Introduction
Human embryonic stem cells (hESCs) are derived from
the inner cell masses of human blastocysts (1). The 2 basic characteristics of hESCs
are pluripotency and the ability to self-renew. hESCs have the
ability to differentiate into any cell type in the body. The
self-renewal ability of hESCs is regulated by a set of
transcription factors, including Oct-4, Nanog and Sox-2 (2). Since their derivation, hESCs hold
great promise for regenerative medicine and are a powerful tool for
basic research (1,3), such as disease research, toxicology
and drug screening. The first hESC line was propagated in a
co-culture system on a layer of mitotically inactivated mouse
embryonic fibroblasts (MEFs) (1).
hESCs are commonly cultured in medium supplemented with knockout
serum-replacement (KSR) together with basic fibroblast growth
factors (bFGF) on inactivated MEF feeders. bFGF is the key growth
factor in maintaining undifferentiated growth in hESCs (4–10).
bFGF has to be exogenously supplemented in the culture medium when
using a mouse-feeder cell line or feeder-free conditions, as the
KSR replacement medium is used instead of an animal serum in the
expansion of hESCs. A number of studies have focused on the
secreted factors released from MEF feeder layers, capable of
maintaining the self-renewal of hESCs, and have identified a number
of factors responsible for maintaining hESC pluripotency (11–14).
The exact mechanisms through which feeder cells support the growth
of hESCs remain only partially understood. In recent years, there
have been various protocols for culturing embryonic stem cells,
with the newer trends moving toward a feeder-free or serum-free
culture. For human and mouse embryonic stem cells, however,
fibroblast feeder layers are often used at some phase during the
culture protocol. The feeders, often MEFs, provide a substrate that
increases the plating efficiency, helps maintain pluripotency and
facilitates both the survival and the growth of stem cells
(15).
We accidentally obtained a spontaneously
immortalized cell line from the mouse fetal livers in the process
of utilizing mouse fetal liver stromal cells to promote the
hematopoietic differentiation of hESCs. These cells appear
fibroblast-like in morphology, namely the KM3 cell line. They are
characterized by growing rapidly and having low nutritional
requirements. We observed that the KM3 cell line does not promote
the hematopoietic differentiation of hESCs. We hypothesized that
the immortalized KM3 cells may be used as feeder cells for hESC
culture. Thus, in the present study, we aimed to examine whether
KM3 cells can support the growth of hESCs, while allowing them to
retain their undifferentiated state.
Materials and methods
MEF culture and establishment of murine
fetal liver-derived stromal cells
Pregnant Kunming female mice were provided by the
Animal Experimentation Center of Jiangsu University and embryos
were dissected from the uteri at 13.5 days post coitum. All
experimental procedures were conducted in accordance with the
Chinese legislation on animal protection. MEFs were isolated and
cultured from the mouse embryos as described previously (16).
The murine fetal livers (FLs) were removed from the
mouse embryos and washed with PBS 3 times, then dissected into 1-mm
pieces using a scissors and treated with 0.25% trypsin/EDTA
(Invitrogen) at 37°C for 3 min. Subsequently, the cells were washed
and seeded in a 25 cm2 flask (NUNC) at a density of 5–6
FLs/flask. The culture medium contained 90% DMEM (Gibco Invitrogen)
and 10% fetal bovine serum (FBS) (Gibco Invitrogen). The medium was
exchanged after 24 h and 3 days thereafter. Cells were split 1:3 to
1:4 and transferred into new flasks. Usually, mouse fetal liver
stromal cells are passaged only for 4–5 generations. However, the
mouse fetal liver stromal cells that were derived from our research
mice became spontaneously immortalized. Cell passaging lasted for
138 days from the 1st to the 5th generation and the cells showed
increased proliferation from the 6th generation. The culture medium
that contained 90% DMEM and 10% newborn bovine serum (NBS)
(Sijiqing, Shandong, China) was changed from the 33rd passage.
hESC culture
hESCs (SHhES2) were donated by Dr Jin Ying, School
of Medicine, Shanghai Jiao Tong University. The hESC culture medium
contained 80% knockout DMEM, 20% KSR, 1 mM L-glutamine, 1%
non-essential amino acid (NEAA), 4 ng/ml bFGF (all from Gibco
Invitrogen) and 0.1 mM β-mercaptoethanol (Sigma). The cells were
incubated at 37°C in 5% CO2 in air and 95% humidity. The
hESCs were initially maintained on MEF feeders and later
transferred to KM3 feeders. According to the instructions of the
manufacturer, hESCs were briefly treated with 1 mg/ml of type IV
collagenase (Sigma) for 30–40 min at 37°C. The hESCs were harvested
and further broken into small clumps using pipette tips and
subcultured through seeding onto mitomycin C (Roche)-treated
feeders (MEFs and KM3 cells) to split the cells (1:3 to 1:4). The
medium was exchanged after 48 h and every day thereafter. The hESC
colonies were passaged every 4 days. To assess hESC proliferation,
the undifferentiated hESC colonies were counted on the MEF and KM3
feeder layer before dissociation during each of the 10 passages and
then passaged in the same proportion. To determine
population-doubling time, cell numbers in 5 selected independent
colonies were counted under an inverted microscope. After the 20th
passage, the morphological characteristics of the hESC colonies
were observed with Giemsa staining.
Alkaline phosphatase and periodic acid
Schiff (PAS) staining
Alkaline phosphatase (ALP) and PAS staining were
carried out in 6-well plates. Prior to analysis, adherent cell
layers were washed twice with PBS and air-dried. Staining was
performed using Cytochemistry Staining kits (Shanghai Sun Biotech
Co., Ltd), according to the manufacturer’s instructions, except for
staining with hematoxylin.
Karyotype analysis
For hESCs that had been cultured on KM3 cells for 47
passages, karyotype analyses were carried out. Briefly, cells were
incubated with a final concentration of 0.2 μg/ml of colcemid
(Sigma) of culture medium for 5–6 h. Subsequently the cells were
washed twice with PBS, trypsinized in 0.25% trypsin/EDTA, treated
with hypotonic 0.075 M KCl at 37°C for 30 min, collected by
centrifugation and then fixed with fresh fixative (methanol/acetic
acid 3:1). After 3 rinses in fixative, the cells were dropped onto
pre-cleaned chilled glass slides. After R-band staining, the
chromosomes were visualized by cytogenetics specialists from the
Center of Clinical Laboratory, the Affiliated Hospital of Jiangsu
University.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted by TRIzol reagent
(Invitrogen) from undifferentiated hESCs grown at least for 19
passages on MEFs or KM3 cells, or from control MEFs or KM3 cells.
