Introduction
Proneurotensin/neuromedin N (proNT/NMN) is the
common precursor of two neuropeptides, namely neurotensin (NT) and
neuromedin N (NMN). It is expressed in the gut, brain, and adrenals
(1), and in the cancer tissues
derived from the pancreas (2) and
colon (3). In these tissues,
proNT/NMN undergoes differential processing, generating different
patterns of maturation products such as NT, NMN, large NT, and
large NMN (4–8). The biological and pharmacological
behaviors of NT have been extensively studied, and NT is known to
regulate digestive processes including gastrointestinal motilities
and pancreatic and biliary secretions (9). In addition, NT stimulates the growth
and proliferation of cancer cells derived from the gut, lungs,
pancreas, and prostate through NT receptors (10–12).
Friry et al (13)
demonstrated that large forms of the processed products are more
resistant to degradation than NT and NMN. Therefore, these products
might function as long-lasting activators of NT receptors. However,
little is known about the behavior of its precursor the
proNT/NMN.
We previously used MS/MS proteomic analysis to
identify secreted proteins produced by lung carcinoma cell lines in
serum-free conditioned medium (14). In addition to the proteins
previously found in serum-free conditioned media, proNT/NMN was
identified in the media from small cell lung carcinoma (SCLC) cell
lines, but not from non-small cell lung carcinoma (NSCLC) cell
lines. These results suggest that proNT/NMN is specifically
secreted from SCLC and might be a potential tumor marker.
To investigate the specific secretion of proNT/NMN
from SCLC, we established in vivo xenograft models of SCLC.
SBC3 cells, which secrete proNT/NMN mostly in SCLC cell lines, were
inoculated into mice, and the plasma proNT/NMN levels of those mice
were detected using specific antibodies. ProNT/NMN was detected in
the plasma and tumor tissues of SBC3 tumor-bearing mice, while it
was not detected in control mice. In addition, plasma proNT/NMN
levels had a high correlation with tumor volume. These results
suggest that SBC3-derived tumors produce and secrete proNT/NMN into
blood.
Materials and methods
Antibodies
We established polyclonal antibodies against the
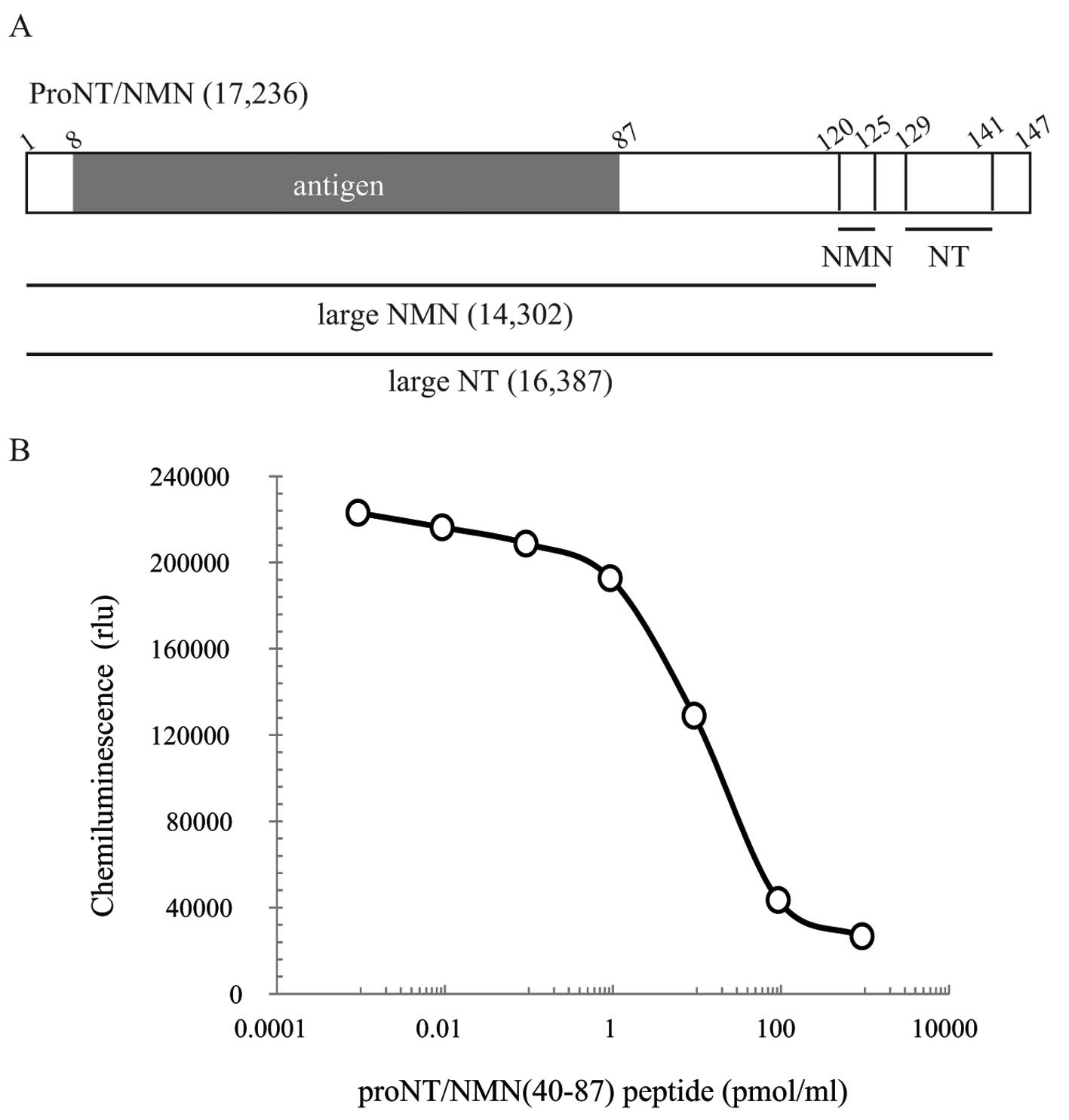
peptide sequences of proNT/NMN (amino acids (aa) 8–87) shown in
Fig. 1A. Chemically synthesized
proNT/NMN (aa 8–87) was conjugated with keyhole limpet hemocyanin
(KLH) and then purified. Antibodies were obtained by immunizing
three SPF Japanese white rabbits with KLH-conjugated peptides.
Cell culture and preparation of
serum-free conditioned medium
