Introduction
Malignant pleural mesothelioma (MPM) is a highly
aggressive tumor that arises from the mesothelial cells of pleural
cavities. MPM is associated with asbestos and develops decades
after exposure. Epidemiological evidence indicates that the
incidence of mesothelioma will increase over the next 10 to 20
years because of the historical use of asbestos and the quantities
consumed (1). Since MPM responds
poorly to conventional treatments, such as chemotherapy, surgery
and radiation therapy, novel approaches for MPM treatment are
urgently required. Therefore, it is critically important to define
the molecular events involved in the induction and progression of
MPM.
To address this issue, we focused our attention on
the transmembrane type I glycoprotein called podoplanin, which
consists of 162 amino acid residues, including 9 that reside in the
cytoplasm. Podoplanin is widely expressed in the body and can be
detected in kidney podocytes, placenta, skeletal muscle, lung,
heart and mesothelial cells (2).
Podoplanin null mice die at birth of lethal respiratory failure
accompanied by immature lymphatic vessel formation (3,4).
Podoplanin is used as a specific marker for lymphatic endothelial
cells (5). Although it is
reasonable to assume that podoplanin plays an important role in the
development of the lymphatic vascular system, its physiological
function is unknown.
Podoplanin is expressed at abnormally high levels in
many types of human cancers, including squamous cell carcinoma of
the oral cavity, larynx, lung, cervix, esophagus and skin, as well
as dysgerminomas of the ovary, granulosa cell tumors, and tumors of
the central nervous system (6–10).
Podoplanin is also expressed by MPM, and therefore is a useful
marker for diagnosing this disease (11–16).
Podoplanin expression also correlates with poor prognosis of oral,
renal and brain tumors (17–19).
Podoplanin promotes the migratory and invasive properties of the
Madin-Darby canine kidney type II epithelial cell line (20).
Podoplanin, also known as Aggrus, possesses
platelet-aggregating activity associated with its PLAG domain.
Moreover, podoplanin-induced platelet aggregation facilitates
hematogenous metastasis. Chinese hamster ovary cell lines
transfected with a podoplanin expression vector are more metastatic
to lung than control cells, as determined using a mouse
experimental metastasis model (21). Based on these findings, we believe
that it is reasonable to conclude that podoplanin enhances the
metastatic potential of tumor cells.
Here, to address the role of podoplanin in MPM and
to determine whether it is a suitable target for designing more
efficacious therapies for MPM, we generated an MPM cell line that
overexpresses podoplanin. We determined the effects of
overexpression of podoplanin in these cells in vitro with
respect to their ability to migrate, invade an artifical matrix,
and withstand the effects of inducers of apoptosis. The tumorigenic
properties of the cells were assessed using a nude mouse xenograft
model.
Materials and methods
Cell lines and animals
The MSTO-211H and NCI-H226 human mesothelioma cell
lines were obtained from the American Type Culture Collection
(ATCC, Manassas, VA, USA). The cells were maintained in RPMI-1640
medium (Sigma, St. Louis, MO, USA) containing 10% fetal bovine
serum (FBS) and antibiotics (100 U/ml penicillin and 100 μg/ml
streptomycin). The medium was replaced every 2–3 days, and the
cells were subcultured by treatment with 0.25% trypsin/0.53 mM
EDTA.
Female BALB/c-nu/nu mice (age, 6 weeks) were
obtained from Clea Japan Inc. (Tokyo, Japan) and housed in laminar
flow cabinets under specific pathogen-free conditions. All
experiments using mice were conducted in accordance with the
guidelines of the National Institutes of Health (NIH, Bethesda, MD,
USA) for the care and use of laboratory animals. The Animal Care
and Experimentation Committee, Gunma University, approved the study
protocol (approval no. 08-30).
Construction of a human podoplanin
expression vector and stable transfection
A vector capable of expressing human podoplanin was
constructed using the p3xFLAG-CMV-14 vector (Invitrogen, Tokyo,
Japan) according to the manufacturer’s instructions. The human
podoplanin coding sequence was cloned using the pGEM®-T
Easy vector (Invitrogen) into which we inserted the full-length
podoplanin cDNA. The cDNA was generated by polymerase chain
reaction (PCR) amplification using high-fidelity
Platinum® TaqDNA polymerase (Invitrogen) and specific
primers (forward, 5′-GGAAGGTGTCAGCTCTGCTC-3′ and reverse, 5′-CGCC
TTCCAAACCTGTAGTC-3′) (6). The
resulting construct was named p3xFLAG-Podoplanin-CMV-14. After
verification by automated nucleotide sequence analysis, recombinant
DNA plasmids were transfected into mesothelioma cells using
Lipofectamine™ 2000 transfection reagent (Invitrogen) according to
the manufacturer’s instructions. The positive colonies were
selected based on their resistance to 400 μg/ml
Geneticin® (Invitrogen) and identified by reverse
transcriptase (RT)-PCR and flow cytometry. In the present study,
clonal cell lines expressing podoplanin were designated PODO1 and
PODO2. Cells transfected with the p3xFLAG-CMV-14 vector were
designated p3x. The transfectants were used before passage 20 in
all cases in order to minimize the potential impacts of clonal
variations and phenotypic instability. Cell cultures were used for
all functional and biological assays upon reaching 70–90%
confluence. The viability of cells in these cultures was
>95%.
RT-PCR
Total RNA was extracted from cultured cells using
TRIzol® reagent (Invitrogen), and RT-PCR was performed
according to the manufacturer’s instructions using the following
specific primers as described previously: matrix
metalloproteinase-1 (MMP-1) (22)
forward, 5′-GAGCAAACACATCTG AGGTACAGGA-3′ and reverse,
5′-TTGTCCCGATGATCT CCCCTGACA-3′; MMP-2 (22) forward, 5′-AGATCTTCTTC
TTCAAGGACCGGTT-3′ and reverse, 5′-GGCTGGTCAGT GGCTTGGGGTA-3′; MMP-7
(23) forward, 5′-AAACTCCC
GCGTCATAGAAAT-3′ and reverse, 5′-TCCCTAGACTGC TACCATCCG-3′; MMP-9
(24) forward, 5′-CACCTTCACTC
GCGTGTAC-3′ and reverse, 5′-CATCTGCGTTTCCAAACC GAG-3′;
platelet-derived growth factor (PDGF-BB) (25) forward, 5′-CTAGGCTCCAAGGGTCTCCT-3′
and reverse, 5′-GAGAAAGATCGAGATTGTGC-3′; PDGFR-β (26) forward, 5′-GTGAACGCAGTGCAGACTGT-3′
and reverse, 5′-AGGTGTAGGTCCCCGAGTCT-3′. As internal controls,
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (27) forward, 5′-CGACCACTTTGTCAAGCTCAT-3′
and reverse, 5′-CCCTGTTGCTGTAGCCAAATT-3′ and β-actin (27) forward, 5′-GATGATGATATCGCCGCGCT-3′
and reverse, 5′-TGGGTCATCTTCTCGCGGTT-3′ were used.