Using the ReverTra Ace kit (Toyobo), 1mg RNA was
reverse-transcribed into cDNA. The PCR primers used are listed in
Table I. PCR was performed by using
the following parameters: denaturing at 94°C for 5 min, 35 cycles
at 94°C for 30 sec, 60°C (56°C for ALP and β-actin) for 30 sec, and
72°C for 30 sec, a final extension at 72°C for 10 min. β-actin was
used as the positive control. The PCR product was stained with 0.1
μg/ml ethidium bromide, followed by electrophoresis on a 1.5%
agarose gel.
 | Table IInformation on RT-PCR primers. |
Table I
Information on RT-PCR primers.
| Genes | Forward (For) and
reverse (Rev) primers (5′-3′) | Size (bp) | Annealing
temperature (°C) |
|---|
| Oct-4 | For:
TATACACAGGCCGATGTGG
Rev: GTGCATAGTCGCTGCTTGA | 397 | 60 |
| Nanog | For:
ATGCCTCACACGGAGACTG
Rev: CTGCGTCACACCATTGCTA | 369 | 60 |
| Sox-2 | For:
ACACCAATCCCATCCACACT
Rev: GCAAACTTCCTGCAAAGCTC | 224 | 60 |
| ALP | For:
AGCTTCAAACCGAGATACAA
Rev: ATTCTGCCTCCTTCCACC | 220 | 56 |
| β-actin | For:
CACGAAAATACCTTCAACTCC
Rev: CATACTCCTGCTTGCTGATC | 265 | 56 |
Embryoid body formation
For embryoid body formation, the undifferentiated
hESC colonies were harvested by treatment with collagenase IV at
the 20th passage on the MEF and KM3 feeders. The clumps of the
cells were transferred onto a bacterial culture dish (NUNC). The
embryoid bodies were grown in medium consisting of 80% knockout
DMEM, 20% KSR, 1 mM L-glutamine, 1% NEAA and 0.1 mM
β-mercaptoethanol. The medium was changed every other day. Embryoid
bodies were cultured for 10 days in suspension cultures.
Teratoma formation
The potential to form derivatives of all 3 embryonic
germ layers was examined in the teratomas. After 26 passages on KM3
feeder cells, approximately 2×106 hESCs with
undifferentiated morphology were harvested and resuspended in a
mixture of PBS. The cell mixtures were subcutaneously injected into
the rear legs of 4-week-old severe combined immunodeficient (SCID)
mice (Laboratory Animal Center of Shanghai, Academy of Sciences,
Shanghai, China). Teratomas were dissected from the mice and fixed
with 4% paraformaldehyde overnight approximately 7 weeks after
injection. Tumors were embedded in paraffin and histologically
examined after hematoxylin and eosin staining. KM3 feeder cells
(2×106) inoculated into the same sites of SCID mice as
the controls did not develop tumors.
Results
Isolation and morphology of KM3
cells
MEFs and mouse fetal liver stromal cells were
isolated and cultured from 13.5-day-old mouse embryos. The primary
MEFs contained a heterogeneous population of cells. After 2
subsequent passages, the majority of the MEFs had a fusiform shape
and were highly uniform in morphology. These cells at 2–5 passages
were used as the hESC feeders. Generally, mouse fetal liver stromal
cells are passaged for 4–5 generations in vitro and contain
a heterogeneous population of cells. We accidentally obtained a
spontaneously immortalized cell line (KM3). The characteristics of
KM3 cells were similar to other mouse fetal liver stromal cells
before passage 4 (Fig. 1A).
Nevertheless, the proliferation of the cells in passage 5
significantly decreased and reached confluence at 92 days and for
cells in passage 6 at 30 days. Morphologically, the majority of the
KM3 cells had a fusiform shape and were highly uniform in
morphology (Fig. 1B). The interval
between passages was gradually increased from 6–11 days at passages
7 to 18. The cells reached confluence every 4–5 days when they were
split 1:4 at passages 19 to 24. The proliferation rate gradually
increased and reached the maximum. Usually, the cells were split
1:10 and reached confluence every 4–5 days after 32 passages.
When KM3 cells were transferred to the same culture
medium supplemented with NBS instead of FBS at the 33rd passage or
higher, their growth rates and morphology were similar to the lines
derived from FBS. KM3 cells were cultured for 132 passages. No cell
senescence or reduction in the growth rate were observed. The
doubling time of MEFs (passages 2 to 4) and KM3 cells (over 50
passages) was approximately 45.4 and 36.0 h, respectively. After
spontaneous immortalization there were 2 cell lines of different
morphologies in the culture. One cell line had a cobblestone-like
morphology, similar to epithelial cells. The other resembled a
fibroblast, similar to MEFs (Fig. 1C
and D). In addition, we found that the fibroblast-like cells
were easily digested with 0.25% trypsin/EDTA. The previously
digested cells were harvested and reseeded to flasks. After
approximately 50 passages, the fibroblast-like cells prevailed in
population (Fig. 1E) and were
similar to MEFs in morphology (Fig.
1F). This prompted us to consider whether the fibroblast-like
cells may function as feeder layers for hESCs. To test this
hypothesis, KM3 cells at the 50th passage or higher were treated
with mitomycin C and used as feeders. We found that mitomycin
C-treated KM3 cells did not proliferate but maintained their
metabolic activity under the culture conditions.
