Introduction
The human epidermal growth factor receptor-2 (HER2),
a member of the epithelial growth factor receptor family,
transduces cell signaling and plays key roles in cell
differentiation, adhesion, and motility (1). Sufficient evidence has suggested that
patients with HER2-overexpressing tumors exhibit a reduced response
to conventional treatments (2). The
HER2 protein is reportedly overexpressed in several human malignant
tumor, including human breast and ovarian cancer (3), salivary gland adenocarcinoma (4), gastric cancer (5) and osteosarcoma (6-9). Since
it is overexpressed in tumor cells but is not detected in normal
cells, HER2 is an ideal target molecular for cancer gene therapy to
exploit differences at the molecular level between normal and
malignant cells (10).
Caspases are vital elements in transferring
apoptotic signals and executing apoptosis in mammalian cells
(11). Caspase-6 is one of
effective caspases during the cell apoptotic program (12). Activation of caspase-6 induces
apoptosis by cleaving lamin A and other substrates (13). Unlike its wild-type zymogen
counterpart, active caspase-6 constructed with subunits in reverse
order, is capable of autocatalytic processing in vitro
independent of apoptotic signals, and can induce apoptosis of tumor
cells, which thereby makes it an attractive candidate for gene
therapy (14).
As a well-recognized Ab, e23sFv, derived from a
mouse mAb against human HER2, has been confirmed to bind the
extracellular domain of HER2 protein with high affinity and to be
internalized by endocytosis (15,16).
The highly specific antibody to antigen suggests that we can
construct a fusion gene, immunocasp-6, consisting of NH2-terminal
leader sequence to promote secretion of the recombinant
immunocasp-6 fused with an anti-HER2 single-chain Ab, the
translocation domain (domain II) of Pseudomonas exotoxin A
(PEA) and an active caspase-6, to specifically and efficiently
suppress the HER2 overexpressing tumors. PEA is a single-chain
toxin consisting of three major domains (I, II and III) responsible
for binding of the molecule to target cells, translocation of the
molecule to the cytosol, and the induction of cell death,
respectively (17). Domain II of
PEA has been reported to efficiently transfer the cellular toxicity
domain to the cytoplasm (18-20).
By replacing the cellular toxicity domain of PEA with active
caspase-6, we sought to translocate the caspase into tumor cells in
which it would induce tumor cell apoptosis. Even though this novel
immunocasp-6 has been proven to be effective in inducing apoptosis
in the HER2-overexpressing human breast tumor cell line, SKBR-3,
their effects on human osteosarcoma is still unclear. Thus the
purpose of the present study is to extend our immunocasp-6 strategy
to the treatment of osteosarcoma in vitro as well as in
vivo and to verify that the immunocasp-6 can specifically and
efficiently suppress the HER2-overexpressing tumors.
Materials and methods
Plasmid and DNA construct
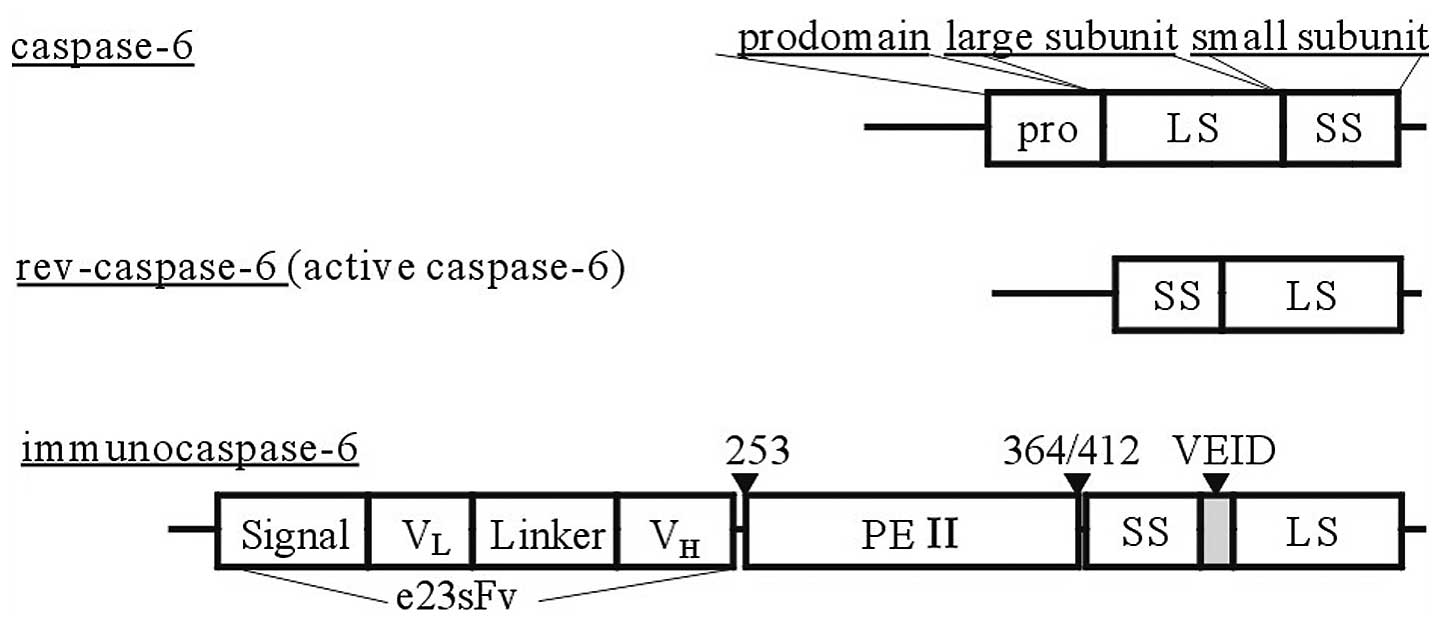
Recombinant immunocasp-6 was generated by sequential
fusion of the genes of a signal peptide (Met-Lys-His-Leu-Trp-Phe-
Phe-Leu-Leu-Leu-Val-Ala-Ala-Pro-Arg-Trp-Val-Leu-Ser-) consisting of
a single chain HER2 antibody (e23sFv), a Pseudomonas
exotoxin A (PEA) translocation domain (from aa 253 to 412) and an
active caspase-6 (Fig. 1). The
immunocasp-6 was cloned into a pCMV plasmid, namely
pCMV-immunocasp-6.
