Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide (1,2). Lung adenocarcinoma is the most common
type of lung cancer, and its incidence has increased in recent
years (3). Surgical resection is
the treatment of first choice for early-stage lung adenocarcinoma,
but the 5-year overall survival (OS) rate remains at ~80% for stage
IA disease (4). Understanding the
histological and biological character of early-stage lung
adenocarcinoma is important for improving the clinical outcome of
this patient population.
Cancer cells often have higher rates of glucose
metabolism than normal cells, producing lactic acid rather than
catabolizing glucose via the tricarboxylic acid (TCA) cycle
(5). The glucose transporter family
is a collection of membrane proteins that are responsible for
glucose uptake. Glucose transporter isoform 1 (GLUT1) is expressed
in the brain and in erythrocytes (6). GLUT1 also maintains a basal level of
glucose uptake in most cell types under hypoxic and hypoglycemic
conditions. The overexpression of GLUT1 has been observed in many
types of human malignancies (7,8). In
non-small cell lung cancers (NSCLC), the overexpression of GLUT1 is
reportedly associated with a poor prognosis (9,10). As
a classical marker of proliferation, the Ki-67 protein is well
known to be present in nuclei during the active phase of the cell
cycle (11–13), and the overexpression of Ki-67 is a
prognostic marker in many types of cancer (14,15). A
high Ki-67 labeling index reportedly predicts poor prognosis in
stage I NSCLCs (16–19).
Since 2004, the role of activating mutations of
epidermal growth factor receptor (EGFR) and KRAS
genes as characteristic somatic mutations in lung adenocarcinoma
has become a topic of interest, since these mutations exhibit a
mutually exclusive relationship and have clinical implications
[i.e., EGFR mutations are associated with responsiveness to
EGFR tyrosine kinase inhibitors (EGFR-TKIs)]
(20–22).
An international multidisciplinary classification
for lung adenocarcinoma was recently proposed by the International
Association for the Study of Lung Cancer (IASLC), the American
Thoracic Society (ATS), and the European Respiratory Society (ERS)
(23). This new classification (the
IASLC/ATS/ERS classification) provides uniform terminology and
diagnostic criteria especially for adenocarcinoma with lepidic
growth which was formerly classified as bronchioloalveolar
carcinoma (BAC), as the definition of BAC was unclear in the
previous classification (24). The
term BAC has been used for a broad spectrum of tumors including
solitary small non-invasive peripheral lung tumors, invasive
adenocarcinomas with minimal invasion, mixed subtype invasive
adenocarcinomas, mucinous and nonmucinous subtypes of tumors and
widespread advanced disease; the clinical outcome of patients with
BAC has also varied. In the new classification, lung
adenocarcinomas are classified into four major categories based on
tumor invasiveness: i) preinvasive lesions, ii) minimally invasive
adenocarcinoma (MIA), iii) invasive adenocarcinoma, and iv)
variants of invasive adenocarcinoma. Invasive adenocarcinoma is the
most common type among surgically resected specimens and is
subdivided into five types according to semiquantitative subtype
patterns, rather than using the term adenocarcinoma, mixed subtype.
Notably, patients with preinvasive lesions or MIAs are expected to
have a 100% or nearly 100% OS following complete resection,
suggesting that this new classification may be useful for
determining suitable therapeutic strategies and predicting patient
outcome.
In this study, we investigated the
clinicopathological impact of GLUT1 and Ki-67 expression levels on
early-stage lung adenocarcinoma classified according to the
IASLC/ATS/ERS classification.
Materials and methods
Patients
We reviewed patients with lung adenocarcinoma who
had undergone complete resections at Okayama University Hospital
between January 2004 and December 2006. A total of 133 consecutive
patients with pathologic stage IA disease, according to the
International Union Against Cancer’s TNM classification for
malignant tumors (25), underwent
complete tumor resection. Among them, patients with recurrent tumor
or second primary lung adenocarcinoma were excluded. As a result,
105 patients with stage IA lung adenocarcinoma were eligible for
this study. The follow-up protocol after surgery was as follows:
chest and abdominal computed tomography (CT) or positron emission
tomography/CT scan and enhanced brain magnetic resonance imaging
were repeated every six months for three years. After three years,
a chest X-ray was, in principle, repeated ever year and additional
examinations were performed as necessary.
Histological classification and
immunohistochemistry for GLUT1 and Ki-67
Two investigators (K.I. and Y.M.) who were unaware
of the clinical data independently classified all the tumors
according to the IASLC/ATS/ERS classification and discussed the
final diagnosis in cases with diagnostic discrepancies. The written
informed consent of each patient and the permission of the
Institutional Review Board were obtained. This study was approved
by the Ethics Committee of Okayama University (approval no.
478).
For the immunohistochemistry (IHC), 4-μm sections
were cut from paraffin-embedded tissue specimens on MAS-GP type A
coated glass slides (S-9901; Matsunami Glass Ind., Ltd., Osaka,
Japan). The slides were deparaffinized in xylene and rehydrated in
a graded series of ethanol (100, 100, 90, 70 and 50%). After
revealing antigens with 10 mM of sodium citrate (pH 6.0), the
slides were incubated in 3% H2O2 for 10 min
to block endogenous peroxidase. To inhibit non-specific binding,
the samples were incubated in diluted normal horse serum for 30
min. After blocking, the slides were incubated with GLUT1 (Abcam,
Cambridge, UK; diluted 1:200 in PBS) and Ki-67 (Novocastra,
Newcastle, UK; diluted 1:2,000 in PBS) antibodies at 4°C overnight.