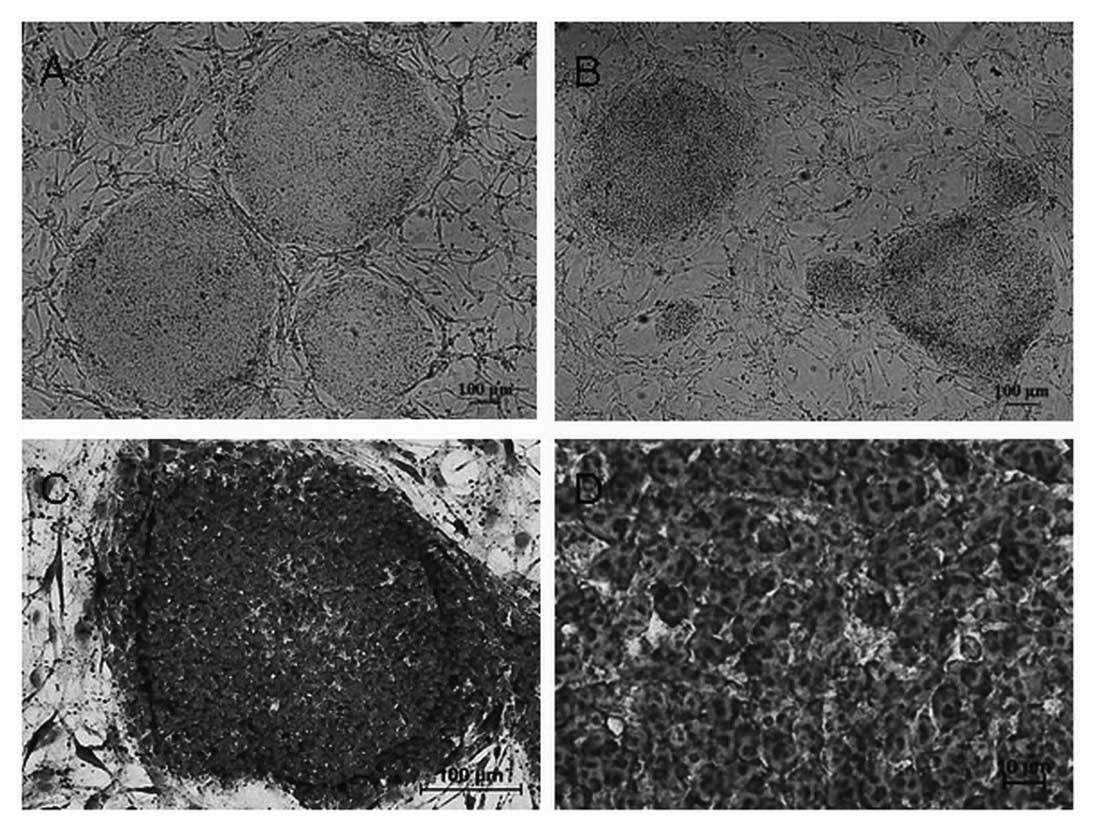
Morphology of hESCs
The SHhES2 cells were cultured on mitotically
inactive MEFs as instructed by the provider. We cultured these
hESCs continuously for 3 months and split the cells (using
collagenase IV) once every 4 days. Being consistent with the
provided protocol, we observed a 3–4-fold expansion in each
passage. SHhES2 cells were then transferred from MEFs to KM3 cells.
The density of KM3 cells was the same as MEFs
(5×105/flask). We observed that hESC colonies that grew
on KM3 feeder layers were slightly thinner and less compact than
colonies grown on MEFs. However, the colonies had the typically
undifferentiated morphology (round, defined colony edges). The
slight difference in the morphology of the colonies grwon on the 2
feeders disappeared when the density of KM3 cells was increased to
7×105/flask (Fig. 2A and
B). The morphology of individual hESCs cultured on KM3 cells
was the same as that of those cultured on MEFs. The cells appeared
to be round and small, with a high nucleus/cytoplasm ratio, a
notable presence of 1–3 nucleoli and typical spacing between the
cells by Giemsa staining (Fig. 2C and
D)
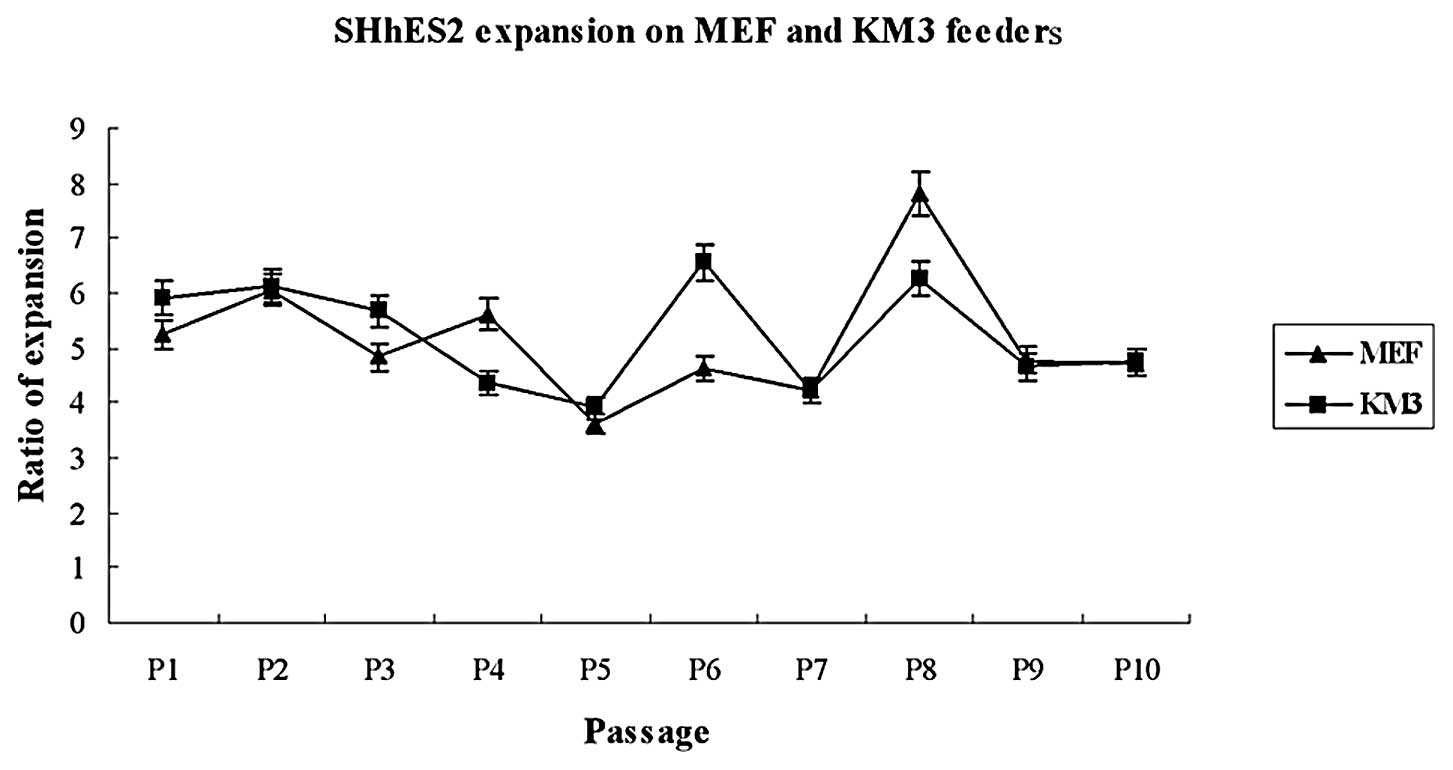
Proliferation rates of hESCs
To examine the proliferation rates of hESCs cultured
on KM3 cells or on MEFs, the same proportion of hESCs was plated,
and the number of colonies was counted before the splitting of
cells in each passage. The expansion ratios of hESCs grown on KM3
cells showed no observable difference compared with those grown on
MEFs, when cultured for up to 10 passages (Fig. 3). The doubling time of hESCs on MEFs
and KM3 cells was 41.4 and 39.8 h, respectively. We subsequently
long-term cultured hESCs on KM3. The SHhES2 line had been
propagated previously and was expanded on KM3 cells for over 96
passages (approximately 380 days). The cells still showed the
typically undifferentiated morphology and similar expansion
ratios.