SBC3, a small cell lung carcinoma cell line, was
purchased from the Japanese Cancer Research Bank. SBC3 cells were
maintained in RPMI-1640 (Sigma, St. Louis, MO, USA) medium
supplemented with 10% FBS (Gibco, Grand Island, NY, USA),
penicillin (100 U/ml), and streptomycin (0.1 mg/ml) in a humidified
5% CO2 incubator. Serum-free conditioned media was
prepared as previously described (14).
Animals
Six-week-old athymic nude mice (BALB/cAJcl- nu/nu)
were purchased from CLEA Japan (Tokyo, Japan), and NRG mice
(NOD.Cg-Rag1tm1MomIl2rgtm1Wjl/SzJ)
were purchased from Charles River (Wilmington, MA, USA). NRG mice
were bred in the Experimental Animal Facility of Shizuoka Cancer
Center Research Institute, and their offspring was used for
experiments. All animal experiments were carried out in accordance
with the general guidelines for animal experiments of Shizuoka
Cancer Center Research Institute.
In vivo experiments
Nude mice were subcutaneously inoculated with SBC3
cells (5×106 cells in 0.1 ml PBS/mouse) into the right
forelimb, and blood samples were collected once a week. The tumor
size was also measured before blood collection to calculate the
tumor volume. NRG mice were intradermally inoculated with SBC3
cells (5×106 cells in 0.1 ml PBS/mouse) to examine the
effect of tumor resection, and divided into two groups (n=5 per
group). Four weeks after inoculation, tumors were surgically
resected from one group and blood was collected from the
non-resected and control mice after 24, 48 and 120 h. Blood samles
were centrifuged, and plasma was collected. Plasma samples were
stored at −80°C until use.
Preparation of mouse tissue lysates
Frozen tumor tissues were treated with lysis buffer
containing 7.5 M urea, 2.5 M thiourea, 12.5% glycerol, 50 mM
Tris-HCl (pH 7.4), 2.5% N-octylglucoside, 6.25 mM Tris-carboxyethyl
phosphine hydrocholine (TCEP), and 1.25 mM protease inhibitor, and
homogenized by TissueLyser II (Qiagen K.K., Tokyo, Japan).
Following rotation for 1 h, the samples were centrifuged at 14,000
× g for 30 min at 4°C, and the supernatants were collected. Protein
concentrations were determined by the Bradford assay (Bio-Rad
Laboratories, Hercules, CA, USA).
Enzyme-linked immunosorbent assay
(ELISA)
Microtiter plates (96-well, Nunc C8 Maxisorp Immuno
module; Nunc, Roskilde, Denmark) were coated with 100 μl of goat
anti-rabbit IgG (Fc) solution (diluted 1:200 in 0.1 M
NaHCO3; MP Biomedicals, Irvine, CA, USA) and incubated
for 24 h at 4°C. After incubation, the antibody solution was
removed, and the wells were blocked overnight at 4°C by using block
ace solution (DS Pharma Biomedical Co., Ltd., Osaka, Japan).