Cell culture and transfection
Human osteosarcoma cell line SOSP-9607-E10, with
relatively high metastatic potential, was derived from a
17-year-old male patient who had been diagnosed of tibial
osteosarcoma and underwent osteotomy and established from these
cells by continuous in vitro cultivation for over 120
transfer generations in one year. SOSP-9607-E10 cells were
maintained in DMEM or RPMI-1640 (Invitrogen) supplemented with 10%
fetal bovine serum (FBS) and 4 mmol/l L-glutamine. At 24 h before
transfection, cells were seeded in 12 or 96-well plates at
1×105 or 5×103 cells per well.
pCMV-immunocasp-6 or pCMV vector alone, 1 μg, encapsulated by 2 μl
Lipofectamine 2000 (Invitrogen) were mixed, incubated for 20 min at
room temperature to form DNA-liposome mixture. Then the mixture was
administered to cells and incubated in a humidified incubator at
37°C with 5% CO2 for 6 h, then the medium was removed
and cells were resuspended in complete medium.
Cell viability assay
Viability of the transiently transfected cells was
tested by using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazo-lium bromide (MTT)
assay. Briefly, after the cells adhered to the low lamber of
96-well plates, the cells were divided randomly into three groups,
namely the mock group, the control group and the immunocasp-6
group, and transfected with pCMV-immunocasp-6 or pCMV vector.
Thereafter, the cells were cultured in 96-well plates for 12 to 96
h, then incubated with 20 μl of 1.5 mg/ml MTT for 4 h. After that,
the cells were treated with 150 μl DMSO. OD at A490 nm were
determined using the Sunrise microplate reader (Tecan). Each assay
was performed in triplicate on at least three independent
occasions.
Flow cytometry assay for detection of
apoptosis
SOSP-9607-E10 cells were seeded at a density of
1×105 cells per well on slides in 12-well Costar
transwell plates, and transfected with pCMV-immunocasp-6 or pCMV
vector when cell number was at a density of 3×105 cells
per well. After 48 h of transfection, cells in the lower chamber
were collected, stained with Annexin V-FITC/PI following standard
procedures and finally analyzed by FCM.
Immunofluorescence
After transfection SOSP-9607-E10 cells were
incubated in complete medium for an additional 48 h. Then, the
cells in the lower chamber were fixed in paraformaldehyde solution
(4% in phosphate buffered saline (PBS), pH 7.4), permeabilized with
PBS containing 0.1% Triton X-100, and blocked with 2% normal rabbit
serum. Then the cells were stained with antibodies recognizing
caspase-6 (C20, 1:200; Santa Cruz Biotechnologies) as the primary
antibodies, with biotin-linked anti-goat IgG (1:100; Santa Cruz
Biotechnologies) and FITC-linked anti-rabbit IgG (1:100; Sigma) as
the secondary antibodies. The staining was examined using a
fluorescence microscope (Japanese Olympus Co.).
Electronic microscopy assay
SOSP-9607-E10 cells were harvested 48 h after
transfection, and then fixed in 2.5% glutaraldehyde, dehydrated and
embedded to observe morphologic change with a transmission electron
microscope.
Immunohistochemistry assay
The transfected cells were cultured on coverslips as
mention above, and then fixed with a freshly prepared
paraformaldehyde solution for 30 min at room temperature, and
permeabilized with 0.1% Triton X-100 for 15 min on ice.
Xenograft tumors and muscle tissues were fixed in
paraformaldehyde solution and embedded in paraffin after treatment,
paraffin-embedded tissue sections were dewaxed, hydrated, and
incubated in 0.3% methanol-H2O2 for 20 min to
remove endogenous peroxidase. Next, they were dried and blocked for
1 h with the appropriate serum in a humidified chamber. Primary
antibody was added overnight at 4°C.
