Introduction
Cancer is a major public health problem around the
world, and epidemiologic and animal studies have indicated that
vegetables and fruits with chemopreventive natural products, alone
or in a mixture, are associated with reducing the risk of
developing cancer (1–3). DNA polymerase [DNA-dependent DNA
polymerase (pol), E.C. 2.7.7.7]1 catalyzes
deoxyribonucleotide addition to the 3′-hydroxyl terminus of primed
double-stranded DNA molecules (4).
DNA replication, recombination and repair in eukaryotes are key
systems in which pols have important maintenance roles (5). Pol inhibitors can thus be employed as
anticancer chemotherapy agents because they in turn inhibit cell
proliferation and, based on this idea, we have been screening for
mammalian pol inhibitors from natural phytochemical products in
vegetables and fruits for over 15 years.
The human genome encodes at least 15 DNA pols that
conduct cellular DNA synthesis (6,7).
Eukaryotic cells contain 3 replicative pols (α, δ and ɛ), 1
mitochondrial pol (γ), and at least 11 non-replicative pols [β, ζ,
η, θ, ι, κ, λ, μ, ν, terminal deoxynucleotidyl transferase
(TdT) and REV1] (8,9). Pols have a highly conserved structure,
with their overall catalytic subunits showing little variance among
species; conserved enzyme structures are usually preserved because
they perform important cellular functions that confer evolutionary
advantages. On the basis of sequence homology, eukaryotic pols can
be divided into four main families, termed A, B, X and Y (9). Family A includes mitochondrial pol γ
as well as pols θ and ν. Family B includes the three replicative
pols α, δ and ɛ and also pol ζ. Family X comprises pols β, λ and
μ as well as TdT; and last, family Y includes pols η, ι and
κ in addition to REV1. Focusing on replicative pol inhibition
supposes a concurrent antitumor effect, because replicative pols,
such as B-family pols, are essential for cancer cell growth. As a
result of this screening, we found that glycoglycerolipids from a
fern and an alga potently inhibited eukaryotic pol activities
(10,11).
In higher plants, particularly in chloroplasts, the
thylakoid membrane contains major glycoglycerolipids, such as
monogalactosyl diacylglycerol (MGDG), digalactosyl diacylglycerol
and sulfoquinovosyl diacylglycerol (12). It is known that glycoglycerolipids
are present in vegetables, fruits and grains (13,14),
and it has been found here that spinach was the best
glycoglycerolipid source, with the highest MGDG content, among the
vegetables tested (15).
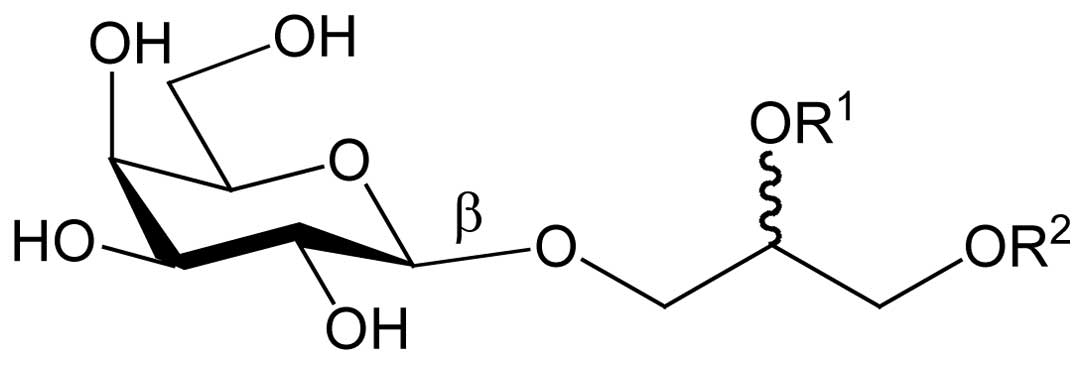
In this study, attention was focused on spinach MGDG
(Fig. 1) and its antitumor effect
on nude mice bearing solid human tumors. In in vivo mouse
experiments, the characteristically fat-soluble MGDG was difficult
to solubilize in water and results were thus liable to fluctuate
relative to the degree of MGDG solubilization. To solve this
problem, liposomes were prepared which had sialyl Lewis X (SLX)
bound to their surfaces and containing spinach MGDG
(SLX-Lipo-MGDG). Liposomes are used as a drug delivery system (DDS)
in medicine due to their unique properties, including the ability
to carry both hydrophobic and hydrophilic molecules. To deliver
cargo molecules at the sites of action, their lipid bilayers can
fuse with other bilayers, such as cell membranes, thus delivering
their liposome contents. By generating liposomes in a solution of
one or more drugs, some of which would normally be unable to
diffuse through the membrane, a medicine can be indiscriminately
packaged inside the liposomes. Thus, liposomes, incorporating
various substances, such as spinach MGDG, and delivering them to a
diseased region, have already been used in practice (16,17).
However, these DDSs control the effective liposome distribution to
target cells by adjusting their particle size and surface electric
charge in a passive manner, lacking cell-type specificity (18–20).
In addition, trials thus far, providing liposomes with an active
targeting ability by surface-binding various ligands, such as
antibodies, transferrin, folic acid or monosaccharides, have had
few successes (20,21). In this research group, the specific
recognition and binding between lectin and sugar chains had been
noticed and used for conferring active targeting ability to
liposomes (22,23). Much research has been done on animal
lectins, such that the molecular recognition mechanisms of sugar
chains by lectins have become clear. Based on lectin primary
structures, they are classified into fourteen types, which include
C-type lectin, galectin, I-type lectin, P-type lectin and pentraxin
(24–26). Among these, the mutual recognition
of E-selectin, classified into the C-type lectin and SLX has been
best clarified in an inflammation model. In a tumor inflammation
region, the vascular endothelial cells are activated by
inflammatory cytokines, IL-1β and TNF-α, and E-selectin then
expressed on the cell surface, such that the sugar chain SLX on
leucocyte surfaces then causes these cells to roll along on the
vascular endothelial cells via gentle binding with E-selectin; this
phenomenon is called ‘rolling’. Leucocytes, rolling down through
vascular endothelium gaps caused by inflammation, thus migrate into
the tissues from the blood vessels; leucocytes appear to accumulate
specifically in inflammation regions by such mechanisms (27,28).