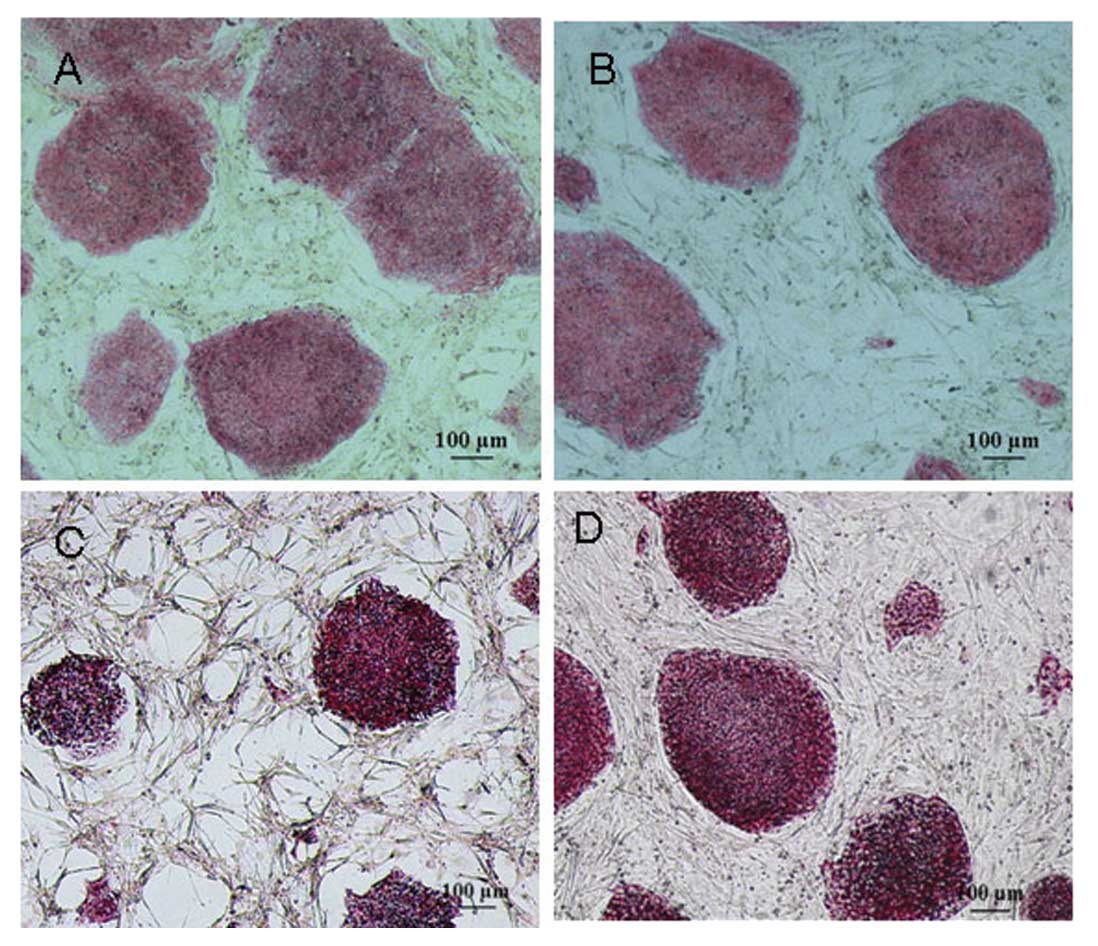
Cytochemical staining of hESCs
After the 20th passage or higher, the hESC colonies
grown on MEFs and KM3 cells were analyzed by cytochemical staining.
Both hESC colonies showed a strong ALP activity, while the MEF and
KM3 control cells were negative for ALP expression (Fig. 4A and B). Additionally, we observed
that the typically undifferentiated hESCs grown on MEFs and KM3
cells were strongly positive for PAS (Fig. 4C and D), whereas the differentiated
hESCs showed a weak PAS activity, while the MEFs and KM3 cells were
negative for PAS.
