Introduction
Gastric cancer is the fourth most common cancer and
the second leading cause of cancer-related death in the world. In
China alone, ~463,000 new patients will be diagnosed with gastric
cancer and 352,000 patients will die annually, accounting for
nearly half of the new cases and deaths of gastric cancer in the
world (1). Clinically, gastric
cancer rarely presents with noticeable early signs and symptoms in
the early stage; thus, most patients are diagnosed at advanced
stages (2). To date, various
treatment options, such as surgery, radiotherapy, and chemotherapy,
are routinely used to manage gastric cancer patients, but the
overall 5-year patient survival rate still remains at only ~26%
(3,4). The significant failure of the
treatment and the poor survival rate are due to diagnosis at the
later stages, which make surgical resection impossible; therefore,
innovative treatment strategies and approaches to early gastric
cancer diagnosis could significantly improve survival and treatment
outcome of this disease.
It has been well documented that angiogenesis plays
an important role in growth and metastasis of gastric cancer
(5,6). Thus, anti-angiogenesis has been used
as a potential and promising strategy in gastric cancer treatment
by developing anti-angiogenesis drugs in clinical trials against
gastric cancer (6–8). A comprehensive understanding of
angiogenesis mechanisms responsible for gastric cancer progression
could lead to novel treatment approaches. To this end, our research
has focused on the crucial molecules involved in gastric cancer
angiogenesis.
Protease-activated receptors (PARs) are a recently
described subfamily of G-protein-coupled receptors with seven
transmembrane-spanning domains and are comprised of four proteins,
i.e., PAR-1, PAR-2, PAR-3, and PAR-4 (9). Among the four receptors, PAR-2 is
mainly activated by trypsin and other trypsin-like serine proteases
through cleavage of the extracellular N-terminal domain, which in
turn enables the N-terminus of the protein to bind to the receptor
itself as a tethered ligand to activate G-protein-coupled signal
transduction pathways (10). PAR-2
is widely expressed in a variety of tissues, with a high expression
level in the gastrointestinal tract, where physiological trypsin is
highly expressed (11).
Functionally, PAR-2 has been implicated in inflammation, pain,
tissue injury, as well as in the regulation of gastrointestinal
functions and diseases (12).
Furthermore, PAR-2 protein is also highly expressed in various
cancers, such as breast, colorectal, pancreatic, gastric,
gallbladder, kidney, lung, uterine, cervical cancer and melanoma,
and glioblastoma (13,14).
Recently, increasing attention has focused on the
role of PAR-2 in angiogenesis (15). For example, PAR-2 activation in
vascular endothelial cells was able to stimulate angiogenesis
during tissue repair and promote neovascularization of the retina
(15,16). PAR-2 activation also was shown to
induce proliferation of endothelial cells, enhance production of
proangiogenesis cytokines (such as vascular endothelial growth
factor, namely VEGF), and upregulate expression of cyclooxygenase-2
(COX-2) protein in human endothelial cells and other types of cells
(17–21). In gastric cancer (22), overexpression of PAR-2 protein could
be the key inducer of angiogenesis. However, the underlying
molecular mechanism responsible for PAR-2 action has yet to be
determined. In the present study, we hypothesized that PAR-2
activation by trypsin contributes to gastric cancer angiogenesis,
which may be through upregulation of VEGF and COX-2 expression.
Materials and methods
Cell lines and culture
A well-differentiated human tubular gastric
adenocarcinoma MKN-28 cell line was kindly provided by Professor
Fang-Dong Men of The Institute of Oncology, China Medical
University, Shenyang, China. The human lung adenocarcinoma A549
cell line was purchased from the Chinese Academy of Sciences,
Shanghai, China. These cell lines were cultured in RPMI-1640 medium
(Gibco Life Technologies, Grand Island, NY, USA) supplemented with
10% fetal bovine serum (FBS; Gibco) at 37˚C in a humidified
atmosphere containing 95% air and 5% CO2. Cells were
seeded at 1×106 cells per well in 6-well plates for
reverse transcription polymerase chain reaction (RT-PCR) and
western blot analysis, or at 5×105 cells per well in
12-well plates for ELISA. In each experiment, MKN-28 cells were
first deprived of FBS for 2 h and then treated with trypsin,
SB20358 (a p38 MAP kinase inhibitor), or PD98059 (a specific
inhibitor to block ERK1/2 phosphorylation). Trypsin was purchased
from Amresco (Solon, OH, USA), and SB20358 and PD98059 were from
Sigma Chemical Co. (St. Louis, MO, USA).
RNA isolation and RT-PCR
Total RNA was isolated from the cultured cells using
the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s protocol. The concentration and purity of the
freshly isolated RNA were measured by the optical densities at 260
and 280 nm using a Beckman Coulter DU 530 spectrophotometer. The
RNA was then reverse transcribed into cDNA using a PrimeScript™ RT
Reagent kit (Takara, Dalian, China) according to the manufacturer’s
instructions. Next, PCR amplification was performed using a Takara
PCR amplification kit (Takara) in a total volume of 25 μl
containing 2 μl of cDNA (0.5 μg), 200 μM each dNTP, 1.25 units of
Taq polymerase, and 0.2 μM each primer (Takara). The PCR was set at
94˚C for 3 min, followed by 30 cycles of denaturation at 94˚C for
30 sec, annealing at 55˚C for 30 sec, and extension at 72˚C for 30
sec, and a final extension at 72˚C for 10 min. The amplified PCR
products were then separated by agarose gel electrophoresis and
visualized by ethidium bromide staining. The primers used for
detection of PAR-2 mRNA were 5′-GGC CAA TCT GGC CTT GGC TGA C-3′
(sense) and 5′-GGG CAG GAA TGA AGA TGG TCT GC-3′ (antisense), and
for β-actin they were 5′-GCC AAC CGT GAA AAG ATG-3′ (sense) and
5′-CCA GGA TAG AGC CAC CAA T-3′ (antisense).