Flow cytometry
The transfected p3x, PODO1 and PODO2 cells were
harvested, and single-cell suspensions (1×106 cells)
were prepared. The cells were treated according to the standardized
protocol as follows. The cells were harvested after brief exposure
to trypsin. After washing with phosphate-buffered saline (PBS), the
cells were treated with anti-podoplanin mouse monoclonal antibody
(ab10288; Abcam, Tokyo, Japan) for 30 min at 4°C and then incubated
with FITC-conjugated anti-mouse immunoglobulin antibody
(DakoCytomation, Glostrup, Denmark) for 30 min at 4°C. Flow
cytometric analysis was performed using a BD FACSCalibur flow
cytometer (Becton-Dickinson, San Jose, CA, USA), and the data were
analyzed using CellQuest software (Becton-Dickinson).
Platelet aggregation assay
Platelet aggregation was measured using a platelet
aggregometer, PA-200 (Kowa Co., Ltd., Nagoya, Japan). Platelet-rich
plasma (PRP) and platelet-poor plasma (PPP) were obtained from the
blood of healthy donors, which was treated with 3.13% sodium
citrate (blood:sodium citrate, 9:1) to inhibit coagulation and then
centrifuged sequentially at 170 × g for 10 min and 1,880 × g for 10
min. When the platelet count of PRP was >200×109/l,
PRP was diluted by PPP to 200×109/l. The sample was
placed in a plastic cuvette containing a magnetic stirring bar at
37°C for 10 min. CaCl2 (200 mM) was added, and 3 min
later, platelet aggregation was initiated by adding PODO1, PODO2,
or p3× cells (5×106 cells/sample), and the samples were
monitored for at least 15 min.
Cell migration and Matrigel invasion
assay
Cell invasiveness was assessed using BioCoat™
Matrigel™ invasion chambers (Becton-Dickinson) according to the
protocol provided by the manufacturer. In brief, 1×104
cells were seeded in the upper compartment in serum-free RPMI-1640
medium. The lower compartment was filled with RPMI-1640 medium
containing 10% FBS as a chemoattractant. After incubation for 48 h
at 37°C in humidified air containing 5% CO2,
non-invading cells remaining on the upper surface of the chamber
were removed by scrubbing with a cotton-tipped swab. The invading
cells that adhered to the bottom surface of the chamber membrane
were fixed and counted after staining with Mayer’s hematoxylin and
eosin. The assays were performed in triplicate, at least 5 fields
were counted/filter, and mean cell numbers and standard deviations
were calculated. For the cell migration analysis, polycarbonate
filters (pore size, 6 μm) were used without Matrigel (BD Falcon™
Cell Culture Inserts; Becton-Dickinson).
Wound migration assay
Mesothelioma cell lines were seeded in a Petri dish
at 1×106 cells/ml and grown in RPMI-1640 medium
containing 10% FBS until the cells were almost confluent. The
medium was removed and two lines were drawn using a pipette tip
through the cell monolayer. The cells were washed twice with PBS
and RPMI-1640 medium. After 0, 6, 12 and 24 h images were captured
using a Nikon TMS microscope at magnification, ×40.
Cell proliferation assay
Cell Counting Kit (CCK)-8 (Dojindo, Tokyo, Japan)
was used to determine cell proliferation. The cells were harvested
and deposited in 96-well plates (1×104 cells/well) and
maintained in a humidified atmosphere containing 5% CO2
at 37°C. At each time point, 10 μl of the cell counting solution
was added to triplicate wells and incubated for 0.5 h. The formazan
dye generated by dehydrogenases in the cells was dissolved in 100
μl/well 1 N HCl (100 μl/well), and the absorbance at 450 nm was
measured using a microtiter plate reader (Molecular Devices,
Sunnyvale, CA, USA) to calculate the numbers of viable cells in
each well.
Cell extraction and western blot
analysis
Lysates were prepared from exponentially growing
cells using a buffer containing the following components: 20 mM
Tris-HCl (pH 7.6), 1 mM EDTA, 140 mM NaCl, 1% Nonidet P-40, 1%
aprotinin, 1 mM phenylmethylsulfonyl fluoride, and 1 mM sodium
vanadate. Protein concentrations were determined using a BCA
protein assay kit (Pierce, Rockford, IL, USA). Protein (40 μg)
samples from each cell line were added to sodium dodecyl sulphate
(SDS) sample buffer [100 mM Tris-HCl (pH 8.8), 0.01% bromophenol
blue, 36% glycerol, 4% SDS and 1 mM dithiothreitol], boiled for 5
min, and electrophoresed through a 5–20% acrylamide gel
(Tris-Tricine Ready Gels; Bio-Rad, Tokyo, Japan). Proteins were
electrophoretically transferred to a Hybond-N+ nitrocellulose
membrane (Amersham Pharmacia Biotech, Buckinghamshire, UK).
Antibodies to PKB/Akt (BD Biosciences, Tokyo, Japan) p44/p42
mitogen-activated protein kinase (MAPK), p38 MAPK and SAPK/JNK
(MAPK family antibody sampler kit, phospho-Akt, phospho-p44/p42
MAPK, phospho-p38 MAPK, and phospho-SAPK/JNK (phospho-MAPK family
antibody sampler kit) (all were from Cell Signaling Technology,
Inc., Danvers, MA, USA) and caspase-3 (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) were used. The antibodies were diluted
(1:1,000) and incubated with the membranes overnight at 4°C. After
washing, the proteins were incubated with the secondary antibody
(horseradish peroxidase-conjugated anti-mouse or anti-rabbit
immunoglobulin G antibody) diluted 1:1,000. The antigen-antibody
complexes were detected using an enhanced chemiluminescence
detection system (Amersham Pharmacia Biotech). Membranes were
reprobed using anti-β-actin (Sigma) as a loading control.
Assays for apoptosis
Mesothelioma cell lines were seeded in complete
growth medium, incubated for 16 h, and treated with
cis-diamminedichloroplatinum(II) (CDDP) (cisplatin) for 48 h
at 80% confluence. The MSTO-211H and NCI-H226 cultures were treated
with 5 μM (IC50) and 25 μM CDDP. CDDP was not added to
the control cells. The IC50 value is defined as the
concentration required to reduce absorbance (calculated from the
growth curve) by 50%. After 48 h, detached cells were collected,
and the adherent cells were harvested after treatment with trypsin.