The samples were probed with primary
antibody-recognizing caspase-6 (C20, 1:200; Santa Cruz
Biotechnologies), followed by biotin-linked antirabbit IgG (1:100;
Santa Cruz Biotechnology) as the secondary antibody and then
processed with the Vectastain Elite ABC kit per the manufacturer’s
instructions prior to digital photography under an Olympus Eclipse
E600 microscope with a Spot RT slider camera and imaging
software.
TUNEL staining
TUNEL staining was performed on paraffin sections,
using the TdT-FragEL™ DNA Fragmentation Detection kit (Calbiochem)
in accordance with the manufacture’s instructions. Hematoxylin was
used to counterstain the sections.
Antitumor activity of immunocasp-6 in
vivo
Six- to eight-week-old BALB/c athymic mice were
purchased from National Rodent Laboratory Animal Resources,
Shanghai Branch (Shanghai, China), and were cared and used in
compliance with institutional guidelines. The mice were inoculated
s.c. with 2×106 SOSP-9607-E10 cells. Tumors were allowed
to grow until they reached a diameter of 5-7 mm (day 0). The mice
were then randomly divided into different two groups, namely the
immunocasp-6 group and vector group.
The mice bearing SOSP-9607-E10 tumors were subjected
to liposome-mediated immunocasp-6 or vector treatments.
pCMV-immunocasp-6 or pCMV vector alone, 10 μg, encapsulated by 20
μl Lipofectamine 2000 was administered i.m. to mice. Nine mice were
utilized for each treatment, each mouse was administered every 3
days for a total of five times. The volume of the tumor, the body
weight of the mice and the net weight of the tumor were observed
and analyzed by statistics.
Assessment of immunocasp-6 effects on
human osteosarcoma lung metastasis
Eighteen athymic, six- to eight-week-old Balb/c mice
were inoculated with 2×105 SOSP-9607-E10 cells into
thighbone marrow. The mice were then divided randomly into two
groups, nine mice in each group, for i.m. liposome-mediated
immunocasp-6 or vector treatments as indicated above. The treatment
was performed once every 3 days for two weeks, then once a week
thereafter for five weeks. The number of the neonatal tumors in
lungs were counted, mouse survival times were recorded and the
neonatal mass was tested by H&E staining.
Statistical analyses
The data are expressed as the mean ± SD. Statistical
analyses were performed with the SPSS13.0 software package for
Windows (SPSS, Chicago, IL). Cell viability assay were analyzed by
the analysis of covariance (ANCOVA, dunnett T3) method. The volume
of the tumor, the body weight of the mice and the net weight of the
tumor were analyzed using independent-samples t-test (the data of
the volume of the tumor and the body weight of the mice were
obtained from the data before treatment subtracted by the data
after treatment). The survival rates were analyzed using the
Kaplan-Meier method, and comparisons among treatment groups were
obtained using the log-rank test. Statistical significance was
based on a value of P<0.05.
Results
Immunocasp-6 effectively and specifically
suppresses the growth of SOSP- 9607-E10 cells in vitro
Active caspase-6 is expressed and secreted from the
transfected cells, binds to HER2-overexpressing breast tumor cells,
internalizes, undergoes autoprocessing between PEA Arg279 and
Gly280, and induces cell apoptosis (21). To investigate whether the same
cytotoxic effect could be achieved in osteosarcoma cells, we first
tested the cell viability of the transiently transfected cells by
MTT assay, Table I shows that
transient expression of the immunocasp-6 led to an apparent delay
in cell viability. In other words, tumors cells in the immunocasp-6
treatment group have weaker viability than those in the mock or
control group (P=0.013, P=0.007, respectively), while there is no
significantly difference between the mock group and control group
(P=0.989).
 | Table ISOSP-9607-E10 cells transiently
transfected with immunocasp-6, and cell OD value (490 nm) generated
using the MTT assay (mean ± SD, h). |
Table I
SOSP-9607-E10 cells transiently
transfected with immunocasp-6, and cell OD value (490 nm) generated
using the MTT assay (mean ± SD, h).
