Introduction
Ovarian cancer is one of the most common
gynecological malignancies and the leading cause of death from
gynecological cancers in women (1).
Despite considerable efforts to improve early detection and the
advances in chemotherapy, metastasis remains a major challenge in
the clinical management of ovarian cancer. Approximately 70% of
patients present with tumors that have spread beyond the ovaries
(2). However, the mechanism through
which a primary ovarian cancer cell develops into a metastatic
phenotype is not well understood.
Ikaros, a member of a family of zinc finger
transcription factors, is encoded by the IKZF1 gene (also known as
ZNFN1A1) that comprises 8 exons. Alternative splicing of exons 3–6
can generate multiple Ikaros isoforms (3,4).
Ikaros was originally found to function as a critical regulator of
lymphocyte differentiation. Subsequent studies reported that Ikaros
also plays a role in hematopoietic stem cells and some myeloid
cells (5–8). Moreover, Ikaros has also been shown to
be expressed in mouse pituitary tissues, where it regulates the
expression of adrenocorticotropic hormone and the adrenocortical
hormone output (9), and several
other tissues including liver, lung, prostate, brain, heart,
placenta and intestine, in which the role of Ikaros is largely
unknown. A recent report showed that the expression of Ikaros was
correlated with the prognosis of several kinds of cancers including
breast, lung, ovarian and skin cancers (10), suggesting a possible role of Ikaros
in solid tumors. However, the expression and functional role of
Ikaros in ovarian cancer have not been well studied.
In this study, through immunohistochemical analysis,
we found that Ikaros is expressed at higher levels in human ovarian
cancer tissues than normal ovarian tissues, and Ikaros expression
level is correlated with the different stages of ovarian serous
adenocarcinoma cancer. Furthermore, we demonstrated that Ikaros may
perform a dual role in ovarian cancer cells, that is, inhibiting
cell proliferation and enhancing cell migration and invasion. We
show that Slug (also known as Snail 2), an epithelial-mesenchymal
transition associated protein, plays an important role in
Ikaros-induced migration and invasion in ovarian cancer cells.
Materials and methods
Cell cultures
Human epithelial ovarian cancer cell line SKOV3 was
obtained from ATCC (Manassas, VA). SKOV3 and HEK293T cell lines
were cultured in RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO) or
Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich)
supplemented with 10% fetal bovine serum (FBS, Gibco BRL,
Gaithersburg, MD). All cell lines were incubated in a 5%
CO2/95% air humidified atmosphere at 37°C.
Retrovirus production and transduction of
target cells
The pMSCV-puro-Flag-IK1 plasmid was obtained as
reported previously (11). To
produce virus, pGag-pol and pVSVG (Clontech, Palo Alto, CA) were
co-transfected with pMSCV-puro-Ikaros or vehicle plasmid into 293T
using FuGENE6 (Roche Applied Science, Basel, Switzerland) according
to the manufacturer’s instructions. Retrovirus-containing
supernatant was harvested 48 h after transfection. The day before
retrovirus infection, 3×105 cells were seeded in 2 ml
growth medium. On the next day, the growth medium was aspirated
from the plate, 0.5 ml growth medium was added, and 2 ml
retrovirus-containing supernatant was mixed with polybrene (Sigma,
St. Louis, MO) to a final concentration of 2 μg/ml. Forty-eight
hours later, 1 μg/ml puromycin (Sigma) was added to the medium.
Positive polyclone population was identified based on Flag-IK1
expression.
Quantitative real-time polymerase chain
reaction
Total cellular RNA was extracted by TRIzol kit
(Invitrogen), followed by treatment with RNase-free DNase (Promega,
Madison, WI). Complementary DNA was synthesized according to the
manufacturer’s instructions (Promega). Fluorescence real-time
quantitative RT-PCR was performed with the double-stranded DNA dye
SYBR Green PCR Core Reagents (PE Biosystems, Warrington, UK) using
the ABI PRISM 7900 system (Perkin-Elmer, Torrance, CA). The
specific primers used are shown in Table I. Real-time quantitative RT-PCR was
performed and data were analyzed according to a previous report
(12).
 | Table IPrimers for real-time PCR. |
Table I
Primers for real-time PCR.