The cells (1×105) were washed twice with PBS and
resuspended in 250 μl of binding buffer (Annexin V/FITC Apoptosis
Detection kit; Sigma) that contained 2 μl of 20 mg/ml propidium
iodide (PI) and 2.5 μl of Annexin V/FITC. The data were collected
using FACSCalibur (Becton-Dickinson). FITC and PI emissions were
detected in the FL-1 and FL-2 channels, respectively.
An Active Caspase-3 Apoptosis kit I (BD Biosciences)
was used to detect the direct activation of caspase-3 according to
the manufacturer’s instructions. In brief, the cells
(1×105) were washed twice with cold PBS, resuspended in
Cytofix/Cytoperm solution for 20 min, and washed twice with
Perm/Wash buffer. The cells were then resuspended in the
FITC-conjugated active caspase-3 monoclonal antibody and incubated
for 30 min. Subsequently, the cell suspension was washed twice with
Perm/Wash buffer and analyzed using a flow cytometer.
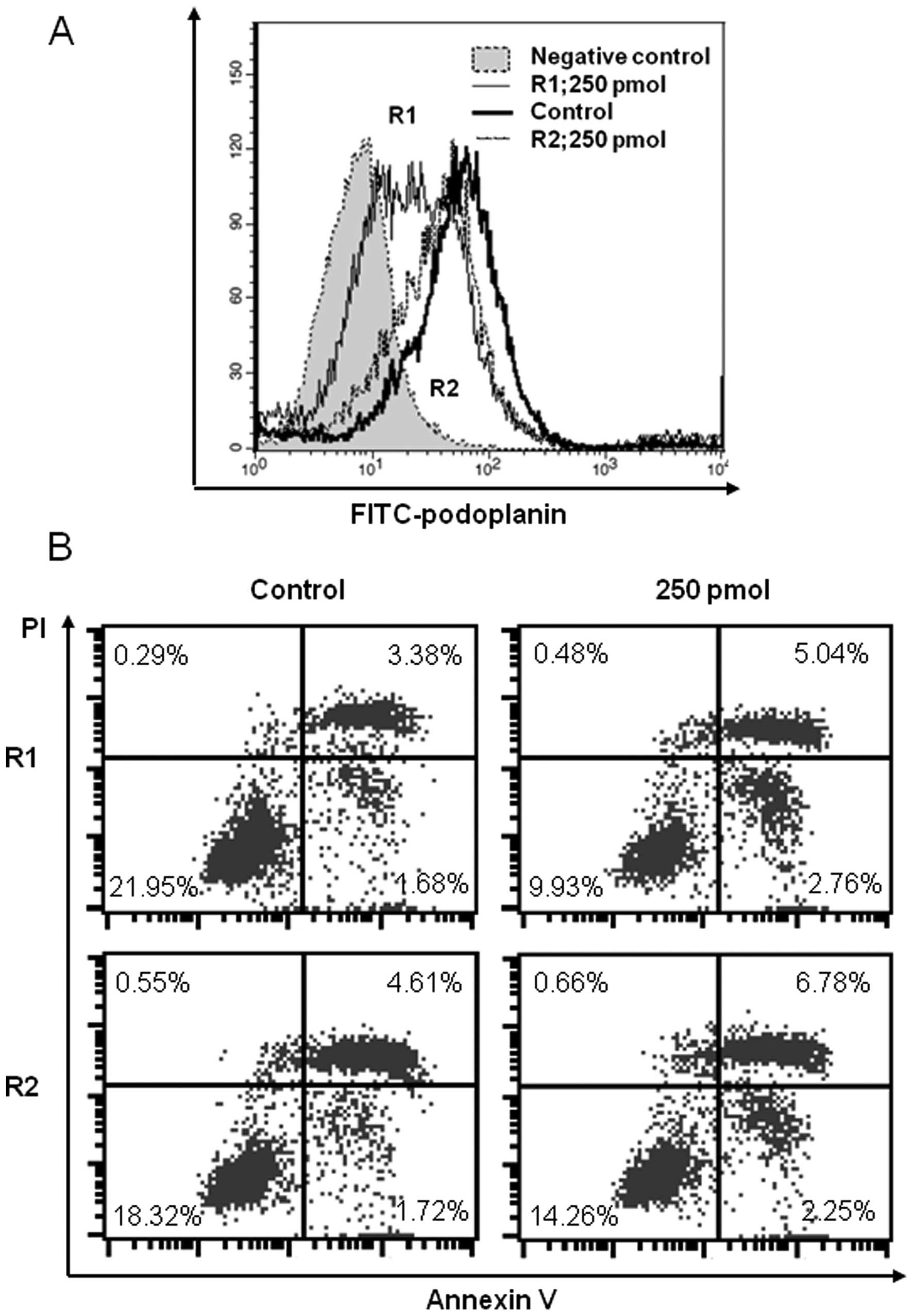
Gene silencing
We synthesized 21-nucleotide-long small interfering
RNAs (siRNAs). Stealth RNAi™ siRNAs were obtained from Invitrogen.
The podoplanin siRNA target sequences synthesized in our laboratory
were as follows (4): (R1) 5′-GCG
AAGACCGCUAUAAGUCdTdT-3′ and (R2) 5′-AAGAUG GUUUGUCAACAGUdTdT-3′.
NCI-H226 cultures were transfected with various concentrations
(50–1,000 pmol) of siRNAs or with equimolar concentrations of the
control vector using Lipofectamine™ 2000 transfection reagent
according to the manufacturer’s instructions. The cells were
harvested 48 h after transfection, and the podoplanin expression
level was confirmed by flow cytometry. CDDP was added, and 48 h
after this treatment, the apoptosis detection assay was
performed.
Mouse model of mesothelioma
PODO1 cells (1×107) in 0.2 ml complete
medium were injected into the dorsum of female BALB/c-nu/nu
mice. Tumor volumes were calculated using the formula (long axis ×
short axis × short axis)/2. The growth curves were generated by
considering the mean tumor volume at different time points.
Statistical analysis
Unpaired Student’s t-test and one-factor analysis of
variance (Scheffe’s post hoc test) were used to determine
statistical significance. Survival rates were calculated by the
Kaplan-Meier method, and the log-rank test was used to assess the
differences in prognosis between two groups. Differences were
considered significant at p-values of <0.05. Statistical
analyses were performed using StatView software (version 5; SAS
Institute, Cary, NC, USA).
