Introduction
Gastric cancer is the second leading cause of cancer
death in the world behind lung cancer (1,2).
Gastric carcinogenesis is a multistep process including many
genetic and epigenetic alterations, such as abnormalities in DNA
mismatch repair genes, growth factors/receptors, angiogenic factors
or cell cycle regulators. These abnormalities can also define
biological characteristics of gastric cancer, which can play a role
in therapy (3,4). Although genetic abnormalities which
include gene mutation and deletion are remarkable in causing
oncogene activation and tumor suppressor gene inactivation,
epigenetic silence of tumor suppressor genes through aberrant
promoter hypermethylation have also been confirmed to be frequent
in gastric carcinoma (5,6). Gene silencing by promoter
hypermethylation has been affirmed in several genes in gastric
cancer, including CDH1, which is involved in cell adhesion, and
hMLH1, which is associated with DNA mismatch repair and the cell
cycle regulator p16. In addition, promoter hypermethylation of MAL
has been shown to be an independent prognostic marker for gastric
cancer (7–9). DNA high methylation of tumor
suppressor genes frequently occurs in the early stage of human
carcinogenesis, so investigating the methylation of these gene
promoters may contribute to the diagnosis, prognosis and target
therapy in gastric carcinoma.
Cadherin molecules are pivotal in producing and
preserving tissue structure in tumorigenesis (10,11).
Such as E-cadherin, it is a classical tumor suppressor which is
mutated in gastric carcinoma and lobular breast carcinoma (12). Accumulating data have suggested that
protocadherins can function as tumor suppressors. Protocadherins 10
and 20 are frequently silenced in carcinomas of the nasopharynx and
lung due to promoter methylation and inhibit cell migration and
proliferation (13,14). Protocadherin 17 in esophageal
squamous cell carcinoma is also frequently silenced because of
promoter methylation (15). The
protocadherin 8 (PCDH8) gene localizes on the human chromosome
13q14.3, is an adhesion protein with six cadherin repeats that
organizes the formation and polarity of developing cellular
structures in embryos (16).
It has been shown that PCDH8 is epigenetically
silenced caused by promoter hypermethylation in most of breast
tumors and it can suppress breast epithelial migration and
proliferation (17). To the best of
our knowledge, the expression of PCDH8 and its promoter
hypermethylation has not yet been shown in gastric cancers.
Therefore, in this study, we first confirmed the expression of
PCDH8 and the methylation of its gene promoter in human gastric
cancer cell lines and determined the role of 5-aza-2′-deoxycytidine
(5-AZA, a drug that inhibits the DNA methyltransferase
(DNMT)-mediated hypermethylation of promoter region CpG islands) in
regulation of PCDH8 expression in gastric cancer cells. We also
detected the methylation of PCDH8 gene promoter in tissue specimens
and found the association between PCDH8 gene promoter methylation
and clinicopathological characteristics of gastric cancer.
Materials and methods
Human gastric samples
A total of 65 primary gastric adenocarcinomas and
their adjacent non-cancer specimens, and 10 normal gastric
specimens from patients with normal endoscopy results were obtained
from the Department of General Surgery, First Affiliated Hospital
of the Medical College of Xi’an Jiaotong University, Xi’an, China
between January 2009 and January 2010. All specimens were
immediately frozen in liquid nitrogen and stored at −80°C until
use. The gastric cancer cases were clinically and pathologically
verified. Standard protocols established by the Hospital’s
Protection of Human Subjects Committee were followed in this
study.
Cell lines and culture
A total of four gastric cancer cell lines (SGC7901,
MKN45, MKN28, and BCG823) and the immortalized gastric mucosal
epithelial cell line (GES-1) were purchased from the Laboratory
Animal Research Centre of the Fourth Military Medical University at
Xi’an, China. All cell lines were cultured in RPMI-1640 medium
(Gibco BRL, Gaithersburg, MD, USA) supplemented with 10% fetal
bovine serum in a humidified incubator with 5% CO2 and
95% air at 37°C.
These cells were passaged at a ratio of 1:3 with
trypsin once they reached confluence (~106 cells) into
752 cm culture flasks (Sarstedt, Newton, NC). For treatment with
5-aza-2′-deoxycytidine, these cell lines were split and cultured at
a low density (30% confluence) overnight and then treated with
5-aza-2′-deoxycytidine (Sigma, St. Louis, MO) at a concentration of
1 μmol/l for up to 72 h. The growth medium was refreshed every 24
h, and at the end of the treatment, DNA and RNA from these cells
were isolated as described below.