| Gene name | Forward
primers | Reverse
primers |
|---|
| Snail1 |
5′-TGCCCTCAAGATGCACATCCGA-3′ |
5′-GGGACAGGAGAAGGGCTTCTC-3′ |
| Slug |
5′-ATCTGCGGCAAGGCGTTTTCCA-3′ |
5′-GAGCCCTCAGATTTGACCTGTC-3′ |
| β-catenin |
5′-CACAAGCAGAGTGCTGAAGGTG-3′ |
5′-GATTCCTGAGAGTCCAAAGACAG-3′ |
| E-cadherin |
5′-GCCTCCTGAAAAGAGAGTGGAAG-3′ |
5′-TGGCAGTGTCTCTCCAAATCCG-3′ |
| N-cadherin |
5′-CCTCCAGAGTTTACTGCCATGAC-3′ |
5′-GTAGGATCTCCGCCACTGATTC-3′ |
| MMP-2 |
5′-AGCGAGTGGATGCCGCCTTTAA-3′ |
5′-CATTCCAGGCATCTGCGATGAG-3′ |
| MMP-9 |
5′-GCCACTACTGTGCCTTTGAGTC-3′ |
5′-CCCTCAGAGAATCGCCAGTACT-3′ |
| GAPDH |
5′-CCACTCCTCCACCTTTGAC-3′ |
5′-ACCCTGTTGCTGTAGCCA-3′ |
Tissue microarrays and
immunohistochemistry
An ovarian cancer tissue array (US Biomax Inc.,
Rockville, MD) containing ovarian tumors from 87 patients and 10
cases of normal tissues were used for this study. Deparaffinization
and antigen retrieval were accomplished by using Trilogy solution
(Cell Marque, Rocklin, CA) and heating/pressure supplied by a
conventional pressure cooker. Endogenous peroxidase activity was
inhibited by using 0.3% hydrogen peroxide. Nonspecific interactions
were blocked by using normal goat serum. Ikaros antibody (ab26083,
Abcam) was diluted (1:100) in PBS and incubated at 4°C overnight.
Bound antibody was detected by using biotin-linked anti-rabbit
secondary antibody and streptavidin-conjugated HRP enzyme in
conjunction with DAB chromagen. Tissue was counterstained with
hematoxylin. The immunoreactivity was defined by discrete brownish
chromogen deposit in the cells. To quantify Ikaros expression, we
further analyzed the cases with Ikaros expression by IPP (Image-Pro
Plus, version 5.0, Media Cybernetics, Silver Spring, MD) using a
method introduced by Xavier et al (13).
Cell proliferation assay
Cell proliferation was determined by Cell Counting
kit-8 assay (Dojindo, Japan), a sensitive nonradioactive
colorimetric assay for determining cell growth, according to the
manufacturer’s instructions.
Cell cycle analysis
Briefly, Cells were fixed in 75% ethanol,
resuspended in staining solution containing 50 μg/ml propidium
iodide (PI) and 100 μg/ml RNase A, and incubated at 37°C for 30
min. DNA content was analyzed by flow cytometry on a FACScan
(Becton-Dickinson).
Scratch-wound assay
Cells were seeded to form a monolayer on 6-well
plate surface and then a scratch wound was performed by dragging a
sterile pipette tip across the layer. Detached cells were washed
away with cell culture medium. An image was captured immediately by
Olympus BX51 fluorescence microscope and the wound distance was
calculated as a basic width. After 24 h, cells were washed 3 times
by PBS and another image was taken and the width of the wound
distance was calculated. The wound closure (%) was determined as
the width migrated after 24 h relative to the basic width.
Transwell migration and invasion
assay
Cell migration and invasion were gauged using a
transwell migration assay and a matrigel invasion assay. Cell
migration was examined using transwell chamber assay according to
the protocol of the manufacturer (Costar). Briefly,
2.0×105 cells in RPMI-1640 plus 1% FBS in 200 μl of
RPMI-1640 were placed on each 8.0-μm pore size upper chamber.
RPMI-1640 plus 10% FBS was placed in the bottom wells as
chemoattractant. The invasion assay used a BD Biocoatt Matrigelt
Invasion Chamber (BD Biosciences, Bedford, MA, USA) with an 8.0-μm
pore size polyethylene terephthalate (PET) membrane coated with
matrigel. The inserts were rehydrated by adding 0.5 ml of warm
culture medium at 37°C for 2 h. The cells were seeded
(2.0×105 cells in 0.5 ml of serum-free medium) in the
upper chamber and cultured as described in the method for the
migration assay. After 24 h, the nonmigrated or noninvaded cells on
top of the membrane were gently removed with a cotton swab. Cells
that had migrated or invaded were stained with 0.1% crystal violet
(Sigma) and were counted and photographed under a microscope at a
magnification of 400 in five to six randomly selected areas.
Western blot analysis
Western blotting was performed as previously
described (11). Antibodies for
western blotting are anti-Slug (9585), anti-Snail1 (3879),
anti-β-catenin (9582), anti-P21 (2947), anti-cyclin D1 (2922) from
Cell Signaling, anti-P27 (Santa Cruz, SC-528), anti-flag (F1804,
Sigma), anti-Ikaros (ab26083, Abcam), anti-β-actin (CP01,
Calbiochem), and anti-β-tubulin (T4026, Sigma). All experiments
were repeated at least three times.
RNA interference and transfection
All RNAi oligonucleotides were chemically
synthesized by GenePharma Co. (Shanghai, China). These RNAi
oligonucleotides were transfected into cells by using the
Lipofectamine 2000 transfection kit (Invitrogen) according to the
manufacturer’s instructions. The siRNA sequences were as follows:
nonspecific control (NC) 5′-UUCUCCGAACGUGUCACGU-3′, Slug-1 siRNA
human (S1) 5′-GGACUACCGCUGCUCCAUU-3′, Slug-2 siRNA human (S2)
5′-GACCCACACAUUACCUUGU-3′ and Slug-3 siRNA human (S3)
5′-GCACAAACAUGAGGAAUCU-3′.
