Introduction
Breast cancer is the leading cancer in women,
affecting millions worldwide. In metropolitan cities it ranks as
the most common cancer (1). The
prognosis for patients with advanced-stage breast cancer is related
to the degree of aggressive metastasis. Once breast cancer has
spread, there are limited effective treatment options (2). Therefore, there is an urgent need to
understand the mechanisms involved in the progression of cancer.
The process of cancer metastasis appears to be regulated by a
variety of gene products, since the precise mechanisms of
dissemination are not well understood.
One important concept of epithelial-mesenchymal
transition (EMT), which has been recognized for several decades as
a fundamental process of embryogenesis, is currently considered a
pivotal event in the initial step of the metastatic cascade that
allows cells to acquire migratory, invasive and stem-like
properties (3). During EMT of
cancer cells in situ, epithelial cell layers lose polarity
together with cell-to-cell contacts and then undergo a dramatic
remodeling of the cytoskeleton. The expression of proteins that
promote cell-to-cell contact, such as E-cadherin and γ-catenin, may
be lost, and the cells may acquire mesenchymal markers such as
vimentin, fibronectin, N-cadherin, and the metalloproteinases MMP-2
and MMP-9, resulting in an enhanced ability for cell migration and
invasion (4). Once migrating to a
suitable site, tumor cells re-express E-cadherin and other
epithelial markers through a process that is often referred to as
mesenchymal-epithelial transition (MET) (5). Induction of EMT is driven through the
complex interplay between cancer cells and the tumor environment.
The mechanisms include the activation of several transcriptional
repressors, notably Snail, Slug and Twist, through multiple
cellular signaling pathways such as NF-κB, Wnt and Hedgehog
(6–10). Thus, reversing or blocking the EMT
process is a promising therapeutic strategy for limiting the spread
of cancer.
Dietary chemopreventive agents have received much
attention in the area of cancer research. It is estimated that one
third of all cancers may be prevented by diet modification,
maintenance of optimum body weight and regular physical activity
(11,12). Among several dietary chemopreventive
agents, curcumin has gained considerable interest. Curcumin, an
active component of the spice turmeric (Curcuma longa), has
been shown to inhibit carcinogen activation, induce
carcinogen-detoxifying and antioxidant enzymes, modulate cell
survival and apoptosis, inhibit angiogenesis and to evoke
anti-invasive and anti-metastatic effects (13). The antitumor mechanisms of curcumin
involve the NF-κB, PI3K/Akt, MAPK, Wnt and Notch signaling pathways
(14,15).
Notably, it has been known that curcumin inhibits
cancer cell invasion by suppressing both constitutive and inducible
NF-κB activation (16). The NF-κB
signaling pathway is critically involved in the acquisition of
Snail-mediated EMT (17). Moreover,
during the progression to metastatic competence, carcinoma cells
have been described to enter into an EMT program, allowing them to
acquire features of mesenchymal-like cells that may significantly
endow invasiveness (18). Whether
the anti-invasive effects of curcumin are a result of its ability
to attack EMT has not yet been demonstrated in breast cancer cells.
Based on a previous study showing that lipopolysaccharide (LPS)
induces EMT in cancer cells (19),
we used LPS to induce EMT in the following experiments. The aim of
this study was to investigate the potential of curcumin to inhibit
LPS-triggered EMT in breast cancer cells. We suggest that curcumin
may inhibit LPS-induced EMT and that this effect is accompanied by
the inhibition of the NF-κB-Snail signaling pathway.
Materials and methods
Cell cultures and treatments
MDA-MB-231 and MCF-7 human breast cancer cell lines
(obtained from the American Tissue Type Collection, USA) were
maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, USA)
supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml),
0.1 mM non-essential amino acids, 0.2 mM glutamine, 1 mM pyruvate,
and 10% heat-inactivated fetal bovine serum (FBS) and incubated in
a 5% CO2 humidified atmosphere at 37°C. Cells were grown
to 80% confluency prior to treatment. The antibodies against NF-κB
p65 subunit, Snail, E-cadherin, vimentin and β-actin were purchased
from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Matrigel, LPS,
curcumin, NF-κB inhibitor PDTC were purchased from Sigma (Beijing,
China).
Proliferation assay
Cell proliferation was determined by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT,
Sigma) uptake method. After the cells were seeded
(5×103/well) in 200 μl of DMEM medium into 96-well
plates and cultured overnight, curcumin (0–70 μM) was added to the
cells and further cultured for 24 h. Then, MTT reagent (5 mg/ml)
was added and incubation was continued for an additional 4 h. The
reaction was terminated with 150 μl dimethyl sulfoxide (DMSO,
Sigma) per well. Absorbance values were determined using an MRX
Revelation 96-well multiscanner (Dynex Technologies, Chantilly, VA,
USA). The cells cultured in DMEM served as the control group. The
cell viability index was calculated according to the following
formula: Experimental OD value/Control OD value. The experiments
were repeated 3 times.
Transmission electron microscopy
The cells treated or untreated with LPS and/or
curcumin were harvested and rinsed with PBS. Cells were fixed for
30 min in 4% paraformaldehyde and 1% glutaraldehyde in 0.1 M
phosphate buffer (pH 7.4) (PB), rinsed in PB, and post-fixed in 1%
osmium tetraoxide for 30 min. After washing in PB, cells were
progressively dehydrated in a 10% graded series of 50–100% ethanol
and then cleared in QY-1 (Nissin EM, Tokyo Japan). Cells were
embedded in Epon 812 resin, and thin sections (70 nm thickness)
were stained with uranyl acetate and lead citrate, and then
examined by transmission electron microscopy.