Following three washes with 0.05% Tween-20 in PBS, the biotinylated
antigen solution [20 ng/ml biotinyl-proNT/NMN (aa 40–87), prepared
in PBS containing 0.1% BSA], the plasma samples, and the antibody
solution [anti-proNT/NMN rabbit polyclonal antibody (105S), 1/3,000
dilution] were added and incubated with shaking for 3 h at room
temperature. Plates were washed with PBS containing 0.05% Tween-20
four times before the addition of labeling solution (streptavidin,
horseradish peroxidase conjugate; Merck, Darmstadt, Germany), and
incubated for 2 h at room temperature. After four final washes, BM
chemiluminescence ELISA substrate (POD) solution (Roche Applied
Science, Mannheim, Germany) was added to each well and allowed to
develop in the dark for 3 min. The chemiluminescence of BM ELISA
was measured using Wallac 1420 ARVO MX (Perkin-Elmer Life Sciences,
Waltham, MA, USA). A standard curve for proNT/NMN levels was
obtained using proNT/NMN (aa 40–87) as the standard antigen.
Immunoblotting
Proteins were separated by SDS-PAGE on 14.25%
polyacrylamide gels and electrophoretically transferred onto
polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA,
USA). Immunoblotting was performed using anti-proNT/NMN rabbit
polyclonal antibodies (105S, 1:1,000 dilution), or anti-neurotensin
goat polyclonal antibodies (N-17 and C-19, 1:1,000 dilution; Santa
Cruz Biotechnology Inc., Santa Cruz, CA, USA) as the primary
antibody, and anti-rabbit IgG-, or anti-goat IgG-horseradish
peroxidase (HRP) conjugate (1:10,000 dilution; Jackson
ImmunoResearch, West Grove, PA, USA) as the secondary antibody.
HRP-dependent luminescence was developed using ECL Prime Western
blotting detection reagent (GE Healthcare UK Ltd., Buckinghamshire,
UK) and detected in a LAS-3000 (Fuji Film, Tokyo, Japan).
Immunohistochemistry
Formalin-fixed tumor tissues were gradually
dehydrated in ethanol and embedded in paraffin. Tissue sections
(3-μm thick) were cut and subjected to immunohistochemistry.
Sections were deparaffinized in xylene, and treated with 1%
H2O2 in methanol for 30 min to block the
endogenous peroxidase activity. After blocking, they were incubated
with anti-proNT/NMN polyclonal antibody (1:200 dilution) or with
the antibody in the presence of the antigen peptide for 30 min at
room temperature. The sections were then washed with PBS and
incubated with peroxidase labeled polymer (goat anti-rabbit IgG;
Dako, Glostrup, Denmark) for 30 min. Following one wash with PBS,
peroxidase activity was initiated with DAB substrate solution
(Dako). The peroxidase reaction was completed, the sections were
washed with PBS, counterstained with hematoxylin, dehydrated,
cleared in xylene, and a coverslip was placed over the
sections.
Statistical analysis
The results of the experiments are expressed as the
mean ± SE values. The statistical significance of differences was
determined according to the Student’s t-test.
Results
Evaluation of the proNT/NMN levels in
tumor-bearing mice
We first established a rabbit polyclonal antibody
against the proNT/NMN (aa 8–87) and developed a competitive ELISA
system. Based on the results of the standard curve with proNT/NMN
(aa 40–87) (Fig. 1B), the proNT/NMN
levels in mouse plasma were measured by ELISA. Every week, plasma
samples were collected from the control and SBC3-inoculated mice
after inoculation. As shown in Fig.
2A, tumor volumes of SBC3-inoculated mouse gradually increased.
Correlating with tumor volumes, the plasma proNT/NMN levels of the
tumor-bearing mice were elevated, while the proNT/NMN levels in
control mouse were not significantly detectable.