Statistical analysis
The χ2 test was applied to test for a
possible association between Ikaros expression and histological
grade and type. Kruskal-Wallis test was performed to examine the
significance of Ikaros expression among the groups as determined by
IPP. Student’s t-test was used to evaluate the difference between
two different groups. All statistical analyses were performed using
the SPSS software package (version 17.0, SPSS, Inc., Chicago, IL).
Tests were 2-sided and P<0.05 was considered as statistically
significant.
Results
Ikaros is expressed at high levels in
human ovarian cancer tissues
To evaluate the expression levels of Ikaros in
ovarian cancer, immunohistochemistry was performed on tissues from
87 cases of ovarian cancer and 10 cases of normal (only intact
tissue samples were included). Compared with the weak positive
staining of IK1 observed in 10% (1/10) of normal ovarian tissues,
significant increase of positive staining of IK1 in 71% (62/87) of
ovarian cancer were observed (Table
II, P<0.001).
 | Table IIClinicopathological features of
ovarian tissue with regard to the relative expression of Ikaros
protein. |
Table II
Clinicopathological features of
ovarian tissue with regard to the relative expression of Ikaros
protein.
| Tissue | No. of
specimens | No. and ratio of
positive expression (%) |
|---|
| Normal ovary | 10 | 1/10 (10) |
| All malignant
tumors | 87 | 62/87 (71)a |
| Serous
adenocarcinoma | 58 | 50/58 (86) |
| Mucous
adenocarcinoma | 6 | 2/6 (33) |
| Adult granulosa
cell tumor | 7 | 3/7 (43) |
| Clear cell
carcinoma | 5 | 2/5 (40) |
| Dysgerminoma | 4 | 2/4 (50) |
| Endometrioid
adenocarcinoma | 3 | 2/3 (67) |
| Sertoli-Leydig
cell tumor | 1 | 0/1 (0) |
| Mixed germ cell
tumors | 1 | 0/1 (0) |
| Malignant
teratoma | 2 | 1/2 (50) |
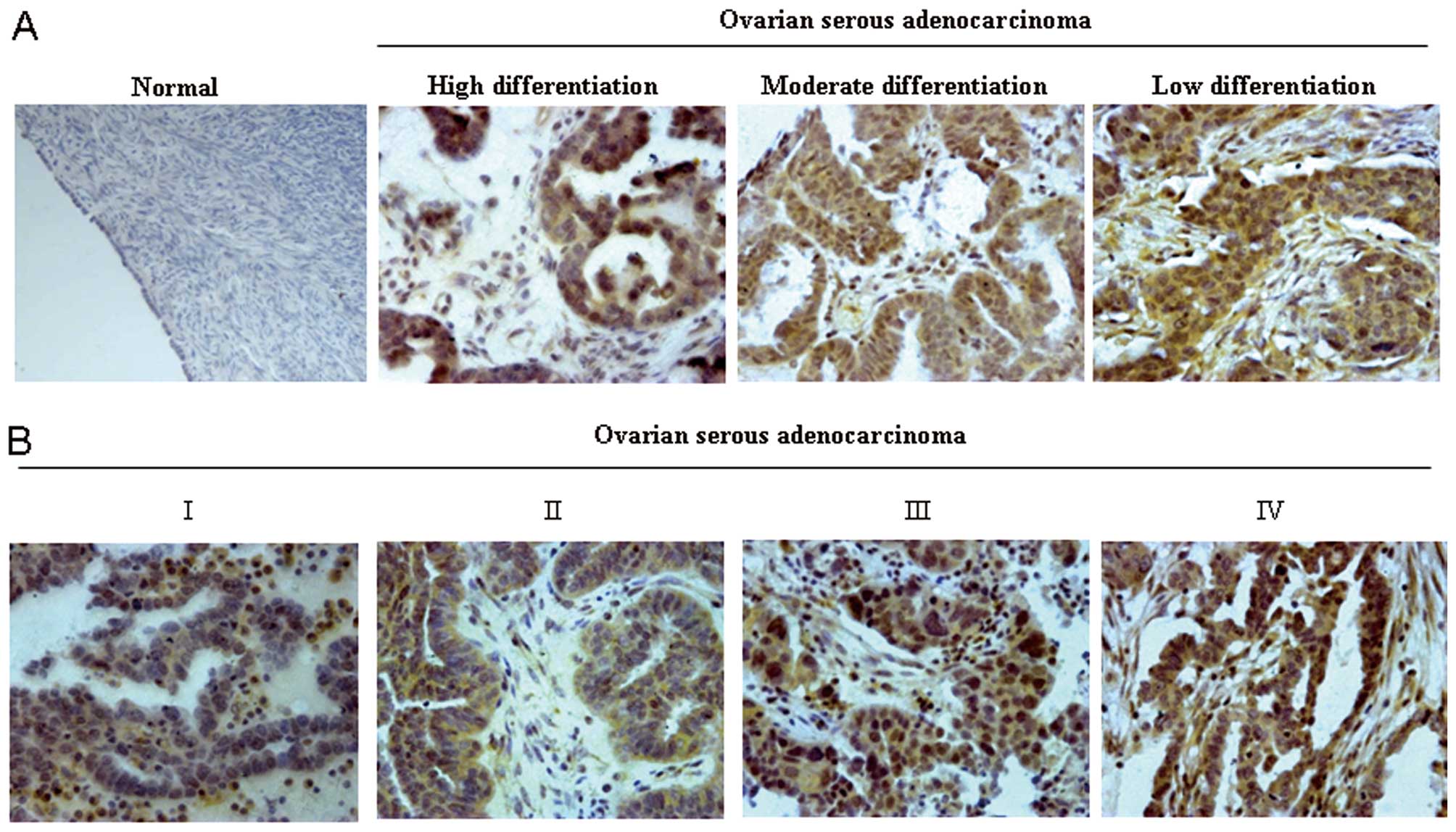
We further analyzed the 10 normal ovarian tissues
and 58 specimens of ovarian serous adenocarcinoma (OSA) tissues.