Cell invasion assay
Cell invasion assay was performed and evaluated as
recently described in detail by employing Boyden chambers equipped
with 8 μm porosity polyvinylpyrrolidone-free polycarbonate filters
that were coated with 50 μg/ml of Matrigel solution (20). The cells were first seeded in
12-well plates at a concentration of 2.5×105/well and
were cultured for 48 h following treatment with LPS (5 μg/ml). For
the co-treatment experiment, 20 μM curcumin was added to the cell
cultures 1 h before the addition of LPS. Normal culture medium was
added at the bottom chamber to induce the cancer cell lines. Cells
which were pretreated were seeded in the top chamber. The Matrigel
invasion chamber was incubated for 24 h in a humidified tissue
culture incubator. After 24 h, the non-invasive cells were removed
from the upper surface of the separating membrane by gentle
scrubbing with a cotton swab, and the invading cells were fixed in
100% methanol and stained with 0.1% crystal violet solution. They
were then counted under a microscope at a magnification of
×200.
RT-PCR
Total RNA from MDA-MB-231 and MCF-7 cells was
isolated using TRIzol reagent (Gibco-BRL), and the quantities were
determined spectrophotometrically. First-strand cDNA was
synthesized from 2 μg of total RNA using the RevertAid kit (MBI
Fermentas, USA). Sequences of the PCR primers were: E-caderin (502
bp) forward 5′-CGC ATT GCC ACA TAC A-3′ and reverse 5′-CGT TAG CCT
CGT TCT CA-3′; Vimentin (690 bp) forward 5′-CGC TTC GCC AAC TAC
AT-3′ and reverse 5′-AGG GCA TCC ACT TCACAG-3; β-actin (179 bp)
forward 5′-ATC GTG CGT GAC ATT AAG GAG AAG-3′ and reverse 5′-AGG
AAG GAA GGC TGG AAG AGT G-3′. The PCR conditions included an
initial cDNA synthesis reaction at 42°C for 1 h using the RevertAid
kit, followed by a denaturation step for 5 min at 95°C and 22
cycles of 30 sec at 95°C, 30 sec at 57°C, and 30 sec at 73°C. After
the last cycle, a final extension was performed at 73°C for 10 min.
The housekeeping gene β-actin was used as an internal control.
Preparation of nuclear extracts
Breast cells from the control and experimental
groups were incubated on ice for 30 min, followed by the
preparation of nuclear extracts using a nuclear extract kit
(Pierce, IL, USA) according to the manufacturer’s instructions.
Samples were washed with ice-cold PBS, scraped, and collected by
centrifugation at 500 × g for 5 min. The pellets were suspended in
500 μl hypotonic buffer, incubated on ice for 20 min, and
centrifuged at 15,000 × g for 30 sec at 4°C. The resulting nuclear
pellet was suspended in 50 μl of complete lysis buffer and
incubated on ice for 30 min with frequent mixing. Finally, the
suspension was centrifuged at 16,000 × g for 30 min at 4°C, and the
supernatant (nuclear extract) was collected and stored at −70°C
until use. Protein concentration was measured by the Bradford assay
with BSA as the standard.
Western blotting
Briefly, 5×105 cells were incubated on
ice for 30 min in 0.5 ml of ice-cold whole-cell lysate buffer.
Debris was removed by centrifugation. The protein content of the
cell was determined, and the cellular lysates were separated by 10%
SDS-PAGE and electro-transferred onto nitrocellulose membranes.
After being blocked with 5% non-fat milk in TBST, the membranes
were incubated with primary antibodies at 4°C overnight, followed
by 1:2,000 horseradish peroxidase (HRP)-conjugated secondary
antibody (Santa Cruz Biotechnology) for 2 h. Immunoreactive bands
were visualized using an enhanced chemiluminescence kit (Amersham
Pharmacia Biotech, Piscataway, NJ, USA). Western blot signals were
quantitated by densitometric analysis using Total Lab Nonlinear
Dynamic Image analysis software (Nonlinear, USA).
Statistical analysis
Each experiment was performed at least 3 times. Data
are indicated as mean values ± standard deviation and differences
were evaluated using a Student’s t-test. A probability value
<0.05 was considered to indicate a statistically significant
result.
