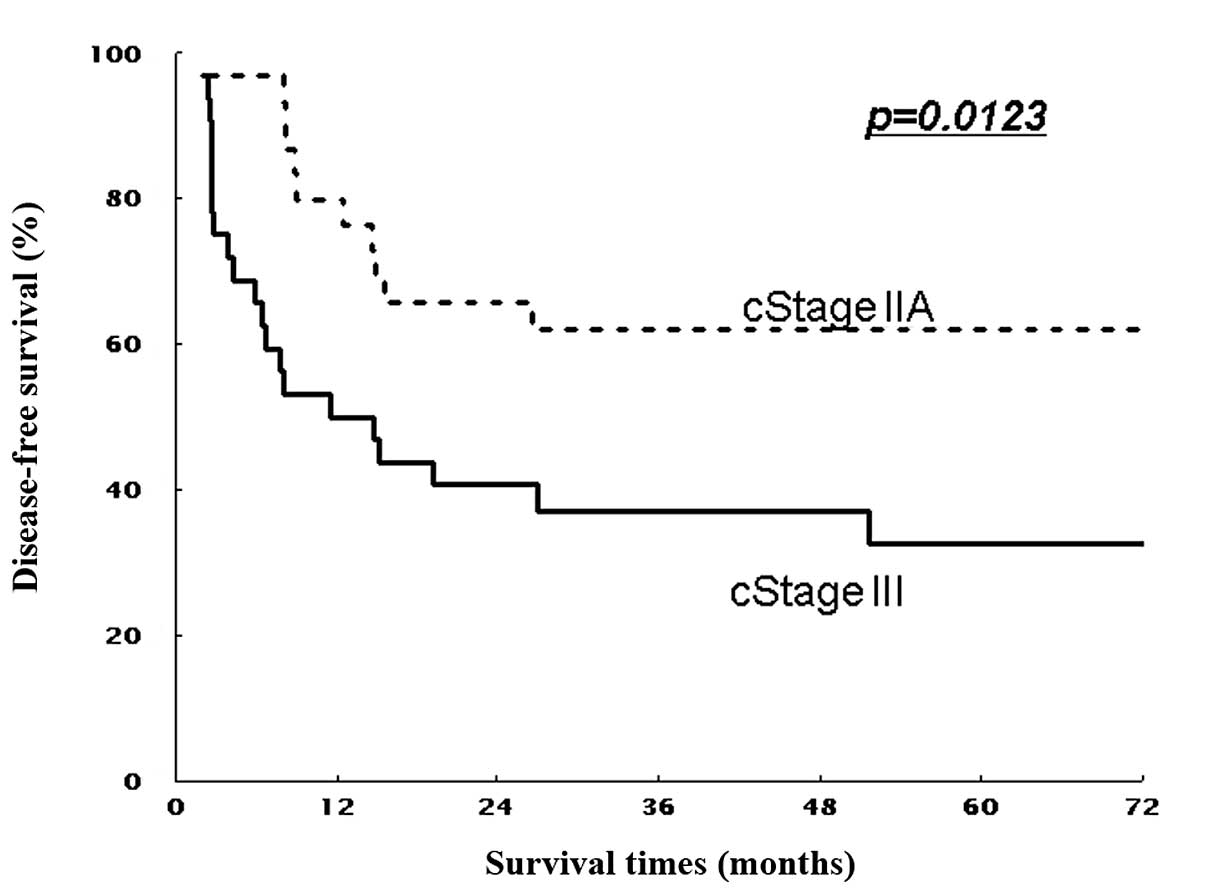
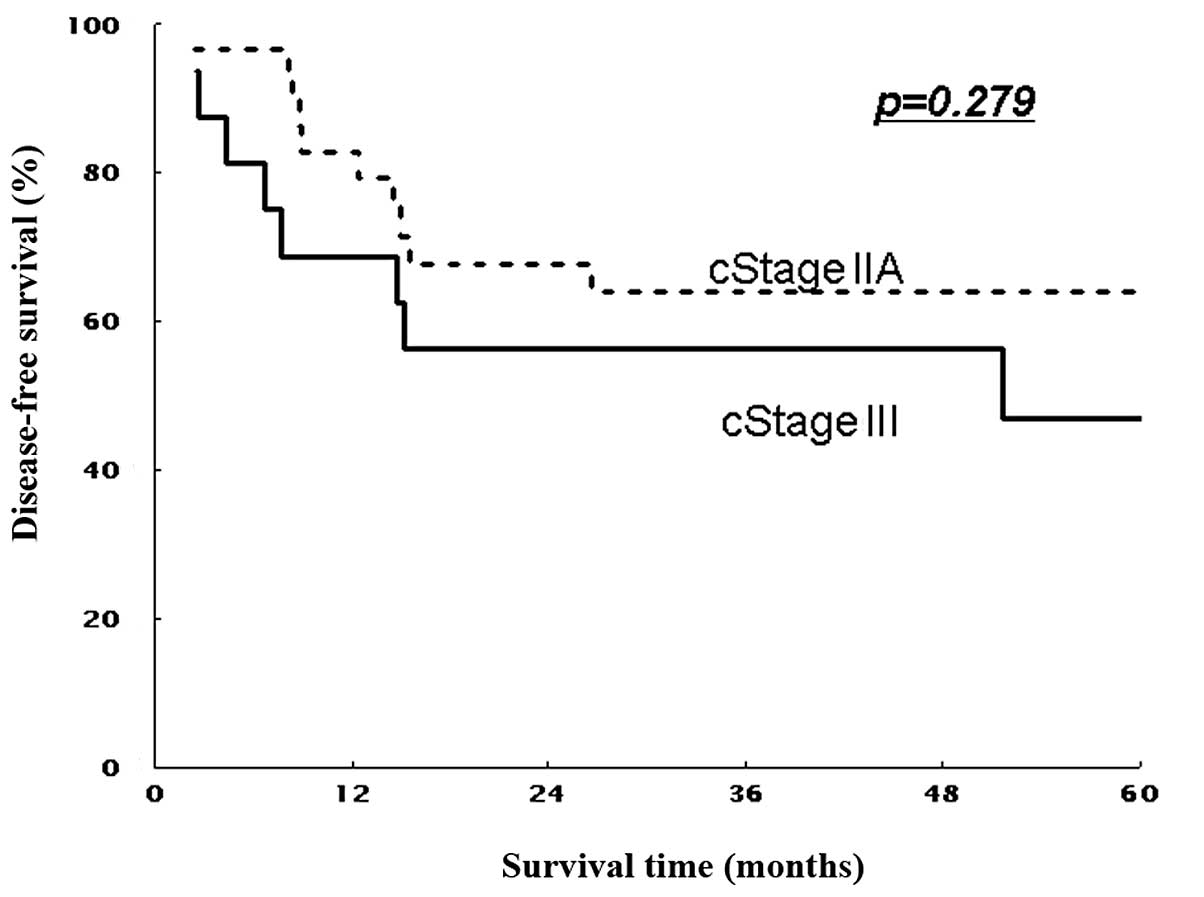
|
1
|
Goseki N, Koike M and Yoshida M:
Histopathologic characteristics of early stage esophageal
carcinoma. A comparative study with gastric carcinoma. Cancer.
69:1088–1093. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roth JA and Putnam JB Jr: Surgery for
cancer of the esophagus. Semin Oncol. 21:453–461. 1994.PubMed/NCBI
|
|
3
|
Sugimachi K, Inokuchi K, Kuwano H, Kai H,
Okamura T and Okudaira Y: Patterns of recurrence after curative
resection for carcinoma of the thoracic part of the esophagus. Surg
Gynecol Obstet. 157:537–540. 1983.PubMed/NCBI
|
|
4
|
Salazar JD, Doty JR, Lin JW, et al: Does
cell type influence post-esophagectomy survival in patients with
esophageal cancer? Dis Esophagus. 11:168–171. 1998.PubMed/NCBI
|
|
5
|
Ando N, Ozawa S, Kitagawa Y, Shinozawa Y
and Kitajima M: Improvement in the results of surgical treatment of
advanced squamous esophageal carcinoma during 15 consecutive years.