The latter represents the most common subtype of epithelial ovarian
cancer. Representative immunohistochemical findings of IK1 in
tissue specimens are shown in Fig.
1. Significantly higher expression of IK1 was observed in
malignant cancer tissues than in normal ovarian tissues and
significant differences were also observed among different
pathology grades (Table III,
P<0.001 and Fig. 1A) and
clinical stages of OSA (Table
III, P=0.005). Further analysis showed that there was a
significant increase of IK1 expression in advanced-stage cancers
(II, III, IV), where lymph nodes or distant metastases were
present, in comparison to early-stage cancers (I) (Table III, P=0.001 and Fig. 1B). These data indicated that IK1 may
be involved in the metastasis of ovarian cancer cells.
 | Table IIICorrelation analysis of Ikaros and
clinical manifestation of ovarian serous adenocarcinoma. |
Table III
Correlation analysis of Ikaros and
clinical manifestation of ovarian serous adenocarcinoma.
| IOD |
|---|
| |
|
|---|
| Variables | N | Mean ± SD | P-value |
|---|
| Histology | | | <0.001 |
| Normal | 10 |
67901.3±38438.2 | |
| G1 | 12 |
204987.6±71071.0 | |
| G2 | 18 |
335607.7±100766.5 | |
| G3 | 28 |
368952.4±92663.5 | |
| Stage | | | 0.005 |
| I | 26 |
270296.2±82500.65 | |
| II | 13 |
341853.1±107934.8 | |
| III | 14 |
408530.3±119316.5 | |
| IV | 5 |
382052.8±63569.11 | |
| Metastasis | | | 0.001 |
| Negative (I) | 26 |
270296.2±82500.7 | |
| Positive (II, III,
IV) | 32 |
377305.6±109417.6 | |
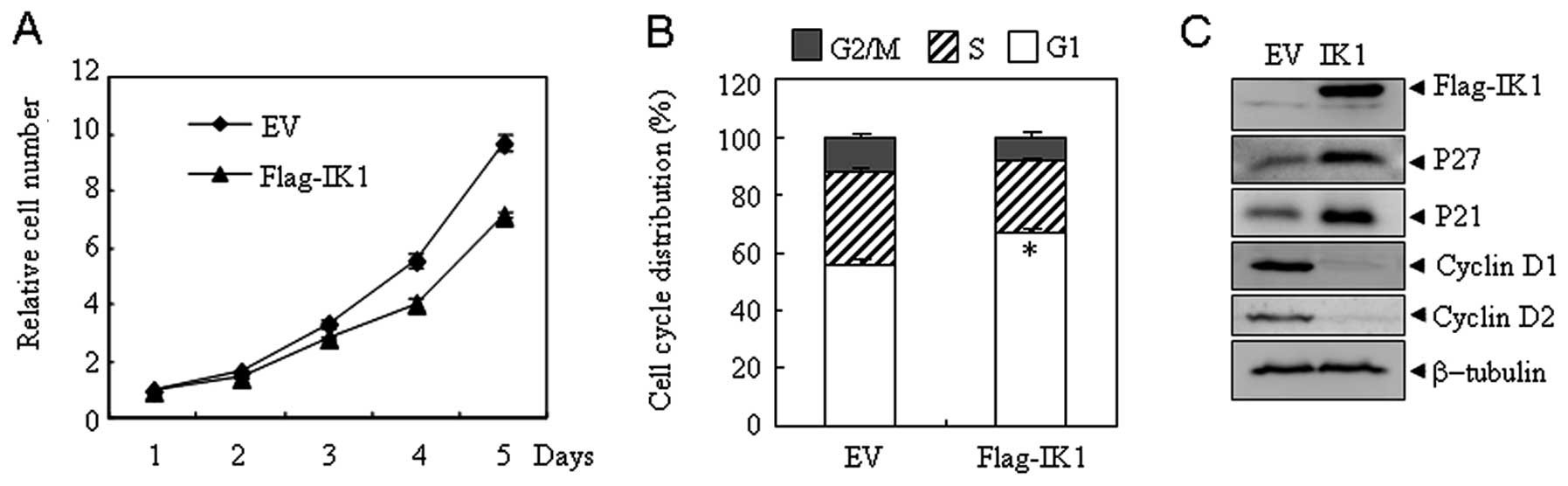
Overexpression of IK1 inhibits the
proliferation of ovarian OSA cells
To investigate the potential role of IK1 in OSA, OSA
cell line SKOV3 expressing low levels of IK1 were stably
transfected with IK1 (SKOV3Flag-IK1) or the empty vector
(SKOV3EV). As shown in Fig.