The slides were washed in PBS for 5 min and incubated in secondary
antibody for 30 min at room temperature (ImmPRESS Anti-Rabbit Ig
peroxidase Polymer Detection kit; Vector Laboratories,
Peterborough, UK). The slides were stained with
3,3′-diaminobenzidine (DAB Substrate kit; Vector Laboratories), and
were counterstained in Mayer’s hematoxylin. Tumor cells were
considered positive for GLUT1 if the cell membrane staining was no
less than that of the erythrocytes in the same section. GLUT1
expression was considered positive in each section if the
percentage of tumor cells with positive staining was >10%, as
previously reported (7,26,27).
Ki-67 staining was evaluated using the labeling index (28,29).
In the area with the strongest Ki-67 staining, positively stained
cells were defined as having a clearly stained nucleus and the
Ki-67 labeling index was considered positive when >15% of the
tumor cells were stained among at least 1,000 tumor cells (30–32).
DNA extraction and mutation analyses of
EGFR and KRAS genes
DNA was extracted from formalin-fixed and
paraffin-embedded tissues (n=51) or frozen tissues (n=54) using the
QIAamp® DNA FFPE Tissue kit (Qiagen, Hilden, Germany) or
by digestion with proteinase K, followed by phenol-chloroform (1:1)
extraction and ethanol precipitation, respectively. EGFR
mutational status was determined using a mutant non-enriched PCR
assay, as previously reported (33). KRAS mutations at codons 12
and 13 were examined using PCR-based direct sequencing using an ABI
PRISM 3130×l Genetic Analyzer (Applied Biosystems, Foster City, CA,
USA), as previously reported (34,35).
Statistical analysis
Differences between the two groups were assessed
using χ2 tests or Fisher’s exact test, as appropriate.
Multiple logistic regression analyses were used to identify
independent factors and to adjust for the influence of other
co-variables. The OS and disease-free survival (DFS) periods were
calculated from the date of operation until the date of death or
the last follow-up for the OS and until confirmed disease
recurrence based on cross-sectional imaging studies or death for
the DFS.
A univariate analysis of the OS and DFS was
performed using the Kaplan-Meier method and the log-rank test. A
multivariate analysis for OS and DFS was performed using the Cox
proportional-hazards model. The stepwise procedure was used to
select independent variables using a backward elimination method
with P-values of 0.10 for entry and 0.15 for rejection. All the
data were analyzed using the JMP, version 9.0.0 (SAS Institute
Inc., Cary, NC, USA).
Results
Patient characteristics
The patient characteristics are shown in Table I. The median age was 65 years
(range, 29–83 years); 49 patients were men, and 56 were women. The
smoking categories were defined as follows: never-smokers, those
with a lifetime exposure of ≤100 cigarettes; and ever-smokers,
those with a lifetime exposure of >100 cigarettes. Eighty-eight
patients underwent a lobectomy, while a segmentectomy or wedge
resection was performed for 17 tumors exhibiting a pure ground
glass opacity during CT imaging. We defined these cases as curative
operations since none of the 17 cases experienced disease
relapse.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Subsets | n | % |
|---|
| Age |
| <65 | 52 | 49.5 |
| ≥65 | 53 | 50.5 |
| Gender |
| Male | 49 | 46.7 |
| Female | 56 | 53.3 |
| Smoking status |
| Never | 56 | 53.3 |
| Ever | 49 | 46.7 |
| Tumor size |
| T1a (≤2 cm) | 73 | 69.5 |
| T1b (>2
cm) | 32 | 30.5 |
| GLUT1
expression |
| Negative | 77 | 73.3 |
| Positive | 28 | 26.7 |
| Ki-67
expression |
| Negative | 72 | 68.6 |
| Positive | 33 | 31.4 |
| EGFR
mutation |
| Mutant | 51 | 48.6 |
| Wild-type | 54 | 51.4 |
| KRAS
mutation |
| Mutant | 5 | 4.8 |
| Wild-type | 100 | 95.2 |
| IASLC/ATS/ERS
classification |
| Preinvasive
lesion |
| Adenocarcinoma
in situ |
| Nonmucinous | 19 | 18.1 |
| Mucinous | 0 | 0.0 |
| Mixed
mucinous/nonmucinous 0 0.0 |
| Minimally
invasive adenocarcinoma |
| Nonmucinous | 12 | 11.4 |
| Mucinous | 0 | 0.0 |
| Mixed
mucinous/nonmucinous | 0 | 0.0 |
| Invasive
adenocarcinoma |
| Lepidic
predominant | 18 | 17.1 |
| Acinar
predominant | 1 | 1.0 |
| Papillary
predominant | 45 | 42.9 |
| Micropapillary
predominant | 0 | 0.0 |
| Solid
predominant with mucin production | 5 | 4.8 |
| Variants of
invasive adenocarcinoma |
| Invasive
mucinous adenocarcinoma | 5 | 4.8 |
| Colloid | 0 | 0.0 |
| Fetal | 0 | 0.0 |
| Enteric | 0 | 0.0 |
IASLC/ATS/ERS classification of lung
adenocarcinoma
The details of the lung adenocarcinoma subtypes
according to the IASLC/ATS/ERS classification are shown in Table I. Among the four categories,
invasive adenocarcinoma (n=69, 65.7%) was the most frequent
diagnosis in this study. Among the invasive adenocarcinomas, 63.8%
would have been diagnosed as mixed subtype, according to the 2004
World Health Organization classification (24). Since adenocarcinoma in situ
(AIS) and minimally invasive adenocarcinoma (MIA) with a diameter
equal to 2 cm or less have previously been reported to have a
5-year OS or DFS rate of 100% (36,37),
we defined these tumors as ‘non-invasive type’ (n=31) and compared
them with the other ‘invasive type’ (n=74) adenocarcinomas,
including invasive adenocarcinoma and invasive mucinous
adenocarcinoma. We then performed further analyses comparing those
two groups.
