Introduction
Head and neck tumors are the common malignant tumors
worldwide, while oral squamous cell carcinoma (OSCC) is the most
prevalent type of head and neck tumor accounting for 2–3% of
malignant tumors and 80% of oral and maxillofacial malignant tumors
(1,2). Although the techniques of surgical
treatment, radiotherapy and chemotherapy for OSCC have progressed
in the past few decades, the 5-year survival rate of OSCC patients
has shown no noticeable improvement (3,4). Thus,
it is crucial to explore the pathological mechanisms of OSCC, and
new biomarkers must be identified and used in the detection of
precancerous lesions, early diagnosis and therapy of OSCC.
Numerous factors, such as genetic alterations,
epigenetic changes and environmental influence, are involved in the
development of OSCC. Inactivation and deletion of tumor-suppressor
genes (TSGs) play important roles in the development of carcinomas
(5). Previous studies have
suggested that analyzing the LOH (loss of heterozygosity) in tumor
tissues can facilitate the identification of relevant TSGs. For
example, RB(6), p53
(7), Wilms tumor
(WT)(8), cyclin-dependent
kinase number 2 (CDKN2)(9),
adenomatous polyposis coli (APC) (10), deleted in pancreatic carcinoma locus
4 (DPC4) (11), fragile
histidine triad (FHIT) (12)
and putative protein tyrosine phosphatase gene (PTEN)
(13,14) have all been identified using LOH
analysis. As for OSCC, a high frequency of LOH has been observed on
chromosomes 3p, 4q, 5q, 7q, 8p, 9p, 11q, 13q, 18q, 20q, 21q and 22q
regions using comparative genomic hybridization and LOH analysis
(15–18). Thus, these chromosome segments
appear to be target regions where TSGs of OSCC may exist.
Roz et al(19) found LOH at one or more loci of
chromosome 3p by investigating 30 oral dysplastic lesions,
presenting clinically as either erythroplakias or leukoplakias with
histopathological features of either severe epithelial dysplasia or
carcinoma in situ. Furthermore, three discontinuous deletion
regions including -3p13-21.1, -3p21.3-5 and -3p25, were detected in
patients with precancerous lesions and OSCC, which suggest that
alterations of these three regions may be early events in the
development of OSCC. Yamamoto et al(20) found different degrees of LOH at
3p21.3 in OSCC patients from Japan and India. At the same time, LOH
at 3p21-22 was present in other common malignant tumors such as
renal carcinoma (21), lung cancer
(22), squamous cell carcinoma of
the head and neck (23), cervical
carcinoma (24) and breast cancer
(25). Several groups have studied
the relevant TSGs for nasopharyngeal carcinoma (NPC) and lung
cancer at region 3p21.3 and found that BLU, RASSF1A,
SEMA3B and SEMA3F play vital roles in the development
and progression of NPC or lung cancer (26–30).
Similar studies performed in OSCCs revealed that the expression
levels of the LTF, LIMD1, CACNA2D2, RASSF1A,
CDC25A and SCOTIN genes located at 3p21.3 were
decreased by 67.6, 53.2, 23.7, 15.1, 5.3 and 0.58%, respectively.
LOH and DNA methylation alterations may account for the abnormal
expression of LTF, LIMD1, CACNA2D2 and
RASSF1A.
In the present study, based on the above-mentioned
research, bioinformatics analysis was carried out to screen
candidate TSGs located at 3p21.3, which to date have not been or
have been inadequately studied in OSCC. The methylation state in
the promoters of TSGs was also analyzed using methylation-specific
PCR (MSP) to explore the role of DNA methylation in the
downregulation of TSGs in OSCCs.
Materials and methods
Selecting target genes located at
3p21.3
On the basis of a database search (PubMed, Gene
Card, OMIM and Gene Map), using ‘3p21.3’ and ‘tumor-suppressor
genes’ as search terms, 10 genes were selected for analysis based
on the following inclusion criteria: i) they were previously
reported to exhibit low expression or no expression in other tumor
types; and ii) the functions of these genes were not previously
studied or inadequately studied in OSCCs.
Cell line and tissues
TCA8113, a tongue cancer cell line, was provided by
the Cancer Research Institute of Central South University. TCA8113
cells were cultured in RPMI-1640 supplemented with 10% fetal calf
serum at 37°C in a humidified atmosphere with 5% CO2.
Thirty-six pairs of normal and tumor tissues from OSCC patients
were obtained from the Hunan Cancer Hospital (Changsha, Hunan,
China) and the Second Xiangya Hospital of Central South University
(Changsha, Hunan, China) with informed patient consent prior to
treatment. All fresh tissues were snap-frozen in liquid nitrogen
and stored until required. Clinicopathological information of the
patients is presented in Table
I.
 | Table IClinicopathological features of the
oral squamous cell carcinoma patients. |
Table I
Clinicopathological features of the
oral squamous cell carcinoma patients.
| | Gender | | | Lymph node
status |
|---|
| |
| | |
|
|---|
| Tumor subsite | Cases | Male | Female | Age (years) | Median age
(years) | Positive | Negative |
|---|
| Tongue | 28 | 24 | 4 | 17–80 | 42.1 | 13 | 15 |
| Gum | 3 | 2 | 1 | 40–61 | 50.3 | 1 | 2 |
| Soft plate | 1 | 1 | 0 | 73 | 73.0 | 0 | 1 |
| Floor of the
mouth | 2 | 2 | 0 | 53 | 53.0 | 1 | 0 |
| Jaw bones | 2 | 1 | 1 | 48–59 | 53.5 | 2 | 0 |
DNA and RNA extraction
gDNA from all specimens as well as from
1×106 cells was extracted using an improved method for
extracting high-molecular-weight DNA with phenol/chloroform
according to previously reported procedures (31). Total RNA was isolated from a 100 mg
sample from each specimen and a total number of 107
cells using TRIzol reagent according to the manufacturer’s protocol
(Invitrogen, USA).