Real-time RT-PCR
To quantify PAR-2 mRNA levels, real-time RT-PCR
(qRT-PCR) was performed with the LightCycler thermal cycling system
(Roche Diagnostics Corp., Indianapolis, IN, USA) using a
SYBR® Premix Ex Taq™ II (Perfect real-time) kit (Takara)
as described by the manufacturer. Briefly, the total qPCR volume
was 20 μl, which contained 2 μl of cDNA template (100 ng), 10 μl of
2X SYBR Premix Ex Taq II, 0.8 μl of primers (10 μM each), and 6.4
μl of ddH2O. After an initial denaturation at 95˚C for 3
min, PCR was performed for 40 cycles of 95˚C for 30 sec and 60˚C
for 30 sec. The primer sequences for human VEGF were 5′-TGA CGG ACA
GAC AGA CAG ACA CC-3′ (sense) and 5′-AGA ACA GCC CAG AAG TTG GAC
GA-3′ (antisense), for COX-2 they were 5′-TCA CAG GCT TCC ATT GAC
CAG-3′ (sense) and 5′-CCG AGG CTT TTC TAC CAG A-3′ (antisense), and
for β-actin they were 5′-TCA TCA CCA TTG GCA ATG AG-3′ (sense) and
5′-CAC TGT GTT GGC GTA CAG GT-3′ (antisense). All primers were
synthesized by Takara. The qPCR data were analyzed by using the
Roche Molecular Biochemicals LightCycler software (version 3.5).
Specificity of the amplification reactions was confirmed by
analyzing their corresponding melting curves. The relative
expression of each gene was normalized with the β-actin control and
then estimated as values of 2-ΔΔCt.
Protein extraction and western
blotting
Total cellular protein was extracted from the
cultured tumor cells with ice-cold lysis buffer containing 50 mM
Tris/HCl (pH 7.5), 150 mM NaCl, 10% glycerol, 10 mM NaF, 1 mM Na3
vanadate, 0.2 mM phenylmethylsulfonyl fluoride (PMSF), 10 μg/ml
aprotinin (pH 7.3), 10 μg/ml leupeptin, 0.1% SDS, 1 mM EGTA, 1 mM
EDTA, 0.5% deoxysodium cholate, and 1% NP-40 for 15 min, followed
by centrifugation at 21,000 × g for 15 min at 4˚C. The
concentrations of these protein samples were determined using a BCA
Protein Assay kit (Bio-Rad Laboratories, Hercules, CA, USA)
standardized to bovine serum albumin (BSA) according to the
manufacturer’s protocol.
The protein samples (50 μg for each lane) were
resolved by electrophoresis in 7.5 or 12.5% SDS-PAGE precast gels
(Bio-Rad). Protein samples were then electro-transferred onto
nitrocellulose membranes. The membranes were incubated in 5% (w/v)
non-fat dry milk in 20 mM Tris-buffered saline with 0.1% Tween
(TBS-T) for 2 h at room temperature and then with the primary
antibodies overnight at 4˚C. These primary antibodies were human
PAR-2 (sc-8205, 1:1000), VEGF (sc-7269, 1:1000), COX-2 (sc-7951,
1:1000), ERK1/2 (sc-93, 1:2000), p38 (sc-7972, 1:2000),
phosphorylated ERK1/2 (p-ERK1/2), phosphorylated p38 (p-p38), the
phospho-specific antibodies targeting p-ERK1/2 (Tyr 204) (sc-7383,
1:1000), p-p38 (Tyr182) (sc-7973, 1:2000), or β-actin (sc-1615,
1:1000), all of which were from Santa Cruz Biotechnology Inc.
(Santa Cruz, CA, USA). The next day, the membranes were washed
three times with TBS-T and then incubated with the HRP-conjugated
anti-rabbit, anti-mouse, or anti-goat secondary antibodies (Santa
Cruz Biotechnology Inc.) at a dilution of 1:2000 for 1 h at room
temperature. The membranes were washed extensively with TBS-T three
times and positive signals were detected by using the enhanced
chemiluminescence detection system (Beyotime, China) and exposed to
X-ray film.
ELISA analysis
The levels of VEGF in the conditioned cell culture
media were determined by using ELISA. Briefly, gastric cancer MNK28
cells were seeded at 5×105 cells per well into 12-well
plates and grown to reach a confluence of 60–70%. Next, the cells
were washed three times with phosphate-buffered saline (PBS), and
the culture medium was replaced with serum-free medium and treated
with increased concentrations of trypsin (0–100 nM; Amresco) for 6
h, or treated with 10 nM trypsin for up to 24 h. At the end of the
experiments, the conditioned cell culture medium was harvested and
centrifuged for ELISA. A commercially available sandwich ELISA kit
(Jinmei Biotechnology Co., Ltd., Shenzhen, China) was used to
assess VEGF concentration in the supernatant of the conditioned
growth medium according to the manufacturer’s instructions. The
data were normalized to the kit controls and the number of
producing cells and then expressed as pg of VEGF protein per
106 cells.
Statistical analysis
All experiments were performed in triplicate and
repeated at least three times. Data are expressed as means ±
standard deviation (SD). The Student’s t-test was used to compare
the means of two groups. P<0.05 was considered statistically
significant. All analyses were performed using SPSS 10.0
statistical packages (SPSS Inc., Chicago, IL, USA).
