Introduction
Colorectal cancer (CRC) is one of the major causes
of cancer-related death worldwide (1). Metastasis to regional lymph nodes
represents a major problem, usually leading to poor survival rate
after radical operation (2). The
investigation of metastasis-associated genes for the early
detection or therapeutic targeting of CRC could, therefore,
potentially improve the survival rate of future CRC patients.
MicroRNAs are non-coding small RNAs (18–22
nucleotides in size) and negatively regulate gene expression by
binding to the 3′UTR of target genes (3). They are involved in a variety of
biological processes including differentiation, morphogenesis,
proliferation, and apoptosis (3).
Studies have reported dysregulation of miRNAs in a number of human
diseases including colon cancer (4,5).
Several miRNAs display tumor suppressive functions while others
have an oncogenic role during carcinogenesis (5–7).
Investigators have linked the levels of expression of these miRNAs
to clinicopathological features of disease. Analysis of their
expression might, therefore, facilitate the detection and diagnosis
of cancer (8–10). The DNA methylation of gene promoters
is critical in the repression of gene expression in various cell
types or development stages. Abnormal methylation of tumor
suppressor genes plays a significant role in tumor development
(11–15). Accumulating evidence has shown that
several tumor-associated miRNAs are overexpressed or downregulated
during CRC progression. Among these dysregulated miRNAs, tumor
suppressive miRNAs, including miR-9, miR-129, miR-134, miR-342 and
miR-34b/c, are frequently silenced by aberrant DNA hypermethylation
in CRC genomic DNA (16–19).
The miR-1 and miR-133a family of miRNAs is encoded
at two paralogous loci and reportedly downregulates and displays
tumor suppressive function in various human cancers, including
bladder cancer, prostate cancer, lung cancer, esophageal squamous
carcinoma and colon cancer (20–24).
Datta et al(25) reported
that DNA methylation can mediate the miR-1 gene and suppress tumor
cell growth by repressing its oncogenic targets MET, FoxP1 and
HDAC4 in hepatocellular carcinoma. However, the relationships
between the DNA methylation status of the miR-1-133a cluster,
levels of expression and biological function in CRC have yet to be
fully elucidated. The present study performed a series of
sequential analyses to evaluate the methylation status and
expression of the miR-1-133a cluster in 64 paired samples of CRC
tissue and 14 liver metastatic tissue samples. Results indicated
that high frequency DNA hypermethylation leads to silencing of the
miR-1-133a cluster expression, and that this silencing is
associated with tumor metastasis.
Materials and methods
Clinical samples
Sixty-four paired tumor and adjacent normal mucosa
samples and 14 metastatic liver tumor, primary tumor and adjacent
mucosa samples were obtained from CRC patients who underwent
surgical operation at the Department of Surgery, Veterans General
Hospital, Taipei, Taiwan. Informed consent was obtained from all
patients.
Extraction of RNA
The total RNA of the tissue was extracted using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the
instruction manual. Briefly, tissue samples were homogenized in 1
ml TRIzol reagent and mixed with 0.2 ml chloroform to extract
protein, before RNA was precipitated using 0.5 ml isopropanol. The
concentration, purity and amount of total RNA were determined using
a Nanodrop 1000 spectrophotometer (Nanodrop Technologues, Inc.,
USA).
Cell lines and 5-Aza-dC treatment
Human colorectal cancer cell lines, HT29 and B77,
were obtained from the American Type Culture Collection and
maintained in Dulbecco’s modified Eagle’s medium supplemented with
10% inactivated FBS (Invitrogen). Colorectal cancer cells were
cultured in the presence or absence of 2.5 μM 5-Aza-dC for 4 days.
The total RNA was then prepared using TRIzol (Invitrogen),
according to the previously described protocol.
Real-time polymerase chain reaction and
statistical analysis
The primers were designed to the mature miRNAs for
miR-1 and miR-133a for reverse transcription according to the
methods described by Chen et al(26). One microgram total RNA was
reverse-transcribed using a stem-loop RT reaction with RT primers
and SuperScript III Reverse Transcriptase according to the user’s
manual (Invitrogen). The reaction was performed with the following
incubation conditions: 30 min at 16°C, followed by 50 cycles of
20°C for 30 sec, 42°C for 30 sec and 50°C for 1 sec. The enzyme was
subsequently inactivated by incubation at 85°C for 5 min. Real-time
PCR reactions were performed using a microRNA-specific forward
primer and a universal reverse primer, and were conducted at 94°C
for 10 min, followed by 40 cycles of 94°C for 15 sec and 60°C for
32 sec. Gene expression was detected using a SYBR-Green I assay
(Applied Biosystems, Foster City, CA, USA) and the expression
levels of miRNAs were normalized to that of U6. Expression of
TAGLN1 and LASP1 was examined using real-time polymerase chain
reaction (PCR) with a gene-specific primer and normalized to GAPDH.
All reactions were performed in triplicate and the differences
between tissue types were analyzed using Student’s t-test. The
difference was considered to be significant at P-value
<0.05.
The individual primers used were as follows:
miR-1-RT, 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGA GATACATAC-3′;
miR-133a-RT, 5′-CTCAACTGGTGTCG TGGAGTCGGCAATTCAGTTGAGCAGCTGGT-3′;
miR-1-GSF, 5′-CGGCGGTGGAATGTAAAGAAGT-3′; miR-133a-GSF,
5′-CGGCGGTTTGGTCCCCTTCAAC-3′; Universal-R,
5′-CTGGTGTCGTGGAGTCGGCAATTC-3′; U6-F, 5′-CTCGCTTCGGCAGCACA-3′;
U6-R, 5′-AACG CTTCACGAATTTGCGT-3′; TAGLN1-F, 5′-CGAGGG
GCAGGCCCCAGTAA-3′; TAGLN1-R, 5′-GTCCGCTG CACACAGGCCAT-3′; LASP1-F,
5′-GCAACAGAGTGAG CTCCAGAG-3′; LASP1-R, 5′-TGAAACCTTTGCCCTTG TTC-3′;
GAPDH-F, 5′-TGCACCACCAACTGCTTAGC-3′; GAPDH-R,
5′-GGCATGGACTGTGGTCATGAG-3′.
