Spandidos Publications style
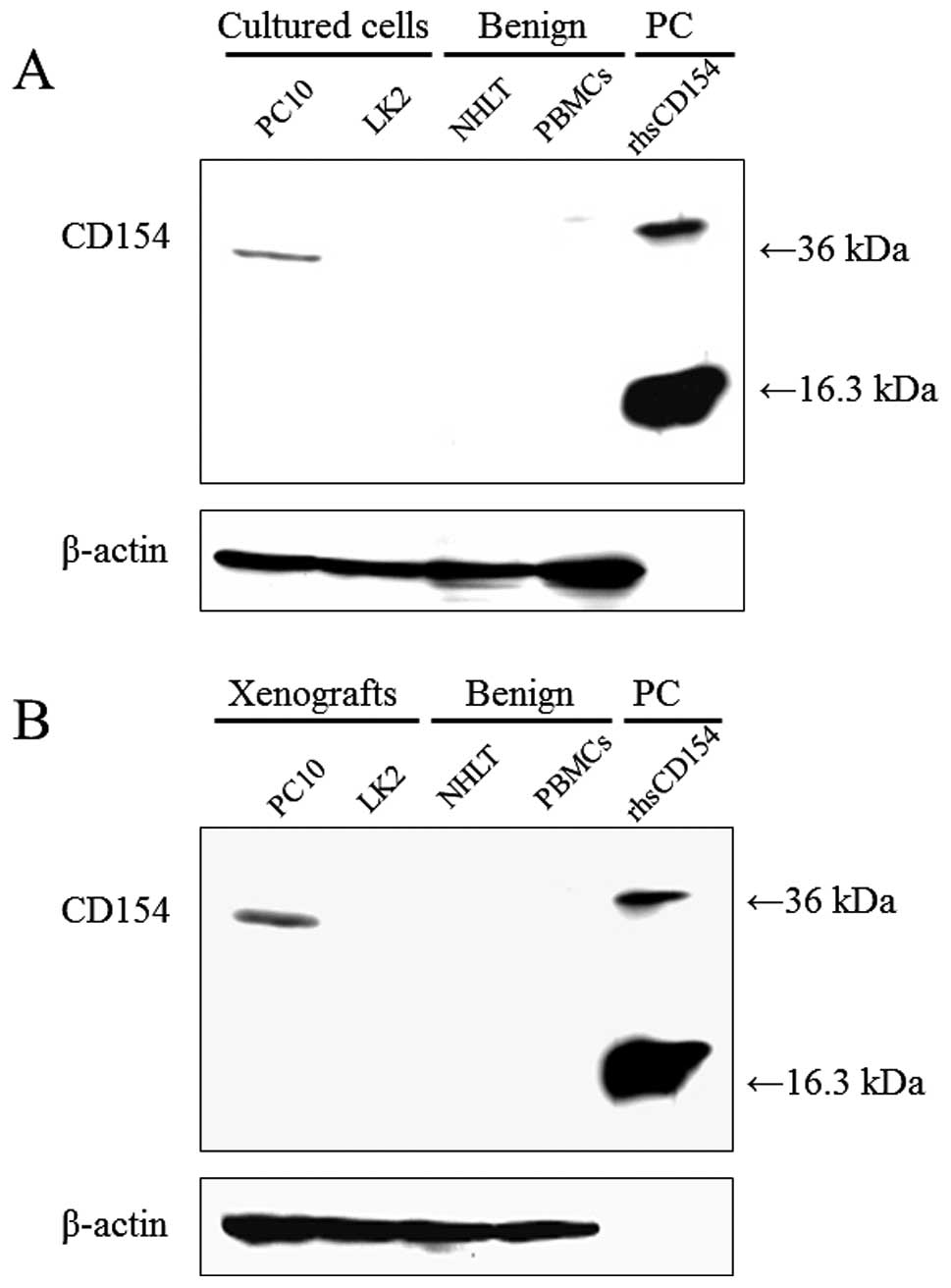
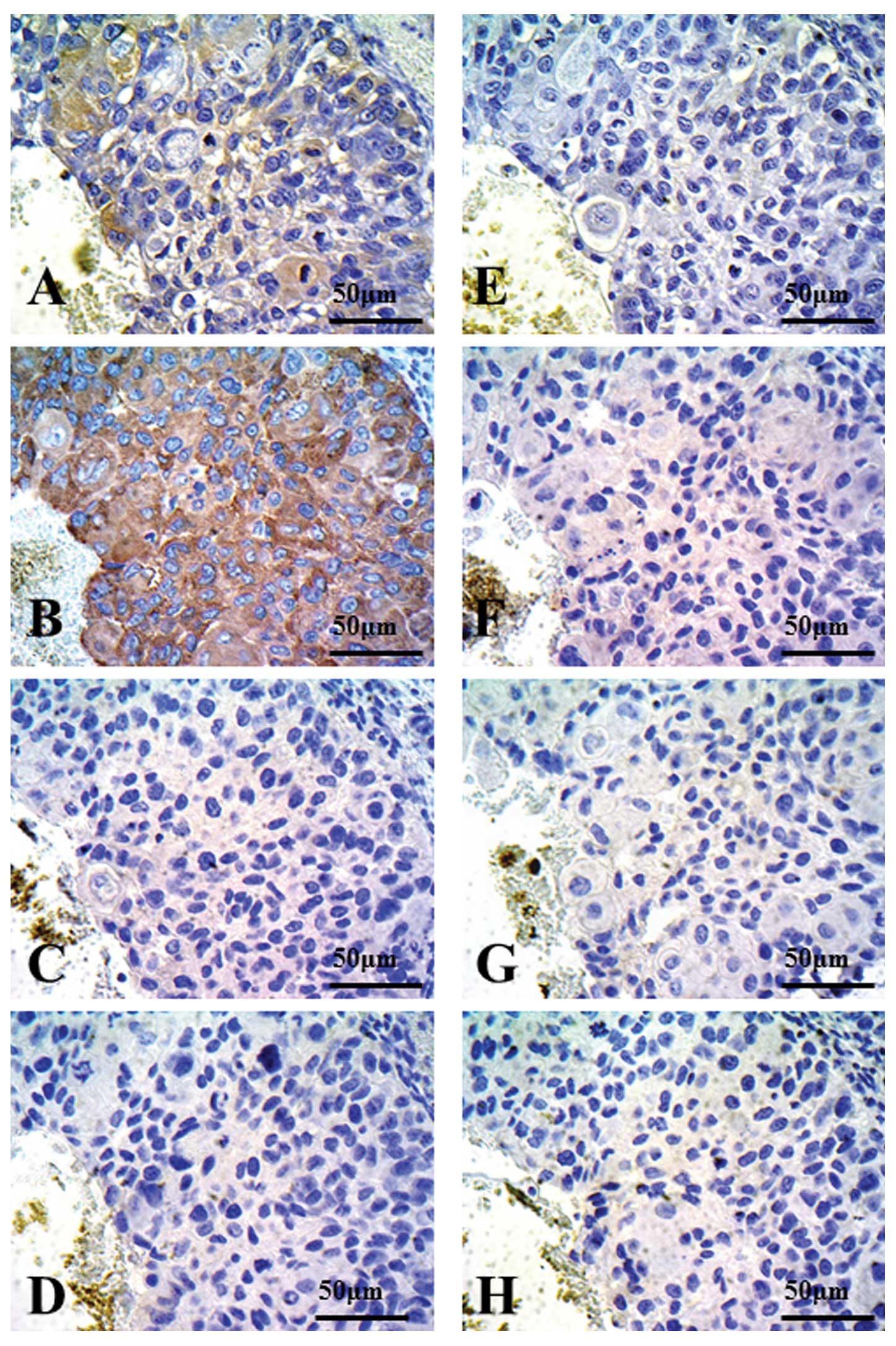
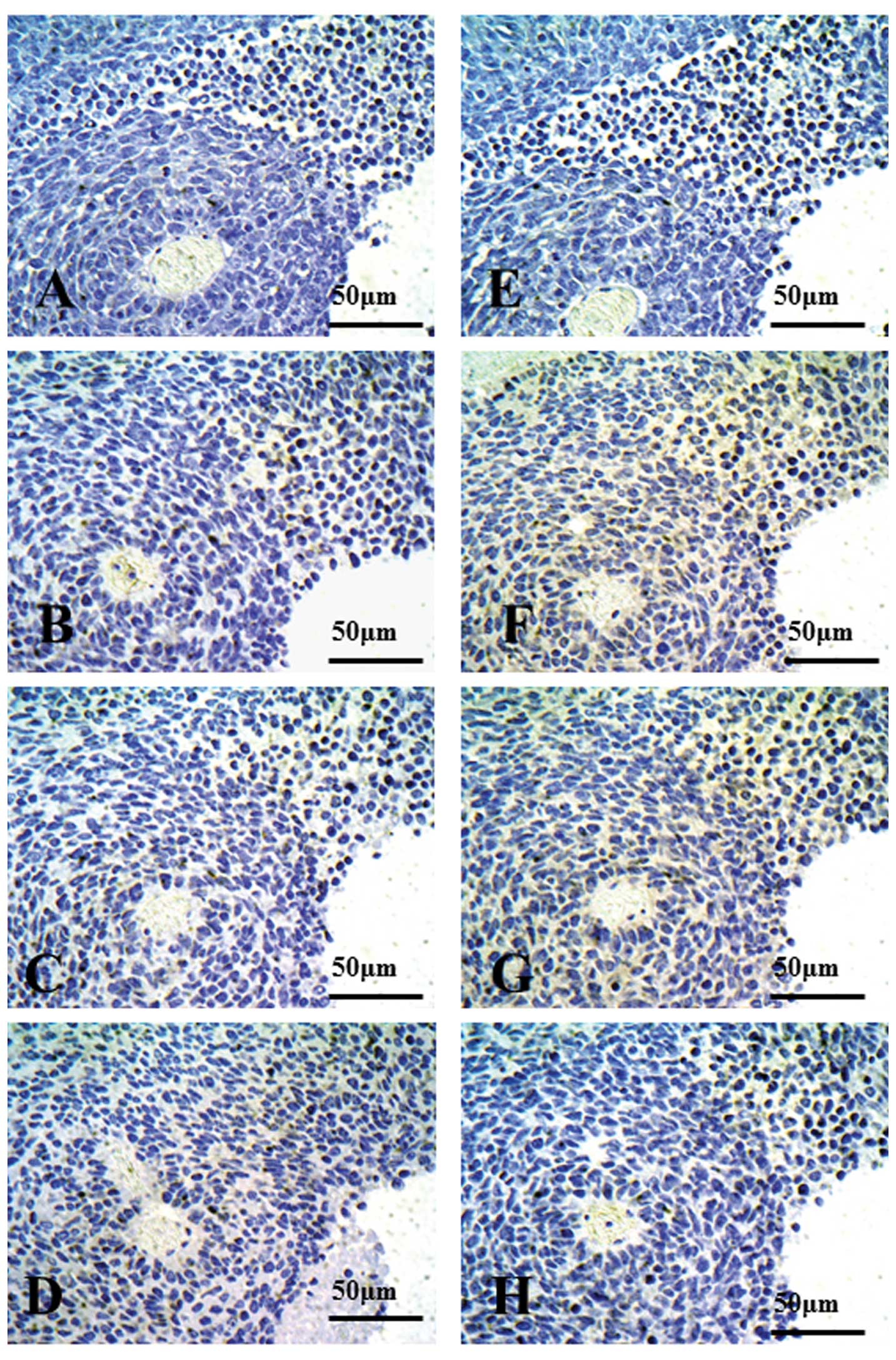
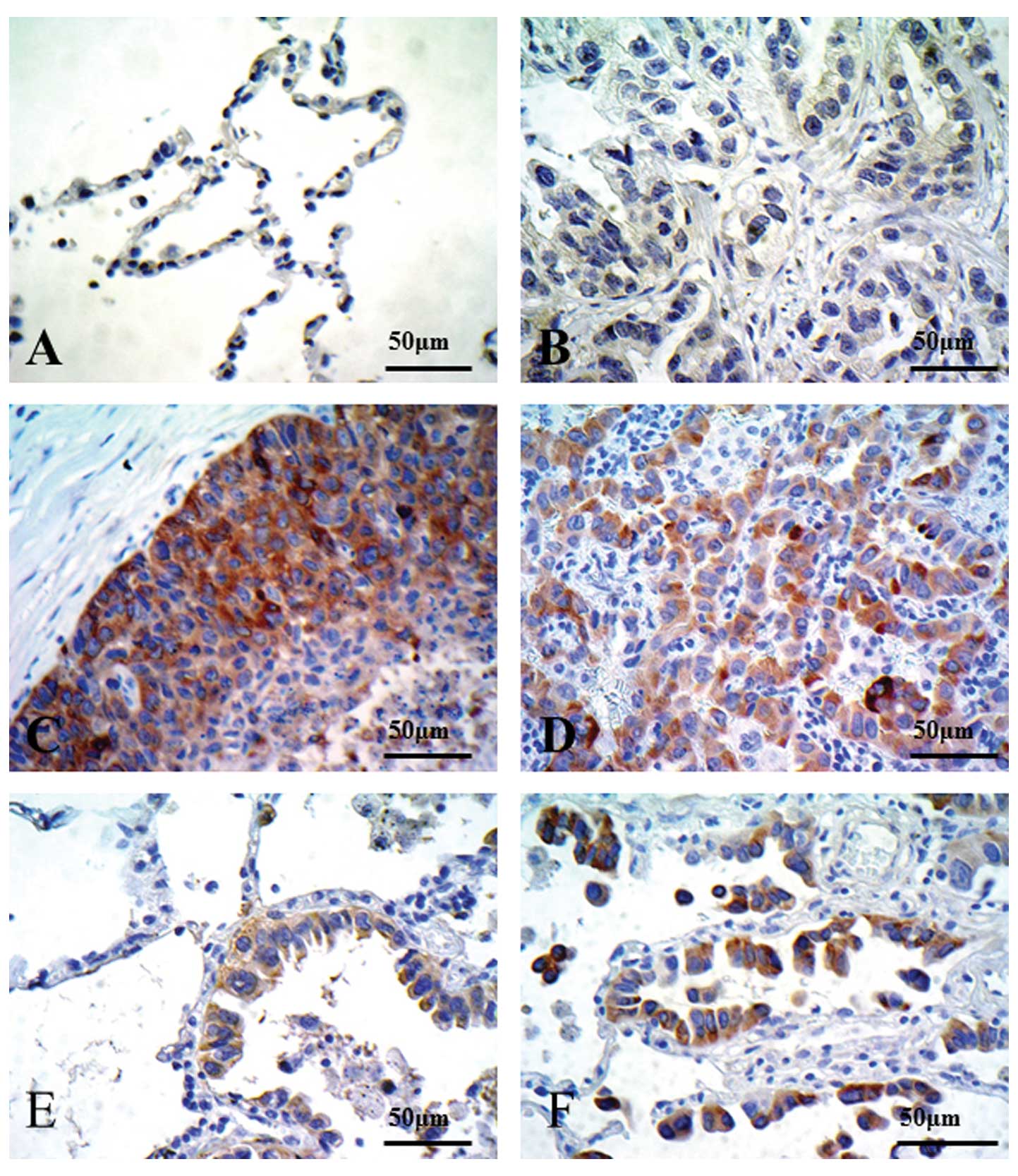
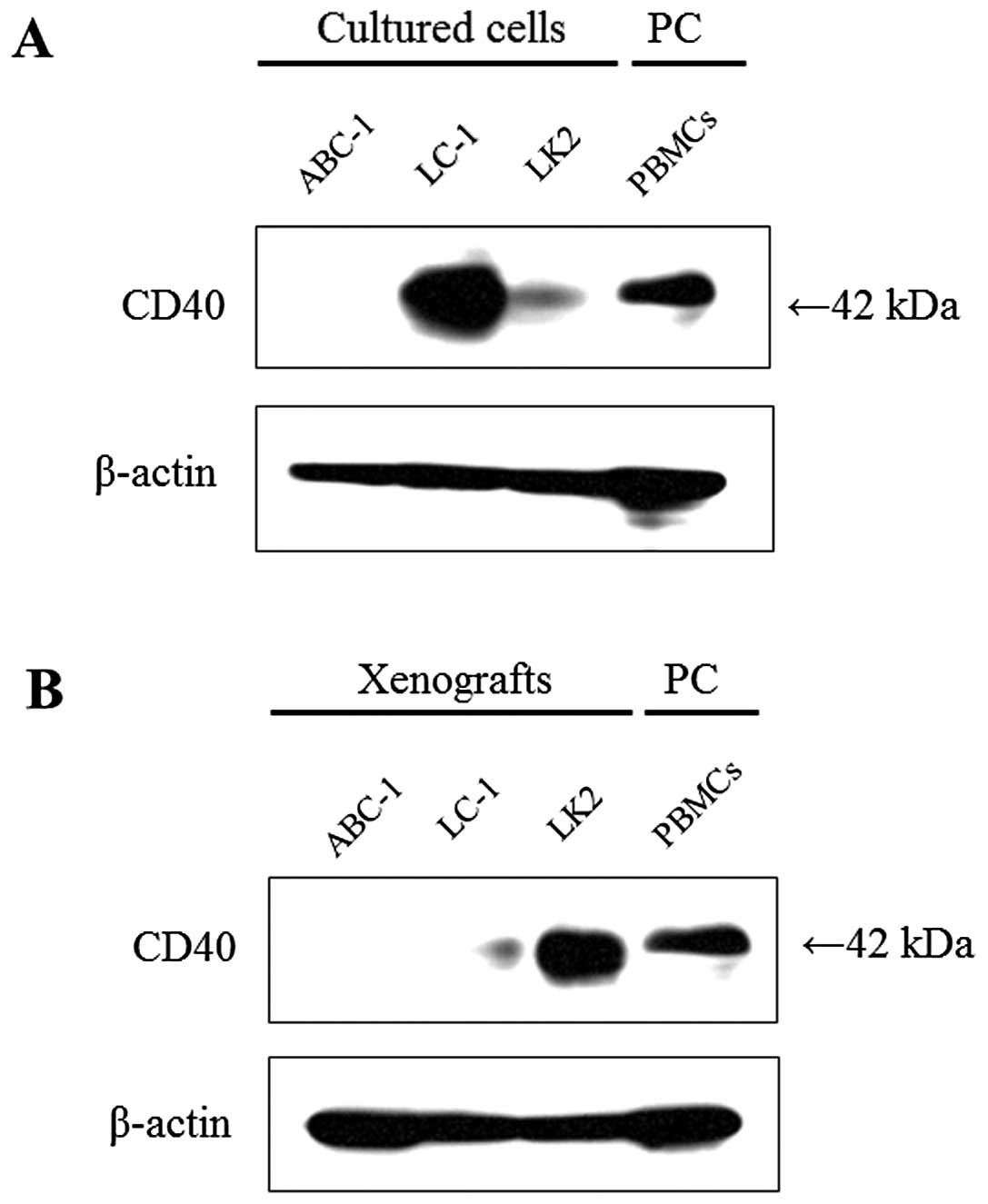
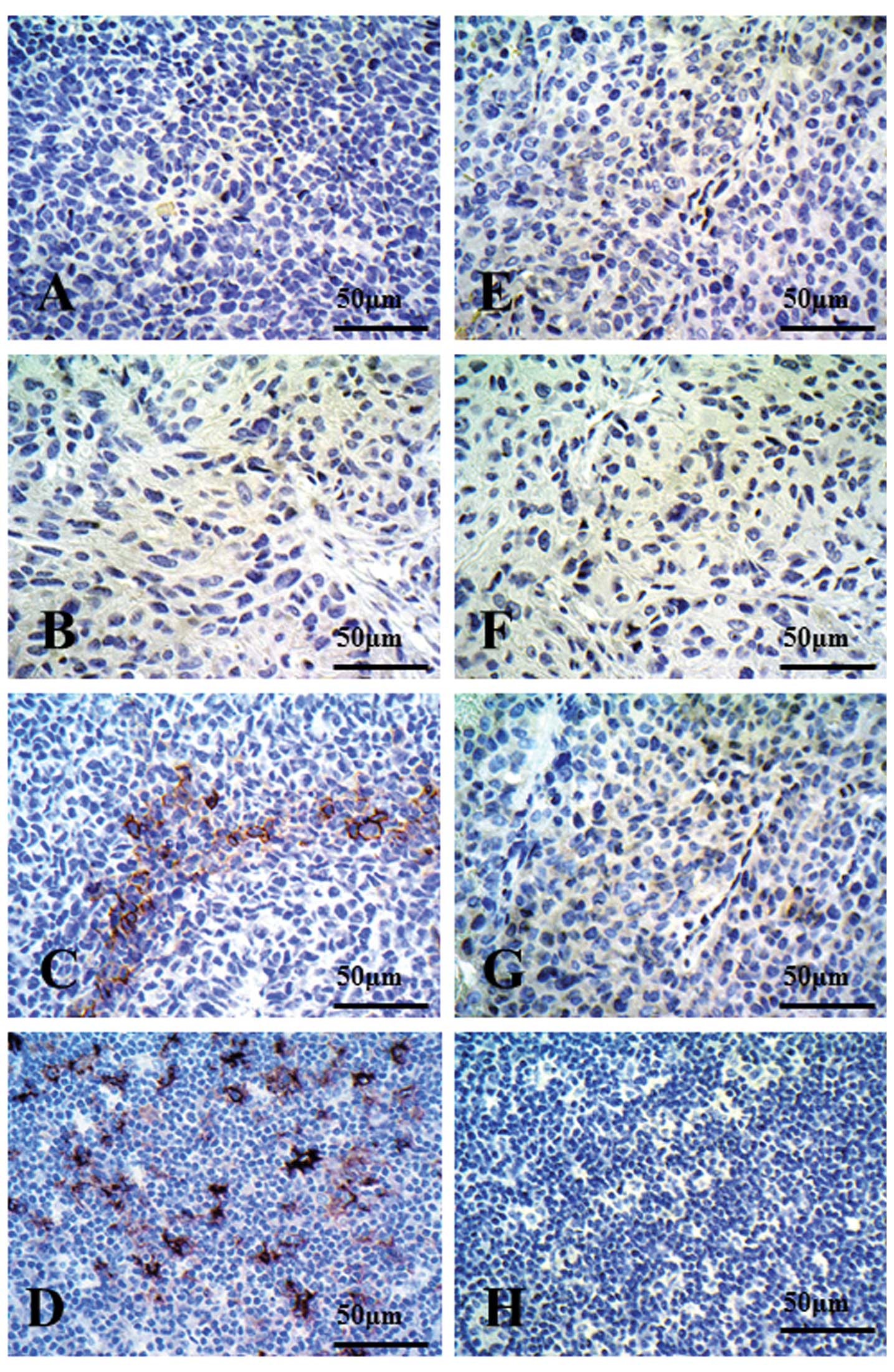