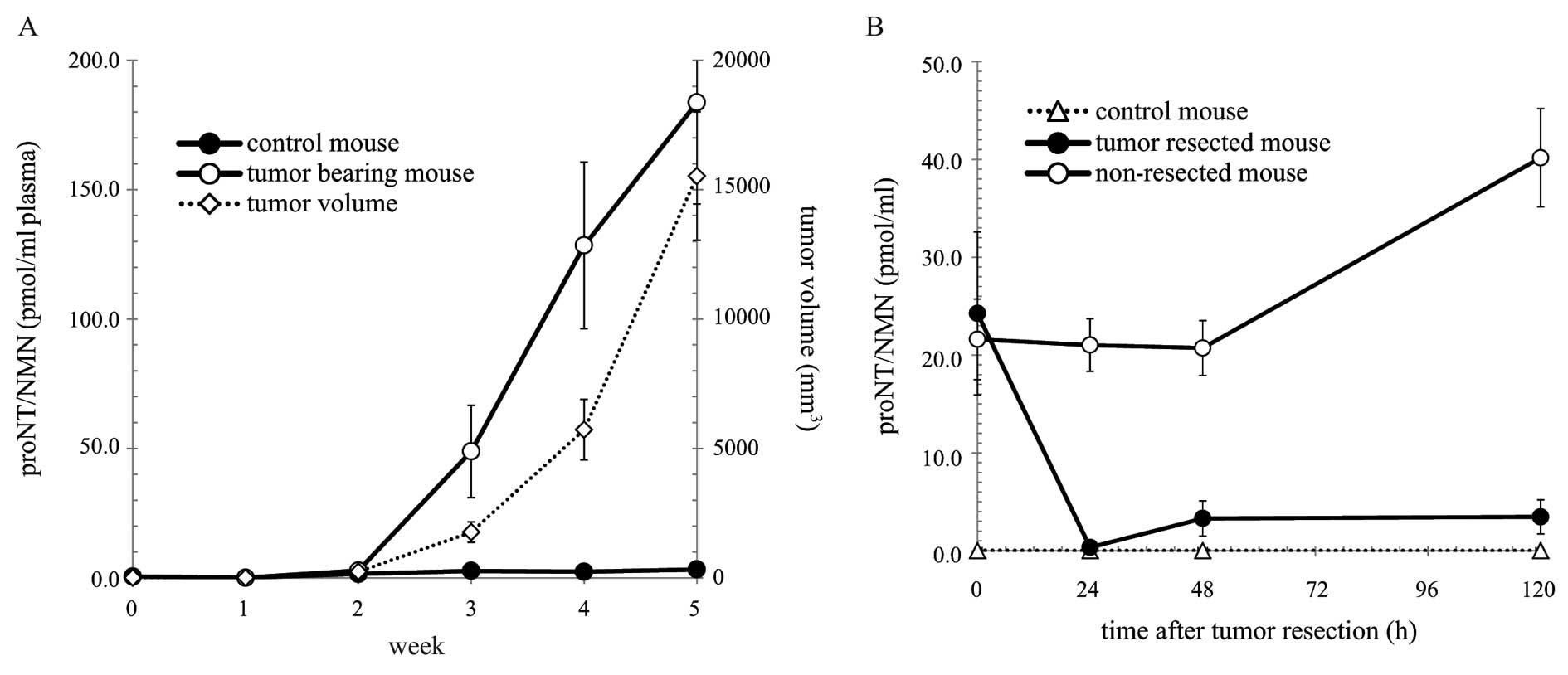 | Figure 2Time course of proNT/NMN levels in the
plasma (A) and the effect of tumor resection on proNT/NMN levels
(B). (A) Plasma was collected from nude mice at 0, 1, 2, 3, 4 and 5
weeks after inoculation. ProNT/NMN levels in the plasma [control
mouse (●) and tumor-bearing mouse (○)] were measured by ELISA, as
described in Materials and methods. The tumor volume (◇) was
calculated using the formula: tumor volume (mm3) =
(length × width2)/2. (B) Four weeks after inoculation,
tumors were resected from the NRG mice, and plasma samples were
collected from all groups of mice at 0, 24, 48 and 120 h after
tumor resection. ProNT/NMN levels in the plasma [control mouse (Δ),
tumor-resected mouse (●) and non-resected mouse (○)] were measured
by ELISA, as described in Materials and methods. Data are expressed
as the means ± SE values in multi-replicate experiments (n=4–5).
Statistical significance (*P<0.05 as compared with
the group of non-resected mouse) was evaluated by Student’s
t-test. |
To further investigate proNT/NMN secretion, tumors
were surgically resected from the NRG mice intradermally inoculated
with SBC3 cells, and the plasma proNT/NMN levels were evaluated
after resection. Compared to nude mice that were subcutaneously
inoculated with SBC3 cells, the tumors of NRG mice grew slowly, and
the plasma proNT/NMN levels were slightly elevated 4 weeks after
inoculation (nude mice, 128.49±32.18 pmol/ml plasma; NRG mice,
22.93±4.67 pmol/ml plasma). The differences in the plasma proNT/NMN
levels between the control and intradermally inoculated NRG mice
were statistically significant. After surgical resection, the
plasma proNT/NMN levels of the tumor-resected mice decreased until
they reached the level identical to that of the control mice
(Fig. 2B). Although the levels of
the tumor-bearing mice (non-resected mice) were significantly
elevated after 120 h, the levels of the tumor-resected mice
remained the same as that of the control mice. These results
clearly suggest that SBC3 tumors secreted proNT/NMN into blood.