Conversion of DNA bisulfite and COBRA
analysis
Genomic DNA was extracted from cultured cells or CRC
tissues using TRIzol reagent (Invitrogen) and then 2 μg genomic DNA
was subjected to bisulfite conversion using the EZ DNA
Methylation-Gold kit (Zymo Research Corp., Orange, CA, USA). The
conditions for the bisulfite conversion reaction were 98°C for 10
min followed by 64°C for 2.5 h, with a final incubation at 4°C for
up to 20 h in a PCR thermocycler. The modified genomic DNA was used
for the methylation analysis of CpG25, CpG81 and CpG26 using the
specific methylation primers. The PCR conditions were as follows:
94°C for 10 min, followed by 35 cycles of 94°C for 1 min, 60°C for
1 min and 72°C for 30 sec, with a final extension at 72°C for 10
min using a PCR thermocycler and HotStart Taq DNA polymerase
(Qiagen, Hilden, Germany). The methylation status of the genomic
DNA of individual samples was also examined using BstUI digestion
(New England Biolabs, MA, USA). The digested PCR fragments were
then separated on 2% agarose gel. The individual primers used were
as follows: CpG25-F, 5′-GGA GGGGTAGGATAGTAGTTTGAGT-3′; CpG25-R,
5′-AAAA AAACCTAACCTAAAAAACCAAAA-3′; CpG81-F, 5′-GGT
GAGTTTTGTTTAGTTTATTATTAT-3′; CpG81-R, 5′-ATC
AAAATTCCTACCTCCCAACTA-3′; CpG26-F, 5′-TGTTTG
TGTTAGTAGGTGGAAGTGT-3′; CpG26-R, 5′-CCTCTA
AACAATTTCTACCCTAACC-3′.
Results
Transcriptional activity of the
miR-1-133a cluster is controlled by DNA methylation
Expression of miR-1 and miR-133a originated from two
genomic loci in chromosome 18 and chromosome 20. This study
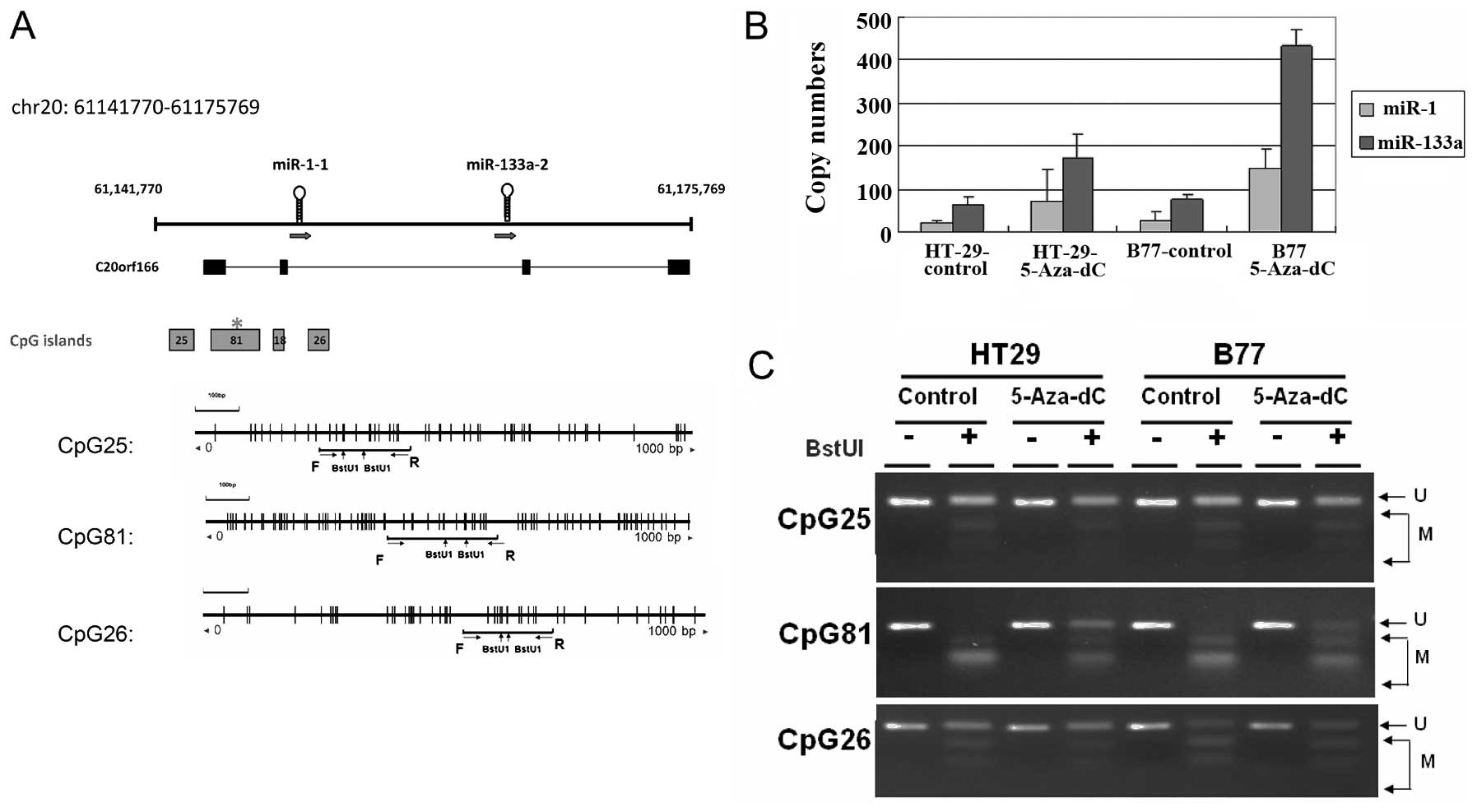
investigated the DNA methylation alterations to regions upstream of
miR-1 and miR-133a. Searches of the UCSC database identified 4 CpG
islands located at the putative transcription start sites of the
miR-1-1 and miR-133a-2 loci, which suggested that DNA methylation
might control their transcriptional activities (Fig. 1A). The methylation status of 3
CpG-rich regions in 2 human CRC cell lines was analyzed using a
COBRA approach. Complete hypermethylation of CpG of the CpG81
occurred in 2 human CRC cell lines (Fig. 1C). After 4 days of treatment with
5-Aza-dC, the transcriptional activities of miR-1 and miR-133a had
reactivated in examined cells (Fig.
1B). These results indicated that the human miR-1-133a cluster
can be regulated epigenetically by DNA methylation in CRC
cells.
