Introduction
Hepatitis B virus (HBV) infects ~350 million
individuals globally each year and 1.2 million people die from
chronic HBV infection, cirrhosis and liver cancer. About 2 billion
of the world’s population has been infected with the hepatotropic
DNA virus at present time (1). HBV
contains four identified open reading frames (ORFs) named C, S, P,
and X coding for hepatitis B core antigen (HBcAg), hepatitis B
surface antigen (HBsAg), hepatitis B envelope antigen (HBeAg), and
X protein, respectively (2,3). HBcAg, HBsAg, HBeAg can be used in the
diagnosis of the infection and to determine the severity of the
infection (4). The X protein (HBX),
which consist of 154 amino acids, contains four regions important
for trans-activation, and modulation of cytoplasmic signal
transduction pathways (5). HBX has
received much attention because it can affect apoptosis, gene
expression, cell cycle, and cell proliferation in host cells
(6–8). Furthermore, the relationship between
HBX and many signaling pathways has been demonstrated, such as
Ras-Raf MAPK signaling pathway (9,10),
JAK-STAT signaling pathway (11,12),
PKC signaling pathway (13,14), and SAPK/JNK signaling pathway
(15,16). However, whether HBx induced or
inhibited apoptosis remains unclear. Previous research has
identified that HBX could upregulate survivin a well-known
anti-apoptosis protein (17).
Others have found HBX is associated with caspase activation and
mitochondrial dysfunction (18).
Although chronic infection of HBV has been linked
epidemiologically to the development of hepatocellular carcinoma
for >40 years (19), we neglect
another basic problem. As known to us, Hepatitis B virus is
transmitted by contact with blood or body fluids of an infected
person. Gastric ulcers may cause vascular injury and bleeding,
providing an important way for HBV infection. In the last decades,
we only pay attention to the relationship between HBV and the
liver. We did not note that the lesions caused by HBV in the
stomach. Whether HBV can aggravate the injury of gastric ulcer by
infecting gastric mucosa epithelial cell remains unclear. This
study aimed to determine the role of HBV X protein in gastric
tissues and cells.
Materials and methods
Study population and ethics
statement
Sixty-four chronic hepatitis B patients (CHB) with
gastric ulcer were recruited from First Hospital of China Medical
University in this study from July 2007 to July 2011. The diagnosis
of CHB was confirmed by the serological examination of HBsAg for
>6 months. Tissue specimens were derived from the patients after
the resection at the Department of General Surgery. This study was
in compliance with the Helsinki Declaration, all patients gave
written informed consent for participation, and the procedure was
approved by Our University Ethics Committee.
Cell lines
Human gastric mucosa cell line, GES-1, was obtained
from the American Type Culture Collection (Bethesda, MD, USA) and
grown in RPMI-1640 medium (Hyclone, UT, USA) supplemented with 10%
fetal bovine serum and antibiotics (100 U/ml penicillin and 100
μg/ml streptomycin). Cells were maintained in a humidified cell
incubator with 5% CO2 at 37°C.
Plasmids and transfection
A full-length ORF of HBX was obtained from
gastric ulcer samples by RT-PCR. The primers of HBX were,
sense: 5′-CGGAATTCATGGCTGCTAGGC TGTGCTG-3′ (EcoRI) and
antisense: 5′-CGCGGATCCGG CAGAGGTGAAAAAGTTGC-3′ (BamHI)
(Takara Dalian, Dalian, China). The cDNA of HBX was cloned
into BamHI and EcoRI sites of mammalian expression
vector pcDNA3.1. All of the constructs were confirmed by DNA
sequencing (Sunbiotech, Beijing, China). Cells were transfected
using Lipofectamine™ 2000 (Invitrogen, CA, USA) according to the
manufacturer’s instructions. HBX-expressing cells were obtained by
transfecting with pcDNA3.1-HBX.
Western blot analysis
Tissues and cells were lysed in lysis buffer (20 mM
Tris-HCl, 150 mM NaCl, 2 mM EDTA, 1% Triton-X100) containing a
protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Cell
extract protein amounts were quantified using the BCA protein assay
kit. Equivalent amounts of protein (20 μg) were separated using 12%
SDS-PAGE and transferred to PVDF membrane (Millipore Corporation,
Billerica, MA). Western blot was performed using primary
antibodies: HBX (Alexis Biochemicals, San Diego, CA),
stress-activated protein kinase/JNK antibody (Cell Signaling
Technology, Beverly, MA), phospho-stress activated protein
kinase/p-JNK (Thr183/Tyr185) (Cell Signaling
Technology), CHOP (abcam), BiP (Cell Signaling Technology), c-jun
(Cell Signaling Technology), phosphorylated c-jun (Ser63) (Cell
Signaling Technology), and β-actin (Millipore). Each specific
antibody binding was detected with horseradish peroxidase
(HRP)-conjugated, respective, secondary antibodies and ECL
solutions (Amersham Biosciences, UK).
