Introduction
Esophageal cancer is one of the most aggressive
malignancies and is associated with a poor prognosis because of
early metastasis to lymph nodes as well as distant organs (1–3).
Esophageal squamous cell carcinomas (ESCCs) are far more common in
Asian countries including Japan, whilst adenocarcinomas of the
lower third of the esophagus are often seen in Western countries.
In 2005, 11,182 Japanese died from esophageal cancer according to
the Japanese Ministry of Health, Labour and Welfare. Surgery has
been considered the treatment of choice for patients with
locoregionally confined esophageal carcinoma. However, the 5-year
survival rate is less than 25% worldwide (4–6).
In Japan, the survival rate has been improving
during the past two decades since three field lymphadenectomy was
advocated by Isono et al(7)
and Akiyama et al(8) and it
is now widely performed. According to the comprehensive registry of
esophageal cancer in Japan (3rd edition) (9), the current survival rates of clinical
stage IIA, IIB and III patients categorized by UICC (10) are reportedly 47.5, 45.1 and 33.3%,
respectively. These results were rather disappointing in spite of
vigorous lymphadenectomy. The bottom-line in esophageal cancer
treatment is locoregional control, and the locoregional failure
rate after esophagectomy has been reported to be approximately 30%
for patients who received R0 resection (11). Likewise, the locoregional recurrence
rate (which included persistent disease and locoregional
recurrence) is 50–55% after definitive chemoradiotherapy (CRT)
without surgery (12,13).
To resolve these locoregional failures,
multimodality therapy involving the combination of surgery and CRT
has been developed. The most common approach is preoperative CRT
followed by esophagectomy, called trimodality therapy (14,15).
This approach offers the potential advantage of tumor downstaging,
less dissemination of malignant cells during surgery and prevention
of micrometastasis. Nine randomized trials have been performed in
patients with locoregionally confirmed esophageal cancer who
received preoperative CRT compared with surgery alone (15–23).
Two of these 9 studies showed an improved outcome despite a small
number of patients (15), while the
other studies showed no survival benefits in the trimodality
therapy group. Therefore, the benefits of preoperative CRT are
still controversial.
There is no randomized study ongoing or being
planned related to preoperative CRT of ESCC in Japan because of
technical difficulties both in surgery and radiotherapy. Since
1996, we have introduced preoperative CRT using 5-fluorouracil
(5-FU) and cisplatin (CDDP) combined with radical surgery for the
treatment of advanced esophageal cancers, and have reported
increased resectability, a reduced incidence of both local
recurrence and distant metastasis, and a more favorable prognosis
for CRT responders (24). In the
present study, we re-evaluated the feasibility and efficacy of
preoperative CRT and investigated whether a survival benefit was
obtained for stage II/III ESCC patients receiving neoadjuvant
CRT.
Patients and methods
Patients
We performed a retrospective review of 80
consecutive patients with esophageal cancer who received
esophagectomy after neoadjuvant CRT between August 1997 and October
2007 at the Department of Surgery, Hyogo College of Medicine,
Japan. Sixty-two of the 80 patients had clinical stage II or III
disease based on the UICC TNM Classification of Malignant Tumors
(5th edition) (10), as determined
by CT scan and/or endoscopic ultrasound examination findings, and
underwent concurrent CRT followed by esophagectomy.
The eligibility criteria of this study were as
follows: <80 years old, adequate organ function (WBC≥3500, Hb≥10
g/dl, ALT/AST≤2× upper limit of normal, platelets ≥100,000, serum
creatinine≤1.3), and a performance status (Eastern Cooperative
Oncology Group) of <2 at the time of admission (Table I).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Stage II (n=30) | Stage III (n=32) |
|---|
| Age, mean | 60.33 | 61.32 |
| Male/Female | 26/4 | 24/8 |
| Location of primary
tumor |
| Cervical | | 2 |
| Upper thoracic | 3 | 4 |
| Middle thoracic | 22 | 17 |
| Lower thoracic | 5 | 7 |
| Abdominal | | 2 |
| T-classification |
| T3 | 30 | 15 |
| T4 | | 17 |
| N-classification |
| N0 | 27 | 16 |
| N1 | 3 | 16 |
Preoperative radiotherapy was performed for 5 days
per week (Monday to Friday, 2 Gy/day) using a linear accelerator
(Mevatron KD2; Siemens, Germany). The radiation field encompassed
the primary tumor volume (as defined by endoscopy, esophagography
and CT scan) with a 3-cm margin in each cephalad and caudal
direction and 4-cm horizontal margins. If the lymph nodes
metastasis was detected by a CT scan, the radiation field was
extended to include the primary tumor and metastatic lesions. The
patients received 20 fractions of 2 Gy for a total of 40 Gy of
radiation. Concurrent chemotherapy consisted of 5-FU (500
mg/m2/day) administration for a 120-h continuous
intravenous infusion starting on Day 1 and CDDP (15–20 mg/day) for
a 2-h intravenous infusion on Days 1–5, repeated after 3 weeks.
Two to three weeks after the completion of
radiotherapy, the effects of CRT on the primary tumor and
metastatic nodes were assessed using chest CT scanning, barium
esophagography, and/or upper gastrointestinal endoscopy. The
response to therapy was defined as confirmed by esophagography or
esophagoscopy and CT scans according to the criteria of the
Japanese Society of Esophageal Disease (9th edition) (25): i) complete response (CR), 100%
regression of cancer; ii) partial response (PR), >50% regression
of the primary tumor and metastatic nodes; iii) progressive disease
(PD), defined as increase of 25% in the size of the primary tumor
or metastatic nodes or the appearance of new lesions; and iv) no
change (NC) defined as a decrease of <50% in the size of the
primary tumor and metastatic nodes and no evidence of tumor
progression. Toxicities were classified according to NCI CTC
Guidelines, version 3 (26).
Esophagectomy was planned for 4–7 weeks after the
completion of CRT. Most patients underwent thoracotomy, laparotomy,
and cervicotomy to perform esophagectomy with 2- or 3-fields
lymphadenectomy, and gastroesophageal anastomosis at the left side
of the neck. Radical resection (R0) was defined as the removal of
all macroscopic tumors, no evidence of distant metastasis, the
absence of a microscopic residual tumor, free resection margins,
and lymphadenectomy extending beyond the involved nodes. Resection
was defined as non-radical when a microscopic (R1) or macroscopic
(R2) residual tumor was found according to the TNM criteria
(10). Informed consents were
obtained in all patients.
