Introduction
Cancer cells escape apoptosis by a number of
mechanisms, among which overexpression of anti-apoptotic genes,
such as some members of the Bcl-2 gene family, the IAP family and
the Mcl-1 family, have been shown to play a critical role (1–3). The
proto-oncogene Bcl-2, discovered in Burkitt’s lymphoma, is a
prominent member of the Bcl-2 family which prevents apoptosis in
various malignancies including Burkitt’s lymphoma. Consequently,
targeting expression of Bcl-2 has a potential value in Burkitt’s
lymphoma therapy, and has led to the development of therapeutic
strategies to selectively inhibit targeted gene expression.
Previous studies have demonstrated that the inhibition of Bcl-2
expression by antisense oligonucleotides (4,5)
reduces the growth of Burkitt’s lymphoma cells. However, their
efficiency is unsatisfactory due to nuclease degradation. Recently,
the successful use of small interfering RNAs (siRNAs) in
downregulating gene expression in several model systems led to many
attempts to explore this methodology in potentially therapeutic
settings. siRNA involves post-transcriptional gene silencing via a
process in which double-stranded RNA (dsRNA) inhibits gene
expression in a sequence-dependent manner through degradation of
the corresponding mRNA. It has been verified as a powerful tool to
knock down the expression of a target gene in mammalian cells
(6–8). At present, siRNA can be synthesized by
chemical synthesis and in vitro transcription. The
limitations of the two methods are high cost and low stability.
Stable gene repression can be achieved in mammalian cells by using
vectors to express a small hairpin RNA (shRNA) structure with a U6
or H1 promoter under the direction of RNA polymerase III (9,10).
In this study, a U6 promoter-based vector was used
to express shRNA targeting Bcl-2 in Burkitt’s lymphoma Raji cells.
We investigated whether this technique could be used for the
specific inhibition of Bcl-2 overexpression and tested whether this
inhibition could result in antitumor effects.
Materials and methods
Cell culture
The human B-cell lymphoma cell line Raji used in
this study was maintained in our laboratory. The cells were grown
in a suspension of RPMI-1640 medium (Gibco, USA) supplemented with
10% heat-inactivated fetal bovine serum (Sijiqing, Hangzhou,
China), 5 mmol/l HEPES, 100 U/ml penicillin and 100 U/ml
streptomycin in 5% atmospheric CO2 at 37°C. The cells
were passaged every three days, checked routinely, and found to be
free of contamination. When the cells grew to exponential phase,
they were harvested and used for in vitro and in vivo
studies.
shRNA design and construction of
recombinant plasmid expressing Bcl-2-shRNA
According to the shRNA design principle (11), two 19 bp sequences in Bcl-2 cDNA
from GenBank accession number NM-000633.2 as our target sites were
designed by using siRNA design software downloaded from the
internet (http://www.ambion.com). One was
5′-GTACATCCATTATAAGCTG-3′, which corresponds to the nucleotides
544–562, and the other was 5′-CATCGCCCT GTGGATGACT-3′, which
corresponds to the nucleotides 1009–1027. The secreted sequences
were submitted to BLAST search to ensure the only secreted gene was
targeted. The negative control scrambled sequence was
5′-GACTTCATAAGG CGCATGC-3′, which has no significant homology to
mouse or human gene sequences. The oligonucleotides contained a
sense strand of 19 nucleotides followed by loop sequence TTC
AAGAGA, an antisense strand, a transcription terminator TTTTT, an
identification restriction enzyme EcoRI site, as well as
terminal BamHI and HindIII restriction enzyme sites
(Table I). Double complementary
shRNA DNA segments were gained through annealing, named Bcl-2-1
shRNA, Bcl-2-2 shRNA and NC shRNA and then inserted into the
BamHI and HindIII sites of plasmid pGenesil-1 vector,
respectively. The recombinant plasmids were evaluated by
restriction enzyme cutting and sequencing, and were then designated
as pGenesil-1-Bcl-2-1, pGenesil-1-Bcl-2-2 and pGenesil-1-NC,
respectively.
 | Table IDNA sequences of insertion fragments
for shRNAs. |
Table I
DNA sequences of insertion fragments
for shRNAs.
| Name | Sequences
(5′-BamHI+sense+loop+antisense+termination
signal+EcoRI+HindIII-3′) | Target site |
|---|
| Bcl-2-1 shRNA |
5′-GATTCGTACATCCATTATAAGCTGTTCAAGACGCAGCTTATAATGGATGTACTTTTTTGAATTCA-3′ | 544–562 |
|
3′-GCATGTAGGTAATATTCGACAAGTTCTGCGTCGAATATTACCTACATGAAAAAACTTAAGTTCGA-5′ | |
| Bcl-2-2 shRNA |
5′-GATTCGCATCGCCCTGTGGATGACTTTCAAGACGAGTCATCCACAGGGCGATGTTTTTTGAATTCA-3′ | 1009–1027 |
|
3′-GCGTAGCGGGACACCTACTGAAAGTTCTGCTCAGTAGGTGTCCCGCTACAAAAAACTTAAGTTCGA-5′ | |
| NC shRNA |
5′-GATTCGACTTCATAAGGCGCATGCTTCAAGACGGCATGCGCCTTATGAAGTCTTTTTTGAATTCA-3′ | |
|
3′-GCTGAAGTATTCCGCGTACGAAGTTCTGCCGTACGCGGAATACTTCAGAAAAAACTTAAGTTCGA-5′ | |
Transfection with the shRNA expression
vector
For transfection, Raji cells in exponential phase of
growth were harvested and washed three times with Opti-MEM
(Invitrogen, USA) to replace the culture medium and then
5×105 cells were seeded into wells of a 6-well plate and
divided into five groups: group 1, normal cultured Raji cells;
group 2, Raji cells transfected with pGenesil-1-Bcl-2-1; group 3,
Raji cells transfected with pGenesil-1-Bcl-2-2; group 4, Raji cells
transfected with negative control plasmid vector pGenesil-1-NC.
Transfection was performed according to the manufacturer’s
protocols. The ratio of plasmid to Lipofectamine™ 2000 (Invitrogen,
USA) was 1:2. Five hours after the transfection, the medium was
replaced by the common complete medium again. After 48 h of
transfection, cells stably expressing shRNA were established by
selection with medium first containing 600 μg/ml G418. The medium
was renewed every 3 days. After 15 days selection, the resistant
colonies were combined in pools in selective medium. Then, the
resistant colonies were further selected by a huge dose G418 (2,000
μg/ml) for 10 days in order to exclude the possibility of
non-transfected but G418-resistant colonies, after the huge dose
selection of G418, the colonies stably transfected with
G418-resistance were amplified and analyzed by RT-PCR, western blot
analysis and flow cytometric assays, for subcutaneous tumorigenesis
assay in nude mice.
Bcl-2 mRNA expression detected by
RT-PCR
Stably transfected and untreated cells were
collected and washed with phosphate-buffered saline (PBS). Total
cellular RNA was extracted with TRIzol reagent (Invitrogen, USA)
according to the manufacturer’s instructions. The purity and
concentration were determined by measuring the absorbance (A) at
260 and 280 nm (A260/A280). To generate
first-strand cDNA, an oligo(dT)18 was used as primer,
and 2 μg RNA was reverse-transcribed based on the MMLV First Strand
cDNA Synthesis kit (Fermentas, USA) protocols. Subsequently,
aliquots of 5 μl cDNA were amplified in a total volume of 25 μl
using the polymerase chain reaction (PCR) kit (Fermentas, USA). The
sense and antisense primers for Bcl-2 were: 5′-CGCGACT
CCTGATTCATT-3′, 5′-TGCATTCTTGGACGAGGG-3′ (316 bp); the sense and
antisense primers for the housekeeping gene β-actin used as an
internal control were: 5′-GGACCTGA CTGACTACCTC-3′,
5′-TCATACTCCTGCTTGCTG-3′ (420 bp), respectively. The cycling
conditions were 94°C for 5 min, followed by 30 cycles of 94°C for 1
min, 51°C for 30 sec, and 72°C for 1 min and a final extension of
72°C for 10 min. PCR products were separated in 1.5% agarose gels,
stained with ethidium bromide and visualized by UV absorption.