Concurrent silencing of miR-1 and
miR-133a by DNA hypermethylation in colon cancer
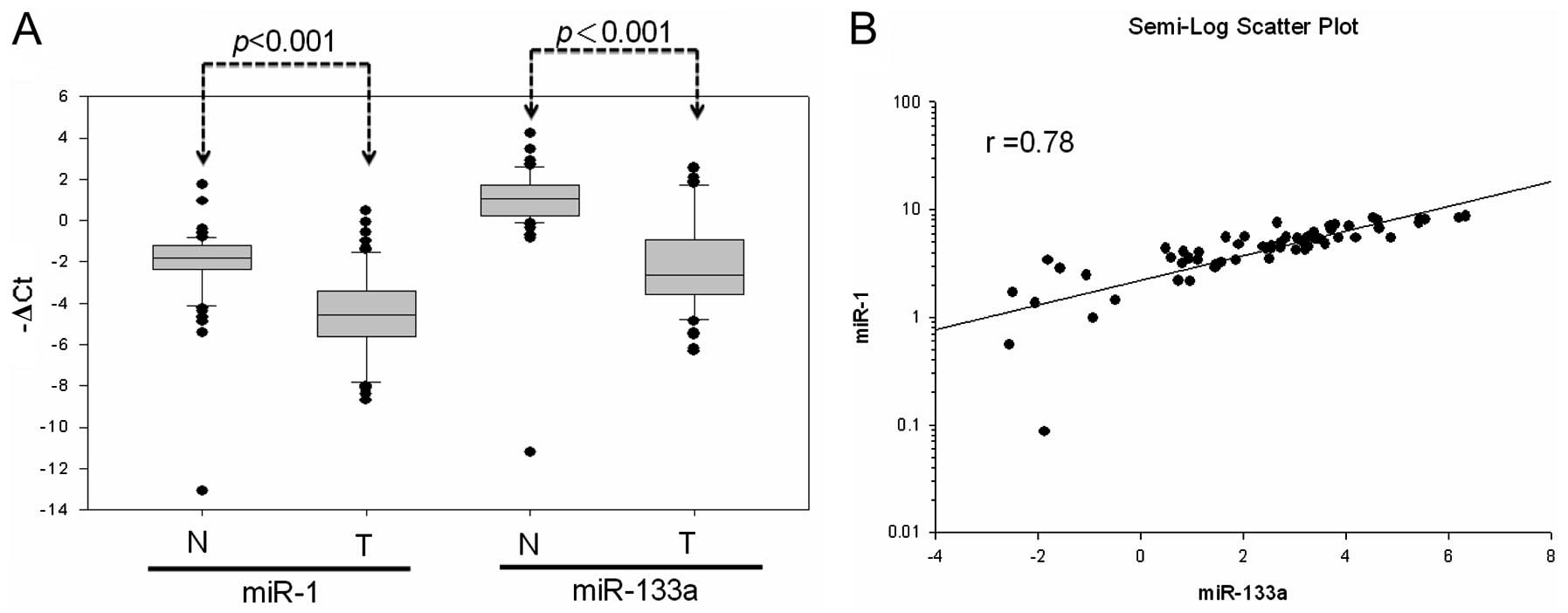
Further analysis of miR-1 and miR-133a expression in
primary CRC tissues of 64 patients revealed the significant
downregulation of mature miR-1 and miR-133a expression compared to
expression in normal mucosa (miR-1, 55 out of 64; miR-133a, 56 out
of 64) (Fig. 2A). Simultaneous
miR-1 and miR-133a downregulation occurred at a high frequency in
the examined samples (84.3%; 54 out of 64). This showed that miR-1
expression well-correlated with that of miR-133a in the 64 CRC
patients (r=0.78) (Fig. 2B). These
results suggested that DNA hypermethylation led to low miR-1-133a
cluster expression in colon cancer cells. Further analyses
evaluated whether downregulation of the miR-1-133a cluster resulted
from DNA hypermethylation of CpG-rich regions upstream of miR-1-1
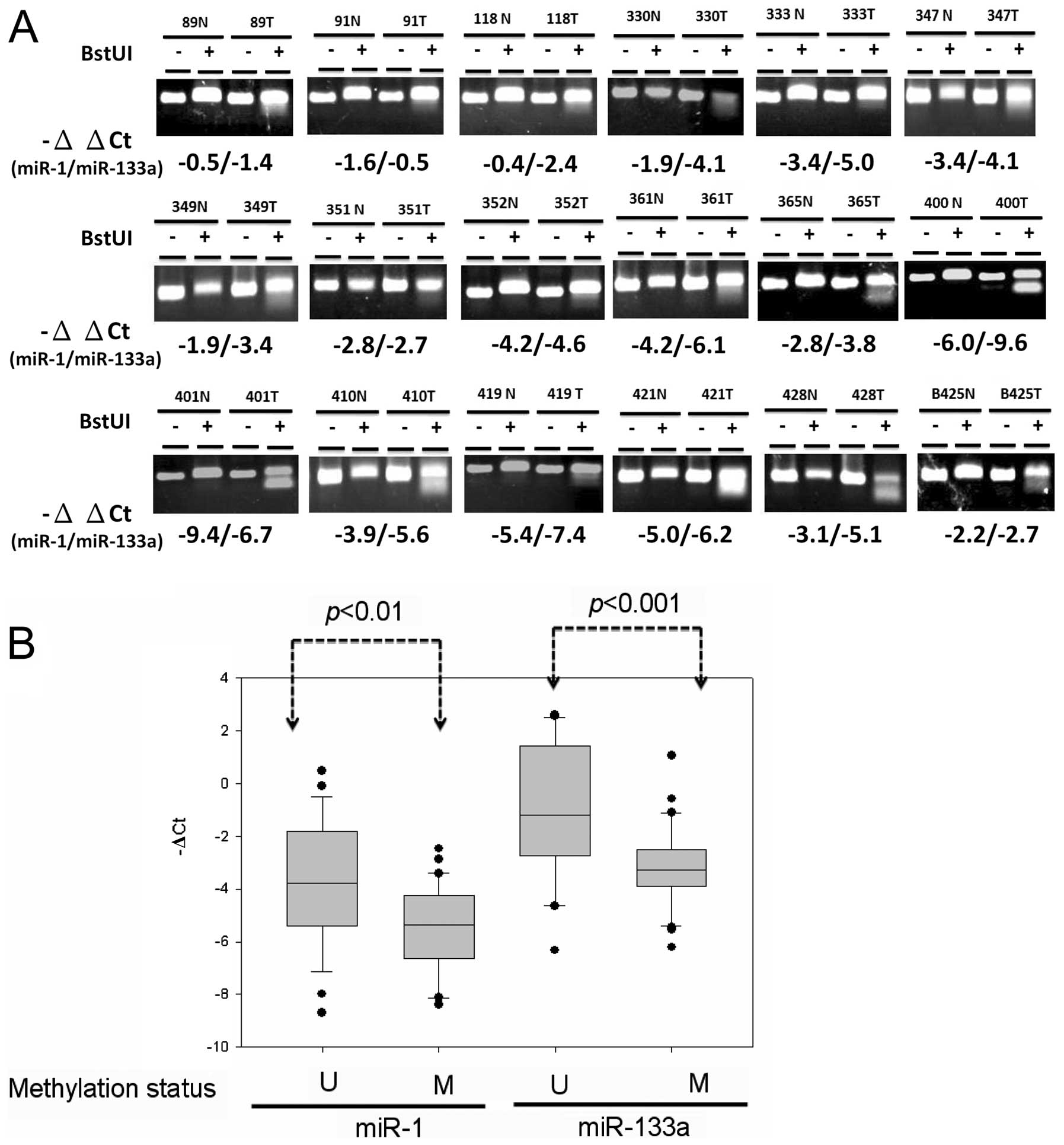
and miR-133a-2. Analysis of the methylation status of CpG81 using
COBRA analysis revealed that tumor-specific DNA methylation of
CpG81 occurred in 35 out of 64 (54.6%) of the primary CRC samples
(Fig. 3A). Expression of miR-1
(P<0.01) and miR-133a (P<0.001) was significantly reduced in
DNA hypermethylation compared with DNA hypomethylation cases of
primary colon cancer tissues (Fig.