Statistical analysis
Overall survival (OS) was defined as the time from
the data of initial treatment to patient death or the data of the
last available information on the vital status. Disease-free
survival (DFS) was defined as the length of time after treatment
during which no cancer was found. Differences between the
cumulative survival rates of the patient groups were calculated by
the log-rank test for comparison using Kaplan-Meier survival
curves. Statistical significance was considered at values of
P<0.05. Univariate analyses were used to examine the patients’
characteristics and other prognostic factors. Multivariate analyses
were employed for the identification of prognostic factors with the
Cox proportional hazard model. Statistical analyses were carried
out using the Statistica software, version 06J (StatSoft, Tulsa,
OK, USA), and SPSS version 16 (SPSS, Tokyo, Japan).
Results
Patient characteristics
The patient characteristics of this study are
summarized in Table I. All tumors
were histologically confirmed to be ESCC. The gender was biased
toward males (male/female, 50:12). The mean age was 60.83 years
old. Fifty-eight of the 62 patients had tumors in the thorax.
Seventeen stage III patients had tumors infiltrating through the
esophageal wall to adjacent structures (T4, 53.1% of stage III
patients). Nineteen patients (30.65%) had lymph nodes metastasis on
a CT scan at the time of diagnosis.
Response and toxicities
The clinical response to CRT is summarized in
Table II. The clinical response
(CR+PR) rates of CRT for the primary tumor and metastatic nodes
were 83.9 and 70%, respectively. The clinical response of both the
primary tumor and metastatic nodes was 82.3%. Major toxicities of
treatment are summarized and laboratory findings were obtained from
59 patients. Leukocytopenia and thrombocytopenia of grade 3 or
higher were noted in 33.9 and 5.1% of the patients, respectively.
Liver dysfunction of grades 1 or 2 was noted in 11.8%. Fatigue,
stomatitis and nausea of grade 1 or 2 were noted in 36, 10 and 26%
of the cases, respectively. Other toxicities were found in 50
patients. CRT-related death was not reported.
 | Table IIEffects of CRT for primary tumor and
metastatic nodes. |
Table II
Effects of CRT for primary tumor and
metastatic nodes.