Densitometric scanning of the bands was performed and relative
amount of Bcl-2 mRNA expression was estimated by normalization to
the β-actin mRNA detected in the same sample.
Western blot analysis
Cytoplasmic proteins of the groups above were
extracted using cytoplasmic extraction reagents (Beyotime
Biotechnology, China). Cell lysates were centrifuged at 15,000 rpm
for 5 min at 4°C. Protein content in the supernatants was
determined by a BCA protein assay kit. Equal amounts of lysate
protein were separated by 12% SDS-PAGE and transferred onto
nitrocellulose membranes. The membrane was blocked in 5% skimmed
milk in TBST buffer at room temperature for 1–2 h with gentle
shaking and then incubated overnight at 4° with mouse anti-human
Bcl-2 mAb (1:1,000) or mouse anti-human β-actin IgG mAb (1:1,000,
Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washing with
TBST, the membrane was incubated with HRP-conjugated goat
anti-mouse IgG antibody (1:5,000, Immunotech, France). Bands were
developed by using ECL substrate and exposed to X-ray film. The
expression levels of Bcl-2 and β-actin protein were quantified by
densitometry. The signal strength of each Bcl-2 signal was
normalized against the corresponding β-actin control.
Bcl-2 protein expression detected by flow
cytometry
Transfected cells, as well as untreated cells, were
collected and washed twice with PBS and then fixed in 1%
paraformaldehyde for 5 min and permeabilized with 0.1% Triton X-100
for 5 min. After two additional rinses with PBS, the cells were
incubated with primary mouse anti-human Bcl-2 IgG mAb (Immunotech)
for 30 min, followed by FITC-conjugated goat anti-mouse IgG mAb
(Immunotech) for 30 min at room temperature in the dark. The cells
were then rinsed twice with PBS containing 2% FBS and analyzed by
flow cytometry (FCM). Controls consisted of incubation with no
primary antibody or incubation but with only the secondary
antibody. The experiments were performed in triplicate and the
results are given as the means ± SD.
Cell proliferation assay
To determine whether Bcl-2 shRNA could inhibit Raji
cell proliferation, Raji cells stably transfected with
pGenesil-1-Bcl-2-1, pGenesil-1-Bcl-2-2, pGenesil-NC and untreated
cells were plated in 96-well plates at a density of
1×104 cells/well and incubated for 9 days in 5%
atmospheric CO2 at 37°C. Cells were stained with trypan
blue and counted using a hemocytometer. Cell numbers of the above
groups were detected on days 1–9. Each experimental condition was
performed three times and all data presented as the means ± SD for
each group were determined to compose the growth curve.
Treatment in vivo
We then investigated whether Bcl-2 shRNA would alter
the tumorigenicity or not. Four-week-old male BALB/c nude mice were
purchased from the Shanghai Experimental Animal Center of Chinese
Academy of Sciences (Shanghai, China) and our animal studies were
carried out in accordance with established institutional guidelines
and approved protocols. An equal number (3×107 cells in
0.1 ml PBS) of Raji cells stably transfected with
pGenesil-1-Bcl-2-1, pGenesil-1-Bcl-2-2, pGenesil-NC control, and
untreated cells were harvested, washed with PBS and injected into
male 4-week-old BALB/c nude mice (six mice for each group)
subcutaneously. The mice were kept in a pathogen-free environment.
Tumor sizes were measured every four days and calculated by the
following formula: volume (mm3) = 1/2
(width)2 × length. Forty days after injection, the mice
were sacrificed and the weights of the tumors were recorded. The
tumors were removed, fixed by 4% polyformaldehyde, paraffin
embedded and sectioned for immunohistochemical analysis and
transmission electron microscopic examination.
Immunohistochemistry
For the immunohistochemistry, 5 μm-thick
paraffin-embedded sample tissue sections were cut, and subsequently
dewaxed, re-hydrated and then subjected to antigen retrieval by
heating in 10 mM citrate buffer in a microwave for 15 min. The
sections were cooled, treated with 3% H2O2,
blocked with 10% goat serum and then incubated overnight at 4°C
with primary antibodies against Bcl-2 (1:100 dilution, Santa Cruz
Biotechnology). Negative control was incubated with an equivalent
volume of diluent solution alone. After 3 washes with PBS, the
standard streptavidin-biotin-peroxidase complex technique using
sequential 20 min incubation with biotinylated goat anti-mouse IgG
(Sigma, USA) and peroxidase-labeled streptavidin (Invitrogen, USA)
was performed. We used 3,3′-diaminobenzidine (DAB) as a substrate
chromogen solution for the development of peroxidase activity.
Hematoxylin was used for nuclear counterstaining, then the sections
were mounted and coverslipped. Images were captured with a
microscope (BX51, Olympus, Japan).
Transmission electron microscopic
examination
Tumor tissues from mice were prefixed in 2.5%
glutaraldehyde, postfixed in 1% osmic acid, dehydrated in gradient
acetone and embedded in the resin. Ultrathin sections were cut,
stained with lead citrate and assessed for the morphological
changes under a transmission electron microscope.
Statistical analysis
All experiments were performed in triplicate and
data are expressed as the means ± SD. Statistical analyses were
conducted with the Student’s t-test and performed with SPSS 10.0
software. P<0.05 was considered to indicate statistically
significant differences.