Results
Effect of curcumin on the growth of
breast cancer cells in vitro
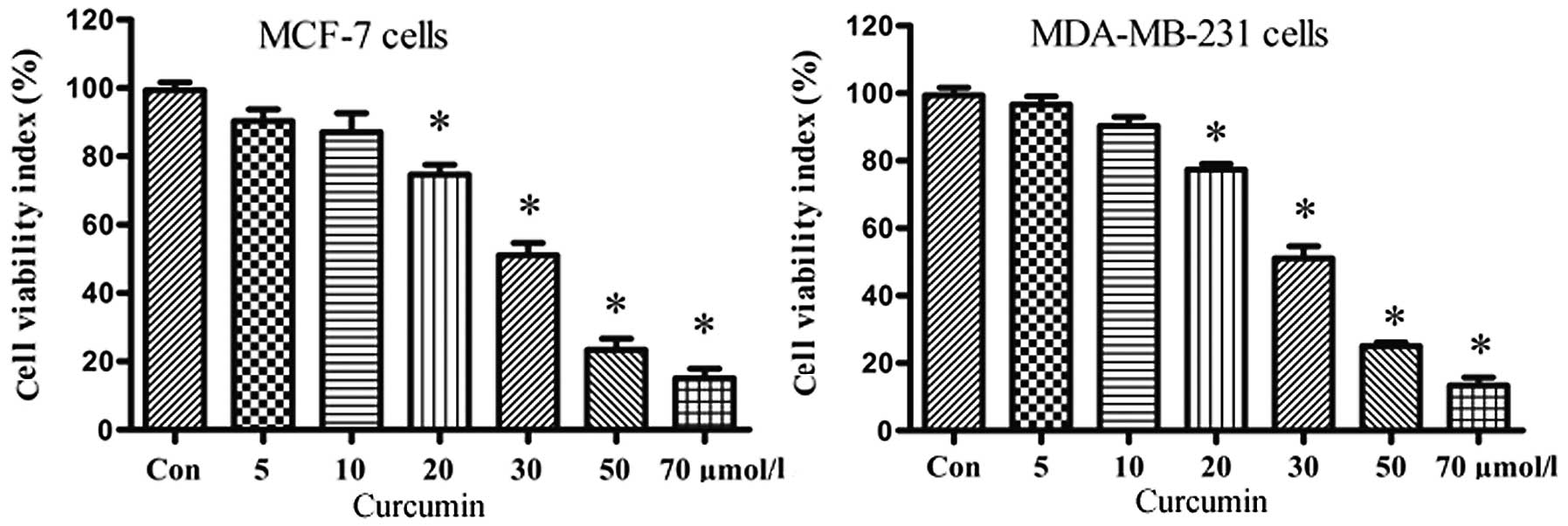
We initially investigated the effect of curcumin on
the proliferation of MCF-7 and MDA-MB-231 cells. Both breast cancer
cell lines were treated for 24 h with graded concentrations of
curcumin (0–70 μmol/l) and cell viability was measured by MTT
assay. The resulting growth curves demonstrated that cell
proliferation was inhibited in a dose-dependent manner by curcumin
(Fig. 1), with pronounced
inhibition at doses ≥30 μmol/l. These results suggest that curcumin
exhibits potent growth inhibition. In light of further experiments,
we considered 20 μmol/l curcumin a suitable dose.
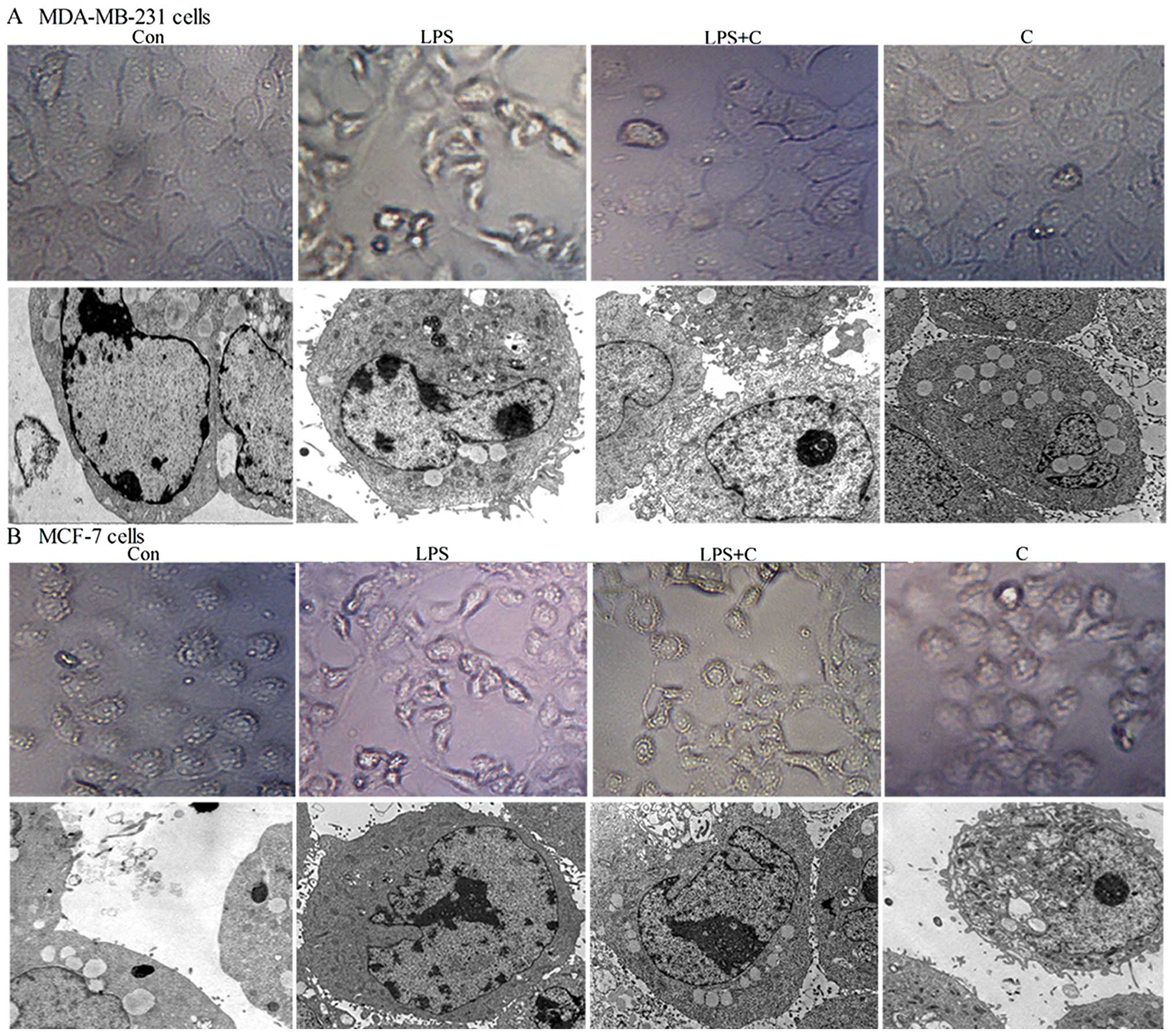
Curcumin inhibits LPS-induced cell
morphological changes of EMT in breast cancer cells
Previous studies suggest that LPS (5 μg/ml) as an
independent factor may trigger the EMT process (19). To verify this phenomenon and to
determine whether curcumin (20 μmol/l) inhibits LPS-induced EMT, we
used optical and transmission electron microscopy to investigate
changes in the morphology of MCF-7 and MDA-MB-231 human breast
cancer cells exposed to LPS in the presence or absence of curcumin.