Reverse transcription-PCR (RT-PCR)
Total RNA was treated with DNAse I (Roche
Diagnostics, USA) and purified in accordance with the
manufacturer’s instructions. Reverse transcription was performed
with 1 μg of treated total RNA using AMV reverse transcriptase
(Promega, Madison, WI, USA). An equal amount of cDNA from each
sample was amplified using specific primers for each gene (Table II). The conditions for PCR
reactions were as follows: 95°C for 5 min, followed by 30–34 cycles
of 94°C for 35 sec, 55–58°C for 35 sec, 72°C for 35 sec, and 72°C
for 10 min for the extension. The PCR products were electrophoresed
on 1% agarose gel and visualized by ethidium bromide staining.
 | Table IISummary of primer sequences,
annealing temperatures and product sizes. |
Table II
Summary of primer sequences,
annealing temperatures and product sizes.
| Squence
(5′-3′) | Product size
(bp) | Annealing
temperature (°C) | Cycles | Ref. |
|---|
| Primer for
RT-PCR |
| GAPDH | F:
GAGATCCCTCCAAAATCAAGTG
R: GAGTCCTTCCACGATACCAAAG | 282 | 58 | 28 | |
| AXUD1 | F:
TGCGTAACAGTCTCCCACTG
R: CCTGATTTCAGCCCTGTCTC | 301 | 58 | 30 | |
| BAP1 | F:
TACGCTACAACCGTGCTGTC
R: TCAGCCTCCACACACTTCAG | 295 | 58 | 30 | |
| FUS1 | F:
GGATCTGGCTCACGAGTTCT
R: GAAGGTTATGGGCCAACAGA | 296 | 55 | 30 | |
| GNAT1 | F:
CATCTGCAACCACCGCTAC
R: CGCACGTCATGTGGGAATA | 221 | 62 | 32 | |
| LARS2 | F:
TTACTGGATGCCTGTGGATT
R: CCCGTTGTGTTTGGACTTAC
R: GCCTGCGACATACTGTGGTC | 295 | 56 | 30 | |
| NPRL2 | F:
CGCATTGCTATCCAGAACCT
R: CTTCATAAGCCCGAACTGGA | 300 | 60 | 30 | |
|
RASSF1A | F:
ACACGTGGTGCGACCTCT
R: GATGAAGCCTGTGTAAGAACCGTCCT | 308 | 58 | 32 | |
| SEMA3B | F:
GTCCTCTTCATTGGCACAGAC
R: GTCGCCATTCCTTACGTCTT | 345 | 56 | 30 | |
| SEMA3F | F:
AGTGTCCGTACGATCCCAAG
R: ATGACAGGGTTCCTCACGTC | 466 | 58 | 32 | |
| ZNF35 | F:
GCGCACATAGGCAGTACTCA
R: TGTCTGAAGACCCTGCACTG | 296 | 58 | 30 | |
| Primer for MSP |
| GNAT1 | MF:
GTAAAAGATATATTTATGGTCGGC
MR: CCTCCCAAATAACTAAAATTACGAA | 198 | 59 | | |
| UF:
AAAAGATATATTTATGGTTGGGTGA
UR: CCTCCCAAATAACTAAAATTACAAA | 195 | 59 | | |
| SEMA3B | MF:
TGGTTAGGCGGGGTATTTTC
MR: TCAACAATAAAAACGAAAACG | 133 | 54 | | (52) |
| UF:
GTGGTTAGGTGGGGTATTTTT
UR: ATCAACAATAAAAACAAAAACA | 135 | 54 | | |
Gray scale scanning and data
analysis
The intensity of each electrophoretic band was
measured using ImageMaster VDS (Pharmacia Biotech Inc., Piscataway,
NJ), and analyzed by VDS software version 3.0 for band
quantification with GAPDH as an internal control. The
expression levels of target genes in the tumors and normal tissues
were investigated after they were normalized by transforming them
into two groups according to the ratios of the band intensity of
the target genes over that of GAPDH for the same sample as
previously described (32). A ratio
>2 indicated that the corresponding gene was upregulated in OSCC
and a ratio <1/2 indicated that the corresponding gene was
downregulated in OSCC and all other ratio values indicated that the
corresponding genes were expressed at a normal level. Each RT-PCR
reaction was carried out in triplicate.
Methylation-specific PCR (MSP)
MSP was performed to analyze the methylation state
in the promoter regions of the target genes both in OSCC tissues
and TCA8113 cells. One microgram gDNA from OSCC tissues and TCA8113
cells was treated with bisulfite, similar to previous methods
(33,34). Each sample was amplified with two
sets of primers, one set for methylated DNA and one set for
unmethylated DNA. When gDNA was treated with bisulphite, the
unmethylated cytosine was tranformed to uracil while methylated
cytosine was unaffected. Thus, the methylated and unmethylated
promoters were distinguished by MSP. The primers used for the
methylated and unmethylated target gene promoter regions are listed
in Table II. The PCR products were
analyzed by 1% agarose gel electrophoresis and ethidium bromide
staining.
Sequencing
DNA sequencing was carried out to identify the
methylated and unmethylated sequences. MSP products were
electrophoresed on a 1% agarose gel, and the products were
recovered by using the QIAquick Gel Extraction kit (Qiagen, USA).
The purified DNA was then cloned into the pMD18-T (Takara, Japan)
vector and transformed into Escherichia coli strain Top 10
according to the manufacturer’s instructions. The recombinant
plasmid DNA extracted from the 5 randomly selected positive clones
was used for sequencing.
Treatment of TCA8113 cells with
5-aza-2′-deoxycytidine (5-Aza-Cdc)
TCA8113 cells were plated in 6-well plates at a
density of 1×105 cells/well and cultured overnight. On
the morning of the next day, the TCA8113 cells were treated with
5-Aza-Cdc, a type of demethylation reagent, at final concentrations
(as control) of 0 1, 5, 10, 50 and 100 μM, respectively. After
changing the medium and treating cells with 5-Aza-Cdc once per 24 h
for 4 consecutive days, TCA8113 cells were collected for RNA and
DNA extraction to perform RT-PCR and MSP analysis.
Statistical analysis
Statistical analysis was performed using the
Chi-square test and the Student’s t-test. In all analyses, SPSS
11.5 statistical software (SPSS, Chicago, IL) was used, and a
P-value <0.05 was considered to indicate a statistically
significant result.
Results
Differential expression of the selected
genes in OSCC and normal tissues
We selected 10 genes using ‘3p21.3’ and
‘tumor-suppressor genes’ as the search terms in several databases
(PubMed, Gene Card, OMIM and Gene Map). The selected genes are
listed in Table II.