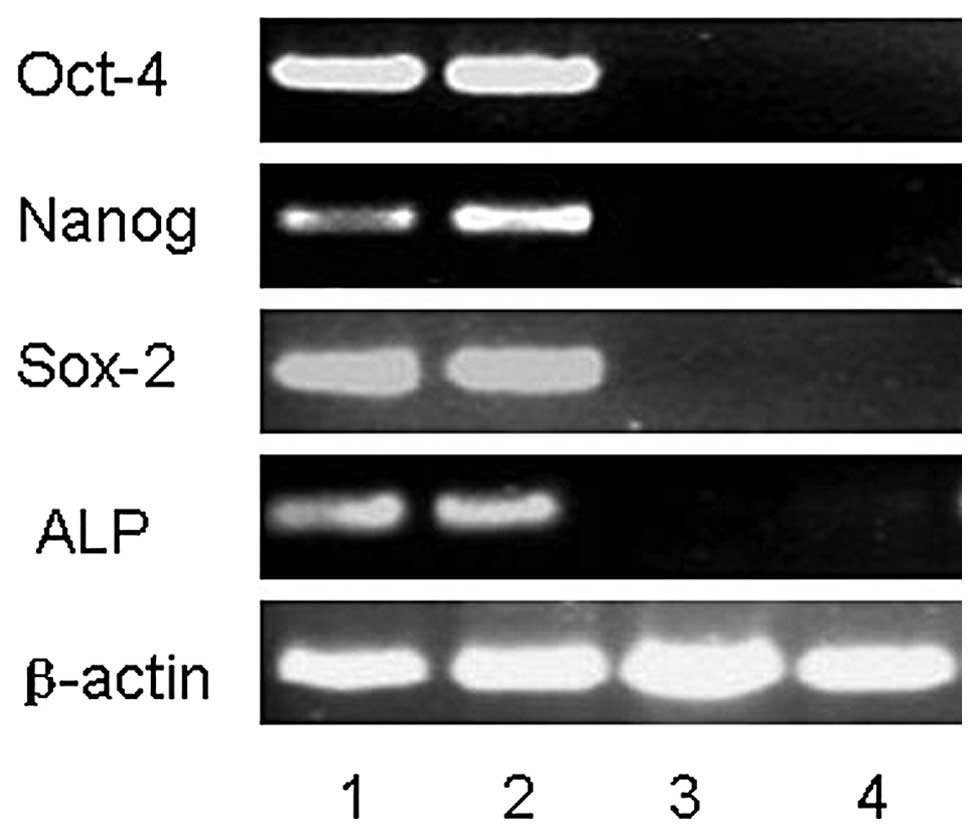
Stem cell marker expression in hESCs
We analyzed the gene expression levels of several
stem cell markers considered to be preferentially expressed in
undifferentiated hESCs by RT-PCR, such as Oct-4, Nanog and Sox-2
using the hESCs that had been cultured on either KM3 cells or MEFs
for 19 passages. These results were indistinguishable from the
results observed for hESCs cultured on MEFs and KM3 cells. The
undifferentiated hESCs were found to be positive for ALP expression
(Fig. 5)
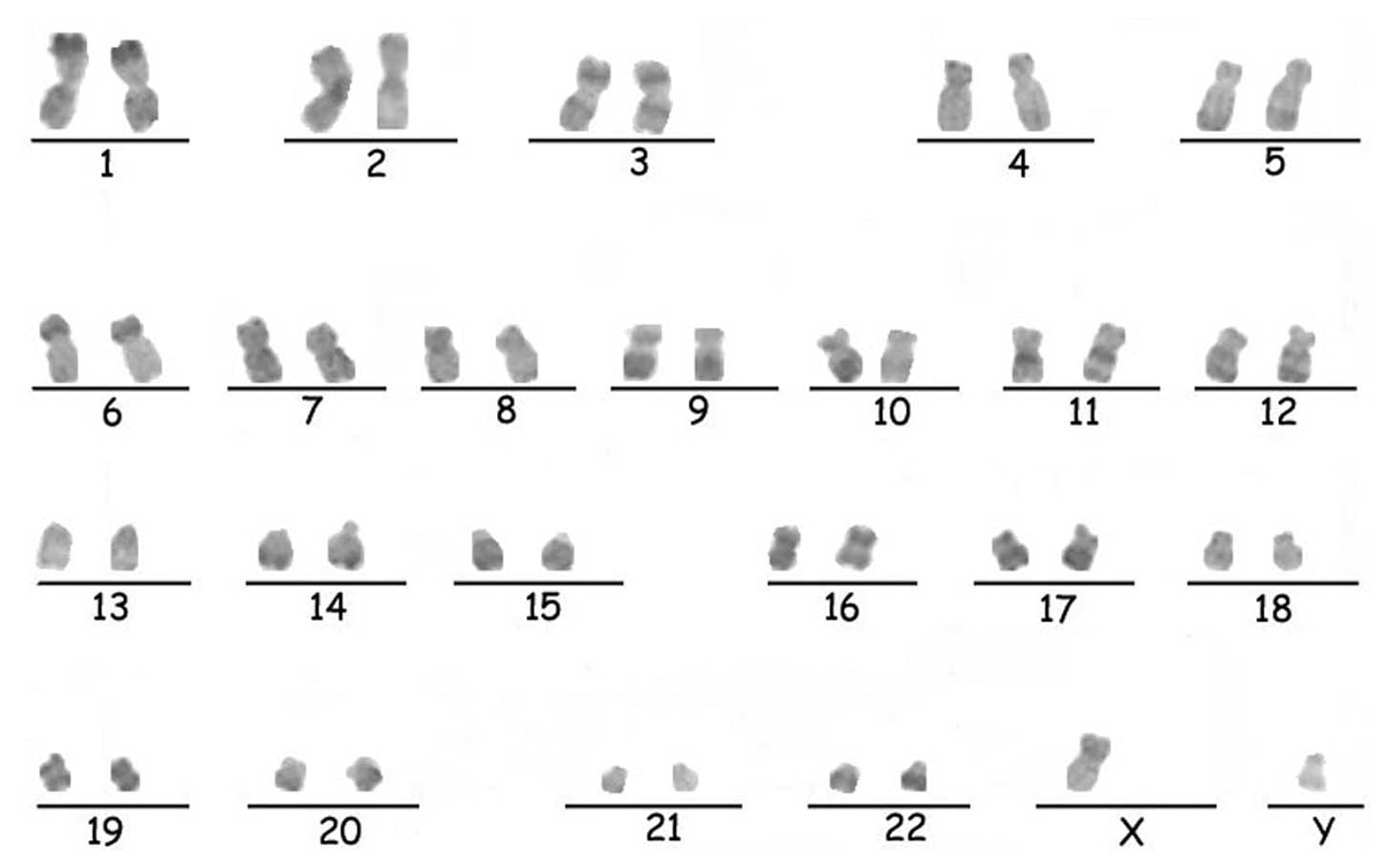
Karyotype analysis
Karyotype analysis was performed on the hESCs
cultured on KM3 cells for more than 47 passages. They were found to
possess the normal human 46, XY karyotype, as analyzed by
R-banding. The result was compatible with the hESCs of the provider
(17). The chromosomes are
illustrated in Fig. 6.