Results
Generation of mesothelioma cell lines
that stably express human podoplanin
To investigate the potential role of podoplanin in
the pathogenesis of MPM, we cloned podoplanin cDNA into an
expression vector and succeeded in establishing mesothelioma cell
lines that stably expressed human podoplanin. We chose to utilize
the MSTO-211H cell line, which was established from the pleural
effusion of a patient with biphasic mesothelioma of the lung and
does not detectably express endogenous podoplanin as assessed by
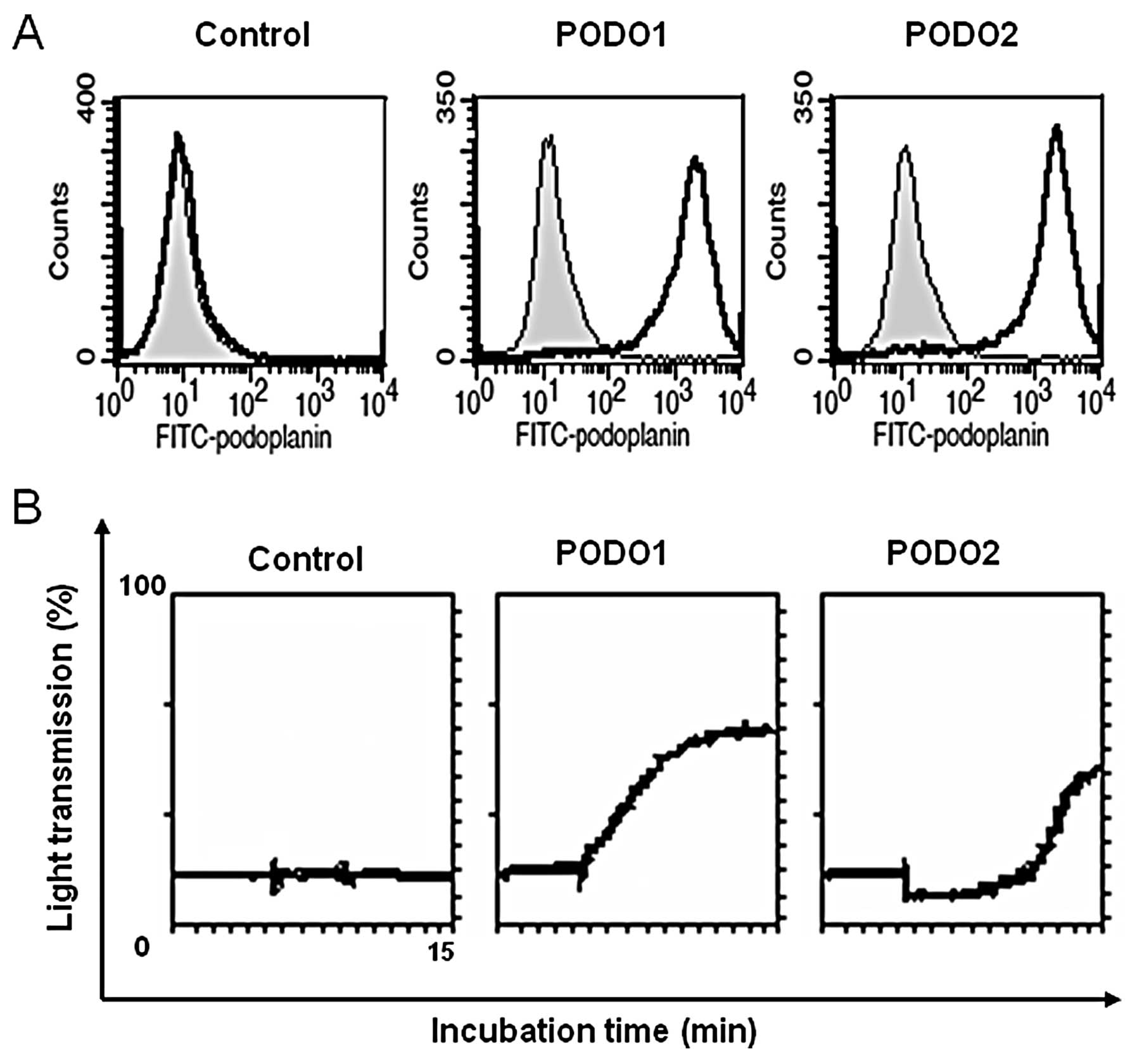
RT-PCR and flow cytometry (Fig. 1A)
(data not shown). This cell line forms tumors in nude mice
(28) and consists of biphasic-type
mesothelioma cells, which exhibit features of epithelial and
sarcomatous cells. Flow cytometry revealed that podoplanin
expression levels in two clones of podoplanin transfectants (PODO1
and PODO2) were significantly higher than those in the cells
transfected with the empty vector (p3x). These two clones were used
in subsequent experiments. Podoplanin induces platelet aggregation,
which we confirmed here using the aggregometer. Platelet
aggregation was detected only when the podoplanin transfectants
were included in the in vitro aggregation assay (Fig. 1B).
Tumors formed by the podoplanin
transfectant reduced the survival of nude mice
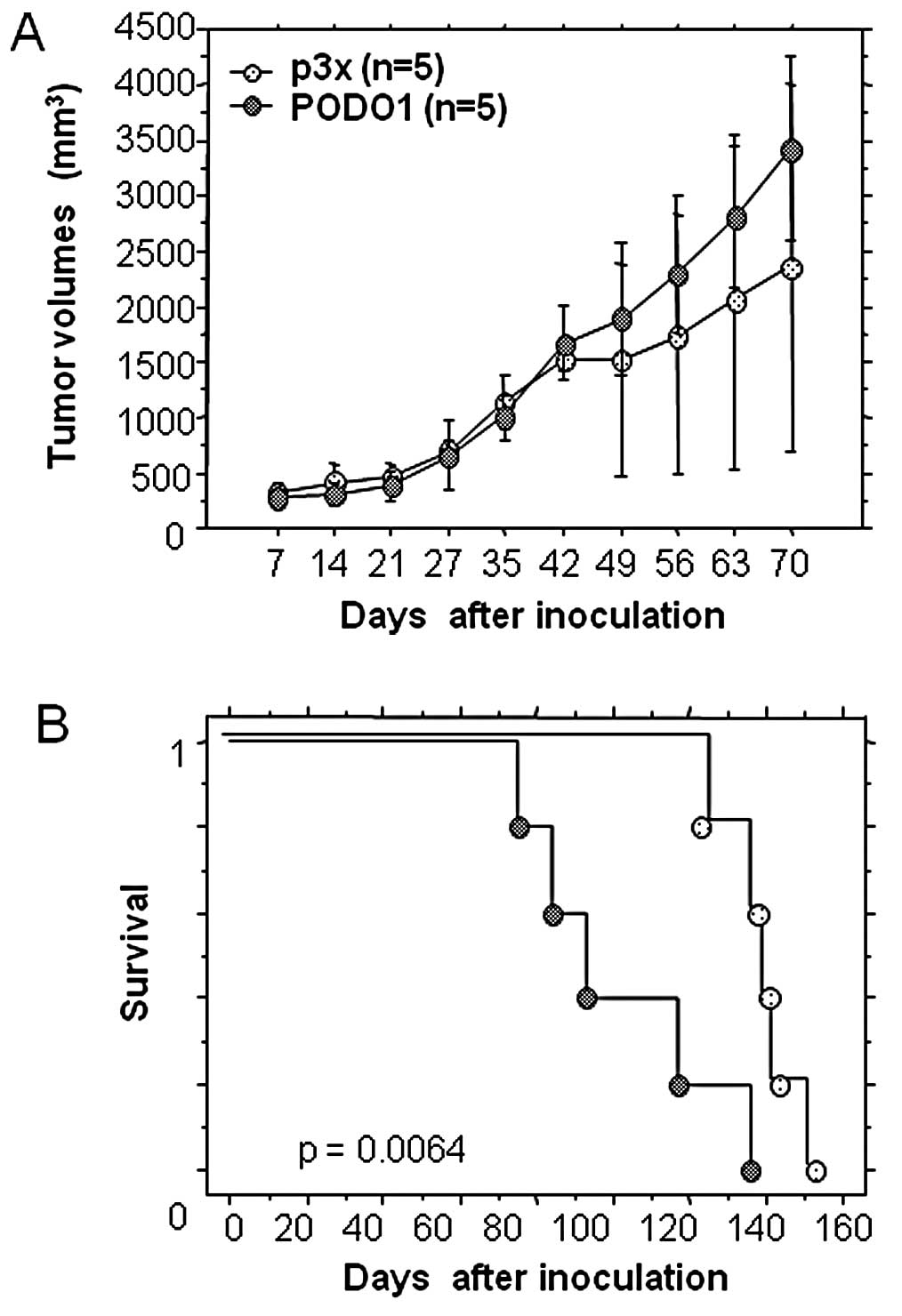