Molecular alterations in clinical
samples
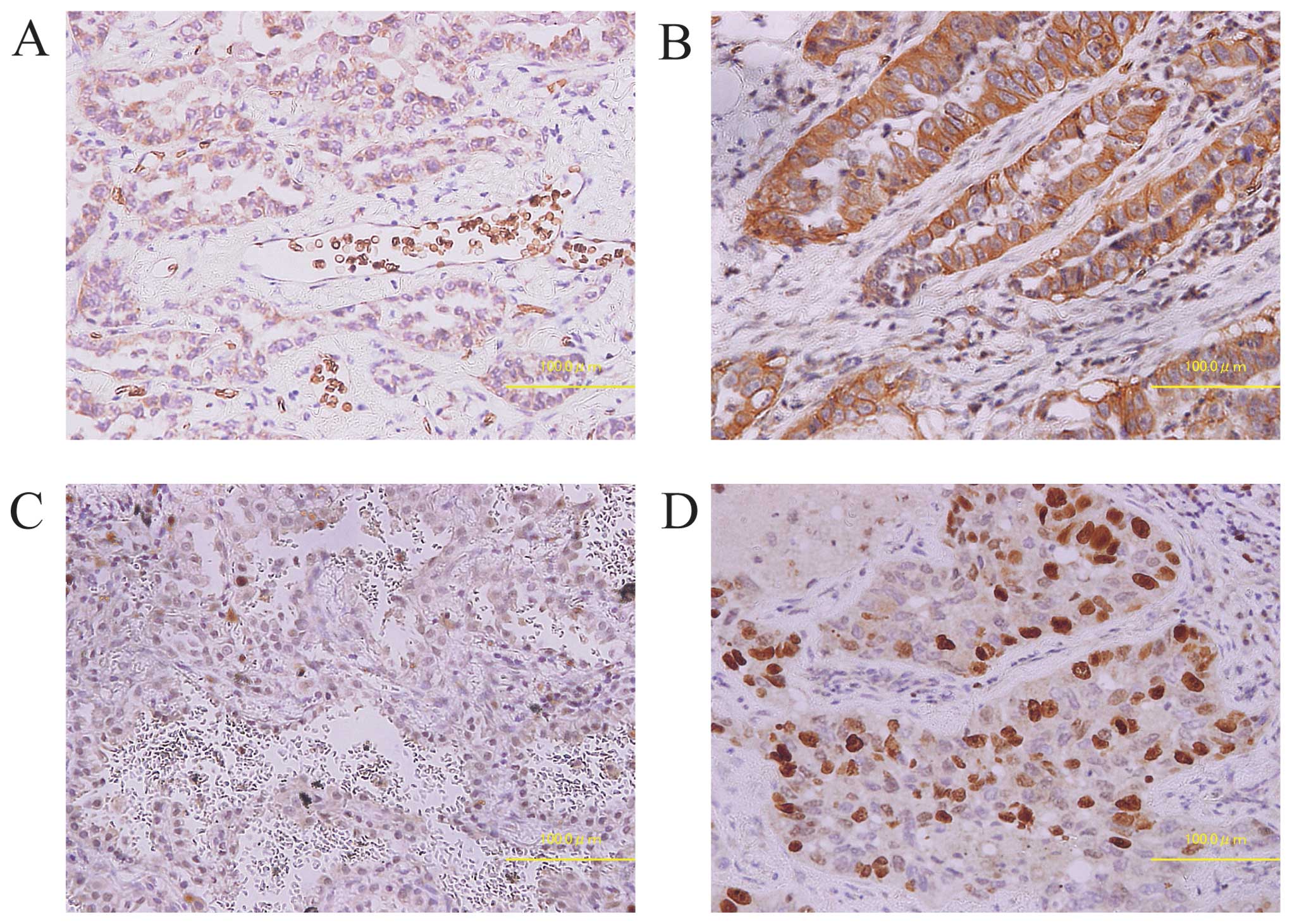
The results and representative samples of the IHC
staining patterns for GLUT1 and Ki-67 are shown in Table II and Fig. 1. Positive GLUT1 and Ki-67
expressions were observed in 28 (26.7%) and 33 (31.4%) of the 105
patients, respectively. Among the 105 tumors, we detected
EGFR mutations in 51 tumors (48.6%). Mutations in
KRAS codons 12 or 13 were detected in 5 of the 105 tumors
(8.3%).