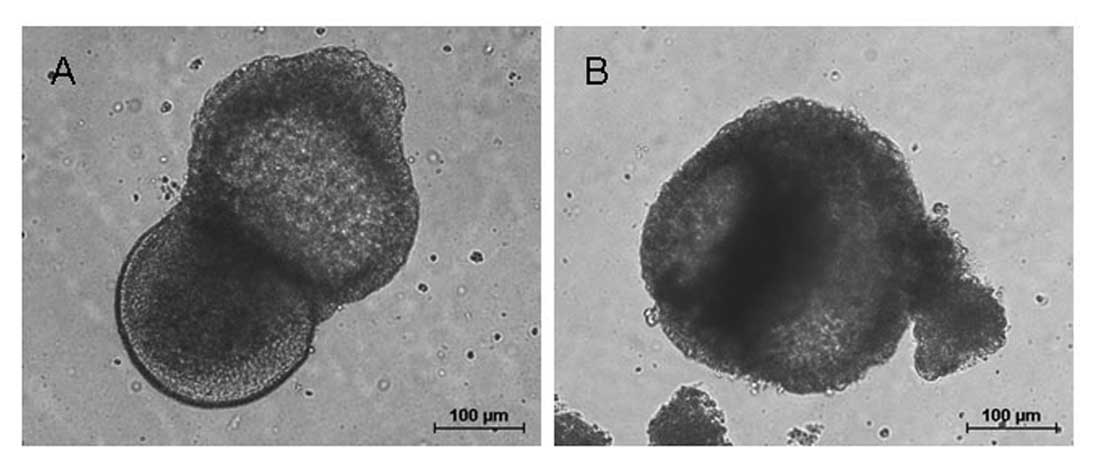
Pluripotency of hESCs
To examine the pluripotency differentiation ability
of hESCs cultured on KM3 cells for 20 passages, we used floating
culture to form embryoid bodies. After 10 days of suspension
cultivation, hESCs formed ball-shaped embryoid bodies (Fig. 7).
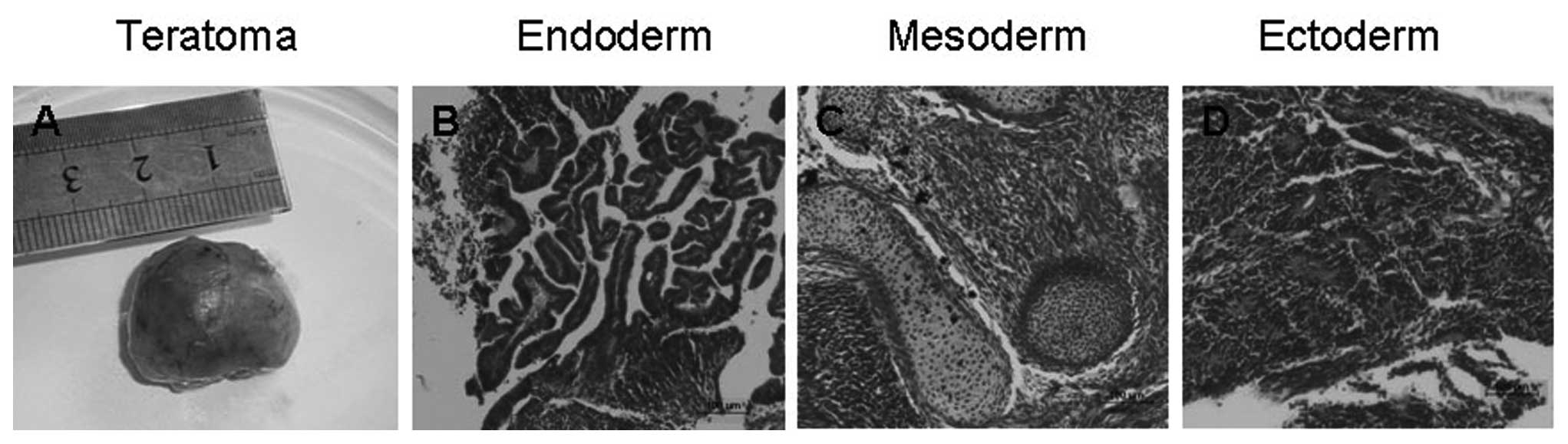
Additionally, the hESCs on KM3 cells for 26 passages
produced typically immature teratomas in vivo after
injection into SCID mice (Fig. 8A).
The teratomas were found to contain tissues of the 3 embryonic germ
layers (including immature tissues with intestinal villi,
cartilages and neural rosettes), further confirming the pluripotent
nature of hESCs (Fig. 8B-D).
Discussion
In this study, we describe a new immortalized cell
line that can function as a feeder layer for the expansion of hESCs
in vitro. The hESCs were cultured on KM3 cells for multiple
passages, while they retained the same morphology of
undifferentiated hESCs as those grown on MEFs. The hESCs were
positive for ALP expression and retained a normal chromosomal
karyotype. The stem cell-associated factor gene expression analysis
of hESCs grown on KM3 cells showed a similarity with the cells
grown on MEFs. The hESCs grown on KM3 cells successfully
differentiated into embryoid bodies in vitro and formed
teratomas in vivo. Furthermore, we observed that the
undifferentiated hESCs were strongly positive for PAS, whereas the
differentiated hESCs demonstrated a weak PAS activity. The hESCs
were stably passaged for 96 passages (approximately 380 days) on
KM3 cells, maintaining the morphology of typically undifferentiated
hESCs. Simultaneously, KM3 cells were expanded for 132 passages
(approximately 2.5 years) with medium containing 10% NBS. As
feeders, they supported the hESC expansion just as efficiently as
MEFs.
Several types of feeder cells have been successfully
used for hESC culture. When the first hESC lines were derived, MEFs
were used as feeder layers to support the propagation of hESCs in
the primitive undifferentiated state (1,3). To
date, hESCs have been cultured mainly by using MEFs as feeder
layers. However, MEFs have many serious limitations as feeder cells
and their proliferation is limited. MEFs often go through
senescence when they are passaged 5–6 times in vitro.
Sufficient MEFs must be freshly prepared, freezed and thawed in
order to fulfil the demand of the long-term and large-scale hESC
culture. In addition, different batches of MEFs may differ in their
growth rate and ability to support hESCs. The immortalization of
feeders would offer a possible solution to this problem, resulting
in a sustainable, standardized and consistent source of feeders for
the culture of hESCs. On the other hand, MEFs are associated with
risks, such as viral infection and transmission of mouse pathogens,
which may prevent the future use of hESCs in clinical trials.
To date, scientific efforts have aimed to expand
hESC-based technology from experimental research into clinical
application. In order to avoid the transmission of animal
pathogens, numerous human feeders from adult, neonatal and fetal
sources have been used as alternative methods to culture and
maintain hESCs, such as human foreskin (18–20),
adult marrow stromal cells (21),
human fetal muscle and adult fallopian tube fibroblasts (22,23),
adult uterine endometrium (24,25),
amniocytes (26–28), placenta and (29) and human fetal liver stromal cells
(30). Several groups have
successfully used fibroblasts differentiated from hESCs as feeders
(8,31–34).