Semi-quantitative real-time PCR
Total tissue and cellular RNA was isolated using
TRIzol reagent (Invitrogen) and was reverse transcribed by using
SuperScript II reverse transcriptase (Invitrogen) according to the
manufacturer’s protocol. Real-time PCR was performed using primers
specific for HBX and GAPDH. Real-time PCR analysis
was performed on the ABI Prism 7500 sequence detection system
(Applied Biosystems, Foster, CA) using the SYBR Green PCR Master
mixture (Takara, Dalian). The PCR conditions were: one cycle at
95°C for 10 min followed by 40 cycles at 95°C for 15 sec and at
60°C for 1 min. The following primer sets were used: HBX sense:
5′-GGCAGAGGTGAAAAAGTTGC-3′, antisense: 5′-GGCAGAGGTGAAAAAGTTGC-3′;
GAPDH sense: 5′-GAA GGTGAAGGTCGGAGT-3′, antisense:
5′-CATGGGTGGAATCATATTGGAA-3′. Relative quantitation was calculated
by ΔΔCt method. Each reaction was repeated independently at three
times in triplicate.
Immunohistochemical staining (IHC)
Immunohistochemistry was used to detect the
expression of HBX protein in gastric ulcer samples. The study
population included 64 patients. Immunohistochemical staining was
performed on 4-μm sections obtained from formalin-fixed,
paraffin-embedded blocks. Endogenous peroxidase activity was
blocked with 3% hydrogen peroxide for 30 min. Antigen retrieval was
carried out in citrate buffer (10 mM, pH 6.0) for 30 min at 95°C in
a pressure cooker. Anti-HBX (Alexis Biochemicals) at 1:500 was
applied incubated at 4°C overnight. Afterward, sections were
incubated with a biotinylated secondary antibody and then exposed
to a streptavidin complex (HRP). Positive reactions were visualized
with 3,3′-diaminobenzidine tetrahydrochloride (DAB, Sigma),
followed by counterstaining with hematoxylin. Normal tissue was
used as a control. Sections treated without primary antibodies were
used as negative controls.
3-[4, 5-Dimethylthiazol-2-yl]-2,
5-diphenyltetrazolium bromide (MTT) assay
The proliferation rate of HBX-expressing cells and
control cells were measured by MTT assay. Briefly, HBX-expressing
cells or control cells were plated at a density of
1×103/well in 96-well plates. After incubation of 48 h,
0.5 mg/ml MTT was added (Sigma). Four hours later, the medium was
replaced with 100 μl dimethylsulfoxide (DMSO) (Sigma). Absorbance
Optical density (OD) of each well was determined at 490 nm of
wavelength with subtraction of baseline reading. Each time point
