Introduction
Medulloblastoma (MB) is the most common malignant
brain tumors of childhood, accounting for >20% of pediatric
brain tumors. Despite recent advances in the treatment of
medulloblastoma including improved surgical resection techniques,
radiation and chemotherapy (1,2), the
prognosis is still relatively poor in infants. Gene therapy,
particularly suicide gene therapy may offer an attractive approach
for the treatment of these patients. However, gene therapy trials
have often produced poor results. Present delivery systems, such as
adenoviruses or retroviruses, are unable to reach the total cancer
population (3,4). Therefore, the enhancement of the
so-called ‘bystander effect’ (BE) in which tumor cells that are not
transduced with the suicide gene are also eliminated along with
gene-transduced cells may have a significant impact on the
therapeutic efficacy.
Gap-junctional intercellular communication (GJIC) is
an important factor in the cell-to-cell communication in cellular
homeostasis, normal embryonic development (5,6),
differentiation, and the regulation of cellular proliferation
(7). Dysfunctional GJIC is
exhibited in most cancer cells (8).
There is evidence that GJIC is important in at least some
prodrug/suicide gene systems (9,10) by
augmenting BE. GJIC is made up of hemichannels (called connexons)
in the membrane of one cell joined in mirror symmetry with the same
number of hemichannels in the opposing cell membrane, which are
composed of six subunits called connexins (Cxs) (11). Among various Cxs, Cx43 is present in
most tissues or cell types and is reduced in cancer and chemically
transformed cells (12–14). Rosolen et al(15) reported a suboptimal in vivo
effect of the suicide gene therapy in treating MB, which might
partially be explained by a limited BE coupled with a low
expression of Cx43 protein. More importantly, the transfection of
Cx43 was found to increase sensitivity to several chemotherapeutic
agents in human glioblastoma U251 cells (16). Our previous study showed that Cx43
expression is increased when Daoy cells are co-cultured with neural
stem C17.2 cells, which may be one way of augmentating BE (17). A large body of experimental evidence
suggests that apoptosis is regulated by both apoptosis blockers and
apoptosis promoters. Bcl-2, an important apoptosis blocker, is
known to be closely associated with the sensitivity to anticancer
drugs. Elevated levels of Bcl-2 protein in gene-transfection
experiments lead to the increased resistance to a wide variety of
chemotherapeutic drugs as well as radiation (18–20).
Therefore, identification of an effective agent which may influence
both Cx43 and Bcl-2 in the treatment of MB is needed.
In the present study, we investigated the effect of
dibutyryl cyclic adenosine monophosphate (db-cAMP) on BE and
chemosensitivity in human medulloblastoma Daoy cells. Our results
showed that db-cAMP upregulates Cx43 protein expression and thus
the GJIC function, which may possibly result in the enhancement of
BE in the herpes simplex virus thymidine kinase
(HSV-tk)/ganciclovir (GCV) system. Meanwhile, db-cAMP increased the
cytotoxicity of temozolomide and teniposide in Daoy cells, possibly
by downregulating the Bcl-2 expression and increasing
apoptosis.
Materials and methods
Cell culture
D283Med, Daoy and D341Med cells (originally obtained
from the American Type Culture Collection, ATCC) were maintained in
an Advanced-Minimal Essential medium (Sigma-Aldrich, St. Louis, MO,
USA) at 37°C under 5% CO2, supplemented with 10% fetal
bovine serum, 2 mmol/l L-glutamine, 2 mmol/l sodium pyruvate, 100
U/ml penicillin, and 100 μg/ml streptomycin. As was indicated,
db-cAMP (Sigma-Aldrich) was added at a final concentration of 0.5
mmol/l and gossypol at a final concentration of 5 μmol/l, both of
which, based on the result of preliminary study, have no toxic
effect on Daoy cells.
Western blot analysis
Cell lysis from different cell lines or brain
tissues were extracted as usual, separated by SDS-polyacrylamide
gel and transferred to polyvinylidene difluoride membranes
(Millipore, Bedford, MA, USA). The membranes were then incubated
with an anti-Cx43 or Bcl-2 monoclonal antibody (Sigma-Aldrich)
diluted to 1:200, followed by incubation with a rabbit anti-mouse
horseradish peroxidase conjugated IgG. The ECL western blot
analysis kit (Amersham, Italy) was used to observe the results.
Scrape-loading and dye transfer
To assess GJIC function under different conditions,
the scrape-loading and dye transfer (SL/DT) assay was carried out.
Daoy cells were treated with db-cAMP for 12, 24 or 72 h, followed
by incubation with gossypol for another 12 or 48 h. Daoy cells
grown on glass chambers were rinsed with PBS. A scrape through the
monolayer was made with a needle in the presence of 0.5%
hydrophilic dye Lucifer yellow in the extracellular solution. After
incubation for 3 min at room temperature, cells were washed with
PBS and then incubated for another 5 min. Cells were then fixed
with 4% paraformaldehyde and observed under a confocal microscope
(Olympus, Tokyo, Japan) to measure the longest distance of Lucifer
yellow from the scrape.
Retroviral vectors and stable
transfection
The HSV-tk retrovirus-producing cells (PA317, mouse
fibroblast cell line with HSV-tk gene) were obtained from
Genetic Therapy, Inc., (Gaithersburg, MD, USA). To produce a
virus-containing supernatant, the cells were plated in
75-cm2 flasks in DMEM with high glucose and 10%
heat-inactivated foetal calf serum. After 24–48 h of incubation,
the supernatant was collected, filtered and stored at −80°C until
use. Parental Daoy cells were incubated in a 75-cm2
flask and then transferred to 6-well plates. Twenty-four hours
later, 2×105 cells were incubated in a supernatant
containing vectors with 4 μg/ml Polybrene (Sigma-Aldrich) for 24 h
before being cultured in normal medium. The cells were then exposed
to 1 mg/ml G-418 (Life Technologies, Carlsbad, CA, USA) for drug
selections. After 2 weeks of G-418 selection, resistant cells were
obtained for consecutive experiments.
Indirect immunofluorescence assay
Diluted serum (20 μl) (1:10) was added onto slides
containing a monolayer of transfected cells, which were fixed in
acetone for 10 min. The slides were incubated in 0.2% Triton X-100
at room temperature for 10 min, washed three times with
phosphate-buffered saline (PBS) and then maintained in non-immune
serum and incubated at 37°C for another 30 min. After the serum was
removed, the antibody against HSV-tk (diluted 1:100) or Myc
(diluted 1:50) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was
added and incubated at 4°C overnight. After being washed three
times with PBS, the slides were incubated with fluorescein
isothiocyanate (FITC)-labeled rabbit anti-mouse IgG for 30 min.
Nuclei were stained with 2 μg/ml Hoechst 33342 at 37°C for 20 min
and then fluorescence was detected.
RT-PCR
Total RNA was isolated from Daoy cells using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA), and cDNA was synthesized
with the cDNA Synthesis kit (Roche, Basel, Switzerland). The
primers for HSV-tk and β-actin were: HSV-tk 5′-GCGC
GTATGGCTTCGTACCC-3′ (sense) and 5′-TCCTTGCGTGT TTCAGTTAGCCTC-3′
(antisense); β-actin, 5′-TCACCCAC ACTGTGCCCATCTACGA-3′ (sense) and
5′-CAGCGGAACC GCTCATTGCCAATGG-3′ (antisense). PCRs were carried out
under optimized conditions. Agarose electrophoresis (2%) was used
for detection. The integrated density values (IDV) were calculated
with β-actin as an internal control.