2A, significant cell proliferation inhibition was observed in
SKOV3Flag-IK1 cells compared with SKOV3EV
cells. In addition, IK1 overexpression elicited a significant
increase in the number of SKOV3 cells in the
G0/G1 phase with a concomitant decline in the
S and G2/M phases (Fig.
2B). Consistent with the G1-arrest phenotype,
SKOV3Flag-IK1 cells showed a dramatic elevation in the
expression of the cell cycle inhibitors (14,15),
P21 and P27, along with a substantial decrease in the expression of
the cell cycle inducers (16,17),
cyclin D1 and D2 (Fig. 2C). Similar
results were observed in another OSA cell line HO8910 (data not
shown), indicating that IK1-induced inhibition of cell
proliferation is not SKOV3 cell specific.
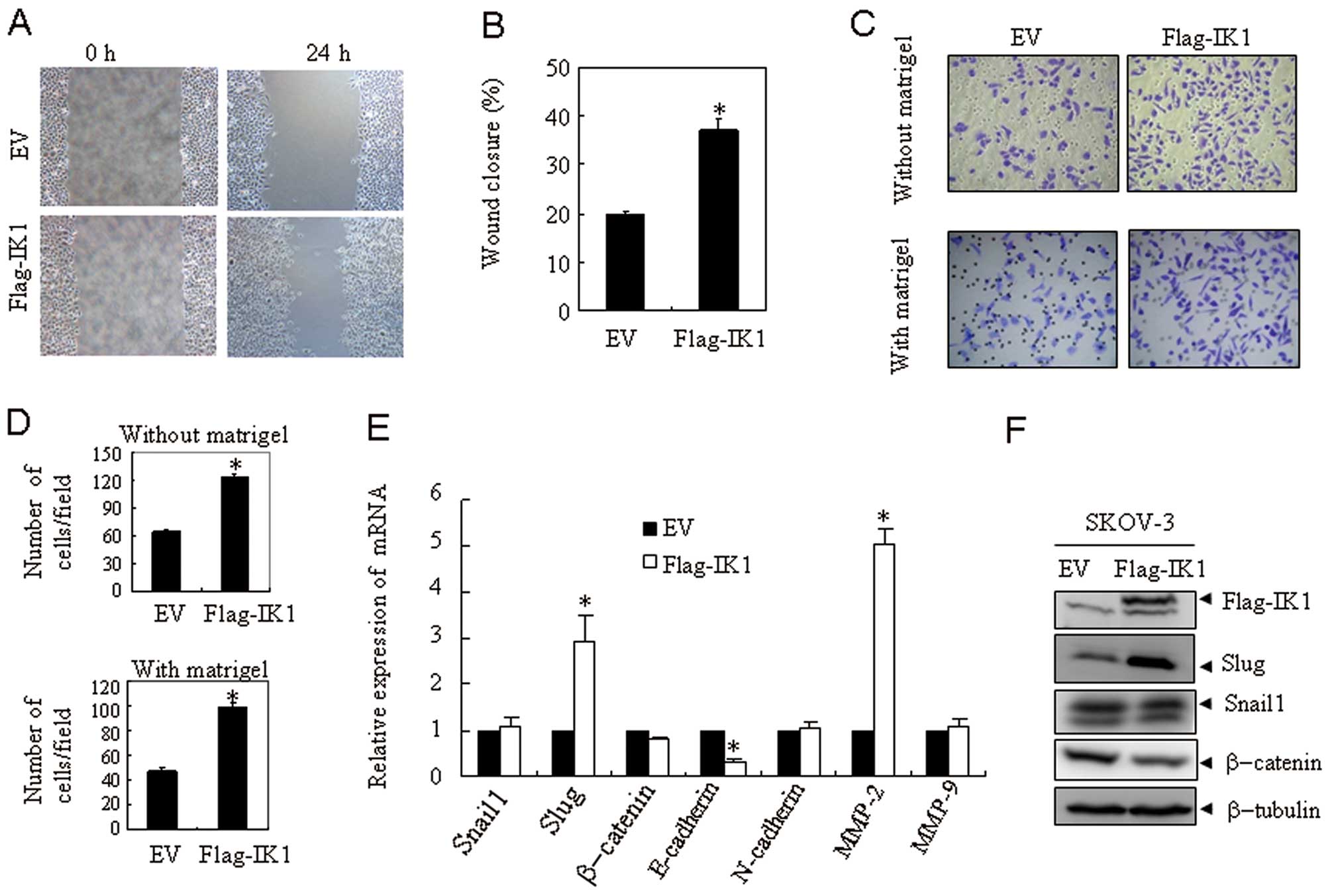
Overexpression of IK1 enhances migration
and invasion of OSA cells
Since tissues analysis suggested that IK1 expression
is associated with progression of ovarian cancer, we next
investigated the effect of IK1 on the metastasis of ovarian cancer.
