Introduction
Breast cancer is one of the leading causes of
cancer-related death in women (1).
Despite successful treatment of the primary malignancy, relapse and
subsequent metastatic spread can still occur at distant sites in
the body through the bloodstream or lymphatic channels, including
bone, lung, liver, kidney, thyroid and brain (2). Invasion and metastasis are fundamental
processes and are the major causes of morbidity and mortality in
patients with breast cancer. These processes require degradation of
the extracellular matrix (ECM), which provides biochemical and
mechanical barriers to cancer cell movement (3). The ECM consists of type IV collagen,
laminin, heparan sulfate proteoglycan, nidogen and fibronectin
(4). Degradation of the ECM
requires extracellular proteinases, of which the matrix
metalloproteinases (MMPs) play a critical role in breast cancer.
Among the MMP family, gelatinases A (72 kDa gelatinase, type IV
collagenase, MMP-2) and B (92 kDa gelatinase, type IV collagenase,
MMP-9) play critical roles in ECM degradation and cell migration,
leading to tumor cell invasion in breast cancer (4,5).
MMP-9 is a key enzyme for degrading type IV
collagen, which is a major component of the basement membrane.
Elevated MMP-9 levels are functionally linked to elevated
metastatic potential in many types of tumors, including brain
(6), prostate (7), bladder (8), and breast (9,10). A
variety of stimuli, including growth factors (e.g. fibroblast
growth factor-2, epidermal growth factor and hepatocyte growth
factor), cytokines (e.g., tumor necrosis factor-α), oncogenes
(e.g., Ras) and 12-O-tetradecanoylphorbol-13-acetate (TPA)
are involved in metastasis (11–14).
Among these stimulators, TPA is a well-known selective activator of
protein kinase C (11) and
stimulates MMP-9 synthesis and secretion during MCF-7 cell invasion
(9,10). Consequently, inhibiting MMP-9
expression and/or its upstream regulatory pathways is critical for
treating malignant tumors, including breast carcinoma. NF-κB and
AP-1 are transcription factors important in the regulation of
MMP-9, as the MMP-9 gene promoter contains binding sites for both
factors (15). Studies have shown
that the mitogen-activated protein kinase (MAPK) signaling pathway
is important for AP-1 activation, and that NF-κB activation
requires I-κB kinase, ERK, JNK and p38 MAPK, depending on the cell
type (14,16–18).
Sulfuretin is a major flavonoid isolated from the
heartwood of Rhus verniciflua Stokes (RVS), which has been
used to reduce oxidative stress (19), platelet aggregation (20), the inflammatory response (21) and mutagenesis (22). A recent study found that sulfuretin
inhibits cytokine-induced β-cell damage and prevents
streptozotocin-induced diabetes by suppressing the NF-κB pathway
(23). Therefore, it was
hypothesized that sulfuretin may have anticancer properties that
inhibit cell invasion. In the present study, sulfuretin was
evaluated for its potential activity against TPA-induced cell
invasion and MMP-9 expression in MCF-7 cells, and the related
molecular mechanisms were investigated. Our results demonstrated
that sulfuretin suppresses TPA-induced MMP-9 expression by blocking
NF-κB activation but not AP-1 activation.
Materials and methods
Cells and materials
MCF-7 cells were obtained from the American Type
Culture Collection (Manassas, VA, USA). The cells were cultured in
Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10%
fetal bovine serum (FBS) and 1% antibiotics at 37°C in a 5%
CO2 incubator. Sulfuretin was purchased from Symrise
GmbH & Co. (Holzminden, Germany). TPA,
3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and anti-β-actin antibodies were obtained from Sigma (St. Louis,
MO, USA). Antibodies for p38, p-p38, JNK, p-JNK, ERK and p-ERK were
purchased from Cell Signaling Technology (Beverly, MA, USA).
Antibodies for MMP-9, p50, p65, proliferating cell nuclear antigen
(PCNA), and horseradish peroxidase (HRP)-conjugated IgG were
purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
α-32P-dCTP was obtained from Amersham (Buckinghamshire,
UK). High glucose-containing DMEM, FBS, and phosphate-buffered
saline (PBS) were obtained from Gibco-BRL (Gaithersburg, MD,
USA).
Determination of cell viability
The effect of sulfuretin on MCF-7 cell viability was
determined using an established MTT assay. Briefly,
3xl04 cells were seeded in wells and incubated at 37°C
for 24 h to allow attachment. The attached cells were untreated or
treated with 1, 5, 10, 30, and 50 μM sulfuretin for 24 h at 37°C.
The cells were washed with PBS prior to adding MTT (0.5 mg/ml PBS)
and incubated at 37°C for 30 min. Formazan crystals were dissolved
with dimethyl sulfoxide (100 μl/well) and detected at 570 nm using
a Model 3550 microplate reader (Bio-Rad, Richmond, CA, USA).
Western blot analysis
MCF-7 cells (5×105) were pretreated with
10 and 30 μM sulfuretin for 1 h and then incubated with TPA for 24
h at 37°C. Cells were lysed with ice-cold M-PER®
Mammalian Protein Extraction reagent (Pierce Biotechnology,
Rockford, IL, USA), and the protein concentration in the lysate was
determined using the Bradford method (24). Samples (20 μg) were separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis with 10%
acrylamide and transferred to Hybond™ polyvinylidene fluoride
membranes (GE Healthcare Life Sciences, Buckinghamshire, UK) using
a western blot apparatus. Each membrane was blocked for 2 h with 2%
bovine serum albumin or 5% skim milk and then incubated overnight
at 4°C with 1 μg/ml of a 1:2000 dilution of primary antibody.