MTT assay
An MTT assay was performed to detect the effect of
db-cAMP on BE, GCV cytotoxicity, and the toxicity of various drugs
in Daoy cells. Briefly, 20 μl of MTT was added to treated or
untreated Daoy cells at a final concentration of 5 mg/ml, and cells
were incubated for another 4 h at 37°C and dissolved by 150 μl DMSO
(Sigma-Aldrich). Finally, plates were read on a microplate reader
at 570 nm. For BE, cells were treated with 0.5 mmol/l db-cAMP for
72 h or with 0.5 mmol/l db-cAMP for 24 h, followed by gossypol for
another 48 h, and mixing experiments were carried out and the ratio
of tk+/tk- cells for 50% cell killing was
detected. For GCV toxicity, cells were treated with 100 μmol/l GCV
combined with or without 0.5 mmol/l db-cAMP for 24 or 72 h, and the
ratio of the OD value of db-cAMP-treated cells to that of untreated
cells was calculated to assess cell survival. For drug toxicity
assay, cells were treated with temozolomide or teniposide combined
with or without 0.5 mmol/l db-cAMP or a Bcl-2 family antagonist
(ABT-737, 0.01 μmol/l) and the inhibition concentration of 50% cell
growth (IC50) was calculated for various drugs.
Flow cytometry
To detect apoptosis, Daoy cells treated with db-cAMP
(combined with or without gossypol) were collected and incubated
with propidium iodide (PI) (Sigma-Aldrich) solution for 45 min at
4°C in the dark. Then the cells were analyzed by flow cytometric
analysis using ModFit LT 3.0 software.
Statistical analysis
Data were compared by Mann-Whitney U and
Kruskal-Wallis non-parametric tests. P<0.05 was considered
significant. The statistical process was completed using SPSS 13.0
software.
Results
Influence of db-cAMP on Cx43 expression
and GJIC function in medulloblastoma cells
To elucidate the role of Cx43 in medulloblastoma, we
first examined its expression in a case of primary human MB and
different medulloblastoma cell lines with normal rat brain tissues
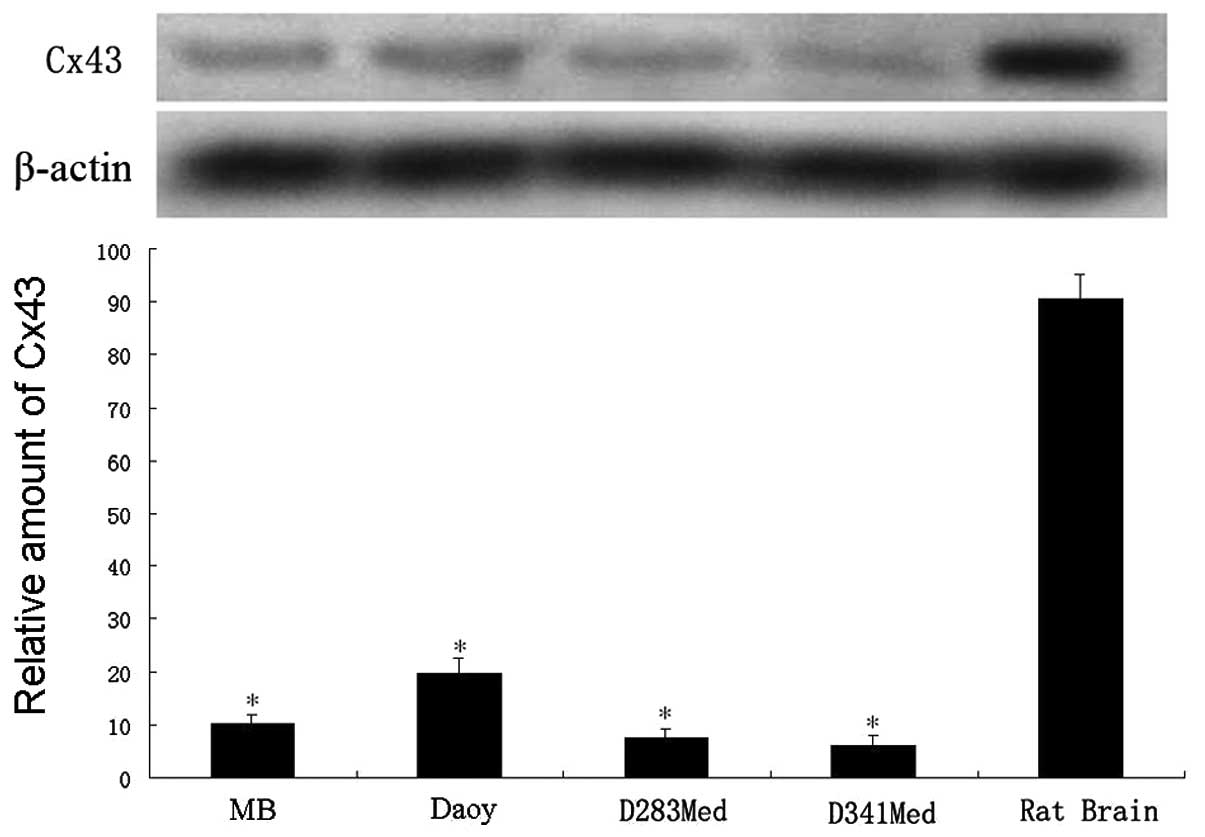
as the control. As expected, the results of western blot analysis
(Fig. 1) showed low expression
levels of Cx43 in MB tissues and in all 3 cell lines (D283Med, Daoy
and D341Med, respectively), indicating the effect of Cx43 on the
development or progression of medulloblastoma. Daoy cells were used
in the following study.