Immunoblot detection of proNT/NMN in
mouse plasma
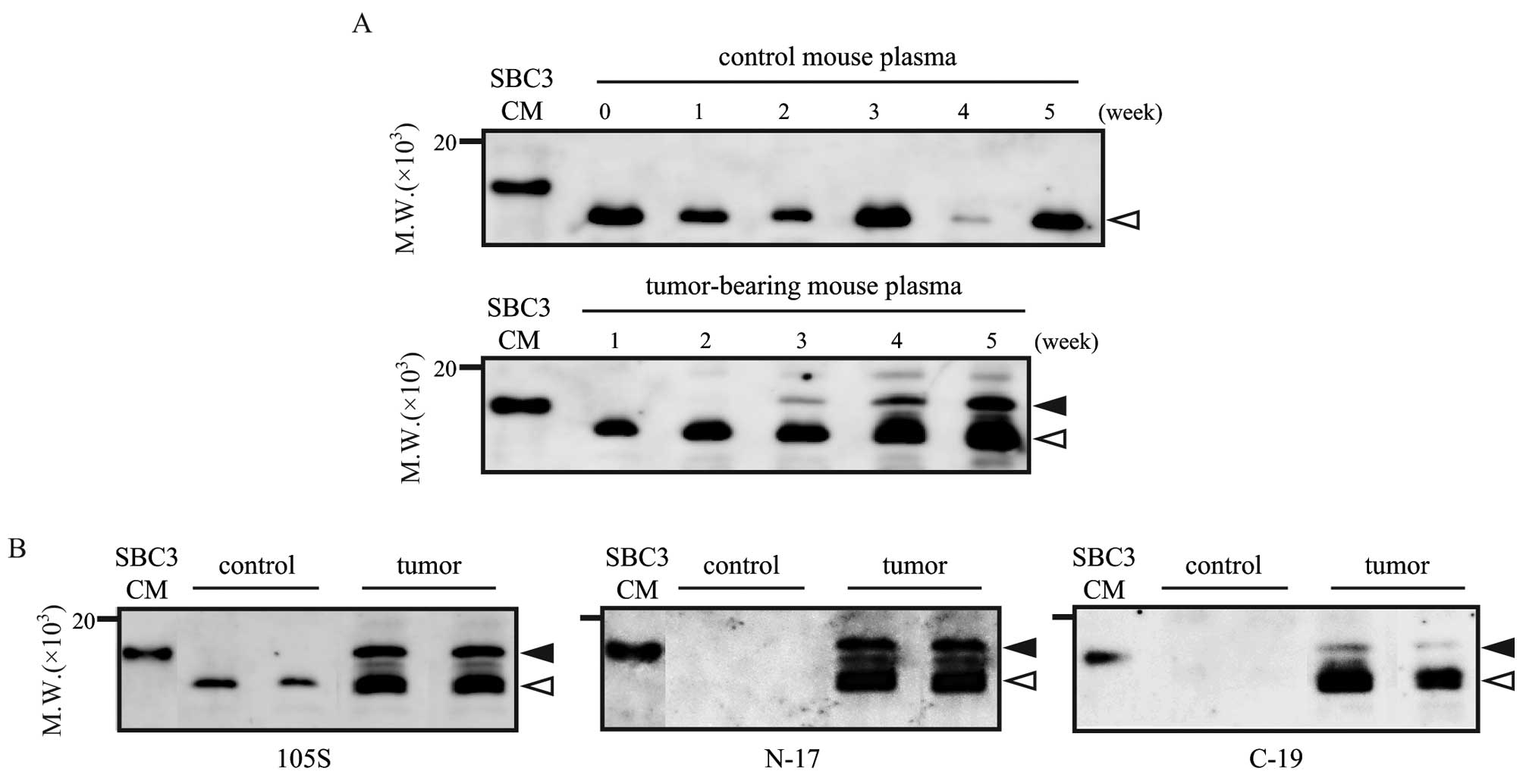
Plasma proNT/NMN was also detected by immunoblotting
using the same antibody as used in the ELISA. In SBC3 serum-free
conditioned medium, the positive band was detected around 17 kDa
(Fig. 3A left line, SBC3-CM). The
medium contains proNT/NMN (14),
and the theoretical molecular mass of proNT/NMN is about 17 kDa,
suggesting that the band detected around 17 kDa is specific to
proNT/NMN. In mouse plasma, several bands were detected around the
molecular mass of 17 kDa. The band at 17 kDa, specific to
full-length proNT/NMN, was only detected in tumor-bearing mouse
plasma; however, the lower bands (~14–16 kDa) were detected in the
plasma samples from both the tumor-bearing and control mice
(Fig. 3A).
To gain more insight into whether the lower bands
are derived from proNT/NMN, immunoblotting was performed using
anti-NT antibodies, N-17 and C-19. Both antibodies can recognize
NT-containing products such as NT, large NT, proNT/NMN, and N-17
can also recognize large NMN. Using these two antibodies, the bands
around 14–16 kDa were detected in the plasma of tumor-bearing
mouse, but not in the plasma from the control mouse (Fig. 3B). These results suggest that the
lower band detected in the control mouse plasma is not derived from
secreted proNT/NMN, but in the tumor-bearing mouse plasma, the
processing products of proNT/NMN such as large NMN and large NT,
were detected around 14–16 kDa.