These studies have shown that human cells can be used to support
hESC growth and to maintain undiffierentiated hESCs. However, human
fetal and adult cells may still be unsuitable feeder layers due to
ethical and practical limitations. We also obtained fibroblast-like
cells which differentiated spontaneously from hESCs. Nevertheless,
it was difficult for these fibroblast-like cells to achieve a high
passage, as they usually went through senescence and apoptosis when
they were passaged 5–6 times after derivation. Since human feeder
cells are unable to maintain continuity, producing sufficient hESCs
for clinical therapy is difficult. Furthermore, it is important to
standardize the source of feeder cells used for research, as this
is a variable that could hamper comparison between results obtained
by different groups.
There have been several reports describing the
maintenance of hESCs on immortalized feeder layers. Park et
al used STO, a permanently growing cell line, as a feeder to
support the growth of hESCs (35,36).
Several human immortalized cell lines have also been used as
feeders to culture and maintain hESCs (37–39).
These immortalized feeder cell lines have not been widely used by
other laboratories, and more studies are required before the MEFs
can be replaced completely.
The other culture systems include feeder-free
(40–42), feeder-conditioned (43) and most recent suspension cultures
(44). Feeder-free systems using
additional growth factors will significantly increase the cost of
culture. These conditions may not be optimal for a wide range of
hESC lines (45). Furthermore, even
though feeder-free and serum-free conditions have been defined for
the maintenance of hESCs, further research is required to determine
the factors responsible for maintaining the pluripotent phenotype
and stability of hESC lines in general (46).
Taken together, each of these culture systems offers
certain advantages and disadvantages. KM3 cells may be used as a
new feeder cells to support hESC expansion in vitro. This
has several advantages compared with other hESC culture systems. A
major advantage is that the KM3 cells were immortalized and growth
proceeded rapidly. The KM3 cells do not need to be freshly
prepared, or frozen and thawed frequently in order to be used as a
hESC feeder layer. KM3 culture only requires medium that consists
of 90% DMEM and 10% NBS to highly propagate. These characteristics
render KM3 cells particularly suitable for the mass production of
standardized feeders to satisfy the large-scale production of
hESCs, while they can decrease the cost of cultures and reduce
operational stress. Moreover, our immortalized cell line was
derived from mouse fetal liver tissues. Richards et al
reported that fetal or embryonic tissues performed better in
vitro than adult tissues when supporting the growth of
undifferentiated hESCs (23).
Further studies are required to determine the nature of KM3 cells
and whether they can function as feeders support to other hESC
lines or induced pluripotent stem cells (iPSCs). Additionally,
future research is required as regards the mechanisms through which
KM3 cells support hESC growth.
In conclusion, our findings suggest that KM3 cells
can support the growth of hESCs (SHhES2) and may used as novel
feeders for the long-term proliferation of hESCs in an
undifferentiated and pluripotent state. Moreover, this expansion
process has the potential for large-scale production with a simple,
low-cost and less labor-intensive manner.
Acknowledgements
The authors are grateful to Dr Jin Ying for
providing the hESC line (SHhES2) and would like to thank Professor
Zhang Zhijian at the School of Medical Science and Laboratory
Medicine at the Jiangsu University for the analysis of the
teratomas. The authors would also like to thank Ba Rong and You
Haiyan at the Center of Clinical Laboratory at the Affiliated
Hospital of the Jiangsu University for their technical support. The
present study was funded by the Startup Foundation for Advanced
Talents, Jiangsu University (grant no. 09JDG037) and the National
Natural Science Foundation of China (grant no.. 31071421).
References
|
1
|
Thomson JA, Itskovitz-Eldor J, Shapiro SS,
et al: Embryonic stem cell lines derived from human blastocysts.
Science. 282:1145–1147. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Niwa H: How is pluripotency determined and
maintained? Development. 134:635–646. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reubinoff BE, Pera MF, Fong CY, Trounson A
and Bongso A: Embryonic stem cell lines from human blastocysts:
somatic differentiation in vitro. Nat Biotechnol. 18:399–404. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu C, Inokuma MS, Denham J, et al:
Feeder-free growth of undifferentiated human embryonic stem cells.
Nat Biotechnol. 19:971–974. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carpenter MK, Rosler ES, Fisk GJ, et al:
Properties of four human embryonic stem cell lines maintained in a
feeder-free culture system. Dev Dyn. 229:243–258. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosler ES, Fisk GJ, Ares X, et al:
Long-term culture of human embryonic stem cells in feeder-free
conditions. Dev Dyn. 229:259–274. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Levenstein ME, Ludwig TE, Xu RH, et al:
Basic fibroblast growth factor support of human embryonic stem cell
self-renewal. Stem Cells. 24:568–574. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saxena S, Hanwate M, Deb K, Sharma V and
Totey S: FGF2 secreting human fibroblast feeder cells: a novel
culture system for human embryonic stem cells. Mol Reprod Dev.
75:1523–1532. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang G, Zhang H, Zhao Y, et al: Noggin and
bFGF cooperate to maintain the pluripotency of human embryonic stem
cells in the absence of feeder layers. Biochem Biophys Res Commun.
330:934–942. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park Y, Kim JH, Lee SJ, et al: Human
feeder cells can support the undifferentiated growth of human and
mouse embryonic stem cells using their own basic fibroblast growth
factors. Stem Cells Dev. 20:1901–1910. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu RH, Peck RM, Li DS, Feng X, Ludwig T
and Thomson JA: Basic FGF and suppression of BMP signaling sustain
undifferentiated proliferation of human ES cells. Nat Methods.
2:185–190. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lim JW and Bodnar A: Proteome analysis of
conditioned medium from mouse embryonic fibroblast feeder layers
which support the growth of human embryonic stem cells. Proteomics.
2:1187–1203. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cai J, Chen J, Liu Y, et al: Assessing
self-renewal and differentiation in human embryonic stem cell
lines. Stem Cells. 24:516–530. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chin AC, Fong WJ, Goh LT, Philp R, Oh SK
and Choo AB: Identification of proteins from feeder conditioned
medium that support human embryonic stem cells. J Biotechnol.