Bisulphite treatment of DNA,
methylation-specific polymerase chain reaction (MSP)
Genomic DNA from these cell lines and tissue
specimens were extracted by a DNA mini kit (Qiagen, Valencia, CA,
USA). Methylation status of PCDH8 in gastric tissues and cell lines
were determined by MSP. Briefly, 2 mg of genomic DNA was
bisulphite-treated with Zymo DNA Modification kit (Zymo Research,
Orange, CA, USA). Bisulphite- treated DNA was used as a template
for MSP and quantitative PCR by ABI 2700 thermocycler (Applied
Biosystems, CA, USA) and LightCycler (Roche Diagnostic),
respectively. Oligo 6 software (Molecular Biology Insights, CO,
USA) was used for designing primers specific for methylated of each
amplification. The extracted DNA was then dissolved in Tris-EDTA
(TE) buffer and stored at −20°C. To assess the methylation levels
of the PCDH8 gene promoter, genomic DNA from gastric cancer cell
lines and tissue specimens were first subjected to bisulfite
treatment and then methylation-specific polymerase chain reaction
(MSP) as described previously (18). The MSP primers for PCDH8 were
designed and synthesized according to genomic sequences skirting
the presumed transcription start sites for PCDH8. The primer
sequences were: PCDH8-UN 5′ CCT ACG CGG GCA GCT ACC T 3′,
PCDH8-UN-AS 5′ CGC GTT GTC GTT CTC GTC G 3′, PCDH8-ME-S 5′ GTG CGT
TGC GTT TTT TAT GG 3′ and PCDH8-ME-AS 5′ CGC GTT ATC GTT CTC GTC G
3′. Each MSP reaction incorporated ~100 ng of bisulfite-treated
DNA, 25 pmol of each primer, 100 pmol dNTPs, 2.5 μl 10X PCR buffer,
and 1 U of JumpStart RedTaq Polymerase (Sigma) in a final reaction
volume of 25 μl. The PCR amplification conditions were an initial
95°C for 5 min and then 35 cycles of 95°C for 30 sec, 60°C for 30
sec, and 72°C for 30 sec and a final extension at 72°C for 5 min
and then stored at 4°C. The MSP products were separated on 2%
agarose gel electrophoresis and visualized under the ultraviolet
(UV) light.
RNA isolation and semi-quantitative
reverse transcription PCR
Total cellular RNA from the cell lines was isolated
using the TRIzol reagent (Invitrogen) according to the WJG
manufacturer’s instructions. RNA quality and quantity were assessed
using agarose gel electrophoresis (1%) and spectrophotometric
analysis of 260/280 ratios. The RNA was stored at −70°C prior to
use. The first strand cDNA was synthesized with oligo-(dT) primer
using a reverse transcriptase kit from Invitrogen. RNA (2 μg) was
subjected to the first strand cDNA synthesis, and 1 μl cDNA from RT
reaction was subjected to PCR amplification of gene expression in a
total 25-μl reaction volume. The PCR amplification was carried out
using primer sets derived from the published PCDH8 gene sequences:
PCDH8 primers: 5′-TGGCGGTGTGGAAAGGACA-3′ and
5′-CGGAGTGACCTGTATATGTG-3′ (17).
This primer set, designed to cross the intronic sequences, can
prevent from amplification of genomic DNA for control of genomic
DNA contamination during RNA isolation. A total of 32 cycles of PCR
amplification were performed based on our pre-experiment for
semi-quantitative measurement of PCDH8 gene expression levels.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified for
25 cycles as an internal control of equal loading and cDNA quality
and quantity. The sequence of GAPDH primers:
5′-GACCACAGTCCATGCCATCAC-3′ and 5′-GTCCACCACCCTGTTGCTGTA-3′. The
PCR products (PCDH8, 251 bp; GAPDH, 150 bp) were then
electrophoresed in 1.5% agarose gels containing ethidium bromide
and reviewed under the UV light. Primers were designed according to
GenBank, NCBI. For the validation, each experiment was done in
triplicate.
Protein extraction and western
blotting
The cells were grown and treated with or without 1
μM 5-aza-2′-deoxycytidine for 72 h and total cellular protein was
then extracted from these cells in 200 μl ice-cold mild lysis
buffer containing 10 μl nonidet P-40, 0.15 mol/l NaCl, 0.01 mol/l
sodium phosphate (pH 7.2), 2 mmol/l EDTA, 50 mmol/l sodium
fluoride, 0.2 mmol/l sodium vanadate, and 1 μg/ml aprotinin. The
cell mixture was centrifuged at 20,000 r/min for 15 min and
supernatants were then collected. The concentration of protein was
quantified by the BCA protein assay from Pierce (Rockford, IL, USA)
and an equal amount of protein was separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then
transferred onto PDVF membranes (Millipore, Billerica, MA, USA).
Western blot analyses were then carried out using mouse monoclonal
anti-PCDH8 antibody (JK-19) (sc-81817 Santa Cruz Biotechnology,
Inc., USA) or an anti-β-actin antibody (Boster, Wuhan, China). The
blots were developed with chemiluminescence substrate solution from
Pierce and exposed to X-ray film.
Immunofluorescence
BGC-823 cells expressing PCDH8 epitope-tagged
proteins were plated onto sterile cover slips in a 6-well dish.
Sixteen hours after plating, cells were fixed in 2%
paraformaldehyde in phosphate-buffered saline (PBS) pH 7.4 for 30
min at room temperature. Cells were washed for 20 min in PBS,
permeabilized for 1 h in buffer A (5% goat serum, 0.1% Triton X-100
in PBS), and incubated with 1:1000 dilution of mouse monoclonal
anti-PCDH8 antibody (JK-19) (sc-81817 Santa Cruz Biotechnology) in
buffer A. Cells were washed in PBS, and incubated with 1:600
dilution of Alexafluor 568 antimouse antibody (Molecular Probes,
Invitrogen Corp., Carlsbad, CA, USA) and counterstained with
40,6-diamidino-2-phenylindole dihydrochloride (DAPI; 0.15 mg/ml in
water).