| Time after
transfection (h) | n | 12 | 24 | 36 | 48 | 60 | 72 | 84 | 96 |
|---|
| Mocka | 3 | 0.580±0.107 | 0.663±0.915 | 0.801±0.099 | 0.992±0.117 | 1.039±0.063 | 1.048±0.177 | 1.205±0.113 | 1.322±0.098 |
| Vectorb | 3 | 0.603±0.132 | 0.663±0.117 | 0.907±0.069 | 1.071±0.115 | 1.065±0.188 | 1.076±0.260 | 1.215±0.116 | 1.341±0.180 |
| Immunocasp-6c | 3 | 0.541±0.053 | 0.564±0.060 | 0.644±0.524 | 0.775±0.081 | 0.601±0.165 | 0.595±0.240 | 0.562±0.123 | 0.458±0.214 |
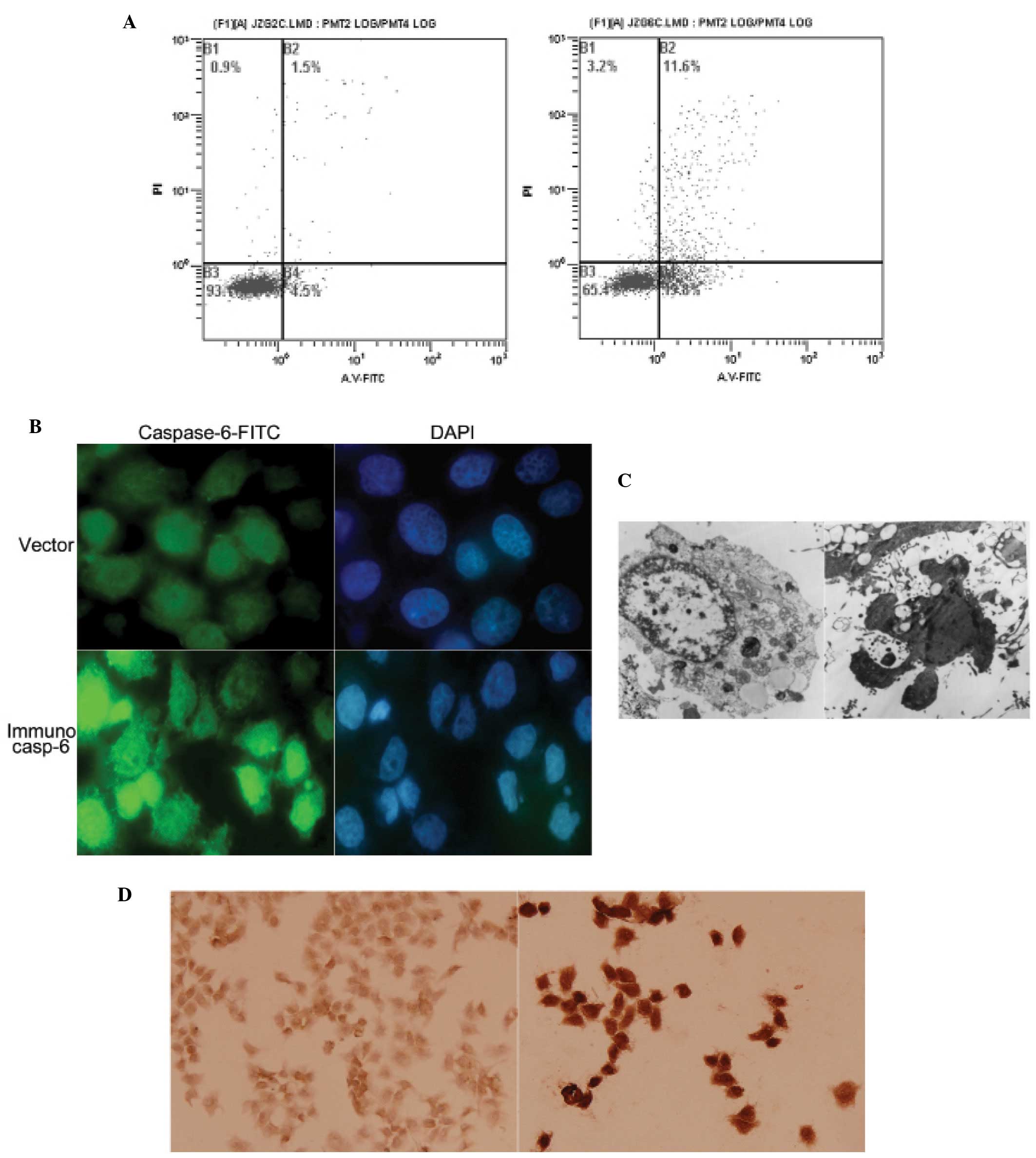
The Annexin V-FITC staining 48 h after the
transfection revealed that the percentages of apoptotic cells in
the immunocasp-6 group were 31.4%, while only 6% in the vector
group (Fig. 2A). When tumor cell
growth was stunted, the morphological change of transfected cells
were tested. Immunofluorescence test discovered that transiently
transfected cells had enriched or chipped nuclear (Fig. 2B). Transmission electron microscopy
presented typical apoptotic changes in cells, including chromatin
condensation and its margination at the nuclear periphery, cellular
shrinkage and blebbing, and formation of so-called apoptotic bodies
(Fig. 2C). Furthermore,
immunohistochemistry staining with anti-caspase-6 antibody revealed
that most of the cells were caspase-6 positive, suggesting that
caspase-6 was effective in inducing tumor cell apoptosis.