In light of these observations, the results of this
study are discussed in terms of the observed properties of
SLX-Lipo-MGDG and their possible use in in vivo experiments,
including clinical anticancer treatments.
Materials and methods
Materials
Dried spinach (Spinacia oleracea L.) was
purchased from Kodama Foods Co., Ltd. (Hiroshima, Japan).
SLX-Lipo-MGDG with and without contained Cy5.5 solution in
distilled phosphate-buffered saline (PBS) were custom-produced by
Katayama Chemical Industries Co., Ltd. (Osaka, Japan) using the
improved cholate dialysis method with some modifications (29). A chemically synthesized DNA
template, poly(dA), was purchased from Sigma-Aldrich (St. Louis,
MO, USA) and a customized oligo(dT)18 DNA primer
produced by Sigma-Aldrich Japan K.K. (Hokkaido, Japan). The
radioactive nucleotide [3H]-deoxythymidine
5′-triphosphate (dTTP, 43 Ci/mmol) was obtained from MP Biomedicals
LLC (Solon, OH, USA). All other reagents were analytical grade from
Nacalai Tesque, Inc. (Kyoto, Japan).
Enzymes
Pols with high activities from mammals, a fish
(cherry salmon), an insect (fruit fly) and plants (cauliflower and
rice) were purified according to our previous report (30). Calf TdT, Taq pol, T4 pol and
T4 polynucleotide kinase were purchased from Takara Bio (Tokyo,
Japan). The Klenow fragment of pol I from E. coli was
purchased from Worthington Biochemical Corp. (Freehold, NJ, USA).
T7 RNA polymerase and bovine pancreas deoxyribonuclease I were
purchased from Stratagene Cloning Systems (La Jolla, CA, USA).
Pol assays
The reaction mixtures for pol α, pol β, plant pols,
and prokaryotic pols have been described previously (31,32);
those for pol γ and for pols δ and ɛ were as described by Umeda
et al (33) and Ogawa et
al (34), respectively; for
pols η, ι and κ the same as for pol α; and for pols λ and μ
the same as for pol β. For pol reactions,
poly(dA)/oligo(dT)18 (A/T, 2/1) and
2′-deoxythymidine-5′-triphosphate (dTTP) were used as the DNA
template-primer substrate and nucleotide (dNTP,
2′-deoxynucleotide-5′-triphosphate) substrate, respectively. For
TdT reactions, oligo(dT)18(3′-OH) and dTTP were used as
the DNA primer and nucleotide substrate, respectively.
MGDG was dissolved in distilled DMSO at various
concentrations and sonicated for 30 sec and 4 μl aliquots
mixed with 16 μl of each enzyme (0.05 units) in 50 mM
Tris-HCl at pH 7.5 containing 1 mM dithiothreitol, 50% glycerol (by
vol), and 0.1 mM EDTA, and held at 0°C for 10 min. These
inhibitor-enzyme mixtures in 8 μl volumes were added to 16
μl of enzyme standard reaction mixture and incubated at 37°C
for 60 min, except for Taq pol, which was incubated at 74°C
for 60 min. Activity without inhibitor was considered 100% and
relative activity determined for each inhibitor concentration. One
unit of pol activity was defined as the amount of each enzyme that
catalyzed incorporation of 1 nmol dNTP (specifically dTTP) into
synthetic DNA template-primers in 60 min, at 37°C and under normal
reaction conditions (31,32).
Other enzyme assays
Activities of primase of pol α, T7 RNA polymerase,
T4 polynucleotide kinase and bovine deoxyribonuclease I were
measured in each of the standard assays according to the
manufacturer’s specifications, as described by Tamiya-Koizumi et
al (35), Nakayama and
Saneyoshi (36), Soltis and
Uhlenbeck (37), and Lu and
Sakaguchi (38), respectively.
Cell culture and cell viability
assessment
Human cancer cells, including lung cancer cells A549
(JCRB0076), acute lymphoblastoid leukemia cells BALL-1 (JCRB0071),
cervical cancer cells HeLa (JCRB9010), promyelocytic leukemia cells
HL60 (JCRB0085) and gastric cancer cells NUGC-3 (JCRB0822), were
supplied by the Health Science Research Resources Bank (Osaka,
Japan). Human colon adenocarcinoma cancer cells HT-29 (ATCC no.
HTB-38) were obtained from American Type Culture Collection
(Manassas, VA, USA). Normal human cells, human umbilical vein
endothelial cells HUVEC (CS-ABI-375) and human dermal fibroblast
HDF (CS-2FO-101), were purchased from Dainippon Sumitomo Pharma
Co., Ltd. (Osaka, Japan).
A549, HeLa and HUGC-3 cells were cultured in Eagle’s
minimum essential medium supplemented with 10% fetal bovine serum
(FBS), penicillin (100 U/ml) and streptomycin (100 μg/ml).
BALL-1 and HL60 cells were cultured in RPMI-1640 medium
supplemented with 10% FBS, penicillin (100 U/ml), streptomycin (100
μg/ml) and 1.6 mg/ml NaHCO3. HT-29 cells were
maintained in McCoy’s 5A medium supplemented with 10% FBS, sodium
bicarbonate (2 g/l) and streptomycin (100 μg/ml). HUVEC and
HDF cells were cultured according to the manufacturer’s
instructions (Dainippon Sumitomo Pharma Co., Ltd.). MGDG
cytotoxicity was investigated by inoculating ~5×103
cells/well in 96-well microtiter plates and the addition of spinach
MGDG solution in DMSO at various concentrations. After incubation
for 48 h at 37°C in a humidified atmosphere of 5%
CO2/95% air, MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide]
solution was added to a final 0.5 mg/ml in PBS for 3 h (39), after which time the medium was
discarded and the cells lysed in acidified 2-propanol. The A570 was
measured in a microplate reader (Molecular Devices, Inc.,
Sunnyvale, CA, USA).
Measurement of particle size and
zeta-potential
Particle size and zeta-potential were measured at
25°C using a Zetasizer Nano-S90 (Malvern Instruments, Ltd.,
Worcestershire, UK) and with the liposome solution diluted 50 times
with distilled PBS.
