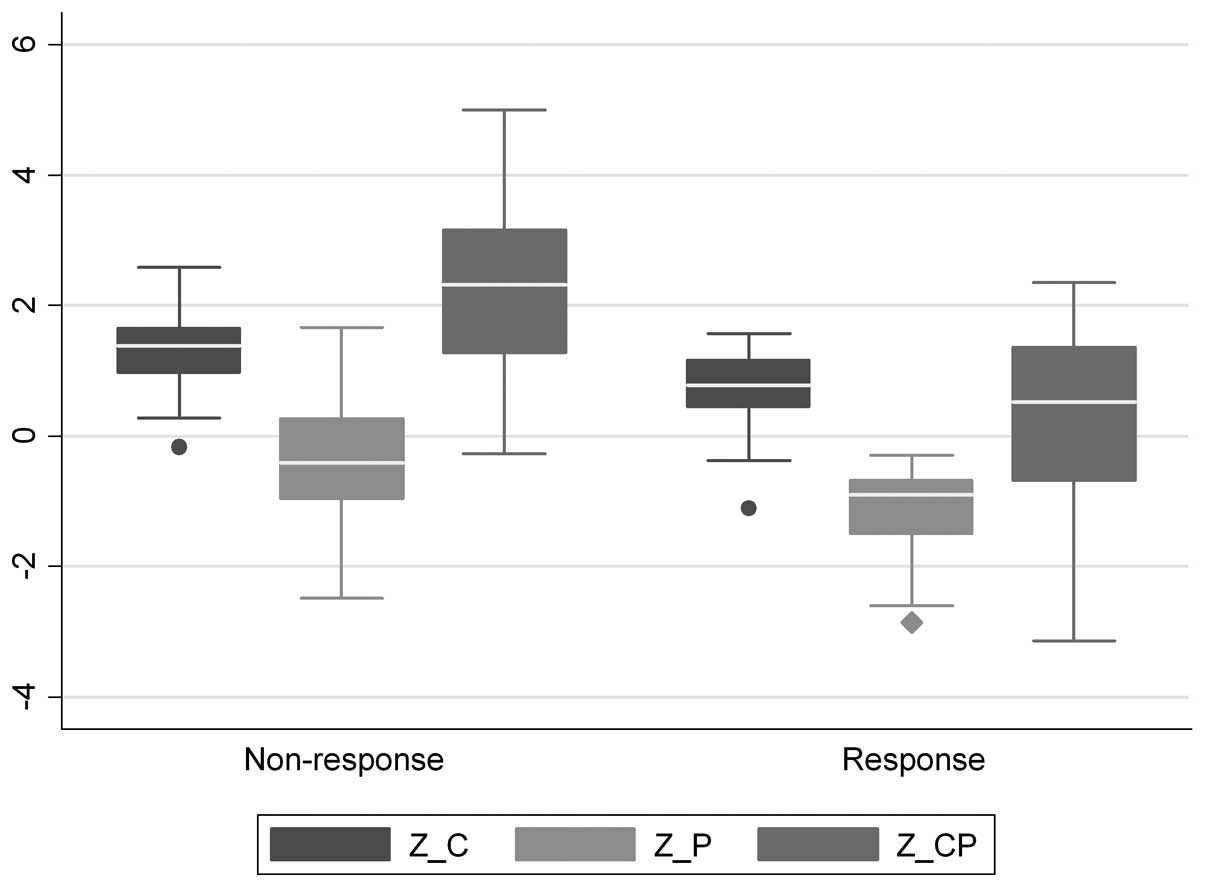
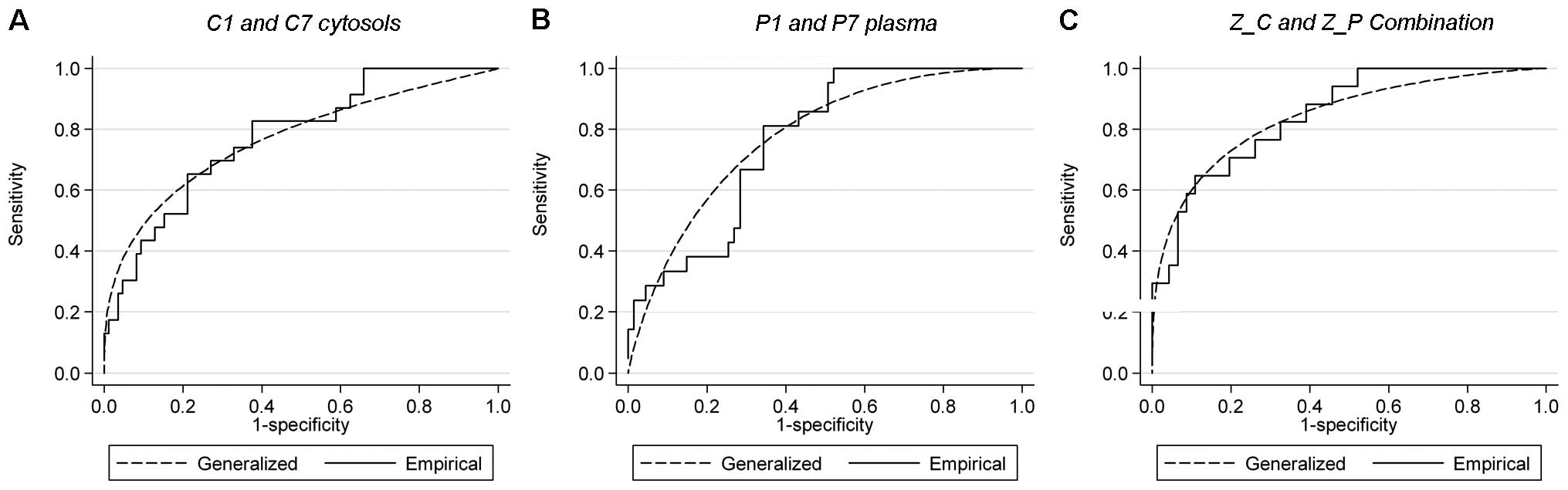
|
1
|
Greenlee RT, Murray T, Bolden S and Wingo
PA: Cancer statistics, 2000. CA Cancer J Clin. 50:7–33. 2000.
View Article : Google Scholar
|
|
2
|
Goldhirsch A, Wood WC, Gelber RD, Coates
AS, Thurlimann B and Senn HJ: Meeting highlights: updated
international expert consensus on the primary therapy of early
breast cancer. J Clin Oncol. 21:3357–3365. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roche H, Fumoleau P, Spielman M, et al:
Sequential adjuvant epirubicin-based and docetaxel chemotherapy for
node-positive breast cancer patients: the FNCLCC PACS 01 Trial. J
Clin Oncol. 24:5664–5671. 2006. View Article : Google Scholar
|
|
4
|
Vercoutter-Edouart AS, Lemoine J, Le
Bourhis X, et al: Proteomic analysis reveals that 14-3-3sigma is
down-regulated in human breast cancer cells. Cancer Res. 61:76–80.
2001.PubMed/NCBI
|
|
5
|
Adam BL, Qu Y, Davis JW, et al: Serum
protein fingerprinting coupled with a pattern-matching algorithm
distinguishes prostate cancer from benign prostate hyperplasia and
healthy men. Cancer Res. 62:3609–3614. 2002.
|
|
6
|
Petricoin EF, Ardekani AM, Hitt BA, et al:
Use of proteomic patterns in serum to identify ovarian cancer.
Lancet. 359:572–577. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Petricoin EF III, Ornstein DK, Paweletz
CP, et al: Serum proteomic patterns for detection of prostate