130:320–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin S and Talbot P: Methods for culturing
mouse and human embryonic stem cells. Methods Mol Biol. 690:31–56.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu J-B, Ma Q-H and Hu S-Q: Preparation and
biological characteristics of feeder cells for human embryonic stem
cells. J Clin Rehabil Tissue Eng Res. 15:4233–4266. 2011.
|
|
17
|
Li C, Yang Y, Lu X, et al: Efficient
derivation of Chinese human embryonic stem cell lines from frozen
embryos. In Vitro Cell Dev Biol Anim. 46:186–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amit M, Margulets V, Segev H, et al: Human
feeder layers for human embryonic stem cells. Biol Reprod.
68:2150–2156. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hovatta O, Mikkola M, Gertow K, et al: A
culture system using human foreskin fibroblasts as feeder cells
allows production of human embryonic stem cells. Hum Reprod.
18:1404–1409. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng G, Liu S, Krawetz R, Chan M, Chernos
J and Rancourt DE: A novel method for generating xeno-free human
feeder cells for human embryonic stem cell culture. Stem Cells Dev.
17:413–422. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng L, Hammond H, Ye Z, Zhan X and
Dravid G: Human adult marrow cells support prolonged expansion of
human embryonic stem cells in culture. Stem Cells. 21:131–142.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Richards M, Fong CY, Chan WK, Wong PC and
Bongso A: Human feeders support prolonged undifferentiated growth
of human inner cell masses and embryonic stem cells. Nat
Biotechnol. 20:933–936. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Richards M, Tan S, Fong CY, Biswas A, Chan
WK and Bongso A: Comparative evaluation of various human feeders
for prolonged undifferentiated growth of human embryonic stem
cells. Stem Cells. 21:546–556. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee JB, Lee JE, Park JH, et al:
Establishment and maintenance of human embryonic stem cell lines on
human feeder cells derived from uterine endometrium under
serum-free condition. Biol Reprod. 72:42–49. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee JB, Song JM, Lee JE, et al: Available
human feeder cells for the maintenance of human embryonic stem
cells. Reproduction. 128:727–735. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang K, Cai Z, Li Y, et al: Utilization
of human amniotic mesenchymal cells as feeder layers to sustain
propagation of human embryonic stem cells in the undifferentiated
state. Cell Reprogram. 13:281–288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lai D, Cheng W, Liu T, Jiang L and Huang
Q: Use of human amnion epithelial cells as a feeder layer to
support undifferentiated growth of mouse embryonic stem cells.
Cloning Stem Cells. 11:331–340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu T, Cheng W, Guo L, et al: Human
amniotic epithelial cell feeder layers maintain mouse embryonic
stem cell pluripotency via epigenetic regulation of the c-Myc
promoter. Acta Biochim Biophys Sin (Shanghai). 42:109–115. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park Y, Choi IY, Lee SJ, et al:
Undifferentiated propagation of the human embryonic stem cell
lines, H1 and HSF6, on human placenta-derived feeder cells without
basic fibroblast growth factor supplementation. Stem Cells Dev.
19:1713–1722. 2010. View Article : Google Scholar
|
|
30
|
Xi J, Wang Y, Zhang P, et al: Human fetal
liver stromal cells that overexpress bFGF support growth and
maintenance of human embryonic stem cells. PLoS One. 5:e144572010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stojkovic P, Lako M, Stewart R, et al: An
autogeneic feeder cell system that efficiently supports growth of
undifferentiated human embryonic stem cells. Stem Cells.
23:306–314. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Q, Fang ZF, Jin F, Lu Y, Gai H and
Sheng HZ: Derivation and growing human embryonic stem cells on
feeders derived from themselves. Stem Cells. 23:1221–1227. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li W, Yamashita H, Hattori F, et al:
Simple autogeneic feeder cell preparation for pluripotent stem
cells. Stem Cell Res. 6:83–89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choo A, Ngo AS, Ding V, Oh S and Kiang LS:
Autogeneic feeders for the culture of undifferentiated human
embryonic stem cells in feeder and feeder-free conditions. Methods
Cell Biol. 86:15–28. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park JH, Kim SJ, Oh EJ, et al:
Establishment and maintenance of human embryonic stem cells on STO,
a permanently growing cell line. Biol Reprod. 69:2007–2014. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park SP, Lee YJ, Lee KS, et al:
Establishment of human embryonic stem cell lines from frozen-thawed
blastocysts using STO cell feeder layers. Hum Reprod. 19:676–684.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu C, Jiang J, Sottile V, McWhir J,
Lebkowski J and Carpenter MK: Immortalized fibroblast-like cells
derived from human embryonic stem cells support undifferentiated
cell growth. Stem Cells. 22:972–980. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cai L, Ye Z, Zhou BY, Mali P, Zhou C and
Cheng L: Promoting human embryonic stem cell renewal or
differentiation by modulating Wnt signal and culture conditions.
Cell Res. 17:62–72. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Unger C, Gao S, Cohen M, et al:
Immortalized human skin fibroblast feeder cells support growth and
maintenance of both human embryonic and induced pluripotent stem
cells. Hum Reprod. 24:2567–2581. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hernandez D, Ruban L and Mason C:
Feeder-free culture of human embryonic stem cells for scalable
expansion in a reproducible manner. Stem Cells Dev. 20:1089–1098.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Thomas RJ, Anderson D, Chandra A, et al:
Automated, scalable culture of human embryonic stem cells in
feeder-free conditions. Biotechnol Bioeng. 102:1636–1644. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rodin S, Domogatskaya A, Strom S, et al:
Long-term self-renewal of human pluripotent stem cells on human
recombinant laminin-511. Nat Biotechnol. 28:611–615. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Escobedo-Lucea C and Stojkovic M: Growth
of human embryonic stem cells using derivates of human fibroblasts.
Methods Mol Biol. 584:55–69. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Larijani MR, Seifinejad A, Pournasr B, et
al: Long-term maintenance of undifferentiated human embryonic and
induced pluripotent stem cells in suspension. Stem Cells Dev.
20:1911–1923. 2011. View Article : Google Scholar
|
|
45
|
Rajala K, Hakala H, Panula S, et al:
Testing of nine different xeno-free culture media for human
embryonic stem cell cultures. Hum Reprod. 22:1231–1238. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vazin T and Freed WJ: Human embryonic stem
cells: derivation, culture, and differentiation: a review. Restor
Neurol Neurosci. 28:589–603. 2010.PubMed/NCBI
|