HRP-conjugated IgG (1:2000 dilution) was used as the secondary
antibody. Protein expression levels were determined by signal
analysis using an image analyzer (Fuji-Film, Tokyo, Japan).
Gelatin zymography assay
Conditioned media were collected after a 24 h
stimulation, mixed with non-reducing sample buffer, and
electrophoresed on a polyacrylamide gel containing 0.1% (w/v)
gelatin. The gel was washed at room temperature for 30 min with
2.5% Triton X-100 solution, and subsequently incubated at 37°C for
16 h in 5 mM CaCl2, 0.02% Brij and 50 mM Tris-HCl (pH
7.5). The gel was stained for 30 min with 0.25% (w/v) Coomassie
Brilliant Blue in 40% (v/v) methanol/7% (v/v) acetic acid and
photographed using an image analyzer (Fuji-Film). Proteolysis was
imaged as the white zone in a dark blue field. Densitometric
analysis was performed using Multi Gauge Image Analysis software
(Fuji-Film).
Quantitative real-time polymerase chain
reaction (PCR)
Total RNA was extracted from cells using a FastPure™
RNA kit (Takara, Shiga, Japan). The RNA concentration and purity
were determined by absorbance at 260/280 nm. cDNA was synthesized
from 1 μg total RNA using a PrimeScript™ RT Reagent kit (Takara).
MMP-9 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA
expression was determined by real-time PCR using the ABI PRISM 7900
Sequence Detection system and SYBR® Green (Applied
Biosystems, Foster City, CA, USA). The primers were: MMP-9 (NM
004994) sense, CCTGGAGACCTGAGAACCAATCT; antisense, CCACCC
GAGTGTAACCATAGC and GAPDH (NM 002046) sense,
ATGGAAATCCCATCACCATCTT; antisense, CGCCCCAC TTGATTTTGG. All results
were normalized to the GAPDH housekeeping gene to control for
variation in mRNA concentrations. Relative quantification was
performed using the comparative ΔΔCT method according to
the manufacturer’s instructions.
Preparation of the nuclear extract
MCF-7 cells (2×106) were treated with
sulfuretin in the presence or absence of TPA for 4 h. Cells were
immediately washed twice, scraped into 1.5 ml of ice-cold PBS (pH
7.5), and pelleted at 1,500 × g for 3 min. Cytoplasmic and nuclear
extracts were prepared from the cells using the NE-PER®
Nuclear and Cytoplasmic Extraction reagent (Pierce
Biotechnology).
Electrophoretic mobility shift assay
(EMSA)
Activation of NF-κB and AP-1 was assessed with a gel
mobility shift assay using nuclear extracts. An oligonucleotide
containing the κ-chain (κB, 5′-AGTTGAGGGGACTTTCCCAGGC-3′) or AP-1
(5′-CGCTTGATGAGTCAGCCGGAA-3′) binding sites was synthesized and
used as a probe for the gel retardation assay. The two
complementary strands were annealed and labeled with
[α-32P]dCTP. Labeled oligonucleotides (10,000 cpm), 10
μg of nuclear extract and binding buffer (10 mM Tris-HCl, pH 7.6,
500 mM KCl, 10 mM EDTA, 50% glycerol, 100 ng poly(dI·dC) and 1 mM
dithiothreitol) were then incubated for 30 min at room temperature
in a final volume of 20 μl. The reaction mixtures were analyzed by
electrophoresis on 4% polyacrylamide gels in 0.5X Tris-borate
buffer. The gels were dried and examined by autoradiography.
Specific binding was controlled by competition with a 50-fold
excess of cold κB and AP-1 oligonucleotide.
Invasion assay
The invasion assay was carried out in 24-well
chambers (8-μm pore size) coated with 20 μl Matrigel-diluted DMEM.
The Matrigel coating was rehydrated in 0.5 ml DMEM for 30 min
immediately before the experiments. Cells (2×105) were
added to the upper chamber with chemoattractant in the bottom well.
Conditioned medium (0.5 ml) was added to the lower compartment of
the invasion chamber, and the chambers were incubated for 24 h.
After the incubation, cells on the upper side of the chamber were
removed using cotton swabs, and cells that had migrated were fixed
and stained with toluidine blue solution. Invading cells were
counted in five random areas of the membrane using a light
microscope. Data are the means ± standard errors from three
individual experiments performed in triplicate.
Statistical analysis
The statistical analysis was performed using
analysis of variance and Duncan’s test. Differences with a
p<0.05 were considered statistically significant.