Results
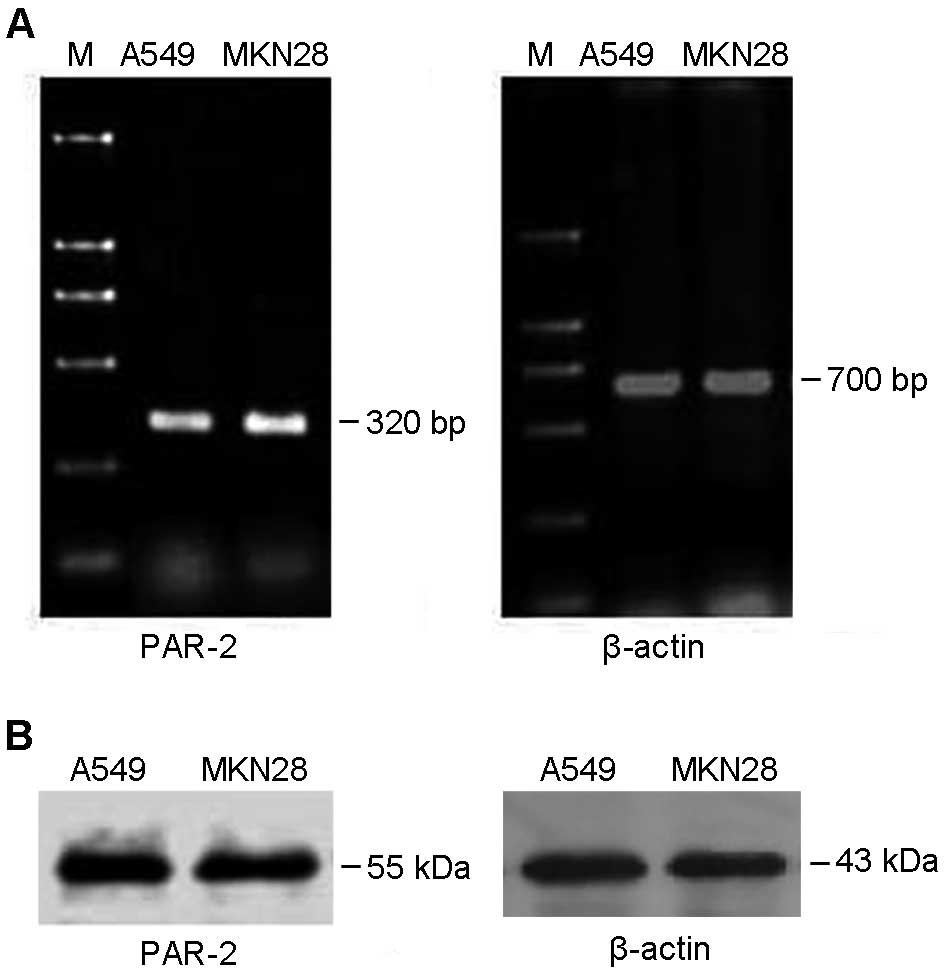
Expression of PAR-2 in gastric cancer
MKN28 cells
Expression of PAR-2 mRNA and protein was determined
in MKN28 gastric cancer cells by using RT-PCR and western blot,
respectively. As shown in Fig. 1A,
a 320-bp PAR-2 target band was amplified by RT-PCR, indicating that
MKE28 expressed PAR-2 mRNA. Moreover, western blot data showed that
the anti-PAR-2 antibody can specifically detect a 55-kDa PAR-2
target band (Fig. 1B). Lung cancer
A549 cells, highly expressing PAR-2, were used as a positive
control.
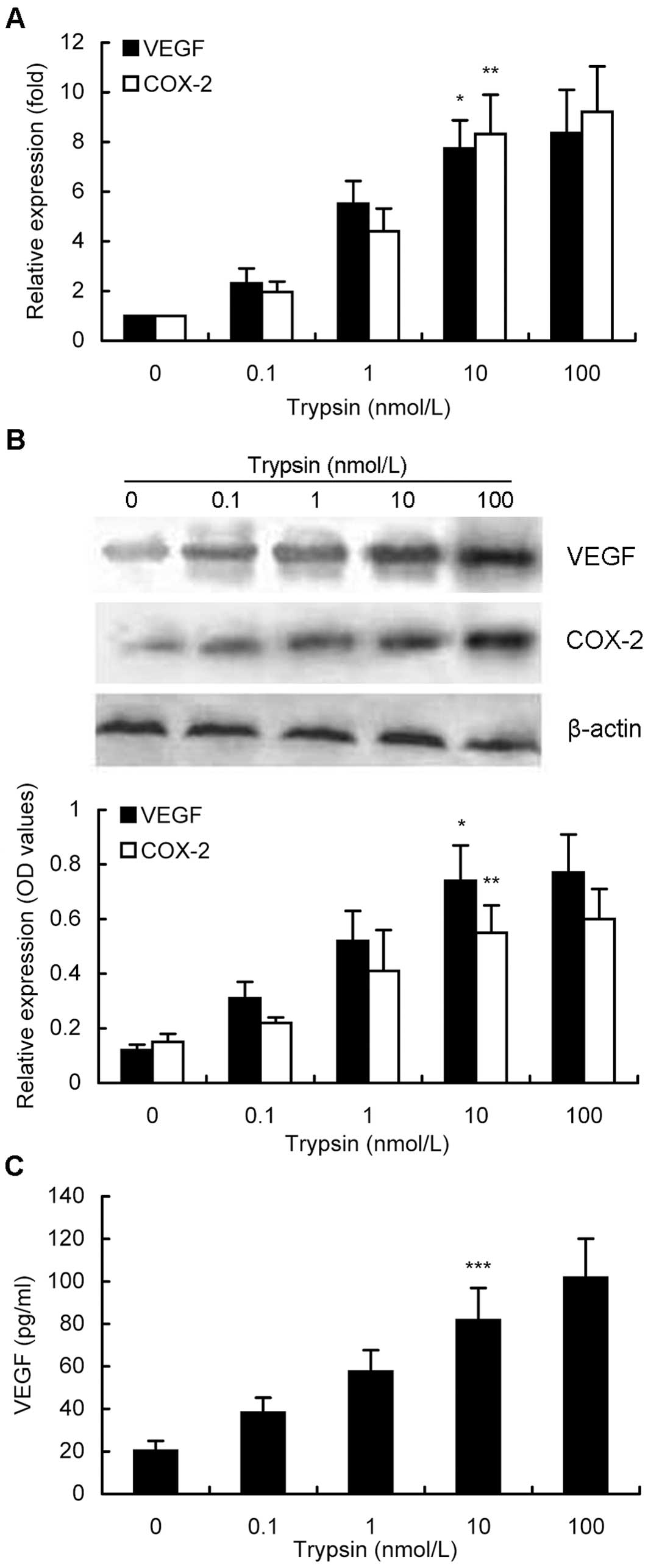
Effects of trypsin-activated PAR-2 on
enhanced expression of VEGF and COX-2 in gastric cancer cells
Next, we determined different gene expressions after
gastric cancer cells were treated with trypsin at different doses
or time points. We found that different treatment doses of trypsin
for 6 h were able to activate PAR-2 expression (Fig. 2A) and in turn to upregulate VEGF and
COX-2 expression in MKN28 cells. As shown in Fig. 2A, with increased concentrations of
trypsin up to 100 nM, expression levels of VEGF and COX-2 mRNA were
increased in a dose-dependent manner, e.g., 10 nM trypsin induced
levels of VEGF and COX-2 mRNA by 7.7-fold and 8.3-fold,
respectively, compared to their corresponding basal levels
(P<0.05). Similar data were confirmed by using western blotting
(Fig. 2B). Furthermore, VEGF
protein expression in the conditioned media was also induced in a
dose-dependent manner (Fig. 2C).