3B). These results indicated that abnormal DNA hypermethylation
concurrently silenced miR-1 and miR-133a expression in CRC.
Expression of the miR-1-133a cluster is
associated with colon cancer metastasis
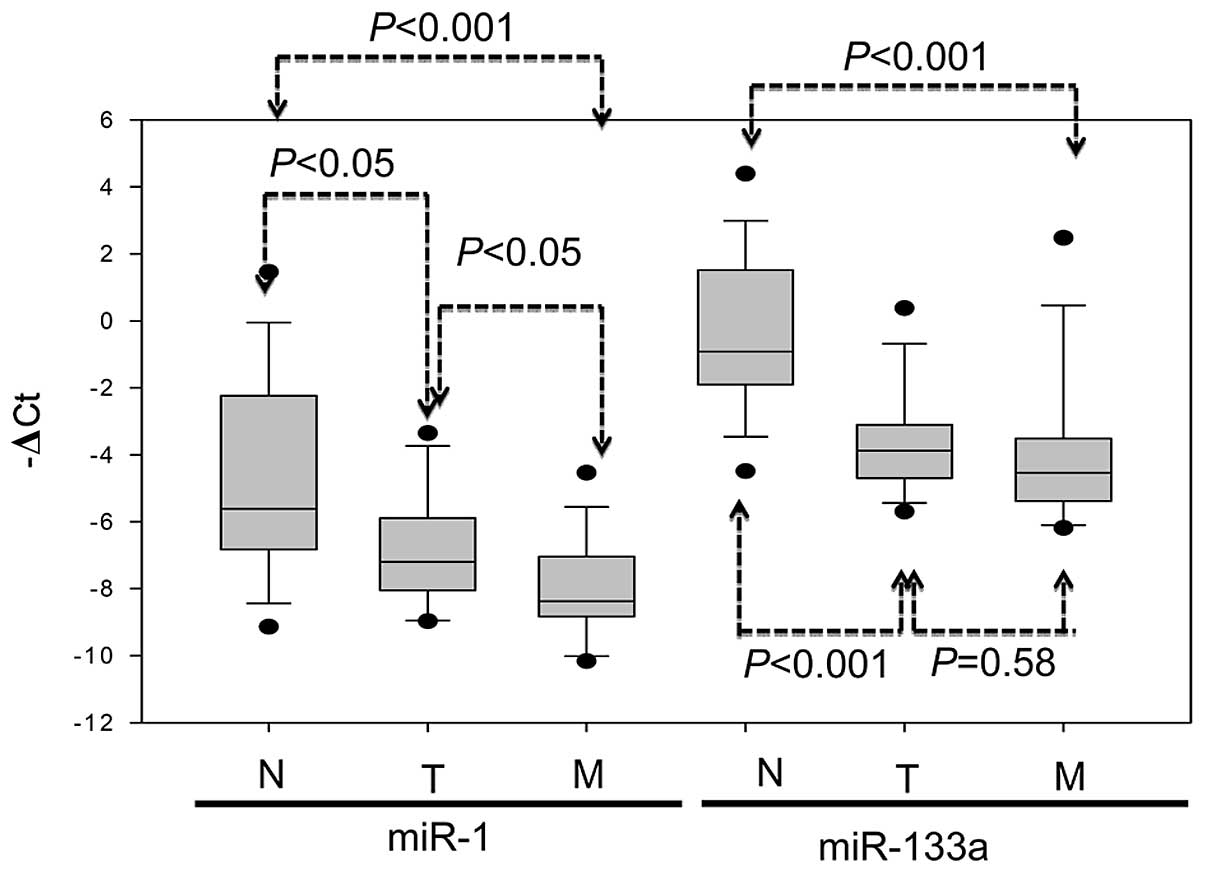
Expression of the miR-1-133a cluster in primary
tumor, metastatic liver tumor and the corresponding normal mucosa
of 14 CRC patients was evaluated using real-time PCR analysis.
Relatively lower miR-1 expression occurred in 10 of 14 (71.4%)
primary tumors and in 12 of 14 (85.7%) metastatic liver tumors in
comparison to the corresponding normal mucosa from the same
patients. Similarly, significantly lower miR-133a expression
occurred in 12 of 14 (85.7%) primary tumors and in 14 of 14 (100%)
metastatic liver tumors in comparison to the corresponding normal
mucosa. Expression of miR-1 was significantly lower in the primary
tumor (P<0.05) and metastatic liver tumor (P<0.001) than in
adjacent normal mucosa; miR-133a expression was also significantly
lower in the primary tumor (P<0.001) and metastatic liver tumor
(P<0.001) than in adjacent normal mucosa (Fig. 4). These data suggested the
involvement of miR-1-133a cluster expression in CRC metastasis.
Reduced repression of TAGLN2 resulting
from DNA hypermethylation of miR-1 and miR-133a
Identification of the target genes of miR-1 and
miR-133a is important for the investigation of their biological
functions. The previously described data indicated that DNA
hypermethylation concurrently silenced miR-1 and miR-133a
expression in the CRC genome, and that low expression of miR-1 and
miR-133a might contribute to CRC invasion and metastasis. This
suggests that miR-1 and miR-133a might share similar biological
functions in the suppression of cancer cell migration during CRC
progression. The putative targets of miR-1 and miR-133a were
identified using the target prediction program (http://www.ebi.ac.uk/enright-srv/microcosm/htdocs/targets/v5/).
