Introduction
Patients with ER-positive tumors are candidates for
endocrine therapy with the anti-estrogen tamoxifen. Unfortunately,
up to 40% of ER-positive patients fail to respond, and a large
fraction of initial responders develop resistance (1–5).
Additional predictive markers for treatment selection are needed,
given that resistant patients are at risk of disease relapse. One
of these potential markers is Wwox, which is considered to be a
tumor suppressor and an inducer of apoptosis (6). Wwox includes two WW domains for
protein-protein interactions and a short-chain alcohol
dehydrogenase-reductase (SDR) domain that may play a part in sex
steroid metabolism (6–8). The expression level of Wwox protein
has shown to be significantly higher in ER-positive than in
ER-negative tumors, suggesting a role in hormone signaling
(9,10). This is also supported by the fact
that, in normal conditions, the highest expression of Wwox is
observed in epithelial cells of hormonally regulated tissues such
as ovaries, testes, prostate and mammary glands while thymus,
adipose and connective tissues seem to lack Wwox (11–13).
Furthermore, the Wwox gene is located on chromosome 16q23 and
contains the fragile site FRA16D, the second most common fragile
site in the human genome (14).
Fragile sites are characterized by DNA instability and are
frequently affected by genomic losses in several types of cancer.
WWOX inactivation due to loss of heterozygosity (LOH) and
hypermethylation has been reported, particularly in breast cancer
(15–25). These events seem to appear early
during carcinogenesis as they are found in ductal carcinoma in
situ(26). Moreover, nearly all
metastatic tissues show lower expression compared to their matched
primary breast tumors, and therefore it is thought that loss of
WWOX expression plays an important role in the growth and
progression of breast cancer (21,27,28).
Intrinsic Wwox is reported mainly in mitochondria,
however, nuclear translocation occurs in response to various stress
signals and sex steroid hormones such as 17β-estradiol (E2). In
breast cancer cells it seems that nuclear translocation occurs to a
lesser extent as compared to prostate cancer cells, possibly due to
interaction with cytoplasmic proteins (27). Through its WW domains, which are
characterized by conserved tryptophan residues, Wwox binds to
proline-rich motifs of several proteins in breast tissue. Wwox
interaction seems to affect the function of other proteins, for
example when bound to p53 it induces apoptosis in a synergistic
manner (29,30). AP2γ, a transcription factor often
upregulated in breast cancer, becomes sequestered in the cytoplasm
by Wwox, preventing its entry into the nucleus (31,32).
Wwox binding to the intracellular domain (4ICD) of human epidermal
growth factor receptor 4 (HER4, ErbB4) has been reported to
generate similar cytoplasmic localization (33,34).
Depletion of WWOX in vitro was found to
result in reduced ER expression and decreased sensitivity to
tamoxifen (35). In a small
non-randomized study correlating Wwox with tamoxifen resistance, it
was shown that loss or reduced expression increased the probability
of resistance more than 4-fold and that Wwox came out as a better
predictor of response than PgR. (31). However, the full mechanism of de
novo and acquired tamoxifen resistance is still largely
unexplained; therefore, it is important to consider new potential
markers. As Wwox has been proposed to predict response to tamoxifen
treatment, we aimed to investigate the expression pattern of Wwox
using a cohort of 912 randomized breast cancer patients. In the
present study, we therefore compared Wwox protein expression with
clinicopathological data of treated and untreated patients, which
may bring us closer to elucidating the predictive value of
Wwox.
Materials and methods
Patients
We analyzed tissues from low-risk breast cancer
patients registered in a randomized tamoxifen trial, conducted in
the Stockholm region between 1976 and 1990 (36). All patients (n=1,780) were
postmenopausal at the time of diagnosis, were required to have a
tumor ≤30 mm in diameter and with no lymph node metastases (N0)
(Table I). The patients were
treated either with modified radical mastectomy or with
breast-conserving surgery plus radiation therapy (50 Gy/5 weeks).
Patients were randomized to tamoxifen therapy (40 mg/day) for 2
years (n=886) or no adjuvant endocrine treatment (n=894). Tamoxifen
treatment was initiated within 2–4 weeks after surgery and thus
administered concurrently with radiation therapy. Patients without
recurrence after 2 years were re-randomized to an additional 3
years of tamoxifen therapy, hence a total treatment period of 5
years, or no further treatment. Standard procedure for tissue
collection was fixation in 4% phosphate-buffered formalin. The ER
status was determined by isoelectric focusing with a cut-off level
set to 0.05 fmol/μg DNA. The mean follow-up period was 17 years
(36). The study was approved by
the Ethics Committee of the Karolinska Institute. The ethical
approval required no informed consent from the patients.
 | Table IComparison of the distribution of
patients in regards to tumor and treatment characteristics. |
Table I
Comparison of the distribution of
patients in regards to tumor and treatment characteristics.
| No. of patients
(%) |
|---|
|
|
|---|
| Patients in the
present study (n=912) | Patients with Wwox
expression (n=686) | Original randomized
study (n=1,780) |
|---|
| Estrogen
receptor |
| Positive | 684 (77) | 519 (77) | 1,183 (80) |
| Negative | 200 (23) | 153 (23) | 296 (20) |
| Unavailable | 28 | 14 | 301 |
| Progesterone
receptor |
| Positive | 379 (48) | 299 (47) | 590 (48) |
| Negative | 416 (52) | 333 (53) | 627 (52) |
| Unavailable | 117 | 54 | 563 |
| Tumor diameter
(mm) |
| ≤20 | 697 (79) | 515 (76) | 1,393 (81) |
| >20 | 189 (21) | 159 (24) | 323 (19) |
| Unavailable | 26 | 12 | 64 |
| Tamoxifen
treatment |
| Yes | 473 (52) | 348 (51) | 886 (50) |
| No | 439 (48) | 338 (49) | 894 (50) |
Breast tissue microarray
Archived breast tumor tissues from 912 of the 1,780
patients participating in the original study were collected.
Representative formalin-fixed and paraffin-embedded tissue was
chosen as a donor block for the tissue microarray (TMA). A section
was cut from each block and stained with hematoxylin and eosin.
