Introduction
Immunohistochemical staining is associated with
several problems related to the sensitivity of the technical
process and standardization of the assessment of potent staining,
including positive control tissue samples. Previous reports have
described ambiguous positive or negative controls when
immunohistochemical staining was performed, particularly when no
internal positive controls were used. One of the reasons for this
ambiguity is that the control tissue specimens themselves were not
objectively confirmed as having target products by another
experimental method.
CD40 is a 42–48-kDa transmembrane glycoprotein
belonging to the tumor necrosis factor (TNF) receptor superfamily
(1,2). Its presence was initially described on
B cells and in bladder carcinoma (3), but it is also reportedly expressed on
monocytes (4), dendritic cells
(5), fibroblasts (6), tonsils (7), thymic epithelial cells (8) and endothelial cells (9).
The ligand of CD40 (CD40L, CD154), a 39-kDa membrane
glycoprotein, is expressed on T cells, basophils and mast cells
(10,11). Interaction between CD40 with CD154
induces proliferation, germinal center formation and allows for the
generation of B cells that secrete IgE following isotype switching
(12–15). Recent reports have demonstrated
CD154 expression in breast cancer (16), thyroid cancer (17) and coronary diseases (18). However, CD154 expression in lung
cancer has not been widely studied (19). Consequently, we performed
immunohistochemistry for CD40 and CD154 in 129 non-small cell lung
cancer (NSCLC) patient tissue samples (20). In the present study, we propose an
approach for standardizing the evaluation of control specimens of
immunohistochemistry.
Materials and methods
Cell lines
Human lung cancer cell lines were obtained from the
Japanese Cancer Research Resources Bank (Tokyo, Japan). PC10, LC-1
and LK2 were grown in RPMI-1640 (Sigma-Aldrich Co., Ltd., Irvine,
CA, USA) with 10% fetal bovine serum (FBS), and 1%
penicillin/streptomycin (p/s). ABC-1 was maintained in minimum
essential medium Eagle (M-EME; Sigma-Aldrich Co., Ltd.,) with 10%
FBS and 1% p/s. All cell lines were maintained in a humidified
incubator with 5% CO2 in air at 37°C.
Mice and tumor xenograft models
CB17/SCID mice were obtained from Charles River
Japan (Yokohama, Japan). All mice were female, 4–6 weeks of age,
and were maintained under specific pathogen-free conditions. All
animal procedures were in accordance with the guidelines of the
Hokkaido University Institutional Animal Care and Use Committee
using an approved protocol. ABC-1, LC-1, LK2 and PC10 cells
(5×106) were subcutaneously injected in a volume of 100
μl of phosphate-buffered saline into the left flank region of each
CB17/SCID mouse. When tumor diameter exceeded 10 mm, mice were
sacrificed and tumors were separated to 2 blocks: one block was
frozen using liquid nitrogen to extract proteins for western blot
analysis, and the other was immersed in formalin for
immunohistological analysis.
Reagents and antibodies
Anti-CD154 rabbit polyclonal antibody (C-20:sc-978)
and anti-CD40 rabbit polyclonal antibody (C-20:sc-975) were
purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and
anti-CD154 mouse monoclonal antibody (TRAP1:IM1842) was purchased
from Immunotech (Marseille Cedex, France). Anti-CD40 mouse
monoclonal antibody (11E9) was purchased from Novocastra
(Newcastle, UK).
Peroxidase-conjugated goat F(ab’)2 anti-rabbit IgG
and peroxidase-conjugated goat F(ab’)2 anti-mouse IgG were
purchased from Jackson ImmunoResearch (West Grove, PA, USA).
Negative control rabbit immunoglobulin fraction (normal) (X0903),
negative control mouse IgG1 (X0941) and negative control mouse
IgG2b (X0944) were purchased from Dako Japan (Kyoto, Japan).
Recombinant human soluble CD40 ligand protein (TRAP1:gp39) was
purchased from Chemicon International Inc. (Billerica, MA,
USA).