Results
Sulfuretin suppresses TPA-induced MMP-9
activation in MCF-7 cells
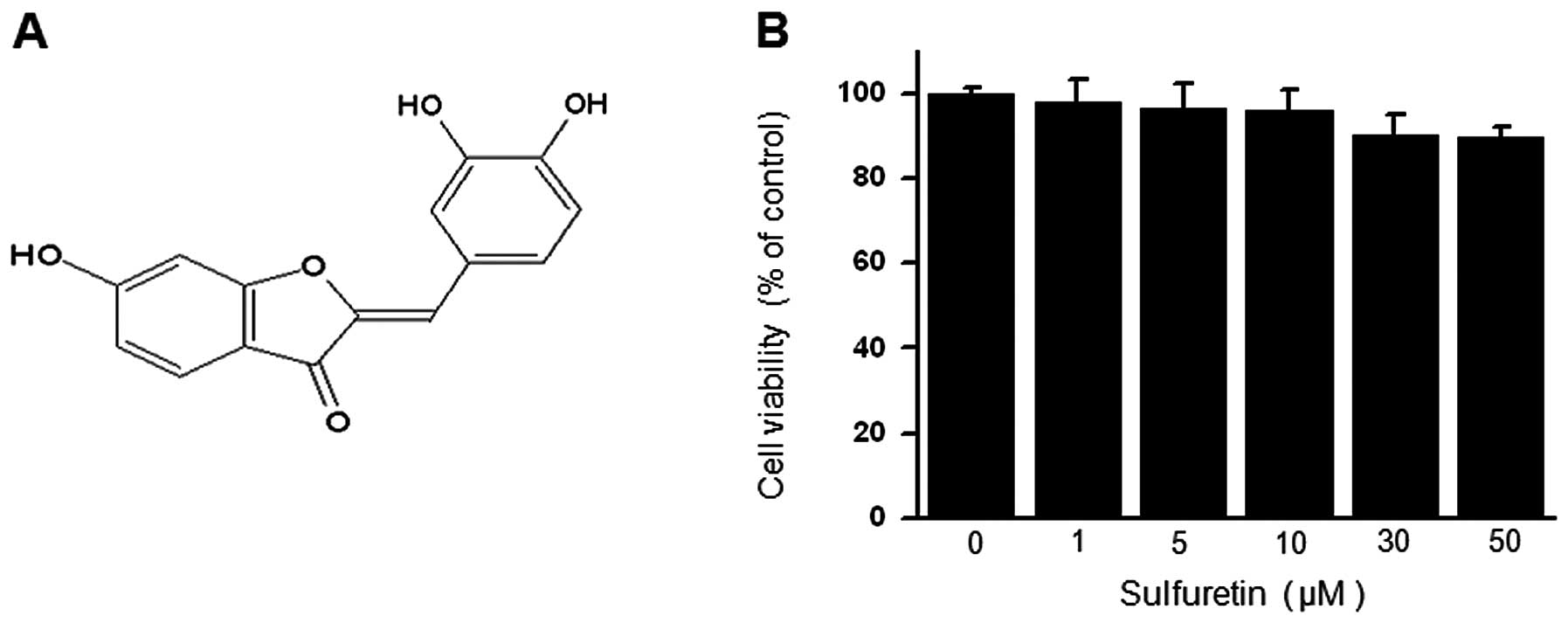
The chemical structure of sulfuretin is shown in
Fig. 1A. To verify the effect of
sulfuretin on cell viability, cells were seeded in 96-well culture
plates at a density of 1×105 cells/well. The cytotoxic
effect of sulfuretin on MCF-7 cells was analyzed using the MTT
assay. Treatment of MCF-7 cells with the indicated concentrations
of sulfuretin for 24 h did not result in any significant change in
cell viability (Fig. 1B).
Therefore, we performed the subsequent experiments using the
optimal non-toxic concentrations (10 and 30 μM) of sulfuretin.
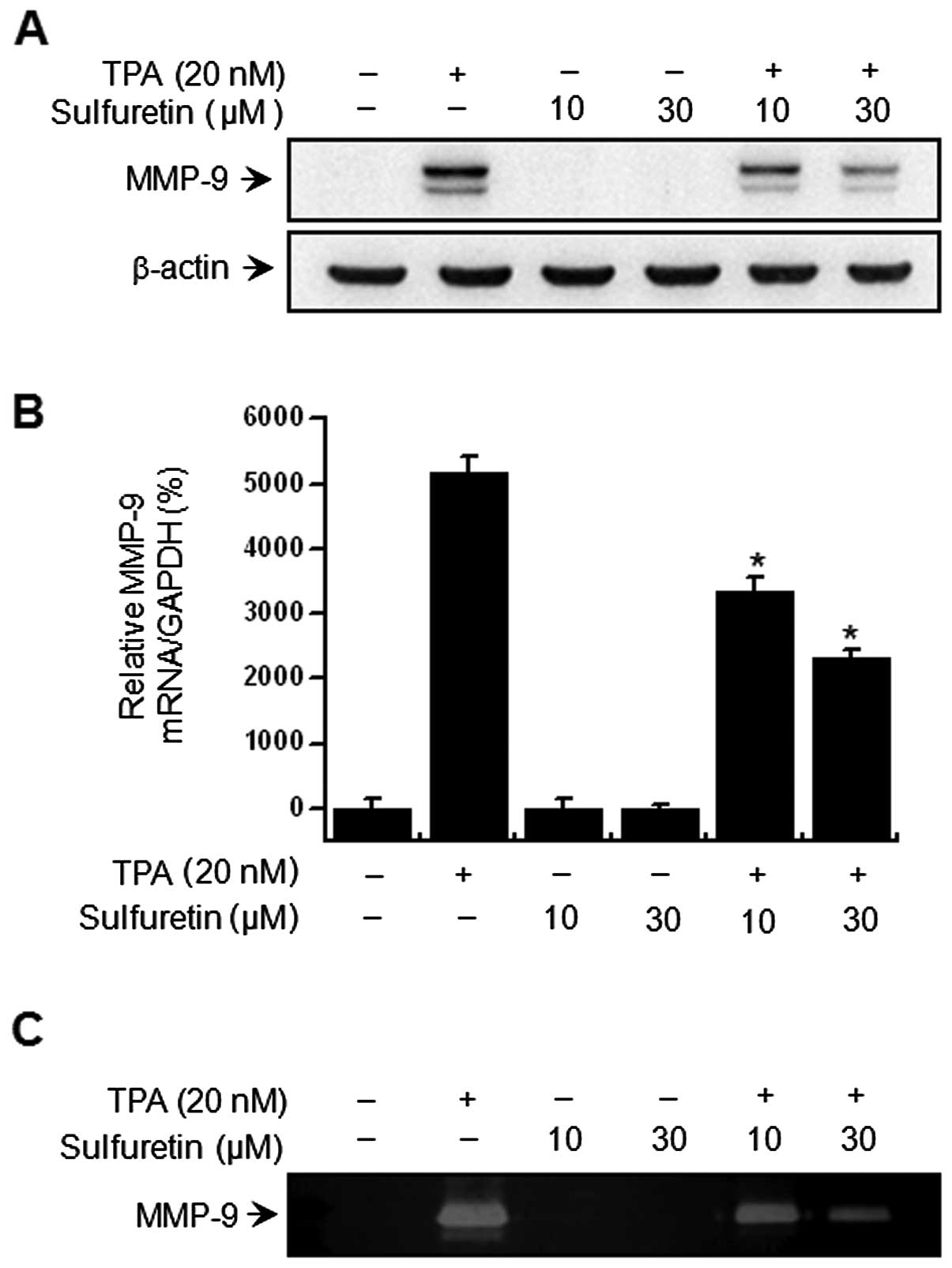
Western blot analysis, real-time PCR and zymography were performed
in MCF-7 cell-containing samples to investigate the effect of
sulfuretin on TPA-induced MMP-9 expression. The western blot
analysis revealed that sulfuretin treatment of MCF-7 cells blocked