Three morphologically representative regions from each section were
chosen, and then cylindrical cores with a diameter of 0.8 mm were
extracted and mounted in a recipient block. For each TMA, cores
from liver tissue were mounted as internal controls. The TMA blocks
were cut into 4-μm sections using a microtome and mounted on glass
slides. The TMA blocks were constructed using a manual arrayer
(Beecher Instrument, Inc.).
Hormone receptor status
The Ventana BenchMark system with prediluted
antibodies (anti-ER clone 6F11 and anti-PgR clone 16) was used to
retrospectively determine the ER and progesterone (PgR) statuses of
the 912 patients. Tumors with >10% positively stained nuclei
were considered positive. The immunohistochemical data regarding
the ER status were used in this study; however, when data were
missing, the results from the cytosol assay were used.
HER2 analysis
Expression of HER2 was analyzed by
immunohistochemistry using the Dako AO0485 polyclonal rabbit
antibody according to the manufacturer’s instructions. The scoring
was limited to the invasive tumor cells and graded 0, 1+, 2+ or 3+.
Patients having a score of 3+ were considered HER2-positive.
Immunohistochemistry
Affinity isolated rabbit polyclonal anti-Wwox
antibodies (cat. no W2143; Sigma-Aldrich, St. Louis, MO, USA) were
used at a dilution of 1:300. Slides were deparaffinized in xylene
and rehydrated in decreasing series of ethanol followed by Milli-Q
water. Heat-induced antigen retrieval was performed in EDTA buffer
(1 mM, pH 8.0) for 1 h in a 99°C water container and cooled to room
temperature (RT). Slides were blocked for endogenous peroxidase
activity and unspecific binding using 3% H2O2
for 10 min followed by 5% horse serum diluted in phosphate-buffered
saline (PBS) for 1 h at RT. The slides were incubated with the
primary antibody at 4°C for 16 h, washed, and subsequent detection
was carried out using the EnVision™ System (DakoCytomation,
Glostrup, Denmark) with secondary horseradish peroxidase (HRP)
polymer reagent for 20 min at RT and subsequently visualized with
3,3′diaminobenzidine (DAB). Sections were counterstained with
hematoxylin and mounted. The positive and negative controls,
prostate and thymus respectively, were stained appropriately. All
washings in-between reactions were performed using PBS with 0.1%
Tween (PBST).
Western blot analysis
To determine the specificity of the Wwox antibody,
western blot analysis was performed. Protein (1 mg/ml) from lysed
MCF-7 cells was separated by 12% SDS-PAGE and transferred to
polyvinylidene difluoride membranes (Bio-Rad Laboratories,
Hercules, CA, USA) for 1 h at 100 V. MCF-7 culturing and protein
extraction were performed according to the standard protocol. The
membranes were blocked for unspecific binding for 1 h at RT using
PBST with 5% horse serum and then incubated with primary antibody
against Wwox (1:3,000) at 4°C for 16 h. For detection, the
membranes were incubated with a HRP-conjugated secondary goat
anti-rabbit antibody 1:50,000 (Santa Cruz Biotechnology, Santa
Cruz, CA, USA) for 1 h at RT and visualized using the ECL Advance
Western Blotting Detection kit (GE Healthcare, Buckinghamshire, UK)
according to the manufacturer’s instructions.
TMA evaluation
The TMA slides were examined with an Olympus BX41
light microscope (Olympus Life Science Europe GMBH, Hamburg,
Germany) connected to a Leica DFC420 digital microscope camera
(Leica Microsystems, Heerbrugg, Switzerland). Two investigators
(A.G.E. and S.W.) evaluated the slides independently and were
blinded to any clinical data or patient outcome. The cut-off level
was set at >10% of positively stained tumor cells. The intensity
of cytoplasmic and nuclear Wwox staining was graded as follows; 0,
negative; 1, weak/moderate or 2, strong. A new category of total
Wwox expression was calculated by adding the scores together: 0,
negative; 1–2, weak/moderate; and 3–4, strong. Cases that were
considered positive for Wwox expression had to have at least an
intensity of 1 in the cytoplasm, in the nucleus or in both. In the
case of non-consistent scoring between investigators, a consensus
score was established after re-evaluation.
Statistical analysis
To examine the relationship between the different
levels of protein expression and tumor characteristics as well as
the correlation between nuclear and cytoplasmic expression, we used
Pearson’s Chi-square tests. The time for recurrence-free survival
was calculated as the time between diagnosis and local recurrence
of breast cancer, distant metastasis or death from breast cancer.
The recurrence-free survival rates and the difference in survival
rate between treatment groups were estimated using the Kaplan-Meier
method and the log-rank test, respectively. The interaction between
Wwox and treatment was calculated with a univariate Cox regression
model. In addition, hazard ratios (relative hazard, HR) with a 95%
confidence interval (95% CI) were assessed using a multivariate Cox
regression model in order to adjust for the tumor characteristics
between the different expression profiles. All P-values <0.05
were considered to indicate a statistically significant result. The
Statistical Package for the Social Sciences (SPSS), version 15.0,
was used to perform all statistical analyses.
