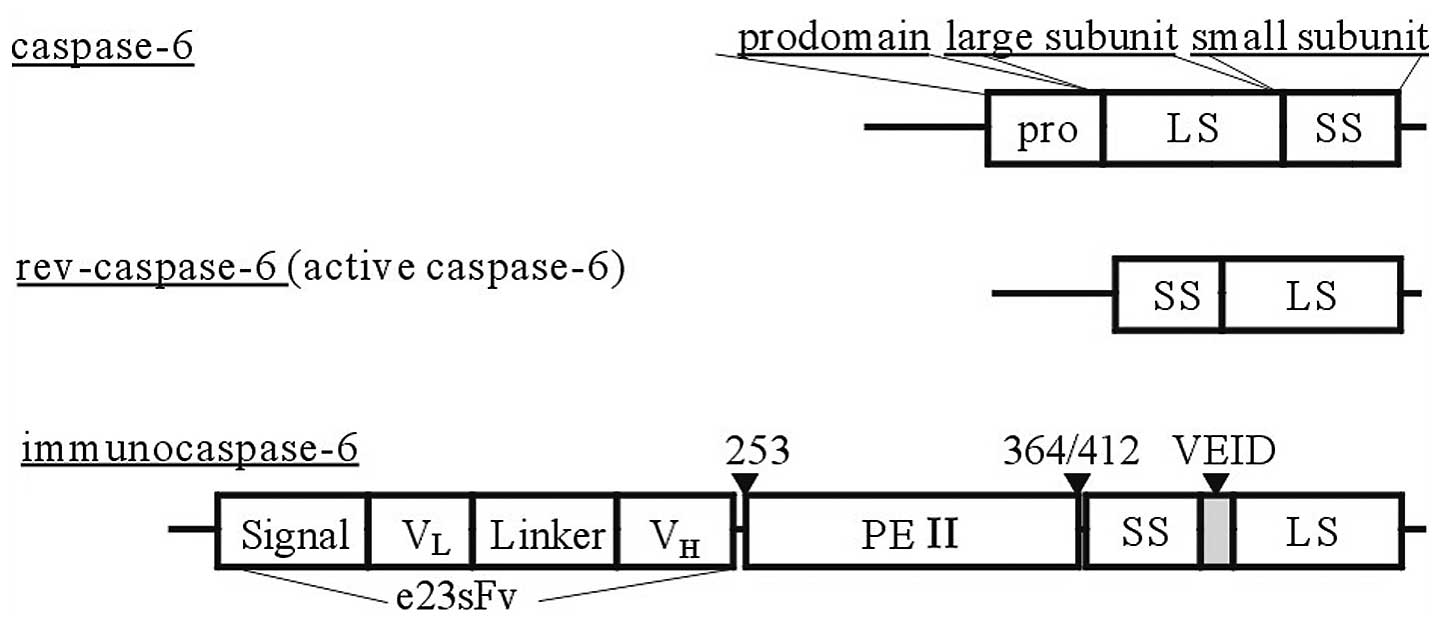
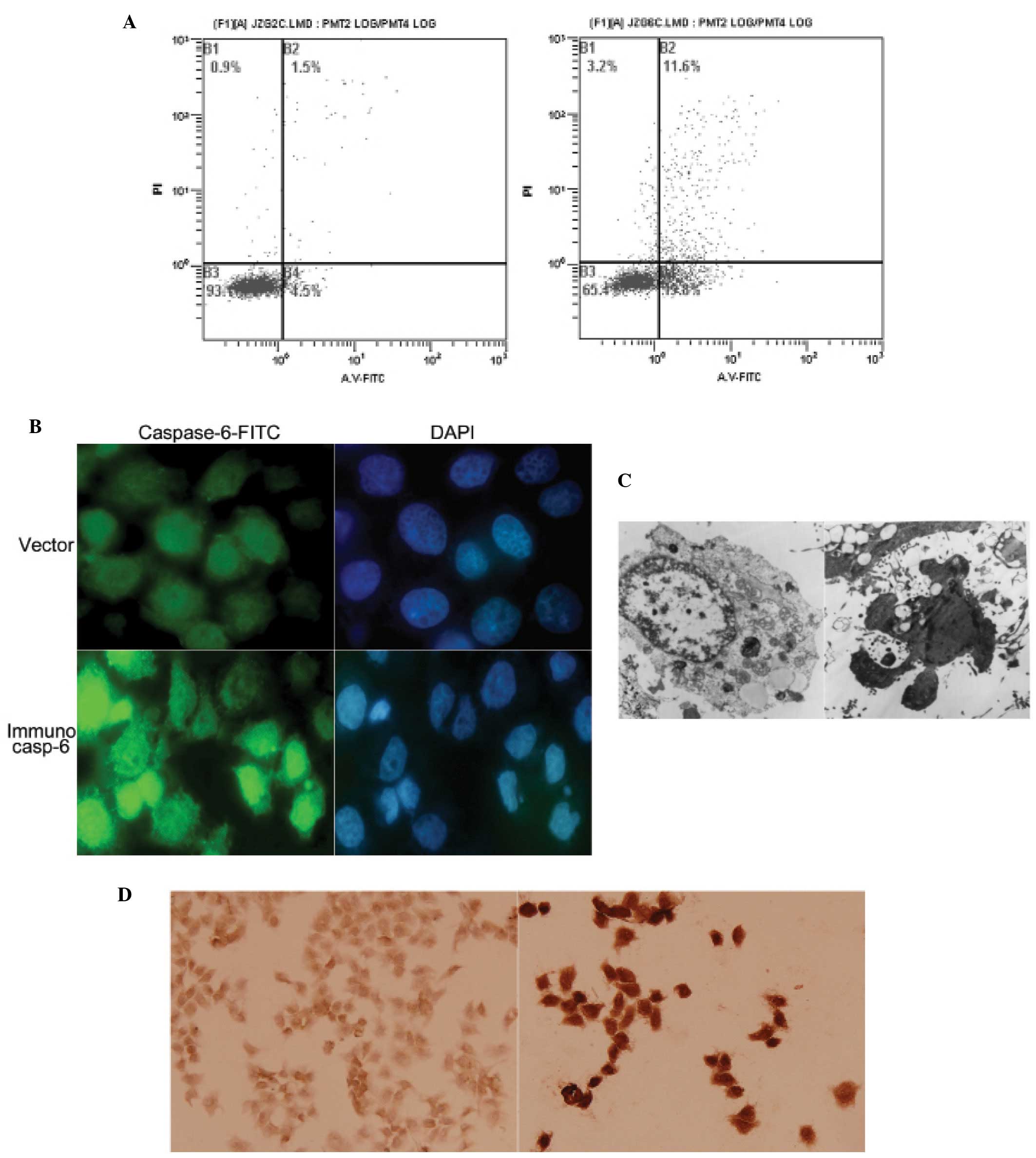
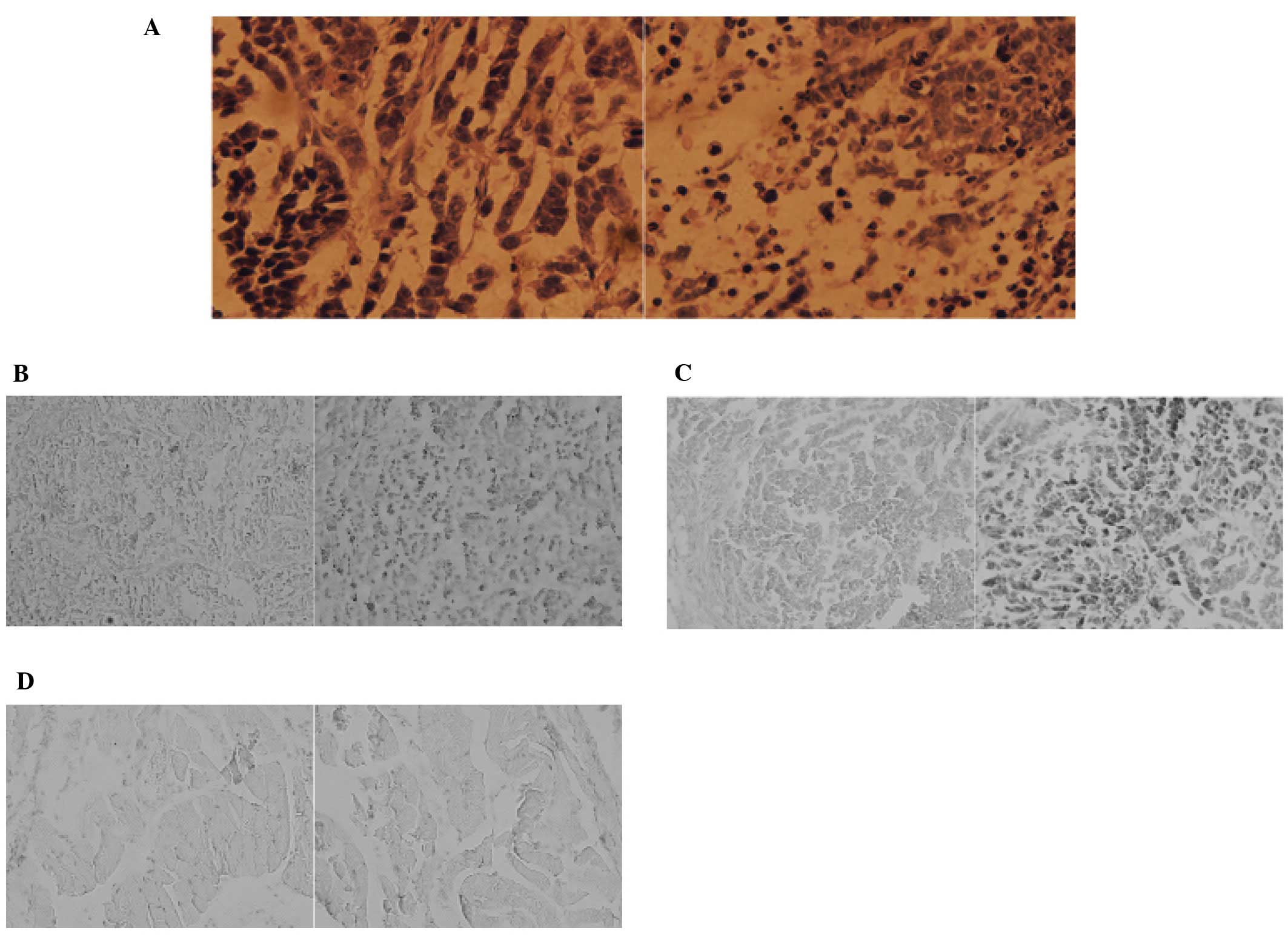
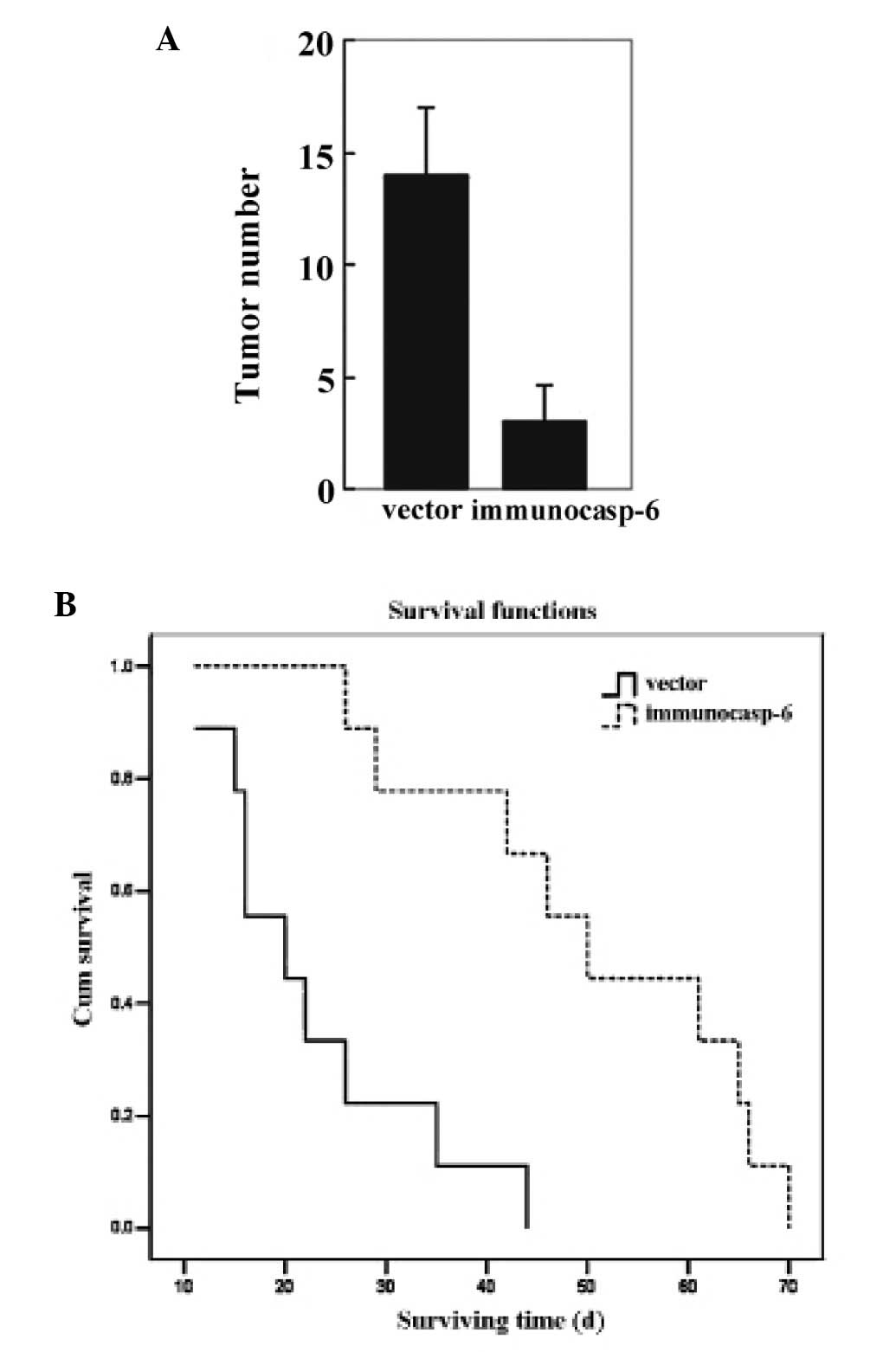
|
1
|
Lupu R, Colomer R, Kannan B and Lippman
ME: Character-ization of a growth factor that binds exclusively to
the erbB-2 receptor and induces cellular responses. Proc Natl Acad
Sci USA. 89:2287–2291. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen JS, Lan K and Hung MC: Strategies to