| Response | Primary tumor | Metastatic
nodes | Clinical response
rate (Primary tumor and metastatic nodes) |
|---|
| CR, n | 14 | 4 | 14 |
| PR, n | 38 | 10 | 37 |
| NC, n | 9 | 4 | 9 |
| PD, n | 1 | 2 | 2 |
| Response rate
(%) | 83.9 | 70 | 82.3 |
Surgery and postoperative
complications
All patients underwent esophagectomy after the
completion of CRT. Radical R0 resection was achieved in 45 patients
(72.6%), R1 resection with a microscopic residual tumor was
achieved in 8 (12.9%), and R2 resection with a macroscopic residual
tumor in 9 (14.5%). The reasons for the failure of radical
resection leading to R2 resection were a residual primary tumor in
4 patients, metastatic nodes in 3, and the occurrence of new
distant metastasis during neoadjuvant CRT in 2. Postoperative
complications are shown in Table
III. One patient died from occlusion of the superior mesenteric
artery (SMA) within 4 weeks of surgery, corresponding to 1.6% of
the operative mortality. Three patients died of respiratory failure
including 2 metastatic lung cancers within 3 months after the
operation corresponding to 6.5% of hospital mortality.
 | Table IIIPostoperative complications after
esophagectomy for patients with stage II, III esophageal
cancer. |
Table III
Postoperative complications after
esophagectomy for patients with stage II, III esophageal
cancer.
| Complications | n (%) |
|---|
| Anastomotic
leakage | 6 (9.7) |
| Recurrent nerve
palsy | 4 (6.5) |
| Respiratory
failure | 4 (6.5) |
| Pleural
effusion | 2 (3.2) |
| Sepsis | 1 (1.6) |
| Arrhythmia | 1 (1.6) |
| Myocardial
infarction | 1 (1.6) |
| SMA occlusion | 1 (1.6) |
Pathological response of the primary
tumor
Fifteen of the 62 patients (24.2%) had no residual
tumor in the resected esophagus, representing pathological CR.
Survival
The mean follow-up period was 46 months (3–169
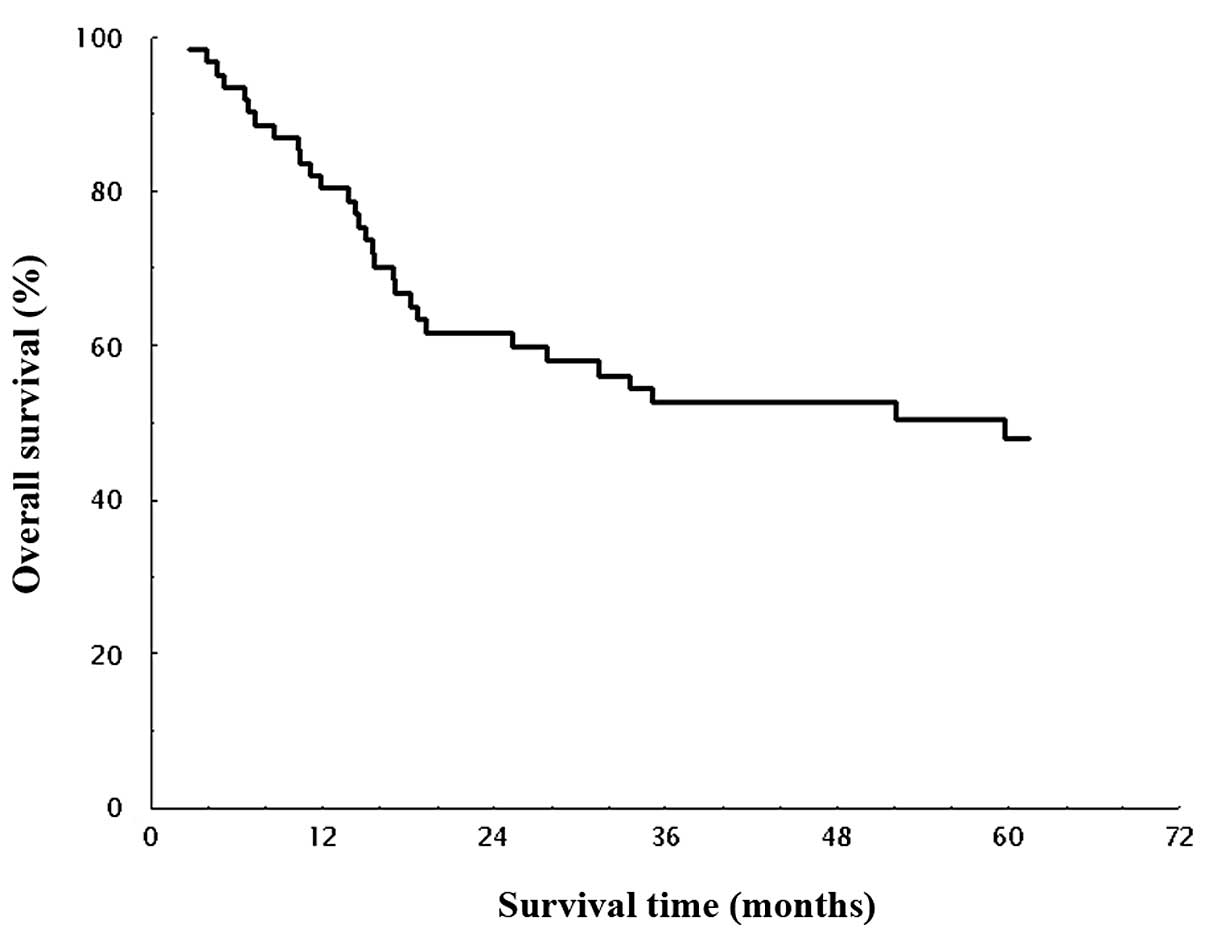
months). OS in all patients is shown in Fig. 1. The median survival time (MST) for
OS was 53.3 months, and the estimated 1-, 2-, 3- and 5-year
survival rates were 80.4, 61.6, 52.6, and 48.0%, respectively. DFS
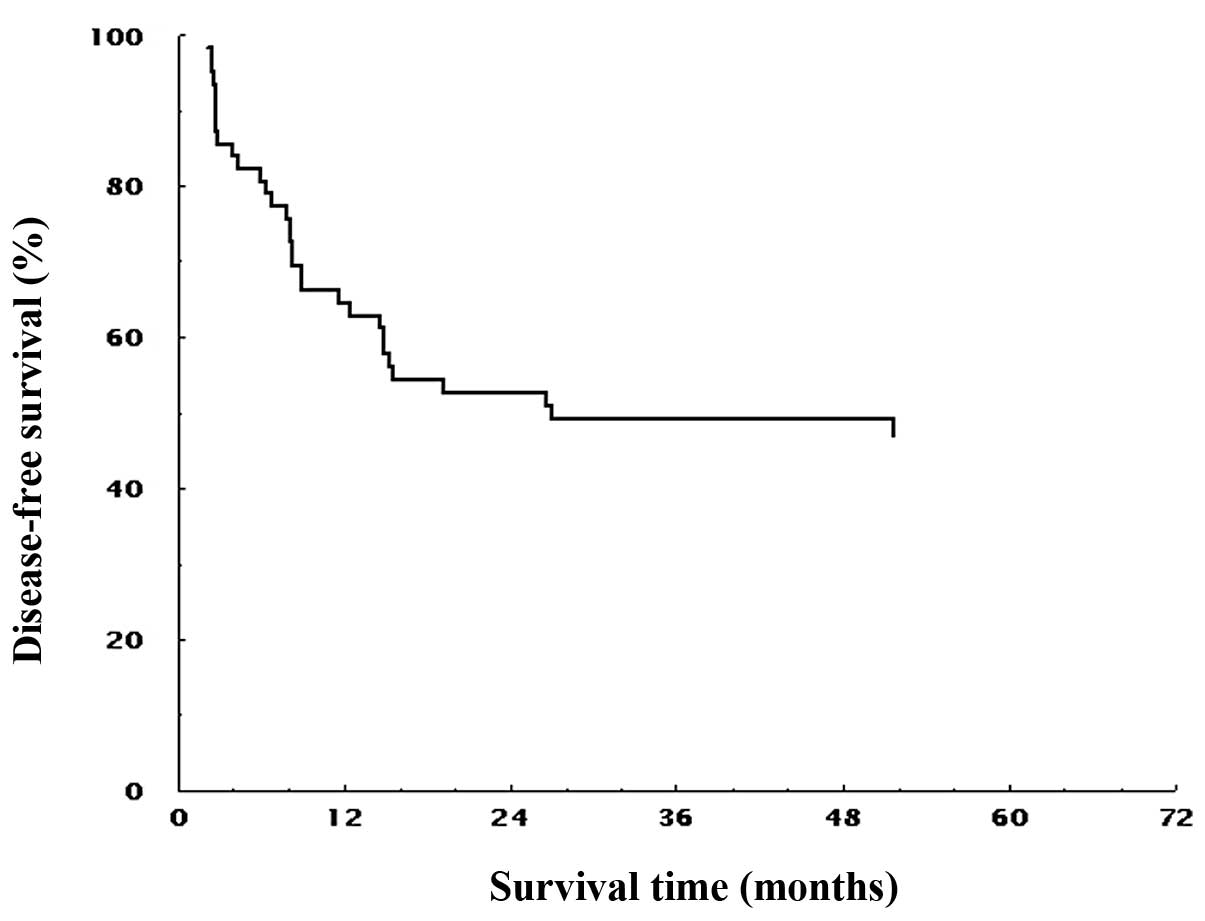
in all patients is shown in Fig. 2.
The MST for DFS was 23.8 months, and the estimated 1-, 2-, 3- and
5-year survival rates were 64.5, 52.7, 49.2 and 47.1%,
respectively.