Western blot analysis
Western blot analysis was performed in order to
analyze CD154 expression in lung cancer cells. Lysates from cell
lines and lysates from SCID mouse xenografts were prepared in SDS
buffer containing 62.5 mm Tris-HCl (pH 6.8), 2% w/v SDS, 10%
glycerol, 50 mm DTT, 0.1% w/v bromphenol blue and 1 mm PMSF. Total
proteins (20 μg) were electrophoresed in 15% SDS-polyacrylamyde
gels and were transferred onto nitrocellulose membranes.
Anti-CD154 rabbit polyclonal antibody (C-20:sc-978,
1:100) and anti-CD154 mouse monoclonal antibody (TRAP1:IM1842,
1:20) were used as primary antibodies for CD154 and anti-CD40
rabbit polyclonal antibody (C-20:sc-975, 1:200) was used as the
primary antibody for CD40. The appropriate peroxidase-conjugated
goat anti-rabbit or anti-mouse IgG was used as the secondary
antibody (1:10,000).
Detection of bound antibodies was performed using
the ECL system (Amersham, Aylesbury, UK). The recombinant human
soluble CD40 ligand (rhsCD40L, rhsCD154) (Chemicon International)
was used as a positive control for CD154. Lysates from normal human
peripheral blood mononuclear cells (PBMCs) were used as a positive
control for CD40.
Patients and tissue specimens
Whole surgical specimens of resected NSCLC were
utilized in this study. Patients enrolled in the study showed no
signs of metastases to secondary sites and had received no prior
anticancer treatments. Cases of in-hospital and non-cancer-related
deaths were excluded. We examined 129 NSCLC surgical specimens
meeting these criteria from patients undergoing curative resection
of the primary tumor, including systematic lymph node dissection.
Resected specimens were examined histopathologically after staining
with hematoxylin and eosin. A single section, from deep in the
tumor specimen, was selected for analysis, and at least
two-independent pathologists performed each diagnosis.
Immunohistochemistry
Immunohistochemical reactions were carried out using
the universal immuno-enzyme polymer method. Tumors from SCID mouse
xenograft models and surgical specimens were fixed in 10% formalin
solution and embedded in paraffin for sectioning at 4 μm. Sections
were then deparaffinized in xylene, dehydrated through a graded
ethanol series, and were either left untreated or treated with a
pressure cooker for 2 min.
Endogenous peroxidase activity was blocked by a
10-min incubation with hydrogen peroxide. Following three washes in
phosphate-buffered saline with 1% Tween-20 (PBS-T), sections were
incubated in 10% normal goat serum (Histofine SAB-PO kit; Nichirei,
Tokyo, Japan) for 30 min.
Samples were then incubated overnight with an
anti-CD154 rabbit polyclonal antibody (1:500 dilution, C-20:sc-978)
at 4°C. In addition, serial tissue sections of each sample were
separately incubated overnight with an anti-CD154 mouse monoclonal
antibody (1:25 dilution, TRAP1:IM1842), and with an anti-CD40 mouse
monoclonal antibody (1:40) at 4°C. Isotype-matched negative control
mouse IgG1 (X0931), negative control rabbit immunoglobulin fraction
(X0903) and negative control mouse IgG2b (X0944) were used as
primary antibodies. A mixture of C-20:sc-978 or TRAP1:IM1842
antibody with 0.1 mg/ml recombinant human soluble CD154 protein
(rhsCD154) boiled for 2 min and incubated for 5 or 90 min at room
temperature was also used as a primary antibody. Following three
additional washes, sections were incubated for 30 min at room
temperature with Histofine MAX-PO (Multi) (Histofine SAB-PO kit;
Nichirei).
Reaction products were visualized by incubation for
~5 min with 3,3′-diaminobenzidine tetrahydrochloride (Nichirei),
followed by washing in distilled water. Sections were
counterstained in hematoxylin for 1 min, and then mounted in
Permount (micro slides; Muto-Glass, Tokyo, Japan). Immunostained
sections were evaluated under a microscope (Olympus Optical Co.,
Ltd., Tokyo, Japan).