was repeated six times and the mean and standard errors were
calculated.
4′-6-Diamidino-2-phenylindole (DAPI)
staining assay
DAPI staining was applied for determining the
apoptotic cells. Cells at a density of 1×105 cells/well
were maintained on six-well plates and then were treated under the
normal culture condition. Cells in each well were individually
fixed in 4% (v/v) paraformaldehyde (Sigma) for 15 min and then
stained using DAPI (Invitrogen) for apoptotic cells. Cells were
then examined and photographed using a fluorescence microscope
(Olympus CX71, Japan).
Cell cycle and apoptosis analysis
The gastric mucosal GES-1 cells
(3×105/well), were plated and incubated overnight. The
control and treated cells were trypsinized, collected in PBS and
fixed on ice with 1% paraformaldehyde, followed by 70% cold
ethanol. After treatment with 10 μg/ml RNase, the cells were
stained with 50 μg/ml propidium iodide (PI, KeyGEN, Nanjing, China)
for 15 min at room temperature for cell cycle analysis. The
apoptotic cells were detected with AnnexinV-FITC/PI double
staining. The cells were trypsinized and stained with Annexin
V-FITC and PI following the manufacturer’s instructions for the
Apoptosis Assay kit (KeyGEN). The stained cells were analyzed by
flow cytometry. Data analysis was performed with CellQuest software
(BD Biosciences, MD).
Statistical analysis
All experiments were done three times in triplicate,
and the results were expressed as means ± SD (standard deviation).
P-values <0.05 were considered to statistically significant. All
statistical analyses were performed with SPSS software (version
16.0; SPSS Inc., Chicago, IL, USA).
Results
The levels of HBX mRNA and protein were
evaluated in gastric ulcer specimens from 64 chronic hepatitis B
patients (CHB)
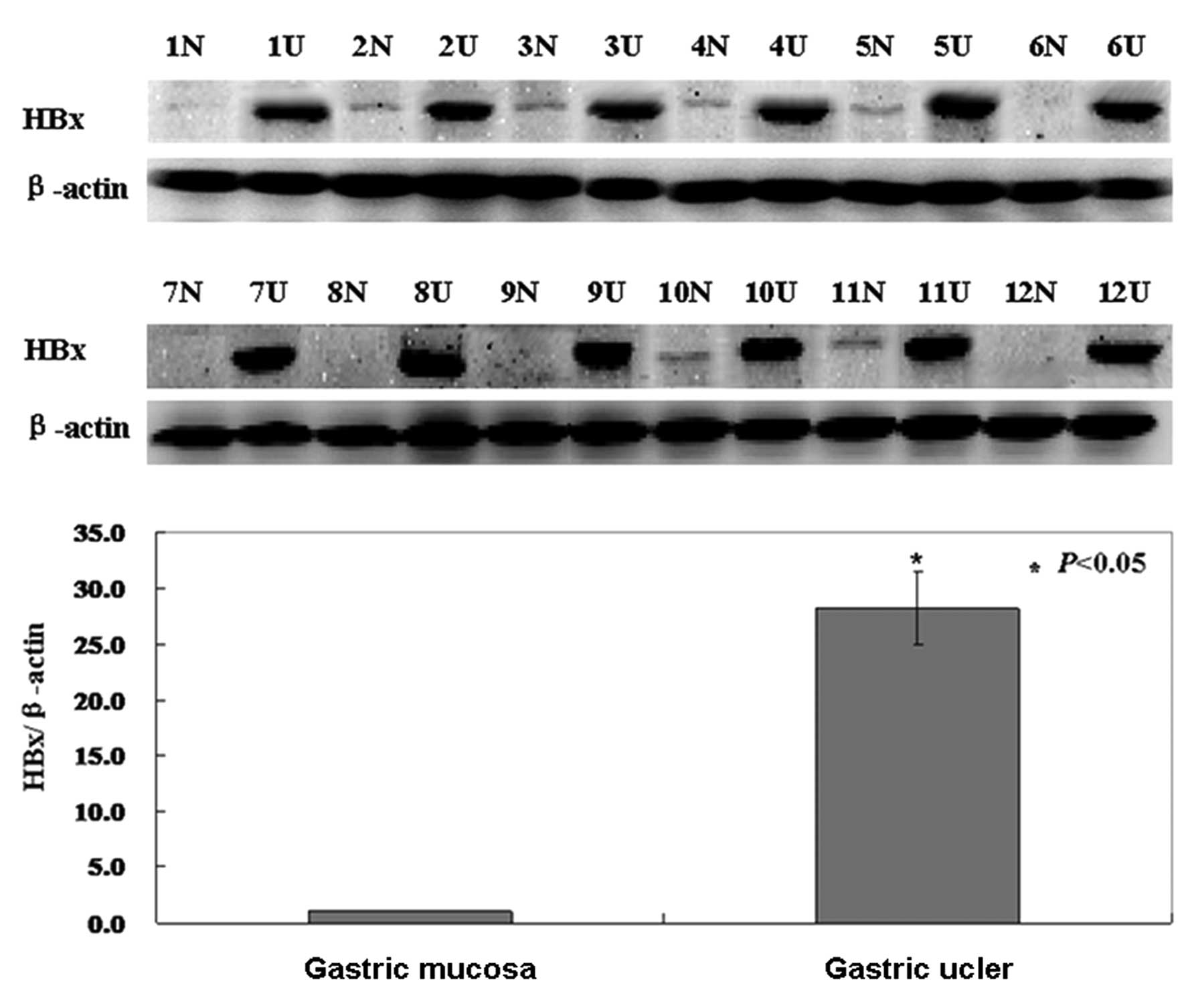
Western blotting was carried out to investigate the
protein status of HBX in gastric ulcer specimens. As shown in the
results, the level of HBX protein was higher in gastric ulcers than
that in normal tissue (P<0.05, Fig.
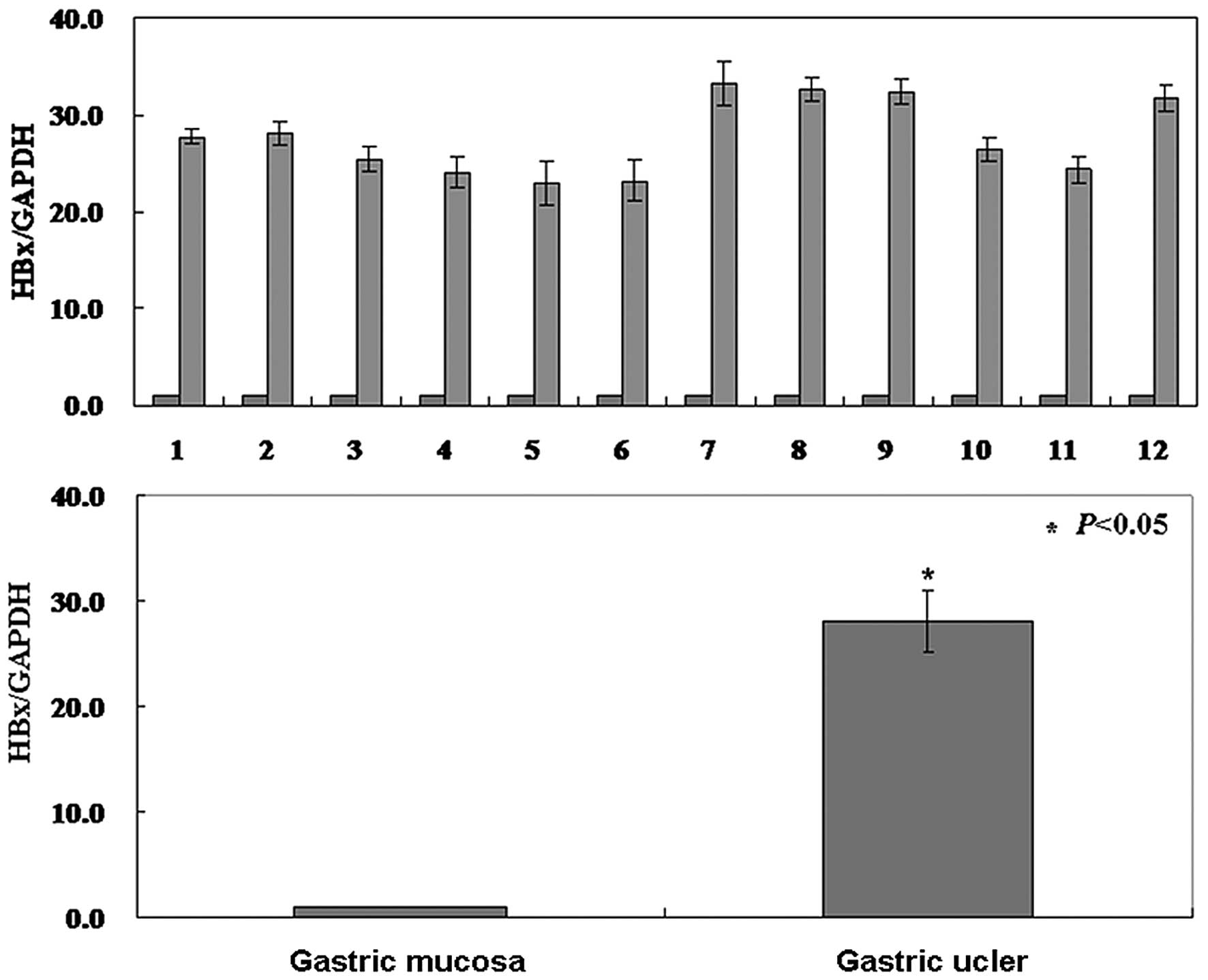
1). To examine the relationship between the level of HBX
protein and the level of HBX transcription, real-time PCR of HBX
mRNA was carried out in gastric ulcer specimens. The results showed
that the level of HBX mRNA was also higher than normal tissue and
coincident with the level of protein (P<0.05, Fig. 2).