Animal experiments
All animal studies were approved by the Kobe-Gakuin
University Animal Committee according to the guidelines for the
‘Care and Use of Laboratory Animals’ of the University. Animals
were anesthetized with pentobarbital before undergoing cervical
dislocation. Five-week-old specific pathogen-free female Balb/c
nu/nu mice (nude mice) were provided by Japan SLC, Inc. (Shizuoka,
Japan), fed a standard diet (MF, Oriental Yeast Co., Ltd., Osaka,
Japan) with water ad libitum, and maintained under a 12-h
light/dark cycle and at 25°C room temperature.
Production of tumor-bearing mice and
SLX-Lipo-MGDG liposome distribution assessment in vivo
HT-29 cells at 5×106 cells/mouse were
subcutaneously inoculated to the right femoral region of nude mice
(male, 7 week of age) and used for experiments 10 days later. For
anesthesia, 200 μl of pentobarbital (Nembutal) solution,
diluted 10 times with saline, was administered into the peritoneal
cavity. Then, 100, 150 or 200 μl of SLX-Lipo-MGDG (20 mg/kg)
containing Cy5.5 in PBS was administered through the tail vein.
Using eXplore Optix (GE Healthcare Bio-Science Corp., Piscataway,
NJ, USA) (Gallant et al, OSA BIOMED Meeting WD2, 2004), the
Cy5.5 fluorescent signal was monitored in the tumor region (right
femoral region) of the same mouse at 0, 24, 48 and 96 h after
injection (excitation and emission, 680 and 700 nm,
respectively).
Assessment of antitumor activity in
vivo
HT-29 cells at 5×106 cells/mouse were
subcutaneously inoculated into nude mice and the resulting
tumor-bearing mice divided randomly into four groups and treatment
started with the SLX-Lipo in PBS, MGDG (4 or 20 mg of MGDG/kg) in
PBS, SLX-Lipo-MGDG (4 or 20 mg of MGDG/kg) in PBS or vehicle
control (PBS only) 5 days after implantation, when tumor volume
[length × (width)2 × 0.5] was 80–90 mm3.
These groups (n=6) were administered ~200 μl of treatment
solution into the tail vein three times at 6-day intervals.
Results
Isolation of pol inhibitor MGDG from
spinach
Vegetables were screened for eukaryotic pol
inhibitors and the ethanol extract from dried spinach (Spinacia
oleracea L.) was found to inhibit the activities of replicative
pol species. The spinach ethanol solution was diluted to 70%
ethanol and subjected to Diaion HP-20 (Sigma-Aldrich) column
chromatography. The eluted solution of 95% ethanol (v/v) was
evaporated to dryness, the residue redissolved in chloroform, and
the resulting solution subjected to silica gel (PSQ60B, Fuji
Silysia, Tokyo, Japan) column chromatography. After washing the
column with chloroform/ethyl acetate (1/1, v/v), the column was
eluted with ethyl acetate and the eluate purified using Sep-Pak
C18 (Waters Corp., Tokyo, Japan) column chromatography
eluted with methanol. The active fractions were evaporated,
yielding the purified material which was ~98% of the chemical
purity that can be obtained by normal-phase silica gel (Shiseido
Co., Ltd., Tokyo, Japan) high performance liquid chromatography
coupled with evaporative light scattering detector, eluted with
chloroform/methanol (1/1, v/v).
The obtained purified compound was a yellow oily
material that was analyzed by nuclear magnetic resonance, mass
spectroscopy and optical rotation, and the chemical structure
characterized as MGDG (Fig. 1)
(data not shown). The acyloxy groups of MGDG (R1 and
R2; Fig. 1) have various
lengths and numbers of double bonds such that a molecular weight
could not be determined.
Effects of the purified MGDG on the
activities of DNA polymerases and other DNA metabolic enzymes
The effects of spinach MGDG on pols from various
species and other DNA metabolic enzymes are shown in Table I. This compound selectively
inhibited the activities of calf pol α, and human pols γ, δ and ɛ,
with the inhibitory effect ranked pol ɛ>pol δ>pol
α>>pol γ. The inhibitory effect on B-family pols α, δ and ɛ
was stronger than on A-family pols, such as pol γ, with 50%
inhibitory concentrations (IC50 values) observed at
doses of 8.0–16.5 μg/ml and 26.3 μg/ml, respectively
by family. On the other hand, MGDG did not influence the activities
of the X-family pols, rat pol β, human pol λ and calf TdT, and the
Y-family pols, human pol η, mouse pol ι and human pol κ, which
suggested that MGDG was a selective inhibitor among mammalian pol
species. This compound also inhibited the activities of fish pol δ,
and insect pols α, δ and ɛ, all B-family, at almost the same
concentrations that inhibited mammalian pols, with IC50
values of 13.0–18.2 μg/ml.
 | Table IIC50 values of spinach
MGDG on the activities of various pols and other DNA metabolic
enzymes. |
Table I
IC50 values of spinach
MGDG on the activities of various pols and other DNA metabolic
enzymes.
| Enzyme | IC50
values (μg/ml) |
|---|
| Mammalian DNA
polymerases |
| A-Family |
| Human DNA
polymerase γ | 26.3±1.4 |
| B-Family |
| Calf DNA
polymerase α | 16.5±1.1 |
| Human DNA
polymerase δ | 14.9±1.0 |
| Human DNA
polymerase ɛ | 8.0±0.6 |
| X-Family |
| Rat DNA
polymerase β | >200 |
| Human DNA
polymerase λ | >200 |
| Human DNA
polymerase μ | >200 |
| Calf terminal
deoxynucleotidyl transferase | >200 |
| Y-Family |
| Human DNA
polymerase η | >200 |
| Mouse DNA
polymerase ι | >200 |
| Human DNA
polymerase κ | >200 |
| Fish DNA
polymerases |
| B-Family |
| Cherry salmon DNA
polymerase δ | 18.2±1.1 |
| Insect DNA
polymerases |
| B-Family |
| Fruit fly DNA
polymerase α | 17.8±1.2 |
| Fruit fly DNA
polymerase δ | 17.6±1.1 |
| Fruit fly DNA
polymerase ɛ | 13.0±0.7 |
| Plant DNA
polymerases |
| B-Family |
| Cauliflower DNA
polymerase α | >200 |
| X-Family |
| Rice DNA
polymerase λ | >200 |
| Prokaryotic DNA
polymerases |
| E. coli
DNA polymerase I | >200 |
| Taq DNA
polymerase | >200 |
| T4 DNA
polymerase | >200 |
| Other DNA metabolic
enzymes |
| Calf primase of
DNA polymerase α | >200 |
| T7 RNA
polymerase | >200 |
| T4 polynucleotide
kinase | >200 |
| Bovine
deoxyribonuclease I | >200 |
In contrast, MGDG exhibited no effect on the plant
pols, cauliflower pol α and rice pol λ, and the prokaryotic pols,
E. coli pol I, Taq pol or T4 pol (Table I); the three-dimensional structures
of eukaryotic pols are likely to differ greatly from prokaryotic
pols. When activated DNA (bovine deoxyribonuclease I-treated DNA)
and dNTPs were used as the DNA template-primer substrate and
nucleotide substrate, respectively, in place of synthesized DNA
[poly(dA)/oligo(dT)18 (A/T=2/1)] and dTTP, respectively,
the inhibitory effects of these compounds did not change (data not
shown).