Ishikawa K, Miyamoto M, Yoshioka T, Kadoya M, Li L, Mishra R, Ichinokawa K, Shoji Y, Matsumura Y, Hida Y, Hida Y, et al: Method for the validation of immunohistochemical staining using SCID mouse xenografts: Expression of CD40 and CD154 in human non-small cell lung cancer. Oncol Rep 29: 1315-1321, 2013.
APA
Ishikawa, K., Miyamoto, M., Yoshioka, T., Kadoya, M., Li, L., Mishra, R. ... Hirano, S. (2013). Method for the validation of immunohistochemical staining using SCID mouse xenografts: Expression of CD40 and CD154 in human non-small cell lung cancer. Oncology Reports, 29, 1315-1321. https://doi.org/10.3892/or.2013.2275
MLA
Ishikawa, K., Miyamoto, M., Yoshioka, T., Kadoya, M., Li, L., Mishra, R., Ichinokawa, K., Shoji, Y., Matsumura, Y., Hida, Y., Kaga, K., Kato, T., Kaji, M., Ohbuchi, T., Itoh, T., Dosaka-Akita, H., Matsui, Y., Hirano, S."Method for the validation of immunohistochemical staining using SCID mouse xenografts: Expression of CD40 and CD154 in human non-small cell lung cancer". Oncology Reports 29.4 (2013): 1315-1321.
Chicago
Ishikawa, K., Miyamoto, M., Yoshioka, T., Kadoya, M., Li, L., Mishra, R., Ichinokawa, K., Shoji, Y., Matsumura, Y., Hida, Y., Kaga, K., Kato, T., Kaji, M., Ohbuchi, T., Itoh, T., Dosaka-Akita, H., Matsui, Y., Hirano, S."Method for the validation of immunohistochemical staining using SCID mouse xenografts: Expression of CD40 and CD154 in human non-small cell lung cancer". Oncology Reports 29, no. 4 (2013): 1315-1321. https://doi.org/10.3892/or.2013.2275