Particularly, 10 nM trypsin treatment of MKN28 cells increased
expression of VEGF protein to 81.7 pg/ml in the conditioned growth
medium, while 100 nM trypsin treatment upregulated VEGF levels to
101.7 pg/ml (P<0.05). Taking all the findings into
consideration, a concentration of 10 nM trypsin was used for
further experiments.
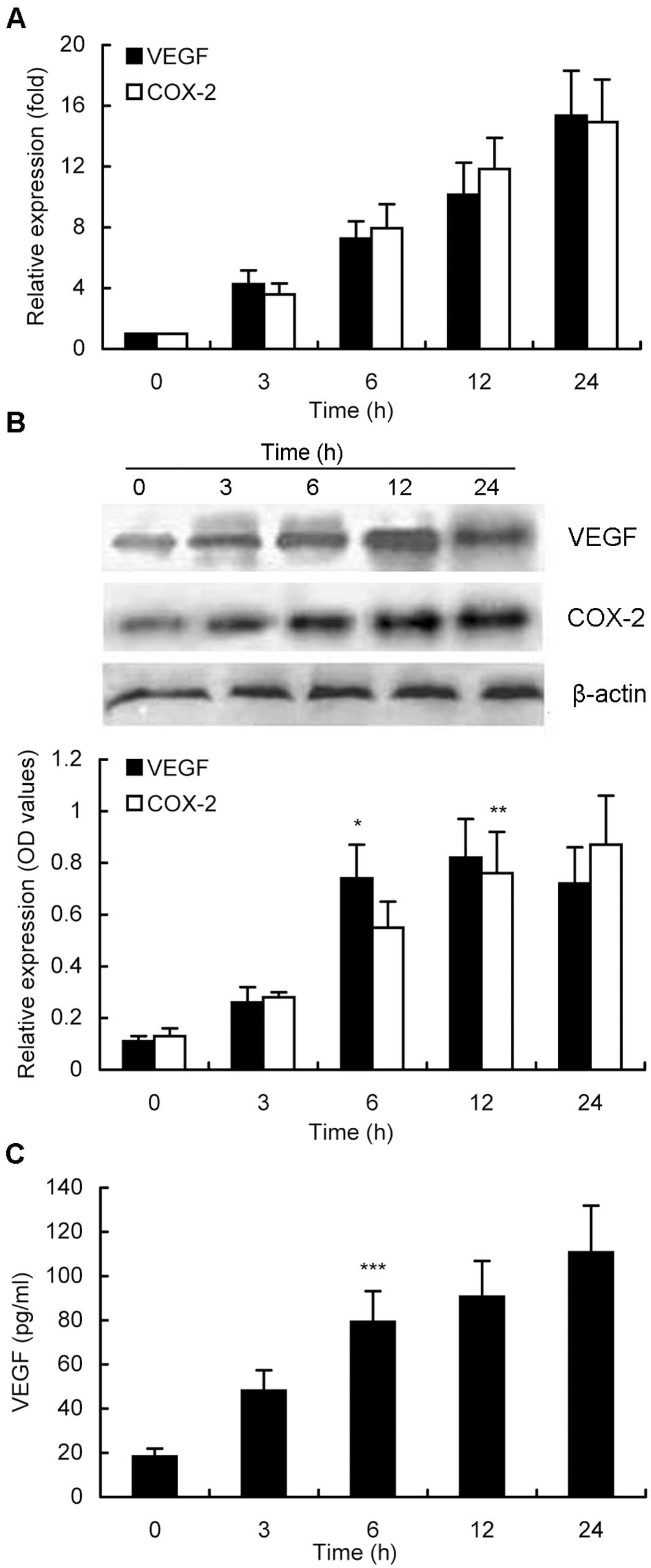
Furthermore, induction of VEGF and COX-2 expression
by activated PAR-2 at different time points was also analyzed in
MKN28 cells. As shown in Fig. 3A,
up to 10 h of treatment of MKN28 cells with 10 nM trypsin
upregulated both mRNA and protein levels of VEGF and COX-2
expression in a time-dependent manner. A 24-h treatment of tumor
cells with trypsin induced expression of VEGF and COX-2 to the peak
mRNA levels, whereas the peak levels of their protein expression
were reached after a 12-h treatment, although there was no
statistical difference in expression of VEGF protein after 6, 12,
or 24 h of treatment (Fig. 3B). The
maximum COX-2 protein expression was found at 24 h of treatment,
without statistical difference between 12 and 24 h (Fig. 3B). The VEGF concentration in the
conditioned media was also time-dependent (Fig. 3C).