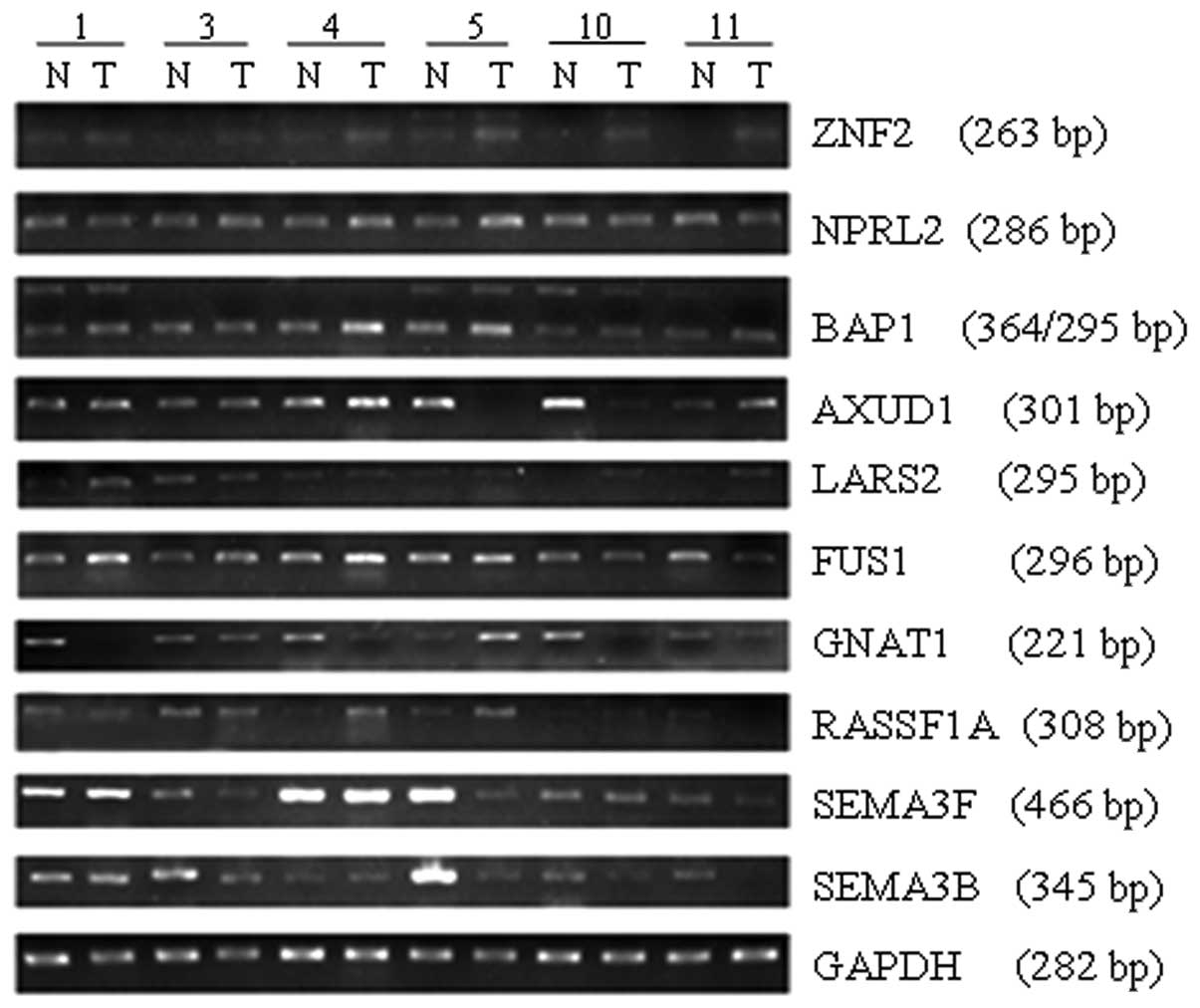
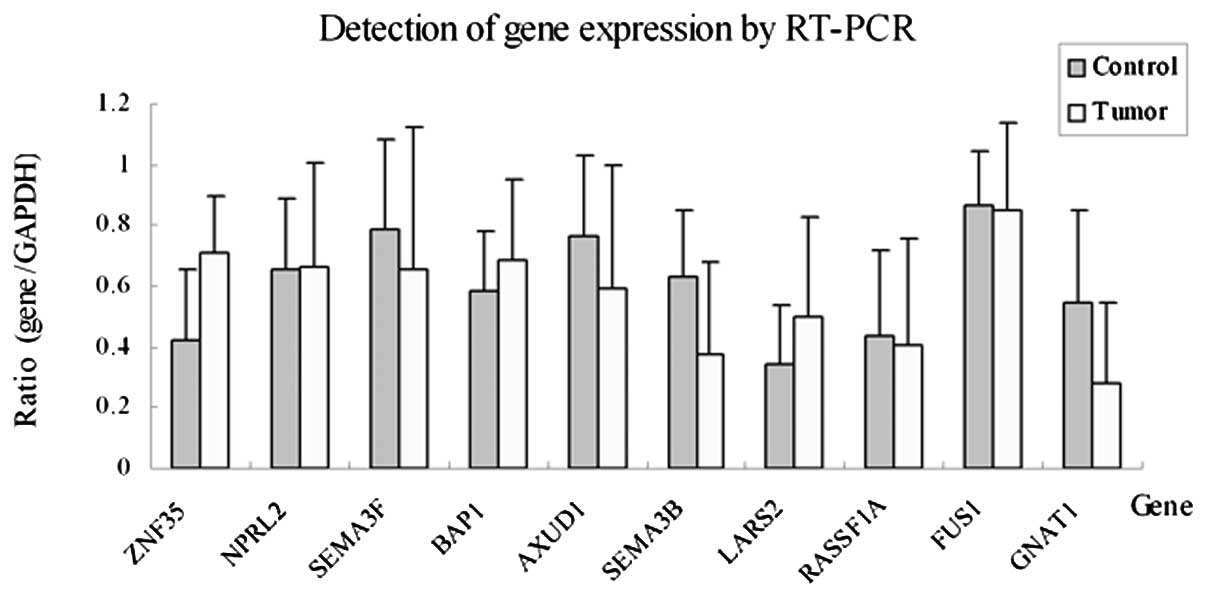
Differential expression of the target genes was
assessed using semi-quantitative RT-PCR combined with Gray Scale
Scanning analysis in 12 pairs of OSCC tissues and contralateral
normal tissues. The results revealed that the expression of
GNAT1, SEMA3B and AXUD1 was downregulated or
deficient in the OSCC tissues with percentages of 58.3 (7/12), 41.7
(5/12) and 41.7% (5/12), respectively (P<0.05). The expression
of ZNF35 was upregulated in 33.3% (4/12) of OSCC tissues. No
significant difference in expression for NPRL2, BAP1,
FUS1, LARS2, RASSF1A and SEMA3F was
observed between the OSCC and normal tissues (P>0.05) (Figs. 1 and 2). We selected three downregulated genes,
GNAT1, SEMA3B and AXUD1, for further
study.