Correlation between HBX expression and
clinicopathological features in gastric ulcers
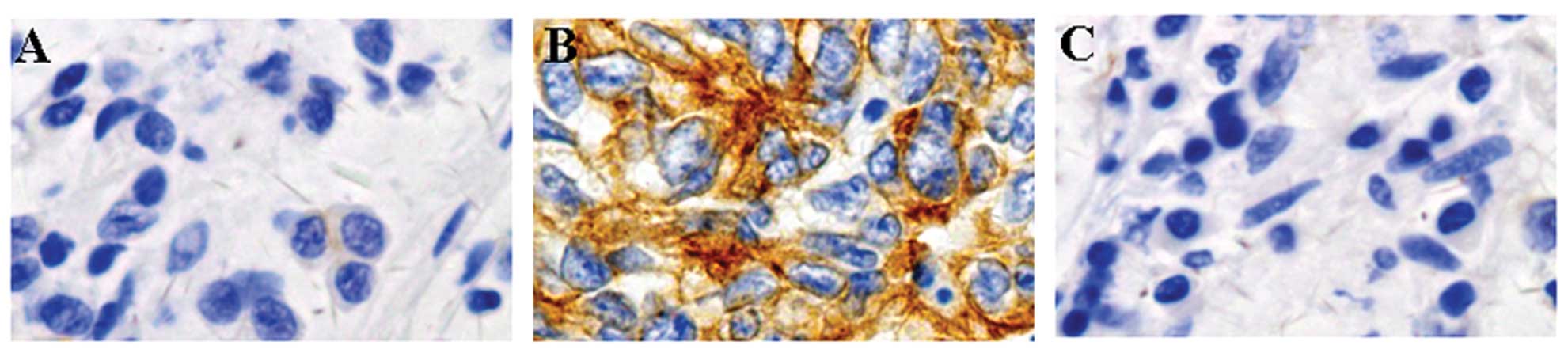
The immunostaining for HBX was only localized in the
cytoplasm. HBX protein was highly expressed in gastric ulcer
specimens, but not in normal parts of the specimens (Fig. 3). We then analyzed the potential
relationship between the expression of HBX and the
clinicopathological characteristics of these patients. The results
are summarized in Table I. No
correlation was found with gender, age, intake of alcohol, ulcer
location, or ulcer stage (P>0.05). However, HBx expression was
significantly associated with atrophy, metaplasia and bleeding
(P<0.05).
 | Table IRelationship between HBx expression
and clinicopathological parameters of gastric ulcers. |
Table I
Relationship between HBx expression
and clinicopathological parameters of gastric ulcers.
| Clinicopathological
features | | HBx expression |
|---|
|
|
|---|
| n | − | + | ++ | +++ | PR (%) | χ2 value | P |
|---|
| Gender | | | | | | | 0.986 | 0.982 |
| Female | 26 | 4 | 2 | 11 | 9 | 84.6 | | |
| Male | 38 | 5 | 6 | 14 | 13 | 86.8 | | |
| Age (years) | | | | | | | 0.882 | 0.498 |
| <45 | 22 | 3 | 3 | 10 | 6 | 86.4 | | |
| ≥45 | 42 | 6 | 5 | 15 | 16 | 85.7 | | |
| Intake of
alcohol | | | | | | | 5.266 | 0.510 |
| No | 23 | 1 | 5 | 8 | 9 | 95.7 | | |
| Yes | 41 | 8 | 3 | 17 | 13 | 80.5 | | |