Detection of apoptosis
Gastric cancer cells were treated with or without 1
μM 5-aza-2′-deoxycytidine for up to 72 h. FCM was performed with PI
and fluorescein isothiocyanate (FITC)-labeled annexin V (Joincare
Biosciences, Zhuhai, China). After the treatment, the remaining
intact cells were incubated at 37°C for 24 h, and then the cells
were washed with cool PBS at 4°C. After a centrifugation at 1500
rpm for 5 min, 500 μl of 1X binding buffer, 5 μl of FITC-labeled
Annexin V and 10 μl of PI were added to the cell suspension and
gently mixed. After incubation at 25°C for 10 min in the dark, the
cells were analyzed by FCM.
Migration assays
Equal numbers of cells were plated on a 6-well
plate. A single wound was introduced using a P20 pipette tip and
media was replaced. Migration was assessed at indicated times.
Statistical analysis
The results were expressed as mean ± SD. The
statistical analyses of the experimental data were carried out
using SPSS 16.0 software for Windows (Chicago, IL). P-values for
dichotomous variables were 2-tailed and based on the Pearson
χ2 test or the Pearson χ2 test with
continuity correction. Statistical differences were estimated by
One-way analysis of variance (ANOVA) followed by Dunnett’s test.
P<0.05 was considered statistically significant.
Results