Comparison of survival between T3 and T4 patients
was additionally performed. The estimated 5-year OS rates were
63.3% for T3 patients and 28.3% for T4 patients. Similarly, the
5-year DFS rates were 61.1% for T3 patients and 26.1% for T4
patients. The clinical T3 patients showed significantly longer OS
and DFS compared with clinical T4 (P=0.006 in OS and P=0.002 in
DFS, respectively). Furthermore, the survival rates between
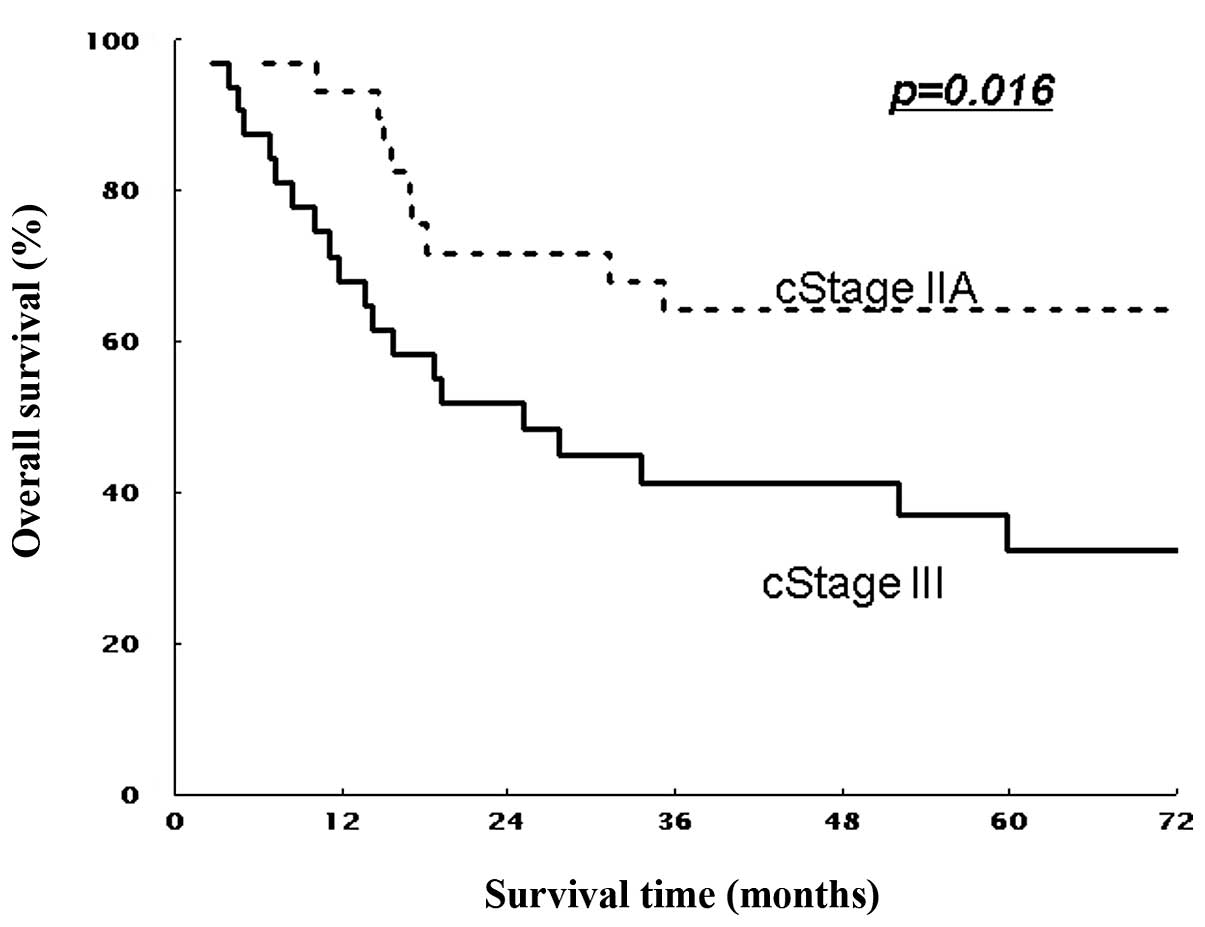
different stages were compared according to the UICC
Classification. The estimated 5-year OS rates were 64.2% for stage
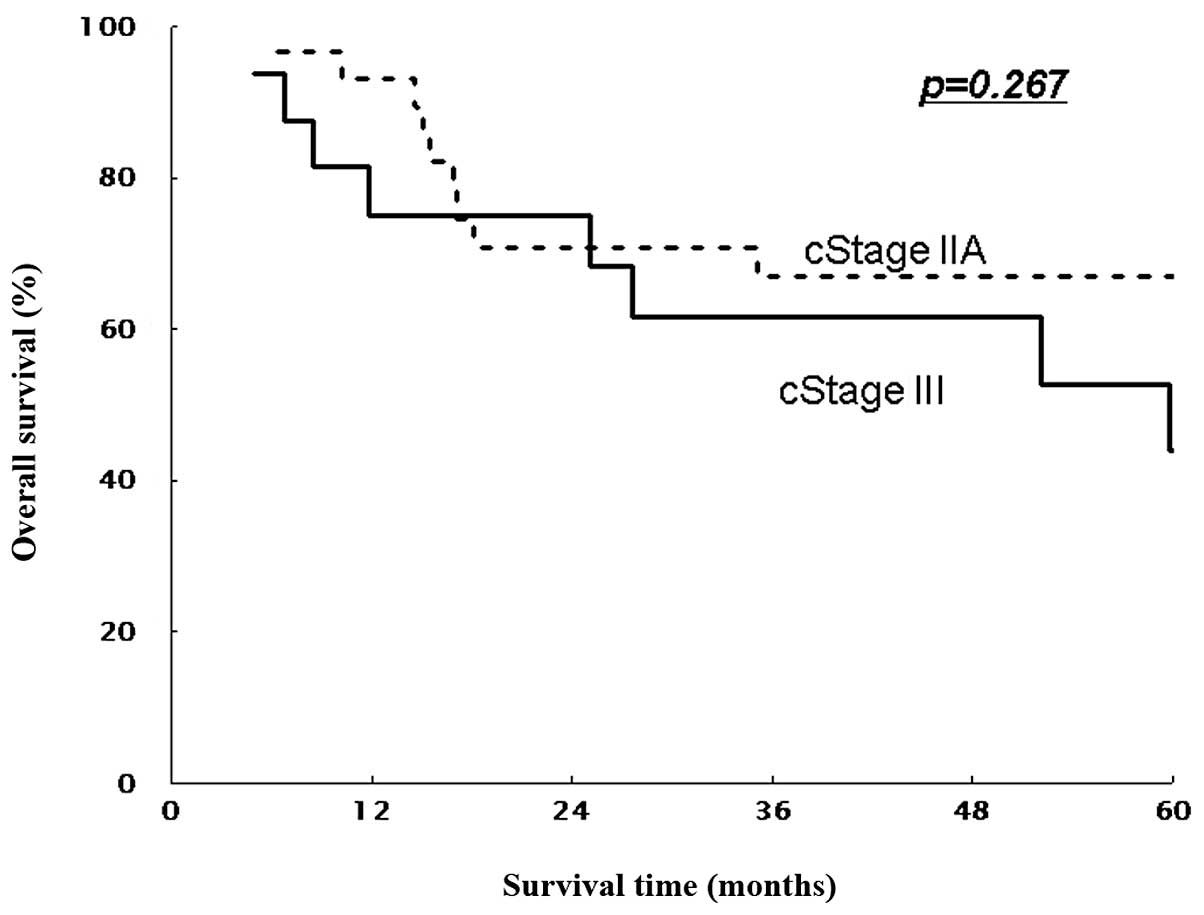