Results
Identification of Bcl-2 shRNA expression
plasmids
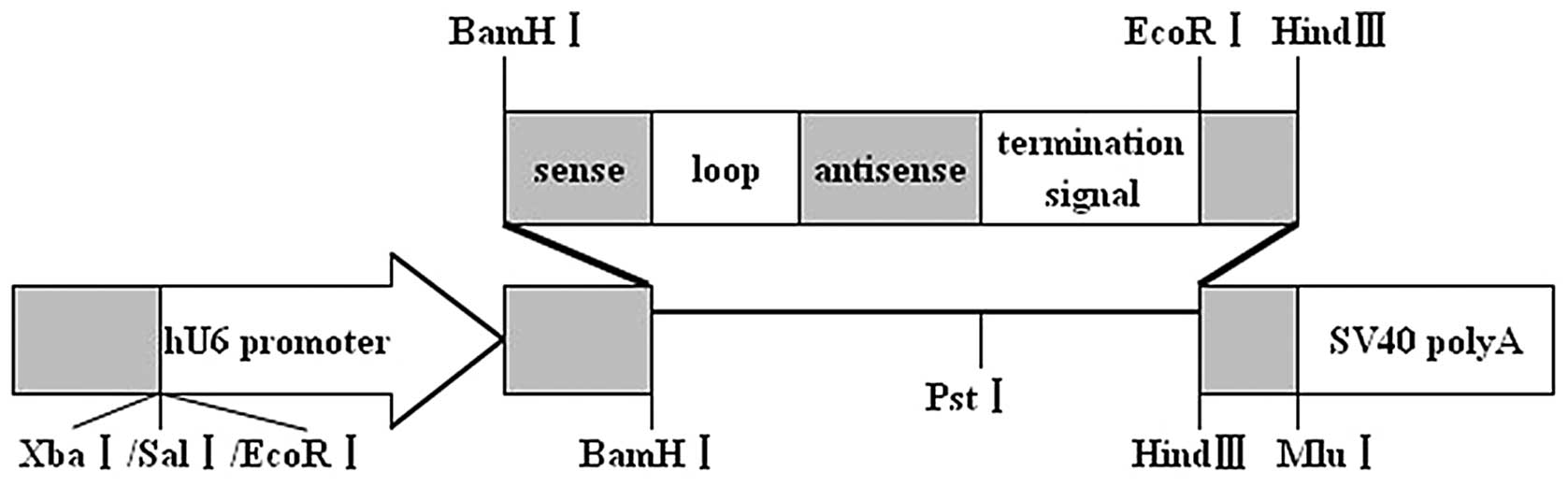
Since the restriction site of enzyme PstI in
plasmid pGenesil-1 (Fig. 1) was
replaced by the inserted DNA sequence, the reconstructed plasmids
could not be digested by PstI. In the inserted target gene
template DNA an EcoRI enzyme digestion site was designed
between BamHI and HindIII. The pGenesil-1 plasmid
itself carried an EcoRI enzyme digestion site. If the
insertion was correct, a band approximately 400 bp should be cut
off by EcoRI. The pGenesil-1-Bcl-2-1, pGenesil-1-Bcl-2-2,
pGenesil-1-NC plasmids were digested by restriction enzyme
PstI and EcoRI, respectively. Agarose gel
electrophoresis (1.0%) showed that EcoRI digestion produced
a fragment of 400 bp, while PstI digestion did not (Fig. 2A). DNA sequencing confirmed that the
plasmids were reconstructed successfully (Fig. 2B).
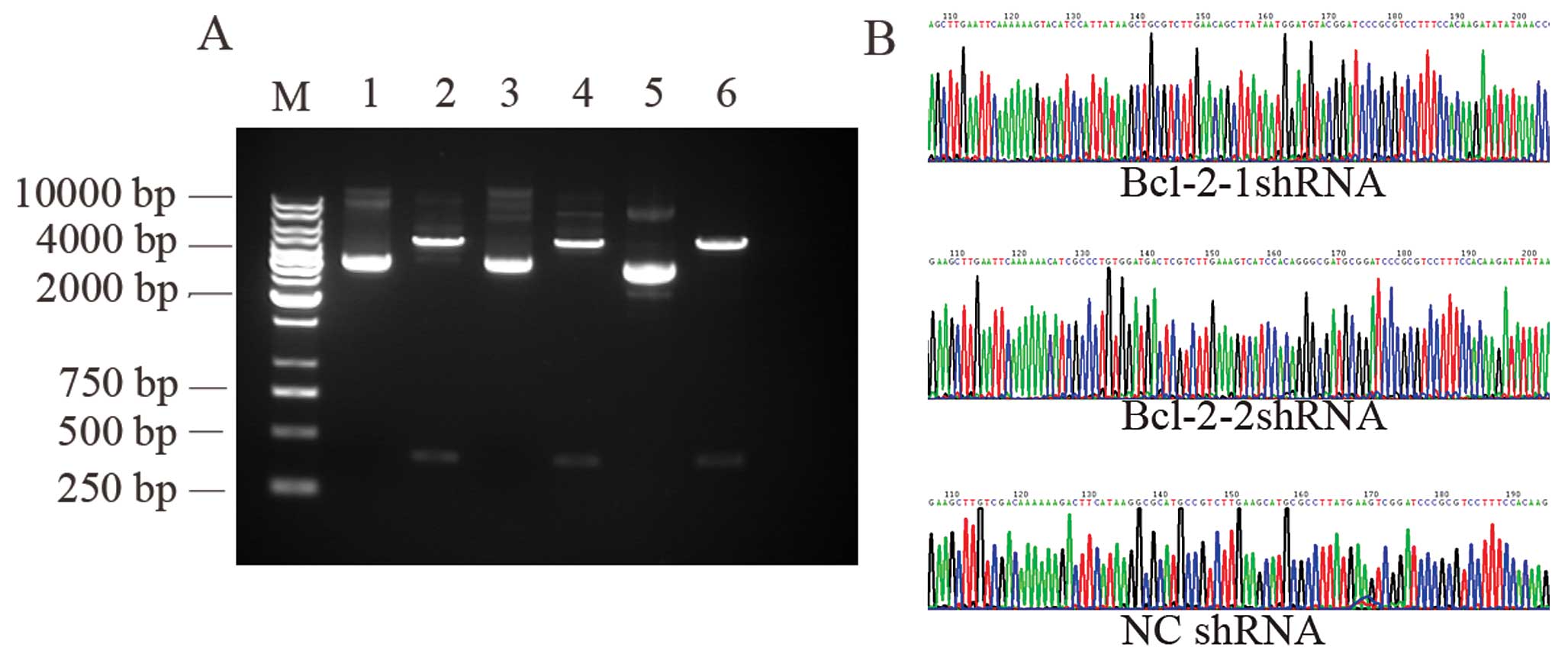 | Figure 2(A) Identification of recombinant
plasmids by restriction enzyme digestion. M: 1 kb DNA marker; 1, 3,
5: pGenesil-1-Bcl-2-1, pGenesil-1-Bcl-2-2 and pGenesil-1-NC
digested by PstI, respectively. Since the restriction site
of enzyme PstI in plasmid pGenesil-1 was replaced by the
inserted shRNA segment, the reconstructed plasmids could not be
digested by PstI; 2, 4, 6: pGenesil-1-Bcl-2-1,
pGenesil-1-Bcl-2-2, pGenesil-1-NC digested by EcoRI,
respectively. An EcoRI enzyme site was designed in each
inserted shRNA segment, and the pGenesil-1 plasmid itself carried
an EcoRI enzyme digestion site. A band approximately 400 bp
was cut off by EcoRI for pGenesil-1-Bcl-2-1,
pGenesil-1-Bcl-2-2 and pGenesil-1-NC, respectively. (B) Recombinant
plasmids identified by DNA sequence analysis (the insertion
segment). |
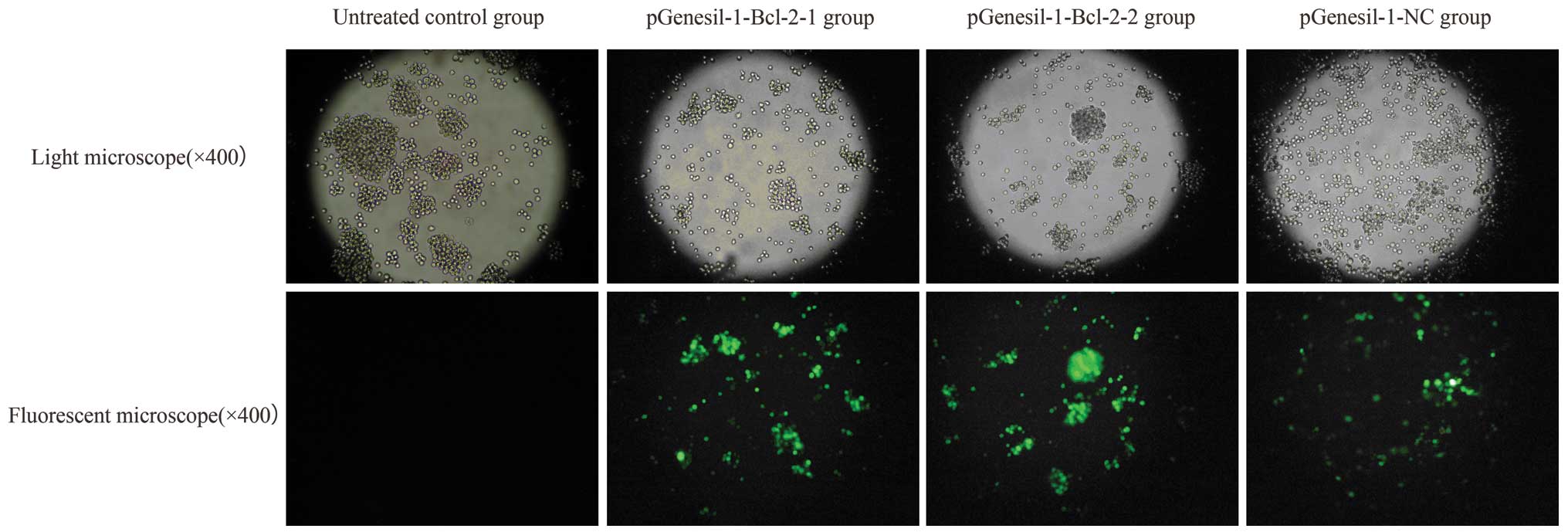
Results of transfection
Twenty-five days after G418 selection, stable
transfection efficiency of recombinant plasmid in Raji cells was
examined by coexpressing enhanced green fluorescent protein (EGFP).