Silence of PCDH8 expression through methylation of
PCDH8 gene promoter and 5-aza-2′-deoxycytidine induction of PCDH8
gene expression in gastric cancer cell lines.
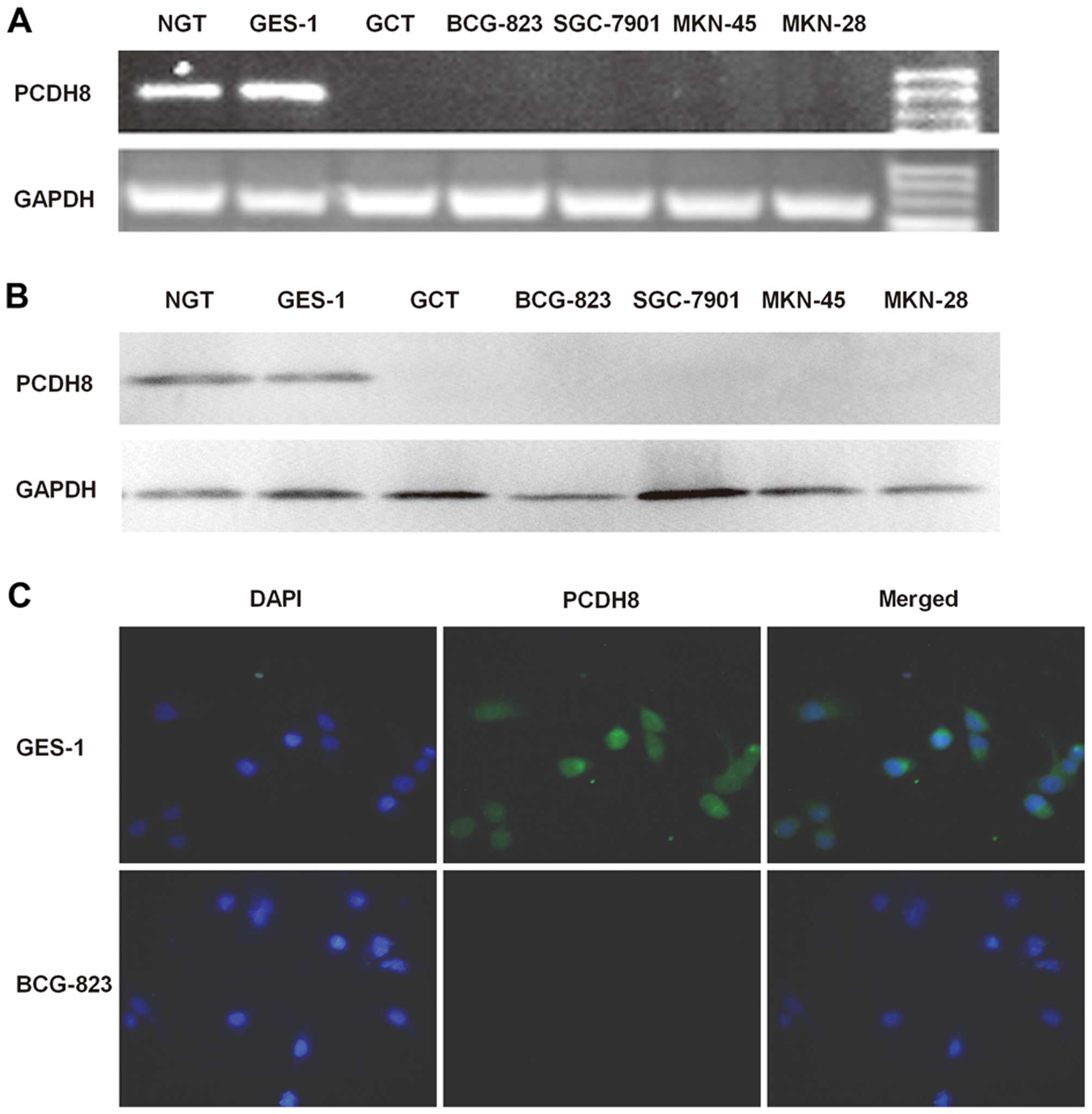
We detected PCDH8 expression in normal gastric and
gastric cancer cell lines or tissues and found that PCDH8 mRNA or
protein was lost in multiple gastric cancer cell lines: SGC7901,
MKN45, MKN28, and BC- G823 cell lines and gastric cancer tissue,
but expressed in GES-1 cell line and normal gastric tissue. The
immunofluorescent results also confirmed the above findings
(Fig. 1). To find out whether the
silence of PCDH8 gene expression is caused by methylation of the
PCDH8 gene promoter, we investigated the methylation status of
PCDH8 promoter in 4 gastric cancer cell lines. The MSP analysis
showed that PCDH8 gene promoter was highly methylated in SGC7901,
MKN45, MKN28, and BCG823 cell lines, but not methylated in GES-1
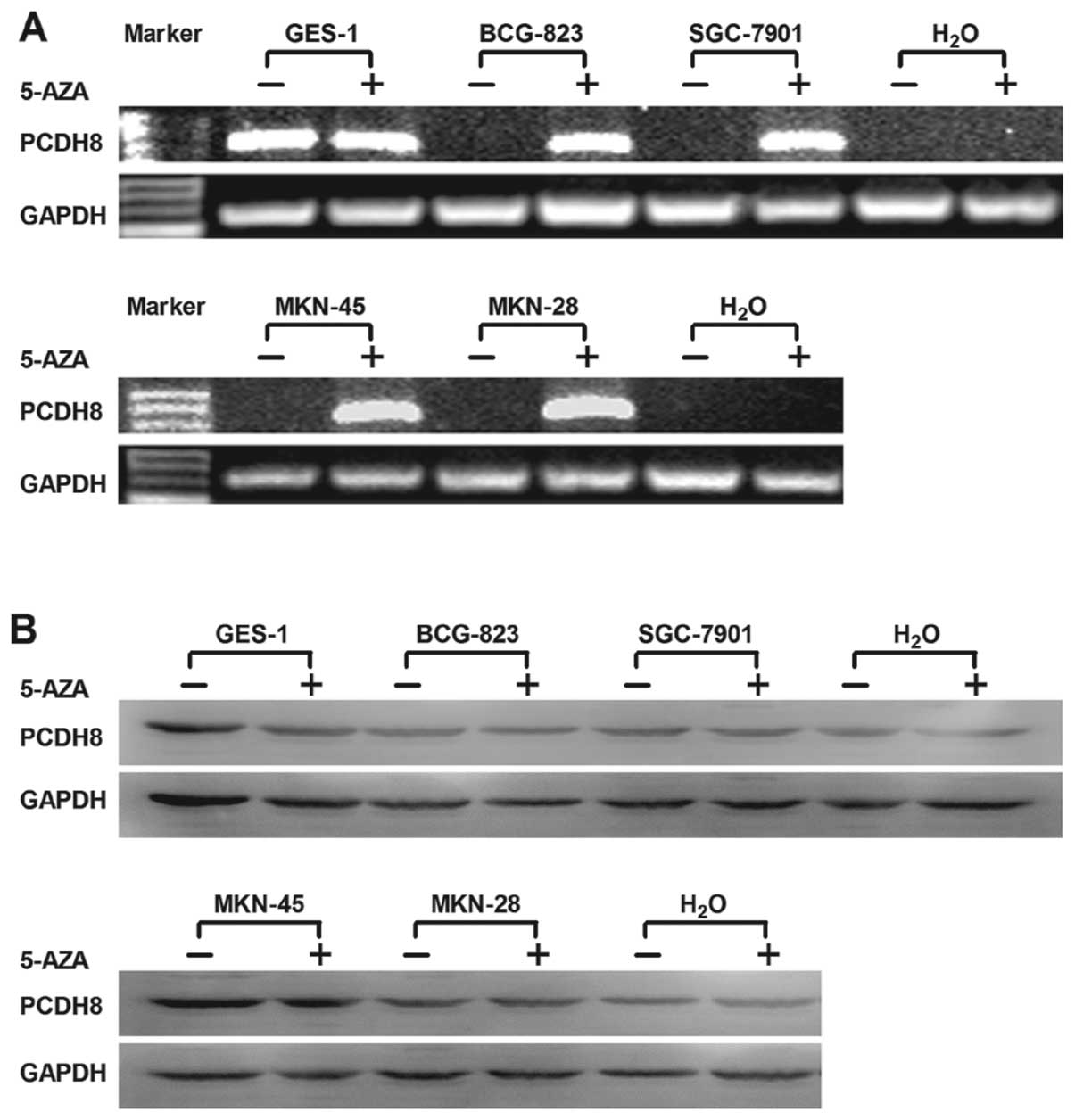
cell line (Fig. 2A). PCDH8 mRNA or
protein expression was induced or upregulated in these cell lines
after we treated them with 5-aza-2′-deoxycytidine (Fig. 3). PCDH8 expression was induced after
the effect of demethylating agent, which demonstrated the silence
of PCDH8 expression in gastric cancer cell lines was due to
methylation of PCDH8 gene promoter.