cancer. J Natl Cancer Inst. 94:1576–1578. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vlahou A, Laronga C, Wilson L, et al: A
novel approach toward development of a rapid blood test for breast
cancer. Clin Breast Cancer. 4:203–209. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koopmann J, Zhang Z, White N, et al: Serum
diagnosis of pancreatic adenocarcinoma using surface-enhanced laser
desorption and ionization mass spectrometry. Clin Cancer Res.
10:860–868. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wadsworth JT, Somers KD, Cazares LH, et
al: Serum protein profiles to identify head and neck cancer. Clin
Cancer Res. 10:1625–1632. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Z, Bast RC Jr, Yu Y, et al: Three
biomarkers identified from serum proteomic analysis for the
detection of early stage ovarian cancer. Cancer Res. 64:5882–5890.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fung ET, Yip TT, Lomas L, et al:
Classification of cancer types by measuring variants of host
response proteins using SELDI serum assays. Int J Cancer.
115:783–789. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fung ET, Thulasiraman V, Weinberger SR and
Dalmasso EA: Protein biochips for differential profiling. Curr Opin
Biotechnol. 12:65–69. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hodgkinson VC, Eagle GL, Drew PJ, Lind MJ
and Cawkwell L: Biomarkers of chemotherapy resistance in breast
cancer identified by proteomics: current status. Cancer Lett.