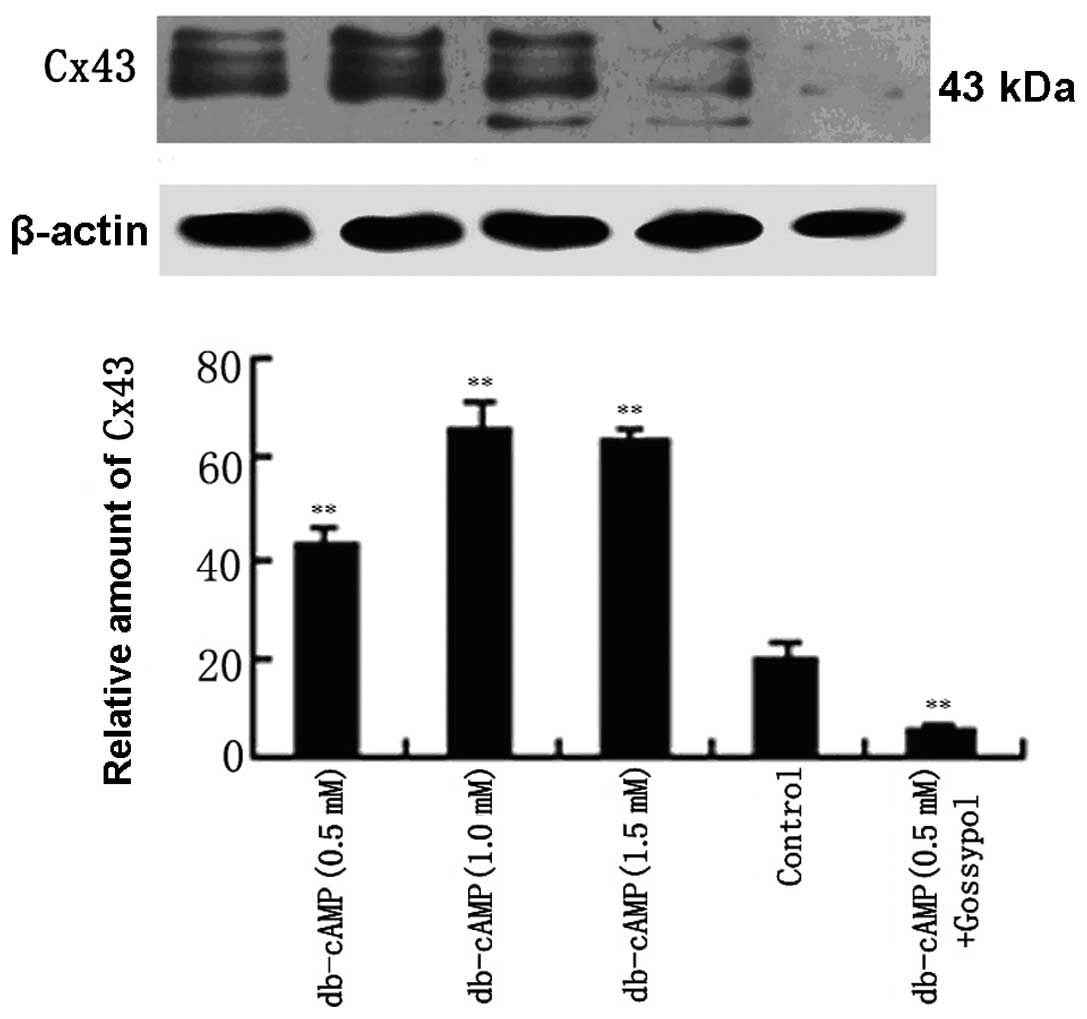
We then detected the changes of Cx43 protein levels
following the treatment of Daoy cells with db-cAMP by western
blotting. As noted in Fig. 2, in
the basal state, Daoy cells expressed a comparatively low level of
Cx43 protein. A non-toxic dose of db-cAMP (0.5 mmol/l) led to an
increase in the Cx43 protein (43 kDa) as well as its phosphorylated
forms (44 and 46 kDa), reaching ~2-fold over the control. When
cells were treated with 1 or 1.5 mmol/l db-cAMP, the Cx43 protein,
as well as its phosphorylation forms, was further increased to
~3-fold of the control. Notably, despite the increasing
concentration of db-cAMP from 1.0 to 1.5 mmol/l the Cx43 protein
was not increased, but slightly reduced. Meanwhile, the Cx43
protein and its phosphorylated forms declined to ~one-fourth of the
control, with the treatment of db-cAMP (0.5 mmol/l) together with
gossypol, a Cx43 inhibitor. These results suggest that db-cAMP
significantly increases the expression of the Cx43 protein and its
phophorylated forms in Daoy cells in a concentration-dependent
manner.
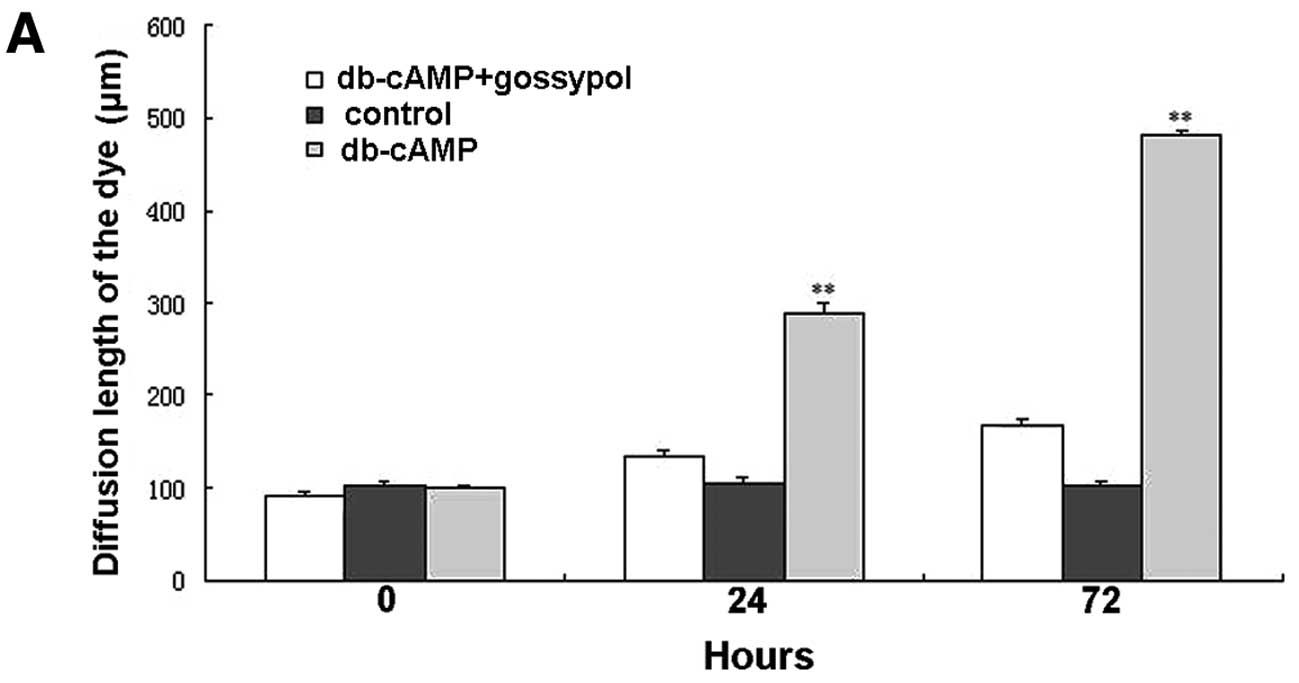
Subsequently, we evaluated the GJIC function of Daoy
cells by conducting a scrape-loading and dye transfer (SL/DT)
assay. Functional GJIC was determined in the intercellular transfer
of Lucifer yellow after using the scrape-loading assay. The
diffusion length of the Lucifer yellow was 501.04±17.76 μm,
measured at 5 min, in cells treated with db-cAMP for 72 h, which
was significantly faster (P<0.01) than 290.22±12.27 μm in cells
treated for 24 h (Fig. 3). Compared
with the control, the GJIC function in cells treated with db-cAMP
was greatly enhanced at both 24 and 72 h (P<0.01), which was
blocked by gossypol, suggesting the involvement of Cx43 in the
effect of db-cAMP on GJIC function.