Results
Expression of CD154 in non-small lung
cancer cell lines in vitro and SCID xenografts in vivo
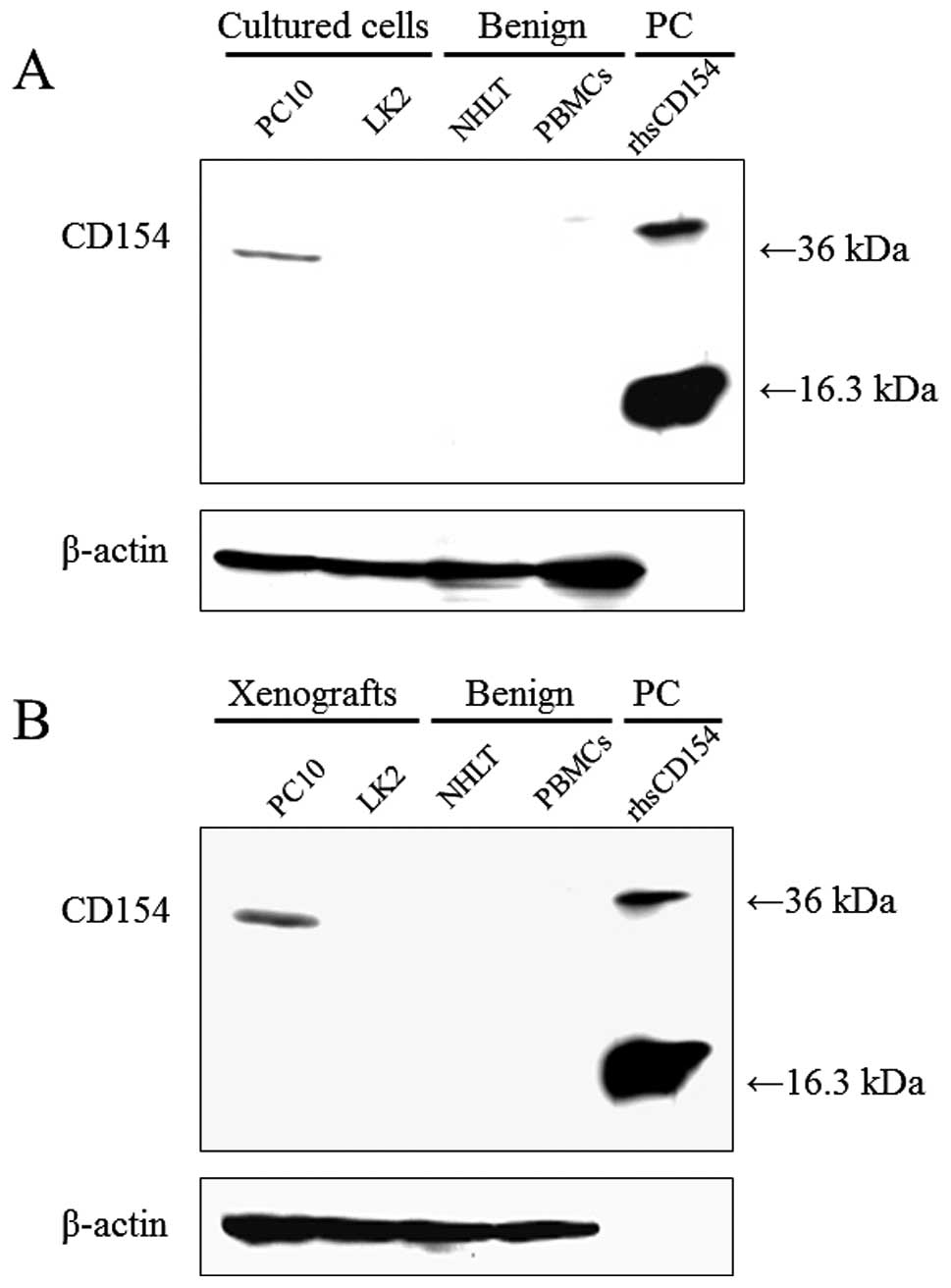
Using western blot analysis, with C-20:sc-978 as a
primary antibody, CD154 expression was detected at 36 kDa in the
lane loaded with the cultured cell line PC10, but was not detected
with LK2, homogenized normal human lung tissue (NHLT) or peripheral
blood mononuclear cells (PBMCs) (Fig.
1A). CD154 expression in lysates from SCID mouse xenografts was
very similar to that in lysates from cultured cell lines (Fig. 1B). Conversely, using TRAP1:IM1842 as
a primary antibody, no bands were detected, even in the lane loaded
with rhsCD154 (data not shown).