As shown in Table I, this provided
49 putative targets for simultaneous silencing by miR-1 and
miR-133a. The targets TAGLN2 and LASP1, which may be involved in
promoting cancer migration, were selected for further analysis
(27,28). Previous studies have shown that
miR-1 and miR-133a can repress the expression of TAGLN2 and LASP1
by targeting the 3′UTR region (20,24).
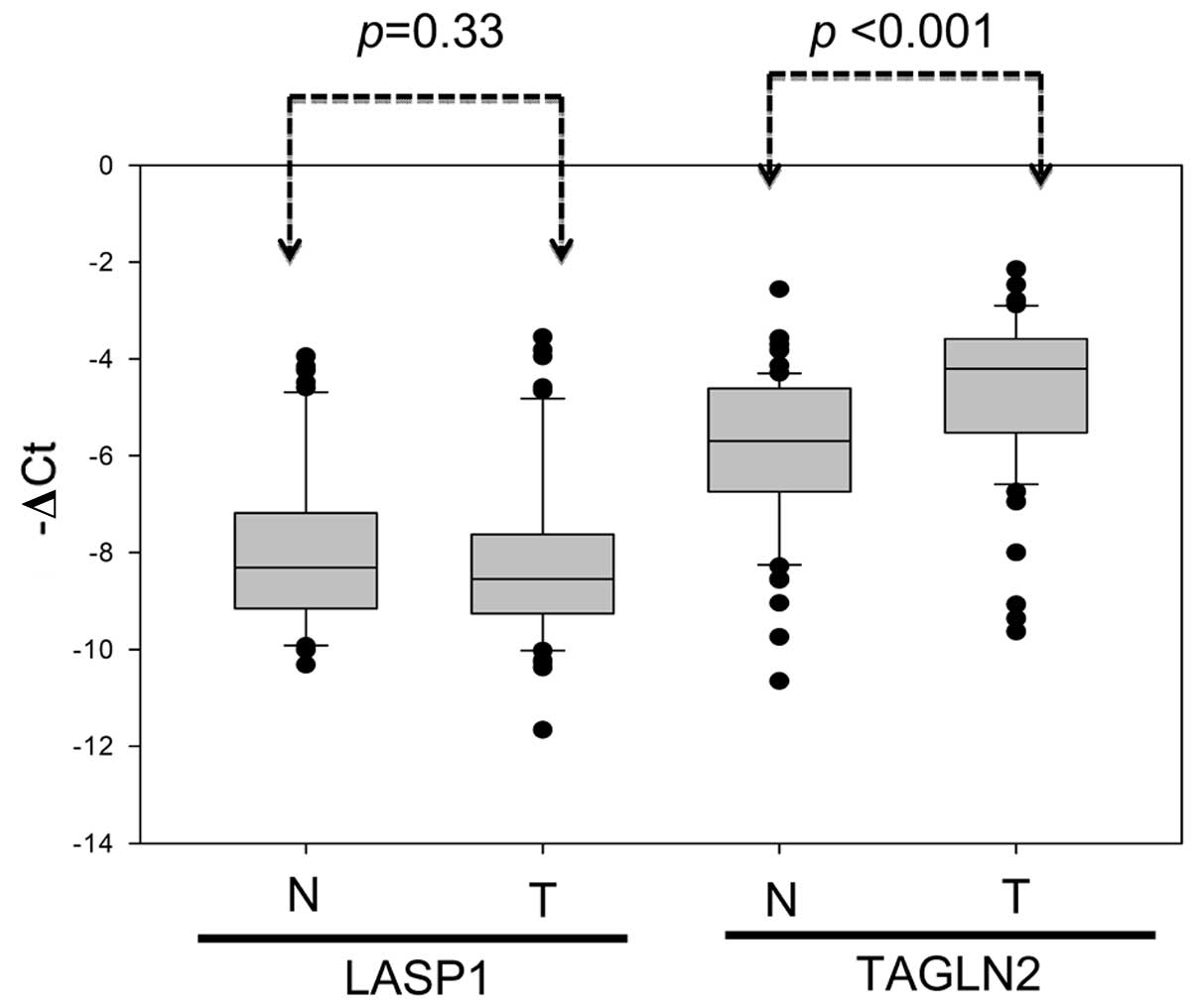
Expression of TAGLN2 and LASP1 in paired adjacent normal and
gastric tumor tissues from 64 patients was, thus, further examined
using real-time quantitative PCR (Fig.
5). Results indicated an inverse correlation between TAGLN2
mRNA and miR-1-133a cluster expression in the tumor specimens. The
TAGLN2 mRNA levels were significantly higher in the CRC tissues
than in the adjacent normal mucosa (Fig. 5, P<0.001). In contrast,
miR-1-133a cluster expression was significantly lower in the tumor
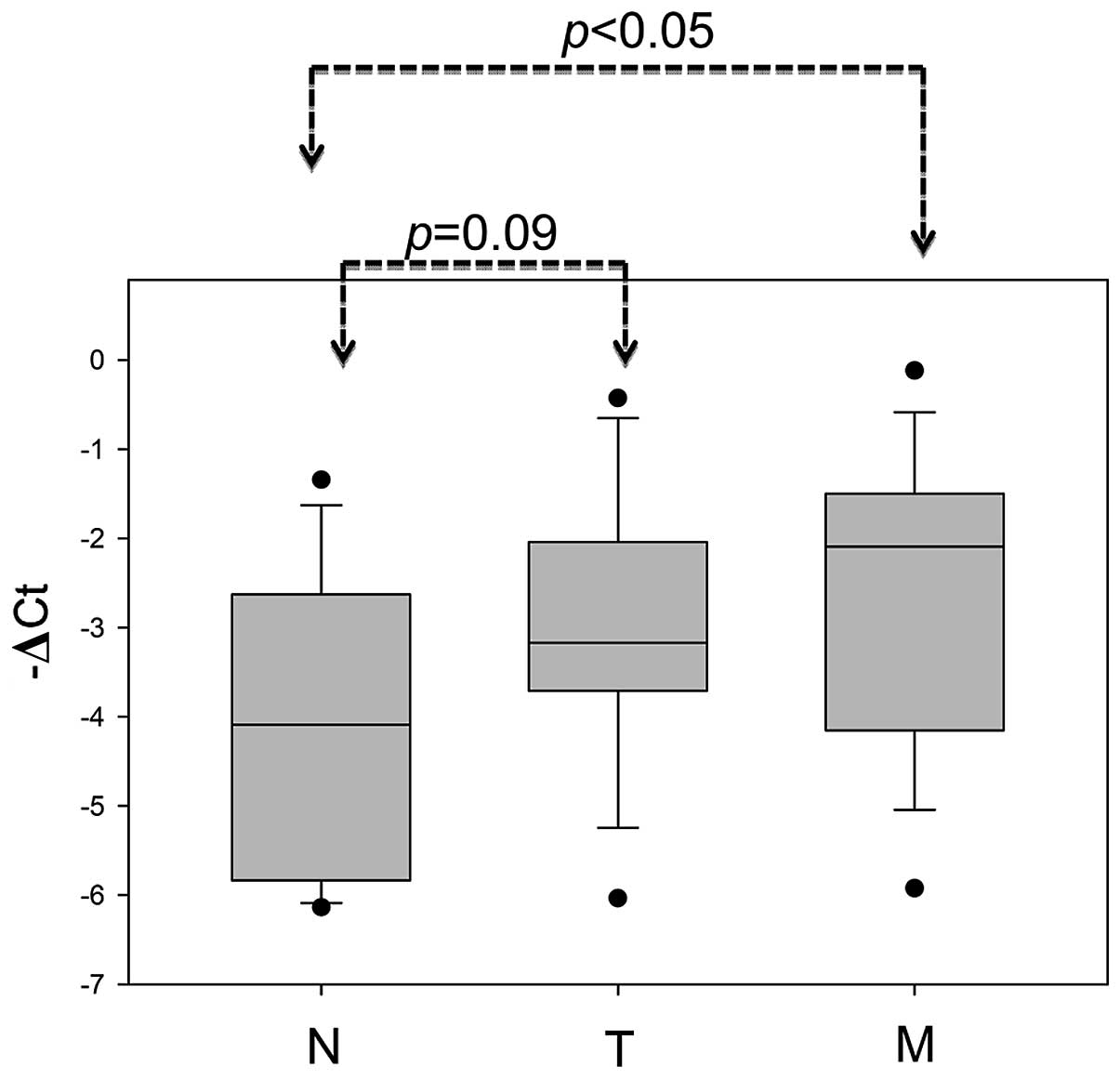
tissues than in normal adjacent mucosa (Fig. 2A; P<0.001). The metastatic liver
tumors also displayed significantly increased TAGLN2 expression in
comparison to the corresponding normal mucosa from the same patient
(Fig. 6; P<0.05). In summary,
DNA hypermethylation in miR-1-1 and miR-133a-2 loci resulted in
their low expression and TAGLN2 overexpression. This process might
have a significant role in colorectal cancer metastasis.
 | Table IIdentify putative targets of
miR-1-133a through target prediction program (MicroCosm Targets
Version 5). |
Table I
Identify putative targets of
miR-1-133a through target prediction program (MicroCosm Targets
Version 5).
| ABL2 | v-abl Abelson
murine leukemia viral oncogene homolog 2 (arg, Abelson-related
gene) |
| ASH1L | ash1 (absent,
small, or homeotic)-like (Drosophila) |
| BCL11A | B-cell CLL/lymphoma
11A (zinc finger protein) |
| CALM1 | Calmodulin 1
(phosphorylase kinase, δ) |
| CAP1 | CAP, adenylate
cyclase-associated protein 1 (yeast) |
| CORO1C | Coronin, actin
binding protein, 1C |
| CREB5 | cAMP responsive
element binding protein 5 |
| CTBP2 | C-terminal binding
protein 2 |
| DDX3X | DEAD
(Asp-Glu-Ala-Asp) box polypeptide 3, X-linked |
| EIF2C1 | Eukaryotic
translation initiation factor 2C, 1 |
| EVI1 | Ecotropic viral
integration site 1 |
| EYA4 | Eyes absent homolog
4 (Drosophila) |