 | Table IIGLUT1 and Ki-67 expression and
clinical and genetic factors. |
Table II
GLUT1 and Ki-67 expression and
clinical and genetic factors.
| GLUT1 positive | Ki-67 positive | EGFR
mutant | KRAS
mutant |
|---|
|
|
|
|
|
|---|
| Subsets | n | % | P-value | n | % | P-value | n | % | P-value | n | % | P-value |
|---|
| Age |
| <65 | 14 | 26.9 | 1 | 14 | 26.9 | 0.3 | 23 | 44.2 | 0.8 | 1 | 1.9 | 0.2 |
| ≥65 | 14 | 26.4 | | 19 | 35.8 | | 28 | 52.8 | | 4 | 7.5 | |
| Gender |
| Male | 21 | 42.9 | <0.001 | 24 | 49.0 | <0.001 | 15 | 30.6 | <0.001 | 2 | 4.1 | 0.8 |
| Female | 7 | 12.5 | | 9 | 16.1 | | 36 | 64.3 | | 3 | 5.4 | |
| Smoking status |
| Never | 9 | 16.1 | 0.008 | 11 | 19.6 | 0.005 | 35 | 62.5 | 0.002 | 2 | 3.6 | 0.4 |
| Ever | 19 | 38.8 | | 22 | 44.9 | | 16 | 32.7 | | 3 | 6.1 | |
| Tumor size |
| T1a (≤2 cm) | 14 | 19.2 | 0.009 | 21 | 28.8 | 0.4 | 32 | 43.8 | 0.14 | 3 | 4.1 | 0.2 |
| T1b (>2
cm) | 14 | 43.8 | | 12 | 37.5 | | 19 | 59.4 | | 2 | 6.3 | |
| GLUT1
expression |
| Negative | - | - | - | 18 | 23.4 | 0.003 | 44 | 57.1 | 0.004 | 1 | 1.3 | 0.02 |
| Positive | - | - | | 15 | 53.6 | | 7 | 25.0 | | 4 | 14.3 | |
| Ki-67
expression |
| Negative | 13 | 18.1 | 0.003 | - | - | - | 38 | 52.8 | 0.2 | 2 | 2.8 | 0.2 |
| Positive | 15 | 45.5 | | - | - | | 13 | 39.4 | | 3 | 9.1 | |
| EGFR
mutation |
| Mutant | 7 | 13.7 | 0.004 | 20 | 37.0 | 0.2 | - | - | - | 0 | 0.0 | 0.03 |
| Wild-type | 21 | 38.9 | | 13 | 25.5 | | - | - | | 5 | 9.8 | |
| KRAS
mutation |
| Mutant | 4 | 80.0 | 0.02 | 3 | 60.0 | 0.2 | 0 | 0.0 | 0.03 | - | - | - |
| Wild-type | 24 | 24.0 | | 30 | 30.0 | | 51 | 51.0 | | - | - | |
The inter-relationships of GLUT1 and Ki-67
expression levels and EGFR and KRAS mutations were
examined. Positive GLUT1 expression was significantly more common
among EGFR wild-type cases (P=0.004), KRAS mutant
cases (P=0.02), and tumors with positive Ki-67 expression (P=0.003)
(Table II).
Relationship between molecular
alterations and clinicopathological factors
The associations between the above-mentioned
molecular alterations and clinical factors are shown in Table II. Positive GLUT1 expression was
significantly more common among men (P<0.001), ever smokers
(P=0.008), and patients with large tumors (T1b) (P=0.009). Positive
Ki-67 expression was significantly more common among men
(P<0.001) and ever smokers (P=0.005). Mutant EGFR was
significantly more common among women (P<0.001) and never
smokers (P=0.002). Mutant KRAS was not significantly
associated with any clinical factor.
Furthermore, we investigated the relationship
between the IASLC/ATS/ERS classification and clinical and molecular
factors (Table III). ‘Invasive
type’ adenocarcinomas were more common among patients with large
tumors (P=0.01), GLUT1 positive tumors (P=0.002), and Ki-67
positive tumors (P=0.01). In a multiple logistic regression
analysis including the significant factors mentioned above,
‘invasive type’ adenocarcinomas were only correlated with positive
GLUT1 expression [odds ratio (OR), 4.85; 95% confidence interval
(CI), 1.21–32.56; P=0.048].
 | Table IIIAssociation between ‘invasive type’
adenocarcinoma and clinical and genetic factors. |
Table III
Association between ‘invasive type’
adenocarcinoma and clinical and genetic factors.
| | | Univariate | Multivariate |
|---|
| | |
|
|
|---|
| Subsets | n | % | P-value | P-value |
|---|
| Age |
| <65 | 37 | 71.2 | 0.9 | |
| ≥65 | 37 | 69.8 | | |
| Gender |
| Male | 37 | 75.5 | 0.3 | |
| Female | 37 | 66.1 | | |
| Smoking status |
| Never | 36 | 64.3 | 0.14 | |
| Ever | 38 | 77.6 | | |
| Tumor size |
| T1a (≤2 cm) | 46 | 63.0 | 0.01 | 0.06 |
| T1b (>2
cm) | 28 | 87.5 | | |
| GLUT1
expression |
| Negative | 48 | 62.3 | 0.002 | 0.048 |
| Positive | 26 | 92.9 | | |
| Ki-67
expression |
| Negative | 45 | 62.5 | 0.01 | 0.06 |
| Positive | 29 | 87.9 | | |
| EGFR
mutation |
| Mutant | 39 | 76.5 | 0.19 | |
| Wild-type | 35 | 64.8 | | |
| KRAS
mutation |
| Mutant | 4 | 80.0 | 1.0 | |
| Wild-type | 70 | 70.0 | | |
Impact of GLUT1 and Ki-67 expression on
clinical outcome
As of September 2011, 4 (3.8%) of the 105 patients
had succumbed and the median follow-up duration was 59.7 months.