Immunocasp-6 transduction-induced
HER2-overexpressing osteosarcoma cell death in subcutaneously
transplanted nude mice
As showed in Table
II, the growth of the tumor in the liposome-mediated
pCMV-immunocasp-6 treatment group was significantly slower than
that of the pCMV vector group (P=0.001), the body weight of the
mice in the immunocasp-6 group was significantly heavier than that
of the vector group (P=0.0002), and the net weight of the tumor in
the immunocasp-6 group was significantly less than that of the
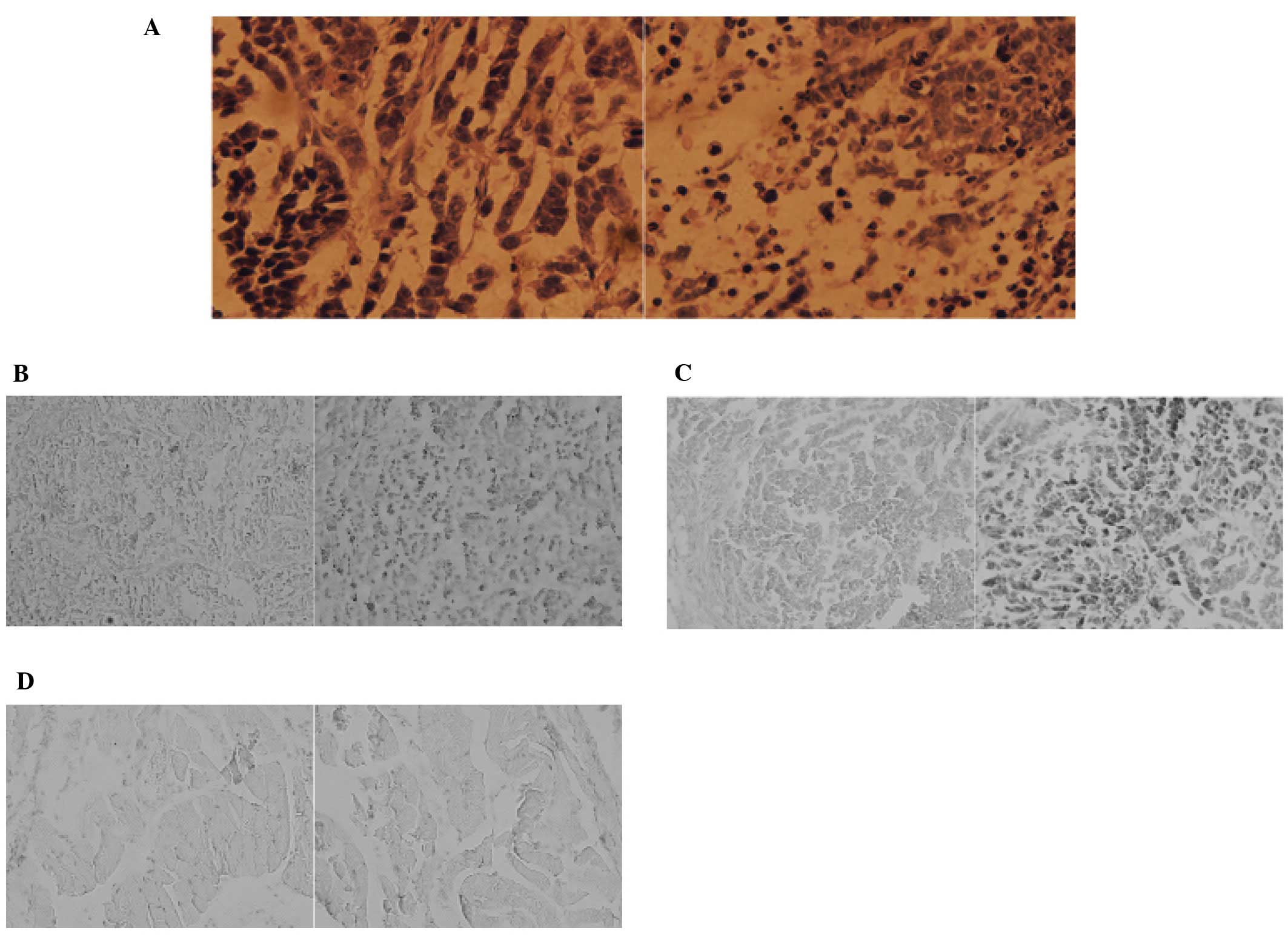
vector group (P=0.0006). Then the osteosarcoma tissues were
collected and H&E (hematoxylin and eosin) stain was performed.
The staining discovered tumor tissues were in poor condition in
which most of tumor tissue was dead (Fig. 3A). Furthermore, terminal
deoxynucleotidyl transferase-mediated dUTP nick-end-labeling
(TUNEL) staining, demonstrated that most of the tumor tissue in the
immunocasp-6 group were in the state of apoptosis (Fig. 3B). Thereafter, immunohistochemistry
analysis confirmed the presence of caspase-6 in tumor tissues in
the immunocasp-6 group, but not in those treated with vector
(Fig. 3C) and muscle tissues in
either immunocasp-6 group or vector group (Fig. 3D). These findings suggest that
immunocasp-6 can specifically and efficiently induce tumor tissue
apoptosis.
 | Table IIComparison between control and
treatment group of the tumor volume, tumor net weight and mouse
body weight (mean ± SD). |
Table II
Comparison between control and
treatment group of the tumor volume, tumor net weight and mouse
body weight (mean ± SD).