upregulation of TPA-induced MMP-9 protein expression (Fig. 2A). Real-time PCR revealed a
TPA-induced increase in the MMP-9 level in MCF-7 cells, and that
sulfuretin blocked TPA-induced MMP-9 upregulation in a
dose-dependent manner (Fig. 2B). A
zymography analysis was carried out to determine the effect of
sulfuretin on TPA-induced MMP-9 secretion. The analysis
demonstrated that treatment of MCF-7 cells with TPA increased MMP-9
secretion, while sulfuretin significantly diminished the
TPA-induced MMP-9 secretion (Fig.
2C). These results were consistent with the potent
sulfuretin-mediated inhibition of TPA-induced MMP-9 expression in
MCF-7 cells.
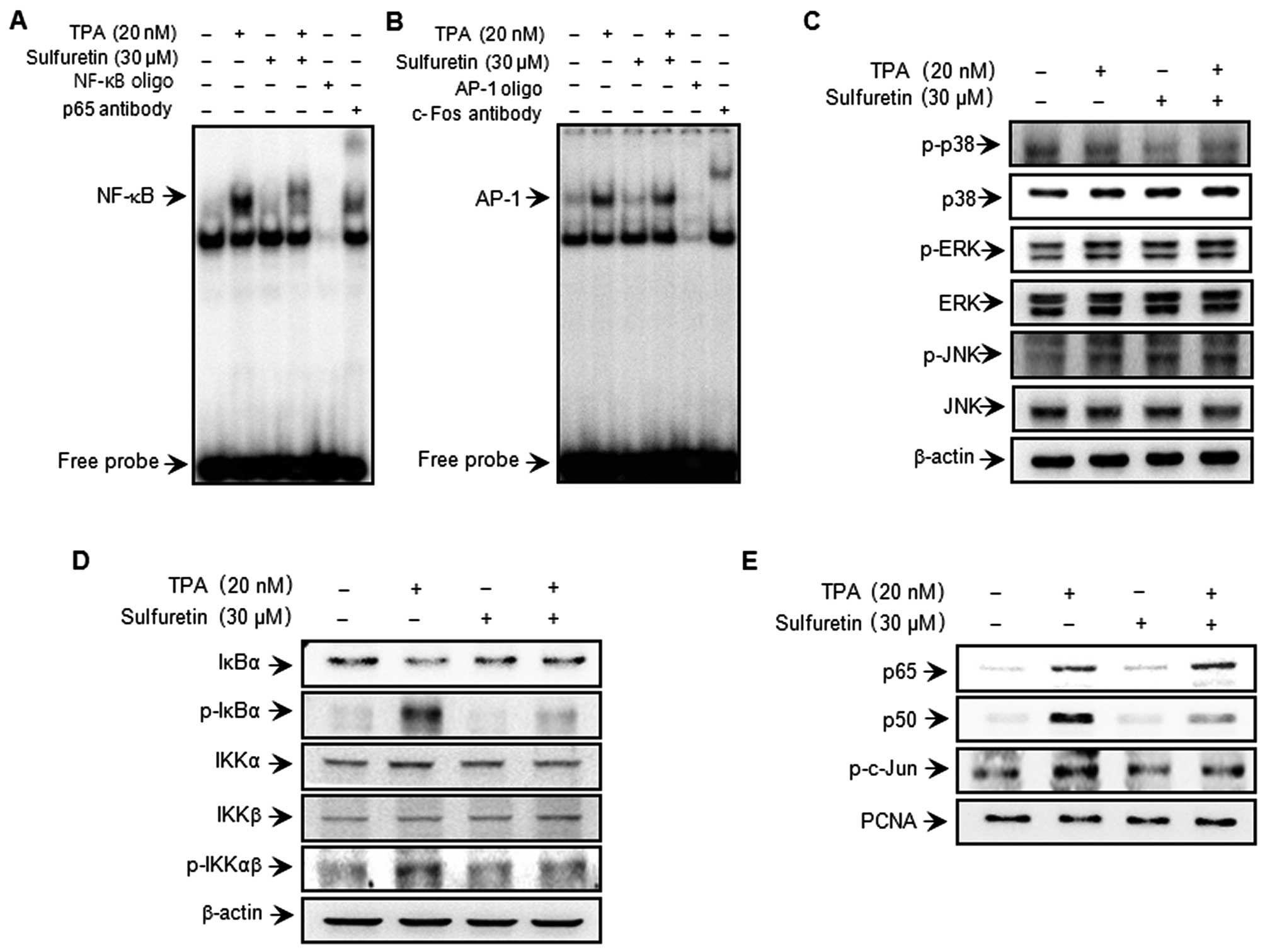
Sulfuretin inhibits TPA-induced NF-κB DNA
binding activity but not the AP-1 and MAPK pathway
To clarify the mechanism involved in the
sulfuretin-mediated inhibition of MMP-9 expression, the effects of
sulfuretin on TPA-induced activation of NF-κB and AP-1 were
evaluated using EMSA. As shown in Fig.