Ten (9.5%) patients had experienced disease relapse. The 5-year OS
rate was 94.6% (95% CI, 86.0–98.0%). The 5-year DFS rate was 90.2%
(95% CI, 81.9–94.8%). The associations between OS or DFS and
clinicopathological and genetic factors are shown in Table IV. The associations between the OS
and clinicopathological and genetic factors showed that positive
Ki-67 expression was the only significant factor of a poor OS
(P=0.002), although positive GLUT1 expression and ‘invasive type’
adenocarcinoma tended to be correlated with a poor OS (P=0.063 and
P=0.098, respectively) according to univariate analysis. In a
multivariate analysis, Ki-67 was the only independent factor
associated with a poor OS [hazard ratio (HR), 2.0×107;
95% CI, 2.00–3.27×1059; P=0.012]. All 10 tumors in
patients with disease relapse were diagnosed as ‘invasive type’
adenocarcinomas. In univariate analyses, a male gender (P=0.02), an
ever smoking status (P=0.02), an ‘invasive type’ classification
(P=0.005), a positive GLUT1 expression (P=0.0003), and a positive
Ki-67 expression (P=0.0005) were significantly associated with a
poor DFS. In a multivariate analysis including the significant
factors mentioned above, positive GLUT1 expression was the only
independent predictor of a poor DFS (HR, 6.02; 95% CI, 1.25–48.22;
P=0.040), although positive Ki-67 expression tended to correlate
with a poor DFS (HR, 6.49; 95% CI, 1.15–57.53; P=0.058).
 | Table IVCox proportional hazards model for
post-operative overall survival and disease-free survival. |
Table IV
Cox proportional hazards model for
post-operative overall survival and disease-free survival.
| OS | DFS |
|---|
|
|
|
|---|
| Univariate | Multivariate | Univariate | Multivariate |
|---|
|
|
|
|
|
|---|
| Subsets | HR | P-value | HR | P-value | HR | P-value | HR | P-value |
|---|
| Age |
| <65 | 0.70 | 0.7 | | | 0.77 | 0.7 | | |
| ≥65 | | | | | | | | |
| Gender |
| Male | 3.58 | 0.2 | | | 4.94 | 0.02 | 0.32 | 0.5 |
| Female | | | | | | | | |
| Smoking status |
| Never | 0.27 | 0.2 | | | 0.19 | 0.02 | 0.3 | 0.4 |
| Ever | | | | | | | | |
| Tumor size |
| T1a (≤2 cm) | 1.28 | 0.8 | | | 0.39 | 0.15 | | |
| T1b (>2
cm) | | | | | | | | |
| IASLC/ATS/ERS
classification |
| ‘Non-invasive
type’ |
4.7×10−7 | 0.098 |
1.9×106 | 0.4 |
4.4×10−7 | 0.005 |
8.4×10−7 | 0.2 |
| ‘Invasive
type’ | | | | | | | | |
| GLUT1
expression |
| Positive | 7.02 | 0.063 | 1.82 | 0.6 | 11.9 | 0.0003 | 6.02 | 0.040 |
| Negative | | | | | | | | |
| Ki-67
expression |
| Positive |
1.3×107 | 0.002 |
2.0×107 | 0.012 | 10.7 | 0.0005 | 6.49 | 0.058 |
| Negative | | | | | | | | |
| EGFR
mutation |
| Mutant | 1.11 | 0.9 | | | 0.91 | 0.9 | | |
| Wild-type | | | | | | | | |
| KRAS
mutation |
| Mutant |
2.1×10−6 | 0.5 | | | 0.40 | 0.4 | | |
| Wild-type | | | | | | | | |
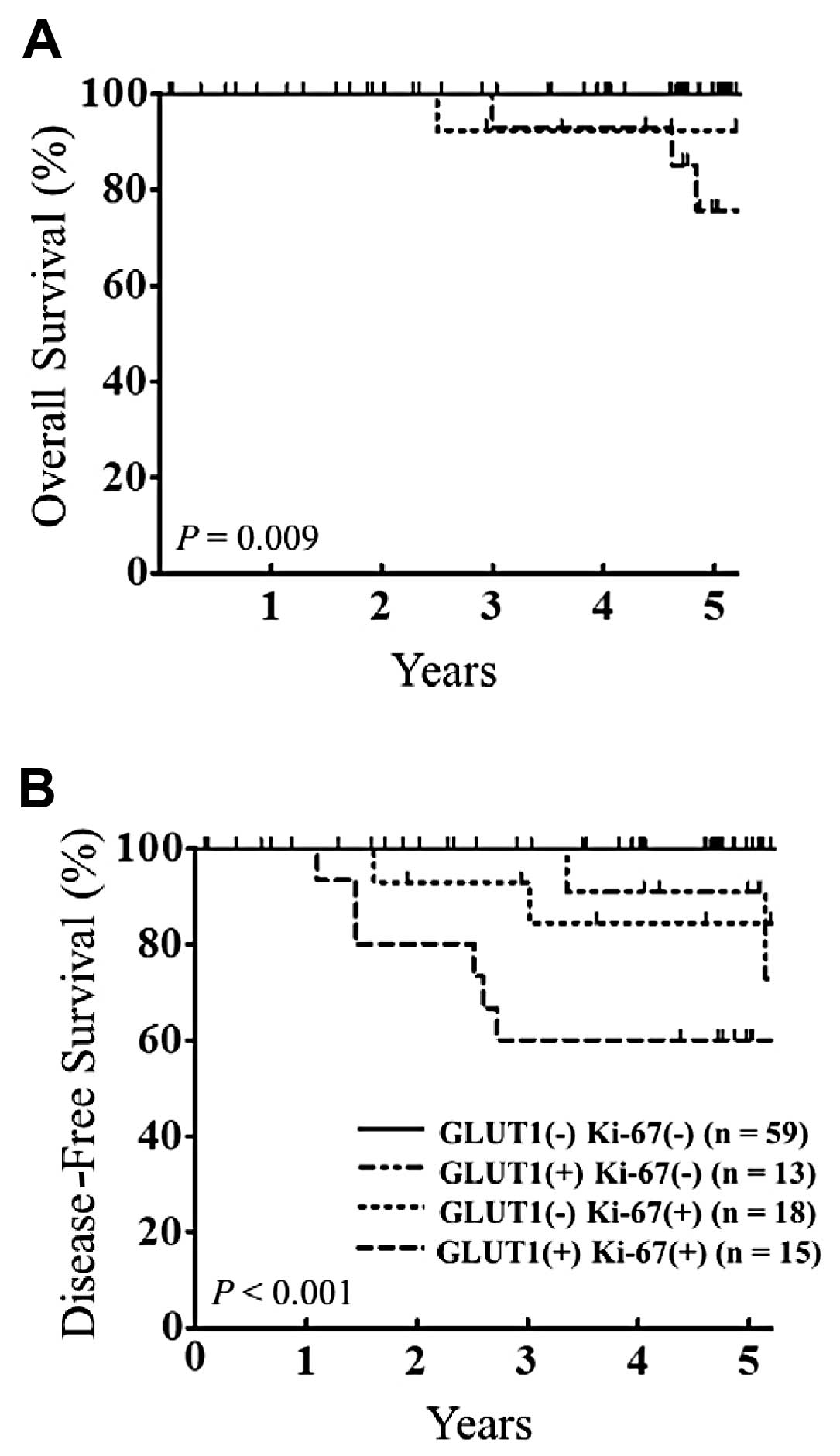
We also investigated the impact of the combined
effect of GLUT1 and Ki-67 expression on OS and DFS. We found that
patients with positive expression for both GLUT1 and Ki-67 had a