Results
Protein expression of Wwox
Protein expression of Wwox was assessed using
immunohistochemical staining performed on TMA slides, comprising
tumor tissues from 912 breast cancer patients. Wwox scoring was
accessible in 686 cases (75.2%), and the patient distribution of
characteristics in comparison to the original cohort is described
in Table I. Between cases available
on TMA and those scored for Wwox expression, there were no
statistical differences regarding the distribution of
characteristics. From the accessible cases, expression of
cytoplasmic Wwox was observed in 560 (81.6%) cases and expression
of nuclear Wwox was observed in 564 (82.2%) cases (Table II). There were 62 (9.0%) cases who
were positive for nuclear but not cytoplasmic Wwox and 58 (8.5%)
who were positive for cytoplasmic but not nuclear expression of
Wwox. However, 502 (73.2%) cases had Wwox expression in both
locations (OR=8.9, 95% CI 5.7–13.9, P<0.0001). Different
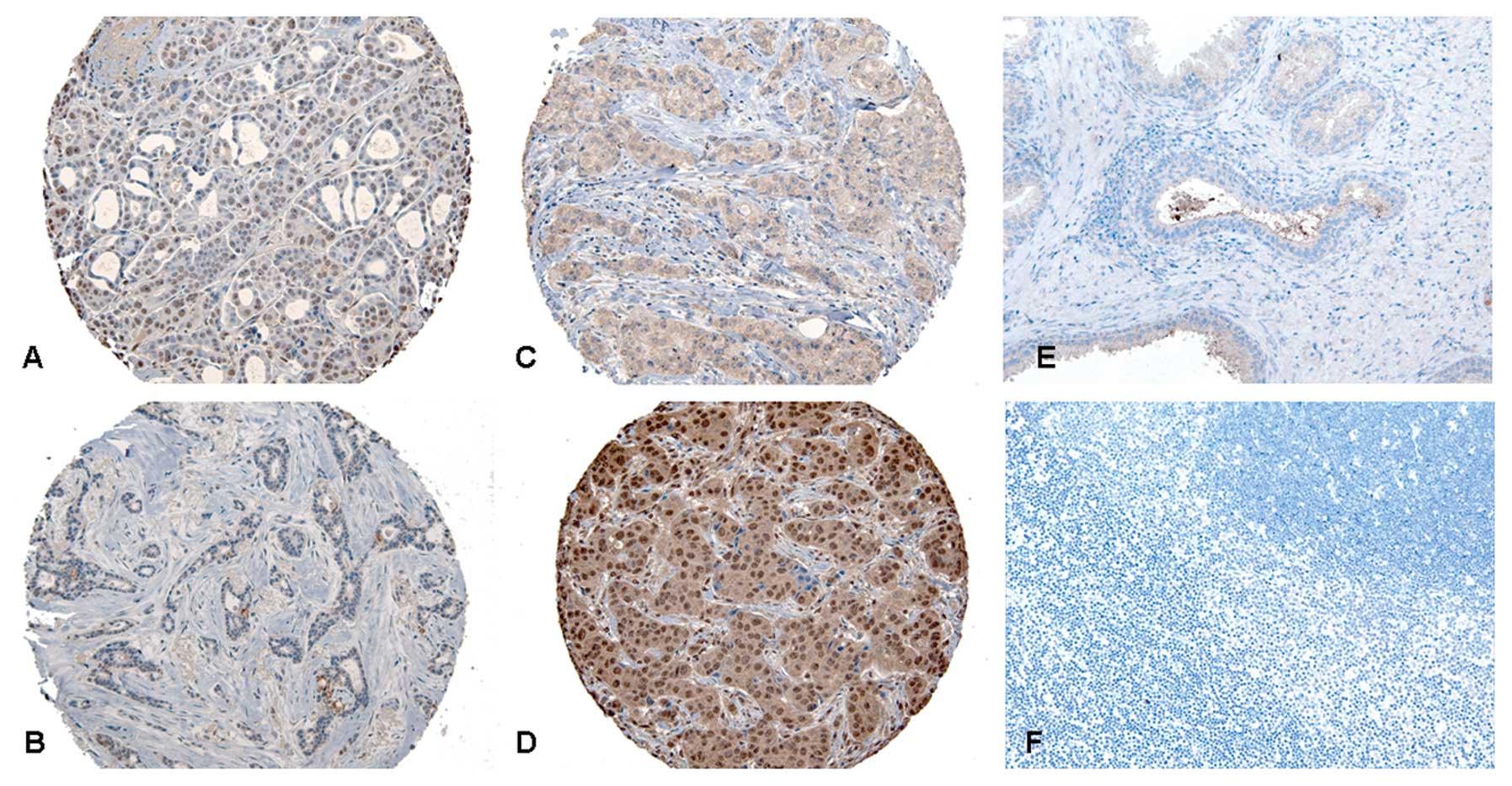
patterns of immunohistochemical staining along with the result from
the positive and negative controls are shown in Fig. 1. The specificity of the antibody was
verified with western blot analysis (data not shown).
 | Table IIStatistical correlations between Wwox
expression and tumor characteristics in the 686 breast cancer
patients included in the study. |
Table II
Statistical correlations between Wwox
expression and tumor characteristics in the 686 breast cancer
patients included in the study.
| No. of patients (%
within each group) |
|---|
|
| |
|---|
| Nuclear Wwox
expression | | Cytoplasmic Wwox
expression | |
|---|
|
| |
| |
|---|
| Negative | Moderate | Strong | P-valuea/b | Negative | Moderate | Strong | P-valuea/b |
|---|
| Total | 122 (17.8) | 366 (53.4) | 198 (28.9) | | 126 (18.4) | 393 (57.3) | 167 (24.3) | |
| Estrogen
receptor |
| Positive | 83 (69.2) | 269 (74.9) | 167 (86.5) | | 97 (78.9) | 295 (76.4) | 127 (77.9) | |
| Negative | 37 (30.8) | 90 (25.1) | 26 (13.5) | 0.001/0.0001 | 26 (21.1) | 91 (23.6) | 36 (22.1) | 0.830/0.899 |
| Progesterone
receptor |
| Positive | 41 (39.4) | 161 (47.2) | 97 (51.9) | | 53 (47.3) | 178 (49.7) | 68 (42.0) | |
| Negative | 63 (60.6) | 180 (52.8) | 90 (48.1) | 0.125/0.045 | 59 (52.7) | 180 (50.3) | 94 (58.0) | 0.261/0.292 |
| Tumor diameter
(mm) |
| ≤20 | 93 (78.2) | 263 (73.1) | 159 (81.5) | | 100 (80.0) | 284 (74.3) | 131 (78.4) | |
| >20 | 26 (21.8) | 97 (26.9) | 36 (18.5) | 0.071/0.286 | 25 (20.0) | 98 (25.7) | 36 (21.6) | 0.336/0.880 |
| Tamoxifen
treatment |
| Yes | 54 (44.3) | 192 (52.5) | 102 (51.5) | | 57 (45.2) | 203 (51.7) | 88 (52.7) | |
| No | 68 (55.7) | 174 (47.5) | 96 (48.5) | 0.282/0.285 | 69 (54.8) | 190 (48.3) | 79 (47.3) | 0.384/0.232 |
| HER2 |
| Positive | 12 (10.5) | 44 (12.8) | 20 (10.8) | | 5 (4.3) | 44 (11.9) | 27 (17.1) | |
| Negative | 102 (89.5) | 300 (87.2) | 166 (89.2) | 0.705/0.928 | 111 (95.7) | 326 (88.1) | 131 (82.9) | 0.005/0.001 |
Protein expression of Wwox in correlation
with prognostic factors
The analysis of Wwox and its relationship with tumor
characteristics demonstrated a correlation between the grade of
nuclear Wwox expression and positive ER and PgR status (P=0.0001
and P=0.045, respectively, Table
II) and the grade of cytoplasmic Wwox was correlated to the
HER2 status (P=0.001). Several HER2-positive patients were also
ER-positive (32 of 76) but the Chi-square test for trend showed no
correlation between Wwox expression and ER status in these patients
(P=0.24). There were no significant correlations to other
characteristics.