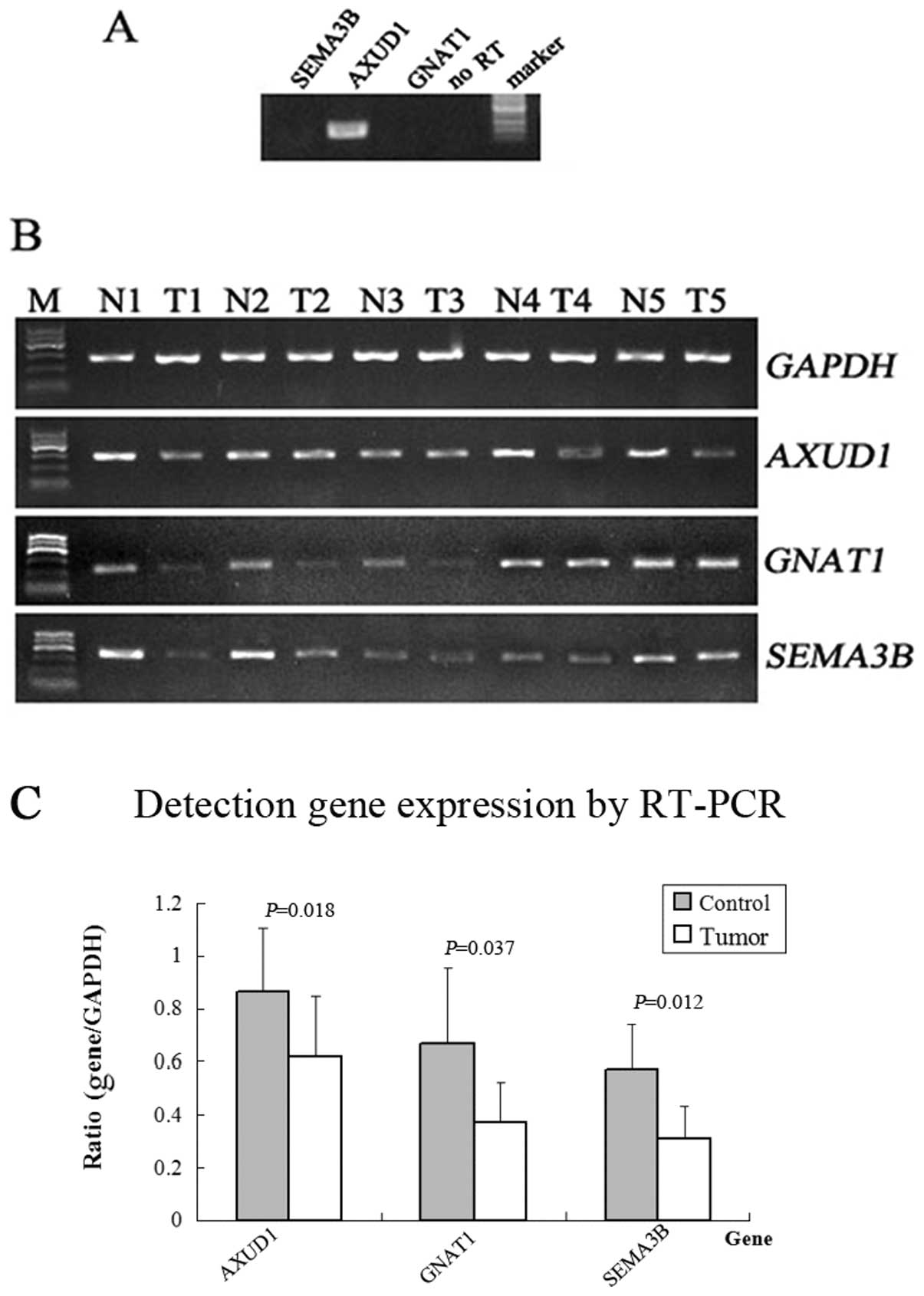
Expression of GNAT1, SEMA3B and AXUD1 in
OSCC tissues and the TCA8113 cell line
RT-PCR was performed to verify the low expression of
GNAT1, SEMA3B and AXUD1 in 36 OSCC tissues,
corresponding adjacent normal tissues and TCA8113 cells. The
results confirmed that the expression of GNAT1,
SEMA3B and AXUD1 was downregulated or undetectable in
44.4 (16/36) 50 (18/36) and 47.2% (17/36) of the OSCC tissues,
respectively, compared with the normal expression of these genes in
adjacent normal tissues (P<0.05) (Fig. 3B and C). No expression of
GNAT1 and SEMA3B was detected and AXUD1 was
expressed at a normal level in the TCA8113 cells (Fig. 4A) as compared with the expression in
OSCC tissues. No significant correlation was observed between the
downregulated genes and OSCC metastasis and patient gender when
compared with the clinical data (Table III).
 | Table IIIStatistical analysis of the
correlation between the downregulated genes and tumor
metastasis. |
Table III
Statistical analysis of the
correlation between the downregulated genes and tumor
metastasis.