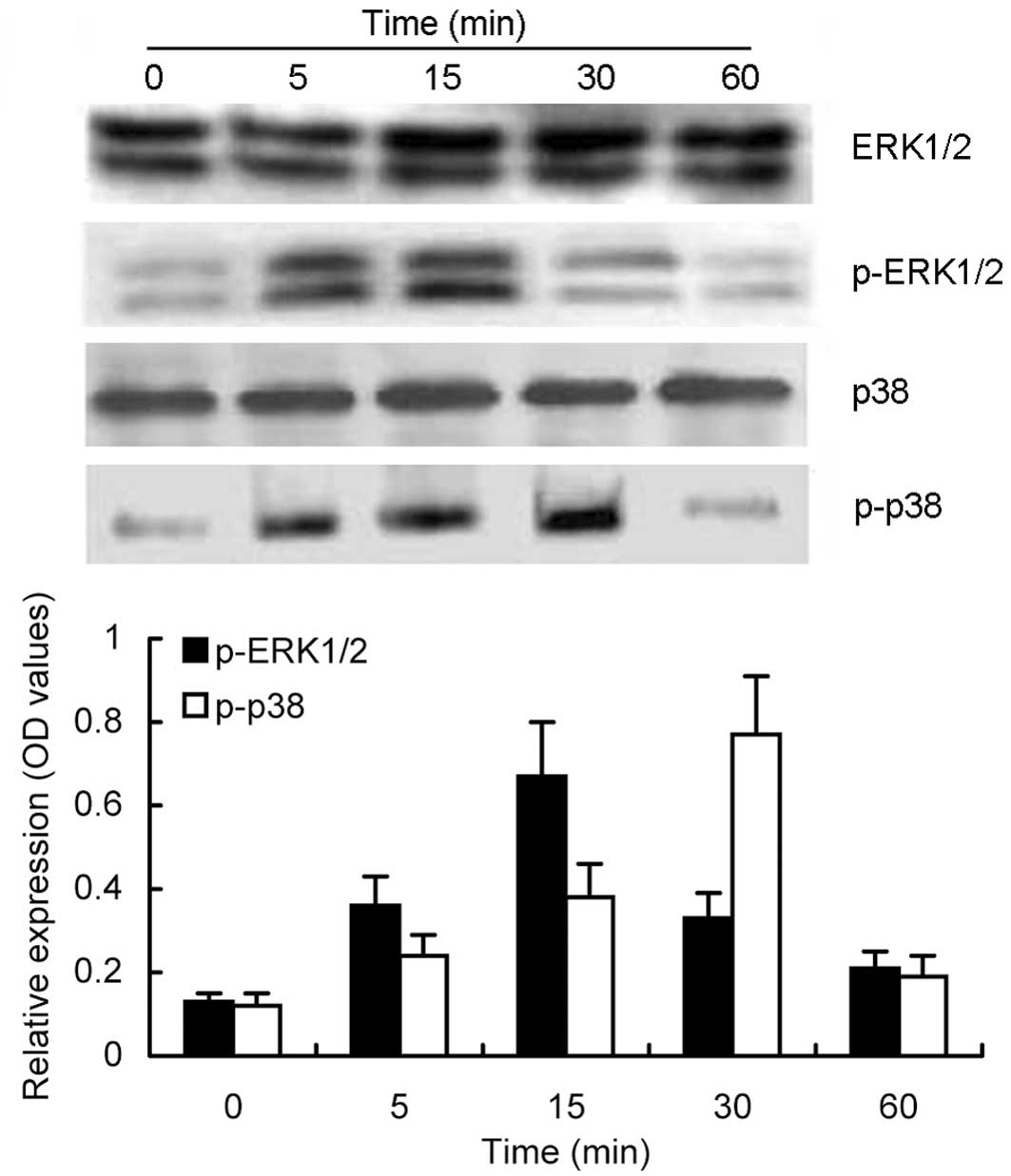
Effects of trypsin-activated PAR-2 on
induction of ERK1/2 and p38 MAP kinase phosphorylation
To determine whether the MAP kinase signaling
pathway participates in the upregulation of VEGF and COX-2
expression, we assessed the total and phosphorylated ERK1/2 and p38
MAP kinase proteins after trypsin-induced PAR-2 activation in MKN28
cells. As shown in Fig. 4,
expression levels of the total ERK1/2 protein showed no significant
changes from 0 to 60 min, whereas phosphorylated ERK1/2 protein was
induced by trypsin-induced PAR-2 activation, which peaked at 15 min
and returned back to the basal level at 60 min. A similar
time-dependent manner for p38 MAP kinase phosphorylation was also
noted, i.e., trypsin-induced PAR-2 activation upregulated
significant and sustained phosphorylation of p38 MAP kinase that
began at 5 min, reached the maximum at 30 min with nearly a 4-fold
increase compared to the untreated controls, and then returned to
the basal level after 60 min.
ERK1/2 and p38 inhibitors antagonize the
effects of trypsin-activated PAR-2 on induction of VEGF and COX-2
expression
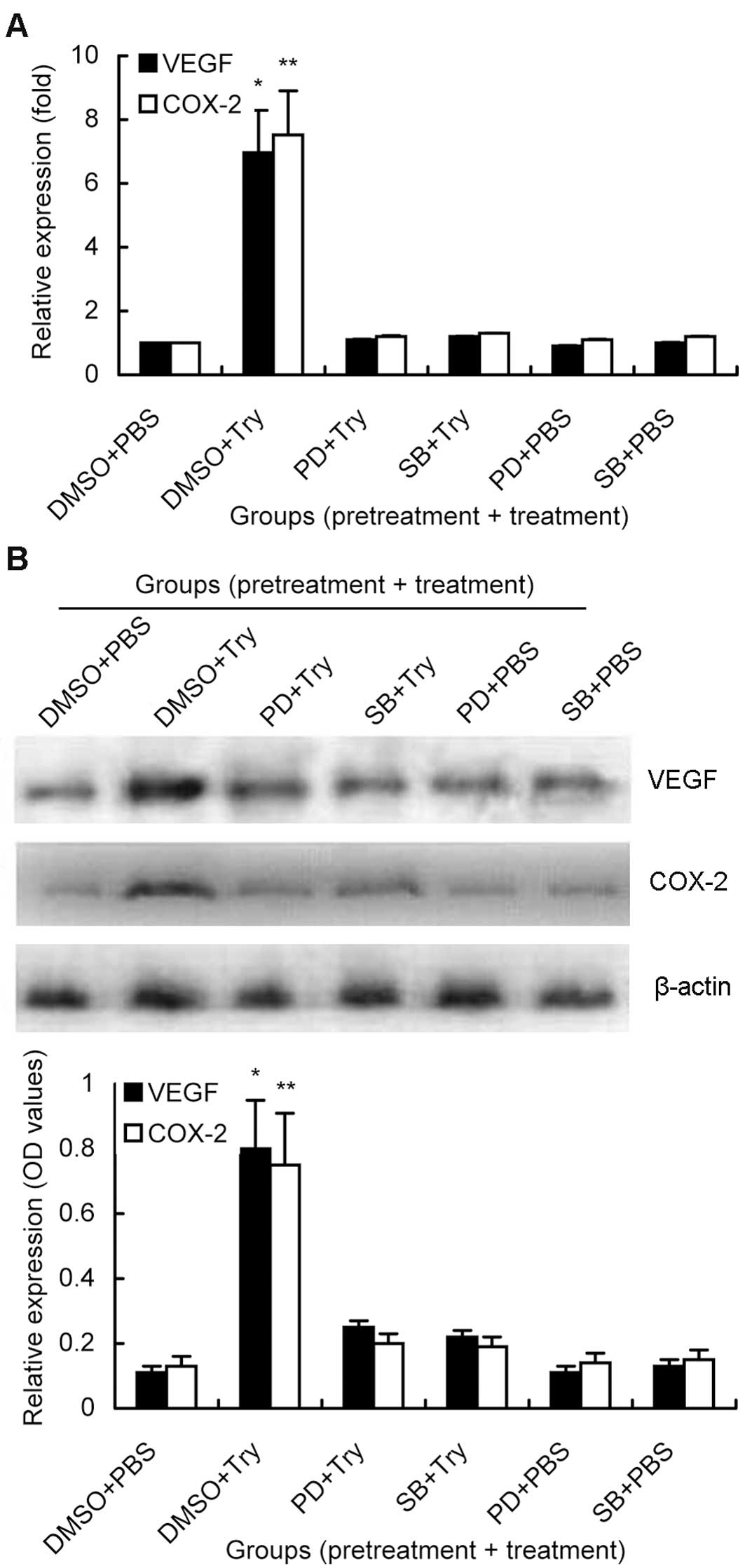
Next, we determined the role of the MAP kinase
signaling pathway in mediating the effects of trypsin-activated
PAR-2 on the induction of VEGF and COX-2 expression by pretreating
MKN28 cells with specific ERK1/2 and p38 MAP kinase inhibitors (25
μM PD98059 or 20 μM SB203580, respectively) 1 h prior to PAR-2
activation with 10 nM trypsin for 6 h. We found that compared to
the PBS control, treatment with 10 nM trypsin resulted in 7.0- and
7.5-fold induction of VEGF and COX-2 mRNA, respectively, whereas
these increases in VEGF and COX-2 mRNA and protein levels were
completely blocked by treatment with PD98059 or SB203580 in gastric
cancer MKN28 cells (Fig. 5).