| Atrophy | | | | | | | 7.866 | 0.048 |
| − | 16 | 2 | 1 | 3 | 10 | 87.5 | | |
| + | 48 | 7 | 7 | 22 | 12 | 85.4 | | |
| Metaplasia | | | | | | | 11.00 | 0.012 |
| − | 19 | 4 | 2 | 2 | 11 | 78.9 | | |
| + | 45 | 5 | 6 | 23 | 11 | 88.9 | | |
| Bleeding | | | | | | | 12.34 | 0.006 |
| − | 24 | 2 | 5 | 4 | 13 | 91.7 | | |
| + | 40 | 7 | 3 | 21 | 9 | 82.5 | | |
| Location | | | | | | | 3.305 | 0.951 |
| Antrum or
angle | 18 | 2 | 2 | 7 | 7 | 88.8 | | |
| Lower body | 15 | 3 | 1 | 6 | 5 | 80.0 | | |
| Mid-body | 16 | 1 | 2 | 7 | 6 | 93.8 | | |
| Upper body | 15 | 3 | 3 | 5 | 4 | 80.0 | | |
| Stage | | | | | | | 6.295 | 0.974 |
| A1 | 11 | 1 | 1 | 4 | 5 | 90.9 | | |
| A2 | 8 | 1 | 1 | 3 | 3 | 87.5 | | |
| H1 | 9 | 1 | 1 | 5 | 2 | 88.8 | | |
| H2 | 12 | 2 | 2 | 2 | 6 | 83.3 | | |
| S1 | 11 | 2 | 2 | 5 | 2 | 81.8 | | |
| S2 | 13 | 2 | 1 | 6 | 4 | 84.6 | | |
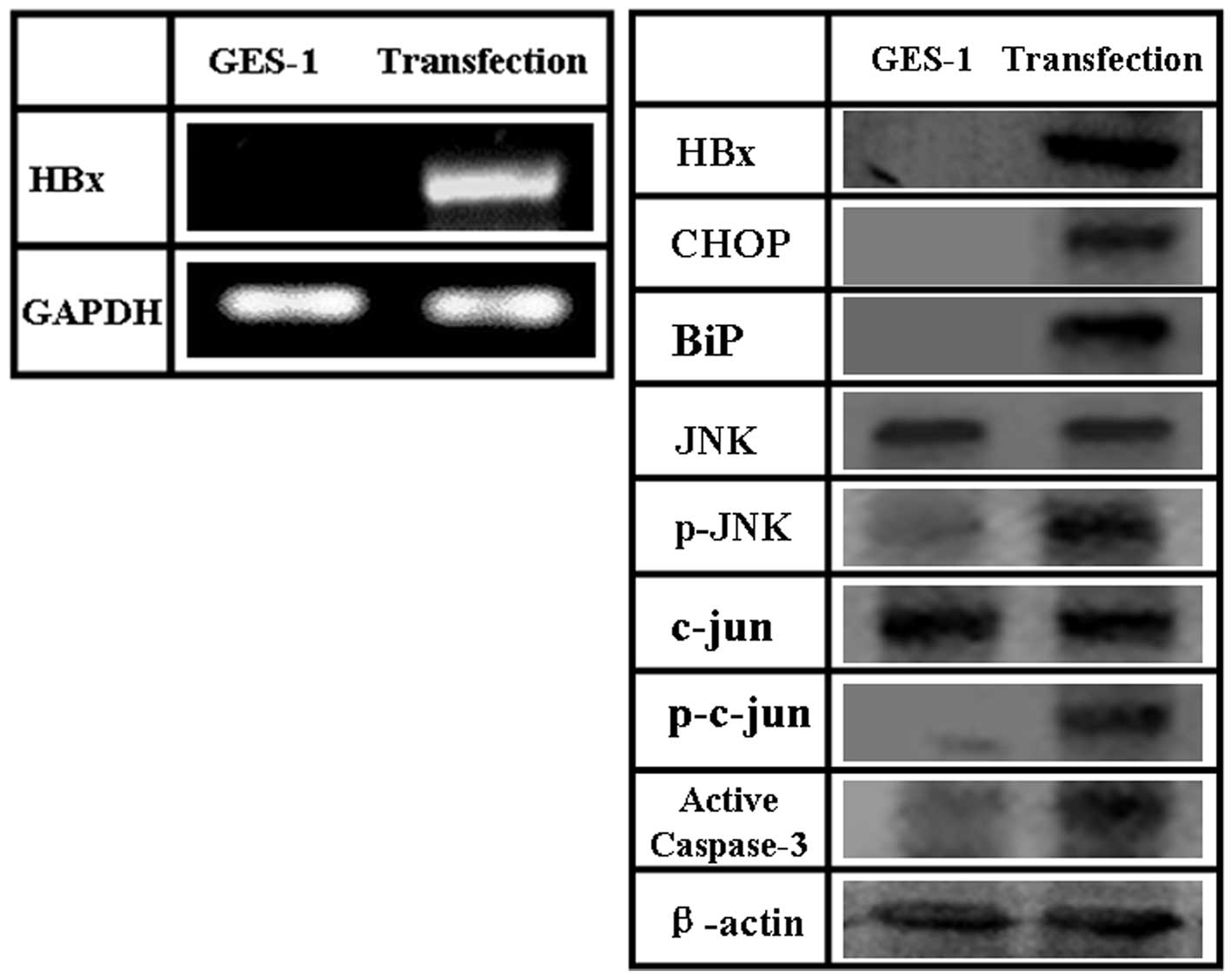
HBX expressed in human gastric mucosa
cell line GES-1
In order to study the role that HBX plays in human
gastric mucosa cell line GES-1, we examined the effects of
exogenous HBX expression in GES-1. To this end, we constructed an
HBX-expressing plasmid, pCDNA-3.1-HBX, and transfected it into
GES-1 cells. The levels of HBX mRNA and protein increased upon
transfection, compared to the levels observed in human gastric
mucosa cell line GES-1 (Fig.
5).