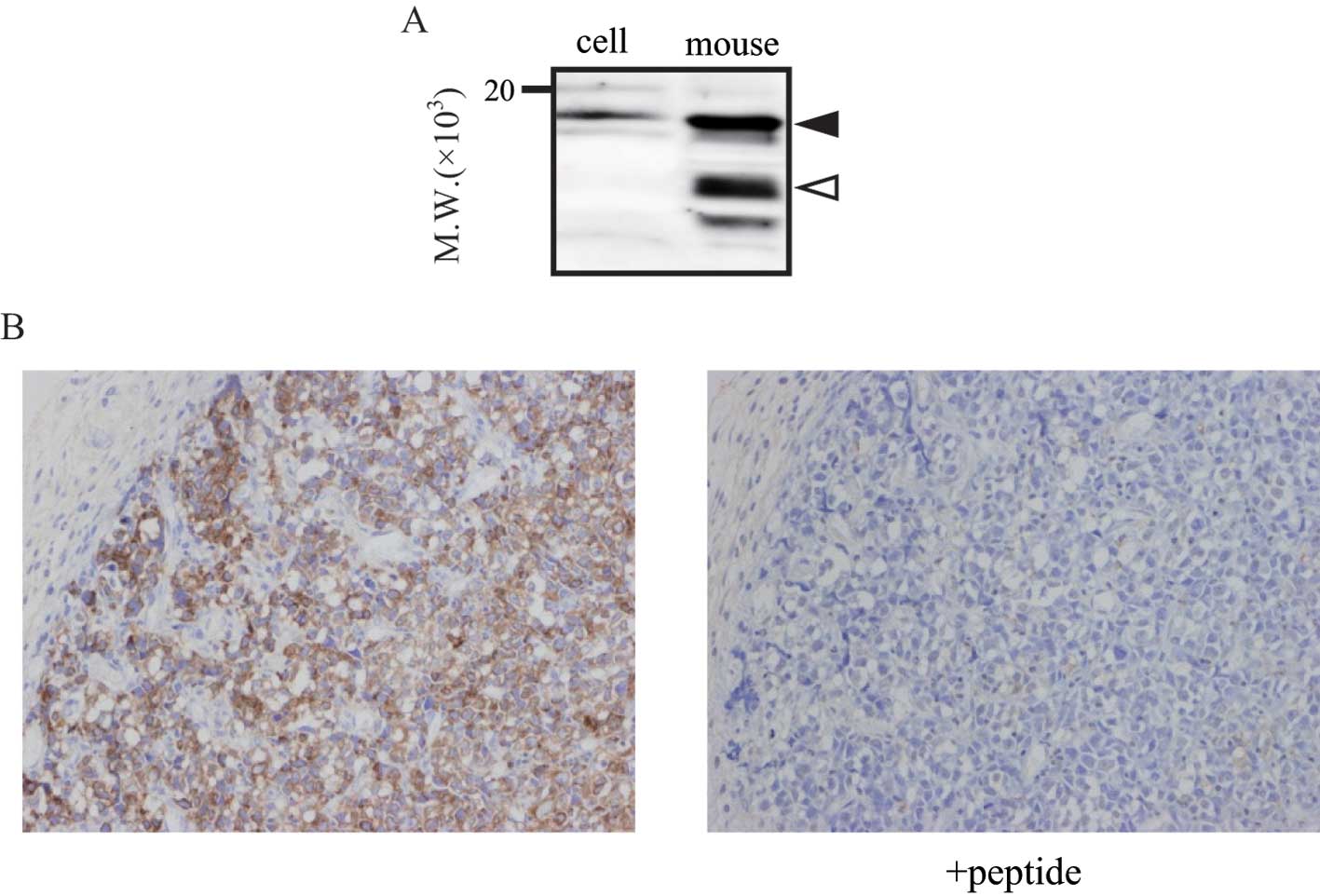
ProNT/NMN expression in tumor
tissues
ProNT/NMN expression in mouse tumor tissues was also
determined by immunoblotting and immunohistochemistry. As with the
plasma samples, the positive bands derived from proNT/NMN were
detected in the SBC3 tumor tissues (Fig. 4A). In addition, Fig. 4B shows that proNT/NMN was expressed
particularly in the superficial layer of the tumor (left panel).
Immunostaining was diminished when the tumor tissue section was
treated with antibody in the presence of the antigen peptide (right
panel). This peptide competition analysis confirmed the expression
of proNT/NMN in the above-mentioned areas in the tumor tissue.
ProNT/NMN expression in the tumor tissues indicated that this
precursor was produced by SBC3 tumors and secreted into plasma as
the tumors grew.