| | Genes |
|---|
| |
|
|---|
| | AXUD1 | GNAT1 | SEMA3B |
|---|
| |
|
|
|
|---|
| Groups | Cases | N (%) | U (%) | N (%) | U (%) | N (%) | U (%) |
|---|
| Metastasis | 17 | 10 (27.8) | 7 (19.4) | 10 (27.8) | 7 (19.4) | 10 (27.8) | 7 (19.4) |
| Non-metastasis | 19 | 9 (25.0) | 10 (27.8) | 9 (25.0) | 10 (27.8) | 8 (22.2) | 11 (30.1) |
| χ2 | | 0.472 | 0.139 | 1.003 |
| P-value | | 0.492 | 0.709 | 0.317 |
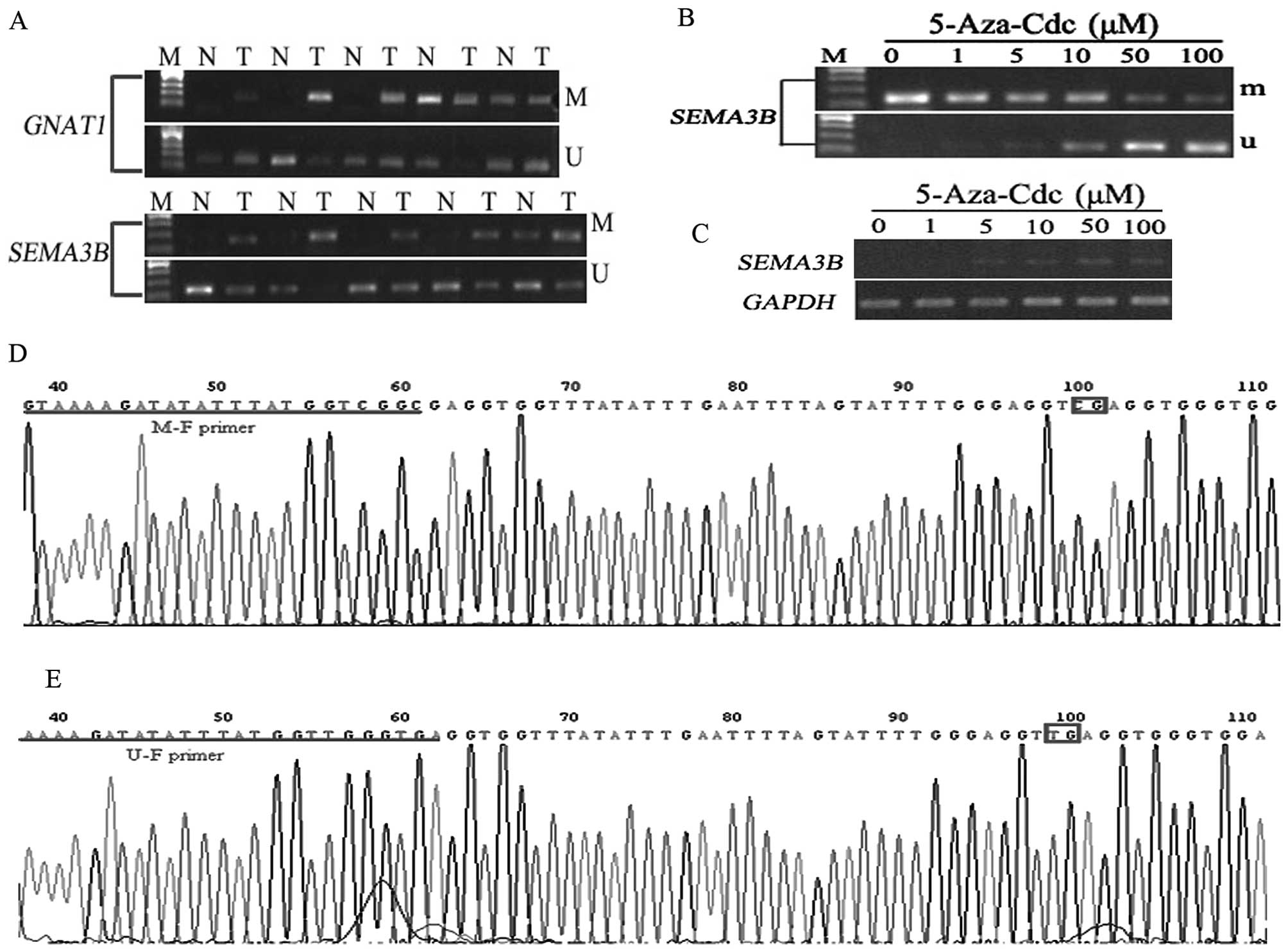
Hypermethylation of GNAT1 and SEMA3B in
OSCC tissues
We analyzed the methylation status of CpG islands in
the promoter region of GNAT1 and SEMA3B in several
OSSC tissue samples and adjacent non-cancerous tissues.
Hypermethylation of GNAT1 and SEMA3B was found in 75
(12/16) and 77.8% (14/18) of OSSC tissues, respectively. In the
non-cancerous tissues, different levels of methylation were also
detected with a percentage of 56.25% (9/16) for GNAT1 and
38.9% (8/18) for SEMA3B (Fig.
4A). The results were also confirmed by the sequencing of the
MSP products (Fig. 4D and E).
Statistical analysis indicated that there was a significant
difference in the frequency of methylation of SEMA3B between
the OSCC and non-cancerous tissues (χ2 test, P=0.018
<0.05) (Table IV), while no
statistical significance was found in the frequency of methylation
of GNAT1 between the OSCC and non-cancerous tissues
(χ2 test, P=0.709 >0.05), suggesting that methylation
of the promoter plays an important role in the downregulation of
the SEMA3B gene in OSSC tissues.
 | Table IVStatistical analysis of the gene
promoter methylation status in OSCC tissues. |
Table IV
Statistical analysis of the gene
promoter methylation status in OSCC tissues.
| Genes |
|---|
|
|
|---|
| GNAT1 | SEMA3B |
|---|
|
|
|
|---|
| Groups | n | M (%) | U (%) | n | M (%) | U (%) |
|---|
| Control | 16 | 9 (56.25) | 7 (43.75) | 18 | 7 (38.9) | 11 (61.1) |
| Tumor | 16 | 12 (75.0) | 4 (25.0) | 18 | 14 (77.8) | 4 (22.2) |
| χ2 | | 1.247 | | 5.60 |
| P-value | | 0.264 | | 0.018 |
5-Aza-Cdc partially recovers the
expression of SEMA3B in TCA8113 cells
RNA and DNA were extracted from TCA8113 cells
treated with different concentrations of 5-Aza-Cdc for 4 days for
expression and methylation analysis of SEMA3B by RT-PCR and
MSP. The results demonstrated that SEMA3B began to be
expressed in the TCA8113 cells when treated with a concentration of
5-Aza-Cdc at 5 μM (Fig. 4C). More
unmethylated products were amplified from the DNA samples of
TCA8113 cells when the higher concentration of 5-Aza-Cdc was
administered (Fig. 4B). This
suggests that methylation of the promoter plays a critical role in
the downregulation of this gene in TCA8113 cells.