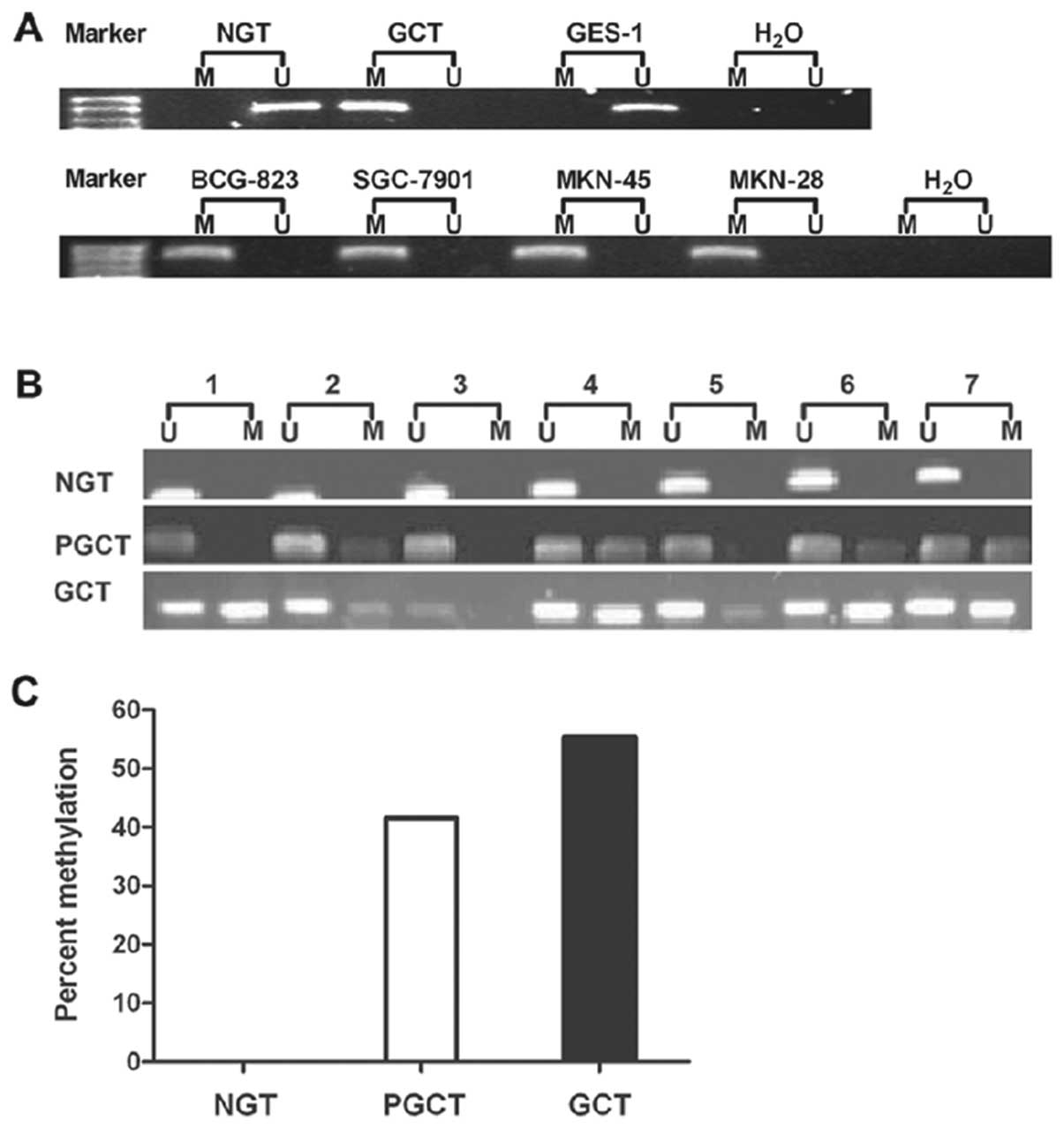 | Figure 2Analysis of the methylation status of
the PCDH8 promoter by MSP in normal gastric and gastric cancer cell
lines or tissues. (A) The MSP analysis showed that PCDH8 gene
promoter was highly methylated in SGC7901, MKN45, MKN28, and BCG823
cell lines and gastric cancer tissue, but not methylated in GES-1
cell line and normal gastric tissue. M, methylated alleles; U,
unmethylated alleles. (B) MSP analysis of PCDH8 gene promoter in 65
patients with human gastric carcinoma. MSP analysis showed that
methylation of the PCDH8 gene promoter was frequently detected in
gastric cancer tissue (GCT) (55.38%, 36/65) and para-carcinoma
tissue of gastric (PGCT) (41.54%, 27/65), but not in normal gastric
tissue (NGT). (C) Comparison of PCDH8 gene promoter methylation
among NGT, PGCT and GCT. Pearson χ2 test by SPSS 16.0
software; a, NGT vs GCT, P=0.005; b, NGT vs PGCT, P=0.002. |
Aberrant hypermethylation of PCDH8 gene
promoter in primary gastric carcinomas
To translate this in vitro finding into ex
vivo tissue specimens, MSP analysis of PCDH8 gene promoter was
conducted in 65 patients with human gastric carcinoma (42 male and
23 female). MSP analysis showed that methylation of the PCDH8 gene
promoter was frequently detected in gastric cancer tissue (55.38%,
36/65) and para-carcinoma tissue of gastric (41.54%, 27/65), but
not in normal gastric tissue. Statistically, there was no
difference in methylation of the PCDH8 gene promoter between
gastric cancer and para-carcinoma tissue. However, there were
statistically significant differences between gastric cancer and
normal gastric tissue, and between para-carcinoma tissue and normal
gastric tissue (Fig. 2B and C,
P=0.002 and 0.005, respectively).