Cells were treated with LPS for 48 h. As shown in Fig. 2, both cell types underwent typical
EMT morphological changes in response to LPS. There was a loss of
cell-to-cell contact, resulting in scattered clusters of cells.
Optical microscopy showed that cells acquired a spindle-shaped and
fibroblast-like phenotype; transmission electron microscopy showed
the appearance of dense core particles in the cell cytoplasm, a
wire-like structure was noted in the nucleus, and extracellular
microvilli were increased in a number of cells. It was subsequently
determined that curcumin inhibited these LPS-induced phenomena. A
mesenchymal phenotype was much less evident in cells co-treated
with PLS and curcumin compared with the cells treated with LPS
alone (Fig. 2). These results
indicate that curcumin inhibits LPS-induced EMT.
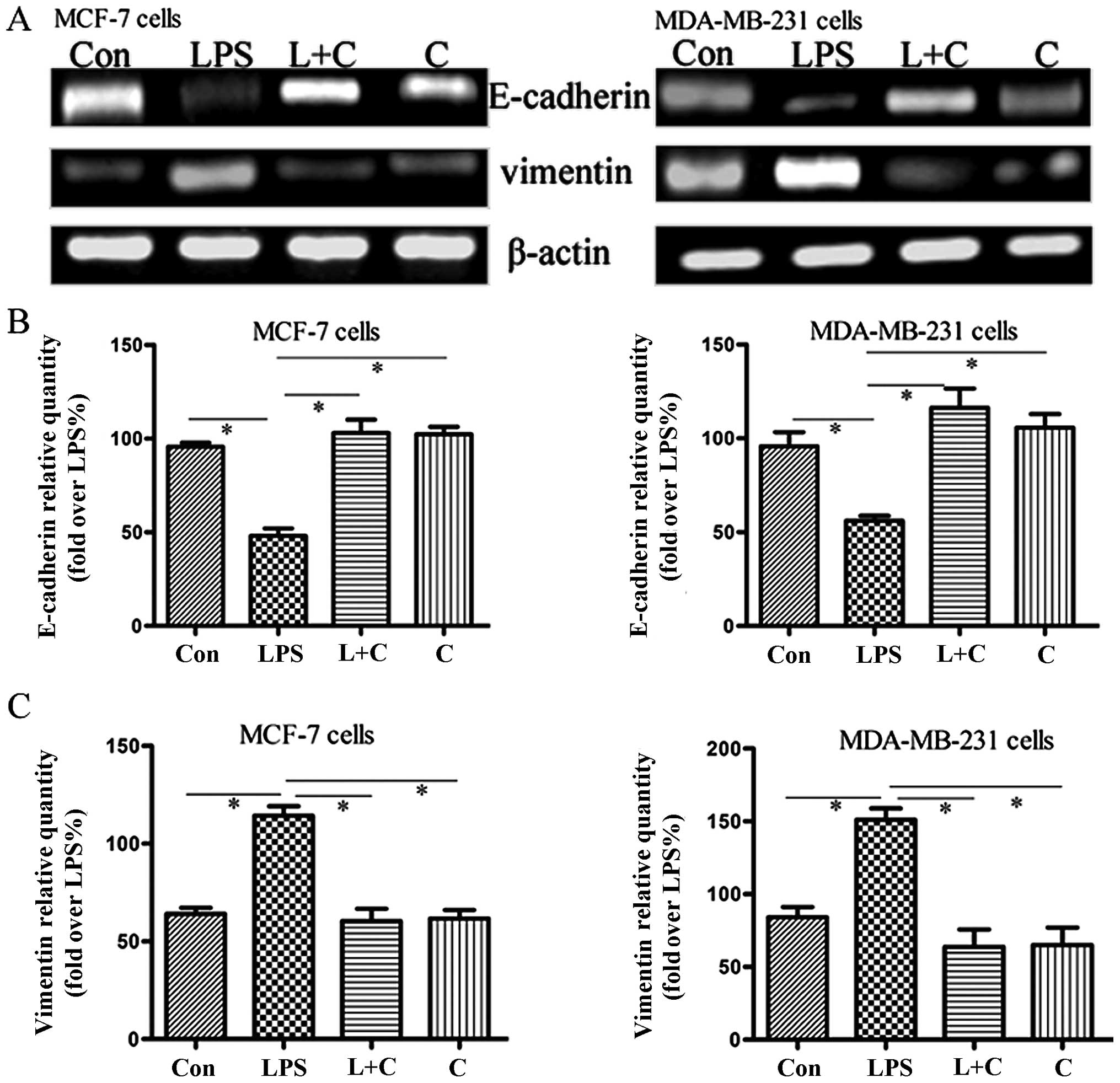
Curcumin inhibits the expression of
markers of EMT in breast cancer cells
To further confirm the effects of curcumin on
LPS-induced EMT, we sequentially analyzed the expression of two EMT
markers, E-cadherin and vimentin. Protein expression was measured
by western blotting, and mRNA expression was measured using
semi-quantitative RT-PCR analysis. Semi-quantitative PCR (Fig. 3A–C) indicated that mRNA levels of
vimentin and E-cadherin were significantly increased and
suppressed, respectively, by LPS treatment. Western blotting
(Fig. 3D–F) showed that the
expression of E-cadherin protein was significantly downregulated in
the LPS group compared to the control, whereas vimentin protein
expression was substantially increased (P<0.05). Notably,
curcumin reversed LPS-induced EMT, causing re-induction of
E-cadherin and inhibition of vimentin expression (Fig. 3). These results further suggest that
curcumin has inhibitory effects on cellular EMT.