| FOXP1 | Forkhead box
P1 |
| GABBR2 | γ-aminobutyric acid
(GABA) B receptor, 2 |
| GCLC | Glutamate-cysteine
ligase, catalytic subunit |
| HIC2 | Hypermethylated in
cancer 2 |
| HIVEP2 | Human
immunodeficiency virus type I enhancer binding protein 2 |
| LASP1 | LIM and SH3 protein
1 |
| LASS2 | Ceramide synthase
2 |
| LETM1 | Leucine
zipper-EF-hand containing transmembrane protein 1 |
| LIN7C | Lin-7 homolog
C |
| MAP3K3 | Mitogen-activated
protein kinase kinase kinase 3 |
| MEIS1 | Meis homeobox
1 |
| MKL2 | MKL/myocardin-like
2 |
| NFAT5 | Nuclear factor of
activated T-cells 5 |
| PDIK1L | PDLIM1 interacting
kinase 1 like |
| PFN2 | Profilin 2 |
| PIK3C2A |
Phosphoinositide-3-kinase, class 2, α
polypeptide |
| PREX1 |
Phosphatidylinositol-3,4,5-trisphosphate-dependent
Rac exchange factor 1 |
| PTBP1 | Polypyrimidine
tract binding protein 1 |
| QKI | QKI, KH domain
containing, RNA binding |
| RARB | Retinoic acid
receptor, β |
| RICTOR | RPTOR independent
companion of MTOR, complex 2 |
| SGK269 | NKF3 kinase family
member |
| SH3PXD2B | SH3 and PX domains
2B |
| SLC25A36 | Solute carrier
family 25, member 36 |
| SP1 | Sp1 transcription
factor |
| SYT1 | Synaptotagmin
I |
| TAGLN2 | Transgelin 2 |
| TFE3 | Transcription
factor binding to IGHM enhancer 3 |
| TTPAL | Tocopherol (α)
transfer protein-like |
| UBXD8 | Fas associated
factor family member 2 |
| VAMP2 | Vesicle-associated
membrane protein 2 |
| VAT1 | Vesicle amine
transport protein 1 homolog |
| WIPF2 | WAS/WASL
interacting protein family, member 2 |
| YES1 | v-yes-1 Yamaguchi
sarcoma viral oncogene homolog 1 |
| YPEL2 | Yippee-like 2 |
| ZBTB4 | Zinc finger and BTB
domain containing 4 |
| ZNF217 | Zinc finger protein
217 |
Discussion
Colorectal carcinogenesis is a multistep process
involving the genetic dysregulation of oncogenes and tumor
suppressor genes. Recent studies have shown that
microRNA-associated transcriptionally regulated gene expression
plays a critical role in the control of various cellular functions,
such as cell proliferation, cell cycle, apoptosis and cell motility
(5–7). Investigators have described miR-1 and
miR-133a as regulators of muscle cell growth and differentiation,
and identified that abnormal miR-1-133a cluster expression
contributes to cardiovascular disease (29–31).