When the cells were examined under a fluorescence microscope after
stable transfection, >95% of cells transfected with shRNA showed
fluorescence in total cells and 0.25% in control. The results
showed a high efficiency of shRNA transfection (Fig. 3).
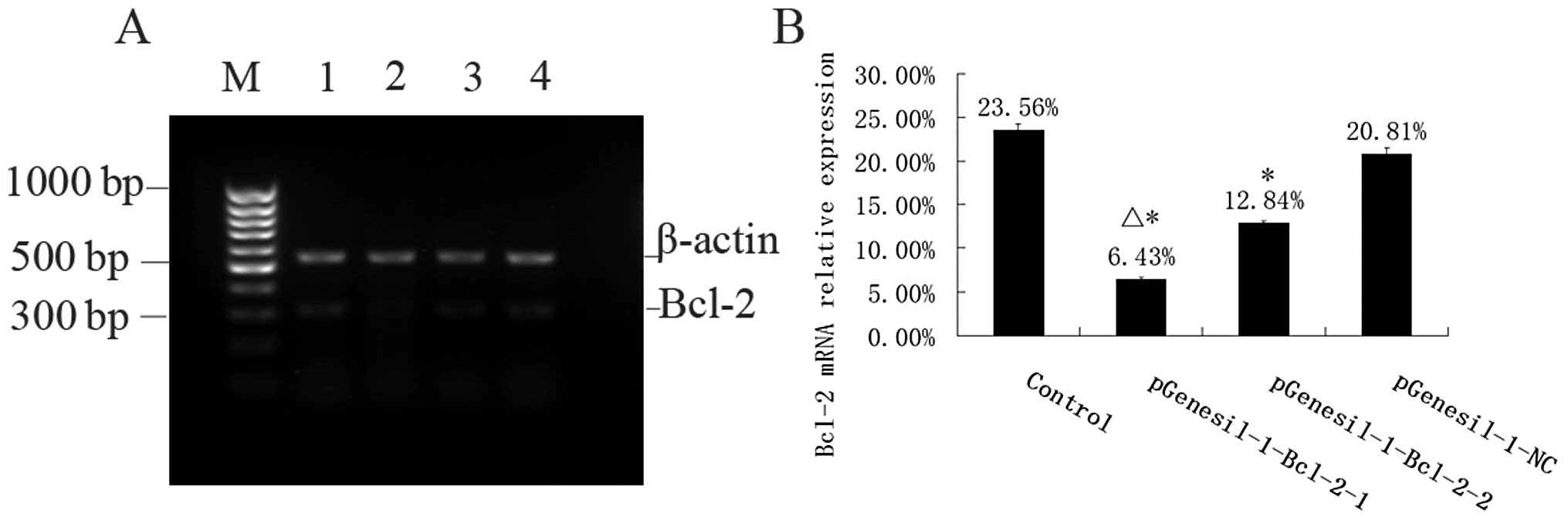
Inhibition of mRNA expression by Bcl-2
shRNA
The mRNA expression intensities of Bcl-2 genes,
inhibited by specific Bcl-2 shRNAs in the Raji cells, were analyzed
by semiquantitative RT-PCR. The mRNA levels were normalized by
internal control β-action (Fig. 4).
The rate of Bcl-2/β-actin mRNA was 23.56±0.68%, 6.43±0.25%,
12.84±0.33%, and 20.81±0.70% for the control, the
pGenesil-1-Bcl-2-1, the pGenesil-1-Bcl-2-2 and the pGenesil-NC
group, respectively. The statistical analysis showed that Bcl-2
mRNAs of Raji cells in the pGenesil-1-Bcl-2-1 and the
pGenesil-1-Bcl-2-2 group were reduced significantly, compared with
those of the control group (P<0.05). The inhibition rate reached
72.71 and 45.50% in the pGenesil-1-Bcl-2-1 and the
pGenesil-1-Bcl-2-2 group, respectively. However, the pGenesil-NC
group showed no significant inhibition for Bcl-2 mRNA expression
(P>0.05, vs. control).
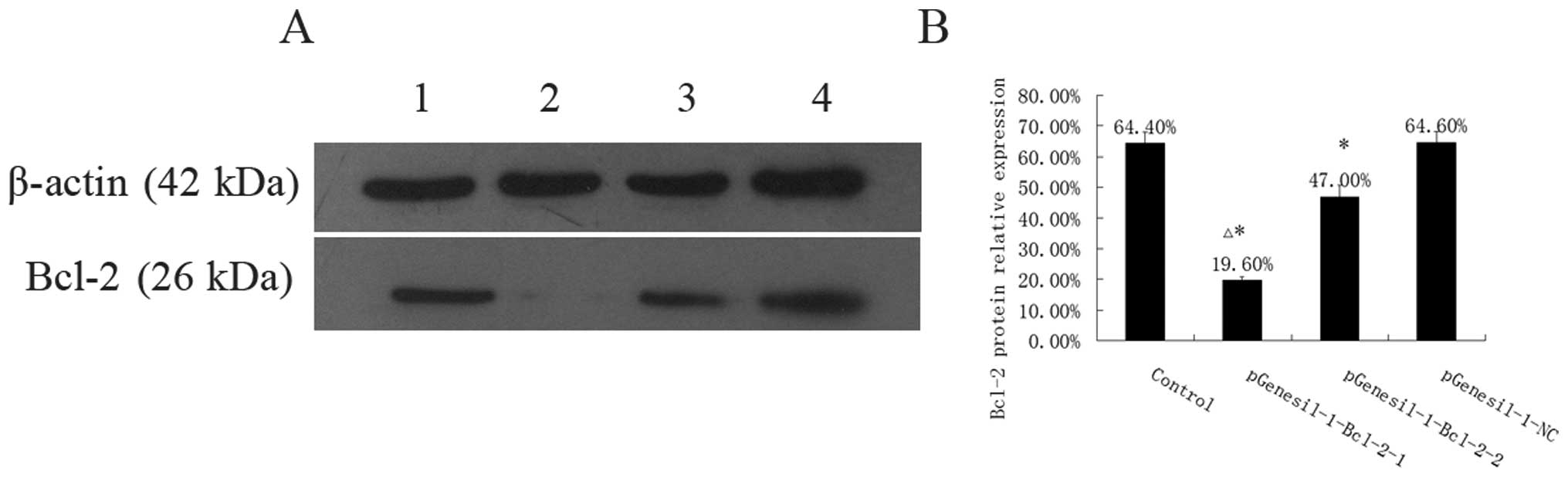
Knockdown of Bcl-2 protein expression by
Bcl-2 shRNA
To confirm whether the inhibition of Bcl-2 mRNA by
Bcl-2 specific shRNA expressing plasmid influences Bcl-2 protein
expression, Bcl-2 protein levels in Raji cells after stable
transfection with Bcl-2 shRNA expressing plasmids were evaluated by
western blotting. As shown in Fig.