Discussion
Most peptide hormones including neuropeptides are
derived from the processing of larger inactive polypeptide
precursors by prohormone convertases (PCs). PC-mediated processing
often generates different combinations of active peptides. NT is
derived from the large precursor proNT/NMN and this precursor is
mainly processed by PC1, PC2 and PC5 (8,15).
Each PC cleaves at a different site of proNT/NMN; therefore, the
pattern of processing products depends on the PC being expressed.
Previous studies have demonstrated that the major products derived
from proNT/NMN are NMN, NT, and large NT in the brain, large NMN in
the gut, and NT, large NMN, and large NT in the adrenals (4,16–18).
Moreover, proNT/NMN processing occurs in only ~50% of a subset of
colon cancer cell lines, because PC1 and PC2, which are mainly
related to the proNT/NMN processing, are not expressed in these
cell lines (3). Most SCLC cell
lines, including SBC3, do not concurrently express PC1, PC2 and PC5
(data not shown). Only the precursor was detected in the SBC3 cells
and the conditioned medium, whereas the large forms of processing
products were not detected (Fig.
3). On the other hand, not only proNT/NMN, but also the large
processing products, was detected in the plasma of SBC3
tumor-bearing mice. These results suggest that proNT/NMN was the
major product secreted from SCLC and the large forms of processing
products were also produced in the SCLC tumor-bearing mice.
Neuropeptides are generally easy to metabolize, and
therefore, have a short half-life in blood. Gastrin-releasing
peptide (GRP) is one of the prototypical neuropeptide hormones.
Although GRP is produced in high amounts and secreted into blood by
SCLC cells, the unstable nature renders its detection in blood
quite difficult. Therefore, the ProGRP (aa 31–98), which is also
secreted into blood, was established as a useful tumor marker for
SCLC (19–21). NT is also known to be more unstable
than its precursor in vivo (22,23),
as well as in vitro (13).
While the production and secretion of NT, as well as the expression
of NT receptors, were detected in many lung cancer cell lines
including SCLC and NSCLC (24–26),
it remains unclear whether NT or NT receptors are potential useful
tumor markers. In fact, the plasma NT levels in the SBC3
tumor-bearing mice were slightly elevated compared with the control
mice and were detected in the tumors. However, the NT levels were
much lower than the proNT/NMN levels (data not shown). To our
knowledge, this is the first report to investigate proNT/NMN
secretion from SCLC tumors and to evaluate the plasma levels of
proNT/NMN itself. Our findings suggest that proNT/NMN might be a
potential tumor marker for SCLC. To confirm this possibility,
further analysis of the blood samples from SCLC patients is
required.
Acknowledgements
This research was partially supported by the
Ministry of Education, Science, Sports and Culture,
grant-in-cooperation of the Regional Innovation Cluster Program
2010. The authors thank T. Ide for kindly providing technical
assistance.
Abbreviations:
|
aa
|
amino acids
|
|
CM
|
conditioned medium
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
FBS
|
fetal bovine serum
|
|
GRP
|
gastrin-releasing peptide
|
|
HRP
|
horseradish peroxidase
|
|
KLH
|
keyhole limpet hemocyanin
|
|
NMN
|
neuromedin N
|
|
NSCLC
|
non-small cell lung carcinoma
|
|
NT
|
neurotensin
|
|
PC
|
prohormone convertase
|
|
proNT/NMN
|
proneurotensin/neuromedin N
|
|
SCLC
|
small cell lung carcinoma
|
References
|
1
|
Kislauskis E, Bullock B, McNeil S and
Dobner PR: The rat gene encoding neurotensin and neuromedin N.
Structure, tissue-specific expression, and evolution of exon
sequences. J Biol Chem. 263:4963–4968. 1988.PubMed/NCBI
|
|
2
|
Carraway RE, Mitra SP, Feurle GE and Hacki
WH: Presence of neurotensin and neuromedin-N within a common
precursor from a human pancreatic neuroendocrine tumor. J Clin
Endocrinol Metab. 66:1323–1328. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rovere C, Barbero P, Maoret JJ, Laburthe M
and Kitabgi P: Pro-neurotensin/neuromedin N expression and
processing in human colon cancer cell lines. Biochem Biophys Res
Commun. 246:155–159. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carraway RE, Mitra SP and Spaulding G:
Posttranslational processing of the neurotensin/neuromedin-N
precursor. Ann N Y Acad Sci. 668:1–16. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carraway RE, Mitra SP and Muraki K:
Isolation and structures of xenopsin-related peptides from rat
stomach, liver and brain. Regul Pept. 29:229–239. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bidard JN, de Nadai F, Rovere C, et al:
Immunological and biochemical characterization of processing
products from the neurotensin/neuromedin N precursor in the rat
medullary thyroid carcinoma 6–23 cell line. Biochem J. 291:225–233.