The scratch wound healing assay (Fig.
3A), and transwell chamber assays (Fig. 3C) were performed to compare the
migration and invasion capability between SKOV3EV and
SKOV3Flag-IK1 cells. At 24 h, the
SKOV3Flag-IK1 cells showed ~2-fold increase in migration
and invasion capability (Fig. 3B and
D). Similar results were also observed in HO8910 cells (data
not shown).
IK1 overexpression influences expression
of metastasis-related genes
To investigate how IK1 affects the metastatic
process, a series of metastasis-related genes including Snail1,
Slug, β-catenin, E-cadherin, N-cadherin, matrix metalloproteinase-2
(MMP-2), and matrix metalloproteinase-9 (MMP-9) were examined in
SKOV3Flag-IK1 and SKOV3EV cells. At mRNA
level, overexpression of IK1 in SKOV3 cells resulted in a
significant increase in the expression of Slug and MMP-2 and a
significant decrease of E-cadherin. A slight downregulation of
β-catenin was also observed (Fig.
3E). At the protein level, overexpression of IK1 in SKOV3 cells
resulted in a marked increase of Slug and a decrease of β-catenin
(Fig. 3F). Similar results were
also observed in HO8910 cells (data not shown). These results
indicated a potential role for Slug in IK1-induced migration and
invasion of OSA cells.
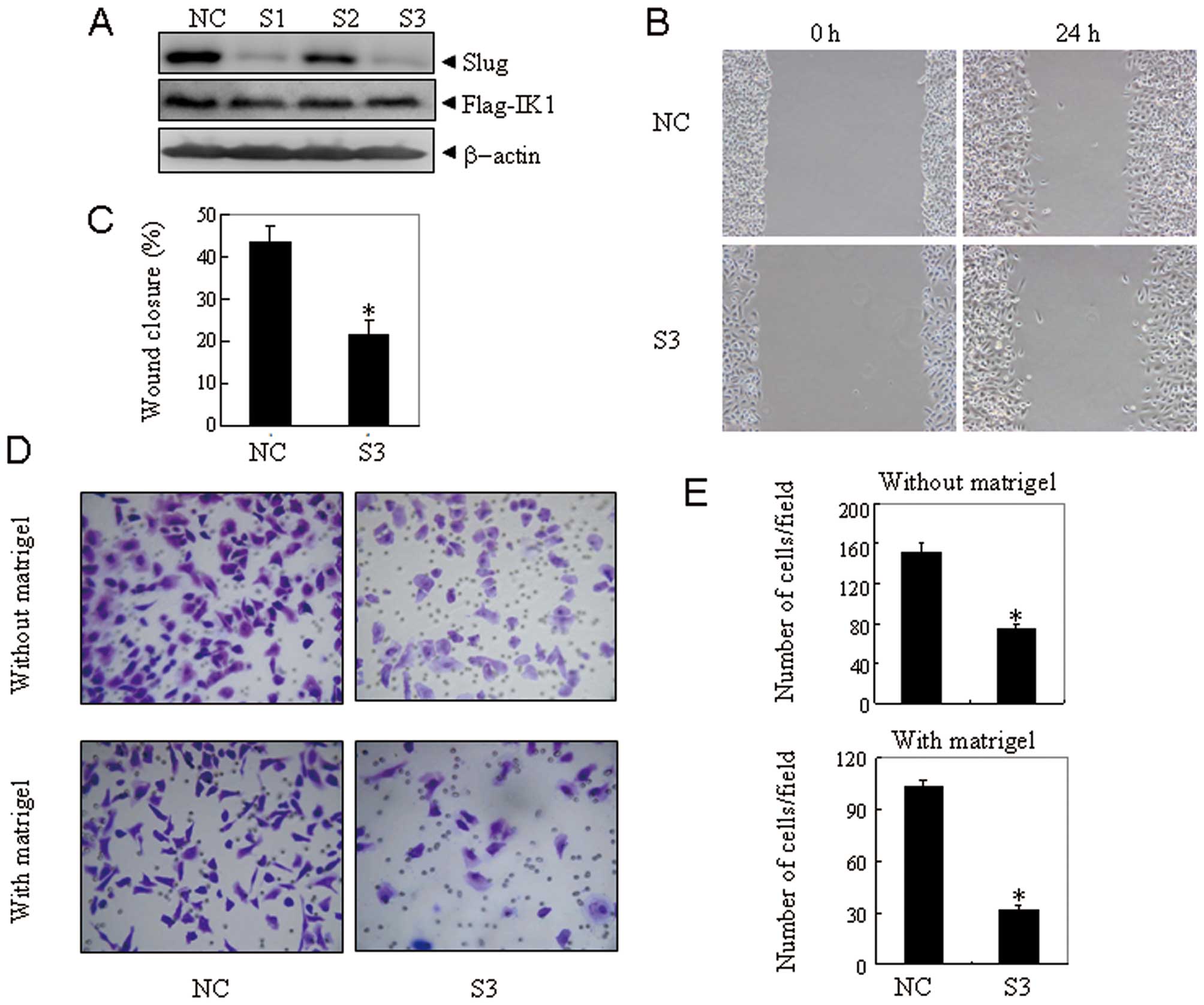
Slug is responsible for IK1-induced
migration and invasion of OSA cells
To illustrate whether Slug mediates IK1-induced
migration and invasion of ovarian cancer, Slug was specifically
knocked down by RNA interference. As shown in Fig. 4A, the target sequence S1 and S3
against Slug were efficient and specific, as the expression of Slug
was efficiently suppressed with no effect on IK1 expression. Next,
nonspecific control vector (NC) and S3 were transiently transfected
into SKOV3Flag-IK1 cells. Seventy-two hours after
transfection, the scratch wound healing assay (Fig. 4B), traswell migration assay, and
matrigel invasion assay (Fig. 4D)
were performed. The results revealed that the suppression of Slug
expression could significantly block IK1-induced migration and
invasion of SKOV3Flag-IK1 cells (Fig. 4C and E). These results indicated
that Slug contributed to IK1-induced cell migration and invasion in
ovarian cancer cells.