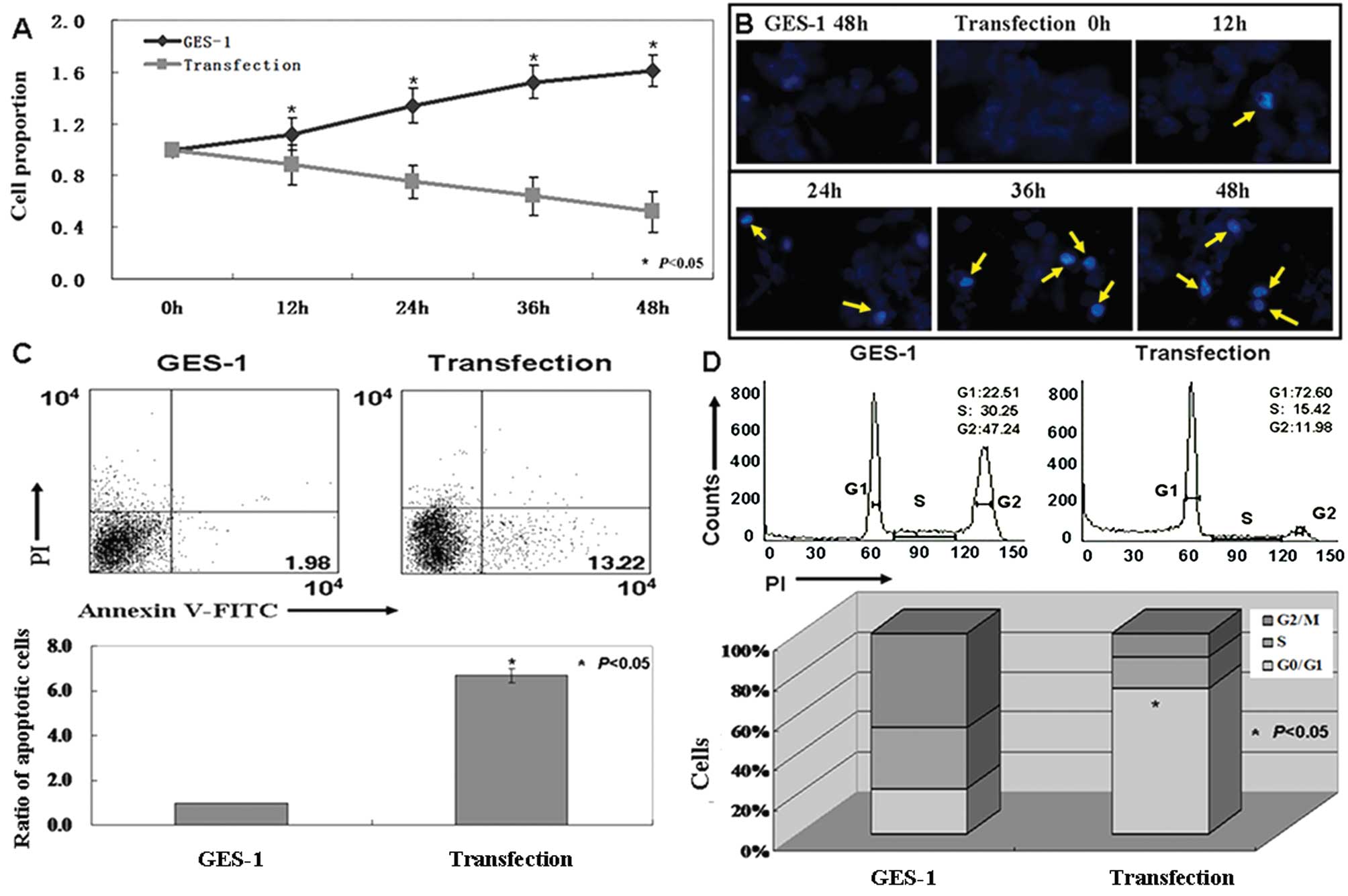
The effects of HBX on GES-1 cells
MTT assays were performed, and growth inhibition
curves were generated (P<0.05, Fig.
4A). Proliferative ratio of the human gastric mucosa GES-1
cells was inhibited by HBX expression. DAPI staining showed
morphological changes in the nucleus after HBX transfection.
Fig. 4B shows nuclear condensation
in apoptotic cells. AnnexinV-FITC and PI double staining was
performed to detect apoptotic cells quantitatively. The apoptotic
ratio of the cells transfected with pCDNA-3.1-HBX was 6–7 times
higher than that of untransfected cells (P<0.05, Fig. 4C). When cell cycles of transfected
and untransfected cells were examined using PI staining, the ratio
of cells in the G1 phase increased in transfected cells
versus untransfected cells (P<0.05, Fig. 4D).
The mechanism of HBX induced-apoptosis in
GES-1 cells
Given that ER stress is highly correlated with the
promotion of apoptosis, in this study we examined the changes of
ER-stress mediated apoptotic pathways in GES-1 after pCDNA-3.1-HBX
transfection. As shown in Fig. 5,
expression of ER stress molecules (BiP and CHOP) was significantly
increased in HBX-transfected cells. Activation of JNK pathway in
GES-1 cells transfected with pCDNA-3.1-HBX was confirmed using a
phosphorylated JNK-specific antibody and by detecting the
phosphorylation of c-jun (Fig. 5).
No significant changes of JNK and c-jun were detected in
transfected cells. These results indicate that JNK phosphorylation
is a crucial event controlling HBX-induced apoptosis. We also
confirmed that caspase-3 was activated in transfected cells.