Ann Surg. 232:225–232. 2000.PubMed/NCBI
|
|
6
|
Earlam R and Cunha-Melo JR: Oesophogeal
squamous cell carcinoms: II. A critical view of radiotherapy. Br J
Surg. 67:457–461. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Isono K, Sato H and Nakayama K: Results of
a nationwide study on the three-field lymph node dissection of
esophageal cancer. Oncology. 48:411–420. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akiyama H, Tsurumaru M, Udagawa H and
Kajiyama Y: Radical lymph node dissection for cancer of the
thoracic esophagus. Ann Surg. 220:364–372. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ozawa S, Baba H, Tachimori Y, et al:
Comprehensive registry of esophageal cancer in Japan, 2003.
Esophagus. 8:9–29. 2011. View Article : Google Scholar
|
|
10
|
International Union Against Cancer (UICC).
TNM Classification of malignant Tumours. Sobin LH, Gospodarowicz MK
and Wittekind C: 5th edition. John Wiley & Sons, Inc; New York:
1997
|
|
11
|
Kelsen DP, Ginsberg R, Pajak TF, et al:
Chemotherapy followed by surgery compared with surgery alone for
localized esophageal cancer. N Engl J Med. 339:1979–1984. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cooper JS, Guo MD, Herskovic A, et al:
Chemoradiotherapy of locally advanced esophageal cancer: long-term
follow-up of a prospective randomized trial (RTOG 85-01). Radiation
Therapy Oncology Group. JAMA. 281:1623–1627. 1999. View Article : Google Scholar
|
|
13
|
Minsky BD, Pajak TF, Ginsberg RJ, et al:
INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial
of combined-modality therapy for esophageal cancer: high-dose
versus standard-dose radiation therapy. J Clin Oncol. 20:1167–1174.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Urba SG, Orringer MB, Perez-Tamayo C,
Bromberg J and Forastiere A: Concurrent preoperative chemotherapy
and radiation therapy in localized esophageal adenocarcinoma.
Cancer. 69:285–291. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Walsh TN, Noonan N, Hollywood D, Kelly A,
Keeling N and Hennessy TP: A comparison of multimodal therapy and
surgery for esophageal adenocarcinoma. N Engl J Med. 335:462–467.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nygaard K, Hagen S, Hansen HS, et al:
Pre-operative radiotherapy prolongs survival in operable esophageal
carcinoma: a randomized, multicenter study of pre-operative
radiotherapy and chemotherapy. The second Scandinavian trial in
esophageal cancer. World J Surg. 16:1104–1110. 1992. View Article : Google Scholar
|
|
17
|
Le Prise E, Etienne PL, Meunier B, et al:
A randomized study of chemotherapy, radiation therapy, and surgery
versus surgery for localized squamous cell carcinoma of the
esophagus. Cancer. 73:1779–1784. 1994.PubMed/NCBI
|
|
18
|
Apinop C, Puttisak P and Preecha N: A
prospective study of combined therapy in esophageal cancer.
Hepatogastroenterology. 41:391–393. 1994.PubMed/NCBI
|
|
19
|
Bosset JF, Gignoux M, Triboulet JP, et al:
Chemoradiotherapy followed by surgery compared with surgery alone
in squamous-cell cancer of the esophagus. N Engl J Med.
337:161–167. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Urba SG, Orringer MB, Turrisi A,
Iannettoni M, Forastiere A and Strawderman M: Randomized trial of
preoperative chemoradiation versus surgery alone in patients with
locoregional esophageal carcinoma. J Clin Oncol. 19:305–313.
2001.PubMed/NCBI
|
|
21
|
Lee JL, Park SI, Kim SB, et al: A single
institutional phase III trial of preoperative chemotherapy with
hyperfractionation radiotherapy plus surgery versus surgery alone
for resectable esophageal squamous cell carcinoma. Ann Oncol.
15:947–954. 2004. View Article : Google Scholar
|
|
22
|
Burmeister BH, Smithers BM, Gebski V, et
al: Surgery alone versus chemoradiotherapy followed by surgery for
resectable cancer of the oesophagus: a randomised controlled phase
III trial. Lancet Oncol. 6:659–668. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tepper J, Krasna MJ, Niedzwiecki D, et al:
Phase III trial of trimodality therapy with cisplatin,
fluorouracil, radiotherapy, and surgery compared with surgery alone
for esophageal cancer: CALGB 9781. J Clin Oncol. 26:1086–1092.