Immunohistochemical staining for CD154 in
SCID mouse xenograft models
SCID mouse xenograft models using two cell lines,
PC10 and LK2, were established. When C-20:sc-978 was used as a
primary antibody, CD154 expression in the PC10 xenograft was weakly
detected without pressure cooker treatment (Fig. 2A). By contrast, CD154 expression was
strongly detected in the cytoplasm and cell membranes in the PC10
xenograft with pressure cooker treatment (Fig. 2B). When rhsCD154 was added to
C-20:sc-978 and incubated for 90 min at room temperature, CD154
staining in the xenograft PC10 was markedly decreased (Fig. 2C). On the other hand, CD154
expression was not detected in the LK2 xenograft, with (Fig. 3B) or without (Fig. 3A) pressure cooker treatment. When
rhsCD154 was added to C-20:sc-978 for 90 min, no expression of
CD154 in the LK2 xenograft was detected (Fig. 3C).
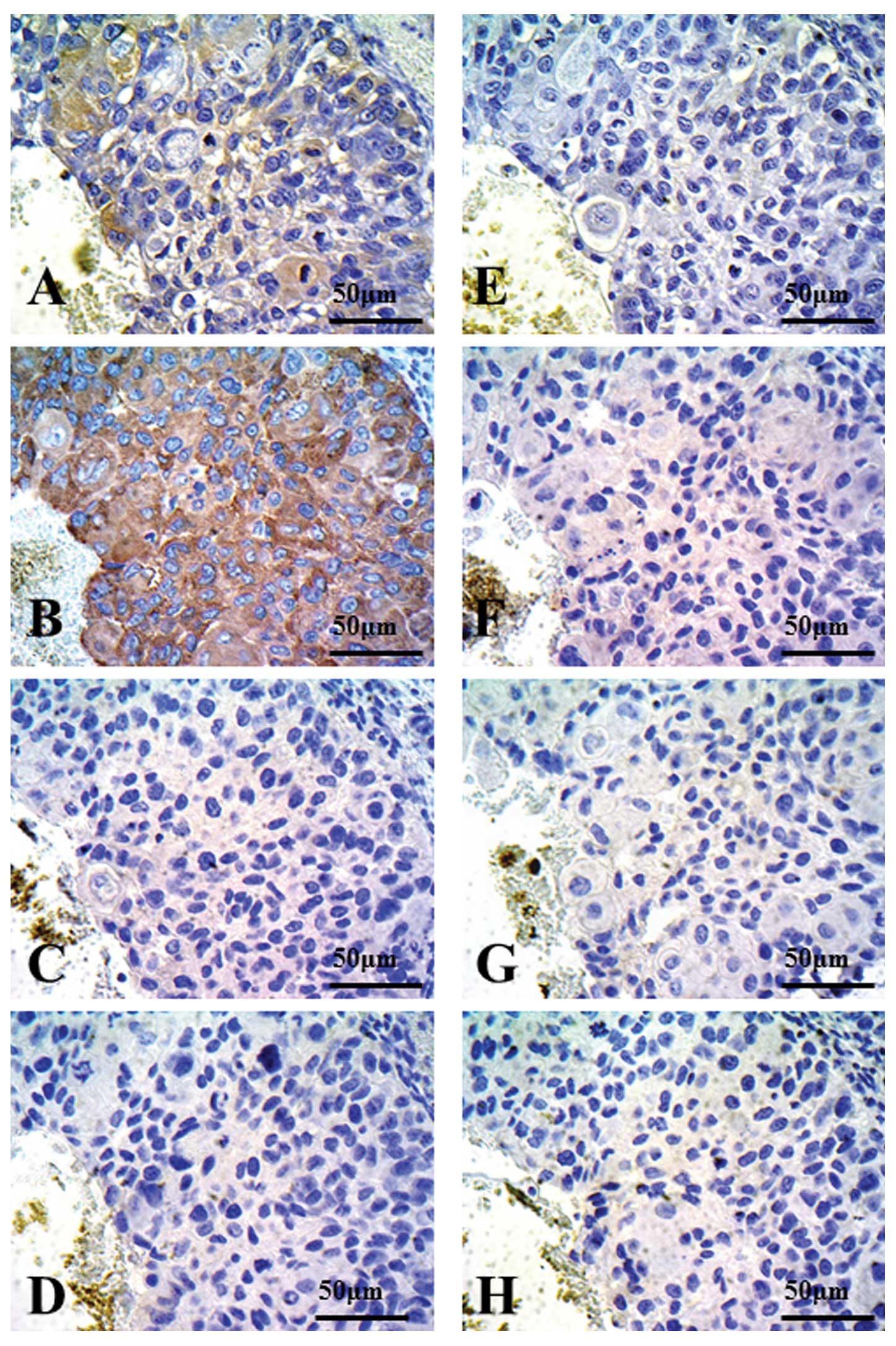 | Figure 2Immunohistochemical staining for
CD154, PC10 xenograft models. Using western blot analysis, PC10
xenografts exhibited CD154-positive cells. Autoclaved or
non-autoclaved refers to the presence of heat treatment using a