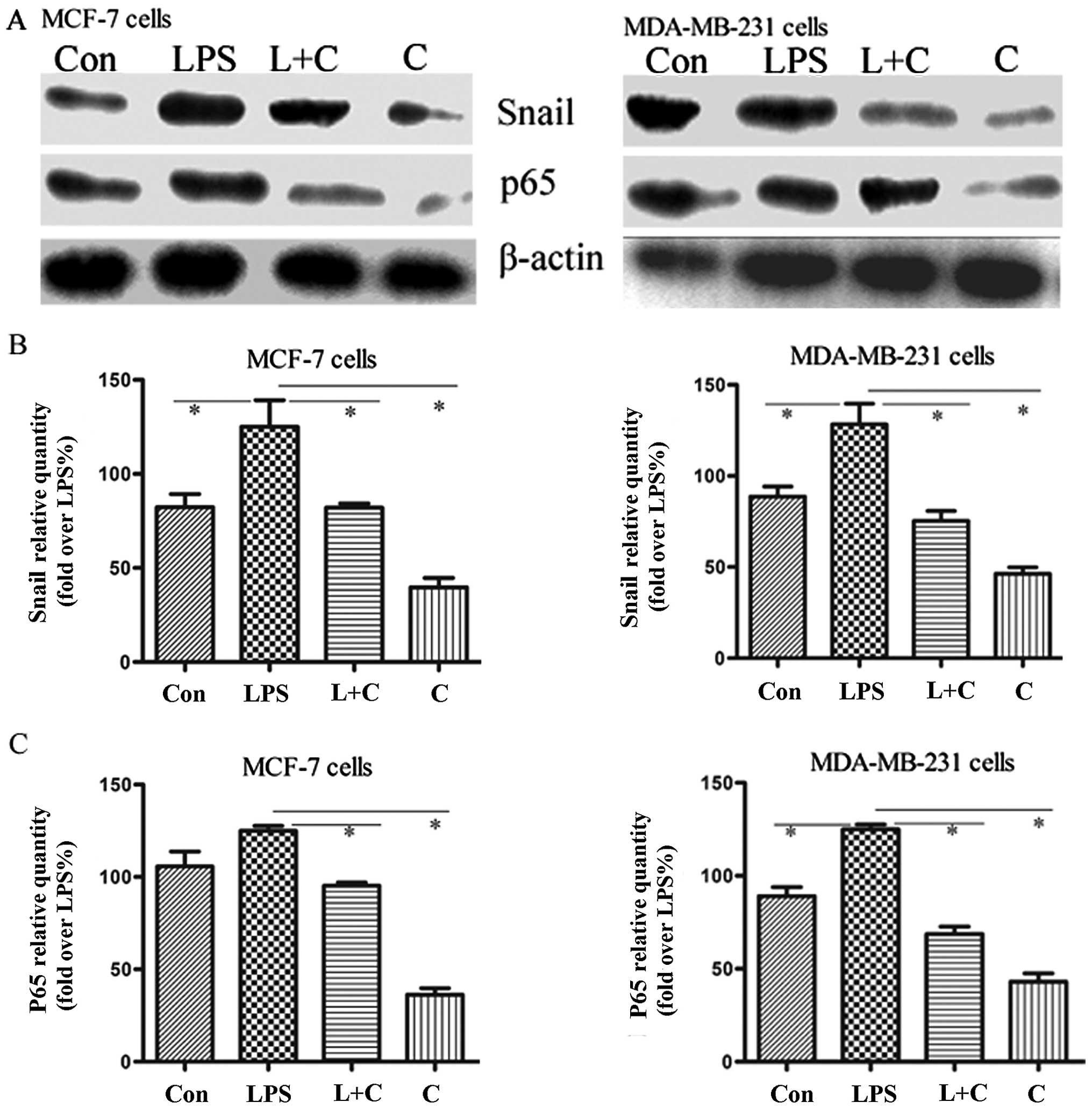
NF-κB-Snail signaling is required to
decrease E-cadherin and increase vimentin expression
As shown in previous studies, curcumin may inhibit
cancer cell invasion by suppressing NF-κB activation (16). Moreover, the NF-κB signaling pathway
is critically involved in the acquisition of EMT mediated by Snail,
the direct downstream transcription factor (17). To explore whether the above effect
of curcumin is associated with the inhibition of NF-κB-Snail
activation, the expression level of the NF-κB p65 subunit, which
represents the active form of NF-κB, and Snail protein were
measured in breast cancer cells by western blot analysis. Our
results demonstrated that LPS promoted the expression of NF-κB p65
and Snail protein, accompanied by a decreased expression of
E-cadherin and an increased expression of vimentin, and this effect
was blocked by curcumin (Fig. 4).
We also showed that NF-κB inhibitor PDTC reversed LPS-induced EMT,
accompanied by the inhibition of Snail and vimentin expression and
an increase in the expression of E-cadherin. These results indicate
that NF-κB-Snail plays a critical role in the EMT process.