target HER2/neu overexpression for cancer therapy. Drug Resist
Updat. 6:129–136. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Slamon DJ, Godolphin W, Jones LA, et al:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Semba K, Kamata N, Toyoshima K and
Yamamoto T: A v-erbB-related protooncogene, c-erbB-2, is distinct
from the c-erbB-1/epidermal growth factor-receptor gene and is
amplified in a human salivary gland adenocarcinoma. Proc Natl Acad
Sci USA. 82:6497–6501. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fukushige S, Matsubara K, Yoshida M, et
al: Localization of a novel v-erbB-related gene, c-erbB-2, on human
chromosome 17 and its amplification in a gastric cancer cell line.
Mol Cell Biol. 6:955–958. 1986.PubMed/NCBI
|
|
6
|
Shan LQ, Ma S, Qiu XC, et al: A novel
recombinant immuno-tBid with a furin site effectively suppresses
the growth of HER2-positive osteosarcoma cells in vitro.
Oncol Rep. 25:325–331. 2011.PubMed/NCBI
|
|
7
|
Scotlandi K, Manara MC, Hattinger CM, et
al: Prognostic and therapeutic relevance of HER2 expression in
osteosarcoma and Ewing’s sarcoma. Eur J Cancer. 41:1349–1361.
2005.PubMed/NCBI
|
|
8
|
Gorlick R, Huvos AG, Heller G, et al:
Expression of HER2/erbB-2 correlates with survival in osteosarcoma.