pressure cooker. (A) Anti-CD154 antibody (C-20:sc-978, 1:500
dilution), non-autoclaved. (B) Anti-CD154 antibody (C-20:sc-978,
1:500 dilution), autoclaved. (C) Anti-CD154 antibody (C-20:sc-978,
1:500 dilution), autoclaved, with *rhsCD154. (D) Rabbit
control IgG. (E) Anti-CD154 antibody (TRAP1:IM1842, 1:25 dilution),
non-autoclaved. (F) Anti-CD154 antibody (TRAP1:IM1842, 1:25
dilution), autoclaved. (G) Anti-CD154 antibody (TRAP1:IM1842, 1:25
dilution), autoclaved, with *rhsCD154. (H) Mouse control
IgG. *rhsCD154, recombinant human soluble CD154 (CD40
ligand). |
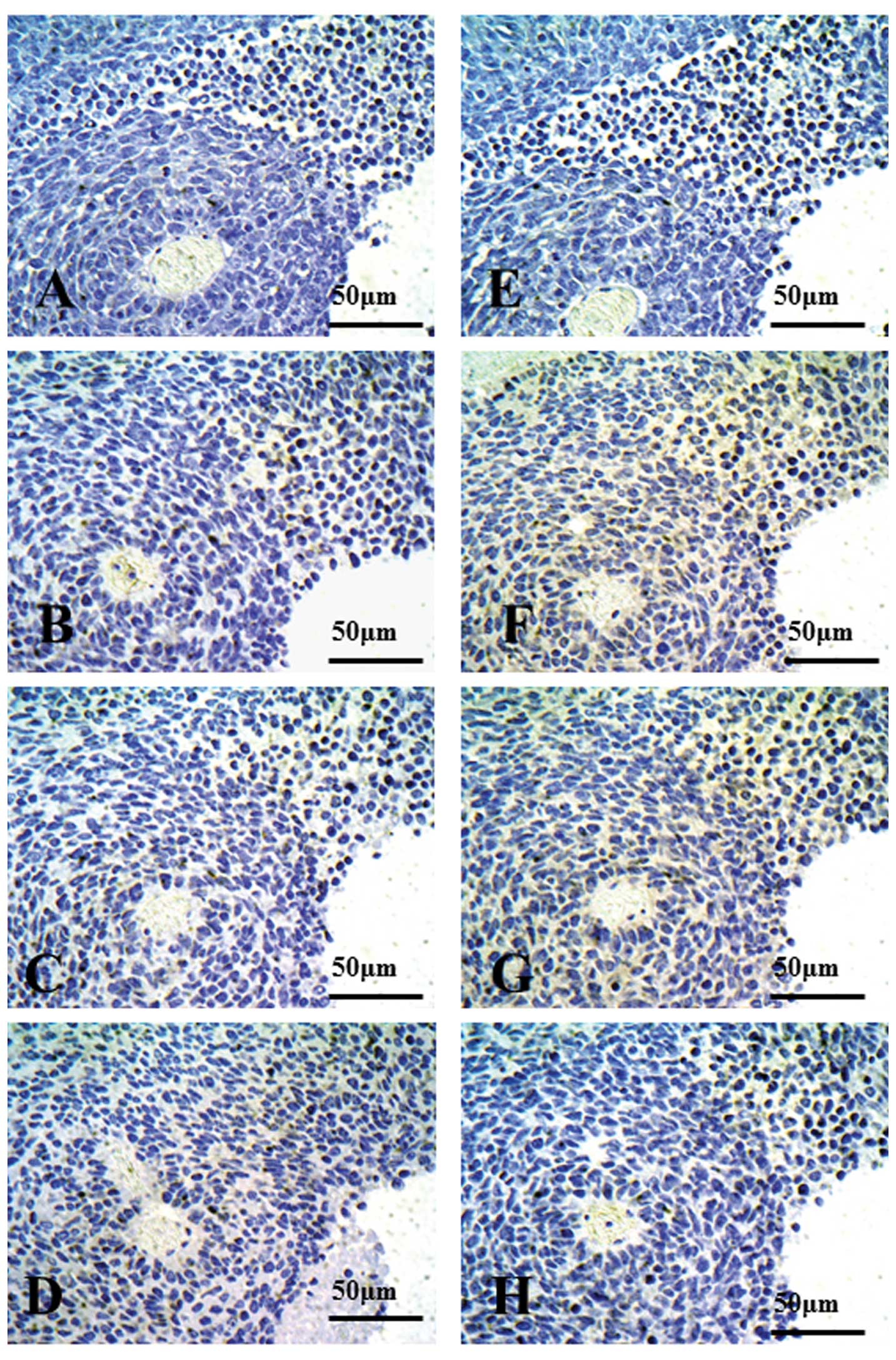 | Figure 3Immunohistochemical staining for
CD154, LK2 xenograft models. Using western blot analysis, LK2
xenografts exhibited CD154-negative cells. Autoclaved or
non-autoclaved refers to the presence of heat treatment using a
pressure cooker. (A) Anti-CD154 antibody (C-20:sc-978, 1:500
dilution), non-autoclaved. (B) Anti-CD154 antibody (C-20:sc-978,
1:500 dilution), autoclaved. (C) Anti-CD154 antibody (C-20:sc-978,
1:500 dilution), autoclaved, with *rhsCD154. (D) Rabbit
control IgG. (E) Anti-CD154 antibody (TRAP1:IM1842, 1:25 dilution),