3A and B, TPA substantially increased NF-κB and AP-1 binding
activity. Pretreatment with sulfuretin inhibited TPA-stimulated
NF-κB binding activity but not that of AP-1. These results were
consistent with the view that sulfuretin specifically blocks NF-κB
activation in MCF-7 cells. In the western blot analysis, TPA
stimulated the phosphorylation of IKKκβ and IκBα in the cytoplasm
and, thereby, the nuclear translocation of NF-κB subunits p50 and
p65. In the case of AP-1, c-Jun expression was considerably
augmented, while c-Fos expression was only negligibly induced in
the TPA-treated cells. Based on our results, the increased p-IKKαβ,
p-IκBα and translocation of p65 and p50 as a result of TPA
stimulation was significantly suppressed by sulfuretin treatment
(Fig. 3D and E). We confirmed that
TPA induced the phosphorylation of c-Jun, a major subunit of AP-1,
and sulfuretin had no effect on c-Jun phosphorylation (Fig. 3E). The effect of sulfuretin on
TPA-induced phosphorylation and activation of MAPKs was
investigated to determine the inhibitory effect of sulfuretin on
MAPK. Sulfuretin had no effect on MAPK phosphorylation 30 min after
TPA treatment (Fig. 3C). These
results suggest that the regulation of TPA-induced MMP-9 expression
by sulfuretin does not involve the MAPK pathway.
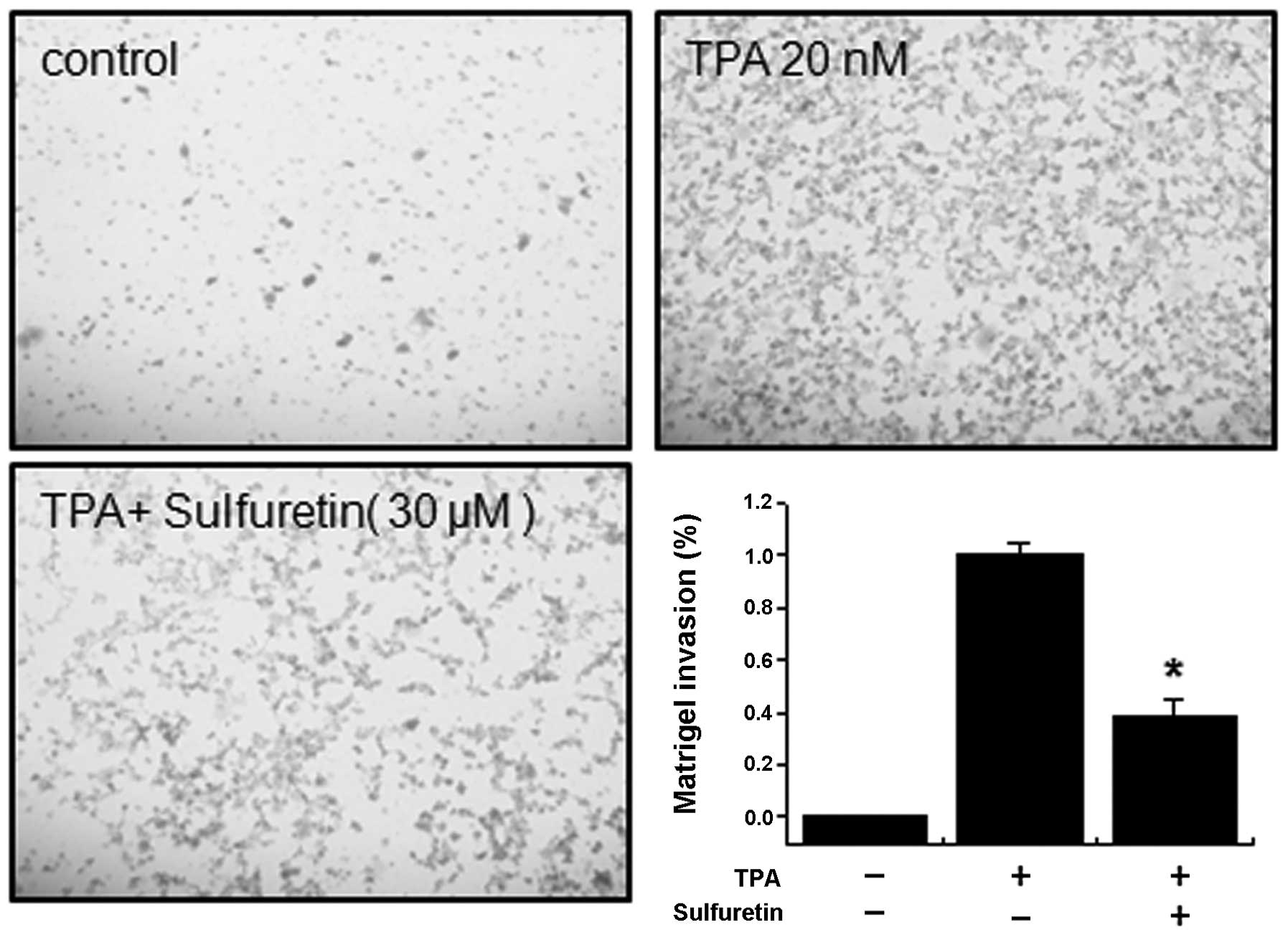
Effect of sulfuretin on TPA-induced MCF-7
cell invasion in vitro
Upregulation of MMP-9 expression contributes to the
increased invasiveness of cancer cells (25,26).
