identification of cancer stem cell-like side population cells
in human nasopharyngeal carcinoma cell line. Cancer Res.
67:3716–3724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi GM, Xu Y, Fan J, et al: Identification
of side population cells in human hepatocellular carcinoma cell
lines with stepwise metastatic potentials. J Cancer Res Clin Oncol.
134:1155–1163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang Q, Li Y and Lin X: A survival
analysis of nasopharyngeal carcinoma from 1995 to 2004 in Sihui,
Guangdong. Bull Chin Cancer. 3:150–151. 2007.
|
|
19
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lino MM, Merlo A and Boulay JL: Notch
signaling in glioblastoma: a developmental drug target. BMC Med.
8:722010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lai EC: Notch signaling: control of cell
communication and cell fate. Development. 131:965–973. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fukaya R, Ohta S, Yamaguchi M, et al:
Isolation of cancer stem-like cells from a side population of a
human glioblastoma cell line, SK-MG-1. Cancer Lett. 291:150–157.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo S, Liu M and Gonzalez-Perez RR: Role
of Notch and its oncogenic signaling crosstalk in breast cancer.
Biochim Biophys Acta. 1815:197–213. 2011.PubMed/NCBI
|
|
24
|
Zhang Y, Li B, Ji ZZ and Zheng PS: Notch1
regulates the growth of human colon cancers. Cancer. 116:5207–5218.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harrison H, Farnie G, Howell SJ, et al:
Regulation of breast cancer stem cell activity by signaling through
the Notch4 receptor. Cancer Res. 70:709–718. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yung WK: Tumor stem cells, notch, and the
news. Neuro Oncol. 12:1152010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu YY, Zheng MH, Cheng G, et al: Notch
signaling contributes to the maintenance of both normal neural stem
cells and patient-derived glioma stem cells. BMC Cancer. 11:822011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang XP, Zheng G, Zou L, et al: Notch
activation promotes cell proliferation and the formation of neural
stem cell-like colonies in human glioma cells. Mol Cell Biochem.
307:101–108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fan X, Khaki L, Zhu TS, et al: NOTCH
pathway blockade depletes CD133-positive glioblastoma cells and
inhibits growth of tumor neurospheres and xenografts. Stem Cells.
28:5–16. 2010.PubMed/NCBI
|
|
30
|
Sikandar SS, Pate KT, Anderson S, et al:
NOTCH signaling is required for formation and self-renewal of
tumor-initiating cells and for repression of secretory cell
differentiation in colon cancer. Cancer Res. 70:1469–1478. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhen Y, Zhao S, Li Q, Li Y and Kawamoto K:
Arsenic trioxide-mediated Notch pathway inhibition depletes the
cancer stem-like cell population in gliomas. Cancer Lett.
292:64–72. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nam Y, Aster JC and Blacklow SC: Notch
signaling as a therapeutic target. Curr Opin Chem Biol. 6:501–509.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tien AC, Rajan A and Bellen HJ: A Notch
updated. J Cell Biol. 184:621–629. 2009. View Article : Google Scholar
|
|
34
|
Kopan R and Ilagan MX: Gamma-secretase:
proteasome of the membrane. Nat Rev Mol Cell Biol. 5:499–504. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Suwanjunee S, Wongchana W and Palaga T:
Inhibition of gamma-secretase affects proliferation of leukemia and
hepatoma cell lines through Notch signaling. Anticancer Drugs.
19:477–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vermeulen L, Sprick MR, Kemper K, Stassi G
and Medema JP: Cancer stem cells - old concepts, new insights. Cell
Death Differ. 947–958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chikazawa N, Tanaka H, Tasaka T, et al:
Inhibition of Wnt signaling pathway decreases
chemotherapy-resistant side-population colon cancer cells.
Anticancer Res. 30:2041–2048. 2010.PubMed/NCBI
|