5, the Bcl-2 protein levels were 64.40±3.58, 19.60±1.14,
47.00±3.74, and 64.60±3.65% in the control, the pGenesil-1-Bcl-2-1,
the pGenesil-1-Bcl-2-2 and the pGenesil-1-NC group, respectively.
Protein levels of Bcl-2 in the control and the pGenesil-NC group
were quite similar (P>0.05), but reduced by 69.57 and 27.02% in
the pGenesil-Bcl-2-1 and the pGenesil-Bcl-2-2 group respectively
(P<0.05 vs. control). We then further quantified the amount of
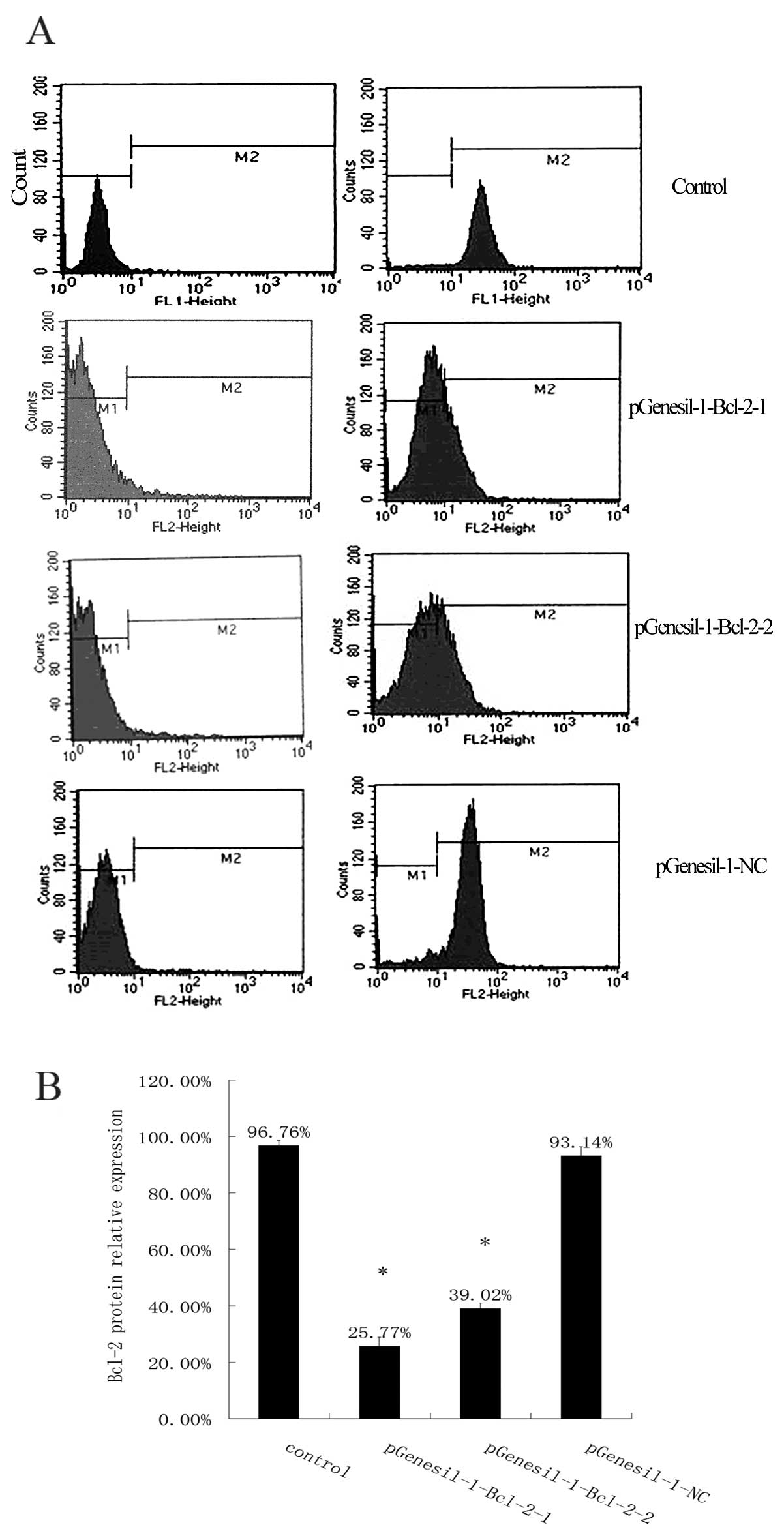
Bcl-2 protein expression in each group by using FCM (Fig. 6). The results showed that Bcl-2
protein levels were 96.76±1.83, 25.77±3.12, 39.02±1.97 and
93.14±3.14% in the control, the pGenesil-1-Bcl-2-1, the
pGenesil-1-Bcl-2-2 and the pGenesil-1-NC group, respectively.
Consistent with the western blot results, the statistical analysis
showed that the expression of the Bcl-2 protein in Raji cells was
downregulated significantly after stable transfection with Bcl-2
shRNA (P<0.05, vs. control) and the inhibition rates were 73.37
and 59.67%, respectively (P<0.05, vs. control). As expected,
there was no difference in the reduction of Bcl-2 protein
expression between the control and the pGenesil-1-NC group
(P>0.05). The above results indicated that Bcl-2 shRNA
significantly decreased the Bcl-2 protein expression levels in Raji
cells following stable transfection.