This MGDG had minimal influence on the activity of
other DNA metabolic enzymes, such as primase of calf pol α, T7 RNA
polymerase, T4 polynucleotide kinase or bovine deoxyribonuclease I.
Collectively, these results suggested that this compound
selectively inhibited the activity of animal family A and B pols,
such as pols α, γ, δ and ɛ.
Effects of MGDG on cultured human cancer
and normal cells
As pols conduct cellular DNA synthesis (4–6) and
are essential for DNA replication, repair and subsequent cell
division, inhibition of these enzymes will lead to cell death,
particularly under proliferative conditions. Thus, pol inhibitors
can be considered potential agents for cancer chemotherapy and,
thus, the effect of MGDG on cell growth was investigated in eight
human cell lines.
MGDG suppressed the growth of all six human cancer
cell lines tested, including A549, BALL-1, HeLa, HL60, HT-29 and
NUGC-3, and showed the strongest growth inhibitory effect on HT-29
cells, with a 50% lethal dose (LD50 value) of 25.9
μg/ml (Table II). Effective
suppression of cell growth involved concentrations similar to those
for inhibition of mammalian pols α, γ, δ and ɛ by MGDG, suggesting
that the cause of MGDG’s influence in cancer cells may be its
effect on pol activities, particularly the replicative pols α, δ
and ɛ. The cytotoxic dose was almost the same or ~2-fold higher
than the enzyme inhibitory concentrations (the range of
LD50 and IC50 values for MGDG were 25.9–36.5
μg/ml and 8.0–26.3 μg/ml, respectively, Tables I and II), suggesting that MGDG could penetrate
human cancer cells and inhibit nuclear and mitochondrial
replicative pol activities.
 | Table IILD50 values of spinach
MGDG on the growth of human cancer and normal cell lines. |
Table II
LD50 values of spinach
MGDG on the growth of human cancer and normal cell lines.
| Human cultured cell
line | LD50
values (μg/ml) |
|---|
| Cancer cells |
| A549 (lung
cancer) | 36.5±3.0 |
| BALL-1 (acute
lymphoblastoid leukemia) | 28.9±2.4 |
| HeLa (cervix
cancer) | 35.3±2.9 |
| HL60
(promyelocytic leukemia) | 27.4±2.2 |
| HT-29 (colon
adenocarcinoma) | 25.9±2.2 |
| NUGC-3 (stomach
cancer) | 35.6±2.9 |
| Normal cells |
| HDF (human dermal
fibroblasts) | >200 |
| HUVEC (human
umbilical vein endothelial cells) | >200 |
In comparison, MGDG did not influence the growth of
normal human cells HUVEC and HDF with a 48-h incubation (Table II), suggesting that MGDG could be a
potent and selective anticancer chemotherapeutic agent.
Properties of SLX-Lipo-MGDG
The contents and properties of SLX-Lipo-MGDG
prepared by the improved cholate dialysis method are shown in
Table III. The particle size of
SLX-Lipo (without MGDG), SLX-Lipo-MGDG (0.4 mg/ml) and
SLX-Lipo-MGDG (2 mg/ml) showed an almost uniform distribution and
mean particle size of 222–285 nm. The zeta-potential, showing the
electric charge of liposome membrane surface, was negatively
charged at -40 mV. After storage at 4°C for 6 months, the particle
size distribution was almost the same as right after preparation,