II and 33.1% for stage III (all T), and 46.9% for stage III
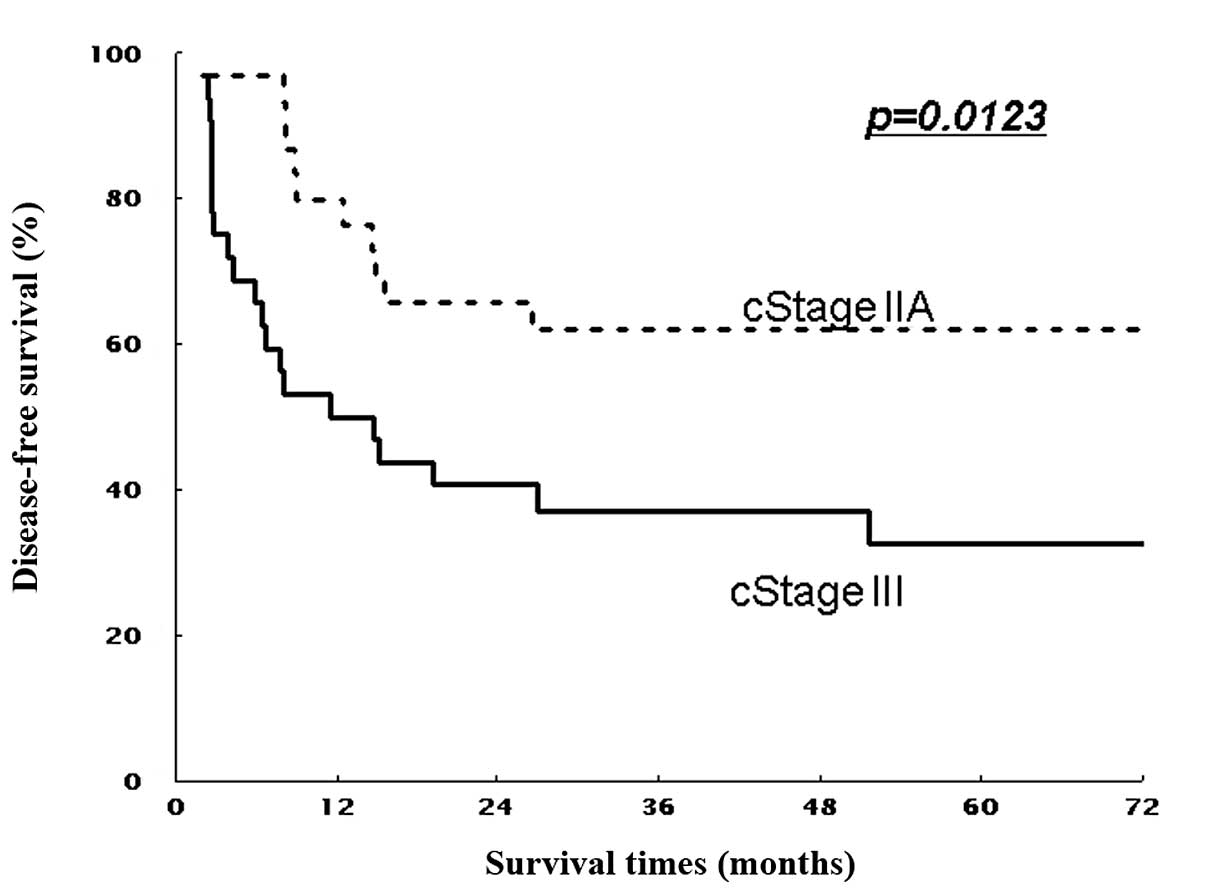
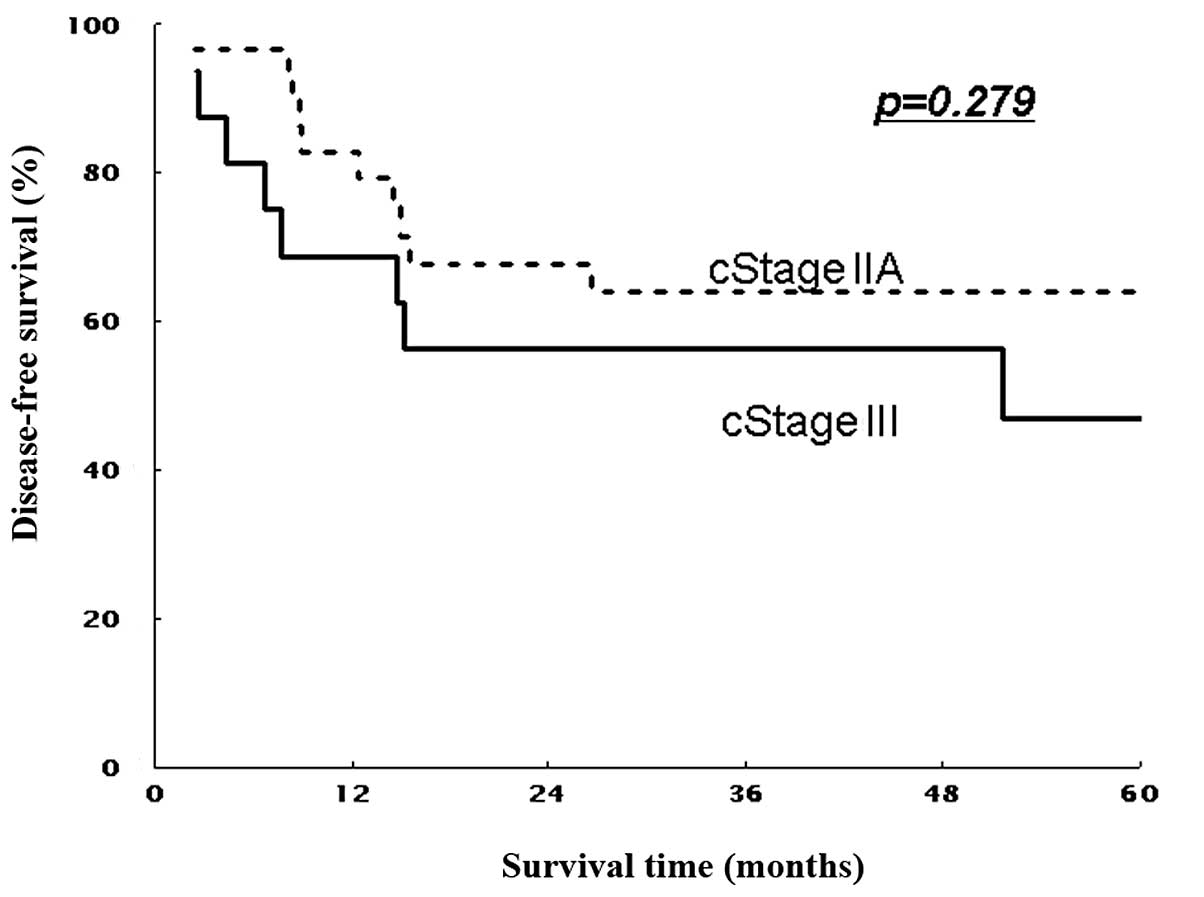
(non-T4) patients (P=0.016 and P=0.267, Figs. 3 and 5). Similarly, 5-year DFS rates were 61.9%
for stage II, 32.3% for stage III (all T), and 43.8% for stage III
(non-T4) patients (P=0.011 and P=0.297; Figs. 4 and 6). Patients with stage II showed
significantly longer OS and DFS than those with stage III. In
subgroup analysis for stage III patients, the estimated 5-year OS
and DFS were 46.9 and 43.9% for T3, and 20.3 and 18.8% for T4,
respectively (P=0.045 and P=0.035, Figs. 5 and 6).