294:13–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He J, Shen D, Chung DU, Saxton RE,
Whitelegge JP, Faull KF and Chang HR: Tumor proteomic profiling
predicts the susceptibility of breast cancer to chemotherapy. Int J
Oncol. 35:683–692. 2009.PubMed/NCBI
|
|
16
|
Sataloff DM, Mason BA, Prestipino AJ,
Seinige VL, Lieber CP and Baloch Z: Pathologic response to
induction chemotherapy in locally advanced carcinoma of the breast:
a determinant of outcome. J Am Coll Surg. 180:297–306.
1995.PubMed/NCBI
|
|
17
|
Bradford MM: Rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kramar A, Farraggi D, Fortuné A and Reiser
B: mROC: a computer program for combining tumour markers in
predicting disease states. Comput Methods Programs Biomed.
66:199–207. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kozak KR, Amneus MW, Pusey SM, et al:
Identification of biomarkers for ovarian cancer using strong
anion-exchange ProteinChips: potential use in diagnosis and
prognosis. Proc Natl Acad Sci USA. 100:12343–12348. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gonçalves A, Esterni B, Bertucci F, et al:
Postoperative serum proteomic profiles may predict metastatic
relapse in high-riskprimary breast cancer patients receiving
adjuvant chemotherapy. Oncogene. 25:981–989. 2006.PubMed/NCBI
|
|
21
|
Diamandis EP: Analysis of serum proteomic
patterns for early cancer diagnosis: drawing attention to potential
problems. J Natl Cancer Inst. 96:353–356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ricolleau G, Charbonnel C, Lodé L, et al:
Surface-enhanced laser desorption/ionization time of flight mass
spectrometry protein profiling identifies ubiquitin and ferritin
light chain as prognostic biomarkers in node-negative breast cancer
tumors. Proteomics. 6:1963–1975. 2006. View Article : Google Scholar
|
|
23
|
Pusztai L, Gregory BW, Baggerly KA, et al:
Pharmacoproteomic analysis of prechemotherapy and postchemotherapy
plasma samples from patients receiving neoadjuvant or adjuvant
chemotherapy for breast carcinoma. Cancer. 100:1814–1822. 2004.
View Article : Google Scholar
|
|
24
|
Zhang K, Yuan K, Wu H, et al:
Identification of potential markers related to neoadjuvant
chemotherapy sensitivity of breast cancer by SELDI-TOF MS. Appl
Biochem Biotechnol. 166:753–763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dang CT and Hudis C: Preoperative
chemotherapy for operable breast cancer. Diseases of the Breast.
Harris JR, Lippman ME, Morrow M and Osborne CK:
WoltersKluwer/Lippincott Williams &Williams; Philadelphia: pp.
715–723. 2010
|
|
26
|
Mailliez A, Baranzelli MC, Giard S, et al:
Is there a reliable method to assess the complete pathologic
response on the tumor after neo-adjuvant chemotherapy in
inflammatory breast cancer toward recommendations for the
pathologic process? Experience in 56 patients treated in a single
institution. Breast J. 16:464–471. 2010. View Article : Google Scholar
|
|
27
|
Penault-Llorca F, Abrial C, Raolelfils I,
et al: Changes and predictive and prognostic value of the mitotic
index, Ki-67, cyclin D1, and cyclo-oxygenase-2 in 710 operable
breast cancer patients treated with neoadjuvant chemotherapy.
Oncologist. 13:1235–1245. 2008. View Article : Google Scholar
|
|
28
|
Liedtke C, Mazouni C, Hess KR, et al:
Response to neoadjuvant therapy and long-term survival in patients
with triple-negative breast cancer. J Clin Oncol. 26:1275–1281.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Katz A, Saad ED, Porter P and Pusztai L:
Primary systemic chemotherapy of invasive lobular carcinoma of the
breast. Lancet Oncol. 8:55–62. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liedtke C, Hatzis C, Symmans WF, et al:
Genomic grade index is associated with response to chemotherapy in
patients with breast cancer. J Clin Oncol. 27:3185–3191. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Straver ME, Glas AM, Hannemann J, et al:
The 70-gene signature as a response predictor for neoadjuvant
chemotherapy in breast cancer. Breast Cancer Res Treat.
119:551–558. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tabchy A, Valero V, Vidaurre T, et al:
Evaluation of a 30-gene paclitaxel, fluorouracil, doxorubicin, and
cyclophosphamide chemotherapy response predictor in a multicenter
randomized trial in breast cancer. Clin Cancer Res. 16:5351–5361.
2010. View Article : Google Scholar
|
|
33
|
Lee JK, Coutant C, Kim YC, et al:
Prospective comparison of clinical and genomic multivariate
predictors of response to neoadjuvant chemotherapy in breast
cancer. Clin Cancer Res. 16:711–718. 2010. View Article : Google Scholar : PubMed/NCBI
|