poorer clinical outcome than the other patients (OS, P=0.009 and
DFS, P<0.0001) (Fig. 2).
Discussion
In this study, we investigated the expression status
of GLUT1 and Ki-67 in early-stage cases of lung adenocarcinoma
classified according to a new international multidisciplinary
classification, the IASLC/ATS/ERS classification, and found that,
i) positive GLUT1 expression was significantly associated with a
wild-type EGFR and mutant KRAS status, ii) GLUT1
expression was independently associated with ‘invasive type’
adenocarcinomas, and iii) positive GLUT1 expression was correlated
with positive Ki-67 expression and double positive expression was
associated with a poor outcome.
We examined the EGFR and KRAS
mutations, which are key somatic mutations of lung adenocarcinoma,
in the patient population and analyzed the correlations between the
mutation status and GLUT1 expression, Ki-67 expression, and
histological features. The frequent overexpression of GLUT1 in
NSCLC has been reported in patients with wild-type EGFR and
mutant KRAS(38). In the
present study, we found that positive GLUT1 expression was
frequently observed in lung adenocarcinomas without EGFR
mutation and in those with KRAS mutation. Although the
association between the GLUT1 expression level and the initiation
of these mutations is unknown, low glucose environments, which
promote GLUT1 expression, have been reported as a driving force
underlying the development of KRAS mutations during colorectal
tumorigenesis (39).
Both GLUT1 and Ki-67 are known to be associated with
tumor invasiveness and proliferation (40). Moreover, Noguchi et
al(41) reported that small
lung adenocarcinomas (with a diameter equal to 2 cm or less) with
pure or minimally invasive ‘BAC’ (Noguchi types A and B) had a
5-year DFS of 100% after complete resection as confirmed by
subsequent reports (42,43). The IASLC/ATS/ERS classification has
also indicated that patients with AIS or MIA have a 100% or nearly
100% DFS. In this study, we classified AIS and MIA as ‘non-invasive
type’ adenocarcinomas and compared them with ‘invasive type’
adenocarcinomas; as a result, positive GLUT1 expression was found
to be the only independent factor associated with ‘invasive’
adenocarcinoma. High expression levels of GLUT1 have previously
been reported as a significant predictor of a poor outcome in 47
cases of stage IA and IB adenocarcinoma (16). In the present study, we demonstrated
that GLUT1 and Ki-67 were the most significant factors associated
with a poor clinical outcome with regard to the DFS and OS,
respectively. Of interest, GLUT1 and Ki-67 double-positive cases
had the poorest DFS and OS time, suggesting that this population
exhibited a high degree of biological malignancy. These findings
also suggest that these markers may be useful for predicting the
recurrence of disease after the complete resection of early-stage
lung adenocarcinoma and may be useful for the selection of patients
requiring adjuvant therapy.