Discussion
The ability to metastasize makes ovarian cancer a
fatal disease and a significant number of patients treated for the
localized disease ultimately present with metastases. A better
understanding of molecular events that contribute to tumor invasion
and metastasis is crucial for developing novel treatment strategies
for ovarian cancer.
Although most studies on Ikaros are restricted to
the hematopoietic system, Ikaros plays a role in other tissues
(9,18–21).
In this study, we show for the first time that Ikaros is involved
in the metastasis and invasion of ovarian cancer cells. First,
higher expression of IK1 was observed in malignant ovarian cancer
tissues and was significantly associated with the high FIGO stage
and low differentiation state in OSA; second, overexpression of IK1
in OSA cells resulted in enhanced cell invasion and metastasis.
Consistent with our results, Yamamoto et al (19) reported that IK1 is involved in
migration and invasion of extravillous trophoblasts in early
placentation, although the underlying mechanism is unknown.
Slug is a member of the Snail family of zinc finger
transcription factors that play a central role in the patterning of
vertebrate (22). Recent evidence
showed that Slug is upregulated in metastatic breast cancer,
mesothelioma, and ovarian cancer, and plays an important role in
cancer invasion (23,24). It is interesting to note that Slug
is also implicated in IK1-induced cell migration and invasion.
While IK1 significantly upregulated the expression of Slug,
knocking down Slug abrogated IK1-induced cell migration and
invasion. It is known that Slug could regulate the cell metastasis
and invasion through regulation of the expression of junctional
proteins such as E-cadherin and matrix metalloproteinase, which can
degrade the ECM components and is believed to play a major role in
invasion and metastasis (25–29).
In support of this, E-cadherin and MMP-2, two Slug target genes,
could also be downregulated and upregulated, respectively. Of note,
the effect of IK1 on Slug is relatively specific, as Snail1,
another member of the Snail family that plays a functional role in
ovarian cancer metastasis, was not altered by IK1
overexpression.
One intriguing finding of this study is that IK1
plays a dual role in ovarian cancer cells, inhibiting cell
proliferation on one hand and increasing metastatic ability on the
other hand. Although apparently conflicting, this kind of
phenomenon is not restricted to the Ikaros family. TGF-β signaling
has been shown to function as a double-edged sword in ovarian
cancer development, a tumor suppressor in early tumorigenesis but a
tumor enhancer in advanced-stage cancer (30,31).
Similar situation may exist for IK1 in the progression of ovarian
cancer. In our microarrays study, we found metastatic, poorly
differentiated cancer tissues tend to express higher levels of IK1
protein, indicative of the contribution of IK1 in ovarian cancer
progression.
In conclusion, we provide evidence that
overexpression of IK1 in ovarian cancer cells play a dual role in
proliferation, migration and invasion and Slug upregulation
contributes to IK1-induced increase of cell migration and invasion.
This study reveals a new link between a hematopoietic transcription
factor and the metastasis of ovarian cancer cells. Further studies
on Ikaros-induced Slug upregulation may provide novel targets for
inhibiting ovarian cancer metastasis.
Acknowledgements
This study was supported in part by grants from
National Basic Research Program of China (973 Program) (No.
2009CB918404, 2010CB912104), National Natural Science Foundation of
China (31100980, 81172322, 81070433, 91013008), Science and
Technology Committee of Shanghai (11JC1406500), Innovation Program
of Shanghai Municipal Education Commission (13YZ028) and SMC
Program of Shanghai Jiao Tong University.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Naora H and Montell DJ: Ovarian cancer
metastasis: integrating insights from disparate model organisms.
Nat Rev. 5:355–366. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Molnar A and Georgopoulos K: The Ikaros
gene encodes a family of functionally diverse zinc finger
DNA-binding proteins. Mol Cell Biol. 14:8292–8303. 1994.PubMed/NCBI
|
|
4
|
Koipally J and Georgopoulos K: A molecular
dissection of the repression circuitry of Ikaros. J Biol Chem.
277:27697–27705. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Georgopoulos K, Bigby M, Wang JH, et al:
The Ikaros gene is required for the development of all lymphoid
lineages. Cell. 79:143–156. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dumortier A, Kirstetter P, Kastner P and
Chan S: Ikaros regulates neutrophil differentiation. Blood.