demonstrating the stability of these liposomes. From electron
microscopic observations, nearly all liposomes appeared spherical
and 200–300 nm in size (data not shown).
 | Table IIIParticle size and formation of
SLX-Lipo-MGDG. |
Table III
Particle size and formation of
SLX-Lipo-MGDG.
| Property | SLX-Lipo-MGDG |
|---|
| Particle
sizea |
| 0 mg/ml MGDG | 222±8.7 nm |
| 0.4 mg/ml
MGDG | 241±9.5 nm |
| 2 mg/ml MGDG | 285±11.6 nm |
| Absorbance (680
nm) | 2.9 |
| Lipid | 0.93 mg/ml |
| Photodynamic
inactivation | 0.287 |
| Protein | 2.95 mg/mi |
| Zeta potential | −40 mV |
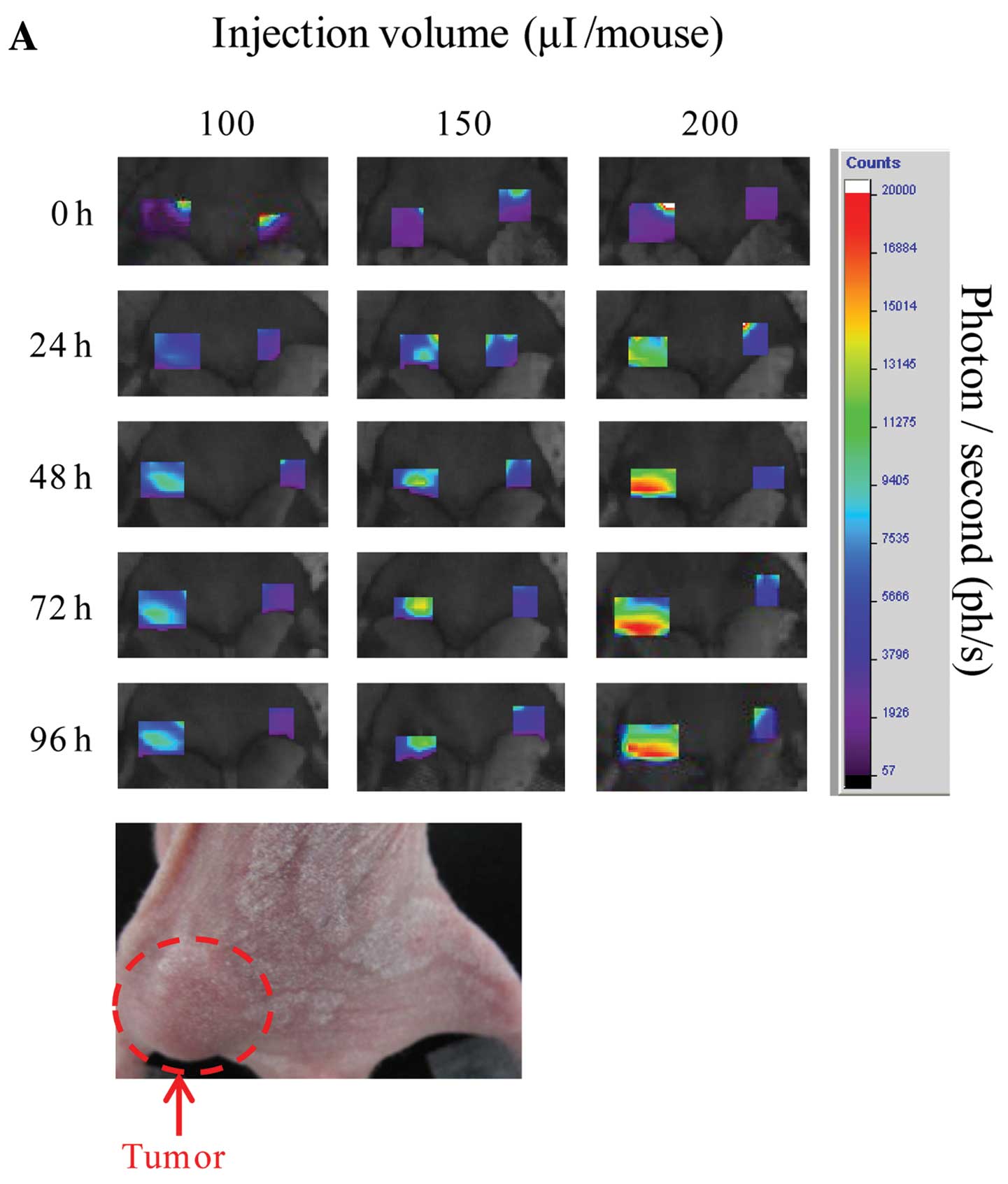
Accumulation of SLX-Lipo MGDG containing
Cy5.5 using in vivo imaging analysis
The HT-29 tumor region of Balb/c nude mouse was
observed at 0, 24, 48, 72 and 96 h after administration using
eXplore Optix. SLX-Lipo-MGDG (2 mg/ml) containing Cy5.5 accumulated
significantly at the tumor region, increasing gradually until 48 h
(Fig. 2A). Accumulations from 200
μl injections were greater than those of 100 and 150
μl injections and were thus dependent on injected amounts
(Fig. 2B). On the other hand,
accumulation of Lipo-MGDG with Cy5.5, with no SLX on the liposome
surfaces, indicated no accumulation (data not shown). To examine
overall fluorescence distribution, the whole body was scanned 96 h
after injection and the liver, bladder and tumor regions exhibited
the strongest fluorescence signals, which were analyzed in terms of
fluorescence lifetime. In the tumor regions, only Cy5.5 bound to
human serum albumin (HSA; lifetime, 1.8 ns) was detected, while in
the liver and bladder, both free (lifetime, 1.5 ns) and HSA-bound
Cy5.5 was identified (lifetime, 1.8 ns, data not shown). In
addition, leucocyte rolling was observed on blood vessel
endothelium in the tumor region by scanning fluorescent microscope,
indicating that SLX-Lipo-MGDG was recognized by E-selectin
expressed during tumor growth and, as a result, it accumulated in
the tumor region. According to previous reports, the E-selectin
expression pattern on human umbilical vein endothelial cells,
induced by lipopolysaccharides, was not uniform but formed partial
and high density aggregates on the cells (40,41).
From these results, it was concluded that SLX-Lipo-MGDG bound
partially, not uniformly, to the vascular endothelium.
Effect of SLX-Lipo MGDG on antitumor
activity in vivo
At 5 days after HT-29 cell implantation, nude mice
bearing solid tumors were intravenously injected with PBS
(control), SLX-Lipo alone (0 mg MGDG/kg body weight), 4 or 20 mg/kg
MGDG alone or 4 or 20 mg/kg SLX-Lipo-MGDG at 6-day intervals for a
total of three injections. As the mouse body weights were ~20 g, 4
and 20 mg of MGDG/kg were equivalent to the administration of ~200
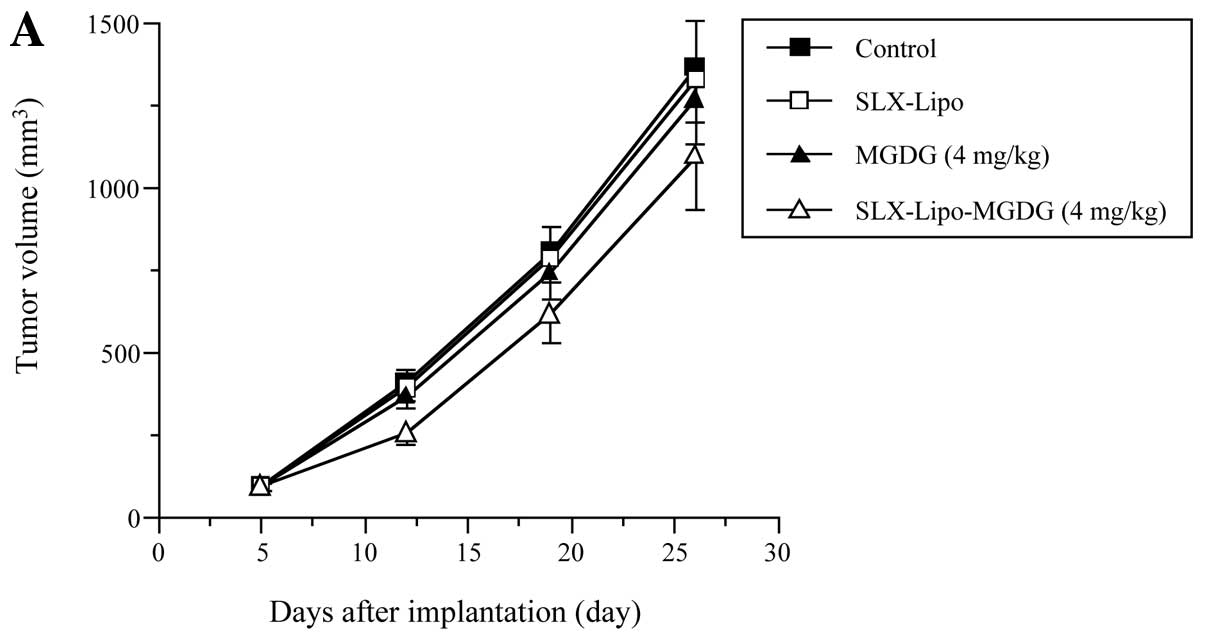
μl of 0.4 and 2 mg/ml MGDG, respectively. Tumor volumes of
the groups administered the control and SLX-Lipo increased
time-dependently, at 1370 and 1271 mm3, respectively, by
26 days after the implantation (Fig.