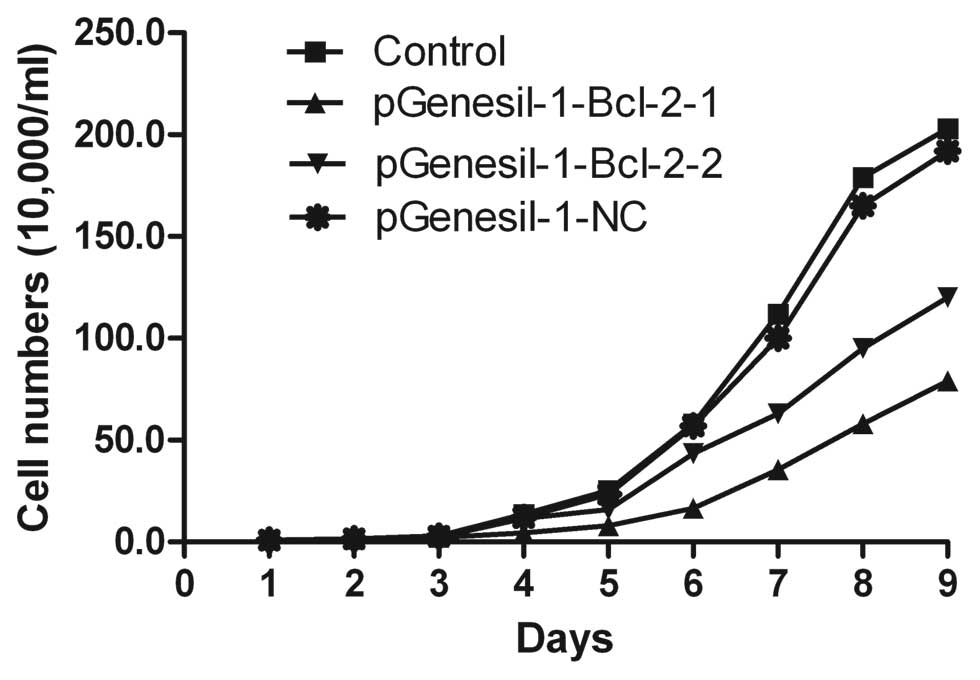
Inhibition of Raji cell proliferation by
Bcl-2 shRNA
Cell proliferation was measured by counting the
number of viable cells using trypan blue staining. As shown in
Fig. 7, when Raji cells were stably
transfected with pGenesil-1-Bcl-2-1 and pGenesil-1-Bcl-2-2,
respectively, the growth of the cells was suppressed as compared
with untreated control cells (P<0.01). However, no statistical
significance was found between the untreated control and the
pGenesil-1-NC group (P>0.05). Raji cell growth was inhibited at
a significantly higher rate in the pGenesil-1-Bcl-2-1 group than in
the pGenesil-1-Bcl-2-2 group at different times (P<0.05).
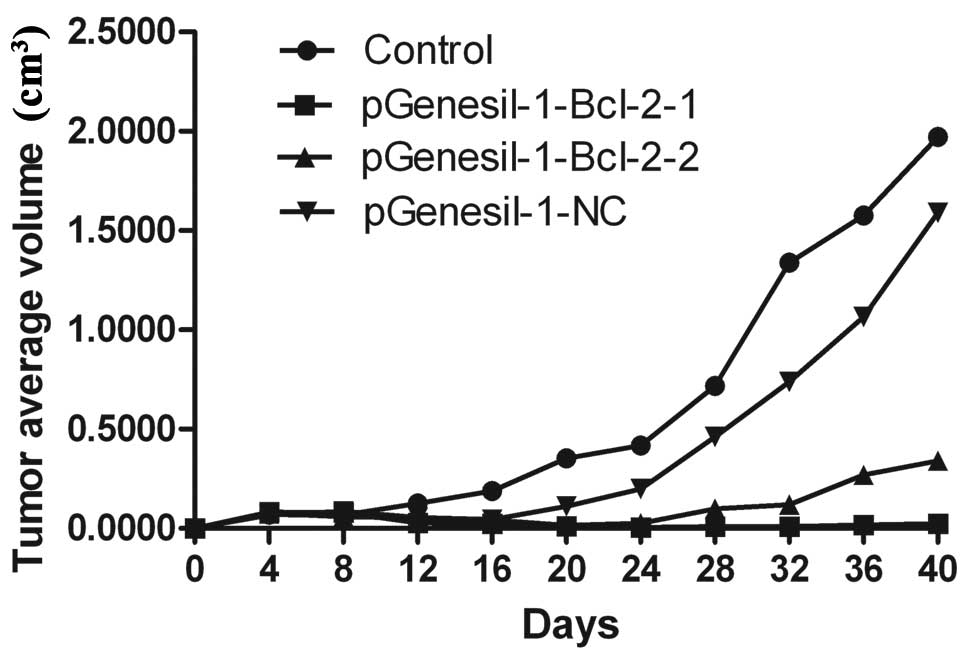
Inhibition of in vivo tumor growth by
Bcl-2 shRNA
To determine the potential effects of Bcl-2 shRNAs
on the inhibition of the growth of Raji cells in vivo, equal
numbers (3×107 cells/ml) of Raji cells stably
transfected with pGenesil-1-Bcl-2-1, pGenesil-1-Bcl-2-2,
pGenesil-1-NC and untreated control were injected into nude mice
subcutaneously. The growth of tumors was measured every four days.
Forty days after injection, mice were sacrificed and the weights of
tumors were recorded. As shown in Table II and Fig. 8, untreated Raji cells and those of
the pGenesil-1-NC group grew rapidly, resulting in palpable tumors
8–12 days following injection. By contrast, tumor formation was
significantly slower after inoculation of pGenesil-1-Bcl-2-1 or
pGenesil-1-Bcl-2-2 clone (P<0.01). The tumors were significantly
smaller than those in either the untreated control or the
pGenesil-1-NC group (P<0.05). These results indicated that Bcl-2
shRNA-mediated Bcl-2 downregulation exerted a strong antitumoral
effect in vivo on B-cell lymphoma.
 | Table IIEffect of Bcl-2 shRNA on tumor growth
in nude mice (mean ± SD). |
Table II
Effect of Bcl-2 shRNA on tumor growth
in nude mice (mean ± SD).
| Groups | n | Days of tumor
formation | Size of tumor
(cm3) | Weight of tumor
(g) |
|---|
| Untreated
control | 6 | 8.2±1.2 | 1.9712±0.3309 | 0.7810±0.2288 |
|
pGenesil-1-Bcl-2-1 | 6 | 24±2.3 |
0.0238±0.0142b |
0.0533±0.0058b |
|
pGenesil-1-Bcl-2-2 | 6 | 20±2.0a |
0.3397±0.0581b |
0.2053±0.0200b |
| pGenesil-1-NC | 6 | 10±1.5a | 1.5910±0.2480 | 0.7533±0.0706 |
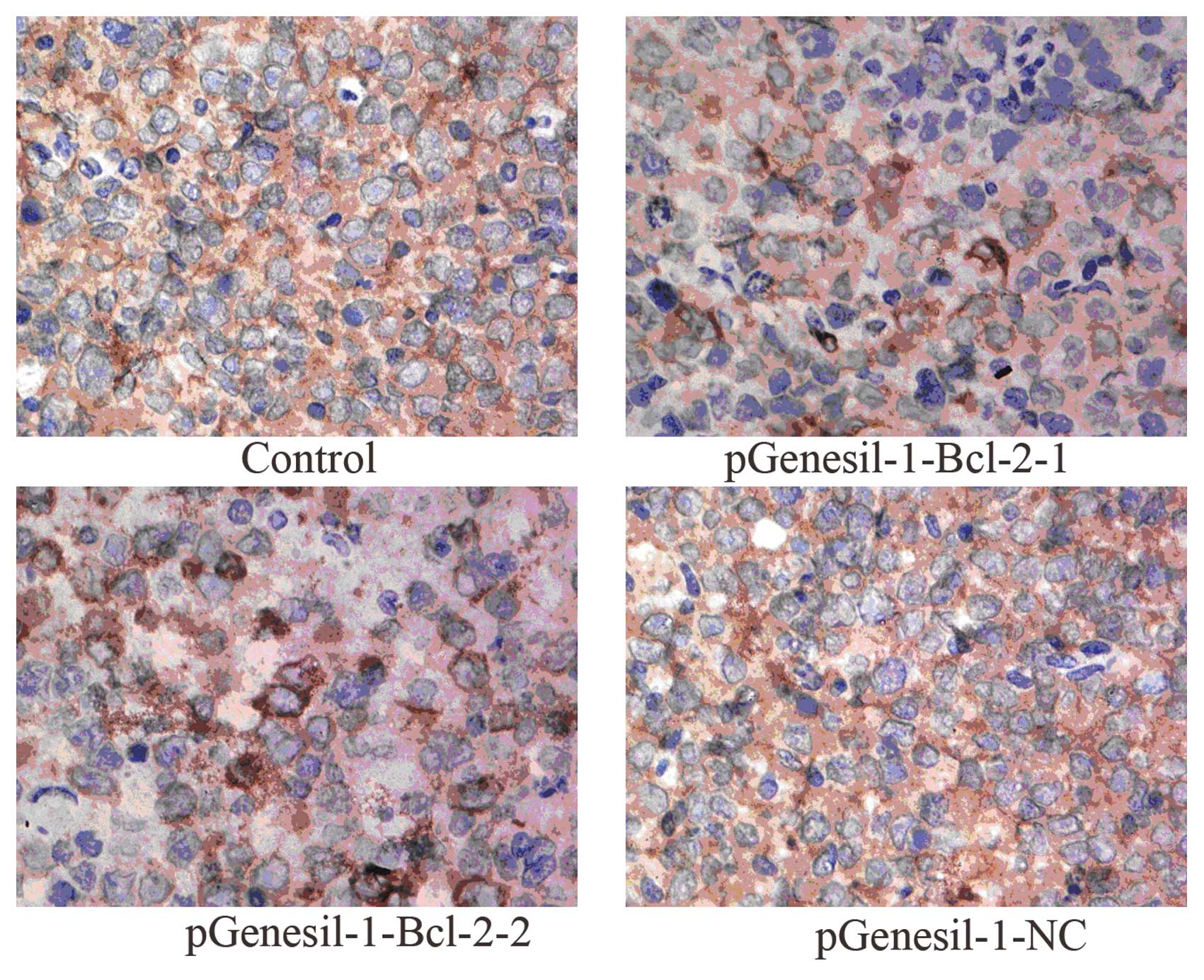
Tumor tissues from mice were excised and
subjected to immunohistochemistry staining
As shown in Fig. 9,
the microscopic examination of stained tumor sections showed that
Bcl-2 expression was strongly detected in the untreated control and
the pGenesil-1-NC group, respectively. However, weak
immunoreactivity was observed in the pGenesil-Bcl-2-1 and the
pGenesil-1-Bcl-2 group, respectively. Bcl-2 expression in the
control and the pGenesil-NC group were markedly higher than in the
pGenesil-1-Bcl-2-1 and the pGenesil-1-Bcl-2 group (both P<0.01),
suggesting Bcl-2 shRNA remarkably downregulated Bcl-2 protein
expression on B-cell lymphoma in vivo.