Induction of gastric cancer cell
apoptosis and inhibiting migration with 5-aza-2′-deoxycytidine
treatment
Previous results showed that treatment with 1 μmol/l
of 5-aza-2′-deoxycytidine for up to 72 h significantly upregulated
expression of PCDH8 in 4 gastric cancer cell lines. To investigate
the relation between PCDH8 expression and cell development. We
evaluated cell apoptosis and migration with or without PCDH8
expression. Fig. 4 shows that
following the 5-aza-2′-deoxycytidine treatment (1 μM for 72 h), FCM
demonstrated an obvious increase in Annexin-FITC positive apoptotic
tumor cells (apoptosis rate: BCG-823, 3.73±0.30 vs 9.27±0.26%;
SGC-7901, 4.82±0.32 vs 10.63±0.26%; MKN-45, 3.92±0.23 and 8.7±2.0%;
MKN-28, 5.4±0.21 and 12.92±2.0%; P<0.05) (Fig. 4A). And after the treatment, the
average number of migrated cells were obviously decreased in four
gastric cancer cell lines [migrated cells (×103):
BCG-823, 33.67±5.38 vs 81.56±6.85; SGC-7901, 38.32±4.25 vs
84.06±7.65; MKN-45, 32.51±3.14, 82.63±7.42; MKN-28, 45.36±6.57,
85.67±6.13; P<0.05] (Fig. 4B).
These data suggested that PCDH8 is able to induce gastric cancer
cell apoptosis and to inhibit cell migration.
Association of PCDH8 gene promoter
methylation with clinicopathological data in gastric cancer
patients
To determine the role of PCDH8 methylation status in
gastric cancer, we examined the correlation of methylation status
with the clinicopathological features. There was no significant
difference in the distribution of patients with methylation or
unmethylation of PCDH8 in terms of age, sex, distant metastasis,
tumor size, or TNM stage. Methylation of the PCDH8 gene was
detected in 80.0% (28 of 35) of the patients who did not have lymph
node metastasis, whereas it was detected in 27.6 (8 of 29) of the
patients who had lymph node metastasis. Methylation of the PCDH8
gene was also detected in 71.0% (22 of 31) of the patients who were
moderate/poor, whereas it was not detected in 39.4% (13 of 33) of
the patients who were well. Our statistical data showed that the
methylated status of the PCDH8 gene was significantly correlated
with the lymph node metastasis and tumor differentiation (Table I).
 | Table IAssociation of PCDH8 gene promoter
methylation with clinicopathological data in gastric cancer
patients. |
Table I
Association of PCDH8 gene promoter
methylation with clinicopathological data in gastric cancer
patients.
| Variable | Patients | PCDH8
methylation | PCDH8
unmethylation | P-value |
|---|
| Sex | | | | 0.43 |
| Male | 42 | 22 | 20 | |
| Female | 23 | 14 | 9 | |
| Age (years) | | | | 0.43 |
| ≤50 | 24 | 14 | 10 | |
| >50 | 41 | 22 | 19 | |
| Tumor size (cm) | | | | 0.24 |
| <5 | 35 | 21 | 14 | |
| ≥5 | 29 | 15 | 14 | |
| Tumor
differentiation | | | | 0.01a |
| Moderate/poor | 31 | 22 | 9 | |
| Well | 33 | 13 | 20 | |
| TNM stage | | | | 0.68 |
| I–II | 23 | 12 | 11 | |
| III–IV | 39 | 22 | 17 | |
| Lymph node
metastasis | | | | 0.0038a |
| − | 29 | 8 | 21 | |
| + | 35 | 28 | 7 | |
| M (distal
metastasis) | | | | 0.86 |
| M0 | 60 | 31 | 29 | |
| M1 | 5 | 5 | 0 | |
In the multivariate logistic regression analysis
with backward selection, independent variables with P<0.05 in
the univariate analyses were included. The variables (tumor
differentiation and lymph node metastasis) were chosen for
multivariate logistic regression analysis. Methylated PCDH8 was
significantly associated with lymph node metastasis in a logistic
regression analysis (odds ration 9.78, CI 1.12–86.84, P=0.026)
(Table II). These results suggest
that negative lymph node metastasis was significantly associated
with methylated PCDH8.