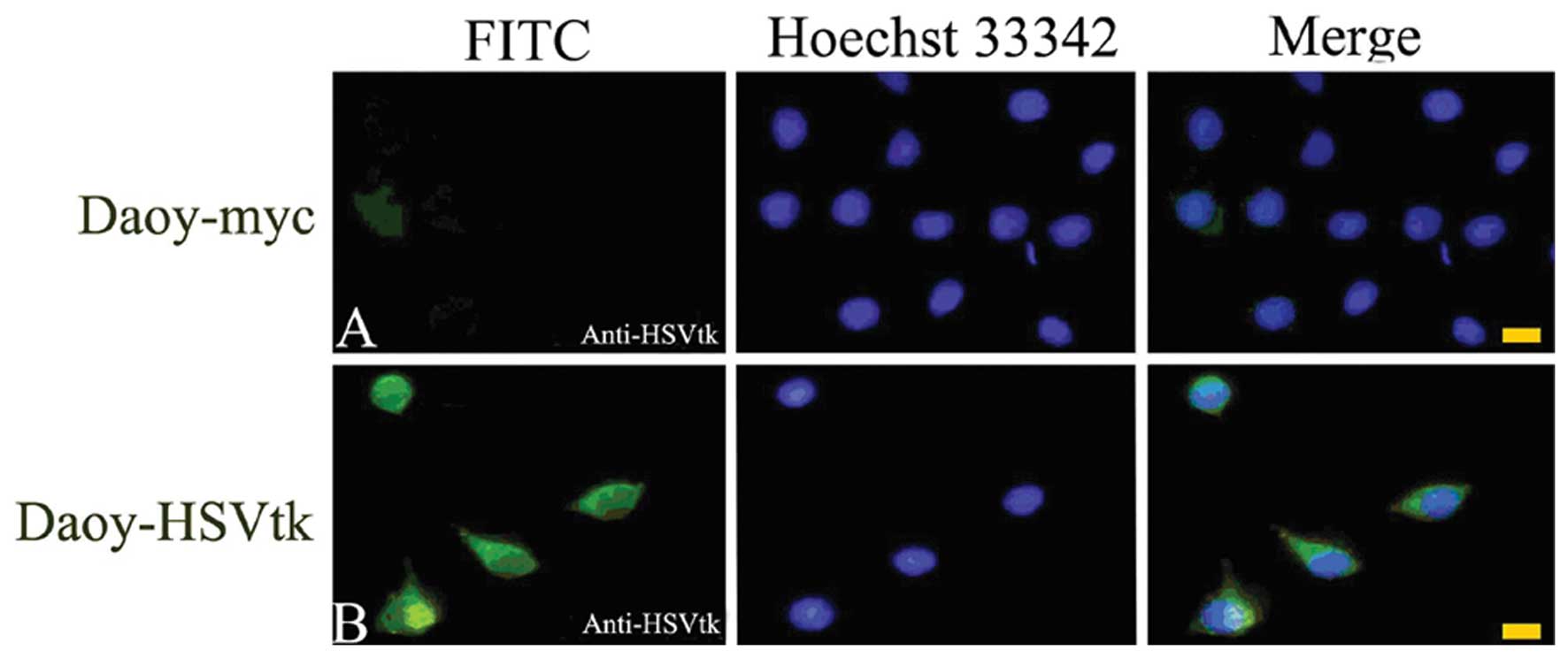
Identification of HSV-tk-transfected
cells
To assess the BE, we transfected Daoy cells with
retroviral vectors containing the HSV-tk gene. The
transfection efficiency was first detected using indirect
immunofluorescence asssy. As shown in Fig. 4, the retroviral vectors were
successfully transfected and HSV-tk was expressed in Daoy
cells.
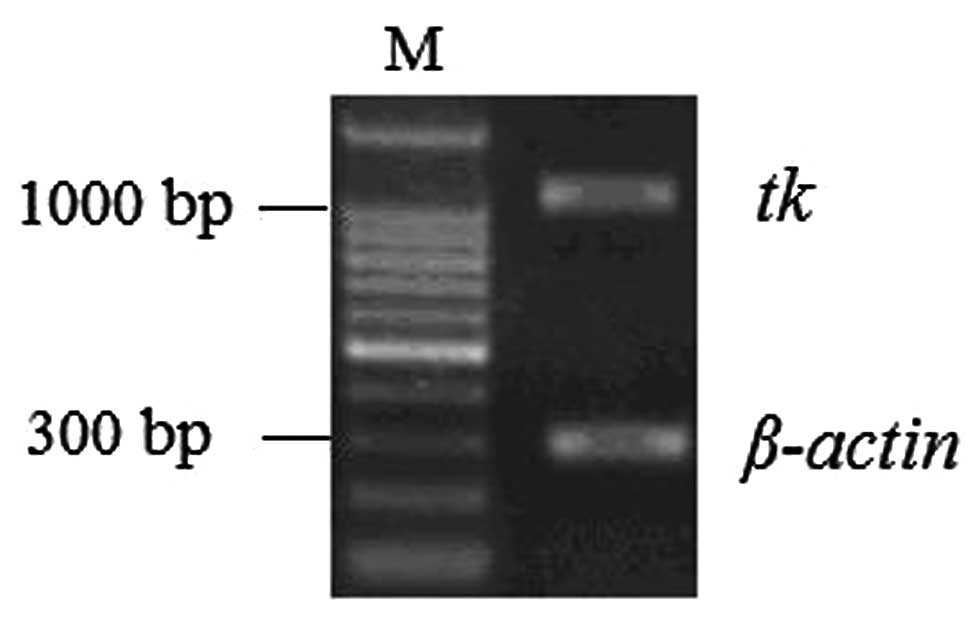
Furthermore, we detected the mRNA expression of the
HSV-tk gene in transfected Daoy cells. RT-PCR results showed
a HSV-tk band (Fig. 5),
suggesting the stable expression of the gene in the cells.
Effect of db-cAMP on BE in the HSV-tk/GCV
system
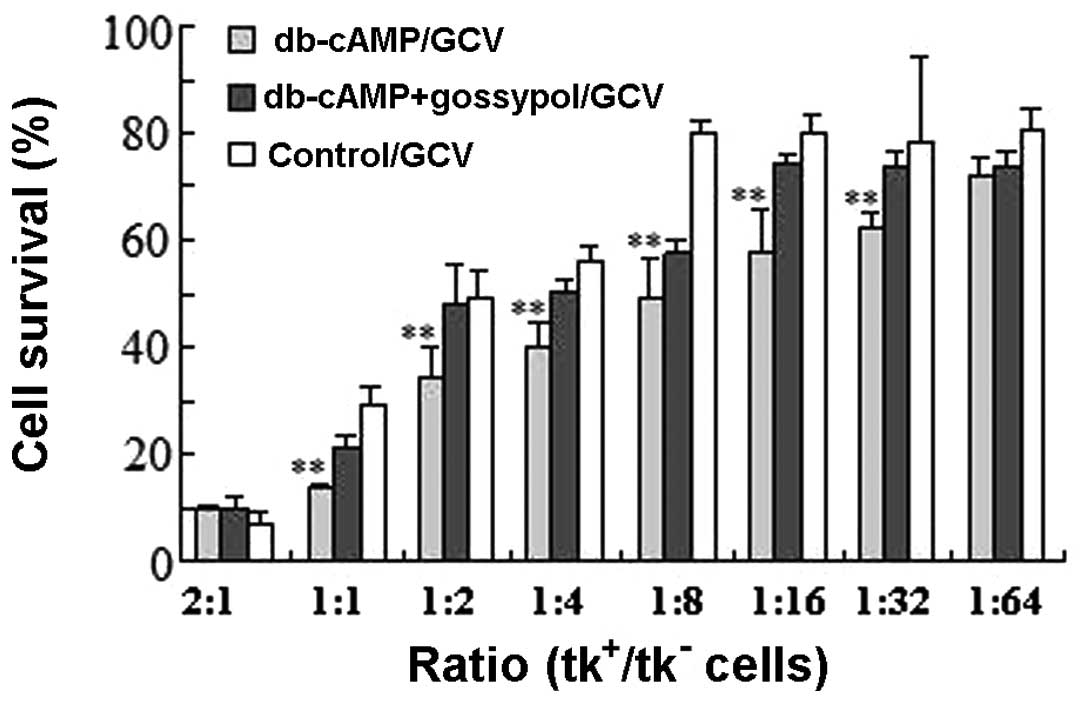
To evaluate the influence of db-cAMP on BE, MTT was
performed. As shown in Fig. 6,
db-cAMP markedly enhanced the BE compared with control cells when
the ratio of tk+/tk- cells was 1:1–1:16
(P<0.05). GCV treatment without db-cAMP obtained 50% cell
killing at the ratio of 1:2 of tk+/tk- cells,
but only the ratio of 1:8 was needed in the presence of db-cAMP
(P<0.05). Significant BE could be observed when the ratio of
tk+/tk- cells was as low as 1:16 in the
presence of db-cAMP, but at the ratio of 1:4 in the absence of
db-cAMP. The cytotoxicity was the same in the presence or absence
of db-cAMP when the ratio of tk+/tk- cells
was 2:1 (P>0.05). Compared with untreated cells, a decrease was
observed in cell killing when Daoy cells were treated with db-cAMP
combined with gossypol, indicating a role of Cx43 in the effect of
db-cAMP on BE.