Discussion
HBV infection is a major risk factor of human
chronic liver disease and is strongly associated with
hepatocellular carcinogenesis (HCC) (20). Although the relationship between HBV
infection and chronic liver disease has been identified, the
effects of HBV infection on gastric problems remain unclear. HBX is
a 17-kDa transcriptional co-activator that plays a significant role
in the regulation of genes involved in inflammation and cell
survival (21). In our studies, HBX
was found to be highly expressed in gastric ulcer specimens from
chronic hepatitis B patients. We found that HBX expression was
significantly associated with atrophy, metaplasia and bleeding.
These results suggested that HBV could infect gastric mucosa and
aggravate gastric mucosal injury.
Some previous studies have suggested that HBX can
activate apoptotic pathways and induce apoptosis (22–25).
However, opposing the pro-apoptotic activity of HBX was observed in
other studies (15,26–28).
The effects of HBX on apoptosis are not entirely understood. In our
studies, we confirmed that HBX could induce GES-1 cells to
apoptosis. GES-1 cells transfected with pCDNA-3.1-HBX exhibited
apoptosis and G1 arrest. The results of our study are
consistent with two other studies which concluded that HBX
inhibited hepatocyte regeneration (29,30).
Wu et al (29) found that
HBX protein blocks G1/S transition of the hepatocyte
cell cycle progression. The results of Gearhart and Bouchard
(31) also suggest that HBX uses
mitochondrial-dependent calcium signaling to cause hepatocytes to
exit G0 but stall in G1.
In previous studies, HBX was shown to be involved in
many cell signaling transduction pathways, such as Ras-Raf MAPK
signaling pathway, JAK-STAT signaling pathway, PKC signaling
pathway, and SAPK/JNK signaling pathway (9–16). HBX
activates AP-1 via a pathway that is mediated by the activation of
ERK and JNK (32,33). Kong et al (34) have demonstrated that activation of
AP-1 and JNK was inhibited in the cells after siRNA treatment. In
order to detect the mechanism of HBX in GES-1, we carried out
western blot to analyze the changes of related signaling pathways.
Consistent with previous studies, we found that HBX induced
apoptosis in GES-1 though the JNK signaling pathway. We confirmed
that HBX was able to efficiently provoke endoplasmic reticulum (ER)
stress in GES-1. ER stress can be induced by the unfolded protein
response (UPR), which is activated in a number of disease
processes, such as obesity, diabetes, heart disease, cancer, and
viral infection (35,36). Previous studies have demonstrated
that alteration of the levels of the ER molecular chaperone
GRP78/BiP can inhibit tumor growth in vivo (37). Others studies also confirmed that ER
stress-induced apoptosis is highly dependent on the upregulation of
the UPR-inducible transcription factor CHOP (38). Consistent with previous studies, we
found that the levels of BiP and CHOP in GES-1 cells were higher
than untreated ones. These results indicated that HBX could provoke
ER stress and further activate JNK signaling pathway in GES-1.
In conclusion, our results demonstrated that the
infection of HBV is involved in progression of gastric ulcers. In
addition, HBX acts as a positive regulator in the JNK signaling
pathway. These findings may provide important information in
understanding the role of the HBV infection in gastric ulcers.
Acknowledgements
We thank Dr Miao Yu for technical assistance.
References
|
1
|
Shepard CW, Simard EP, Finelli L, et al:
Hepatitis B virus infection: epidemiology and vaccination.
Epidemiol Rev. 28:112–125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Robinson WS: Molecular events in the
pathogenesis of hepaDNA virus-associated hepatocellular carcinoma.
Annu Rev Med. 45:297–323. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yee JK: A liver-specific enhancer in the
core promoter region of human hepatitis B virus. Science.
246:658–661. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gitlin N: Hepatitis B: diagnosis,
prevention, and treatment. Clin Chem. 43:1500–1506. 1997.PubMed/NCBI
|
|
5
|
Tang H, Oishi N, Kaneko S, et al:
Molecular functions and biological roles of hepatitis B virus X
protein. Cancer Sci. 97:977–983. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang X, Liu Y, Zhang Q, et al: Hepatitis
B virus sensitizes hepatocytes to TRAIL-induced apoptosis through
Bax. J Immunol. 178:503–510. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mukherji A, Janbandhu VC and Kumar V:
HBx-dependent cell cycle deregulation involves interaction with
cyclin E/A-cdk2 complex and destabilization of p27Kip1. Biochem J.
401:247–256. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shan C, Xu F, Zhang S, et al: Hepatitis B
virus X protein promotes liver cell proliferation via a positive
cascade loop involving arachidonic acid metabolism and p-ERK1/2.
Cell Res. 20:563–575. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stockl L, Berting A, Malkowski B, et al:
Integrity of c-Raf-1/MEK signal transduction cascade is essential
for hepatitis B virus gene expression. Oncogene. 22:2604–2610.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tarn C, Lee S, Hu Y, et al: Hepatitis B
virus X protein differentially activates RAS-RAF-MAPK and JNK
pathways in X-transforming versus non-transforming AML12
hepatocytes. J Biol Chem. 276:34671–34680. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee YH and Yun Y: HBx protein of hepatitis
B virus activates Jak1-STAT signaling. J Biol Chem.
273:25510–25515. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bock CT, Toan NL, Koeberlein B, et al:
Subcellular mislocalization of mutant hepatitis B X proteins
contributes to modulation of STAT/SOCS signaling in hepatocellular
carcinoma. Intervirology. 51:432–443. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cong YS, Yao YL, Yang WM, et al: The
hepatitis B virus X-associated protein, XAP3, is a protein kinase
C-binding protein. J Biol Chem. 272:16482–16489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kekulé AS, Lauer U, Weiss L, et al:
Hepatitis B virus transactivator HBx uses a tumor promoter
signaling pathway. Nature. 361:742–745. 1993.PubMed/NCBI
|
|
15
|
Diao J, Khine AA, Sarangi F, et al: X
protein of hepatitis B virus inhibits Fas-mediated apoptosis and is
associated with up-regulation of the SAPK/JNK pathway. J Biol Chem.