| Group | n | Tumor volume
(mm3) | Tumor net weight
(g) | Mouse body weight
(g) |
|---|
| Control | 9 | 975.09±49.76 | 1.08±0.16 | 6.20±1.14 |
| Treatment | 9 |
376.01±265.18a | 0.64±0.18b | 4.07±0.49c |
Inhibitory effect of immunocasp-6 on lung
metastasis of HER2-overexpressing osteosarcoma in vivo
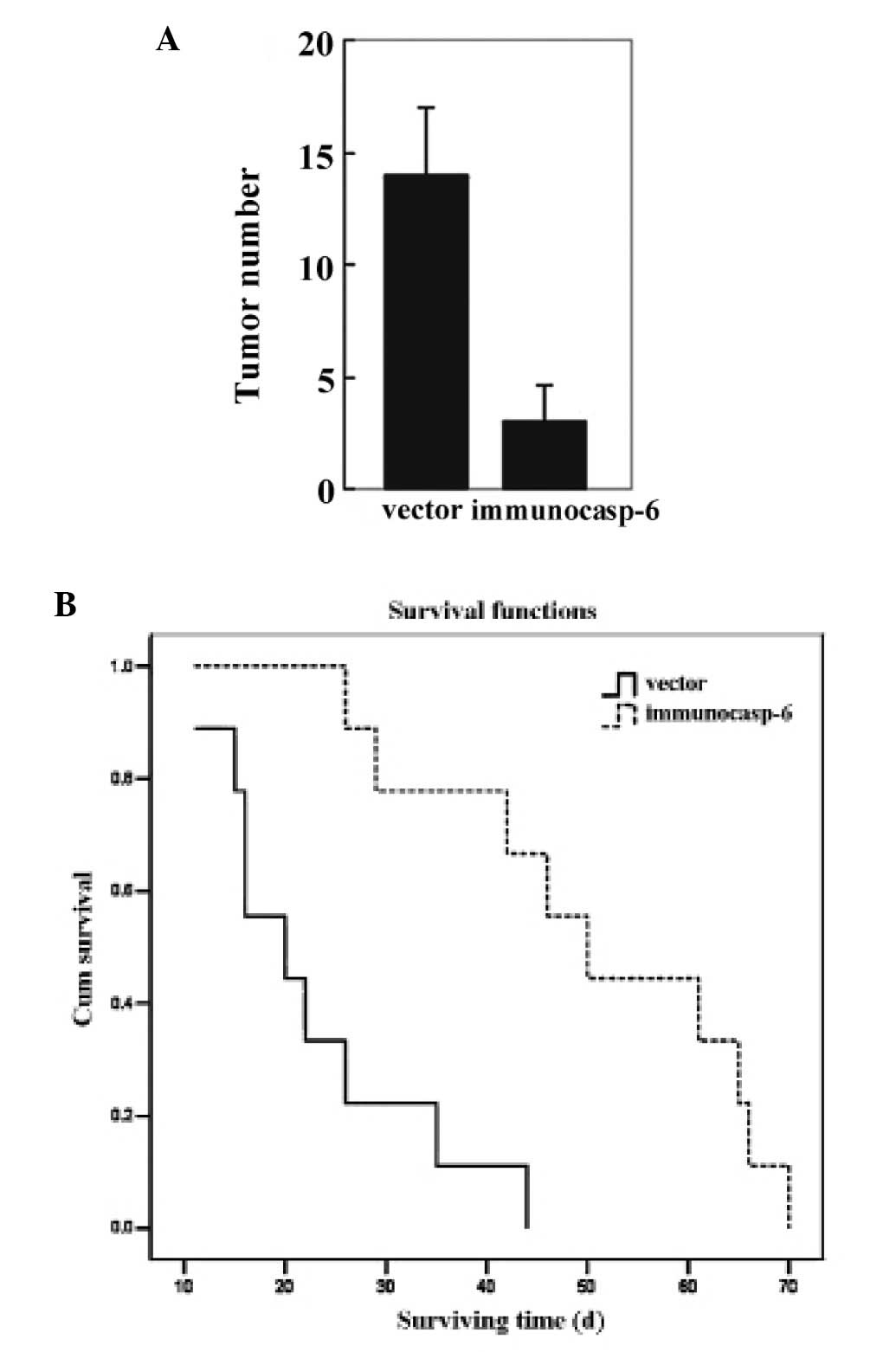
The neonatal mass were diagnosed as osteosarcoma by
H&E staining (data not shown). As shown in Fig. 4A, the number of neonatal tumors in
lungs in the immunocasp-6 group was significantly less than that in
the vector group. Furthermore, the mice in the immunocasp-6 group
survived longer than that in the vector group (Fig. 4B). SOSP-9607-E10 tumors were more
likely to metastasize to the lung in the absence of immunocasp-6
treatment, suggesting that immunocasp-6 can prevent or slow
osteosarcoma metastasis.