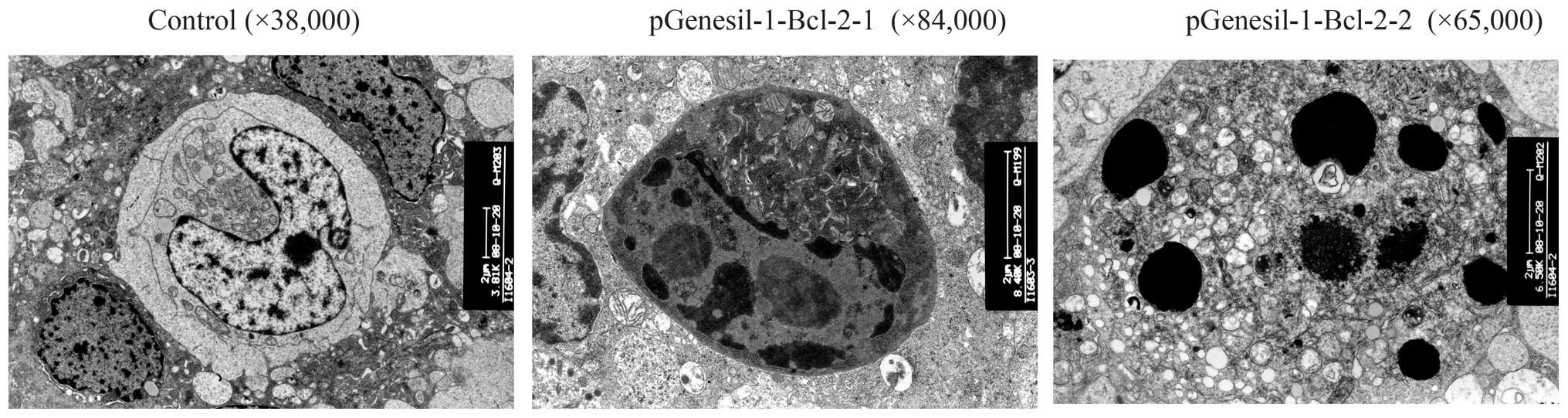
Morphological change under transmission
electron microscopy
With the help of the trans-nuclear membrane,
distributed nuclear chromosome, distinct organelle, big nuclei and
mission electron microscope, we found that the normal Raji cell had
intact cell membrane and excessive nuclei division, which indicated
that the Raji cell was relatively highly malignant. When the Raji
cells were transfected with Bcl-2 shRNA, some changes, such as
apoptosis, cell shrinkage, separation from neighboring cells,
plasma condensation, plasma vacuolation, karyopyknosis, margination
of condensed chromatin and membrane-bound apoptotic bodies were
observed (Fig. 10).
Discussion
The worldwide incidence of lymphoma is increasing
and lymphoma accounts for approximately 3–4% of all types of cancer
(11). The conventional treatments
including surgery, chemotherapy, radiation therapy and
immunotherapies do not achieve satisfactory effects due to drug
resistance and lymphoma recurrence. In addition, many conventional
treatments usually lead to toxicity that affects therapeutic
outcome and quality of life. Thus, it is urgent to find a novel
approach to overcome these short comings in cancer therapy. With
the development of molecular biotechnology, gene therapy becomes a
new potential approach for the treatment of cancer, as it has a
highly efficient, specific effect and only slight drug resistance.
To date, RNAi has attracted much attention. RNAi is one of the most
commonly used approaches for genes targeting cutting edge
technology (12). RNAi is mediated
by small interfering RNAs of approximately 21 nucleotides or by
continually expressed short hairpin RNAs that are cut into siRNAs
by Dicer (13,14). Then, the produced siRNA is
incorporated into nuclease complex, forming the RNA-inducing
silencing complex, which degrades mRNA containing a sequence
homologous to that of the small RNA fragment (15–19).
As a post-transcriptional gene silencing mechanism, RNAi has been
demonstrated to have prospects for cancer therapy.
The development of B-cell lymphoma is correlated
with multiple genes. Among these genes, Bcl-2 plays an important
role (20). Bcl-2 is a member of
the Bcl-2 family that is key in the regulation of the intrinsic
pathway by controlling mitochondrial membrane permeability and the
release of the proapoptotic factor cytochrome c. Bcl-2
overexpression has been demonstrated in B-cell lymphoma (21,22).
Bcl-2 overexpression has been reported to be involved in the
progression of tumors (23,24). Xu et al noted that
pGenesil-1-Bcl-2 shRNA could significantly inhibit Bcl-2
expression, it suppressed the growth of human bladder cancer cells
T24 and induced apoptosis of the cells (25). Lei et al(26) demonstrated that Bcl-2 shRNA reduced
the level of Bcl-2 mRNA and Bcl-2 protein expression in HL-60 cells
and induced cell apoptosis. Therefore, specific downregulation of
Bcl-2 may be a potential therapeutic strategy against human
cancer.