J Clin Oncol. 17:2781–2788. 1999.PubMed/NCBI
|
|
9
|
Akatsuka T, Wada T, Kokai Y, et al: ErbB2
expression is correlated with increased survival of patients with
osteosarcoma. Cancer. 94:1397–1404. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qiu XC, Xu YM, Wang F, et al: Single-chain
antibody/activated BID chimeric protein effectively suppresses
HER2-positive tumor growth. Mol Cancer Ther. 7:1890–1899. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Slee EA, Adrain C and Martin SJ:
Executioner caspase-3, -6, and -7 perform distinct, non-redundant
roles during the demolition phase of apoptosis. J Biol Chem.
276:7320–7326. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ruchaud S, Korfali N, Villa P, et al:
Caspase-6 gene disruption reveals a requirement for lamin A
cleavage in apoptotic chromatin condensation. EMBO J. 21:1967–1977.
2002. View Article : Google Scholar
|
|
14
|
Srinivasula SM, Ahmad M, MacFarlane M, et
al: Generation of constitutively active recombinant caspases-3 and
-6 by rearrangement of their subunits. J Biol Chem.
273:10107–10111. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen SY, Yang AG, Chen JD, et al: Potent
antitumour activity of a new class of tumour-specific killer cells.
Nature. 385:78–80. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Batra JK, Kasprzyk PG, Bird RE, Pastan I
and King CR: Recombinant anti-erbB2 immunotoxins containing
Pseudomonas exotoxin. Proc Natl Acad Sci USA. 89:5867–5871.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hwang J, Fitzgerald DJ, Adhya S and Pastan
I: Functional domains of Pseudomonas exotoxin identified by
deletion analysis of the gene expressed in E. coli. Cell.
48:129–136. 1987.
|
|
18
|
Siegall CB, Chaudhary VK, FitzGerald DJ
and Pastan I: Functional analysis of domains II, Ib, and III of
Pseudomonas exotoxin. J Biol Chem. 264:14256–14261.
1989.PubMed/NCBI
|
|
19
|
Jinno Y, Ogata M, Chaudhary VK, et al:
Domain II mutants of Pseudomonas exotoxin deficient in
translocation. J Biol Chem. 264:15953–15959. 1989.PubMed/NCBI
|
|
20
|
Siegall CB, Ogata M, Pastan I and
FitzGerald DJ: Analysis of sequences in domain II of
Pseudomonas exotoxin A which mediate translocation.
Biochemistry. 30:7154–7159. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu YM, Wang LF, Jia LT, et al: A caspase-6
and anti-human epidermal growth factor receptor-2 (HER2) antibody
chimeric molecule suppresses the growth of HER2-overexpressing
tumors. J Immunol. 173:61–67. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pastan I, Chaudhary V and FitzGerald DJ:
Recombinant toxins as novel therapeutic agents. Annu Rev Biochem.
61:331–354. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Springer CJ and Niculescu-Duvaz I:
Prodrug-activating systems in suicide gene therapy. J Clin Invest.
105:1161–1167. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yazawa K, Fisher WE and Brunicardi FC:
Current progress in suicide gene therapy for cancer. World J Surg.
26:783–789. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Horowitz J: Adenovirus-mediated p53 gene
therapy: overview of preclinical studies and potential clinical
applications. Curr Opin Mol Ther. 1:500–509. 1999.PubMed/NCBI
|
|
26
|
Jia LT, Zhang LH, Yu CJ, et al: Specific
tumoricidal activity of a secreted proapoptotic protein consisting
of HER2 antibody and constitutively active caspase-3. Cancer Res.
63:3257–3262. 2003.
|
|
27
|
Zhao J, Zhang LH, Jia LT, et al: Secreted
antibody/granzyme B fusion protein stimulates selective killing of
HER2-overexpressing tumor cells. J Biol Chem. 279:21343–21348.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu CJ, Jia LT, Meng YL, et al: Selective
proapoptotic activity of a secreted recombinant antibody/AIF fusion
protein in carcinomas overexpressing HER2. Gene Ther. 13:313–320.
2006. View Article : Google Scholar : PubMed/NCBI
|