Discussion
Many strategies of gene therapy have been designed
to kill cancer cells, including their transduction with suicide
genes or tumor suppressor genes or activation of the immune system
against the tumor cells (22-25).
Although many problems have impeded the practical use of gene-based
therapy for human cancers, the search for safe, effective and
tissue-specific gene therapies continues. In the past decade, we
carried out a series of studies on antibody-directed and
cell-mediated cancer immunotherapy by combining the specificity of
antibodies and the potent cytotoxicity of pro-aoptotic proteins. Up
to now, a number of pro-apoptotic effectors, including caspase-3
(26), caspase-6 (21), granzyme B (27), tBid (10) and apoptosis inducing factor (AIF)
(28), have been used to construct
immunoproapoptotic proteins and have been confirmed to be efficient
in inducing targeted apoptosis both in vitro and in
vivo. In the present study, we generated a novel immunocasp-6
gene construct by fusing a leader sequence, single-chain HER2
(e23sFv) Ab and the translocation domain of PEA to the active
caspase-6 and extended the strategy to HER2 overexpression
osteosarcoma.
In the study, we verified that the HER2-targeted
suppressing effect of the novel immunocap-6 on osteosarcoma cells
in vitro. Firstly, we transfected the fusion gene into
SOSP-9607-E10 cells and compared the vitality of these tumor cells.
The effective destruction of SOSP-9607-E10 cells by the novel
immunocasp-6 was confirmed by both MTT assay and flow cytometry
assay. These findings suggested that the immunocasp-6 can strongly
inhibit the growth of SOSP-9607-E10 cells. Morphological
examination including immunofluorescence assay and electron
microscopy verified that SOSP-9607-E10 cells in the immunocasp-6
group presented the typical characteristics of apoptosis, which
suggested that immunocasp-6 might inhibit the growth of the tumor
cells by inducing tumor cell apoptosis. In order to further explore
the factors responsible for tumor cell apoptosis, the
immunohistochemistry analysis were applied. The findings verified
that caspase-6 can lead to tumor cell apoptosis. Thus, it can be
concluded that the novel immunocasp-6 can specifically recognize
the HER2-overexpressing osteosarcoma cells, induce tumor cell
apoptosis and strongly inhibit the growth of tumor cells.
In order further to verify HER2-targeted suppressing
effect of the novel immunocap -6 on the osteosarcoma cells in
vivo, the mouse SOSP-9607-E10 tumor xenograft model was
constructed. The mice in the immunocasp-6 group showed better
condition than that in the vector group, the growth of the tumor
became slower (P=0.001), the weight of the nude mice was heavier
(P=0.0006), the net weight of the tumor was lighter (P=0.0002),
which suggested the immunocasp-6 can suppress the growth of the
tumors. Thereafter, H&E staining and TUNEL staining revealed
the tumor tissue treated by immunocasp-6 was also in poor condition
and presented the character of apoptosis, suggesting that
immunocasp-6 suppressed the growth of the tumor by inducing tumor
tissue apoptosis. Furthermore, immunohistochemistry analysis
confirmed the presence of caspase-6 in tumor tissues treated with
immunocasp-6, while caspase-6 were not found in those treated with
pCMV vector and muscle tissues in either treatment group or control
group. Thus, it can be concluded that the novel immunocasp-6 can
specifically recognize and efficiently suppress the growth of
HER2-overexpressing osteosarcoma, without damaging the normal
tissues.
Moreover, in order to verify if the immunocasp-6 can
prevent the metastasis of SOSP-9607-E10 tumors, we counted the
number of neonatal tumors in lungs after mice died and recorded the
survival time. The findings showed that the neonatal tumors in
lungs in the immunocasp-6 group were significantly less than that
in the vector group. Furthermore, the mice in the immunocasp-6
group survived longer than that in the vector group. SOSP-9607-E10
tumors were more likely to metastasize to the lung in the absence
of immunocasp-6 treatment suggesting that immunocasp-6 prevents or
slows osteosarcoma metastasis.
In summary, we described a novel immunocasp-6
therapeutic gene construct, which can kill HER2-overexpressing
osteosarcoma specifically and efficiently; this novel immunocasp-6
holds promise for the generation of a novel therapy for
HER2-overexpressing tumors.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (No. 30330610).
References
|
1
|
Lupu R, Colomer R, Kannan B and Lippman
ME: Character-ization of a growth factor that binds exclusively to
the erbB-2 receptor and induces cellular responses. Proc Natl Acad
Sci USA. 89:2287–2291. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen JS, Lan K and Hung MC: Strategies to
target HER2/neu overexpression for cancer therapy. Drug Resist
Updat. 6:129–136. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Slamon DJ, Godolphin W, Jones LA, et al:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Semba K, Kamata N, Toyoshima K and
Yamamoto T: A v-erbB-related protooncogene, c-erbB-2, is distinct
from the c-erbB-1/epidermal growth factor-receptor gene and is
amplified in a human salivary gland adenocarcinoma. Proc Natl Acad
Sci USA. 82:6497–6501. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fukushige S, Matsubara K, Yoshida M, et
al: Localization of a novel v-erbB-related gene, c-erbB-2, on human
chromosome 17 and its amplification in a gastric cancer cell line.