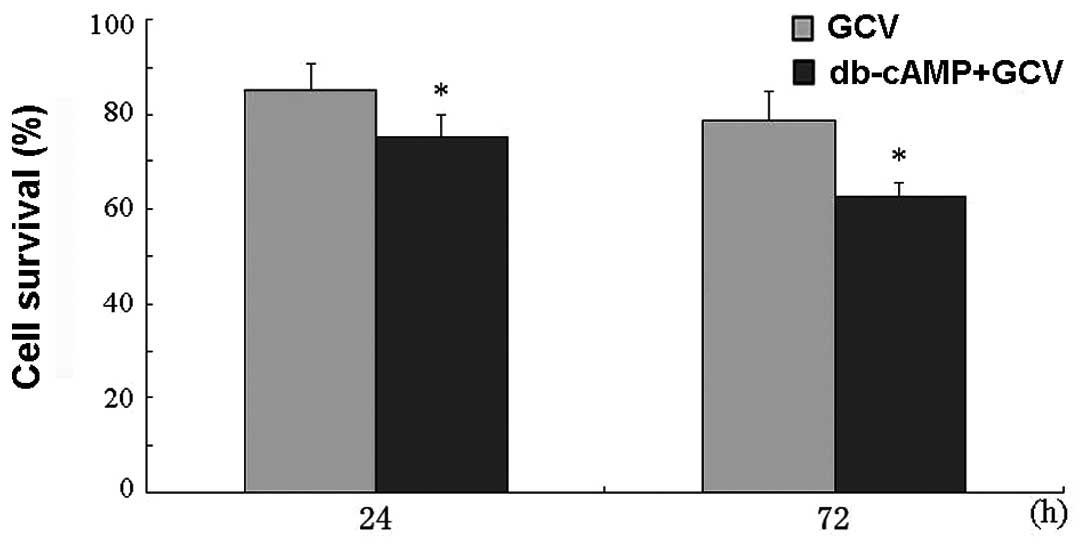
Moreover, we also investigated whether db-cAMP has a
synergistic effect when co-administering Daoy cells with GCV.
Results showed that there was no significant difference in cell
survival between cells with or without db-cAMP treatment for 24 or
72 h after GCV administration (P>0.05) (Fig. 7), which excluded the influence of
db-cAMP on GCV cytotoxicity.
Effect of db-cAMP on Bcl-2 expression and
drug cytotoxicity
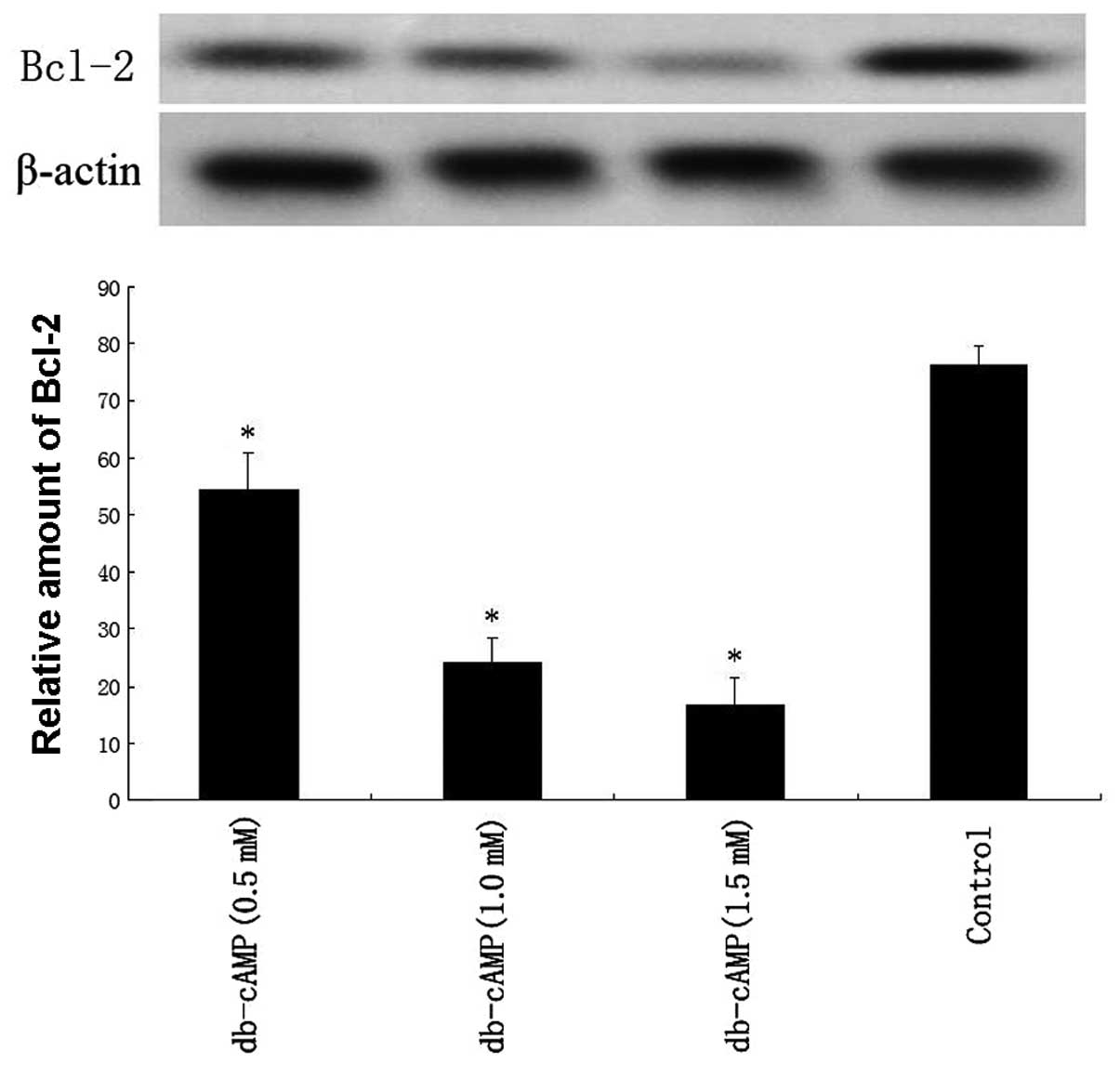
The expression of Bcl-2 protein was detected by
western blot analysis, and results showed that db-cAMP treatment
downregulated the Bcl-2 level in a concentration-dependent manner
in Daoy cells (Fig. 8).