 | Table IISignificant variables associated with
the methylation of PCDH8 gene by the logistic regression
analysis. |
Table II
Significant variables associated with
the methylation of PCDH8 gene by the logistic regression
analysis.
| Variable | Odds ratio | (95% CI) | P-value |
|---|
| Tumor
differentiation | | | 0.128 |
| Moderate/poor | 1 | | |
| Well | 3.832 | (0.68–21.10) | |
| Lymph node
metastasis | | | 0.026a |
| − | 1 | | |
| + | 9.783 | (1.12–86.84) | |
Discussion
In this study, we determined PCDH8 gene expression
and the methylation status of the PCDH8 gene promoter in gastric
cancer cells. We found that the expression of PCDH8 mRNA was lost
in gastric cancer cells. MSP analysis revealed high methylation of
the PCDH8 gene promoter in these tumor cells.
5-aza-2′-deoxycytidine treatment induced PCDH8 expression, but
reduced viability of gastric cancer cells. Furthermore, ex
vivo data demonstrated that the PCDH8 gene promoter is
frequently methylated in gastric cancer and the para-carcinoma
tissues, but not in normal gastric tissue. Therefore, the PCDH8
gene promoter methylation may be further evaluated as a biomarker
for early detection of gastric cancer. Furthermore, methylated
PCDH8 was significantly associated with lymph node metastasis in a
logistic regression analysis. It also revealed that PCDH8
restrained tumor metastasis in vivo.
Inactivation of tumor suppressor genes contributes
to cancer development. Such inactivation may be caused by genetic
or epigenetic alterations, including gene mutation, deletion,
promoter methylation, abnormal splicing, deregulation of imprinting
and haploinsufficiency (4). Among
these abnormalities, loss of heterozygosity (LOH) was shown to
cause inactivation of most candidate tumor suppressor genes in the
critical regions of chromosomes 3p, 5q, 8p and 9p (19–22).
However, changes in methylation status of these genes also
frequently occur. The PCDH8 gene plays an important role in
organizing the formation and polarity of developing cellular
structures in embryos. PCDH8 can also suppress tumor cell migration
and invasion, and induces apoptosis in breast cancer cell lines
(17).
PCDH8 is located on chromosome 13q14.3 and is within
a cluster of protocadherins (PCDH8, PCDH9, PCDH17 and PCDH20)
spanning 13q14-21 that is conserved between humans and mice. It is
interesting to note that PCDH20 is methylated and homozygously
deleted in lung cancer, and when reintroduced into an altered tumor
cell line reduces proliferation (13). PCDH17 is methylated and homozygously
deleted in esophageal squamous cell carcinoma (15).
Our present data demonstrated aberrant methylation
of PCDH8 gene promoter regions and subsequent loss of PCDH8
expression in gastric cancer cell lines and tumor tissue specimens.
These results are consistent with previous studies on other cancers
(17). PCDH8 gene promoter is
frequently methylated in gastric cancer 55.38% (36/65) and the
para-carcinoma tissues 41.54% (27/65), but not in normal gastric
tissue which indicated that it is a common feature of gastric
cancer and may be the early stage accident in gastric
carcinogenesis.
Aberrant promoter hypermethylation has been shown to
be a common event in human cancer mainly due to the loss of
function of tumor suppressor. This neoplasia-related event is
thought to occur early in carcinogenesis, and hence, promoter
hypermethylation is being widely studied as a biomarker for the
diagnosis and detection of early lesions. In this context, PCDH8
was frequently methylated in gastric carcinoma adjacent tissues but
not in normal gastric mucosa. It suggests that PCDH8 methylation
may represent the field defect of gastric carcinoma. PCDH8 gene
promoter methylation may be an early event in gastric cancer
(23–25).
We found that methylation of PCDH8 gene promoter
only occurred in gastric cancer but not in normal gastric tissue.
5-aza-2′-deoxycytidine induced expression of PCDH8, which is
associated with reduced viability of gastric cells, indicating that
PCDH8 plays an important role in suppressing gastric
carcinogenesis. However, we cannot rule out whether other tumor
suppressor genes are also induced and restored by
5-aza-2′-deoxycytidine, which plays a role in regulation of tumor
cell viability. The latter warrants further studies because it has
been shown that epigenetic modification of pro-apoptotic genes is
one of the mechanisms by which the tumor cells are resistant to
chemotherapy (26). Therefore,
treatment with a demethylating agent such as 5-aza-2′-deoxycytidine
prior to chemotherapy may help improve the therapeutic efficacy for
gastric cancer.
In conclusion, silence of PCDH8 expression is
achieved through the gene methylation in gastric cancer. PCDH8 can
suppress gastric tumorigenesis in vitro and in vivo.
Thus, PCDH8 is a candidate tumor suppressor in gastric cancer.
Future studies will evaluate whether PCDH8 gene promoter
methylation can be used as a biomarker for the early detection of
gastric cancer.
Acknowledgements
The authors acknowledge Dr Xue Yang of the
Hepatobiliary Department of First Affiliated Hospital and the
Institution of Genetic Disease Research and, Xi’an Jiaotong
University for technical assistance.