Wwox prognostic relevance in breast
cancer
Among the 348 patients treated with tamoxifen, 271
(77.9%) had an ER-positive tumor and 5 (1.4%) had an unknown ER
status. Among the 338 patients who did not received endocrine
therapy, 248 (73.4%) were ER-positive and 9 (2.7%) had an unknown
ER status. To uncover any prognostic relevance of Wwox in breast
cancer, the group of patients without endocrine treatment was
selected for analysis. These results showed no difference in
recurrence-free survival between patients with or without
cytoplasmic Wwox (P=0.98), nuclear Wwox (P=0.74) or total Wwox
(P=0.38). Subanalysis of the few cases with single nuclear or
single cytoplasmic expression did not show any difference in
recurrence-free survival (P=0.69). When selecting only ER-positive
patients, there was still no difference in survival observed in
regards to Wwox staining (P=0.74, P=0.26 and P=0.69, respectively).
In the multivariate analysis including ER, PgR and tumor size,
moderate or strong Wwox expression were not independent predictive
markers (P=0.83 and P=0.93, respectively).
Wwox expression and response to tamoxifen
treatment
When tamoxifen was introduced into the statistical
model, the ER-positive patients were selected for further analyses,
as this group of patients currently receive tamoxifen therapy. The
patients were divided in regards to Wwox tumor expression, and
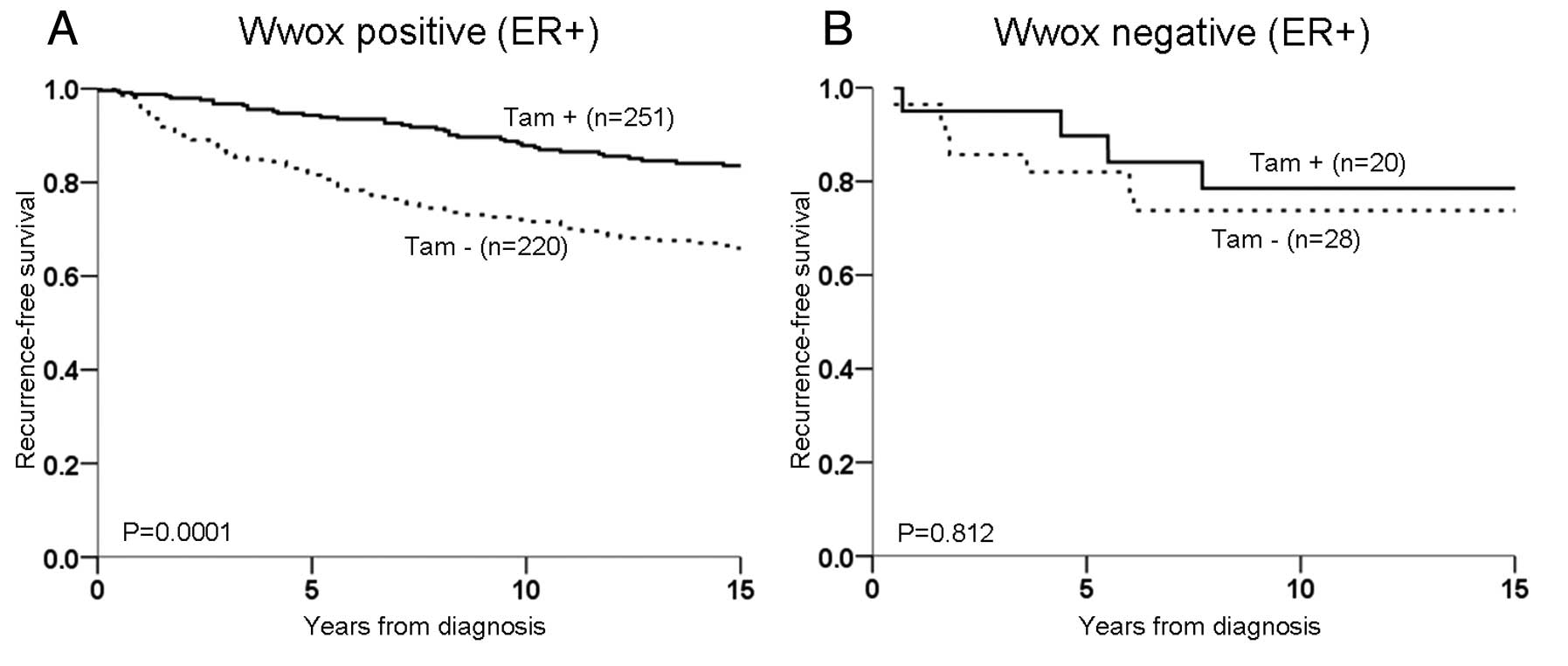
tamoxifen treatment was found to result in an improved
recurrence-free survival for those expressing Wwox (P=0.0001;
moderate and strong expression P=0.001 and P=0.003, respectively,
Fig. 2A). However, in patients
lacking Wwox expression no difference in recurrence-free survival
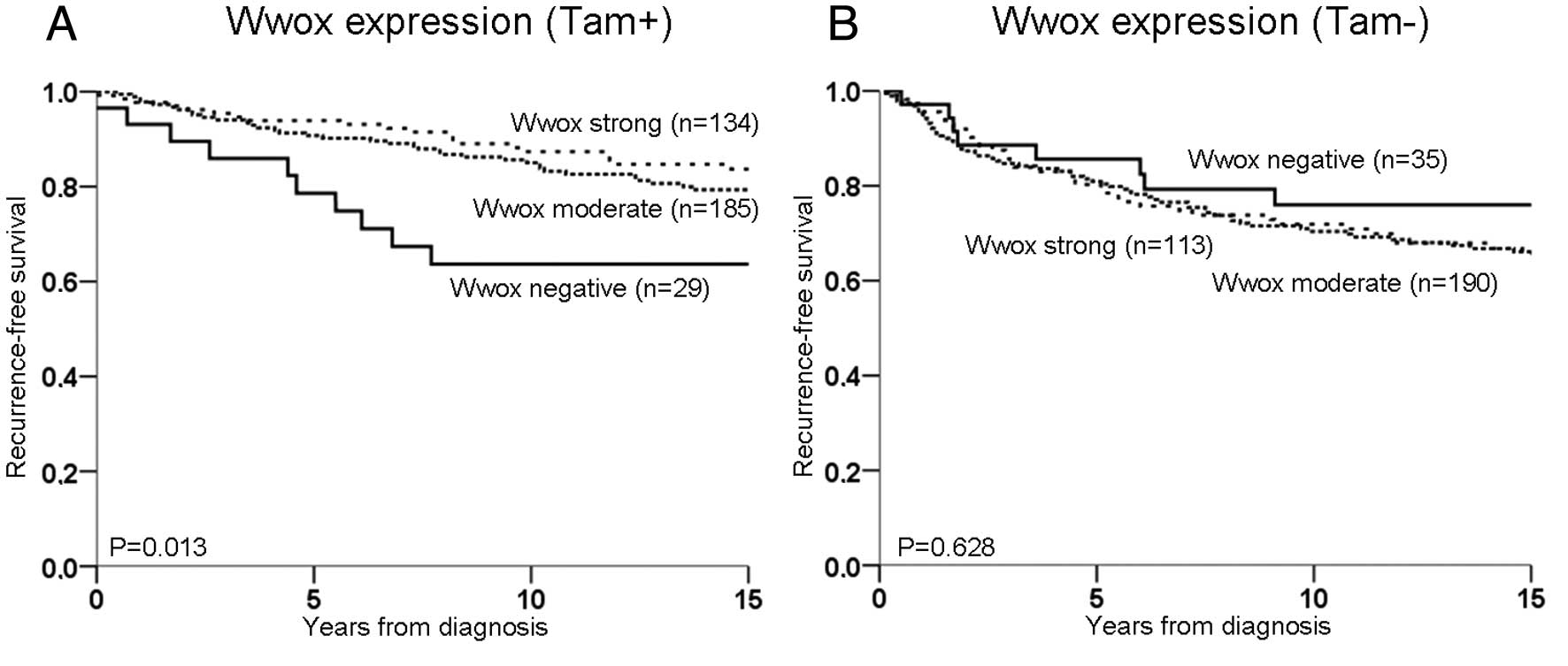
was noted regardless of treatment (P=0.812, Fig. 2B). Irrespective of ER,
tamoxifen-treated patients with a moderate or strong Wwox
expression had an improved recurrence-free survival compared to
patients without Wwox expression (P=0.013, Fig. 3A). When moderate and strong
expression were grouped together and termed Wwox-positive, the
difference was even greater (P=0.005). By contrast, no difference
in recurrence-free survival was noted among untreated patients
according to different levels of Wwox expression (P=0.63, Fig. 3B). The test for the interaction
between Wwox and tamoxifen treatment demonstrated a decreased risk