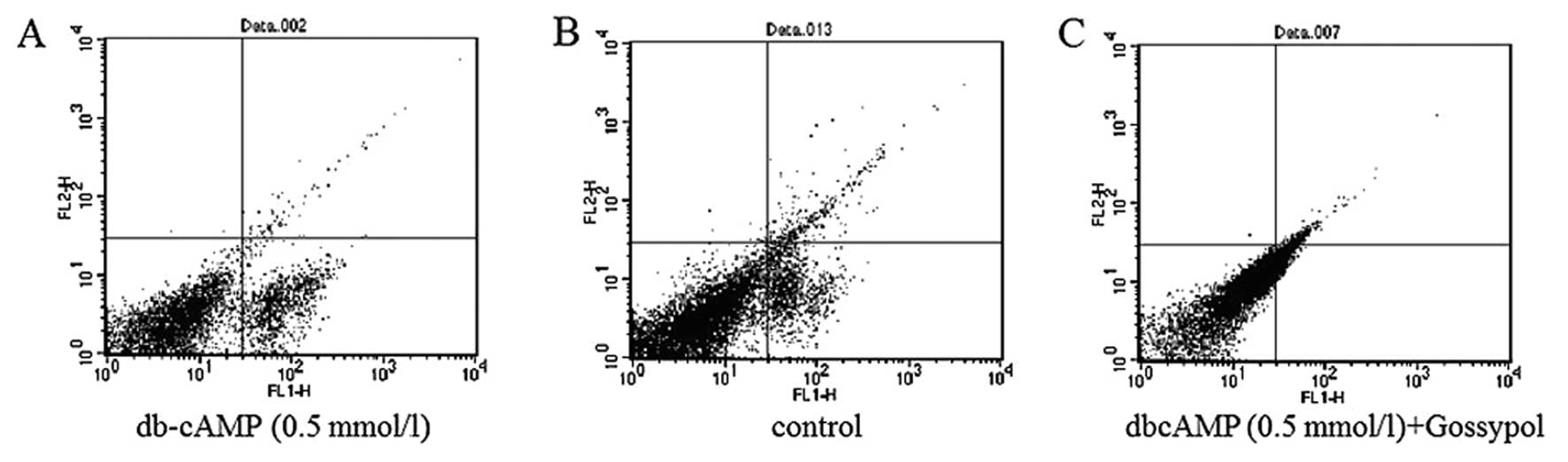
Meanwhile, the results of flow cytometry (Fig. 9) showed db-cAMP greatly increased
the early apoptosis rate of Daoy cells, and when cells were treated
with db-cAMP combined with gossypol, the early apoptosis rate was
only slightly augmented compared with the control. These data
indicated that db-cAMP may induce early apoptosis in Daoy cells,
which was blocked by gossypol.
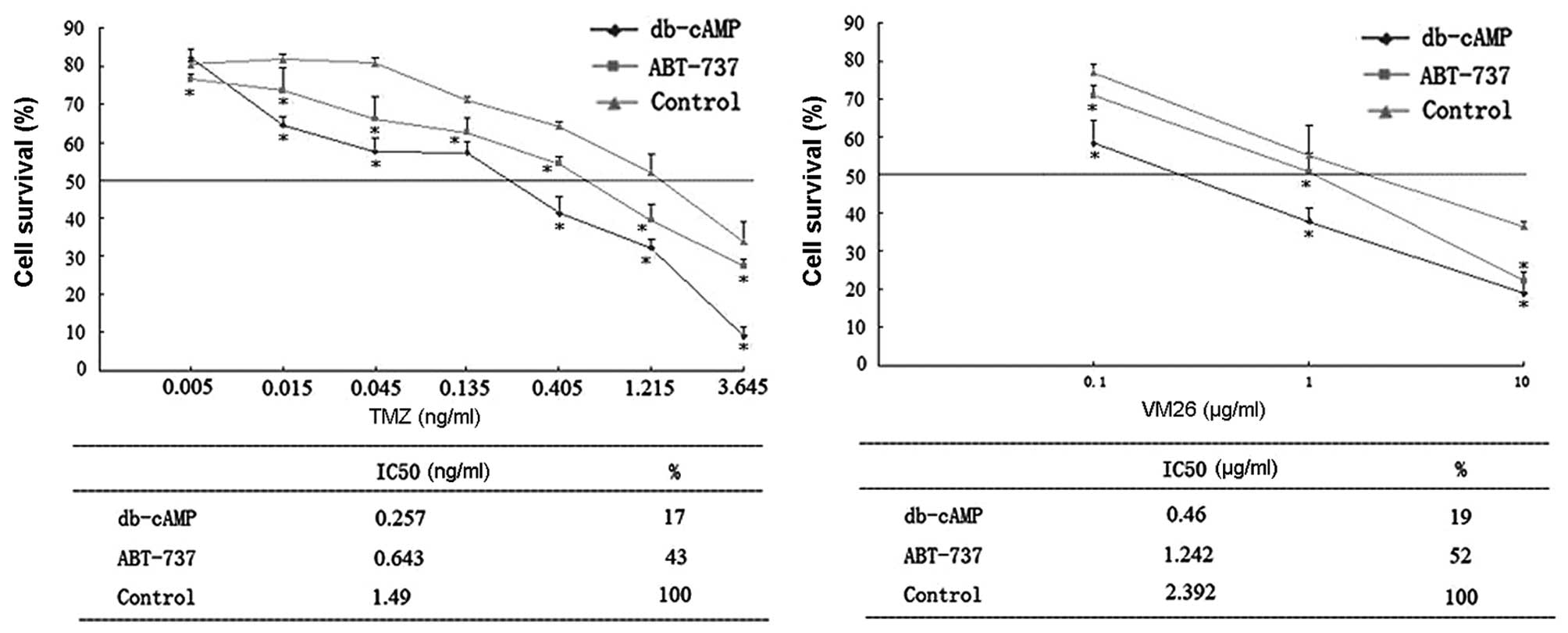
Cytotoxicity of each chemotherapeutic drug in Daoy
cells was measured by determing the IC50 value (Fig. 10). The IC50 values of
temozolomide (TMZ) and teniposide (VM26) were significantly
decreased in cells treated with db-cAMP, indicating the
cytotoxicity of both agents was greatly enhanced by db-cAMP
(reaching >5-fold of the control, P<0.05). Importantly, a
Bcl-2 antagonist, ABT-737, also augmented their chemosensitivity
(reaching ~2.5-fold over control, P<0.05), indicating that a
decrease in Bcl-2 may increase the sensitivity of the two agents,
and the upregulated chemosensitivity by db-cAMP may partly result
from the repression of Bcl-2 expression.