We assessed the tumorigenic properties of PODO1 by
injecting the cells into nude mice and compared tumor size and
survival rates with mice inoculated with a culture of p3x. The
survival rate of mice inoculated with PODO1 was significantly lower
than that of mice inoculated with p3× (Fig. 2). We did not observe the presence of
metastases to other organs, including the lung. No significant
difference was observed between the growth of the podoplanin
transfectant compared with p3× for 42 h after inoculation. After
this duration, the central mass of the tumor necrotized in the
control mice, but the tumor volume did not change. These results
suggest that podoplanin expression did not contribute to the growth
of mesothelioma cell lines in nude mice. Therefore, podoplanin
expression reduced the survival of the mice but did not affect the
growth potential of the transfectants.
Podoplanin promotes migration and
invasive properties of mesothelioma cell lines in
vitro.
Evidence suggests that podoplanin expression is
associated with the enhanced ability of cancer cells to migrate
(10,20). We, therefore, performed in
vitro motility assays to determine whether our podoplanin
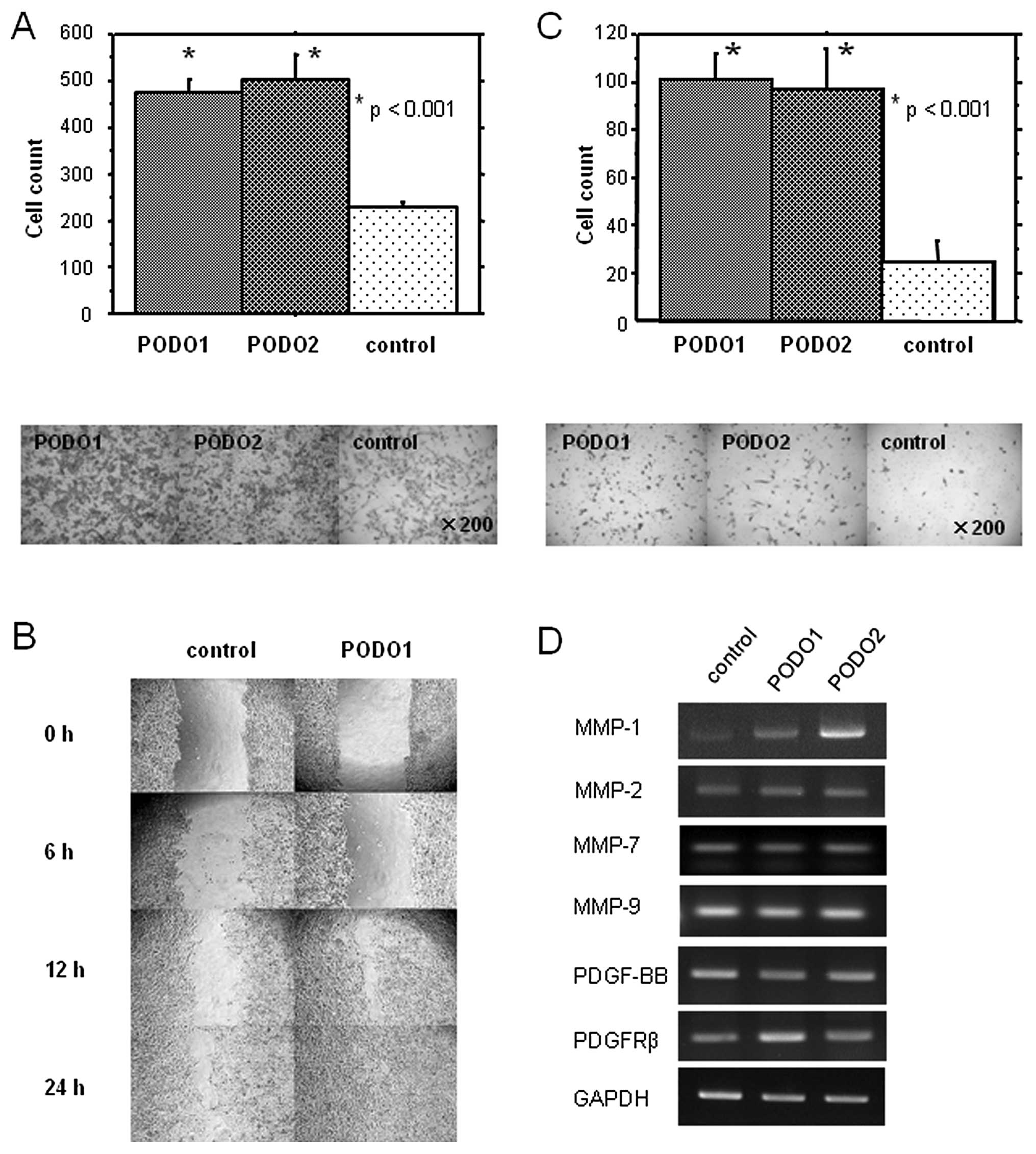
transfectants possessed this property. PODO1 and PODO2 cells
exhibited higher migratory activities than p3× (p<0.05)
(Fig. 3A).