An in vitro invasion assay was used to investigate the
inhibitory effects of sulfuretin on the invasive ability of MCF-7
cells. Treatment with TPA increased MCF-7 cell invasion when
compared with that in untreated control cells, as determined by a
Matrigel invasion assay. Incubating MCF-7 cells with TPA resulted
in a 10-fold increase in cell invasiveness. However, treatment with
sulfuretin diminished TPA-induced cell invasion by 65% (Fig. 4).
Discussion
Studies involved in the development of effective
anti-invasive strategies have focused mainly on the utilization of
natural bioactive agents in MCF-7 cells. In the present study, we
isolated sulfuretin from the heartwood of Rhus verniciflua
Stokes (RVS) and examined its effects on TPA-induced MMP-9
expression and cellular invasion in MCF-7 cells. RVS exerts a
remarkable spectrum of biological activities affecting basic
cellular functions, and the identification of sulfuretin as an
active principle agent has been demonstrated (19,22,23,27–34).
In previous reports, sulfuretin exerted anticancer and
antiproliferative effects in various types of cells (28,30).
Additionally and consistent with these results, our previous
observations indicated that sulfuretin inhibits MMP expression and
inflammatory responses by interfering with the transcriptional
activation of NF-κB (23,27,35).
However, no study has reported on the anti-invasive effects of
sulfuretin in MCF-7 cells. Thus, we designed this study to estimate
the anti-invasive potential of sulfuretin and to explore the
molecular mechanisms underlying its activity.
Metastasis is the primary cause of breast cancer
mortality. Tumor metastasis is a multistep process by which a
subset of individual cancer cells disseminates from a primary tumor
to distant secondary organs or tissues. This process involves cell
proliferation, ECM degradation, cell migration and tumor growth at
metastatic sites (10,36). Tumor cell invasion is an early step
in the metastatic cascade, representing the beginning of the
transition from the benign stage to malignancy. Tumor invasion is
morphologically associated with a distorted edge of the primary
tumor where individual or cohorts of tumor cells actively invade
the ECM surrounding the primary tumor (37). MMP-9 has been regarded as a major
critical molecule during tumor invasion and metastasis. MMP-9
activation is particularly associated with tumor progression and
invasion, including that in mammary tumors (38). Inflammatory cytokines, growth
factors and phorbol esters stimulate MMP-9 by activating different
intracellular-signaling pathways in breast cancer cells (39–41).
The inhibitory effect on MMP-9 expression is important for
developing a therapeutic experimental model of tumor
metastasis.
NF-κB and AP-1 are transcription factors important
in regulating MMP-9, as the MMP-9 gene promoter contains NF-κB and
AP-1 binding sites (15). AP-1,
which belongs to the bZIP group of DNA-binding proteins, associates
to form a variety of homodimers and heterodimers through a
combination of signaling events, leading to increased activity of
proteins that directly potentiate Jun and Fos family members or
activate transcription factors that regulate c-jun and c-fos
expression (42–45). NF-κB comprises a family of inducible
transcription factors that regulate host inflammatory and immune
responses (46). Diverse signal
transduction cascades mediate stimulation of the NF-κB pathway
(46). NF-κB and AP-1 elements are
centrally involved in TPA-mediated MMP-9 gene induction (36,47).
Additionally, the MAPK signaling pathway is important for NF-κB
activation, which requires I-κB kinase depending on cell type
(14,16–18,48).
However, our results showed that sulfuretin inhibited activation of
NF-κB but not MAPK or AP-1 in MCF-7 cells. This result suggests
that sulfuretin inhibited TPA-induced MMP-9 expression through the
NF-κB pathway.
These experiments confirmed that TPA-stimulated cell
invasion was suppressed by sulfuretin. Data obtained from the
Matrigel invasion assay showed that sulfuretin inhibited the
TPA-induced invasive potential of MCF-7 cells (Fig. 4). These data suggest that the
inhibition cell invasion caused by sulfuretin was correlated with
inhibition of MMP-9 expression and the NF-κB signaling pathway.
In conclusion, our results demonstrated that
sulfuretin is a potent inhibitor of TPA-induced MMP-9 expression,
and that it strongly blocked the NF-κB signaling pathway in breast
carcinoma cells. This is the first study to show that sulfuretin
suppresses TPA-stimulated cancer cell invasion by inhibiting MMP-9
expression. We also detailed the molecular mechanisms of the NF-κB
pathway in breast cancer cells responsible for this inhibitory
effect. Thus, sulfuretin may be a potential candidate for
preventing breast tumor invasion and metastasis in vivo.