non-autoclaved. (F) Anti-CD154 antibody (TRAP1:IM1842, 1:25
dilution), autoclaved. (G) Anti-CD154 antibody (TRAP1:IM1842, 1:25
dilution), autoclaved, with *rhsCD154. (H) Mouse control
IgG. *rhsCD154, recombinant human soluble CD154 (CD40
ligand). |
When TRAP1:IM1842 was used as a primary antibody,
slight expression of CD154 in the PC10 xenograft was seen without
pressure cooker treatment (Fig.
2E). CD154 expression was weakly detected in the cytoplasm in
the PC10 xenograft with pressure cooker treatment (Fig. 2F). After adding rhsCD154 to
TRAP1:IM1842 for 90 min, slight expression of CD154 in the PC10
xenograft was detected (Fig.
2G).
Expression of CD154 in the LK2 xenograft was
scarcely seen under all conditions; without pressure cooker
treatment (Fig. 3E), with pressure
cooker treatment (Fig. 3F) or after
adding rhsCD154 to TRAP1:IM1842 for 90 min (Fig. 3G). In addition, after adding rhCD154
to C-20:sc978 or TRAP1:IM1842 for only 5 min, no difference was
seen in CD154 staining (data not shown). There were no
CD154-positive cells seen in xenografts when control IgG was used
as a primary antibody (Figs. 2D and
H, and 3D and H).