However, there was no significant difference in VEGF and COX-2
expression between the control cells and PD98059 or
SB203580-treated tumor cells alone (Fig. 5). The data suggested that the role
of PAR-2 was mediated by the MAP kinase signaling pathway to
upregulate expression of VEGF and COX-2 mRNA and protein.
Discussion
In this study, we chose gastric cancer cells to
demonstrate that PAR-2 activation was able to upregulate expression
of proangiogenesis genes and defined the underlying molecular
pathway. Our data showed that trypsin-activated PAR-2 induced
expression of VEGF and COX-2 in gastric cancer cells in a dose- and
time-dependent manner. The ability of PAR-2 to induce VEGF and
COX-2 expression was mediated by ERK1/2 and p38 gene signaling,
i.e., pretreatment with ERK1/2 and p38 inhibitors blocked the
effects of trypsin-activated PAR-2 on the induction of VEGF and
COX-2 expression. Since angiogenesis plays an important role in
gastric cancer development and progression, this study might
provide a novel anti-angiogenesis target for future treatment of
gastric cancer patients.
PAR-2 is a member of the protease-activated receptor
family. Various proteases can cleave PAR-2 within the extracellular
N-terminal domain to expose a tethered ligand that binds to and
activates the cleaved receptor. Among these different proteases,
trypsin is a potent PAR-2 activator that cleaves and triggers PAR-2
activation, although trypsin was traditionally regarded as an
enzyme with the principal function of degrading dietary proteins
(23). A wide expression of PAR-2
has been observed in the gastrointestinal tract in physiological
conditions and malignant gastrointestinal diseases (24–26).
Trypsin, the most prominent ligand of PAR-2, is not only maintained
at a higher concentration in the gastrointestinal tract naturally,
but also can be secreted by a few types of cancer cells derived
from the gastrointestinal tract by an autocrine mechanism (27–29).
Accumulating evidence has demonstrated that PAR-2 is
involved in the development and progression of human cancers. For
example, PAR-2 overexpression has been associated with the depth of
tumor invasion, lymphatic involvement, early metastasis, and poor
prognosis of gastric cancer and other types of cancer (22,26,30,31).
Activated PAR-2 enhanced the growth of gastric cancer cells via
transactivation of epidermal growth factor receptor (EGFR)
(24). Activated PAR-2 also
promoted the proliferation of colon cancer cells with a similar
mechanism (25). In addition to the
mutagenic function, a potential proangiogenesis effect of PAR-2 has
been confirmed in breast cancer and uterine endometrial cancer
(19,30). Particularly, in human breast cancer
cells, activation of PAR-2 with an agonist peptide, trypsin, or
FVIIa resulted in a robust increase in expression of VEGF mRNA and
protein, the most prominent inducer of angiogenesis (19). Additionally, in human bile duct
cancer cells, PAR-2 agonist peptide activated expression of COX-2
mRNA and protein, and the latter is responsible for prostaglandin
biosynthesis, which also contributes to tumor angiogenesis
(32). Moreover, COX-2 can increase
VEGF production, and a link between COX-2, PGE2, and VEGF was
proposed in AGS gastric cancer cells (33,34).
Therefore, VEGF and COX-2 were presumed to mediate the
proangiogenesis capacity of PAR-2 in gastric cancer.
In the present study, we first determined the effect
of PAR-2 activation on the intracellular expression of VEGF and
COX-2 in MKN28 gastric cancer cells and secreted VEGF in the
conditioned medium. Our data revealed that trypsin-activated PAR-2
was able to upregulate the expression of COX-2 mRNA and protein in
a dose- and time-dependent manner. Thereafter, we demonstrated that
trypsin-activated PAR-2 could also increase the expression of VEGF
mRNA and protein in gastric cancer cells. These data demonstrated
and confirmed the effects of PAR-2 on increasing the expression of
angiogenesis-related genes in gastric cancer cells.
Furthermore, previous studies have reported that
PAR-2 activated by trypsin modulated the downstream signal
transduction pathway through the activation of the MAP kinase
cascade (19,32,35,36).
In this study, we showed that trypsin-activated PAR-2 increased the
phosphorylation of ERK1/2 and p38 MAP kinase. It is to be
determined whether the MAP kinase cascade is the upstream gene
pathway that regulates the expression of VEGF and COX-2. As shown
in Fig. 5, at both the mRNA and
protein level, PD98059 (ERK1/2 inhibitor) and SB203580 (p38 MAP
kinase inhibitor) dramatically blocked the expression of VEGF and
COX-2 induced by trypsin. These data indicate that PAR-2 induced
VEGF and COX-2 expression is through the MAP kinase pathway.