of recurrence for treated patients with a moderate or strong Wwox
expression (HR=0.31, 95% CI 0.10–0.98 and HR=0.28, 95% CI
0.08–0.97, respectively).
Discussion
The Wwox protein is a relatively newly discovered
tumor suppressor with a complex biological behavior that is still
under investigation. Wwox has been shown to interact with a growing
list of proteins and seems to be involved in numerous cellular
pathways. High expression of Wwox has been correlated with a more
favorable survival of breast cancer patients in several studies
(10,12,26,37).
As Guler et al(31)
demonstrated an association between Wwox protein expression and
tamoxifen resistance, we decided to further investigate the
potential of Wwox to predict tamoxifen response. In the present
study, we evaluated Wwox protein expression in tumors from 686
randomized breast cancer patients and correlated the results to
clinicopathological data as well as to tamoxifen treatment. Breast
cancer patients treated with tamoxifen had a significantly
prolonged recurrence-free survival when their tumor expressed
higher levels of the Wwox protein. We also found a significant
difference in recurrence-free survival between Wwox-positive and
-negative cases among the patients treated with tamoxifen but not
the untreated patients. The test of interaction using Cox’s
regression demonstrated that the risk of recurrence decreased in
tamoxifen-treated patients when their tumor expressed the Wwox
protein. This is in line with Guler’s cohort where the probability
of tamoxifen resistance was increased more than 4-fold when Wwox
protein expression was reduced. According to Guler et al,
the predictive value of Wwox was better than PgR (31); however, multivariate analysis of our
results did not reveal such a scenario. Furthermore, our results
did not support that Wwox has any prognostic relevance but our
findings may indicate that Wwox is involved in pathways interfering
with tamoxifen response. There are several known mechanisms leading
to tamoxifen resistance, such as drug metabolism, changes in
co-regulatory proteins, adaption and tolerance, but to date, there
are difficulties in predicting the outcome of treatment based on
these factors (1). In addition, ER
and sex hormone steroids can directly interact with cytoplasmic
signaling molecules such as the tyrosine kinases Src and PI3K,
initiate extranuclear pathways leading to gene expression (38,39).