Acknowledgements
This study was supported by a National Research
Foundation of Korea (NRF) grant funded by the Korea Government
(MEST) (no. 2011-0030716), Republic of Korea.
References
|
1
|
Jemal A, Murray T, Ward E, et al: Cancer
statistics, 2005. CA Cancer J Clin. 55:10–30. 2005. View Article : Google Scholar
|
|
2
|
Friedel G, Pastorino U, Ginsberg RJ, et
al: Results of lung metastasectomy from breast cancer: prognostic
criteria on the basis of 467 cases of the International Registry of
Lung Metastases. Eur J Cardiothorac Surg. 22:335–344. 2002.
View Article : Google Scholar
|
|
3
|
Woessner JF Jr: Matrix metalloproteinases
and their inhibitors in connective tissue remodeling. FASEB J.
5:2145–2154. 1991.PubMed/NCBI
|
|
4
|
Nakajima M, Welch DR, Belloni PN and
Nicolson GL: Degradation of basement membrane type IV collagen and
lung subendothelial matrix by rat mammary adenocarcinoma cell
clones of differing metastatic potentials. Cancer Res.
47:4869–4876. 1987.PubMed/NCBI
|
|
5
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saito N, Hatori T, Murata N, et al: A
double three-step theory of brain metastasis in mice: the role of
the pia mater and matrix metalloproteinases. Neuropathol Appl
Neurobiol. 33:288–298. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Castellano G, Malaponte G, Mazzarino MC,
et al: Activation of the osteopontin/matrix metalloproteinase-9
pathway correlates with prostate cancer progression. Clin Cancer
Res. 14:7470–7480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanayama H: Matrix metalloproteinases and
bladder cancer. J Med Invest. 48:31–43. 2001.PubMed/NCBI
|
|
9
|
Lin CW, Hou WC, Shen SC, et al: Quercetin
inhibition of tumor invasion via suppressing PKC
delta/ERK/AP-1-dependent matrix metalloproteinase-9 activation in
breast carcinoma cells. Carcinogenesis. 29:1807–1815. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee SO, Jeong YJ, Kim M, Kim CH and Lee
IS: Suppression of PMA-induced tumor cell invasion by capillarisin
via the inhibition of NF-kappaB-dependent MMP-9 expression. Biochem
Biophys Res Commun. 366:1019–1024. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Newton AC: Regulation of protein kinase C.
Curr Opin Cell Biol. 9:161–167. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zeigler ME, Chi Y, Schmidt T and Varani J:
Role of ERK and JNK pathways in regulating cell motility and matrix
metalloproteinase 9 production in growth factor-stimulated human
epidermal keratinocytes. J Cell Physiol. 180:271–284. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hozumi A, Nishimura Y, Nishiuma T, Kotani
Y and Yokoyama M: Induction of MMP-9 in normal human bronchial
epithelial cells by TNF-alpha via NF-kappa B-mediated pathway. Am J
Physiol Lung Cell Mol Physiol. 281:L1444–L1452. 2001.PubMed/NCBI
|
|
14
|
Weng CJ, Chau CF, Hsieh YS, Yang SF and
Yen GC: Lucidenic acid inhibits PMA-induced invasion of human
hepatoma cells through inactivating MAPK/ERK signal transduction
pathway and reducing binding activities of NF-kappaB and AP-1.
Carcinogenesis. 29:147–156. 2008. View Article : Google Scholar
|
|
15
|
Eberhardt W, Huwiler A, Beck KF, Walpen S
and Pfeilschifter J: Amplification of IL-1 beta-induced matrix
metalloproteinase-9 expression by superoxide in rat glomerular
mesangial cells is mediated by increased activities of NF-kappa B
and activating protein-1 and involves activation of the
mitogen-activated protein kinase pathways. J Immunol.
165:5788–5797. 2000.
|
|
16
|
Yao J, Xiong S, Klos K, et al: Multiple
signaling pathways involved in activation of matrix
metalloproteinase-9 (MMP-9) by heregulin-beta1 in human breast
cancer cells. Oncogene. 20:8066–8074. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karin M: The regulation of AP-1 activity
by mitogen-activated protein kinases. J Biol Chem. 270:16483–16486.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Madrid LV, Mayo MW, Reuther JY and Baldwin
AS Jr: Akt stimulates the transactivation potential of the RelA/p65
subunit of NF-kappaB through utilization of the IkappaB kinase and
activation of the mitogen-activated protein kinase p38. J Biol
Chem. 276:18934–18940. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JC, Lim KT and Jang YS: Identification
of Rhus verniciflua Stokes compounds that exhibit free
radical scavenging and anti-apoptotic properties. Biochim Biophys
Acta. 1570:181–191. 2002.
|
|
20
|
Jeon WK, Lee JH, Kim HK, et al:
Anti-platelet effects of bioactive compounds isolated from the bark
of Rhus verniciflua Stokes. J Ethnopharmacol. 106:62–69.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jung CH, Kim JH, Hong MH, et al:
Phenolic-rich fraction from Rhus verniciflua Stokes (RVS)
suppress inflammatory response via NF-kappaB and JNK pathway in
lipopolysaccharide-induced RAW 264.7 macrophages. J Ethnopharmacol.
110:490–497. 2007.
|
|
22
|
Park KY, Jung GO, Lee KT, et al:
Antimutagenic activity of flavonoids from the heartwood of Rhus
verniciflua. J Ethnopharmacol. 90:73–79. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song MY, Jeong GS, Kwon KB, et al:
Sulfuretin protects against cytokine-induced beta-cell damage and
prevents streptozotocin-induced diabetes. Exp Mol Med. 42:628–638.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chambers AF and Matrisian LM: Changing
views of the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stetler-Stevenson WG, Hewitt R and
Corcoran M: Matrix metalloproteinases and tumor invasion: from
correlation and causality to the clinic. Semin Cancer Biol.