To confirm and extend these findings, we performed
an in vitro wound-healing assay. As shown in Fig. 3B, PODO1 exhibited increased cell
migration to a greater extent than p3x. Moreover, the abilities of
PODO1 and PODO2 cells to invade a layer of Matrigel were also
significantly enhanced compared with those of p3× cells (Fig. 3C). These results suggest that
podoplanin overexpression promotes the migration and invasion of
mesothelioma cells.
Members of the MMP family play a crucial role in the
migration and invasiveness of cancer cells. Signaling by the
PDGF-BB (25,29) isoform through its receptor, PDGFR-β,
also promotes the migration and invasiveness of mesothelioma cells.
Therefore, we examined the expression of MMP-1, -2, -7, -9 and PDGF
in the podoplanin transfectants. We found that the expression
levels of MMP-1 mRNA, but not of MMP-2, -7 and -9 mRNA, were
increased in PODO1 and PODO2 compared with p3× cells (Fig. 3D). No differences were observed in
the expression levels of PDGF-BB and PDGFR-β mRNA between PODO1 and
PODO2 compared with p3× cells. These results suggest that
podoplanin promotes tumor cell invasion in vitro via
upregulation of MMP-1 expression.
Podoplanin overexpression enhances cell
proliferation and attenuates induction of apoptosis
We assessed the proliferative capacity of the
podoplanin transfectants using an MTT assay. No significant
differences were observed between the proliferative capacities of
the two podoplanin transfectants and p3× after 24 h, indicating
that podoplanin expression was dispensable for the proliferation of
mesothelioma cell lines in vitro during this period
(Fig. 4A). However, the
proliferative capacities of the podoplanin transfectants after 48
and 96 h were significantly greater than those of p3×
(p<0.001).
Cell number is regulated by the balance between cell
proliferation and apoptosis, and this raised the possibility that
the abilities of the podoplanin transfectants to undergo apoptosis
may have been altered after 48–96 h in culture. Therefore, we next
examined apoptosis in the transfectants after 96 h of culture.
Twice the number of apoptotic cells was present in the p3× cultures
compared with the PODO1 cultures (Fig.
4B). These results indicate that podoplanin overexpression
inhibited serum starvation-induced apoptosis.
Podoplanin inhibits induction of
apoptosis by CDDP
We next determined whether podoplanin overexpression
inhibited the induction of apoptosis by CDDP (cisplatin), a drug
widely used to treat mesothelioma. We determined the induction of
apoptosis 48 h after CDDP treatment. The number of apoptotic cells
in the PODO1 cultures was reduced compared with that in the p3×
cultures (Fig. 5A). This effect was
confirmed by determining active caspase expression (Fig. 5B). Taken together, these results
suggest that podoplanin overexpression (in a mesothelioma cell
line) plays an important role in inhibiting apoptosis induced by
serum starvation and CDDP.
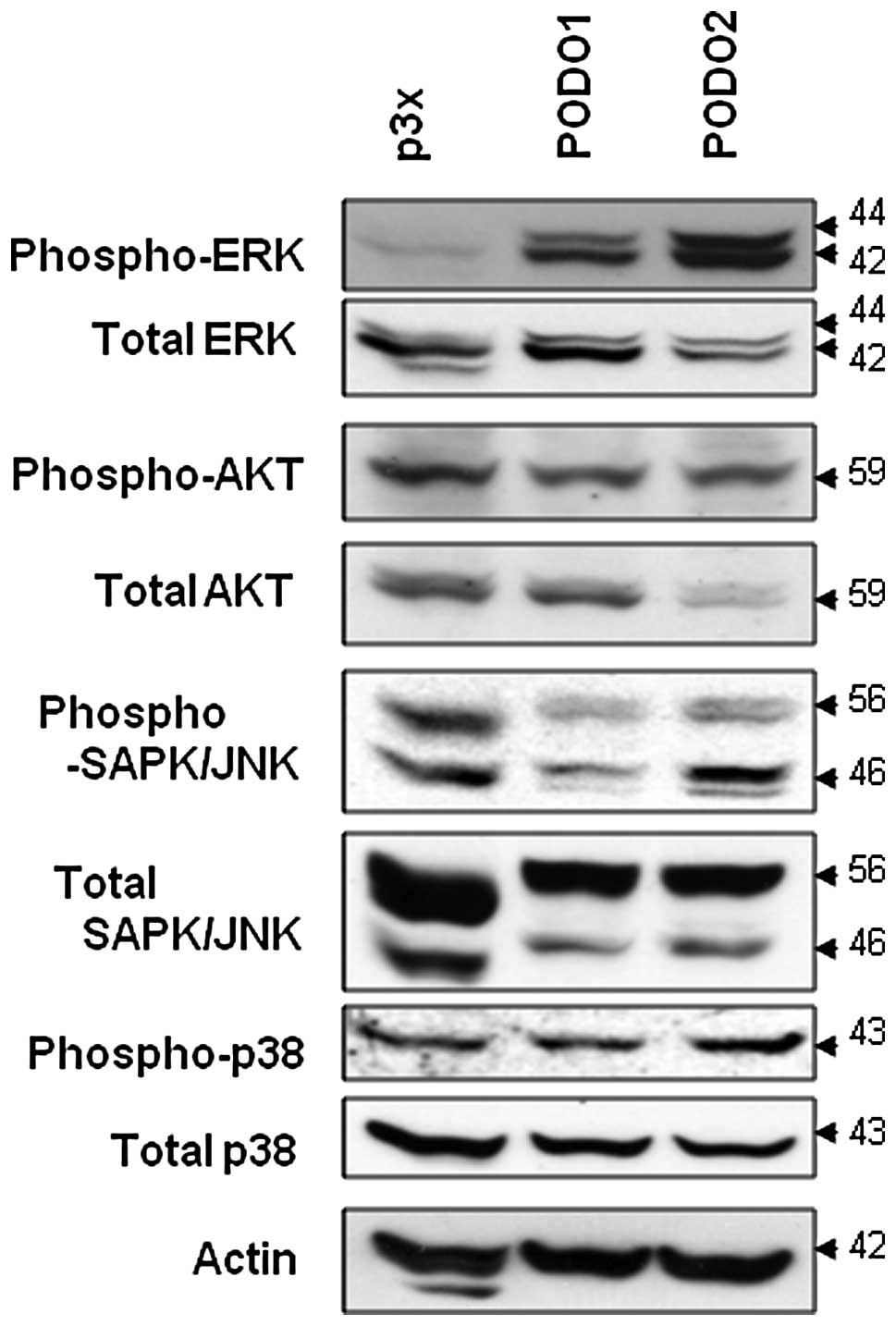
Podoplanin overexpression induces ERK
phosphorylation in mesothelioma cells
To identify the molecular mechanism responsible for
the anti-apoptotic effect of podoplanin overexpression in
mesothelioma cells, we examined the activation of the ERK signaling
pathway. The phosphorylation levels of JNK, p38 and AKT did not
differ between PODO1 and PODO2 compared with p3× cells (Fig. 6). In contrast, the phosphorylation
level of ERK was dramatically enhanced in the two podoplanin
transfectants. Thus, podoplanin-induced ERK activation may be
inhibited by starvation-induced apoptosis in mesothelioma
cells.