This crosstalk between signaling pathways and ER further
complicates the understanding of resistance mechanisms. It is
unclear how Wwox contributes to tamoxifen response given that its
normal functions are yet to be fully explored. Wwox appears mainly
to be involved in protein-protein interactions in the cytoplasm,
yet there are indications of nuclear translocation. This event has
been shown in COS7 fibroblasts after exposure to E2, which suggests
a hormonally influenced nuclear function (27,30).
Watanabe et al(40) carried
out an immunohistochemical analysis of Wwox and observed nuclear
staining in mammary epithelia and in cell lines during confluent
culture conditions. Conversely, when Nunez et al(13) performed a survey on the topographic
distribution of Wwox protein expression they found only cytoplasmic
staining. Importantly, this survey was carried out in normal, not
cancerous, human tissues. As immunohistochemical studies are
subject to the specificity of the antibodies, we used the survey
constructed by Nunez et al to preference control tissues for
validation of our selected antibody (Fig. 1). Moreover, western blot analysis
identified one single band of predicted size also suggesting
specificity. We found that most of our cases had a combination of
nuclear and cytoplasmic expression of Wwox and that the
localization of Wwox did not seem to influence recurrence-free
survival of breast cancer patients. Nevertheless, there was a
correlation between nuclear Wwox and ER and a correlation between
cytoplasmic Wwox and HER2. As ER is a nuclear receptor and HER2 is
a membrane-bound receptor, the correlations noted may reflect a
relationship between cellular positions more than a functional
association. Even so, the correlations may also indicate a possible
Wwox involvement in both ER and HER2 signaling as the pathways
intersect (39,41). The highest normal expression of Wwox
is found in hormonally influenced tissues including the prostate,
and therefore it has been suggested to play a part in prostate
cancer (25,41). In the event of prostate cells
transforming to a cancerous and metastatic state, Wwox is
phosphorylated and translocate to the nucleus (27).
First, the interactive ability of Wwox is modified
via phosphorylation at Tyr33 by Src, which is induced by stress
stimuli and sex steroid hormones such as estradiol (29). Src is frequently upregulated in
cancer and is also known to interact with HER2, promoting growth
and survival of breast cancer cells (42). It has also been implied that higher
levels of cytoplasmic Src are associated with poor response to
tamoxifen therapy (43). In a
normal state, an elevated Src expression could lead to Wwox
activation resulting in Wwox-mediated tumor suppression. Thus, the
loss of Wwox may therefore increase the oncogenic effect of Src
which may result in a worse outcome of cancer.
Secondly, Wwox expression has also been shown to
correlate with activator protein 2 γ (AP2γ), a transcription factor
often upregulated in breast tumors. Reduced Wwox protein expression
enhances the AP2γ transcriptional effect in the nucleus, as AP2γ is
retained to a lesser extent in the cytoplasm without Wwox
interaction (31,32). AP2γ regulates HER2 expression and
other genes involved in tumor growth, and loss of Wwox could
theoretically be biologically associated with increased HER2
expression (42–44). Qin et al(41) reported that ectopically expressed
Wwox reduced HER2 expression via AP2γ interaction in prostate
cancer cells, although it required signaling through the androgen
receptor. The HER2-positive cases in the present study appeared to
be associated with higher Wwox expression, which is in contrast to
the report by Qin et al(41). This might be explained by the fact
that our cohort included low-risk patients with a fewer number of
HER2-positive cases.
A third binding partner to Wwox is HER4, which is
another member of the epidermal growth factor receptor family. HER4
and Wwox interact through the intracellular domain (4ICD) of HER4.
Keeping 4ICD in the cytoplasm by Wwox promotes apoptosis as 4ICD
harbors a pro-apoptotic BH3-domain. 4ICD located in the nucleus
instead acts as a co-activator to ER and contributes to growth and
proliferation (33,34,45,46).
Co-expression of HER4 and Wwox is associated with a favorable
outcome in breast cancer compared to when HER4 is expressed in the
absence of Wwox (34). As one of
its drug effects, tamoxifen obstructs the association between ER
and its co-activators by changing ER conformation (4). It is suggested that HER4 might be
important in drug resistance in breast cancer, as tamoxifen reduces
the possibility of 4ICD binding to the ER complex. When Wwox
expression is lost or reduced, more 4ICD is available in the
nucleus for promoting cell survival. Taken together, it is
plausible that loss of Wwox expression may affect the response of
endocrine therapy in breast cancer. As tamoxifen is one of the most
commonly used cancer drugs and breast cancer affects one in ten
women in the Western world, there is a considerable need for
reliable predictive markers for treatment selection. Since the
present cohort was built up by historically exclusive material
placed on TMA, there are obvious limitations to the use of the
methods. Nevertheless, we were able to evaluate the influence of
Wwox on tamoxifen therapy in a large number of randomized patients
using immunohistochemistry. Collectively, we found that Wwox
expression corresponds to improved recurrence-free survival even
though our results need further validation in other trials. In
conclusion, the present study emphasizes the existing theory of
Wwox as a possible predictor of tamoxifen response.
Acknowledgements
This study was supported by grants from the Percy
Falk Foundation for Research in Breast and Prostate Cancer,
Stockholm, Sweden.
References
|
1
|
Ring A and Dowsett M: Mechanisms of
tamoxifen resistance. Endocr Relat Cancer. 11:643–658. 2004.