2008. View Article : Google Scholar
|
|
24
|
Fujiwara Y, Kamikonya N, Inoue T, et al:
Chemoradiotherapy for T3 and T4 squamous cell carcinoma of the
esophagus using low-dose FP and radiation: a preliminary report.
Oncol Rep. 14:1177–1182. 2005.PubMed/NCBI
|
|
25
|
Japanese Society for Esophageal Disease.
Guidelines for Clinical and Pathologic Studies on Carcinoma of the
Esophagus. 9th edition. Kanehara & Co., Ltd; Tokyo: 2001
|
|
26
|
National Cancer Institute. Cancer Therapy
Evaluation Program, Common Toxicity Criteria Version 3.0.
http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
August 9–2006
|
|
27
|
Courrech Staal EF, Aleman BM, Boot H, van
Velthuysen ML, van Tinteren H and van Sandick JW: Systematic review
of the benefits and risks of neoadjuvant chemoradiation for
oesophageal cancer. Br J Surg. 97:1482–1496. 2010.PubMed/NCBI
|
|
28
|
Keller SM, Ryan LM, Coia LR, et al: High
dose chemoradiotherapy followed by esophagectomy for adenocarcinoma
of the esophagus and gastroesophageal junction: results of a phase
II study of the Eastern Cooperative Oncology Group. Cancer.
83:1908–1916. 1998. View Article : Google Scholar
|
|
29
|
De Vita F, Di Martino N, Orditura M, et
al: Preoperative chemoradiotherapy for squamous cell carcinoma and
adenocarcinoma of the esophagus: a phase II study. Chest.
122:1302–1308. 2002.PubMed/NCBI
|
|
30
|
Hsu FM, Lee JM, Huang PM, et al:
Retrospective analysis of outcome differences in preoperative
concurrent chemoradiation with or without elective nodal
irradiation for esophageal squamous cell carcinoma. Int J Radiat
Oncol Biol Phys. 81:e593–e599. 2011. View Article : Google Scholar
|
|
31
|
Morota M, Gomi K, Kozuka T, et al: Late
toxicity after definitive concurrent chemoradiotherapy for thoracic
esophageal carcinoma. Int J Radiat Oncol Biol Phys. 75:122–128.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Onozawa M, Nihei K, Ishikura S, et al:
Elective nodal irradiation (ENI) in definitive chemoradiotherapy
(CRT) for squamous cell carcinoma of the thoracic esophagus.
Radiother Oncol. 92:266–269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao KL, Ma JB, Liu G, Wu KL, Shi XH and
Jiang G: Three-dimensional conformal radiation therapy for
esophageal squamous cell carcinoma: is elective nodal irradiation
necessary? Int J Radiat Oncol Biol Phys. 76:446–451. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Button MR, Morgan CA, Croydon ES, Roberts
SA and Crosby TD: Study to determine adequate margins in
radiotherapy planning for esophageal carcinoma by detailing
patterns of recurrence after definitive chemoradiotherapy. Int J
Radiat Oncol Biol Phys. 73:818–823. 2009. View Article : Google Scholar
|
|
35
|
Kranzfelder M, Schuster T, Geinitz H,
Friess H and Buchler P: Meta-analysis of neoadjuvant treatment
modalities and definitive non-surgical therapy for oesophageal
squamous cell cancer. Br J Surg. 98:768–783. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ando N, Kato H, Igaki H, et al: A
randomized trial comparing postoperative adjuvant chemotherapy with
cisplatin and 5-fluorouracil versus preoperative chemotherapy for
localized advanced squamous cell carcinoma of the thoracic
esophagus (JCOG9907). Ann Surg Oncol. 19:68–74. 2011. View Article : Google Scholar
|
|
37
|
Ando N, Iizuka T, Ide H, et al: Surgery
plus chemotherapy compared with surgery alone for localized
squamous cell carcinoma of the thoracic esophagus: a Japan Clinical
Oncology Group Study - JCOG9204. J Clin Oncol. 21:4592–4596. 2003.
View Article : Google Scholar : PubMed/NCBI
|