Silencing of the gene encoding podoplanin
induces apoptosis in mesothelioma cells that express high levels of
endogenous podoplanin
To confirm that podoplanin overexpression affects
apoptosis, we studied a mesothelioma cell line that expresses high
levels of endogenous podoplanin (NCI-H226). A siRNA targeted to the
mRNA encoding podoplanin significantly reduced the expression level
of endogenous podoplanin after 48 h (Fig. 7A). The proportion of apoptotic cells
was slightly but significantly increased in the podoplanin
transfectant compared with the control. Apoptosis was detected 48 h
after CDDP treatment (Fig. 7B).
Discussion
In the present study, we examined the potential role
of podoplanin in the pathophysiological properties of MPM using
mesothelioma cell lines. We found that the survival rate of nude
mice inoculated with a mesothelioma cell line that stably
overexpressed podoplanin (PODO1) was significantly reduced compared
with the survival of mice inoculated with the same cell line
transfected with the empty expression vector (p3x). Podoplanin
overexpression in this mesothelioma cell line augmented motility
and invasive properties in vitro. Moreover, podoplanin
overexpression imparted resistance to apoptosis, and reduction of
podoplanin expression using RNA interference led to increased
apoptosis in vitro. These results suggest that podoplanin
plays an important role in the pathogenesis of MPM.
The physiological function of podoplanin is unknown.
We found that the survival of nude mice inoculated with PODO1 cells
was reduced compared with that of mice inoculated with p3x.
However, tumor growth induced by PODO1 was comparable with that
induced by p3× at an early stage (24 h) after inoculation. A
significant difference in survival was observed at 48 and 96 h.
However, podoplanin, also called Aggrus, induces platelet
aggregation, and this activity is associated with its PLAG domain.
Platelet aggregation protects tumor cells from immunological
assault in the circulation and facilitates the formation of
hematogenous metastases (30).
Podoplanin-transfected CHO cells were found to increase the number
of metastases to the lung in an experimental mouse model of
metastatic growth (21). Therefore,
podoplanin may enhance the metastatic potential of mesothelioma
cell lines in nude mice by promoting platelet aggregation. However,
in the present study, we did not detect obvious metastatic lesions
(data not shown), indicating that another mechanism is responsible
for the significantly poorer survival rates of nude mice inoculated
with PODO1 when compared with mice inoculated with p3× cells.
Podoplanin overexpression increases cell migration
and invasion of the MCF7 breast cancer and HaCaT keratinocyte cell
lines (10,20,31).
Consistent with these findings, we found that podoplanin
overexpression in a mesothelioma cell line increased its in
vitro invasive and migratory potential. Therefore, podoplanin
may play a critical role in the motility and invasion of cancer
cells.
There are several possible explanations that account
for how podoplanin expression serves to induce the motility and
invasive property of the mesothelioma cell line studied here.
Podoplanin complexed with CLEC-2 on platelets enhanced the release
of PDGF from cancer cells (32).
PDGF enhanced cell invasion and motility of a mesothelioma cell
line in vitro(25). This
supports the first possibility that the induction of PDGF
expression by podoplanin may affect the motility and invasiveness
of mesothelioma cells. However, we found that podoplanin
overexpression did not affect the expression of PDGF mRNA, making
this possibility unlikely.
MMPs, in particular MMP-2 and MMP-9, are expressed
by most cancer cells, including MPM, and they play a key role in
influencing cell motility and invasiveness (33). Treatment of podoplanin-transfected
breast cancer cell lines with tissue inhibitor of MMPs (TIMP2), a
general MMP inhibitor, almost completely abrogates cellular
invasiveness (10). Therefore,
taken together with our present findings, it is also possible that
MMP-1 is induced by podoplanin and acts to enhance the motility and
invasiveness of mesothelioma cell lines.
Another notable finding of the present study is that
podoplanin overexpression inhibited the induction of apoptosis by
starvation and the chemotherapeutic drug CDDP (cisplatin). Two
major apoptosis pathways are initiated by cell-surface proteins of
the TNFR family, while other pathways are initiated by stress
signals (34,35). MAPK cascades are essential
components of the latter apoptotic signaling pathways. In the
present study, we found that podoplanin overexpression led to
constitutive activation of ERK in PODO1 and PODO2 cells. Moreover,
the activity of the MEK-ERK signaling pathway reduces sensitivity
of ovarian carcinoma cells to CDDP (36). Taken together, these findings
indicate that diminished levels of podoplanin increased the
sensitivity of malignant mesothelioma cells to CDDP by
downregulating ERK activation.
In summary, we discovered a new functional role of
podoplanin in mesothelioma cell lines. Podoplanin overexpression
led to anti-apoptotic effects and increased tumor malignancy in
vitro and in vivo. Moreover, silencing the expression of
the gene encoding podoplanin increased the number of cells
undergoing apoptosis. These findings lead us to conclude that
podoplanin may serve as an important target for developing more
efficacious treatments for MPM.
Acknowledgements
We thank Dr Hideki Uchiumi (Department of Medicine
and Clinical Science, Gunma University) for the excellent technical
assistance in the platelet aggregation analysis.
References
|
1
|
Fennell DA, Gaudino G, O’Byrne KJ, Mutti L
and van Meerbeeck J: Advances in the systemic therapy of malignant
pleural mesothelioma. Nat Clin Pract Oncol. 5:136–147. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wicki A and Christofori G: The potential
role of podoplanin in tumour invasion. Br J Cancer. 96:1–5. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramirez MI, Millien G, Hinds A, Cao Y,
Seldin DC and Williams MC: T1alpha, a lung type I cell
differentiation gene, is required for normal lung cell
proliferation and alveolus formation at birth. Dev Biol. 256:61–72.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schacht V, Ramirez MI, Hong YK, et al:
T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature
formation and causes lymphedema. EMBO J. 22:3546–3556. 2003.
View Article : Google Scholar
|
|
5
|
Breiteneder-Geleff S, Soleiman A, Kowalski
H, et al: Angiosarcomas express mixed endothelial phenotypes of
blood and lymphatic capillaries: podoplanin as a specific marker
for lymphatic endothelium. Am J Pathol. 154:385–394. 1999.