To investigate whether Bcl-2 shRNA could suppress
the development of Raji cells from B-cell lymphoma by the above
RNAi method, we constructed two types of Bcl-2 shRNA and stably
transfected them into Raji cells. Then, we detected Bcl-2 mRNA and
its corresponding protein expression in the pGensil-1-Bcl-2-1 and
the pGensil-1-Bcl-2-2 group. Meanwhile, Raji cell proliferation was
successfully inhibited by Bcl-2 shRNA, compared with the control
group (P<0.05). In order to further investigate whether Bcl-2
shRNA mediated downregulation in vivo, we chose 4-week-old
BACB/C nude mice lacking T cell immune function as the animal
model. When compared with the pGenesil-1-NC and the untreated Raji
cell group, tumor formation was significantly slower after
inoculation of pGenesil-1-Bcl-2-1 or pGenesil-1-Bcl-2-2 clone
(P<0.01) and the tumors were significantly smaller (P<0.05).
The results of immunohistochemistry showed that Bcl-2 shRNA
markedly reduced the expression of Bcl-2 protein in Raji cells and
the transmission electron microscope demonstrated that special
Bcl-2 shRNA successfully induced Raji cell apoptosis. These data
indicated that Bcl-2 shRNA-mediated Bcl-2 downregulation blocked
the transduction of survival signals and trigger apoptosis, as
previously reported (27), and
displayed a strong growth inhibition on B-cell lymphoma in
vitro and in vivo. Bcl-2 shRNA could be applied for
treatment of tumors with overexpression of Bcl-2. However, we
observed that the inhibition effect of the pGenesil-1-Bcl-2-1 group
was better than that of the pGenesil-1-Bcl-2-2 group. The possible
reasons were that the nucleotides 544–562 of mRNA contained few
cytosine and guanine and its space structure was easy to open
compared to the nucleotides 1009–1027. However, the underlying
mechanism requires further examination.
In this study, we also observed that BCL-2 protein
levels were reduced by 69.57 and 27.02% in the pGenesil-Bcl-2-1 and
the pGenesil-Bcl-2-2 group compared with untreated control by using
western blot analysis. The inhibition rates were accordingly lower
than 73.37 and 59.67% by flow cytometry. We considered a possible
cause that detectability and accuracy of western blot analysis were
different from flow cytometry. However, the two test methods
consistently indicated that Bcl-2 shRNA was able to decrease the
Bcl-2 protein expression levels in Raji cells.
In summary, our study indicated that Bcl-2 performed
a fundamental role in the progression of the tumor, and
plasmid-mediated Bcl-2 shRNA inhibited the growth and apoptosis of
human B-cell lymphoma in vitro and in vivo. RNAi may
have potential therapeutic utility in cancer and other
diseases.
Acknowledgements
This study was supported by grants from the Second
Affiliated Hospital of Soochow, China.
References
|
1
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kanwar RK, Cheung CH, Chang JY and Kanwar
JR: Recent advances in anti-survivin treatments for cancer. Curr
Med Chem. 17:1509–1515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akgul C: Mcl-1 is a potential therapeutic
target in multiple types of cancer. Cell Mol Life Sci.
66:1326–1336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Szegedi I, Katona K, Horváth A, Molnár A,
Aradi J and Kiss C: Bcl-2 antisense oligonucleotide inhibits the
proliferation of childhood leukemia/lymphoma cells of the B-cell
lineage. Pathol Oncol Res. 14:275–279. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chanan-Khan A: Bcl-2 antisense therapy in
hematologic malignancies. Curr Opin Oncol. 16:581–585. 2004.
View Article : Google Scholar
|
|
6
|
Chen Y and Huang L: Tumor-targeted
delivery of siRNA by non-viral vector: safe and effective cancer
therapy. Expert Opin Drug Deliv. 5:1301–1311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Walchli S and Sioud M: Vector-based
delivery of siRNAs: in vitro and in vivo challenges. Front Biosci.
13:3488–3493. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gondi CS and Rao JS: Concepts in in vivo
siRNA delivery for cancer therapy. J Cell Physiol. 220:285–291.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roelz R, Pilz IH, Mutschler M and Pahl HL:
Of mice and men: human RNA polymerase III promoter U6 is more
efficient than its murine homologue for shRNA expression from a
lentiviral vector in both human and murine progenitor cells. Exp
Hematol. 38:792–797. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang F, Tong X, Fu L and Zhang R:
Knockdown of STAT3 by shRNA inhibits the growth of CAOV3 ovarian
cancer cell line in vitro and in vivo. Acta Biochim Biophys Sin.
40:519–525. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baris D and Zahm SH: Epidemiology of
lymphomas. Curr Opin Oncol. 12:383–394. 2000. View Article : Google Scholar
|
|
12
|
Lai SR and Andrews LG: Tollefsbol To RNA
interference using a plasmid construct expressing short-hairpin
RNA. Methods Mol Biol. 405:31–37. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brummelkamp TR, Bemards R and Agami R: A
system for stable expression of short interfering RNAs in mammalian
cells. Science. 296:550–553. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paddision PJ, Caudy AA, Bernstein E, et
al: Short hairpin RNAs (shRNAs) induce sequence-specific silencing
in mammalian cells. Genes Dev. 16:948–958. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao Y, Zhang CL, Zeng BF, et al: Enhanced
chemosensitivity of drug-resistant osteosarcoma cells by
lentivirus-mediated Bcl-2 silencing. Biochem Biophys Res Commun.
390:642–647. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sastry L, Johnson T, Hobson MJ, et al:
Titering lentiviral vectors: comparison of DNA, RNA and marker
expression methods. Gene Ther. 9:1155–1162. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuhn DM, Balkis M, Chandra J, et al: Uses
and limitation of the XTT assay in studies of Candidar growth and
metabolism. J Clin Microbiol. 41:506–508. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seol JY, Park KH, Hwang C, et al:
Adenovirus-TRAIL can overcome TRAIL resistance and induce a
bystander effect. Cancer Gene Ther. 10:540–548. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang X, Lin T, Gu J, et al: Cell to cell
contact required for bystander effect of the TNF-related
apoptosis-inducing ligand (TRAIL) gene. Int J Oncol. 22:1241–1245.
2003.PubMed/NCBI
|
|
20
|
Vaux DL, Cory S and Adams JM: BCL-2 gene
promotes haemopoietic cell survival and cooperates with c-myc to
immortalize pre-B cells. Nature. 335:440–442. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paoluzzi L and O’Connor OA: Targeting
survival pathways in lymphoma. Adv Exp Med Biol. 687:79–96. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chipuk JE, Moldoveanu T, Llambi F, et al:
The BCL-2 family reunion. Mol Cell. 37:299–310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gross A: BCL-2 proteins: regulators of the
mitochondrial apoptotic program. IUBMB Life. 52:231–236. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Llambi F and Green DR: Apoptosis and
oncogenesis: give and take in the BCL-2 family. Curr Opin Genet
Dev. 21:12–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu KW, Huang J, Lin TX, et al: The effects
of RNA interference to Bcl-2 gene on proliferation of human bladder
cancer cell line T24. Chin J Urol. 28:26–29. 2007.
|
|
26
|
Lei XY, Yan CY, Tu YL, et al: Inhibition
of Bcl-2 gene expression by siRNA in HL-60 cells. Chinese Pharmacol
Bull. 21:1445–1449. 2005.
|
|
27
|
Ramanarayanan J, Hernandez-Ilizalitu FJ,
Chanan-Khan A and Czuczman MS: Pro-apoptotic therapy with the
oligonucleotide Genasense (oblimersen sodium) targeting Bcl-2
protein expression enhances the biological anti-tumour activity of
rituximab. Br J Haematol. 127:519–530. 2004. View Article : Google Scholar
|