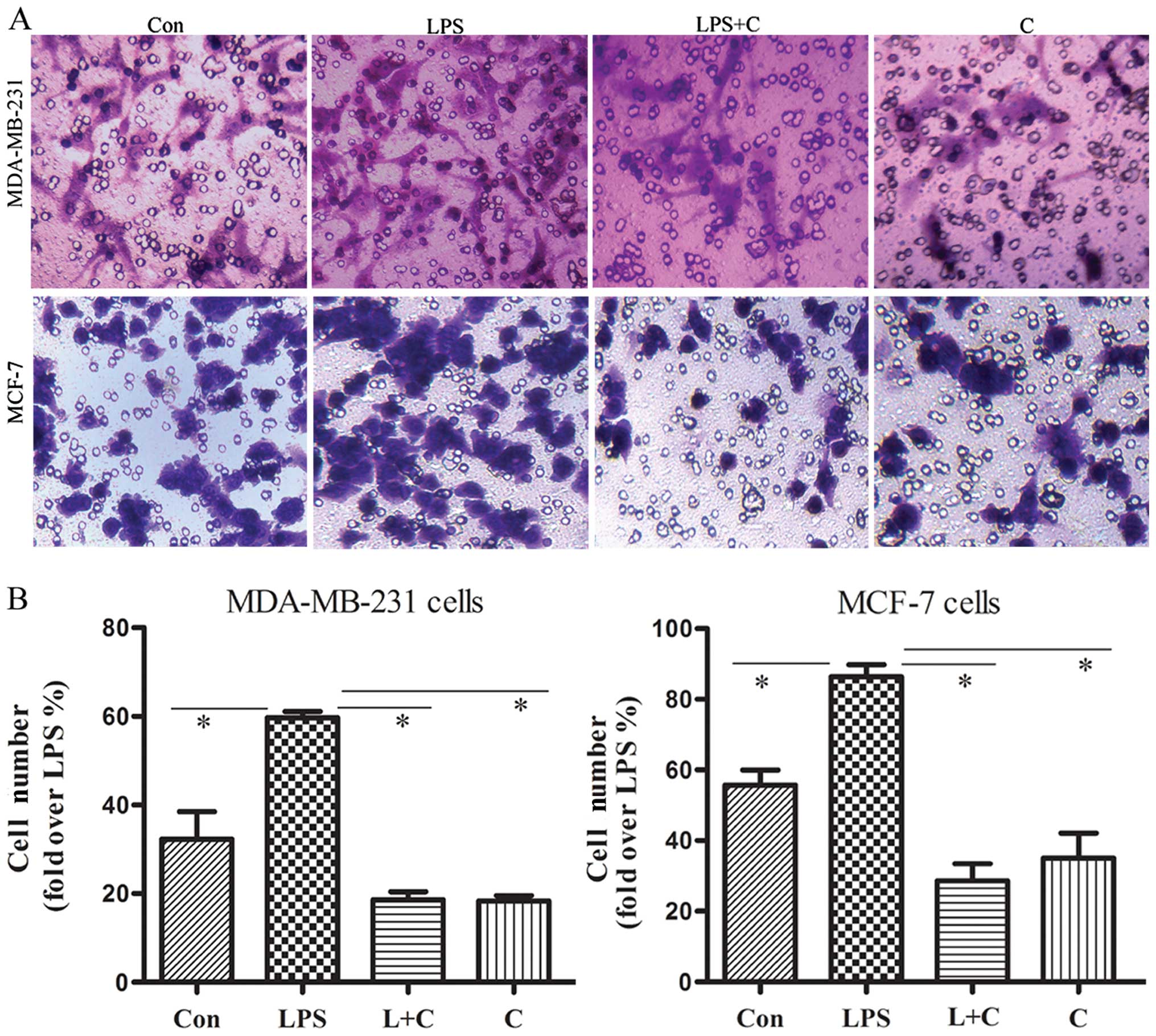
Curcumin inhibits invasion of breast
cancer cells
EMT is associated with enhanced cellular
progression. Our observation that curcumin inhibits the EMT process
prompted us to examine whether curcumin affects the invasion of
breast cancer cells stimulated with LPS. The motile phenotypes of
cells treated with LPS and the combination of LPS plus curcumin
were evaluated by invasion assay. After treatment with LPS alone,
the number of invasive cells increased significantly compared to
the untreated cells. The number of invasive cells was significantly
reduced in cells co-treated with LPS and curcumin (Fig. 5). These results suggest that
curcumin blocks the effect of LPS to increase the invasiveness of
human breast cancer cells.
Discussion
EMT has been proposed as a key process in embryonic
development and cancer progression, by which epithelial cells
acquire mesenchymal, fibroblast-like phenotypes with reduced
cell-to-cell adhesion, loss of cell polarity, with increased
migration and invasiveness (21).
EMT facilitates the migration of tumor cells from their site of
origin and dissemination to distant tissues through the activation
of a specific genetic program (18). This process is triggered by
autocrine and paracrine signals. Thus, agents that may block or
reverse this process may offer a promising therapeutic strategy to
limit cancer diffusion.
Previous studies have implicated a role for LPS in
mediating EMT in breast cancer cells by modifying NF-κB signaling
(19). In this study, we showed
that MDA-MB-231 and MCF-7 cells may be induced by LPS to undergo
representative EMT, characterized by the acquisition of mesenchymal
phenotype, the disappearance of E-cadherin, and the appearance of
vimentin. Treatment with curcumin inhibited LPS-induced EMT.
Curcumin not only restored an epithelial phenotype in mesenchymal
cells, but also blocked the expression of LPS-induced EMT markers.
We further showed that NF-κB-Snail signaling was required for
LPS-induced EMT in MDA-MB-231 and MCF-7 human breast cancer cells.
These findings extend our understanding of the mechanism by which
curcumin may act to inhibit cancer cell invasiveness.
Curcumin is an antioxidant polyphenol derived from
several curcuma species, commonly known as turmeric (Curcuma
longa), which has been shown to inhibit carcinogen activation
and angiogenesis, modulate cell survival and apoptosis, with
anti-invasive and anti-metastatic effects on breast, lung, colon
and prostate cancer (15).