101:2219–2226. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang JH, Nichogiannopoulou A, Wu L, et al:
Selective defects in the development of the fetal and adult
lymphoid system in mice with an Ikaros null mutation. Immunity.
5:537–549. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Payne KJ, Huang G, Sahakian E, et al:
Ikaros isoform x is selectively expressed in myeloid
differentiation. J Immunol. 170:3091–3098. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ezzat S, Mader R, Yu S, Ning T, Poussier P
and Asa SL: Ikaros integrates endocrine and immune system
development. J Clin Invest. 115:1021–1029. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang L, Luo Y and Wei J: Integrative
genomic analyses on Ikaros and its expression related to solid
cancer prognosis. Oncol Rep. 24:571–577. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He LC, Xu HZ, Gu ZM, et al: Ikaros is
degraded by proteasome-dependent mechanism in the early phase of
apoptosis induction. Biochem Biophys Res Commun. 406:430–434. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao KW, Li X, Zhao Q, et al: Protein
kinase Cdelta mediates retinoic acid and phorbol myristate
acetate-induced phospholipid scramblase 1 gene expression: its role
in leukemic cell differentiation. Blood. 104:3731–3738. 2004.
View Article : Google Scholar
|
|
13
|
Xavier LL, Viola GG, Ferraz AC, et al: A
simple and fast densitometric method for the analysis of tyrosine
hydroxylase immunoreactivity in the substantia nigra pars compacta
and in the ventral tegmental area. Brain Res Brain Res Protoc.
16:58–64. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sherr CJ and Roberts JM: CDK inhibitors:
positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alt JR, Gladden AB and Diehl JA: p21(Cip1)
promotes cyclin D1 nuclear accumulation via direct inhibition of
nuclear export. J Biol Chem. 277:8517–8523. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Masamha CP and Benbrook DM: Cyclin D1
degradation is sufficient to induce G1 cell cycle arrest despite
constitutive expression of cyclin E2 in ovarian cancer cells.
Cancer Res. 69:6565–6572. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ely S, Di Liberto M, Niesvizky R, et al:
Mutually exclusive cyclin-dependent kinase 4/cyclin D1 and
cyclin-dependent kinase 6/cyclin D2 pairing inactivates
retinoblastoma protein and promotes cell cycle dysregulation in
multiple myeloma. Cancer Res. 65:11345–11353. 2005. View Article : Google Scholar
|
|
18
|
Ezzat S and Asa SL: The emerging role of
the Ikaros stem cell factor in the neuroendocrine system. J Mol
Endocrinol. 41:45–51. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamamoto E, Ito T, Abe A, et al: Ikaros is
expressed in human extravillous trophoblasts and involved in their
migration and invasion. Mol Hum Reprod. 11:825–831. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ezzat S, Yu S and Asa SL: Ikaros isoforms
in human pituitary tumors: distinct localization, histone
acetylation, and activation of the 5′ fibroblast growth factor
receptor-4 promoter. Am J Pathol. 163:1177–1184. 2003.PubMed/NCBI
|
|
21
|
Elliott J, Jolicoeur C, Ramamurthy V and
Cayouette M: Ikaros confers early temporal competence to mouse
retinal progenitor cells. Neuron. 60:26–39. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alves CC, Carneiro F, Hoefler H and Becker
KF: Role of the epithelial-mesenchymal transition regulator Slug in
primary human cancers. Front Biosci. 14:3035–3050. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kurrey NK, KA and Bapat SA: Snail and Slug
are major determinants of ovarian cancer invasiveness at the
transcription level. Gynecol Oncol. 97:155–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Catalano A, Rodilossi S, Rippo MR, Caprari
P and Procopio A: Induction of stem cell factor/c-Kit/slug signal
transduction in multidrug-resistant malignant mesothelioma cells. J
Biol Chem. 279:46706–46714. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liotta LA, Abe S, Robey PG and Martin GR:
Preferential digestion of basement membrane collagen by an enzyme
derived from a metastatic murine tumor. Proc Natl Acad Sci USA.
76:2268–2272. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Batlle E, Sancho E, Franci C, et al: The
transcription factor snail is a repressor of E-cadherin gene
expression in epithelial tumour cells. Nat Cell Biol. 2:84–89.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shih JY and Yang PC: The EMT regulator
slug and lung carcinogenesis. Carcinogenesis. 32:1299–1304. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kenny HA and Lengyel E: MMP-2 functions as
an early response protein in ovarian cancer metastasis. Cell cycle.
8:683–688. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Akhurst RJ and Derynck R: TGF-beta
signaling in cancer - a double-edged sword. Trends Cell Biol.
11:S44–S51. 2001.PubMed/NCBI
|
|
31
|
Chou JL, Chen LY, Lai HC and Chan MW:
TGF-beta: friend or foe? The role of TGF-beta/SMAD signaling in
epigenetic silencing of ovarian cancer and its implication in
epigenetic therapy. Expert Opin Ther Targets. 14:1213–1223. 2010.
View Article : Google Scholar : PubMed/NCBI
|