Mol Cell Biol. 6:955–958. 1986.PubMed/NCBI
|
|
6
|
Shan LQ, Ma S, Qiu XC, et al: A novel
recombinant immuno-tBid with a furin site effectively suppresses
the growth of HER2-positive osteosarcoma cells in vitro.
Oncol Rep. 25:325–331. 2011.PubMed/NCBI
|
|
7
|
Scotlandi K, Manara MC, Hattinger CM, et
al: Prognostic and therapeutic relevance of HER2 expression in
osteosarcoma and Ewing’s sarcoma. Eur J Cancer. 41:1349–1361.
2005.PubMed/NCBI
|
|
8
|
Gorlick R, Huvos AG, Heller G, et al:
Expression of HER2/erbB-2 correlates with survival in osteosarcoma.
J Clin Oncol. 17:2781–2788. 1999.PubMed/NCBI
|
|
9
|
Akatsuka T, Wada T, Kokai Y, et al: ErbB2
expression is correlated with increased survival of patients with
osteosarcoma. Cancer. 94:1397–1404. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qiu XC, Xu YM, Wang F, et al: Single-chain
antibody/activated BID chimeric protein effectively suppresses
HER2-positive tumor growth. Mol Cancer Ther. 7:1890–1899. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Slee EA, Adrain C and Martin SJ:
Executioner caspase-3, -6, and -7 perform distinct, non-redundant
roles during the demolition phase of apoptosis. J Biol Chem.
276:7320–7326. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ruchaud S, Korfali N, Villa P, et al:
Caspase-6 gene disruption reveals a requirement for lamin A
cleavage in apoptotic chromatin condensation. EMBO J. 21:1967–1977.
2002. View Article : Google Scholar
|
|
14
|
Srinivasula SM, Ahmad M, MacFarlane M, et
al: Generation of constitutively active recombinant caspases-3 and
-6 by rearrangement of their subunits. J Biol Chem.
273:10107–10111. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen SY, Yang AG, Chen JD, et al: Potent
antitumour activity of a new class of tumour-specific killer cells.
Nature. 385:78–80. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Batra JK, Kasprzyk PG, Bird RE, Pastan I
and King CR: Recombinant anti-erbB2 immunotoxins containing
Pseudomonas exotoxin. Proc Natl Acad Sci USA. 89:5867–5871.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hwang J, Fitzgerald DJ, Adhya S and Pastan
I: Functional domains of Pseudomonas exotoxin identified by
deletion analysis of the gene expressed in E. coli. Cell.
48:129–136. 1987.
|
|
18
|
Siegall CB, Chaudhary VK, FitzGerald DJ
and Pastan I: Functional analysis of domains II, Ib, and III of
Pseudomonas exotoxin. J Biol Chem. 264:14256–14261.
1989.PubMed/NCBI
|
|
19
|
Jinno Y, Ogata M, Chaudhary VK, et al:
Domain II mutants of Pseudomonas exotoxin deficient in
translocation. J Biol Chem. 264:15953–15959. 1989.PubMed/NCBI
|
|
20
|
Siegall CB, Ogata M, Pastan I and
FitzGerald DJ: Analysis of sequences in domain II of
Pseudomonas exotoxin A which mediate translocation.
Biochemistry. 30:7154–7159. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu YM, Wang LF, Jia LT, et al: A caspase-6
and anti-human epidermal growth factor receptor-2 (HER2) antibody
chimeric molecule suppresses the growth of HER2-overexpressing
tumors. J Immunol. 173:61–67. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pastan I, Chaudhary V and FitzGerald DJ:
Recombinant toxins as novel therapeutic agents. Annu Rev Biochem.
61:331–354. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Springer CJ and Niculescu-Duvaz I:
Prodrug-activating systems in suicide gene therapy. J Clin Invest.
105:1161–1167. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yazawa K, Fisher WE and Brunicardi FC:
Current progress in suicide gene therapy for cancer. World J Surg.
26:783–789. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Horowitz J: Adenovirus-mediated p53 gene
therapy: overview of preclinical studies and potential clinical
applications. Curr Opin Mol Ther. 1:500–509. 1999.PubMed/NCBI
|
|
26
|
Jia LT, Zhang LH, Yu CJ, et al: Specific
tumoricidal activity of a secreted proapoptotic protein consisting
of HER2 antibody and constitutively active caspase-3. Cancer Res.
63:3257–3262. 2003.
|
|
27
|
Zhao J, Zhang LH, Jia LT, et al: Secreted
antibody/granzyme B fusion protein stimulates selective killing of
HER2-overexpressing tumor cells. J Biol Chem. 279:21343–21348.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu CJ, Jia LT, Meng YL, et al: Selective
proapoptotic activity of a secreted recombinant antibody/AIF fusion
protein in carcinomas overexpressing HER2. Gene Ther. 13:313–320.
2006. View Article : Google Scholar : PubMed/NCBI
|