Bachmeier et al demonstrated that curcumin inhibits cancer
metastases by downregulating the inflammatory cytokines CXCL-1 and
CXCL-2 through modifying NF-κB signaling in breast cancer cells
(22). It was also reported that
curcumin inhibits integrin (α6β4)-dependent breast cancer cell
motility and invasion via PI3K/AKT signaling (23). Lin et al(24) also reported that curcumin inhibits
the migration and invasion of human A549 lung cancer cells through
the inhibition of MMP-2, MMP-9 and VEGF. Unfortunately, these
strong anti-metastatic effects have not been linked to the EMT
process. Most importantly, to the best of our knowledge, our study
is the first to demonstrate that the anti-metastatic effects of
curcumin are associated with the EMT process in cultured breast
cancer cells. Our results offer a new perspective on the role of
curcumin in preventing the progression of cancer.
Our data also demonstrated that the mechanism of
action for curcumin may involve the suppression of NF-κB-Snail
signaling. NF-κB is a structurally conserved family of dimeric
transcription factors, which contain sequences mediating
dimerization, DNA binding, nuclear localization, and interaction
with inhibitory IκB proteins (25,26).
NF-κB plays pivotal roles in both promoting and maintaining an
invasive phenotype (27). Moreover,
numerous sources of evidence have identified NF-κB as an essential
central mediator of EMT. For example, studies showed that Snail
transcription, well established as playing critical roles in EMT,
is directly activated by NF-κB (17). NF-κB was identified as the upstream
regulator of Snail expression during EMT in human mammary
epithelial MCF10A cells. Specifically, the induction of Snail mRNA
during EMT may be reversed by the inhibition of NF-κB signaling
(28). Our results support previous
findings that NF-κB is the upstream regulator of Snail and
indirectly mediates EMT and provides the basis for a new theory for
the inhibition of tumor progression by curcumin.
E-cadherin plays a major role in cell-to-cell
adhesion of epithelial cells and acts as a metastatic suppressor in
epithelial carcinomas. Loss of E-cadherin is significantly
associated with advanced diseases (29). Vimentin is the major intermediate
filament protein found in mesenchymal cells. Vimentin expression
has often been noted in the end stage progression in EMT,
representing the completely dedifferentiated state in tumor cells
that are highly proliferative and invasive (30). These two important markers of EMT
are directly regulated by Snail (31,32).
Many preventive agents have been verified to effectively inhibit
EMT through the inhibition of Snail transcription factors.
Sulforaphane was shown to decrease the self-renewal capacity of
pancreatic cancer stem cells by inhibiting the EMT process. The
combination of quercetin with sulforaphane was found to have
further synergistic effects on Snail-induced downregulated and
upregulated expression of E-cadherin and vimentin, respectively
(33). Vergara et
al(34) reported that
resveratrol inhibited epidermal growth factor-induced EMT by
inhibiting the expression of vimentin and Slug in MCF-7 cells, and
Chen et al(19) also
reported that resveratrol inhibits LPS-induced EMT in a mouse
melanoma model. In the present study curcumin inhibited the
expression of Snail and downregulated or upregulated, respectively,
the expression of the EMT markers E-cadherin and vimentin, and
retarded cancer cell invasion. Although these agents were primarily
verified as effective in retarding EMT, further study will be
required to clarify the regulatory mechanism in vivo.
Taken together, these results suggest that the
ability of curcumin to inhibit tumor invasion is associated with
the EMT process, possibly by inhibiting the activation of
NF-κB-Snail signaling and regulating the expression of the
important downstream EMT markers E-cadherin and vimentin. This
result provides new mechanistic bases for the therapeutic
application of curcumin in breast cancer patients.
Acknowledgements
This study was funded by the Foundation of the
General Hospital of Nanjing Military Region, China.
References
|
1
|
Beiki O, Hall P, Ekbom A and Moradi T:
Breast cancer incidence and case fatality among 4.7 million women
in relation to social and ethnic background: a population-based
cohort study. Breast Cancer Res. 14:1–13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malfettone A, Saponaro C, Paradiso A,
Simone G and Mangia A: Peritumoral vascular invasion and NHERF1
expression define an immunophenotype of grade 2 invasive breast
cancer associated with poor prognosis. BMC Cancer. 12:106–117.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cowin P and Welch DR: Breast cancer
progression: controversies and consensus in the molecular
mechanisms of metastasis and EMT. J Mammary Gland Biol Neoplasia.
12:99–102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moreno-Bueno G, Portillo F and Cano A:
Transcriptional regulation of cell polarity in EMT and cancer.
Oncogene. 27:6958–6969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Creighton CJ, Chang JC and Rosen JM:
Epithelial-mesenchymal transition (EMT) in tumor-initiating cells
and its clinical implications in breast cancer. J Mammary Gland
Biol Neoplasia. 15:253–260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vuoriluoto K, Haugen H and Kiviluoto S:
Vimentin regulates EMT induction by Slug and oncogenic H-Ras and
migration by governing Axl expression in breast cancer. Oncogene.
30:1436–1448. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hardy KM, Booth BW, Hendrix MJ, Salomon DS
and Strizzi L: ErbB/EGF signaling and EMT in mammary development
and breast cancer. J Mammary Gland Biol Neoplasia. 15:191–199.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vincan E and Barker N: The upstream
components of the Wnt signalling pathway in the dynamic EMT and MET
associated with colorectal cancer progression. Clin Exp Metastasis.