1993.PubMed/NCBI
|
|
7
|
Rovere C, Barbero P and Kitabgi P:
Evidence that PC2 is the endogenous pro-neurotensin convertase in
rMTC 6–23 cells and that PC1- and PC2-transfected PC12 cells
differentially process pro-neurotensin. J Biol Chem.
271:11368–11375. 1996.PubMed/NCBI
|
|
8
|
Kitabgi P: Differential processing of
pro-neurotensin/neuromedin N and relationship to pro-hormone
convertases. Peptides. 27:2508–2514. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao D and Pothoulakis C: Effects of NT on
gastrointestinal motility and secretion, and role in intestinal
inflammation. Peptides. 27:2434–2444. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Davis TP, Burgess HS, Crowell S, Moody TW,
Culling-Berglund A and Liu RH: Beta-endorphin and neurotensin
stimulate in vitro clonal growth of human SCLC cells. Eur J
Pharmacol. 161:283–285. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tallett A, Chilvers ER, MacKinnon AC,
Haslett C and Sethi T: Neuropeptides stimulate tyrosine
phosphorylation and tyrosine kinase activity in small cell lung
cancer cell lines. Peptides. 17:665–673. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moody TW, Chiles J, Casibang M, Moody E,
Chan D and Davis TP: SR48692 is a neurotensin receptor antagonist
which inhibits the growth of small cell lung cancer cells.
Peptides. 22:109–115. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Friry C, Feliciangeli S, Richard F,
Kitabgi P and Rovere C: Production of recombinant large
proneurotensin/neuromedin N-derived peptides and characterization
of their binding and biological activity. Biochem Biophys Res
Commun. 290:1161–1168. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ogura S, Kaneko K, Miyajima S, Ohshima K,
Yamaguchi K and Mochizuki T: Proneurotensin/neuromedin N secreted
from small cell lung carcinoma cell lines as a potential tumor
marker. Proteomics Clin Appl. 2:1620–1627. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Villeneuve P, Feliciangeli S, Croissandeau
G, et al: Altered processing of the neurotensin/neuromedin N
precursor in PC2 knock down mice: a biochemical and
immunohistochemical study. J Neurochem. 82:783–793. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shaw C, McKay D, Johnston CF, et al:
Differential processing of the neurotensin/neuromedin N precursor
in the mouse. Peptides. 11:227–235. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carraway RE, Mitra SP and Paradise C:
Characterization of large neuromedin-N using antisera towards
regions of the neurotensin/neuromedin-N precursor. Peptides.
12:601–607. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Nadai F, Rovere C, Bidard JN, Cuber JC,
Beaudet A and Kitabgi P: Post-translational processing of the
neurotensin/neuromedin N precursor in the central nervous system of
the rat. I Biochemical characterization of maturation products.
Neuroscience. 60:159–166. 1994.
|
|
19
|
Yamaguchi K, Abe K, Kameya T, et al:
Production and molecular size heterogeneity of immunoreactive
gastrin-releasing peptide in fetal and adult lungs and primary lung
tumors. Cancer Res. 43:3932–3939. 1983.PubMed/NCBI
|
|
20
|
Miyake Y, Kodama T and Yamaguchi K:
Pro-gastrin-releasing peptide(31–98) is a specific tumor marker in
patients with small cell lung carcinoma. Cancer Res. 54:2136–2140.
1994.
|
|
21
|
Stieber P, Dienemann H, Schalhorn A, et
al: Pro-gastrin-releasing peptide (ProGRP) - a useful marker in
small cell lung carcinomas. Anticancer Res. 19:2673–2678.
1999.PubMed/NCBI
|
|
22
|
Barelli H, Fox-Threlkeld JE, Dive V,
Daniel EE, Vincent JP and Checler F: Role of endopeptidase
3.4.24.16 in the catabolism of neurotensin, in vivo, in the
vascularly perfused dog ileum. Br J Pharmacol. 112:127–132. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Checler F, Amar S, Kitabgi P and Vincent
JP: Catabolism of neurotensin by neural (neuroblastoma clone
N1E115) and extraneural (HT29) cell lines. Peptides. 7:1071–1077.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moody TW, Carney DN, Korman LY, Gazdar AF
and Minna JD: Neurotensin is produced by and secreted from classic
small cell lung cancer cells. Life Sci. 36:1727–1732. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moody TW: Peptide hormones and lung
cancer. Panminerva Med. 48:19–26. 2006.
|
|
26
|
Alifano M, Souaze F, Dupouy S, et al:
Neurotensin receptor 1 determines the outcome of non-small cell
lung cancer. Clin Cancer Res. 16:4401–4410. 2010. View Article : Google Scholar : PubMed/NCBI
|