Immunohistochemical staining for CD154 in
human lung tissues
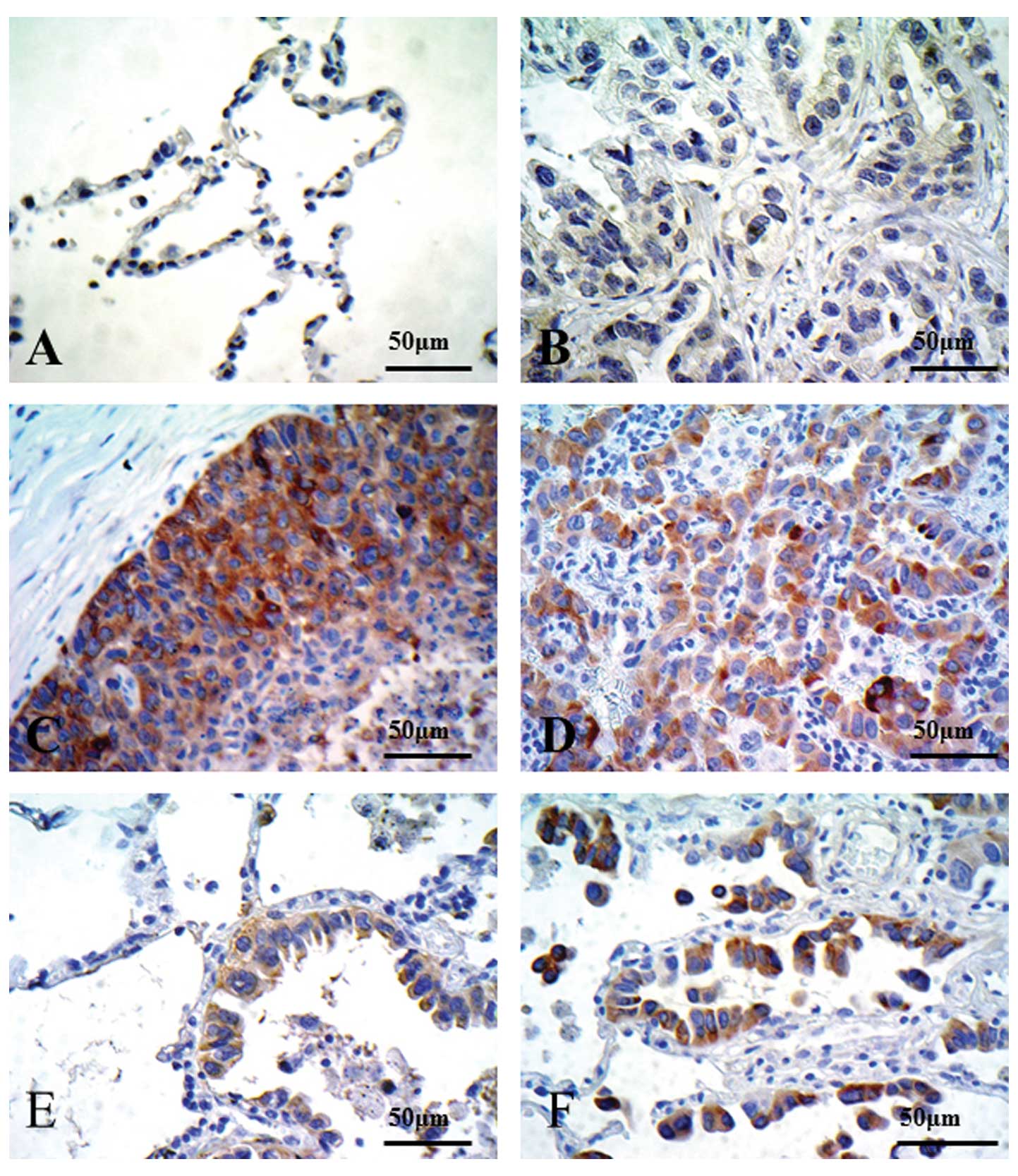
Preliminary immunohistochemical staining of lung
tissues from 30 patients was performed using C-20:sc-978 as a
primary antibody. We used the PC10 xenograft as the positive
control for CD154, and the LK2 xenograft as the negative control.
Representative photomicrographs are shown in Fig. 3. No CD154 staining was observed in
any of the normal alveolar cells (Fig.
4A). Although some adenocarcinoma cases showed no CD154
staining (Fig. 4B), CD154 staining
was observed in squamous cell carcinoma (Fig. 4C), adenocarcinoma (Fig. 4D) and bronchioloalveolar carcinomas
(BACs) (Fig. 4E and F).
Immunostaining patterns in CD154-positive cases in human lung
tissue were very similar to the PC10 SCID xenograft, with strong
staining in the cytoplasm, particularly in the cell membranes.
Thus, there were numerous differences in staining intensity in lung
tissues.