25:657–663. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Javle MM, Gibbs JF and Iwata KK:
Epithelial-mesenchymal transition (EMT) and activated extracellular
signal-regulated kinase (p-Erk) in surgically resected pancreatic
cancer. Ann Surg Oncol. 14:3527–3533. 2007. View Article : Google Scholar
|
|
10
|
Yin T, Wang C, Liu T, Zhao G and Zhou F:
Implication of EMT induced by TGF-beta1 in pancreatic cancer. J
Huazhong Univ Sci Technolog Med Sci. 26:700–702. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Naithani R, Huma LC, Moriarty RM,
McCormick DL and Mehta RG: Comprehensive review of cancer
chemopreventive agents evaluated in experimental carcinogenesis
models and clinical trials. Curr Med Chem. 15:1044–1071. 2008.
View Article : Google Scholar
|
|
12
|
Verschoyle RD, Steward WP and Gescher AJ:
Putative cancer chemopreventive agents of dietary origin - how safe
are they? Nutr Cancer. 59:152–162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Basnet P and Skalko-Basnet N: Curcumin: an
anti-inflammatory molecule from a curry spice on the path to cancer
treatment. Molecules. 16:4567–4598. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shehzad A, Wahid F and Lee YS: Curcumin in
cancer chemoprevention: molecular targets, pharmacokinetics,
bioavailability, and clinical trials. Arch Pharm. 343:489–499.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jagtap S, Meganathan K, Wagh V, Winkler J,
Hescheler J and Sachinidis A: Chemoprotective mechanism of the
natural compounds, epigallocatechin-3-o-gallate, quercetin and
curcumin against cancer and cardiovascular diseases. Curr Med Chem.
16:1451–1462. 2009. View Article : Google Scholar
|
|
16
|
Notarbartolo M, Poma P, Perri D, Dusonchet
L, Cervello M and D’Alessandro N: Antitumor effects of curcumin,
alone or in combination with cisplatin or doxorubicin, on human
hepatic cancer cells. Cancer Lett. 224:53–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chua HL, Bhat-Nakshatri P, Clare SE,
Morimiya A, Badve S and Nakshatri H: NF-kappaB represses E-cadherin
expression and enhances epithelial to mesenchymal transition of
mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2.
Oncogene. 26:711–724. 2007. View Article : Google Scholar
|
|
18
|
Mulholland DJ, Kobayashi N and Ruscetti M:
Pten loss and RAS/MAPK activation cooperate to promote EMT and
metastasis initiated from prostate cancer stem/progenitor cells.
Cancer Res. 72:1878–1889. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen MC, Chang WW, Kuan YD, Lin ST, Hsu HC
and Lee CH: Resveratrol inhibits LPS-induced epithelial-mesenchymal
transition in mouse melanoma model. Innate Immun. 32:1–9.
2012.PubMed/NCBI
|
|
20
|
Takata M, Maniwa Y and Doi T:
Double-layered collagen gel hemisphere for cell invasion assay:
successful visualization and quantification of cell invasion
activity. Cell Commun Adhes. 14:157–167. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tiwari N, Gheldof A, Tatari M and
Christofori G: EMT as the ultimate survival mechanism of cancer
cells. Semin Cancer Biol. 22:194–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bachmeier BE, Mohrenz IV and Mirisola V:
Curcumin downregulates the inflammatory cytokines CXCL1 and -2 in
breast cancer cells via NFkappaB. Carcinogenesis. 29:779–789. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim HI, Huang H, Cheepala S, Huang S and
Chung J: Curcumin inhibition of integrin (alpha6beta4)-dependent
breast cancer cell motility and invasion. Cancer Prev Res (Phila).
1:385–391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin SS, Lai KC, Hsu SC, et al: Curcumin
inhibits the migration and invasion of human A549 lung cancer cells
through the inhibition of matrix metalloproteinase-2 and -9 and
vascular endothelial growth factor (VEGF). Cancer Lett.
285:127–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-κB in development and progression of human
cancer. Virchows Arch. 446:475–482. 2005.
|
|
26
|
Giuliani C, Napolitano G, Bucci I, Montani
V and Monaco F: NF-κB transcription factor: role in the
pathogenesis of inflammatory, autoimmune, and neoplastic diseases
and therapy implications. Clin Ter. 152:249–253. 2001.(In
Italian).
|
|
27
|
Baud V and Jacque E: The alternative NF-κB
activation pathway and cancer: friend or foe? Med Sci.
24:1083–1088. 2008.(In French).
|
|
28
|
Min C, Eddy SF, Sherr DH and Sonenshein
GE: NF-kappaB and epithelial to mesenchymal transition of cancer. J
Cell Biochem. 104:733–744. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pinho SS, Oliveira P and Cabral J: Loss
and recovery of Mgat3 and GnT-III mediated E-cadherin
N-glycosylation is a mechanism involved in epithelial-mesenchymal
transitions. PLoS One. 7:e331912012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ivaska J: Vimentin: Central hub in EMT
induction? Small GTPases. 2:51–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu Y and Zhou BP: Snail: More than EMT.
Cell Adh Migr. 4:199–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fendrich V, Waldmann J and Feldmann G:
Unique expression pattern of the EMT markers Snail, Twist and
E-cadherin in benign and malignant parathyroid neoplasia. Eur J
Endocrinol. 160:695–703. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shankar S, Nall D and Tang SN: Resveratrol
inhibits pancreatic cancer stem cell characteristics in human and
KrasG12D transgenic mice by inhibiting pluripotency maintaining
factors and epithelial-mesenchymal transition. PLoS One.
6:e165302011. View Article : Google Scholar
|
|
34
|
Vergara D, Valente CM and Tinelli A:
Resveratrol inhibits the epidermal growth factor-induced epithelial
mesenchymal transition in MCF-7 cells. Cancer Lett. 310:1–8. 2011.
View Article : Google Scholar : PubMed/NCBI
|