Recent studies have also described downregulation of miR-1
expression and its tumor suppressor activity in human cancers,
including prostate cancer, hepatocellular carcinoma, bladder
urothelial carcinoma, esophageal squamous cell carcinoma, lung
cancer, rhabdomyosarcoma and colorectal carcinoma. In addition,
miR-1 acts as a tumor suppressor by suppressing oncotargets in
human cancer, including Met, Foxp1, HDAC4, F-actin, TAGLN2, SRSF9,
fibronectin and LASP1 (25,32–37).
Several studies have identified the downregulation of miR-133a
expression in cancers and suggested that miR-133a has a tumor
suppressive function in esophageal squamous cell carcinoma and
bladder, head, breast and lung cancers by repressing FSCN1, GSTP1,
MSN, ARPC5 and CAV1 (38–42). Some studies have also reported the
concurrent silencing of miR-1 and miR-133a in prostate cancer,
squamous cell carcinoma and bladder cancer (20–22,24).
The restoration of miR-1 and miR-133a in the bladder cells led to
significant inhibition of cell proliferation and migration by way
of repression of TAGLN2 and LASP1 expression (20,24).
Nohata et al observed similar trends in squamous cell
carcinoma; identifying that miR-1 and miR-133a overexpression
inhibited cell proliferation and induced apoptosis by regulating
TAGLN2 and PNP (22). In the
present study, miR-1 (P<0.01) and miR-133a (P<0.001)
expression was significantly lower in liver metastatic tissue than
in adjacent normal mucosa, suggesting that these miRNAs are
crucially involved in colorectal cancer metastasis. Results
indicated downregulation of the miR-1-133a cluster expression in
84.3% of the CRC samples (54 out of 64) and TAGLN2 overexpression
in 83.3% of the samples (45 out of 54). The TAGLN2 protein is a
member of the calponin family of actin-binding proteins. Several
cancer types, including HCC, lung cancer and head and neck squamous
cell carcinoma, exhibit TAGLN2 overexpression and promoted cancer
cell migration and invasion (22,27,43,44).
Zhang et al(27) further
reported the correlation between TAGLN2 expression and lymph node
metastasis in colorectal cancer. Overall, these data indicate that
TAGLN2 might have an oncogenic function and promote cancer
migration, which is regulated by the miR-1-133a cluster in CRC.
Aberrant DNA methylation is a common feature of
colorectal carcinoma, leading to aberrant expression of tumor
suppressive miRNAs through the hypermethylation of their promoters.
Datta et al first observed DNA hypermethylation in miR-1-1
in human HCC cells and primary HCC, identifying that ectopic
expression of DNA-hypermethylated miR-1-1 suppressed tumor cell
growth by targeting MET, FoxP1 and HDAC4 (25). Hudson et al(32) also demonstrated the epigenetic
silencing of miR-1 in human prostate cancer following 5-Aza-dC
treatment. In the present study, results indicated the concurrent
downregulation of miR-1 and miR-133a expression in colon cancer
tissue compared to adjacent normal mucosa. Tumor genomic DNA
displayed a high frequency of DNA hypermethylation compared with
that of the adjacent normal mucosa (53.2%; Fig. 3). During the preparation of this
manuscript, Suzuki et al(45) and Migliore et al(33) both reported the frequent methylation
of miR-1-1 in colorectal cancer, in which it might function as a
tumor suppressor.
In conclusion, detailed analyses of DNA methylation
status in the miR-1-1 and miR-133a-2 upstream regions identified
that DNA hypermethylation concurrently represses miR-1 and miR-133a
expression. This reduces the microRNA-associated repression of
TAGLN2 in colorectal carcinoma. In the future, DNA hypermethylation
of the miR-1-133a cluster, along with identification of their
target genes, could provide a promising strategy for the treatment
of patients with CRC.
Acknowledgements
This study was supported in part by research funding
from National Sciences Council (NSC 99-2320-B-010-021) and
Kaohsiung Veterans General Hospital (VGHKS 101-010 and
VGHKS101-118).
References
|
1
|
Shike M, Winawer SJ, Greenwald PH, Bloch
A, Hill MJ and Swaroop SV: Primary prevention of colorectal cancer.
The WHO Collaborating Centre for the Prevention of Colorectal
Cancer Bull World Health Organ. 68:377–385. 1990.
|
|
2
|
Sasaki H, Miura K, Horii A, et al:
Orthotopic implantation mouse model and cDNA microarray analysis
indicates several genes potentially involved in lymph node
metastasis of colorectal cancer. Cancer Sci. 99:711–719. 2008.
View Article : Google Scholar
|
|
3
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu WK, Law PT, Lee CW, et al: MicroRNA in
colorectal cancer: from benchtop to bedside. Carcinogenesis.
32:247–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Babashah S and Soleimani M: The oncogenic
and tumour suppressive roles of microRNAs in cancer and apoptosis.
Eur J Cancer. 47:1127–1137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lima RT, Busacca S, Almeida GM, Gaudino G,
Fennell DA and Vasconcelos MH: MicroRNA regulation of core
apoptosis pathways in cancer. Eur J Cancer. 47:163–174. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilmott JS, Zhang XD, Hersey P and Scolyer
RA: The emerging important role of microRNAs in the pathogenesis,
diagnosis and treatment of human cancers. Pathology. 43:657–671.
2011.PubMed/NCBI
|
|
9
|
Luo X, Burwinkel B, Tao S and Brenner H:
MicroRNA signatures: novel biomarker for colorectal cancer? Cancer
Epidemiol Biomarkers Prev. 20:1272–1286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dhayat S, Mardin WA, Mees ST and Haier J:
Epigenetic markers for chemosensitivity and chemoresistance in
pancreatic cancer - a review. Int J Cancer. 129:1031–1041. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim TY, Jong HS, Jung Y, Kim TY, Kang GH
and Bang YJ: DNA hypermethylation in gastric cancer. Aliment
Pharmacol Ther. 20(Suppl 1): S131–S142. 2004. View Article : Google Scholar
|
|
12
|
Oue N, Kuraoka K, Kuniyasu H, et al: DNA
methylation status of hMLH1, p16(INK4a), and CDH1 is not associated
with mRNA expression levels of DNA methyltransferase and DNA
demethylase in gastric carcinomas. Oncol Rep. 8:1085–1089.
2001.PubMed/NCBI
|
|
13
|
Momparler RL: Epigenetic therapy of cancer
with 5-aza-2′-deoxycytidine (decitabine). Semin Oncol. 32:443–451.
2005.
|
|
14
|
Kozaki K, Imoto I, Mogi S, Omura K and
Inazawa J: Exploration of tumor-suppressive microRNAs silenced by
DNA hypermethylation in oral cancer. Cancer Res. 68:2094–2105.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lewis A, Mitsuya K, Umlauf D, et al:
Imprinting on distal chromosome 7 in the placenta involves
repressive histone methylation independent of DNA methylation. Nat
Genet. 36:1291–1295. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Balaguer F, Link A, Lozano JJ, et al:
Epigenetic silencing of miR-137 is an early event in colorectal
carcinogenesis. Cancer Res. 70:6609–6618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bandres E, Agirre X, Bitarte N, et al:
Epigenetic regulation of microRNA expression in colorectal cancer.