Univariate analysis for overall survival in stage
II/III esophageal cancer patients is shown in Table IV. Lymph node metastasis, depth of
tumor invasion and resectability showed significant differences in
the prognostic value (P<0.01). Furthermore, the patients who
were CRT responders showed significantly longer OS compared to
those who were not (P<0.001). Using multivariate analysis,
resectability and the effect of CRT were independent prognostic
factors for OS (Table V).
 | Table IVUnivariate analysis for OS. |
Table IV
Univariate analysis for OS.
|
Characteristics | No. of
patients | Hazard ratio | OS P-value | 95% CI |
|---|
| Age (years) |
| <70 | 48 | 1.367 | 0.53 | 0.514–3.65 |
| ≥70 | 14 | | | |
| Gender |
| Male | 51 | 0.841 | 0.489 | 0.515–1.373 |
| Female | 11 | | | |
| Effect of CRT |
| Effective | 51 | 0.29 | 0.00051b | 0.091–0.476 |
| Not effective | 11 | | | |
| Lymph nodes
metastasis |
| Positive | 19 | 2.855 | 0.00075b | 1.3–6.27 |
| Negative | 43 | | | |
| Depth of tumor
invasion |
| T3 | 45 | 3.463 | 0.0078b | 1.55–7.719 |
| T4 | 17 | | | |
| Tumor
locationa |
| Upper | 9 | 0.767 | 0.628 | 0.262–2.241 |
| Lower | 53 | | | |
| Counts of lymph
nodes metastasis |
| >4 | 6 | 23.77 | 0.00001b | 6.93–81.57 |
| <3 | 56 | | | |
| Resectability |
| R0 | 45 | 10.23 | 0.00001b | 4.34–24.1 |
| R1, R2 | 17 | | | |
| Pathological
complete response |
| Yes | 15 | 25.17 | 0.005b | 6.83–43.5 |
| No | 47 | | | |
 | Table VMultivariate analysis of factors
associated with OS of ESCC. |
Table V
Multivariate analysis of factors
associated with OS of ESCC.
| P-value | HR | 95% CI |
|---|
| Age | 0.28 | 1.633 | 0.671–3.972 |
| Lymph nodes
metastasis | 0.659 | 0.819 | 0.337–1.990 |
| Depth of tumor
invasion | 0.126 | 2.155 | 0.805–5.770 |
| Clinical stage | 0.752 | 1.176 | 0.432–3.199 |
| Resectability | 0.001a | 5.072 | 2.059–12.497 |
| Effect of CRT | 0.01a | 0.279 | 0.106–0.733 |
Discussion
We have previously reported that preoperative CRT
contributes to improve the resectability in patients with ESCC, and
that surgical esophagectomy remains the standard therapy for CRT
responders (24). In this study, we
focused on the characteristics of the UICC stage II/III ESCC, and
analyzed whether this trimodality therapy combined with neoadjuvant
CRT and esophagectomy improved the outcome of the patients.
This retrospective study showed that the 5-year OS
rates of cstage II/III esophageal cancer patients were 64.2 and
33.1%, respectively. On the other hand, those of UICC clinical
stage II/III patients after esophagectomy were reported to be from
47.5% (stage IIA) to 33.3% (stage III) by the Comprehensive
Registry of Esophageal Cancer in Japan (9). These data suggest that the addition of
neoadjuvant CRT is beneficial regarding the outcome of stage II
patients. We failed to show the survival benefit of neoadjuvant CRT
in stage III patients. However, this is thought to be due to the
biased demographics in our study; more advanced T4 patients
comprised approximately 50% in stage III patients. Actually,
subgroup analysis showed that trimodality therapy improved the
outcome of T3 more than T4 patients in stage III.
There have been nine randomized trials of
preoperative CRT following surgery vs. surgery alone (15–23).
Of the nine randomized trials, three studies showed survival
benefits in preoperative CRT group compared to those receiving
surgery alone (15,22,23).
However, patient numbers in two randomized trials were too small to
be evaluated objectively (15,23).
Notably, a large-scale study by Burmeister et al revealed
that 5-FU/CDDP (FP) plus radiation (35 Gy) followed by
esophagectomy for ESCC improves DFS, but not for all patients
including those with adenocarcinoma (22). This report has encouraged us to
continue trimodality therapy for ESCC in Japan. In any case, it is
difficult to evaluate these randomized studies unitarily, because
all these randomized phase III reports have flaws due to their wide
variation in CRT protocols, short follow-up duration, different
histological types, different stages, and different operative
procedures. Moreover, we are urged to standardize the regimen of
chemotherapeutic agents and radiation dose. Courrech Staal et
al systematically reviewed the benefits and risks of
neoadjuvant CRT for esophageal cancer, and reported that FP was the
widely used mainstay in CRT regimens all over the world (27). Therefore, it sounds reasonable that
the standard chemotherapeutic regimen needs to be established based
on FP regimen in Asia as well as in Western countries. The standard
regimen of definitive CRT advocated by Intergroup INT0123
(RTOG9405) consists of 2 cycles of 5-FU (1,000 mg/m2/24
h for 4 days) and CDDP (100 mg/m2/bolus on Day 1) with
50.4 Gy irradiation (13). In
Japan, the regimen of neoadjuvant CRT should also be determined on
the basis of the INT123 study, and the concurrent radiation dose
should be discussed considering the safety of surgery. In this
study, CRT consists of 5-FU (500 mg/m2/24 h for 5 days)
and CDDP (15–20 mg/bolus for 5 days) with 40 Gy irradiation as a
result of discussion with radiologists. The chemotherapeutic and
radiation doses in our regimen were lower than those in the INT0123
study, but our setting dose was sufficient to show the efficacy and
safety with tolerability. Hospital mortality after esophagectomy
following CRT was reported to be 5.2% in Courrech Staal’s review,
which was compatible with that in our study (27).