In conclusion, positive GLUT1 expression is
frequently observed in ‘invasive type’ early-stage lung
adenocarcinoma, as classified according to the IASLC/ATS/ERS
classification. Our results strongly suggest that GLUT1, together
with Ki-67, plays an important role in the acquisition of
biological malignant potential in early-stage lung
adenocarcinoma.
Acknowledgements
The authors thank Ayako Isobe (General Thoracic,
Breast and Endocrinological Surgery, Okayama University Graduate
School of Medicine) for preparing the pathological materials. The
authors also thank the Central Research Laboratory, Okayama
University Medical School, for their technical support with the
immunohistochemical staining.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
3
|
Devesa SS, Bray F, Vizcaino AP and Parkin
DM: International lung cancer trends by histologic type:
male:female differences diminishing and adenocarcinoma rates
rising. Int J Cancer. 117:294–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Rens MT, de la Riviere AB, Elbers HR
and van Den Bosch JM: Prognostic assessment of 2,361 patients who
underwent pulmonary resection for non-small cell lung cancer, stage
I, II, and IIIA. Chest. 117:374–379. 2000.PubMed/NCBI
|
|
5
|
Koike T, Kimura N, Miyazaki K, et al:
Hypoxia induces adhesion molecules on cancer cells: A missing link
between Warburg effect and induction of selectin-ligand
carbohydrates. Proc Natl Acad Sci USA. 101:8132–8137. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wood IS and Trayhurn P: Glucose
transporters (GLUT and SGLT): expanded families of sugar transport
proteins. Br J Nutr. 89:3–9. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kalir T, Wang BY, Goldfischer M, et al:
Immunohistochemical staining of GLUT1 in benign, borderline, and
malignant ovarian epithelia. Cancer. 94:1078–1082. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carvalho KC, Cunha IW, Rocha RM, et al:
GLUT1 expression in malignant tumors and its use as an
immunodiagnostic marker. Clinics (São Paulo). 66:965–972.
2011.PubMed/NCBI
|
|
9
|
Koukourakis MI, Giatromanolaki A,
Bougioukas G and Sivridis E: Lung cancer: a comparative study of
metabolism related protein expression in cancer cells and tumor
associated stroma. Cancer Biol Ther. 6:1476–1479. 2007.PubMed/NCBI
|
|
10
|
Wang K, Sun Y and Wang T: The prognostic
significance of GLUT1 expression in stage I and II NSCLC. Zhongguo
Fei Ai Za Zhi. 5:451–453. 2002.(In Chinese).
|
|
11
|
Brown DC and Gatter KC: Ki67 protein: the
immaculate deception? Histopathology. 40:2–11. 2002. View Article : Google Scholar
|
|
12
|
Jalava P, Kuopio T, Juntti-Patinen L,
Kotkansalo T, Kronqvist P and Collan Y: Ki67 immunohistochemistry:
a valuable marker in prognostication but with a risk of
misclassification: proliferation subgroups formed based on Ki67
immunoreactivity and standardized mitotic index. Histopathology.
48:674–682. 2006. View Article : Google Scholar
|
|
13
|
Scholzen T and Gerdes J: The Ki-67
protein: from the known and the unknown. J Cell Physiol.
182:311–322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goldhirsch A, Ingle JN, Gelber RD, Coates
AS, Thurlimann B and Senn HJ: Thresholds for therapies: highlights
of the St Gallen International Expert Consensus on the primary
therapy of early breast cancer 2009. Ann Oncol. 20:1319–1329. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Klintman M, Bendahl PO, Grabau D, Lovgren
K, Malmstrom P and Ferno M: The prognostic value of Ki67 is
dependent on estrogen receptor status and histological grade in
premenopausal patients with node-negative breast cancer. Mod
Pathol. 23:251–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Minami K, Saito Y, Imamura H and Okamura
A: Prognostic significance of p53, Ki-67, VEGF and Glut-1 in
resected stage I adenocarcinoma of the lung. Lung Cancer. 38:51–57.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tungekar MF, Gatter KC, Dunnill MS and
Mason DY: Ki-67 immunostaining and survival in operable lung
cancer. Histopathology. 19:545–550. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Simony J, Pujol JL, Radal M, Ursule E,
Michel FB and Pujol H: In situ evaluation of growth fraction
determined by monoclonal antibody Ki-67 and ploidy in surgically
resected non-small cell lung cancers. Cancer Res. 50:4382–4387.
1990.PubMed/NCBI
|
|
19
|
Mehdi SA, Etzell JE, Newman NB, Weidner N,
Kohman LJ and Graziano SL: Prognostic significance of Ki-67
immunostaining and symptoms in resected stage I and II non-small
cell lung cancer. Lung Cancer. 20:99–108. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cappuzzo F, Hirsch FR, Rossi E, et al:
Epidermal growth factor receptor gene and protein and gefitinib
sensitivity in non-small-cell lung cancer. J Natl Cancer Inst.