Int J Cancer. 125:2737–2743. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Toyota M, Suzuki H, Sasaki Y, et al:
Epigenetic silencing of microRNA-34b/c and B-cell translocation
gene 4 is associated with CpG island methylation in colorectal
cancer. Cancer Res. 68:4123–4132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lujambio A, Ropero S, Ballestar E, et al:
Genetic unmasking of an epigenetically silenced microRNA in human
cancer cells. Cancer Res. 67:1424–1429. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chiyomaru T, Enokida H, Kawakami K, et al:
Functional role of LASP1 in cell viability and its regulation by
microRNAs in bladder cancer. Urol Oncol. Sep 14–2010.(Epub ahead of
print).
|
|
21
|
Kojima S, Chiyomaru T, Kawakami K, et al:
Tumour suppressors miR-1 and miR-133a target the oncogenic function
of purine nucleoside phosphorylase (PNP) in prostate cancer. Br J
Cancer. 106:405–413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nohata N, Hanazawa T, Kikkawa N, et al:
Identification of novel molecular targets regulated by tumor
suppressive miR-1/miR-133a in maxillary sinus squamous cell
carcinoma. Int J Oncol. 39:1099–1107. 2011.PubMed/NCBI
|
|
23
|
Rao PK, Missiaglia E, Shields L, et al:
Distinct roles for miR-1 and miR-133a in the proliferation and
differentiation of rhabdomyosarcoma cells. FASEB J. 24:3427–3437.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoshino H, Chiyomaru T, Enokida H, et al:
The tumour-suppressive function of miR-1 and miR-133a targeting
TAGLN2 in bladder cancer. Br J Cancer. 104:808–818. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Datta J, Kutay H, Nasser MW, et al:
Methylation mediated silencing of MicroRNA-1 gene and its role in
hepatocellular carcinogenesis. Cancer Res. 68:5049–5058. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen C, Ridzon DA, Broomer AJ, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acids Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Ye Y, Shen D, et al:
Identification of transgelin-2 as a biomarker of colorectal cancer
by laser capture microdissection and quantitative proteome
analysis. Cancer Sci. 101:523–529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao L, Wang H, Liu C, et al: Promotion of
colorectal cancer growth and metastasis by the LIM and SH3 domain
protein 1. Gut. 59:1226–1235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuwabara Y, Ono K, Horie T, et al:
Increased microRNA-1 and microRNA-133a levels in serum of patients
with cardiovascular disease indicate myocardial damage. Circ
Cardiovasc Genet. 4:446–454. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He B, Xiao J, Ren AJ, et al: Role of miR-1
and miR-133a in myocardial ischemic postconditioning. J Biomed Sci.
18:222011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bostjancic E, Zidar N, Stajer D and Glavac
D: MicroRNAs miR-1, miR-133a, miR-133b and miR-208 are dysregulated
in human myocardial infarction. Cardiology. 115:163–169. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hudson RS, Yi M, Esposito D, et al:
MicroRNA-1 is a candidate tumor suppressor and prognostic marker in
human prostate cancer. Nucleic Acids Res. 40:3689–3703. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Migliore C, Martin V, Leoni VP, et al:
MiR-1 downregulation cooperates with MACC1 in promoting MET
overexpression in human colon cancer. Clin Cancer Res. 18:737–747.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nasser MW, Datta J, Nuovo G, et al:
Down-regulation of micro-RNA-1 (miR-1) in lung cancer. Suppression
of tumorigenic property of lung cancer cells and their
sensitization to doxorubicin-induced apoptosis by miR-1. J Biol
Chem. 283:33394–33405. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sarver AL, French AJ, Borralho PM, et al:
Human colon cancer profiles show differential microRNA expression
depending on mismatch repair status and are characteristic of
undifferentiated proliferative states. BMC Cancer. 9:4012009.
View Article : Google Scholar
|
|
36
|
Yan D, da Dong XE, Chen X, et al:
MicroRNA-1/206 targets c-Met and inhibits rhabdomyosarcoma
development. J Biol Chem. 284:29596–29604. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yoshino H, Enokida H, Chiyomaru T, et al:
Tumor suppressive microRNA-1 mediated novel apoptosis pathways
through direct inhibition of splicing factor serine/arginine-rich 9
(SRSF9/SRp30c) in bladder cancer. Biochem Biophys Res Commun.
417:588–593. 2012. View Article : Google Scholar
|
|
38
|
Kano M, Seki N, Kikkawa N, et al: miR-145,
miR-133a and miR-133b: Tumor-suppressive miRNAs target FSCN1 in
esophageal squamous cell carcinoma. Int J Cancer. 127:2804–2814.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ma Y, Zhang P, Yang J, Liu Z, Yang Z and
Qin H: Candidate microRNA biomarkers in human colorectal cancer:
Systematic review profiling studies and experimental validation.
Int J Cancer. 130:2077–2087. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Moriya Y, Nohata N, Kinoshita T, et al:
Tumor suppressive microRNA-133a regulates novel molecular networks
in lung squamous cell carcinoma. J Hum Genet. 57:38–45. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nohata N, Hanazawa T, Kikkawa N, et al:
Caveolin-1 mediates tumor cell migration and invasion and its
regulation by miR-133a in head and neck squamous cell carcinoma.
Int J Oncol. 38:209–217. 2011.PubMed/NCBI
|
|
42
|
Wu ZS, Wang CQ, Xiang R, et al: Loss of
miR-133a expression associated with poor survival of breast cancer
and restoration of miR-133a expression inhibited breast cancer cell
growth and invasion. BMC Cancer. 12:512012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rho JH, Roehrl MH and Wang JY: Tissue
proteomics reveals differential and compartment-specific expression
of the homologs transgelin and transgelin-2 in lung adenocarcinoma
and its stroma. J Proteome Res. 8:5610–5618. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Leung WK, Ching AK, Chan AW, et al: A
novel interplay between oncogenic PFTK1 protein kinase and tumor
suppressor TAGLN2 in the control of liver cancer cell motility.
Oncogene. 30:4464–4475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Suzuki H, Takatsuka S, Akashi H, et al:
Genome-wide profiling of chromatin signatures reveals epigenetic
regulation of MicroRNA genes in colorectal cancer. Cancer Res.
71:5646–5658. 2011. View Article : Google Scholar
|