The clinical response rates were assessed in this
study. Those of the primary tumor ranged from 59 to 87% in previous
preoperative randomized or non-randomized studies (17,18,21,28,29).
Meanwhile, our study showed that the clinical response rate using
the Japanese Guidelines for Esophageal Disease was 83.9% for the
primary tumor and 70% for metastatic nodes.
Regarding the radiation field, the optimal radiation
field design remains controversial (30–34).
Hsu et al(30) compared the
patients with AJCC stage II/III ESCC undergoing preoperative CRT
(median, 36 Gy) followed by radical esophagectomy with or without
elective nodal irradiation (ENI). As a result, ENI reduced the M1a
failure rate, but was not associated with improved outcomes in the
patients undergoing preoperative CRT. Zhao et al(33) also evaluated 3D-CRT (irradiating
only the primary tumor and positive lymph nodes) for ESCC, and
concluded that the omission of elective nodal irradiation was not
associated with a significant failure in lymph node regions not
included in the planned target volume. In our study, we planned a
radiation field including both the primary tumor and metastatic
lymph nodes which were identified by an enhanced CT scan. Namely,
we planned the irradiation field minimally to prevent operative and
postoperative complications. Consequently, CRT minimized
postoperative complications as we expected and improved the
prognosis beyond our expectations, especially with the marked
clinical response for metastatic nodes. In China, Zhao et al
also used the same radiation field setting (33). In this way, the minimum setting for
the primary tumor and metastatic nodes may be promising to achieve
fewer complications and more prognostic benefits.
A recent meta-analysis revealed that a significant
survival benefit for neoadjuvant CRT was evident for patients with
resectable esophageal cancer with no increase in the morbidity rate
[hazard ratio (HR), 0.81], and that definitive CRT did not
demonstrate any survival benefit over other curative strategies
(35). Intriguingly, neoadjuvant
chemotherapy (without radiation) did not show any survival benefit
(HR, 0.93). In Japan, preoperative chemotherapy with FP has been
regarded as the standard treatment for patients with stage II/III
(non-T4) ESCC by the JCOG 9204 and 9907 trials (36,37).
However, some critical problems were pointed out in these
prospective randomized studies. First, there was a significant
difference in subject numbers between pre- and postoperative
chemotherapy groups (P=0.04) Secondly, patients with the pN0 status
did not undergo postoperative chemotherapy in reality. Therefore,
future clinical trials should resolve these above-mentioned
problems. The 5-year OS in stage II/III (T3) patients in our study
was higher than that in the JCOG study (63.3 vs. 55%,
respectively). We strongly propose that preoperative CRT be
included in the next JCOG study to evaluate the efficacy of CRT
more objectively in Japan.
In conclusion, preoperative CRT for cstage II/III
(non-T4) ESCC patients contributed to high response rates for both
the primary tumor and metastatic nodes and showed satisfactory
outcome with tolerable morbidity and mortality. A phase II study is
needed to better clarify the standard neoadjuvant CRT regimen
through a large prospective randomized trial.
References
|
1
|
Goseki N, Koike M and Yoshida M:
Histopathologic characteristics of early stage esophageal
carcinoma. A comparative study with gastric carcinoma. Cancer.
69:1088–1093. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roth JA and Putnam JB Jr: Surgery for
cancer of the esophagus. Semin Oncol. 21:453–461. 1994.PubMed/NCBI
|
|
3
|
Sugimachi K, Inokuchi K, Kuwano H, Kai H,
Okamura T and Okudaira Y: Patterns of recurrence after curative
resection for carcinoma of the thoracic part of the esophagus. Surg
Gynecol Obstet. 157:537–540. 1983.PubMed/NCBI
|
|
4
|
Salazar JD, Doty JR, Lin JW, et al: Does
cell type influence post-esophagectomy survival in patients with
esophageal cancer? Dis Esophagus. 11:168–171. 1998.PubMed/NCBI
|
|
5
|
Ando N, Ozawa S, Kitagawa Y, Shinozawa Y
and Kitajima M: Improvement in the results of surgical treatment of
advanced squamous esophageal carcinoma during 15 consecutive years.
Ann Surg. 232:225–232. 2000.PubMed/NCBI
|
|
6
|
Earlam R and Cunha-Melo JR: Oesophogeal
squamous cell carcinoms: II. A critical view of radiotherapy. Br J
Surg. 67:457–461. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Isono K, Sato H and Nakayama K: Results of
a nationwide study on the three-field lymph node dissection of
esophageal cancer. Oncology. 48:411–420. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akiyama H, Tsurumaru M, Udagawa H and
Kajiyama Y: Radical lymph node dissection for cancer of the
thoracic esophagus. Ann Surg. 220:364–372. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ozawa S, Baba H, Tachimori Y, et al:
Comprehensive registry of esophageal cancer in Japan, 2003.
Esophagus. 8:9–29. 2011. View Article : Google Scholar
|
|
10
|
International Union Against Cancer (UICC).
TNM Classification of malignant Tumours. Sobin LH, Gospodarowicz MK
and Wittekind C: 5th edition. John Wiley & Sons, Inc; New York:
1997
|
|
11
|
Kelsen DP, Ginsberg R, Pajak TF, et al:
Chemotherapy followed by surgery compared with surgery alone for
localized esophageal cancer. N Engl J Med. 339:1979–1984. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cooper JS, Guo MD, Herskovic A, et al:
Chemoradiotherapy of locally advanced esophageal cancer: long-term
follow-up of a prospective randomized trial (RTOG 85-01). Radiation
Therapy Oncology Group. JAMA. 281:1623–1627. 1999. View Article : Google Scholar
|
|
13
|
Minsky BD, Pajak TF, Ginsberg RJ, et al:
INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial
of combined-modality therapy for esophageal cancer: high-dose
versus standard-dose radiation therapy. J Clin Oncol. 20:1167–1174.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Urba SG, Orringer MB, Perez-Tamayo C,
Bromberg J and Forastiere A: Concurrent preoperative chemotherapy
and radiation therapy in localized esophageal adenocarcinoma.