7:147–154. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song MY, Jeong GS, Lee HS, et al:
Sulfuretin attenuates allergic airway inflammation in mice. Biochem
Biophys Res Commun. 400:83–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jang HS, Kook SH, Son YO, et al:
Flavonoids purified from Rhus verniciflua Stokes actively
inhibit cell growth and induce apoptosis in human osteosarcoma
cells. Biochim Biophys Acta. 1726:309–316. 2005.PubMed/NCBI
|
|
29
|
Jang DS, Park EJ, Hawthorne ME, et al:
Potential cancer chemopreventive constituents of the seeds of
Dipteryx odorata (tonka bean). J Nat Prod. 66:583–587. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee JC, Lee KY, Kim J, et al: Extract from
Rhus verniciflua Stokes is capable of inhibiting the growth
of human lymphoma cells. Food Chem Toxicol. 42:1383–1388. 2004.
|
|
31
|
Son YO, Lee KY, Lee JC, et al: Selective
antiproliferative and apoptotic effects of flavonoids purified from
Rhus verniciflua Stokes on normal versus transformed hepatic
cell lines. Toxicol Lett. 155:115–125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ko JH, Lee SJ and Lim KT: 36 kDa
glycoprotein isolated from Rhus verniciflua Stokes fruit has
a protective activity to glucose/glucose oxidase-induced apoptosis
in NIH/3T3 cells. Toxicol In Vitro. 19:353–363. 2005.
|
|
33
|
Lim KT, Hu C and Kitts DD: Antioxidant
activity of a Rhus verniciflua Stokes ethanol extract. Food
Chem Toxicol. 39:229–237. 2001.
|
|
34
|
Choi J, Yoon BJ, Han YN, et al:
Antirheumatoid arthritis effect of Rhus verniciflua and of
the active component, sulfuretin. Planta Med. 69:899–904. 2003.
View Article : Google Scholar
|
|
35
|
Lee YR, Hwang JK, Koh HW, et al:
Sulfuretin, a major flavonoid isolated from Rhus
verniciflua, ameliorates experimental arthritis in mice. Life
Sci. 90:799–807. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chung TW, Moon SK, Chang YC, et al: Novel
and therapeutic effect of caffeic acid and caffeic acid phenyl
ester on hepatocarcinoma cells: complete regression of hepatoma
growth and metastasis by dual mechanism. FASEB J. 18:1670–1681.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar
|
|
38
|
Scorilas A, Karameris A, Arnogiannaki N,
et al: Overexpression of matrix-metalloproteinase-9 in human breast
cancer: a potential favourable indicator in node-negative patients.
Br J Cancer. 84:1488–1496. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cho HJ, Kang JH, Kwak JY, et al:
Ascofuranone suppresses PMA-mediated matrix metalloproteinase-9
gene activation through the Ras/Raf/MEK/ERK- and Ap1-dependent
mechanisms. Carcinogenesis. 28:1104–1110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kajanne R, Miettinen P, Mehlem A, et al:
EGF-R regulates MMP function in fibroblasts through MAPK and AP-1
pathways. J Cell Physiol. 212:489–497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Srivastava AK, Qin X, Wedhas N, et al:
Tumor necrosis factor-alpha augments matrix metalloproteinase-9
production in skeletal muscle cells through the activation of
transforming growth factor-beta-activated kinase 1 (TAK1)-dependent
signaling pathway. J Biol Chem. 282:35113–35124. 2007. View Article : Google Scholar
|
|
42
|
Frigo DE, Tang Y, Beckman BS, et al:
Mechanism of AP-1-mediated gene expression by select
organochlorines through the p38 MAPK pathway. Carcinogenesis.
25:249–261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shaulian E and Karin M: AP-1 in cell
proliferation and survival. Oncogene. 20:2390–2400. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yokoo T and Kitamura M: Dual regulation of
IL-1 beta-mediated matrix metalloproteinase-9 expression in
mesangial cells by NF-kappa B and AP-1. Am J Physiol.
270:F123–F130. 1996.PubMed/NCBI
|
|
45
|
Lungu G, Covaleda L, Mendes O,
Martini-Stoica H and Stoica G: FGF-1-induced matrix
metalloproteinase-9 expression in breast cancer cells is mediated
by increased activities of NF-kappaB and activating protein-1. Mol
Carcinog. 47:424–435. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yamamoto Y and Gaynor RB: Therapeutic
potential of inhibition of the NF-kappaB pathway in the treatment
of inflammation and cancer. J Clin Invest. 107:135–142. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hong S, Park KK, Magae J, et al:
Ascochlorin inhibits matrix metalloproteinase-9 expression by
suppressing activator protein-1-mediated gene expression through
the ERK1/2 signaling pathway: inhibitory effects of ascochlorin on
the invasion of renal carcinoma cells. J Biol Chem.
280:25202–25209. 2005. View Article : Google Scholar
|
|
48
|
Ruhul Amin AR, Senga T, Oo ML, Thant AA
and Hamaguchi M: Secretion of matrix metalloproteinase-9 by the
proinflammatory cytokine, IL-1beta: a role for the dual signalling
pathways, Akt and Erk. Genes Cells. 8:515–523. 2003.PubMed/NCBI
|