References
|
1
|
Herszényi L and Tulassay Z: Epidemiology
of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci.
14:249–258. 2010.
|
|
2
|
Brenner H, Rothenbacher D and Arndt V:
Epidemiology of stomach cancer. Methods Mol Biol. 472:467–477.
2009. View Article : Google Scholar
|
|
3
|
Arkenau HT: Gastric cancer in the era of
molecularly targeted agents: current drug development strategies. J
Cancer Res Clin Oncol. 135:855–866. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yasui W, Sentani K, Motoshita J, et al:
Molecular pathobiology of gastric cancer. Scand J Surg. 95:225–231.
2006.
|
|
5
|
Herman JG and Baylin SB: Gene silencing in
cancer in association with promoter hypermethylation. N Engl J Med.
349:2042–2054. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang JF and Dai DQ: Metastatic suppressor
genes inactivated by aberrant methylation in gastric cancer. World
J Gastroenterol. 13:5692–5698. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Machado JC, Oliveira C, Carvalho R, et al:
E-cadherin gene (CDH1) promoter methylation as the second hit in
sporadic diffuse gastric carcinoma. Oncology. 20:1525–1528.
2001.PubMed/NCBI
|
|
8
|
Esteller M, Corn PG, Baylin SB, et al: A
gene hypermethylation profile of human cancer. Cancer Res.
61:3225–3229. 2001.PubMed/NCBI
|
|
9
|
Buffart TE, Overmeer RM, Steenbergen RD,
et al: MAL promoter hypermethylation as a novel prognostic marker
in gastric cancer. Br J Cancer. 99:1802–1807. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishikawa Y, Miyazaki T, Nakashiro K, et
al: Human FAT1 cadherin controls cell migration and invasion of
oral squamous cell carcinoma through the localization of β-catenin.
Oncol Rep. 26:587–592. 2011.PubMed/NCBI
|
|
11
|
Gumbiner BM: Regulation of
cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol.
6:622–634. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Batlle E, Sancho E, Franci C, et al: The
transcription factor snail is a repressor of E-cadherin gene
expression in epithelial tumour cells. Nat Cell Biol. 2:84–89.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Imoto I, Izumi H, Yokoi S, Hosoda H, et
al: Frequent silencing of the candidate tumor suppressor PCDH20 by
epigenetic mechanism in non-small-cell lung cancers. Cancer Res.
66:4617–4626. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ying J, Li H, Seng TJ, Langford C, et al:
Functional epigenetics identifies a protocadherin PCDH10 as a
candidate tumor suppressor for nasopharyngeal, esophagealand
multiple other carcinomas with frequent methylation. Oncogene.
25:1070–1080. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haruki S, Imoto I, Kozaki K, et al:
Frequent silencing of protocadherin 17, a candidate tumour
suppressor for esophageal squamous-cell carcinoma. Carcinogenesis.
31:1027–1036. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Strehl S, Glatt K, Liu QM, et al:
Characterization of two novel protocadherins (PCDH8 and PCDH9)
localized on human chromosome 13 and mouse chromosome 14. Gemomics.
53:81–89. 1998.PubMed/NCBI
|
|
17
|
Yu JS, Koujak S, Nagase S, et al: PCDH8,
the human homolog of PAPC, is a candidate tumor suppressor of
breast cancer. Oncogene. 27:4657–4665. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Herman JG, Graff JR, Myöhänen S, et al:
Methylation-specific PCR: a novel PCR assay for methylation status
of CpG islands. Proc Natl Acad Sci USA. 93:9821–9826. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gorringe KL, Ramakrishna M, Williams LH,
et al: Are there any more ovarian tumor suppressor genes? A new
perspective using ultra high-resolution copy number and loss of
heterozygosity analysis. Genes Chromosomes Cancer. 48:931–942.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Franko J, Krasinskas AM, Nikiforova MN, et
al: Loss of heterozygosity predicts poor survival after resection
of pancreatic adenocarcinoma. J Gastrointest Surg. 12:1664–1672.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang YC, Yeh KT, Liu TC, et al: Molecular
cytogenetic characterization of esophageal cancer detected by
comparative genomic hybridization. J Clin Lab Anal. 24:167–174.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ye Y, McDevitt MA, Guo M, et al:
Progressive chromatin repression and promoter methylation of CTNNA1
associated with advanced myeloid malignancies. Cancer Res.
69:8482–8490. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hukriede NA, Tsang TE, Habas R, et al:
Conserved requirement of Lim1 function for cell movements during
gastrulation. Dev Cell. 4:83–94. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Medina A, Swain RK, Kuerner KM and
Steinbeisser H: Xenopus paraxial protocadherin has signaling
functions and is involved in tissue separation. EMBO J.
23:3249–3258. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Unterseher F, Hefele JA, Giehl K, et al:
Paraxial protocadherin coordinates cell polarity during convergent
extension via Rho A and JNK. EMBO J. 23:3259–3269. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tian K, Jurukovski V, Wang XP, et al:
Epigenetic regulation of WTH3 in primary and cultured drugresistant
breast cancer cells. Cancer Res. 65:10024–10031. 2005. View Article : Google Scholar : PubMed/NCBI
|