Discussion
Inactivation of TSGs and overexpression of oncogenes
are the main causes for tumorigenesis. LOH and hypermethylation in
promoter regions are the common reasons accounting for the
inactivation of TSGs. The short arm of human chromosome 3, a region
with a high frequency of LOH, is considered to be rich in
TSG-containing sites as indicated by previous studies on lung
cancer, breast cancer, ovarian cancer and other malignant tumors
(35). Screening tumor-related TSG
candidates is an available method based on loss of a chromosome
region. Numerous candidate TSGs have been identified in high
frequency chromosome-deleted regions such as DLC-1, a
potential TSG at 8p21, a region frequently deleted in various types
of cancers (33,36) and DMRT1, DMRT3 and
DOCK8 located at 9p24.3, a deleted region in squamous cell
lung carcinoma (37). In this
study, we selected 10 genes including AXUD1, BAP1,
FUS1, GNAT1, LARS2, NPRL2,
RASSF1A, SEMA3F, SEMA3B and ZNF35 which
were located on region 3p21.3 and analyzed the expression of these
genes using RT-PCR to find candidate TSGs in OSCC. Three
downregulated genes, SEMA3B, AXUD1 and GNAT1,
were identified and both SEMA3B and GNAT1 were
downregulated in TCA8113 cells.
AXUD1, also termed cysteine-serine-rich
nuclear protein 1 gene (CSRNP1), was cloned by Ishiguro
et al(38) while studying
the functions of AXIN1 which encodes one of the apoptotic proteins
induced by TGF-β in hepatoma carcinoma cells. The full length
AXUD1 cDNA is 3188 bp and encodes a 64-kDa protein
containing 596 amino acids and is located mainly in the nucleus.
AXUD1, a downstream responsive protein for AXIN1, negatively
regulates the Wnt pathway which plays an important role in early
embryonic development. Studies have shown that the abnormal
regulation of the Wnt pathway is related to tumorigenesis. The
expression of AXUD1, which is high in normal tissues such as
lung, placenta, skeletal muscle, pancreas and leukocytes, was found
to be downregulated in lung cancer hepatoma and colorectal
carcinoma, suggesting that AXUD1 may be a candidate TSG
(38). Simultaneously, knockdown of
the expression of DAXUD1, a orthologue of the
AXUD1/CSRNP family in Drosophila, was found to induce
imaginal cells to proliferate. Conversely, overexpression of
DAXUD1 retarded cell cycle progression at mitosis behaving
similar to a TSG (39). Our study
showed that the expression of AXUD1 was downregualted in
47.2% (17/36) of the OSCC tissues. However, this downregulation had
no correlation with patient gender and tumor metastasis, indicating
that AXUD1 may be involed in the early malignant
transformation of the oral mucous membrane. The function of this
gene in OSCC warrants further study.
Guanine nucleotide-binding protein G (G protein)
subunit α-1a encoded by GNAT1 is an intracellular protein
involved in light signal transduction in the retina (40). G protein, as an energy regulator,
participates in the regulation of different transmembrane signal
transduction pathways (40). Yi
et al(41) found that
expression of GNAT1 was downregulated in 72.7% (24/33) of
nasopharyngeal carcinoma tissues, which was markedly lower than
that in chronic nasopharyngitis tissues (100%, 15/15). The study
also demonstrated that downregulation of GNAT1 was
associated with LOH, but not with abnormal methylation of the
promoter region. In our study, in comparison with the expression in
normal control tissues, the expression of GNAT1 was
significantly downregulated in 44.4% of the OSCC tissues (P=0.037
<0.05) and in TCA8113 cells. However, the downregulation of
GNAT1 had no correlation with patient gender and tumor
metastasis, suggesting that GNAT1 may be involved in the
early development of OSCC.
SEMA3B, a member of the human semaphoring
family is involved in apoptosis (42,43)
and in antitumor pathways regulated by p53 (44). SEMA3B was cloned by Sekido
et al(45). SEMA3B
cDNA encodes an 83-kDa protein of 749 amino acids. Using Northern
blot analysis, Lerman and Minna (46) detected wide expression of a 3.4-kb
SEMA3B transcript that was strongest in placenta, weaker in
other tissues including lung and testis and not detectable in
small-cell lung cancer cell lines. Missense mutations were found in
non-small cell lung cancer cell lines. The authors also showed that
SEMA3B is likely to be an extracellular secreted protein. Lerman
and Minna concluded that SEMA3B is an attractive candidate
tumor-suppressor gene for methylation and functional analysis. In
the present study, we detected the expression of SEMA3B mRNA
in OSCC tissues and adjacent control tissues. The results showed
that the expression level of SEMA3B was significantly lower
in OSCC tissues than that in control tissues, which was confirmed
by undetectable expression of SEMA3B in TCA8113 cells. The
χ2 test confirmed that no significant correlation
existed between the downregulated expression of SEMA3B and
patient gender and tumor metastasis.
DNA hypermethylation, one of the most common
epigenetic alterations, is involved in inactivation of the
expression of many TSGs (47–49).
MSP is a technique that has facilitated the detection of promoter
hypermethylation at CpG islands in cell lines and clinical samples,
including fresh and frozen tissues (50). Riquelme et al(51) found that the methylation ratio of
the promoter region of SEMA3B is 92% (46/50) in gallbladder
carcinoma, suggesting that the methylation of SEMA3B may
participate in the carcinogenesis and progression of gallbladder
carcinoma. The hypermethylation status of SEMA3B was also
recorded in non-small cell lung cancer decreasing the expression of
SEMA3B(52,53) and in nasopharyngeal carcinoma for
GNAT1, however, it did not account for the low expression of
GNAT1(41).
Thus, MSP was performed with DNA samples from OSCC
tissues, control tissues and 5-Aza-Cdc-treated TCA8113 cells.
Hypermethylation status of SEMA3B in promoter regions was
assessed in OSCC tissues, and the expression of SEMA3B was
partly recovered in TCA8113 cells treated with 5-Aza-Cdc,
demonstrating that the hypermethylation status in the promoter
region led to, at least partially, low expression of SEMA3B
in OSCC in accordance with studies in other cancer types (51–53).