97:643–655. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ichihara S, Toyooka S, Fujiwara Y, et al:
The impact of epidermal growth factor receptor gene status on
gefitinib-treated Japanese patients with non-small-cell lung
cancer. Int J Cancer. 120:1239–1247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Toyooka S, Mitsudomi T, Soh J, et al:
Molecular oncology of lung cancer. Gen Thorac Cardiovasc Surg.
59:527–537. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Travis WD, Brambilla E, Noguchi M, et al:
International association for the study of lung cancer/american
thoracic society/european respiratory society international
multidisciplinary classification of lung adenocarcinoma. J Thorac
Oncol. 6:244–285. 2011. View Article : Google Scholar
|
|
24
|
Travis WD, Brambilla E, Muller-Hermelink
HK and Harris CC: World Health Organization Classification of
Tumours. Pathology and genetics of tumours of the lung, pleura,
thymus and heart. IARC Press; Lyon: 2004
|
|
25
|
Rami-Porta R, Crowley JJ and Goldstraw P:
The revised TNM staging system for lung cancer. Ann Thorac
Cardiovasc Surg. 15:4–9. 2009.PubMed/NCBI
|
|
26
|
Jun YJ, Jang SM, Han HL, Lee KH, Jang KS
and Paik SS: Clinicopathologic significance of GLUT1 expression and
its correlation with Apaf-1 in colorectal adenocarcinomas. World J
Gastroenterol. 17:1866–1873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Parente P, Coli A, Massi G, Mangoni A,
Fabrizi MM and Bigotti G: Immunohistochemical expression of the
glucose transporters Glut-1 and Glut-3 in human malignant melanomas
and benign melanocytic lesions. J Exp Clin Cancer Res. 27:342008.
View Article : Google Scholar
|
|
28
|
Yerushalmi R, Woods R, Ravdin PM, Hayes MM
and Gelmon KA: Ki67 in breast cancer: prognostic and predictive
potential. Lancet Oncol. 11:174–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dowsett M, Nielsen TO, A’Hern R, et al:
Assessment of Ki67 in Breast Cancer: recommendations from the
international Ki67 in Breast Cancer working group. J Natl Cancer
Inst. 103:1656–1664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thurlimann B and Senn HJ: Strategies for subtypes - dealing
with the diversity of breast cancer: highlights of the St. Gallen
International Expert Consensus on the Primary Therapy of Early
Breast Cancer 2011. Ann Oncol. 22:1736–1747. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Colozza M, Sidoni A and Piccart-Gebhart M:
Value of Ki67 in breast cancer: the debate is still open. Lancet
Oncol. 11:414–415. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jonat W and Arnold N: Is the Ki-67
labelling index ready for clinical use? Ann Oncol. 22:500–502.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Asano H, Toyooka S, Tokumo M, et al:
Detection of EGFR gene mutation in lung cancer by mutant-enriched
polymerase chain reaction assay. Clin Cancer Res. 12:43–48. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tokumo M, Toyooka S, Kiura K, et al: The
relationship between epidermal growth factor receptor mutations and
clinicopathologic features in non-small cell lung cancers. Clin
Cancer Res. 11:1167–1173. 2005.PubMed/NCBI
|
|
35
|
Suehisa H, Toyooka S, Hotta K, et al:
Epidermal growth factor receptor mutation status and adjuvant
chemotherapy with uracil-tegafur for adenocarcinoma of the lung. J
Clin Oncol. 25:3952–3957. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Watanabe S, Watanabe T, Arai K, Kasai T,
Haratake J and Urayama H: Results of wedge resection for focal
bronchiolo-alveolar carcinoma showing pure ground-glass attenuation
on computed tomography. Ann Thorac Surg. 73:1071–1075. 2002.
View Article : Google Scholar
|
|
37
|
Yamada S and Kohno T: Video-assisted
thoracic surgery for pure ground-glass opacities 2 cm or less in
diameter. Ann Thorac Surg. 77:1911–1915. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sasaki H, Shitara M, Yokota K, et al:
Overexpression of GLUT1 correlates with Kras mutations in lung
carcinomas. Mol Med Rep. 5:599–602. 2012.PubMed/NCBI
|
|
39
|
Yun J, Rago C, Cheong I, et al: Glucose
deprivation contributes to the development of KRAS pathway
mutations in tumor cells. Science. 325:1555–1559. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Furudoi A, Tanaka S, Haruma K, et al:
Clinical significance of human erythrocyte glucose transporter 1
expression at the deepest invasive site of advanced colorectal
carcinoma. Oncology. 60:162–169. 2001. View Article : Google Scholar
|
|
41
|
Noguchi M, Morikawa A, Kawasaki M, et al:
Small adenocarcinoma of the lung. Histologic characteristics and
prognosis. Cancer. 75:2844–2852. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yokose T, Suzuki K, Nagai K, Nishiwaki Y,
Sasaki S and Ochiai A: Favorable and unfavorable morphological
prognostic factors in peripheral adenocarcinoma of the lung 3 cm or
less in diameter. Lung Cancer. 29:179–188. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yoshida J, Nagai K, Yokose T, et al:
Limited resection trial for pulmonary ground-glass opacity nodules:
fifty-case experience. J Thorac Cardiovasc Surg. 129:991–996. 2005.
View Article : Google Scholar : PubMed/NCBI
|