However, a striking difference has been noted in the mechanism
responsible for activation of the MAP kinase signal transduction
pathway by PAR-2 among a variety of cells and tissues. For example,
trypsin-activated PAR-2 was able to strongly activate ERK1/2
protein but weakly activate p38 by trypsin-activated PAR-2 in rat
aortic smooth-muscle cells with both endogenous and exogenous PAR-2
expression (35,37,38).
In contrast, in human keratinocytes with PAR-2 overexpression,
trypsin-activated PAR-2 strongly induced p38 MAP kinase activation,
but weakly induced ERK1/2 activation (39).
Additionally, ERK1/2, p38, and JNK MAP kinases also
could simultaneously be involved in the signal transduction
following trypsin-induced PAR-2 activation, with independence or
interdependence among them (40,41).
In this study, we found that both ERK1/2 and p38 MAP kinase were
involved in trypsin-activated PAR-2 upregulation of COX-2 and VEGF
expression in MKN28 cells. Our current data were consistent with
findings in dental pulp cells and intestinal epithelial cells
(42,43). Furthermore, although the downstream
signaling of PAR-2 in MKN28 cells also involved these two MAP
kinases (i.e., ERK1/2 and p38 MAP kinase), inhibition of either one
did eliminate the PAR-2-mediated upregulation of VEGF and COX-2
expression. Each of these MAP kinases appeared to modulate
expression of VEGF and COX-2 independently.
It is generally believed that VEGF plays a key role
in tumor angiogenesis and VEGF production is regulated by the tumor
microenvironment. Hypoxia is known to be a potent inducer of VEGF,
which then contributes to angiogenesis in many types of cancer
(44). However, in the present
study, the MKN28 cells were cultured with sufficient oxygen and a
greatly enhanced expression of VEGF still could be observed. The
data suggest that gastric cancer cells continue to produce VEGF
under normal oxygen levels through a PAR-2-dependent MAP kinase
pathway. Therefore, trypsin-PAR-2-MAP kinase-COX-2/VEGF signaling
might present a novel mechanism of angiogenesis in gastric cancer,
and further investigations are required. More importantly, a few
PAR-2 antagonists and inhibitors have been described recently and
PAR-2 might serve as a novel target for anti-angiogenesis therapy
for patients with gastric cancer (45).
Acknowledgements
We thank Medjaden Bioscience Limited for assisting
in the preparation of this manuscript.
References
|
1
|
Ferlay J, Shin H, Bray F, Forman D,
Mathers C and Parkin D: GLOBOCAN 2008, Cancer Incidence and
Mortality Worldwide: IARC CancerBase no. 10. International Agency
for Research on Cancer; Lyon: http://globocan.iarc.fr.
Accessed Nov. 19, 2010
|
|
2
|
Koh T and Wang T: Tumors of the Stomach.
Saunders; Philadelphia, PA: 2002
|
|
3
|
Japanese Gastric Cancer Association.
Japanese Gastric Cancer Treatment Guidelines 2010 (version 3).
Gastric Cancer. 14:113–123. 2011. View Article : Google Scholar
|
|
4
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kitadai Y: Angiogenesis and
lymphangiogenesis of gastric cancer. J Oncol. 2010:4687252010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kakeji Y, Maehara Y, Sumiyoshi Y, Oda S
and Emi Y: Angiogenesis as a target for gastric cancer. Surgery.
131:S48–S54. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iwasaki J and Nihira S: Anti-angiogenic
therapy against gastrointestinal tract cancers. Jpn J Clin Oncol.
39:543–551. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okines AF, Reynolds AR and Cunningham D:
Targeting angiogenesis in esophagogastric adenocarcinoma.
Oncologist. 16:844–858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Trejo J: Protease-activated receptors: new
concepts in regulation of G protein-coupled receptor signaling and
trafficking. J Pharmacol Exp Ther. 307:437–442. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cottrell GS, Amadesi S, Schmidlin F and
Bunnett N: Protease-activated receptor 2: activation, signalling
and function. Biochem Soc Trans. 31:1191–1197. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vergnolle N: Review article:
proteinase-activated receptors - novel signals for gastrointestinal
pathophysiology. Aliment Pharmacol Ther. 14:257–266. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ossovskaya VS and Bunnett NW:
Protease-activated receptors: contribution to physiology and
disease. Physiol Rev. 84:579–621. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schaffner F and Ruf W: Tissue factor and
protease-activated receptor signaling in cancer. Semin Thromb
Hemost. 34:147–153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elste AP and Petersen I: Expression of
proteinase-activated receptor 1-4 (PAR 1-4) in human cancer. J Mol
Histol. 41:89–99. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Milia AF, Salis MB, Stacca T, et al:
Protease-activated receptor-2 stimulates angiogenesis and
accelerates hemodynamic recovery in a mouse model of hindlimb
ischemia. Circ Res. 91:346–352. 2002. View Article : Google Scholar
|
|
16
|
Belting M, Dorrell MI, Sandgren S, et al:
Regulation of angiogenesis by tissue factor cytoplasmic domain
signaling. Nat Med. 10:502–509. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mirza H, Yatsula V and Bahou WF: The
proteinase activated receptor-2 (PAR-2) mediates mitogenic
responses in human vascular endothelial cells. J Clin Invest.
97:1705–1714. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen J, Bierhaus A, Schiekofer S, et al:
Tissue factor - a receptor involved in the control of cellular
properties, including angiogenesis. Thromb Haemost. 86:334–345.
2001.PubMed/NCBI
|
|
19
|
Liu Y and Mueller BM: Protease-activated
receptor-2 regulates vascular endothelial growth factor expression
in MDA-MB-231 cells via MAPK pathways. Biochem Biophys Res Commun.
344:1263–1270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seymour ML, Binion DG, Compton SJ,
Hollenberg MD and MacNaughton WK: Expression of
proteinase-activated receptor 2 on human primary gastrointestinal
myofibroblasts and stimulation of prostaglandin synthesis. Can J
Physiol Pharmacol. 83:605–616. 2005. View
Article : Google Scholar
|
|
21
|
Syeda F, Grosjean J, Houliston RA, et al:
Cyclooxygenase-2 induction and prostacyclin release by
protease-activated receptors in endothelial cells require
cooperation between mitogen-activated protein kinase and NF-kappaB
pathways. J Biol Chem. 281:11792–11804. 2006. View Article : Google Scholar
|
|
22
|
Fujimoto D, Hirono Y, Goi T, Katayama K,
Hirose K and Yamaguchi A: Expression of protease activated
receptor-2 (PAR-2) in gastric cancer. J Surg Oncol. 93:139–144.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dery O, Corvera CU, Steinhoff M and
Bunnett NW: Proteinase-activated receptors: novel mechanisms of
signaling by serine proteases. Am J Physiol. 274:C1429–C1452.