Discussion
In the present study, we first demonstrated the
direct influence of db-cAMP on BE and chemosensitivity in human
medulloblastoma. A Cx43 inhibitor, gossypol, blocked the effect of
db-cAMP by downregulating the Cx43 expression and thus GJIC
function, suggesting a role of Cx43 in db-cAMP-induced increase of
BE. Meanwhile, db-cAMP decreased the Bcl-2 expression and increased
apoptosis, which may be a possible mechanism of db-cAMP-augmented
chemosensitivity in medulloblastoma.
Previous studies demonstrated that the upregulation
of Cx43, one of the major GJIC components, leads to an increased BE
in suicide gene therapy and the sensitivity of several
chemotherapeutic agents (16,21,22).
Expression of Cx43 was found to be reduced in many human
carcinomas, including glioblastoma (7). In this study we found comparatively
low levels of Cx43 protein in 3 MB cell lines (D283Med, Daoy and
D341Med), indicating a role in the development or progression of
MB. Chemical induction of Cx43 has been more effective compared
with gene delivery, since a larger cancer cell population is
obtained. Therefore, we aimed to identify potential chemicals to
enhance BE in a Cx43-mediated manner in MB. A number of classes of
chemicals were found to increase Cx43, including retinoids, cAMP,
and carotenoids (23). Numerous
studies have demonstrated that cAMP is a potent inducer of
differentiation, which may also decrease proliferation of
neoplastic cells (24). Therefore,
we selected db-cAMP as the potent chemical to treat Daoy cells. We
used Daoy cells in the present study since Daoy cells grow in
adhesion and are more suitable for in vitro experiments.
Meanwhile, Cx43 expressed low levels in all 3 cell lines, which is
a common phenomenon in tumor cells, and db-cAMP increased the Cx43
expression in D283Med and D341Med cells (data not shown).
Therefore, to the best of our knowledge, these cells may similarly
express the Cx43 gene and we selected Daoy cells due to their
growth characteristic advantages.
Our results demonstrated that db-cAMP increased the
expression of the Cx43 protein as well as its phosphorylated forms
in Daoy cells, in a dose-dependent manner. The GJIC function was
also greatly enhanced by db-cAMP. However, the effect was blocked
by the Cx43 inhibitor, gossypol. Cx43 protein is phosphorylated by
various protein kinases (25,26),
including PKC and MAP kinases. Previous studies have shown that
activation of cAMP-dependent protein kinase leads to a rapid
augmentation in Cx43 phosphorylation (27), and increases intercellular
communication (28,29). Our results correspond with these
reports, which demonstrated the treatment of db-cAMP in Daoy cells
leads to an evident increase in phosphorylated Cx43 and thus GJIC
function. Meanwhile, we noted that in cells treated with both
db-cAMP and gossypol, the Cx43 protein level was even lower than
that of the control, but the GJIC function in these cells was
almost the same as the control. A possible explanation is that
gossypol may inhibit the expression of both the basal and
db-cAMP-induced Cx43 expression in Daoy cells, but the GJIC
function may also be influenced by an other effect of db-cAMP apart
from the upregulation of Cx43, which cannot be completely blocked
by gossypol.
There are a number of suicide gene systems for
treating different tumors, among which the HSV-tk/GCV is used the
most widely. Interestingly, studies show that the enhancement of BE
may further increase the therapeutic effect of HSV-tk as a more
toxic effect can spread from the transduced cells to the
neighboring untransduced cells. Several hypotheses have been
proposed for its mechanism, including the involvement of the immune
response (30,31), apoptosis (32), endocytosis of toxic cell debris
(33) or blood vessel destruction
(34). In the present study, we
first transfected Daoy cells with retroviral vectors containing the
HSV-tk gene, and an indirect immunofluorescence assay and
RT-PCR showed that HSV-tk was stably expressed. To investigate the
effects of db-cAMP on BE, an MTT assay was performed, which showed
that BE was enhanced by db-cAMP in Daoy cells. Cell killing was
significantly increased when the ratio of
tk+/tk- cells was 1:1–1:16, which suggest
that db-cAMP increased cell killing significantly when
Daoy-tk+ cells were in a small proportion, which is a
common situation found in suicide gene therapy. In this study cell
killing was no longer increased when the ratio of
tk+/tk- cells was 2:1. The reason may be that
when the GJIC function (the most important factor affecting BE)
reached a maximum, BE was no longer enhanced, even when gap
junction assembly was further enhanced. When the Cx43 inhibitor,
gossypol, which downregulates Cx43 and decreases GJIC specifically,
was added subsequently, cell killing decreased significantly,
regardless of the proportion of tk+ cells. These results
suggest that by regulating the Cx43 expression and GJIC function,
db-cAMP may greatly enhance the BE in Daoy cells.
Furthermore, we investigated the role of db-cAMP in
the cytotoxicity of chemotherapeutic agents temozolomide (TMZ) and
teniposide (VM26). Essentially the mechanism used to kill cancer
cells by inducing apoptosis with cytotoxic anticancer drugs is
commonly used in the treatment of MB. Thus, we examined the
expression of Bcl-2, an apoptosis blocker, and revealed db-cAMP
downregulated the levels of Bcl-2 protein in a
concentration-dependent manner. db-cAMP induced early apoptosis in
Daoy cells, which was blocked by gossypol. Our results showed that
db-cAMP induced the cytoxicity of the two agents, which partly
resulted from its downregulation of Bcl-2 expression and induction
of apoptosis. We presume that the downregulation of Bcl-2 by
db-cAMP could re-start the apoptosis pathway it blocked, activate
the apoptosis-related gene and increase apoptosis of tumor cells.
Another important factor is our selection of temozolomide and
teniposide, whose molecular weight was <1 kDa. These agents can
pass through gap junctions more easily, avoiding the influence of
drug particle size on the experimental results.
It is also possible that the db-cAMP effect on BE
was also a result of its repressing Bcl-2 expression by the
activation of apotosis. At the same time, db-cAMP augmented the
cytotoxicity of TMZ and VM26 (>5-fold of the control) more
significantly than the effect of the Bcl-2 antagonist (~2.5-fold
over control), indicating that the increase in chemosensitivity by
db-cAMP may also result from other factors besides the suppression
of Bcl-2, such as the augmentation of Cx43 expression and the GJIC
function, which could spread the effect of the chemical agents more
widely.
In summary, our study demonstrates the beneficial
effect of db-cAMP in the treatment of human medulloblastoma through
its upregulation of BE and increased chemosensitivity through Cx43
and Bcl-2-mediated pathways. The data revealed that db-cAMP
increased the Cx-43 expression and GJIC function in Daoy cells. As
a result, it enhanced the BE in the HSV-tk/GCV system. Meanwhile,
db-cAMP repressed the Bcl-2 expression and induced apoptosis, which
may be a possible way to increase the sensitivity of temozolomide
and teniposide. The present study provides certain molecular
mechanisms for clinical trials in the gene therapy of
medulloblastoma.
Acknowledgements
This study was supported by the Natural Science
Foundation of China (nos. 30800451, 30872656, 30700861, 30670723,
30973079 and 30772246).
References
|
1
|
Taylor RE, Bailey CC, Robinson K, et al:
Results of a randomized study of preradiation chemotherapy versus
radiotherapy alone for non-metastatic medulloblastoma: the
International Society of Paediatric Oncology/United Kingdom
Children’s Cancer Study Group PNET-3 Study. J Clin Oncol.