As for GNAT1, although hypermethylation in the promoter
regions was observed in OSSC tissues, there was no significant
difference in expression between OSSC tissues and control samples.
Futher studies should be conducted to explore the reasons for the
low expression of GNAT1.
In conclusion, our study demonstrated that
AXUD1, GNAT1 and SEMA3B, three candidate TSGs
located at 3p21.3, may be involved in the development of OSCC, and
methylation in the promoter region plays a critical role in the low
expression of SEMA3B. These findings may lead to new avenues
for the early diagnosis and therapy of OSCC.
References
|
1
|
Massano J, Regateiro FS, Januario G and
Ferreira A: Oral squamous cell carcinoma: review of prognostic and
predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 102:67–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
3
|
Lippman SM and Hong WK: Molecular markers
of the risk of oral cancer. N Engl J Med. 344:1323–1326. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vokes EE, Weichselbaum RR, Lippman SM and
Hong WK: Head and neck cancer. N Engl J Med. 328:184–194. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou W, Feng X, Li H, et al: Inactivation
of LARS2, located at the commonly deleted region 3p21.3, by both
epigenetic and genetic mechanisms in nasopharyngeal carcinoma. Acta
Biochim Biophys Sin. 41:54–62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Friend SH, Bernards R, Rogelj S, et al: A
human DNA segment with properties of the gene that predisposes to
retinoblastoma and osteosarcoma. Nature. 323:643–646. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chiba I, Shindoh M, Yasuda M, et al:
Mutations in the p53 gene and human papillomavirus infection as
significant prognostic factors in squamous cell carcinomas of the
oral cavity. Oncogene. 12:1663–1668. 1996.PubMed/NCBI
|
|
8
|
Call KM, Glaser T, Ito CY, et al:
Isolation and characterization of a zinc finger polypeptide gene at
the human chromosome 11 Wilms’ tumor locus. Cell. 60:509–520.
1990.
|
|
9
|
Kamb A, Shattuck-Eidens D, Eeles R, et al:
Analysis of the p16 gene (CDKN2) as a candidate for the chromosome
9p melanoma susceptibility locus. Nat Genet. 8:23–26. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aoki K and Taketo MM: Adenomatous
polyposis coli (APC): a multi-functional tumor suppressor gene. J
Cell Sci. 120:3327–3335. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hahn SA, Schutte M, Hoque AT, et al: DPC4,
a candidate tumor suppressor gene at human chromosome 18q21.1.
Science. 271:350–353. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohta M, Inoue H, Cotticelli MG, et al: The
FHIT gene, spanning the chromosome 3p14.2 fragile site and renal
carcinoma-associated t(3;8) breakpoint, is abnormal in digestive
tract cancers. Cell. 84:587–597. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Yen C, Liaw D, et al: PTEN, a
putative protein tyrosine phosphatase gene mutated in human brain,
breast, and prostate cancer. Science. 275:1943–1947. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Steck PA, Pershouse MA, Jasser SA, et al:
Identification of a candidate tumour suppressor gene, MMAC1, at
chromosome 10q23.3 that is mutated in multiple advanced cancers.
Nat Genet. 15:356–362. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Imai FL, Uzawa K, Miyakawa A, Shiiba M and
Tanzawa H: A detailed deletion map of chromosome 20 in human oral
squamous cell carcinoma. Int J Mol Med. 7:43–47. 2001.PubMed/NCBI
|
|
16
|
Miyakawa A, Wang XL, Nakanishi H, et al:
Allelic loss on chromosome 22 in oral cancer: Possibility of the
existence of a tumor suppressor gene on 22q13. Int J Oncol.
13:705–709. 1998.PubMed/NCBI
|
|
17
|
Sparano A, Quesnelle KM, Kumar MS, et al:
Genome-wide profiling of oral squamous cell carcinoma by
array-based comparative genomic hybridization. Laryngoscope.
116:735–741. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamamoto N, Mizoe J, Numasawa H, Tsujii H,
Shibahara T and Noma H: Allelic loss on chromosomes 2q, 3p and 21q:
possibly a poor prognostic factor in oral squamous cell carcinoma.
Oral Oncol. 39:796–805. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roz L, Wu CL, Porter S, et al: Allelic
imbalance on chromosome 3p in oral dysplastic lesions: an early
event in oral carcinogenesis. Cancer Res. 56:1228–1231.
1996.PubMed/NCBI
|
|
20
|
Yamamoto N, Kuroiwa T, Katakura A,
Shibahara T and Choudhury C: Loss of heterozygosity (LOH) on
chromosomes 2q, 3p and 21q in Indian oral squamous cell carcinoma.
Bull Tokyo Dent Coll. 48:109–117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van den Berg A, Dijkhuizen T, Draaijers
TG, et al: Analysis of multiple renal cell adenomas and carcinomas
suggests allelic loss at 3p21 to be a prerequisite for malignant
development. Genes Chromosomes Cancer. 19:228–232. 1997.PubMed/NCBI
|
|
22
|
Hung J, Kishimoto Y, Sugio K, et al:
Allele-specific chromosome 3p deletions occur at an early stage in
the pathogenesis of lung carcinoma. JAMA. 273:19081995. View Article : Google Scholar
|
|
23
|
Zhu Y, Spitz MR, Zheng YL, Hong WK and Wu
X: BPDE-induced lymphocytic 3p21.3 aberrations may predict head and
neck carcinoma risk. Cancer. 95:563–568. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Senchenko V, Liu J, Braga E, et al:
Deletion mapping using quantitative real-time PCR identifies two
distinct 3p21.3 regions affected in most cervical carcinomas.
Oncogene. 22:2984–2992. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Senchenko VN, Liu J, Loginov W, et al:
Discovery of frequent homozygous deletions in chromosome 3p21.3
LUCA and AP20 regions in renal, lung and breast carcinomas.
Oncogene. 23:5719–5728. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baylin SB, Esteller M, Rountree MR,
Bachman KE, Schuebel K and Herman JG: Aberrant patterns of DNA
methylation, chromatin formation and gene expression in cancer. Hum
Mol Genet. 10:687–692. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bird A: DNA methylation patterns and
epigenetic memory. Genes Dev. 16:6–21. 2002. View Article : Google Scholar
|
|
28
|
Bird AP: CpG-rich islands and the function
of DNA methylation. Nature. 321:209–213. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Merlo A, Herman JG, Mao L, et al: 5′ CpG
island methylation is associated with transcriptional silencing of
the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med.