View Article : Google Scholar
|
|
2
|
Greenspan EM: Tamoxifen in early breast
cancer. Lancet. 1:6551983. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rose C, Thorpe SM, Andersen KW, Pedersen
BV, Mouridsen HT, Blichert-Toft M and Rasmussen BB: Beneficial
effect of adjuvant tamoxifen therapy in primary breast cancer
patients with high oestrogen receptor values. Lancet. 1:16–19.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shiau AK, Barstad D, Loria PM, Cheng L,
Kushner PJ, Agard DA and Greene GL: The structural basis of
estrogen receptor/coactivator recognition and the antagonism of
this interaction by tamoxifen. Cell. 95:927–937. 1998. View Article : Google Scholar
|
|
5
|
Stewart HJ and Prescott R: Adjuvant
tamoxifen therapy and receptor levels. Lancet. 1:5731985.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paige AJ, Taylor KJ, Taylor C, Hillier SG,
Farrington S, Scott D, Porteous DJ, Smyth JF, Gabra H and Watson
JE: WWOX: a candidate tumor suppressor gene involved in multiple
tumor types. Proc Natl Acad Sci USA. 98:11417–11422. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aqeilan RI, Trapasso F, Hussain S,
Costinean S, Marshall D, Pekarsky Y, Hagan JP, Zanesi N, Kaou M,
Stein GS, et al: Targeted deletion of Wwox reveals a tumor
suppressor function. Proc Natl Acad Sci USA. 104:3949–3954. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bednarek AK, Laflin KJ, Daniel RL, Liao Q,
Hawkins KA and Aldaz CM: WWOX, a novel WW domain-containing protein
mapping to human chromosome 16q23.3-24.1, a region frequently
affected in breast cancer. Cancer Res. 60:2140–2145.
2000.PubMed/NCBI
|
|
9
|
Nunez MI, Ludes-Meyers J, Abba MC, Kil H,
Abbey NW, Page RE, Sahin A, Klein-Szanto AJ and Aldaz CM: Frequent
loss of WWOX expression in breast cancer: correlation with estrogen
receptor status. Breast Cancer Res Treat. 89:99–105. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Płuciennik E, Kusińska R, Potemski P,
Kubiak R, Kordek R and Bednarek AK: WWOX - the FRA16D cancer gene:
expression correlation with breast cancer progression and
prognosis. Eur J Surg Oncol. 32:153–157. 2006.PubMed/NCBI
|
|
11
|
Driouch K, Prydz H, Monese R, Johansen H,
Lidereau R and Frengen E: Alternative transcripts of the candidate
tumor suppressor gene, WWOX, are expressed at high levels in human
breast tumors. Oncogene. 21:1832–1840. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guler G, Uner A, Guler N, Han SY,
Iliopoulos D, Hauck WW, McCue P and Huebner K: The fragile genes
FHIT and WWOX are inactivated coordinately in invasive breast
carcinoma. Cancer. 100:1605–1614. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nunez MI, Ludes-Meyers J and Aldaz CM:
WWOX protein expression in normal human tissues. J Mol Histol.
37:115–125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bednarek AK, Keck-Waggoner CL, Daniel RL,
Laflin KJ, Bergsagel PL, Kiguchi K, Brenner AJ and Aldaz CM: WWOX,
the FRA16D gene, behaves as a suppressor of tumor growth. Cancer
Res. 61:8068–8073. 2001.PubMed/NCBI
|
|
15
|
Aqeilan RI, Kuroki T, Pekarsky Y, Albagha
O, Trapasso F, Baffa R, Huebner K, Edmonds P and Croce CM: Loss of
WWOX expression in gastric carcinoma. Clin Cancer Res.
10:3053–3058. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baykara O, Demirkaya A, Kaynak K, Tanju S,
Toker A and Buyru N: WWOX gene may contribute to progression of
non-small-cell lung cancer (NSCLC). Tumour Biol. 31:315–320. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bloomston M, Kneile J, Butterfield M,
Dillhoff M, Muscarella P, Ellison EC, Melvin WS, Croce CM,
Pichiorri F, Huebner, et al: Coordinate loss of fragile gene
expression in pancreatobiliary cancers: correlations among markers
and clinical features. Ann Surg Oncol. 16:2331–2338. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fabbri M, Iliopoulos D, Trapasso F,
Aqeilan RI, Cimmino A, Zanesi N, Yendamuri S, Han SY, Amadori D,
Huebner K, et al: WWOX gene restoration prevents lung cancer growth
in vitro and in vivo. Proc Natl Acad Sci USA. 102:15611–15616.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Finnis M, Dayan S, Hobson L,
Chenevix-Trench G, Friend K, Ried K, Venter D, Woollatt E, Baker E
and Richards RI: Common chromosomal fragile site FRA16D mutation in
cancer cells. Hum Mol Genet. 14:1341–1349. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iliopoulos D, Guler G, Han SY, Johnston D,
Druck T, McCorkell KA, Palazzo J, McCue PA, Baffa R and Huebner K:
Fragile genes as biomarkers: epigenetic control of WWOX and FHIT in
lung, breast and bladder cancer. Oncogene. 24:1625–1633. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iliopoulos D, Fabbri M, Druck T, Qin HR,
Han SY and Huebner K: Inhibition of breast cancer cell growth in
vitro and in vivo: effect of restoration of Wwox expression. Clin
Cancer Res. 13:268–274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuroki T, Yendamuri S, Trapasso F,
Matsuyama A, Aqeilan RI, Alder H, Rattan S, Cesari R, Nolli ML,
Williams NN, et al: The tumor suppressor gene WWOX at FRA16D is
involved in pancreatic carcinogenesis. Clin Cancer Res.
10:2459–2465. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O’Keefe LV, Liu Y, Perkins A, Dayan S,
Saint R and Richards RI: FRA16D common chromosomal fragile site
oxido-reductase (FOR/WWOX) protects against the effects of ionizing
radiation in Drosophila. Oncogene. 24:6590–6596.
2005.PubMed/NCBI
|
|
24
|
Park SW, Ludes-Meyers J, Zimonjic DB,
Durkin ME, Popescu NC and Aldaz CM: Frequent downregulation and
loss of WWOX gene expression in human hepatocellular carcinoma. Br
J Cancer. 91:753–759. 2004.PubMed/NCBI
|
|
25
|
Qin HR, Iliopoulos D, Semba S, Fabbri M,
Druck T, Volinia S, Croce CM, Morrison CD, Klein RD and Huebner K:
A role for the WWOX gene in prostate cancer. Cancer Res.