1998.PubMed/NCBI
|
|
24
|
Caruso R, Pallone F, Fina D, et al:
Protease-activated receptor-2 activation in gastric cancer cells
promotes epidermal growth factor receptor trans-activation and
proliferation. Am J Pathol. 169:268–278. 2006. View Article : Google Scholar
|
|
25
|
Darmoul D, Gratio V, Devaud H and Laburthe
M: Protease-activated receptor 2 in colon cancer: trypsin-induced
MAPK phosphorylation and cell proliferation are mediated by
epidermal growth factor receptor transactivation. J Biol Chem.
279:20927–20934. 2004. View Article : Google Scholar
|
|
26
|
Chang JH, Park JM, Kim SW, Jung CK, Kang
WK and Oh ST: Expression of protease activated receptor-2 in human
colorectal cancer and its association with tumor progression. Dis
Colon Rectum. 53:1202–1208. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kong W, McConalogue K, Khitin LM, et al:
Luminal trypsin may regulate enterocytes through
proteinase-activated receptor 2. Proc Natl Acad Sci USA.
94:8884–8889. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ichikawa Y, Koshikawa N, Hasegawa S, et
al: Marked increase of trypsin(ogen) in serum of linitis plastica
(gastric cancer, borrmann 4) patients. Clin Cancer Res.
6:1385–1388. 2000.PubMed/NCBI
|
|
29
|
Nakanuma S, Tajima H, Okamoto K, et al:
Tumor-derived trypsin enhances proliferation of intrahepatic
cholangiocarcinoma cells by activating protease-activated
receptor-2. Int J Oncol. 36:793–800. 2010. View Article : Google Scholar
|
|
30
|
Jahan I, Fujimoto J, Alam SM, Sato E,
Sakaguchi H and Tamaya T: Expression of protease activated
receptor-2 related to angiogenesis in tumor advancement of uterine
endometrial cancers. Oncol Rep. 17:345–350. 2007.PubMed/NCBI
|
|
31
|
Jahan I, Fujimoto J, Alam SM, Sato E,
Sakaguchi H and Tamaya T: Role of protease activated receptor-2 in
tumor advancement of ovarian cancers. Ann Oncol. 18:1506–1512.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Eguchi H, Iwaki K, Shibata K, Ogawa T,
Ohta M and Kitano S: Protease-activated receptor-2 regulates
cyclooxygenase-2 expression in human bile duct cancer via the
pathways of mitogen-activated protein kinases and nuclear factor
kappa B. J Hepatobiliary Pancreat Sci. 18:147–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Joo YE, Rew JS, Seo YH, et al:
Cyclooxygenase-2 overexpression correlates with vascular
endothelial growth factor expression and tumor angiogenesis in
gastric cancer. J Clin Gastroenterol. 37:28–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang SP, Wu MS, Shun CT, et al:
Cyclooxygenase-2 increases hypoxia-inducible factor-1 and vascular
endothelial growth factor to promote angiogenesis in gastric
carcinoma. J Biomed Sci. 12:229–241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Belham CM, Tate RJ, Scott PH, et al:
Trypsin stimulates proteinase-activated receptor-2-dependent and
-independent activation of mitogen-activated protein kinases.
Biochem J. 320:939–946. 1996.PubMed/NCBI
|
|
36
|
Yang XP, Li Y, Wang Y and Wang P:
Beta-Tryptase up-regulates vascular endothelial growth factor
expression via proteinase-activated receptor-2 and
mitogen-activated protein kinase pathways in bone marrow stromal
cells in acute myeloid leukemia. Leuk Lymphoma. 51:1550–1558. 2010.
View Article : Google Scholar
|
|
37
|
DeFea KA, Zalevsky J, Thoma MS, Dery O,
Mullins RD and Bunnett NW: Beta-arrestin-dependent endocytosis of
proteinase-activated receptor 2 is required for intracellular
targeting of activated ERK1/2. J Cell Biol. 148:1267–1281. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stalheim L, Ding Y, Gullapalli A, et al:
Multiple independent functions of arrestins in the regulation of
protease-activated receptor-2 signaling and trafficking. Mol
Pharmacol. 67:78–87. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kanke T, Macfarlane SR, Seatter MJ, et al:
Proteinase-activated receptor-2-mediated activation of
stress-activated protein kinases and inhibitory kappa B kinases in
NCTC 2544 keratinocytes. J Biol Chem. 276:31657–31666. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lidington EA, Steinberg R, Kinderlerer AR,
et al: A role for proteinase-activated receptor 2 and PKC-epsilon
in thrombin-mediated induction of decay-accelerating factor on
human endothelial cells. Am J Physiol Cell Physiol.
289:C1437–C1447. 2005. View Article : Google Scholar
|
|
41
|
Xiao YQ, Malcolm K, Worthen GS, et al:
Cross-talk between ERK and p38 MAPK mediates selective suppression
of pro-inflammatory cytokines by transforming growth factor-beta. J
Biol Chem. 277:14884–14893. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tancharoen S, Sarker KP, Imamura T, et al:
Neuropeptide release from dental pulp cells by RgpB via
proteinase-activated receptor-2 signaling. J Immunol.
174:5796–5804. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fyfe M, Bergstrom M, Aspengren S and
Peterson A: PAR-2 activation in intestinal epithelial cells
potentiates interleukin-1beta-induced chemokine secretion via MAP
kinase signaling pathways. Cytokine. 31:358–367. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Weis SM and Cheresh DA: Tumor
angiogenesis: molecular pathways and therapeutic targets. Nat Med.
17:1359–1370. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
45
|
Barry GD, Suen JY, Le GT, Cotterell A,
Reid RC and Fairlie DP: Novel agonists and antagonists for human
protease activated receptor 2. J Med Chem. 53:7428–7440. 2010.
View Article : Google Scholar : PubMed/NCBI
|