3); therefore, SLX-Lipo alone showed no effect on tumor growth.
SLX-Lipo-MGDG at 4 mg/kg significantly suppressed tumor growth by
12 days compared to the control and SLX-Lipo groups, and tumor
volume decreased 20.3% by 26 days (Fig.
3A). In contrast, 4 mg/kg of MGDG alone exhibited barely any
tumor suppression, suggesting that the DDS of MGDG in SLX-Lipo must
be very important for an effective antitumor impact. Both 20 mg/kg
of MGDG and SLX-Lipo-MGDG (20 mg/kg) suppressed tumor formation in
a time-dependent manner, and the tumor volume decreases were 25.5
and 33.6%, respectively, at 26 days compared to the control
(Fig. 3B). Thus, as MGDG inhibited
the activities of replicative pols, this compound might also
suppress tumor activity.
In the present study, none of the nude mice showed
any significant loss of body weight throughout the experimental
period (Fig. 3C and D), and it was
also noted that the main visceral organs, such as the liver, lung,
kidney, spleen, heart, stomach, small intestine, large intestine,
pancreas and testes of all groups showed no toxic or degenerative
histological appearance (data not shown). These observations
suggested that SLX-Lipo-MGDG did not have detectable side effects,
such as animal death or evident toxicity, loss of body weight
and/or major organ damage, in these mice and that this liposome
system should be of great interest as a DDS candidate for
anticancer treatment.
Discussion
MGDG is a non-nutrient compound found in vegetables,
grains and fruits and, although its content varies among these
plants (13), it is ingested daily
in food. MGDG’s chemical structure includes two acyloxy groups
consisting of two fatty acid molecules (R1 and
R2; Fig. 1). In the
present study, spinach MGDG was rich in n-3 α-linolenic acid
(26.3% of total fatty acids in spinach MGDG, data not shown). MGDG
ingested from wheat flour includes non-n-3 fatty acids, such
as n-6 linoleic acid, and saturated fatty acids (42), and it appears that the fatty acyl
component influences the antitumor effects. Therefore, the present
findings suggest that researchers should observe and pay attention
to the lipid content and fatty acid composition in MGDG
studies.
In this study, MGDG, isolated from a vegetable,
spinach (Spinacia oleracea L.), was found to a selective
inhibitor of mammalian pols α, γ, δ and ɛ, while having no effect
on other mammalian pols, such as repair-related pols β, η, ι, κ, λ,
μ and TdT (Table I). This
MGDG prevented cell growth in 6 human cancer cell lines but had no
effect on normal human cell proliferation (Table II). As the MGDG LD50
values on human cancer cell growth were almost the same or within
2-fold of MGDG’s IC50 values on pol activities, this
inhibition was concluded to be mostly led by effects on pol
functions and, therefore, MGDG was able to penetrate cancer cells
and reach the nucleus and mitochondria, thus inhibiting mammalian
pols α, γ, δ and ɛ activities and leading to human cancer cell
growth suppression. The mechanism of selective cell growth
suppression between cancer and normal cell lines by MGDG remains
unclear, and it may be considered that the expression amounts and
activities of pols α, δ and ɛ, which are nuclear DNA replicative
pols, as well as pol γ, which is a mitochondrial DNA replicative
pol, in cancer cells are higher than in normal cells and, thus,
MGDG could only inhibit cancer cell proliferation.
It has been reported that liposome retention in
blood vessels significantly influences their accumulation in tumor
regions and this accumulation increases by extending their
retention in blood vessels (43).
Such extension of blood vessel liposome residence would require
‘avoidance of taking into reticuloendothelial system in the liver
and the spleen’, ‘avoidance of the phagocytosis with the
macrophage’ and ‘avoidance of non-specific adsorption with vascular
endothelial cells and the cells such as leucocytes’ (44). SLX-Lipo-MGDG is negatively charged
(Table III), as are vascular
endothelial cells and others, such as erythrocytes and leucocytes,
and are thus electrically repulsed and do not adsorb
non-specifically to such cells. In addition, hydrophilization of
the liposome surface can prevent opsonin protein adsorption from
blood plasma as well as macrophage phagocytosis and, consequently,
extend liposome retention in blood stream.
The experiments here using MGDG in vivo were
relatively difficult to perform because of its solubility. With the
use of liposomes with SLX, high concentrations of MGDG
(SLX-Lipo-MGDG) and particle sizes of 200–300 nm (Table III), this problem was easily
solved and thus should prove helpful in the pharmaceutical
application of MGDG. Indeed, liposomes stabilized with emulsifiers,
such as phospholipids, have been receiving considerable attention
as DDSs (28,44), and as drug carriers, these liposomes
have many appealing properties, such as biodegradability and
biocompatibility. Nanoparticulate systems using nanosomes (the
lower nanometer range of liposome size) have been developed for
pharmaceutical use and their application as drug carriers for
anticancer therapies has recently attracted a great deal of
attention. In the present results, SLX-Lipo-MGDG containing Cy5.5
was found to accumulate specifically and efficiently in
murine-carried human tumor regions (Fig. 2), showing an affinity for E-selectin
and excellent blood vessel retention. The antitumor effect in
vivo of SLX-Lipo-MGDG was stronger than that of MGDG alone
(Fig. 3). From the present
findings, it was concluded that, by selecting appropriate sugar
chains and densities, liposomes carrying sugar chains, such as SLX,
could deliver MGDG, an effective replicative pol inhibitor, to the
specific and desired (disease) region in the body, indicating that
these liposome types might be useful as active-targeting DDSs.