1:686–692. 1995.
|
|
30
|
Steinmann K, Sandner A, Schagdarsurengin U
and Dammann RH: Frequent promoter hypermethylation of tumor-related
genes in head and neck squamous cell carcinoma. Oncol Rep.
22:1519–1526. 2009.PubMed/NCBI
|
|
31
|
Zhang H, Feng X, Liu W, et al: Underlying
mechanisms for LTF inactivation and its functional analysis in
nasopharyngeal carcinoma cell lines. J Cell Biochem. 112:1832–1843.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou W, Feng X, Li H, et al: Functional
evidence for a nasopharyngeal carcinoma-related gene BCAT1 located
at 12p12. Oncol Res. 16:405–413. 2007.PubMed/NCBI
|
|
33
|
Peng D, Ren CP, Yi HM, et al: Genetic and
epigenetic alterations of DLC-1, a candidate tumor suppressor gene,
in nasopharyngeal carcinoma. Acta Biochim Biophys Sin. 38:349–355.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou L, Feng X, Shan W, et al: Epigenetic
and genetic alterations of the EDNRB gene in nasopharyngeal
carcinoma. Oncology. 72:357–363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tuhkanen H, Anttila M, Kosma VM, et al:
Genetic alterations in the peritumoral stromal cells of malignant
and borderline epithelial ovarian tumors as indicated by allelic
imbalance on chromosome 3p. Int J Cancer. 109:247–252. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liao YC and Lo SH: Deleted in liver
cancer-1 (DLC-1): a tumor suppressor not just for liver. Int J
Biochem Cell Biol. 40:843–847. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kang JU, Koo SH, Kwon KC and Park JW:
Frequent silence of chromosome 9p, homozygous DOCK8, DMRT1 and
DMRT3 deletion at 9p24.3 in squamous cell carcinoma of the lung.
Int J Oncol. 37:327–335. 2010.PubMed/NCBI
|
|
38
|
Ishiguro H, Tsunoda T, Tanaka T, Fujii Y,
Nakamura Y and Furukawa Y: Identification of AXUD1, a novel human
gene induced by AXIN1 and its reduced expression in human
carcinomas of the lung, liver, colon and kidney. Oncogene.
20:5062–5066. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Glavic A, Molnar C, Cotoras D and de Celis
JF: Drosophila Axud1 is involved in the control of
proliferation and displays pro-apoptotic activity. Mech Dev.
126:184–197. 2009. View Article : Google Scholar
|
|
40
|
Ngo JT, Bateman JB, Klisak I, Mohandas T,
Van Dop C and Sparkes RS: Regional mapping of a human rod
alpha-transducin (GNAT1) gene to chromosome 3p22. Genomics.
18:724–725. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yi HM, Ren CP, Peng D, Zhou L, Li H and
Yao KT: Expression, loss of heterozygosity, and methylation of
GNAT1 gene in nasopharyngeal carcinoma. Ai Zheng. 26:9–14. 2007.(In
Chinese).
|
|
42
|
Tomizawa Y, Sekido Y, Kondo M, et al:
Inhibition of lung cancer cell growth and induction of apoptosis
after reexpression of 3p21.3 candidate tumor suppressor gene
SEMA3B. Proc Natl Acad Sci USA. 98:13954–13959. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tse C, Xiang RH, Bracht T and Naylor SL:
Human semaphorin 3B (SEMA3B) located at chromosome 3p21.3
suppresses tumor formation in an adenocarcinoma cell line. Cancer
Res. 62:542–546. 2002.PubMed/NCBI
|
|
44
|
Ochi K, Mori T, Toyama Y, Nakamura Y and
Arakawa H: Identification of semaphorin3B as a direct target of
p53. Neoplasia. 4:82–87. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sekido Y, Bader S, Latif F, et al: Human
semaphorins A(V) and IV reside in the 3p21.3 small cell lung cancer
deletion region and demonstrate distinct expression patterns. Proc
Natl Acad Sci USA. 93:4120–4125. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lerman MI and Minna JD: The 630-kb lung
cancer homozygous deletion region on human chromosome 3p21.3:
identification and evaluation of the resident candidate tumor
suppressor genes. The International Lung Cancer Chromosome 3p213
Tumor Suppressor Gene Consortium. Cancer Res. 60:6116–6133.
2000.
|
|
47
|
Li W, Li X, Wang W, et al: NOR1 is an
HSF1- and NRF1-regulated putative tumor suppressor inactivated by
promoter hypermethylation in nasopharyngeal carcinoma.
Carcinogenesis. 32:1305–1314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mao WM, Li P, Zheng QQ, et al:
Hypermethylation-modulated downregulation of RASSF1A expression is
associated with the progression of esophageal cancer. Arch Med Res.
42:182–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu WB, Ao L, Zhou ZY, et al: CpG island
hypermethylation of multiple tumor suppressor genes associated with
loss of their protein expression during rat lung carcinogenesis
induced by 3-methylcholanthrene and diethylnitrosamine. Biochem
Biophys Res Commun. 402:507–514. 2010. View Article : Google Scholar
|
|
50
|
An JH, Choi A, Shin KJ, Yang WI and Lee
HY: DNA methylation-specific multiplex assays for body fluid
identification. Int J Legal Med. 402:507–514. 2012.
|
|
51
|
Riquelme E, Tang M, Baez S, et al:
Frequent epigenetic inactivation of chromosome 3p candidate tumor
suppressor genes in gallbladder carcinoma. Cancer Lett.
250:100–106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kuroki T, Trapasso F, Yendamuri S, et al:
Allelic loss on chromosome 3p21.3 and promoter hypermethylation of
semaphorin 3B in non-small cell lung cancer. Cancer Res.
63:3352–3355. 2003.PubMed/NCBI
|
|
53
|
Ito M, Ito G, Kondo M, et al: Frequent
inactivation of RASSF1A, BLU, and SEMA3B on 3p21.3 by promoter
hypermethylation and allele loss in non-small cell lung cancer.
Cancer Lett. 225:131–139. 2005. View Article : Google Scholar : PubMed/NCBI
|