276:8328–8340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oh JC, Jeong DL, Kim IK, et al: Activation
of calcium signaling by hepatitis B virus-X protein in liver cells.
Exp Mol Med. 35:301–309. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X, Dong N, Yin L, et al: Hepatitis B
virus X protein upregulates survivin expression in hepatoma
tissues. J Med Virol. 77:374–381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takada S, Shirakata Y, Kaneniwa N, et al:
Association of hepatitis B virus X protein with mitochondria causes
mitochondrial aggregation at the nuclear periphery, leading to cell
death. Oncogene. 18:6965–6973. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sherlock S, Fox RA, Niazi SP, et al:
Chronic liver disease and primary liver cell cancer with
hepatitis-associated (Australia) antigen in serum. Lancet.
1:1243–1247. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim CM, Koike K, Saito I, et al: HBx gene
of hepatitis B virus induces liver cancer in transgenic mice.
Nature. 351:317–320. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gottlob K, Pagano S, Levrero M, et al:
Hepatitis B virus X protein transcription activation domains are
neither required nor sufficient for cell transformation. Cancer
Res. 58:3566–3570. 1998.PubMed/NCBI
|
|
22
|
Miao J, Chen GG, Chun SY, et al: Hepatitis
B virus X protein induces apoptosis in hepatoma cells through
inhibiting Bcl-xL expression. Cancer Lett. 236:115–124. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chirillo P, Pagano S, Natoli G, et al: The
hepatitis B virus X gene induces p53-mediated programmed cell
death. Proc Natl Acad Sci USA. 94:8162–8167. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shintani Y, Yotsuyanagi H, Moriya K, et
al: Induction of apoptosis after switch-on of the hepatitis B virus
X gene mediated by the Cre/loxP recombination system. J Gen Virol.
80:3257–3265. 1999.PubMed/NCBI
|
|
25
|
Su F and Schneider RJ: Hepatitis B virus
HBx protein sensitizes cells to apoptotic killing by tumor necrosis
factor alpha. Proc Natl Acad Sci USA. 94:8744–8749. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee YI, Kang-Park S, Do SI, et al: The
hepatitis B virus-X protein activates a phosphatidylinositol
3-kinase-dependent survival signaling cascade. J Biol Chem.
276:16969–16977. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan J, Duan LX, Sun BS, et al: Hepatitis B
virus X protein protects against anti-Fas-mediated apoptosis in
human liver cells by inducing NF-kappa B. J Gen Virol. 82:171–182.
2001.PubMed/NCBI
|
|
28
|
Shih WL, Kuo ML, Chuang SE, et al:
Hepatitis B virus X protein inhibits transforming growth
factor-beta-induced apoptosis through the activation of
phosphatidylinositol 3-kinase pathway. J Biol Chem.
275:25858–25864. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu BK, Li CC, Chen HJ, et al: Blocking of
G1/S transition and cell death in the regenerating liver of
Hepatitis B virus X protein transgenic mice. Biochem Biophys Res
Commun. 340:916–928. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tralhao JG, Roudier J, Morosan S, et al:
Paracrine in vivo inhibitory effects of hepatitis B virus X protein
(HBx) on liver cell proliferation: An alternative mechanism of
HBx-related pathogenesis. Proc Natl Acad Sci USA. 99:6991–6996.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gearhart TL and Bouchard MJ: Replication
of the hepatitis B virus requires a calcium-dependent HBx-induced
G1 phase arrest of hepatocytes. Virology. 407:14–25. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jacqueline B, Fei S, Margherita D, et al:
Hepatitis B virus HBx protein induces transcription factor AP-1 by
activation of extracellular signal-regulated and c-Jun N-terminal
mitogen-activated protein kinases. J Virol. 70:4978–4985. 1996.
|
|
33
|
Nijhara R, Jana SS, Goswami SK, et al:
Sustained activation of mitogen-activated protein kinases and
activator protein 1 by the hepatitis B virus X protein in mouse
hepatocytes in vivo. J Virol. 75:10348–10358. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kong GY, Zhang JP, Zhang S, et al:
Hepatitis B virus X protein promotes hepatoma cell proliferation
via upregulation of MEKK2. Acta Pharmacol Sin. 32:1173–1180. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim I, Xu W and Reed JC: Cell death and
endoplasmic reticulum stress: disease relevance and therapeutic
opportunities. Nat Rev Drug Discov. 7:1013–1030. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Medigeshi GR, Lancaster AM, Hirsch AJ, et
al: West Nile virus infection activates the unfolded protein
response, leading to CHOP induction and apoptosis. J Virol.
81:10849–10860. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Moenner M, Pluquet O, Bouchecareilh M, et
al: Integrated endoplasmic reticulum stress responses in cancer.
Cancer Res. 67:10631–10634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tamaki N, Hatano E, Taura K, et al: CHOP
deficiency attenuates cholestasis-induced liver fibrosis by
reduction of hepatocyte injury. Am J Physiol Gastrointest Liver
Physiol. 294:G498–G505. 2008. View Article : Google Scholar : PubMed/NCBI
|