66:6477–6481. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guler G, Uner A, Guler N, Han SY,
Iliopoulos D, McCue P and Huebner K: Concordant loss of fragile
gene expression early in breast cancer development. Pathol Int.
55:471–478. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang NS, Schultz L, Hsu LJ, Lewis J, Su M
and Sze CI: 17beta-Estradiol upregulates and activates WOX1/WWOXv1
and WOX2/WWOXv2 in vitro: potential role in cancerous progression
of breast and prostate to a premetastatic state in vivo. Oncogene.
24:714–723. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guler G, Himmetoglu C, Jimenez RE, Geyer
SM, Wang WP, Costinean S, Pilarski RT, Morrison C, Suren D, Liu J,
et al: Aberrant expression of DNA damage response proteins is
associated with breast cancer subtype and clinical features. Breast
Cancer Res Treat. 129:421–432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aqeilan RI, Pekarsky Y, Herrero JJ,
Palamarchuk A, Letofsky J, Druck T, Trapasso F, Han SY, Melino G,
Huebner K, et al: Functional association between Wwox tumor
suppressor protein and p73, a p53 homolog. Proc Natl Acad Sci USA.
101:4401–4406. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang NS, Doherty J, Ensign A, Schultz L,
Hsu LJ and Hong Q: WOX1 is essential for tumor necrosis factor-, UV
light-, staurosporine-, and p53-mediated cell death, and its
tyrosine 33-phosphorylated form binds and stabilizes serine
46-phosphorylated p53. J Biol Chem. 280:43100–43108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guler G, Iliopoulos D, Guler N, Himmetoglu
C, Hayran M and Huebner K: Wwox and Ap2gamma expression levels
predict tamoxifen response. Clin Cancer Res. 13:6115–6121. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guler G, Huebner K, Himmetoglu C, Jimenez
RE, Costinean S, Volinia S, Pilarski RT, Hayran M and Shapiro CL:
Fragile histidine triad protein, WW domain-containing
oxidoreductase protein Wwox, and activator protein 2gamma
expression levels correlate with basal phenotype in breast cancer.
Cancer. 115:899–908. 2009. View Article : Google Scholar
|
|
33
|
Aqeilan RI, Donati V, Palamarchuk A,
Trapasso F, Kaou M, Pekarsky Y, Sudol M and Croce CM: WW
domain-containing proteins, WWOX and YAP, compete for interaction
with ErbB-4 and modulate its transcriptional function. Cancer Res.
65:6764–6772. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aqeilan RI, Donati V, Gaudio E, Nicoloso
MS, Sundvall M, Korhonen A, Lundin J, Isola J, Sudol M, Joensuu H,
et al: Association of Wwox with ErbB4 in breast cancer. Cancer Res.
67:9330–9336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abdeen SK, Salah Z, Maly B, Smith Y,
Tufail R, Abu-Odeh M, Zanesi N, Croce CM, Nawaz Z and Aqeilan RI:
Wwox inactivation enhances mammary tumorigenesis. Oncogene.
30:3900–3906. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rutqvist LE, Johansson H and Group SBCS:
Long-term follow-up of the randomized Stockholm trial on adjuvant
tamoxifen among postmenopausal patients with early stage breast
cancer. Acta Oncol. 46:133–145. 2007. View Article : Google Scholar
|
|
37
|
Wang X, Chao L, Ma G, Chen L, Zang Y and
Sun J: The prognostic significance of WWOX expression in patients
with breast cancer and its association with the basal-like
phenotype. J Cancer Res Clin Oncol. 137:271–278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fox EM, Andrade J and Shupnik MA: Novel
actions of estrogen to promote proliferation: integration of
cytoplasmic and nuclear pathways. Steroids. 74:622–627. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang Z, Barnes CJ and Kumar R: Human
epidermal growth factor receptor 2 status modulates subcellular
localization of and interaction with estrogen receptor alpha in
breast cancer cells. Clin Cancer Res. 10:3621–3628. 2004.
View Article : Google Scholar
|
|
40
|
Watanabe A, Hippo Y, Taniguchi H, Iwanari
H, Yashiro M, Hirakawa K, Kodama T and Aburatani H: An opposing
view on WWOX protein function as a tumor suppressor. Cancer Res.
63:8629–8633. 2003.PubMed/NCBI
|
|
41
|
Qin HR, Iliopoulos D, Nakamura T,
Costinean S, Volinia S, Druck T, Sun J, Okumura H and Huebner K:
Wwox suppresses prostate cancer cell growth through modulation of
ErbB2-mediated androgen receptor signaling. Mol Cancer Res.
5:957–965. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Belsches-Jablonski AP, Biscardi JS, Peavy
DR, Tice DA, Romney DA and Parsons SJ: Src family kinases and HER2
interactions in human breast cancer cell growth and survival.
Oncogene. 20:1465–1475. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Morgan L, Gee J, Pumford S, Farrow L,
Finlay P, Robertson J, Ellis I, Kawakatsu H, Nicholson R and Hiscox
S: Elevated Src kinase activity attenuates Tamoxifen response in
vitro and is associated with poor prognosis clinically. Cancer Biol
Ther. 8:1550–1558. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pellikainen J, Naukkarinen A, Ropponen K,
Rummukainen J, Kataja V, Kellokoski J, Eskelinen M and Kosma VM:
Expression of HER2 and its association with AP-2 in breast cancer.
Eur J Cancer. 40:1485–1495. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Junttila TT, Sundvall M, Lundin M, Lundin
J, Tanner M, Härkönen P, Joensuu H, Isola J and Elenius K:
Cleavable ErbB4 isoform in estrogen receptor-regulated growth of
breast cancer cells. Cancer Res. 65:1384–1393. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Naresh A, Thor AD, Edgerton SM, Torkko KC,
Kumar R and Jones FE: The HER4/4ICD estrogen receptor coactivator
and BH3-only protein is an effector of tamoxifen-induced apoptosis.
Cancer Res. 68:6387–6395. 2008. View Article : Google Scholar : PubMed/NCBI
|