Acknowledgements
We are grateful for the donation of calf pol α by Dr
M. Takemura of Tokyo University of Science (Tokyo, Japan); rat pol
β and human pols δ and ɛ by Dr K. Sakaguchi of Tokyo University of
Science (Chiba, Japan); human pol γ by Dr M. Suzuki of Nagoya
University School of Medicine (Nagoya, Japan); mouse pol η and
human pol ι by Dr F. Hanaoka and Dr C. Masutani of Osaka University
(Osaka, Japan); human pol κ by Dr H. Ohmori of Kyoto University
(Kyoto, Japan); and human pols λ and μ by Dr O. Koiwai of
Tokyo University of Science (Chiba, Japan). We thank M. Hirai of
Katayama Chemical Industries Co., Ltd., for preparing SLX-Lipo-MGDG
with and without Cy5.5. Y.M. acknowledges Grant-in-Aids from
Grant-in-Aid for Scientific Research (C) (No. 24580205) from MEXT
(Ministry of Education, Culture, Sports, Science and Technology,
Japan), Takeda Science Foundation (Japan), and the Nakashima
Foundation (Japan). This research was supported by Adaptable and
Seamless Technology Transfer Program through Target-driven R&D
(A-STEP), Japan Science and Technology Agency (JST) and the
MEXT-Supported Program for the Strategic Research Foundation at
Private Universities, 2012–2016.
Abbreviations:
|
pol
|
DNA-dependent DNA polymerase (E.C.
2.7.7.7)
|
|
MGDG
|
monogalactosyl diacylglycerol
|
|
SLX
|
sialyl Lewis X
|
|
SLX-Lipo-MGDG
|
SLX-liposome containing MGDG
|
|
DDS
|
drug delivery system
|
|
PBS
|
phosphate-buffered saline
|
|
dTTP
|
2′-deoxythymidine-5′-triphosphate
|
|
dNTP
|
2′-deoxynucleotide-5′-triphosphate
|
|
FBS
|
fetal bovine serum
|
|
IC50
|
50% inhibitory concentration
|
|
LD50
|
50% lethal dose
|
|
HSA
|
human serum albumin
|
References
|
1
|
Terry P, Giovannucci E, Michels KB,
Bergkvist L, Hansen H, Holmberg L and Wolk A: Fruit, vegetables,
dietary fiber, and risk of colorectal cancer. J Natl Cancer Inst.
93:525–533. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu RH: Potential synergy of
phytochemicals in cancer prevention: mechanism of action. J Nutr.
134:S3479–S3485. 2004.PubMed/NCBI
|
|
4
|
Kornberg A and Baker TA: DNA replication.
W.D. Freeman and Co; New York, NY: Chapter 6. 2nd edit. pp.
197–225. 1992
|
|
5
|
DePamphilis ML: DNA replication in
eukaryotic cells. Cold Spring Harbor Laboratory Press; Cold Spring
Harbor, NY: 1996
|
|
6
|
Hübscher U, Maga G and Spadari S:
Eukaryotic DNA polymerases. Annu Rev Biochem. 71:133–163. 2002.
|
|
7
|
Bebenek K and Kunkel TA: Functions of DNA
polymerases. Adv Protein Chem. 69:137–165. 2004. View Article : Google Scholar
|
|
8
|
Takata K, Shimizu T, Iwai S and Wood RD:
Human DNA polymerase N (POLN) is a low fidelity enzyme capable of
error-free bypass of 5S-thymine glycol. J Biol Chem.
281:23445–23455. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Loeb LA and Monnat RJ Jr: DNA polymerases
and human disease. Nat Rev Genet. 9:594–604. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mizushina Y, Watanabe I, Ohta K, Takemura
M, Sahara H, Takahashi N, Gasa S, Sugawara F, Matsukage A, Yoshida
S and Sakaguchi K: Studies on inhibitors of mammalian DNA
polymerase α and β: sulfolipids from a pteridophyte, Athyrium
niponicum. Biochem Pharmacol. 55:537–541. 1998.
|
|
11
|
Ohta K, Mizushina Y, Hirata N, Takemura M,
Sugawara F, Matsukage A, Yoshida S and Sakaguchi K:
Sulfoquinovosyldiacylglycerol, KM043, a new potent inhibitor of
eukaryotic DNA polymerases and HIV-reverse transcriptase type 1
from a marine red alga, Gigartina tenella. Chem Pharm Bull
(Tokyo). 46:684–686. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roughan PG and Batt RD: The glycerolipid
composition of leaves. Phytochemistry. 8:363–369. 1969. View Article : Google Scholar
|
|
13
|
Sugawara T and Miyazawa T: Separation and
determination of glycolipids from edible plant sources by
high-performance liquid chromatography and evaporative
light-scattering detection. Lipids. 34:1231–1237. 1999.PubMed/NCBI
|
|
14
|
Yunoki K, Sato M, Seki K, Ohkubo T, Tanaka
Y and Ohnishi M: Simultaneous quantification of plant
glyceroglycolipids including sulfoquinovosyldiacylglycerol by
HPLC-ELSD with binary gradient elution. Lipids. 44:77–83. 2009.
View Article : Google Scholar
|
|
15
|
Kuriyama I, Musumi K, Yonezawa Y, Takemura
M, Maeda N, Iijima H, Hada T, Yoshida H and Mizushina Y: Inhibitory
effects of glycolipids fraction from spinach on mammalian DNA
polymerase activity and human cancer cell proliferation. J Nutr
Biochem. 16:594–601. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gabison A, Shmeeda H and Barenholz Y:
Pharmacokinetics of pegylated liposomal Doxorubicin: review of
animal and human studies. Clin Pharmacokinet. 42:419–436. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Unezaki S, Maruyama K, Ishida O, Takahashi
N and Iwatsuru M: Enhanced tumor targeting of Doxorubicin by
ganglioside GM1-bearing long-circulating liposomes. J Drug Target.