View Article : Google Scholar
|
|
6
|
Kato Y, Kaneko M, Sata M, Fujita N, Tsuruo
T and Osawa M: Enhanced expression of Aggrus (T1alpha/podoplanin),
a platelet-aggregation-inducing factor in lung squamous cell
carcinoma. Tumour Biol. 26:195–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martin-Villar E, Scholl FG, Gamallo C, et
al: Characterization of human PA2.26 antigen (T1alpha-2,
podoplanin), a small membrane mucin induced in oral squamous cell
carcinomas. Int J Cancer. 113:899–910. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schacht V, Dadras SS, Johnson LA, Jackson
DG, Hong YK and Detmar M: Up-regulation of the lymphatic marker
podoplanin, a mucin-type transmembrane glycoprotein, in human
squamous cell carcinomas and germ cell tumors. Am J Pathol.
166:913–921. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shibahara J, Kashima T, Kikuchi Y, Kunita
A and Fukayama M: Podoplanin is expressed in subsets of tumors of
the central nervous system. Virchows Arch. 448:493–499. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wicki A, Lehembre F, Wick N, Hantusch B,
Kerjaschki D and Christofori G: Tumor invasion in the absence of
epithelial-mesenchymal transition: podoplanin-mediated remodeling
of the actin cytoskeleton. Cancer Cell. 9:261–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kimura N and Kimura I: Podoplanin as a
marker for mesothelioma. Pathol Int. 55:83–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ordonez NG: Podoplanin: a novel diagnostic
immunohistochemical marker. Adv Anat Pathol. 13:83–88. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hinterberger M, Reineke T, Storz M, Weder
W, Vogt P and Moch H: D2-40 and calretinin - a tissue microarray
analysis of 341 malignant mesotheliomas with emphasis on
sarcomatoid differentiation. Mod Pathol. 20:248–255. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Padgett DM, Cathro HP, Wick MR and Mills
SE: Podoplanin is a better immunohistochemical marker for
sarcomatoid mesothelioma than calretinin. Am J Surg Pathol.
32:123–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mimura T, Ito A, Sakuma T, et al: Novel
marker D2-40, combined with calretinin, CEA, and TTF-1: an optimal
set of immunodiagnostic markers for pleural mesothelioma. Cancer.
109:933–938. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marchevsky AM: Application of
immunohistochemistry to the diagnosis of malignant mesothelioma.
Arch Pathol Lab Med. 132:397–401. 2008.PubMed/NCBI
|
|
17
|
Kawaguchi H, El-Naggar AK,
Papadimitrakopoulou V, et al: Podoplanin: a novel marker for oral
cancer risk in patients with oral premalignancy. J Clin Oncol.
26:354–360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Horiguchi A, Ito K, Sumitomo M, Kimura F,
Asano T and Hayakawa M: Intratumoral lymphatics and lymphatic
invasion are associated with tumor aggressiveness and poor
prognosis in renal cell carcinoma. Urology. 71:928–932. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mishima K, Kato Y, Kaneko MK, Nishikawa R,
Hirose T and Matsutani M: Increased expression of podoplanin in
malignant astrocytic tumors as a novel molecular marker of
malignant progression. Acta Neuropathol. 111:483–488. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martin-Villar E, Megias D, Castel S,
Yurrita MM, Vilaro S and Quintanilla M: Podoplanin binds ERM
proteins to activate RhoA and promote epithelial-mesenchymal
transition. J Cell Sci. 119:4541–4553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kunita A, Kashima TG, Morishita Y, et al:
The platelet aggregation-inducing factor aggrus/podoplanin promotes
pulmonary metastasis. Am J Pathol. 170:1337–1347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deroanne CF, Hamelryckx D, Ho TT, et al:
Cdc42 downregulates MMP-1 expression by inhibiting the ERK1/2
pathway. J Cell Sci. 118:1173–1183. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeng ZS, Shu WP, Cohen AM and Guillem JG:
Matrix metalloproteinase-7 expression in colorectal cancer liver
metastases: evidence for involvement of MMP-7 activation in human
cancer metastases. Clin Cancer Res. 8:144–148. 2002.PubMed/NCBI
|
|
24
|
Tutton MG, George ML, Eccles SA, Burton S,
Swift RI and Abulafi AM: Use of plasma MMP-2 and MMP-9 levels as a
surrogate for tumour expression in colorectal cancer patients. Int
J Cancer. 107:541–550. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Klominek J, Baskin B and Hauzenberger D:
Platelet-derived growth factor (PDGF) BB acts as a chemoattractant
for human malignant mesothelioma cells via PDGF receptor
beta-integrin alpha3beta1 interaction. Clin Exp Metastasis.
16:529–539. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mueller L, Goumas FA, Himpel S, Brilloff
S, Rogiers X and Broering DC: Imatinib mesylate inhibits
proliferation and modulates cytokine expression of human
cancer-associated stromal fibroblasts from colorectal metastases.
Cancer Lett. 250:329–338. 2007. View Article : Google Scholar
|
|
27
|
Faried A, Faried LS, Kimura H, et al: RhoA
and RhoC proteins promote both cell proliferation and cell invasion
of human oesophageal squamous cell carcinoma cell lines in vitro
and in vivo. Eur J Cancer. 42:1455–1465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Spugnini EP, Cardillo I, Verdina A, et al:
Piroxicam and cisplatin in a mouse model of peritoneal
mesothelioma. Clin Cancer Res. 12:6133–6143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhong J, Gencay MM, Bubendorf L, et al:
ERK1/2 and p38 MAP kinase control MMP-2, MT1-MMP, and TIMP action
and affect cell migration: a comparison between mesothelioma and
mesothelial cells. J Cell Physiol. 207:540–552. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gorelik E, Bere WW and Herberman RB: Role
of NK cells in the antimetastatic effect of anticoagulant drugs.
Int J Cancer. 33:87–94. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Scholl FG, Gamallo C, Vilaró S and
Quintanilla M: Identification of PA2.26 antigen as a novel
cell-surface mucin-type glycoprotein that induces plasma membrane
extensions and increased motility in keratinocytes. J Cell Sci.
112:4601–4613. 1999.PubMed/NCBI
|
|
32
|
Suzuki-Inoue K, Kato Y, Inoue O, et al:
Involvement of the snake toxin receptor CLEC-2, in
podoplanin-mediated platelet activation, by cancer cells. J Biol
Chem. 282:25993–26001. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Edwards JG, McLaren J, Jones JL, Waller DA
and O’Byrne KJ: Matrix metalloproteinases 2 and 9 (gelatinases A
and B) expression in malignant mesothelioma and benign pleura. Br J
Cancer. 88:1553–1559. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baker SJ and Reddy EP: Modulation of life
and death by the TNF receptor superfamily. Oncogene. 17:3261–3270.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee S, Yoon S and Kim DH: A high nuclear
basal level of ERK2 phosphorylation contributes to the resistance
of cisplatin-resistant human ovarian cancer cells. Gynecol Oncol.
104:338–344. 2007. View Article : Google Scholar : PubMed/NCBI
|