21:1581–1591. 2003.
|
|
2
|
Zeltzer PM, Boyett JM, Finlay JL, et al:
Metastasis stage, adjuvant treatment, and residual tumor are
prognostic factors for medulloblastoma in children: conclusions
from the Children’s Cancer Group 921 Randomized Phase III Study. J
Clin Oncol. 17:832–845. 1999.PubMed/NCBI
|
|
3
|
Kozarsky KF and Wilson JM: Gene therapy:
adenovirus vectors. Curr Opin Genet Dev. 3:499–503. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pützer BM, Bramson JL, Addison CL, et al:
Combination therapy with interleukin-2 and wild-type p53 expressed
by adenoviral vectors potentiates tumor regression in a murine
model of breast cancer. Hum Gene Ther. 9:707–718. 1998.PubMed/NCBI
|
|
5
|
Nishi M, Kumar NM and Gilula NB:
Developmental regulation of gap junction gene expression during
mouse embryonic development. Dev Biol. 146:117–130. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Houghton FD: Role of gap junctions during
early embryo development. Reproduction. 129:129–135. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vinken M, Vanhaecke T, Papeleu P, et al:
Connexins and their channels in cell growth and cell death. Cell
Signal. 18:592–600. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trosko JE and Chang CC: Modulation of
cell-cell communication in the cause and
chemoprevention/chemotherapy of cancer. Biofactors. 12:259–263.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mesnil M, Piccoli C, Tiraby G, et al:
Bystander killing of cancer cells by herpes virus thymidine kinase
gene is mediated by connexins. Proc Natl Acad Sci USA.
93:1831–1835. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elshami AA, Saavedra A, Zhang H, et al:
Gap junctions play a role in the ‘bystander effect’ of the herpes
simplex thymidine kinase/ganciclovir system in vitro. Gene Ther.
3:85–92. 1996.
|
|
11
|
Alexander DB and Goldberg GS: Transfer of
biologically important molecules between cells through gap junction
channels. Curr Med Chem. 10:2045–2058. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamasaki H: Gap junction intercellular
communication carcinogenesis. Carcinogenesis. 11:1051–1058. 1990.
View Article : Google Scholar
|
|
13
|
Yamasaki H, Mesnil M, Omori Y, et al:
Intercellular communication and carcinogenesis. Mutat Res.
333:181–188. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou DR, Zhou YC, Cui GH, et al: Gossypol
repressed the gap junctional intercellular communication between
Sertoli cells by decreasing the expression of Connexin43. Toxicol
In Vitro. 22:1719–1725. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rosolen A, Frascella E, di Francesco C,
Todesco A, et al: In vitro and in vivo antitumor effect of
retrovirus mediated herpes simplex thymidine kinase gene transfer
in human medulloblastoma. Gene Ther. 5:113–120. 1998. View Article : Google Scholar
|
|
16
|
Huang RP, Hossain MZ, Huang R, et al:
Connexin 43 (cx43) enhances chemotherapy-induced apoptosis in human
glioblastoma cells. Int J Cancer. 92:130–138. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pu K, Li SY, Gao Y, et al: Bystander
effect in suicide gene therapy using immortalized neural stem cells
transduced with herpes simplex virus thymidine kinase gene on
medulloblastoma regression. Brain Res. 1369:245–252. 2011.
View Article : Google Scholar
|
|
18
|
Miyashita T and Reed JC: Bcl-2 oncoprotein
blocks chemotherapy-induced apoptosis in a human leukemia cell
line. Blood. 81:151–157. 1993.PubMed/NCBI
|
|
19
|
Piche A, Grim J, Rancourt C, et al:
Modulation of Bcl-2 protein levels by an intracellular anti-Bcl-2
single-chain antibody increases drug-induced cytotoxicity in the
breast cancer cell line MCF-7. Cancer Res. 58:2134–2140.
1998.PubMed/NCBI
|
|
20
|
Reed JC, Miyashita T, Takayama S, et al:
BCL-2 family proteins: regulators of cell death involved in the
pathogenesis of cancer and resistance to therapy. J Cell Biochem.
60:23–32. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jimenez T, Fox WP, Naus CC, et al:
Connexin over-expression differentially suppresses glioma growth
and contributes to the bystander effect following HSV-thymidine
kinase gene therapy. Cell Commun Adhes. 13:79–92. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang A, Wang Qy, Han Z, et al:
Relationship between the expression of connexin43 and bystander
effect of suicide gene therapy in ovarian cancer. J Huazhong Univ
Sci Technolog Med Sci. 24:476–479. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carystinos GD, Alaoui-Jamali MA, Phipps J,
et al: Upregulation of gap junctional intercellular communication
and connexin 43 expression by cyclic-AMP and all-trans-retinoic
acid is associated with glutathione depletion and chemosensitivity
in neuroblastoma cells. Cancer Chemother Pharmacol. 47:126–132.
2001. View Article : Google Scholar
|
|
24
|
Chen TC, Hinton DR, Zidovetzki R and
Hofman FM: Upregulation of the cAMP/PKA pathway inhibits
proliferation, induces differentiation, and leads to apoptosis in
malignant gliomas. Lab Invest. 78:165–174. 1998.PubMed/NCBI
|
|
25
|
Goodenough DA, Goliger JA and Paul DL:
Connexins, connexons, and intercellular communication. Annu Rev
Biochem. 65:475–502. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Laird DW, Puranam KL and Revel JP:
Turnover and phosphorylation dynamics of connexin43 gap junction
protein in cultured cardiac myocytes. Biochem J. 273:67–72.
1991.PubMed/NCBI
|
|
27
|
Granot I and Dekel N: Phosphorylation and
expression of connexin-43 ovarian gap junction protein are
regulated by luteinizing hormone. J Biol Chem. 269:30502–30509.
1994.PubMed/NCBI
|
|
28
|
Burt JM and Spray DC: Ionotropic agents
modulate gap junctional conductance between cardiac myocytes. Am J
Physiol. 254:H1206–H1210. 1988.PubMed/NCBI
|
|
29
|
Godwin AJ, Green LM, Walsh MP, et al: In
situ regulation of cell-cell communication by the cAMP-dependent
protein kinase and protein kinase C. Mol Cell Biochem.
127–128:293–307. 1993.PubMed/NCBI
|
|
30
|
Vile RG, Nelson JA, Castleden S, et al:
Systemic gene therapy of murine melanoma using tissue specific
expression of the HSVtk gene involves an immune component.
Cancer Res. 54:6228–6234. 1994.PubMed/NCBI
|
|
31
|
Caruso M, Panis Y, Gagandeep S, et al:
Regression of established macroscopic liver metastases after in
situ transduction of a suicide gene. Proc Natl Acad Sci USA.
90:7024–7028. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Samejima Y and Meruelo D: ‘Bystander
killing’ induces apoptosis and is inhibited by forskolin. Gene
Ther. 2:50–58. 1995.
|
|
33
|
Freeman SM, Abboud CN, Whartenby KA, et
al: The ‘bystander effect’: tumor regression when a fraction of the
tumor mass is genetically modified. Cancer Res. 53:5274–5283.
1993.
|
|
34
|
Ram Z, Walbridge S, Shawker T, et al: The
effect of thymidine kinase transduction and ganciclovir therapy on
tumor vasculature growth of 9L gliomas in rats. J Neurosurg.
81:256–260. 1994. View Article : Google Scholar : PubMed/NCBI
|