Cancer. 69:285–291. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Walsh TN, Noonan N, Hollywood D, Kelly A,
Keeling N and Hennessy TP: A comparison of multimodal therapy and
surgery for esophageal adenocarcinoma. N Engl J Med. 335:462–467.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nygaard K, Hagen S, Hansen HS, et al:
Pre-operative radiotherapy prolongs survival in operable esophageal
carcinoma: a randomized, multicenter study of pre-operative
radiotherapy and chemotherapy. The second Scandinavian trial in
esophageal cancer. World J Surg. 16:1104–1110. 1992. View Article : Google Scholar
|
|
17
|
Le Prise E, Etienne PL, Meunier B, et al:
A randomized study of chemotherapy, radiation therapy, and surgery
versus surgery for localized squamous cell carcinoma of the
esophagus. Cancer. 73:1779–1784. 1994.PubMed/NCBI
|
|
18
|
Apinop C, Puttisak P and Preecha N: A
prospective study of combined therapy in esophageal cancer.
Hepatogastroenterology. 41:391–393. 1994.PubMed/NCBI
|
|
19
|
Bosset JF, Gignoux M, Triboulet JP, et al:
Chemoradiotherapy followed by surgery compared with surgery alone
in squamous-cell cancer of the esophagus. N Engl J Med.
337:161–167. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Urba SG, Orringer MB, Turrisi A,
Iannettoni M, Forastiere A and Strawderman M: Randomized trial of
preoperative chemoradiation versus surgery alone in patients with
locoregional esophageal carcinoma. J Clin Oncol. 19:305–313.
2001.PubMed/NCBI
|
|
21
|
Lee JL, Park SI, Kim SB, et al: A single
institutional phase III trial of preoperative chemotherapy with
hyperfractionation radiotherapy plus surgery versus surgery alone
for resectable esophageal squamous cell carcinoma. Ann Oncol.
15:947–954. 2004. View Article : Google Scholar
|
|
22
|
Burmeister BH, Smithers BM, Gebski V, et
al: Surgery alone versus chemoradiotherapy followed by surgery for
resectable cancer of the oesophagus: a randomised controlled phase
III trial. Lancet Oncol. 6:659–668. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tepper J, Krasna MJ, Niedzwiecki D, et al:
Phase III trial of trimodality therapy with cisplatin,
fluorouracil, radiotherapy, and surgery compared with surgery alone
for esophageal cancer: CALGB 9781. J Clin Oncol. 26:1086–1092.
2008. View Article : Google Scholar
|
|
24
|
Fujiwara Y, Kamikonya N, Inoue T, et al:
Chemoradiotherapy for T3 and T4 squamous cell carcinoma of the
esophagus using low-dose FP and radiation: a preliminary report.
Oncol Rep. 14:1177–1182. 2005.PubMed/NCBI
|
|
25
|
Japanese Society for Esophageal Disease.
Guidelines for Clinical and Pathologic Studies on Carcinoma of the
Esophagus. 9th edition. Kanehara & Co., Ltd; Tokyo: 2001
|
|
26
|
National Cancer Institute. Cancer Therapy
Evaluation Program, Common Toxicity Criteria Version 3.0.
http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
August 9–2006
|
|
27
|
Courrech Staal EF, Aleman BM, Boot H, van
Velthuysen ML, van Tinteren H and van Sandick JW: Systematic review
of the benefits and risks of neoadjuvant chemoradiation for
oesophageal cancer. Br J Surg. 97:1482–1496. 2010.PubMed/NCBI
|
|
28
|
Keller SM, Ryan LM, Coia LR, et al: High
dose chemoradiotherapy followed by esophagectomy for adenocarcinoma
of the esophagus and gastroesophageal junction: results of a phase
II study of the Eastern Cooperative Oncology Group. Cancer.
83:1908–1916. 1998. View Article : Google Scholar
|
|
29
|
De Vita F, Di Martino N, Orditura M, et
al: Preoperative chemoradiotherapy for squamous cell carcinoma and
adenocarcinoma of the esophagus: a phase II study. Chest.
122:1302–1308. 2002.PubMed/NCBI
|
|
30
|
Hsu FM, Lee JM, Huang PM, et al:
Retrospective analysis of outcome differences in preoperative
concurrent chemoradiation with or without elective nodal
irradiation for esophageal squamous cell carcinoma. Int J Radiat
Oncol Biol Phys. 81:e593–e599. 2011. View Article : Google Scholar
|
|
31
|
Morota M, Gomi K, Kozuka T, et al: Late
toxicity after definitive concurrent chemoradiotherapy for thoracic
esophageal carcinoma. Int J Radiat Oncol Biol Phys. 75:122–128.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Onozawa M, Nihei K, Ishikura S, et al:
Elective nodal irradiation (ENI) in definitive chemoradiotherapy
(CRT) for squamous cell carcinoma of the thoracic esophagus.
Radiother Oncol. 92:266–269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao KL, Ma JB, Liu G, Wu KL, Shi XH and
Jiang G: Three-dimensional conformal radiation therapy for
esophageal squamous cell carcinoma: is elective nodal irradiation
necessary? Int J Radiat Oncol Biol Phys. 76:446–451. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Button MR, Morgan CA, Croydon ES, Roberts
SA and Crosby TD: Study to determine adequate margins in
radiotherapy planning for esophageal carcinoma by detailing
patterns of recurrence after definitive chemoradiotherapy. Int J
Radiat Oncol Biol Phys. 73:818–823. 2009. View Article : Google Scholar
|
|
35
|
Kranzfelder M, Schuster T, Geinitz H,
Friess H and Buchler P: Meta-analysis of neoadjuvant treatment
modalities and definitive non-surgical therapy for oesophageal
squamous cell cancer. Br J Surg. 98:768–783. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ando N, Kato H, Igaki H, et al: A
randomized trial comparing postoperative adjuvant chemotherapy with
cisplatin and 5-fluorouracil versus preoperative chemotherapy for
localized advanced squamous cell carcinoma of the thoracic
esophagus (JCOG9907). Ann Surg Oncol. 19:68–74. 2011. View Article : Google Scholar
|
|
37
|
Ando N, Iizuka T, Ide H, et al: Surgery
plus chemotherapy compared with surgery alone for localized
squamous cell carcinoma of the thoracic esophagus: a Japan Clinical
Oncology Group Study - JCOG9204. J Clin Oncol. 21:4592–4596. 2003.
View Article : Google Scholar : PubMed/NCBI
|