1:287–292. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maruyama K, Yuda T, Okamoto A, Kojima S,
Suginaka A and Iwatsuru M: Prolonged circulation time in
vivo of large unilamellar liposomes composed of distearoyl
phosphatidylcholine and cholesterol containing amphipathic poly
(ethylene glycol). Biochim Biophys Acta. 1128:44–49. 1992.
|
|
19
|
Gabizon A and Papahadjopoulos D: Liposome
formulation with prolonged circulation time in blood and enhanced
uptake by tumors. Proc Natl Acad Sci USA. 85:6949–6953. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vyas SP, Singh A and Sihorkar V:
Ligand-receptor-mediated drug delivery: an emerging paradigm in
cellular drug targeting. Crit Rev Ther Drug Carrier Syst. 18:1–76.
2001.PubMed/NCBI
|
|
21
|
Willis M and Forssen E: Ligand-targeted
liposomes. Adv Drug Deliv Rev. 29:249–271. 1998. View Article : Google Scholar
|
|
22
|
Yamazaki N, Kojima S and Yokoyama H:
Biomedical nanotechnology for active drug delivery systems by
applying sugar-chain molecular functions. Curr Appl Phys.
5:112–117. 2005. View Article : Google Scholar
|
|
23
|
Yamazaki N: Active targeting DDS.
Farumashia. 42:125–129. 2006.
|
|
24
|
Ehrhardt C, Kneuer C and Bakowsky U:
Selectin-an emerging target for drug delivery. Adv Drug Deliv Rev.
56:527–549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dodd RB and Drickamer K: Lectin-like
proteins in model organisms: implications for evolution of
carbohydrate-binding activity. Glycobiology. 11:R71–R79. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kilpatrick DC: Animal lectins: a
historical introduction and overview. Biochim Biophys Acta.
1572:187–197. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bevilacqua MP, Stengelin S, Gimbrone MA Jr
and Seed B: Endothelial leukocyte adhesion molecule 1: an inducible
receptor for neutrophils related to complement regulatory proteins
and lectins. Science. 243:1160–1165. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vestweber D and Blanks JE: Mechanisms that
regulate the function of the selectins and their ligands. Physiol
Rev. 79:181–213. 1999.PubMed/NCBI
|
|
29
|
Hirai M, Minematsu H, Kondo N, Oie K,
Igarashi K and Yamazaki N: Accumulation of liposome with Sialyl
Lewis X to inflammation and tumor region: application to in
vivo bio-imaging. Biochem Biophys Res Commun. 353:553–558.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Myobatake Y, Takeuchi T, Kuramochi K,
Kuriyama I, Ishido T, Hirano K, Sugawara F, Yoshida H and Mizushina
Y: Pinophilins A and B, inhibitors of mammalian A-, B-, and
Y-family DNA polymerases and human cancer cell proliferation. J Nat
Prod. 75:135–141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mizushina Y, Tanaka N, Yagi H, Kurosawa T,
Onoue M, Seto H, Horie T, Aoyagi N, Yamaoka M, Matsukage A, et al:
Fatty acids selectively inhibit eukaryotic DNA polymerase
activities in vitro. Biochim Biophys Acta. 1308:256–262. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mizushina Y, Yoshida S, Matsukage A and
Sakaguchi K: The inhibitory action of fatty acids on DNA polymerase
β. Biochim Biophys Acta. 1336:509–521. 1997.
|
|
33
|
Umeda S, Muta T, Ohsato T, Takamatsu C,
Hamasaki N and Kang D: The D-loop structure of human mtDNA is
destabilized directly by 1-methyl-4-phenylpyridinium ion (MPP+), a
parkinsonism-causing toxin. Eur J Biochem. 267:200–206.
2000.PubMed/NCBI
|
|
34
|
Ogawa A, Murate T, Suzuki M, Nimura Y and
Yoshida S: Lithocholic acid, a putative tumor promoter, inhibits
mammalian DNA polymerase β. Jpn J Cancer Res. 89:1154–1159.
1998.PubMed/NCBI
|
|
35
|
Tamiya-Koizumi K, Murate T, Suzuki M,
Simbulan CG, Nakagawa M, Takamura M, Furuta K, Izuta S and Yoshida
S: Inhibition of DNA primase by sphingosine and its analogues
parallels with their growth suppression of cultured human leukemic
cells. Biochem Mol Biol Int. 41:1179–1189. 1997.PubMed/NCBI
|
|
36
|
Nakayama C and Saneyoshi M: Inhibitory
effects of 9-β-D-xylofuranosyladenine 5′-triphosphate on
DNA-dependent RNA polymerase I and II from cherry salmon
(Oncorhynchus masou). J Biochem. 97:1385–1389. 1985.
|
|
37
|
Soltis DA and Uhlenbeck OC: Isolation and
characterization of two mutant forms of T4 polynucleotide kinase. J
Biol Chem. 257:11332–11339. 1982.PubMed/NCBI
|
|
38
|
Lu BC and Sakaguchi K: An endo-exonuclease
from meiotic tissues of the basidiomycete Coprinus cinereus:
its purification and characterization. J Biol Chem.
266:21060–21066. 1991.PubMed/NCBI
|
|
39
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Welply JK, Abbas SZ, Scudder P, Keene JL,
Broschat K, Casnocha S, Gorka C, Steininger C, Howard SC and
Schmuke JJ: Multivalent sialyl-LeX: potent inhibitors of
E-selectin-mediated cell adhesion; reagent for staining activated
endothelial cells. Glycobiology. 4:259–265. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pober JS, Bevilacqua MP, Mendrick DL,
Lapierre LA, Fiers W and Gimbrone MA Jr: Two distinct monokines,
interleukin 1 and tumor necrosis factor, each independently induce
biosynthesis and transient expression of the same antigen on the
surface of cultured human vascular endothelial cells. J Immunol.
136:1680–1687. 1986.
|
|
42
|
Sugawara T and Miyazawa T: Digestion of
plant monogalactosyldiacylglycerol and digalactosyldiacylglycerol
in rat alimentary canal. J Nutr Biochem. 11:147–152. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Drummond DC, Meyer O, Hong K, Kirpotin DB
and Papahadjopoulos D: Optimizing liposome for delivery of
chemotherapeutic agents to solid tumors. Pharmacol Rev. 51:691–743.
1999.PubMed/NCBI
|
|
44
|
Yamazaki N, Kodama M and Gabius HJ:
Neoglycoprotein–liposome and lectin-liposome conjugates as tools
for carbohydrate recognition research. Methods Enzymol. 242:56–65.
1994.
|