Consistency of CD40 expression results
between western blotting and immunostaining
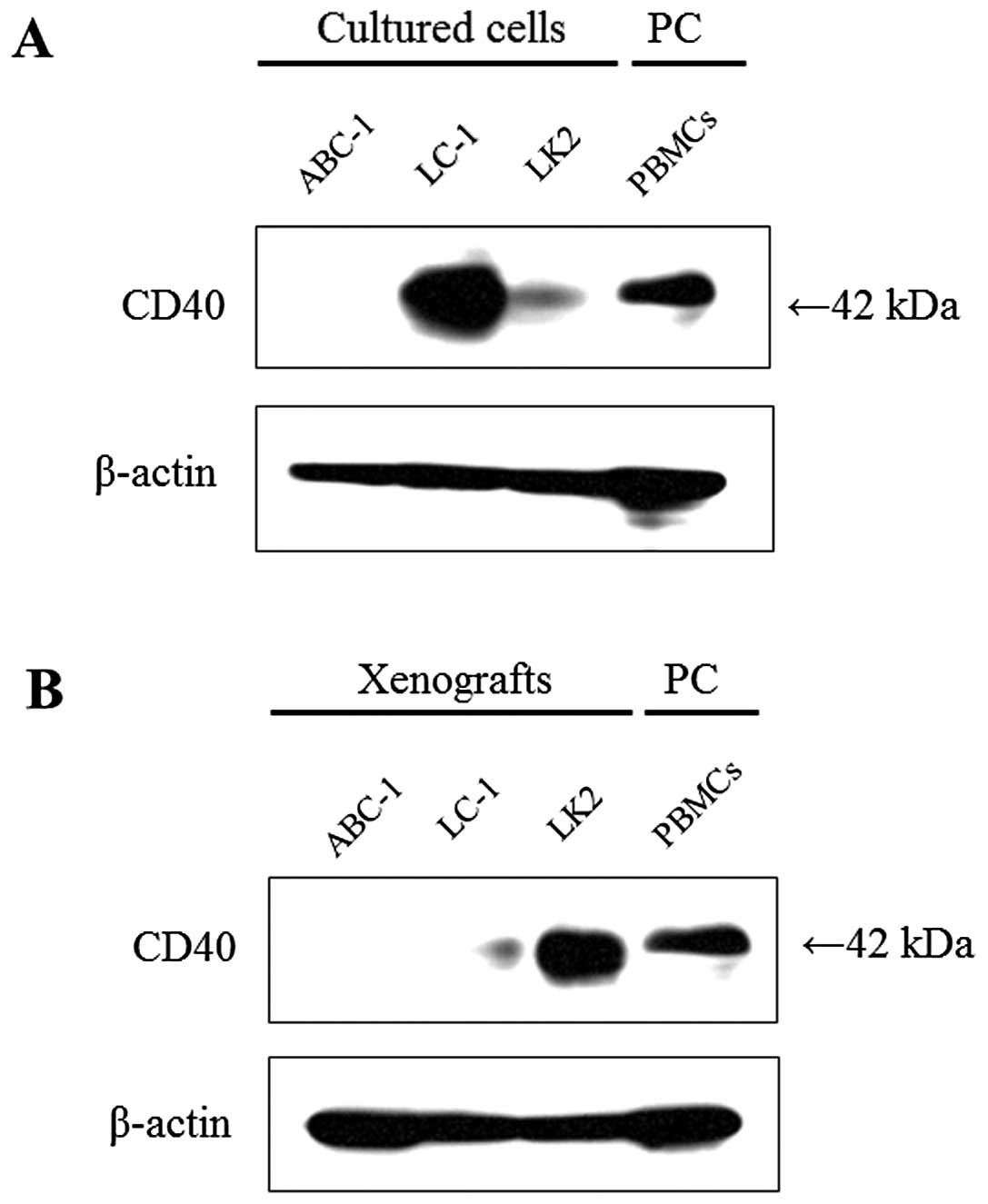
CD40 expression was strongly detected at 42 kDa in
cultured LC-1 cells; it was moderately detected in cultured LK2
cells, but it was not detected in ABC-1 cells (Fig. 5A). When compared with the results
from cultured cell lines, CD40 expression was markedly
downregulated in the LC-1 xenograft, upregulated in the LK2
xenograft, but it was not detected in the ABC-1 xenograft (Fig. 5B).
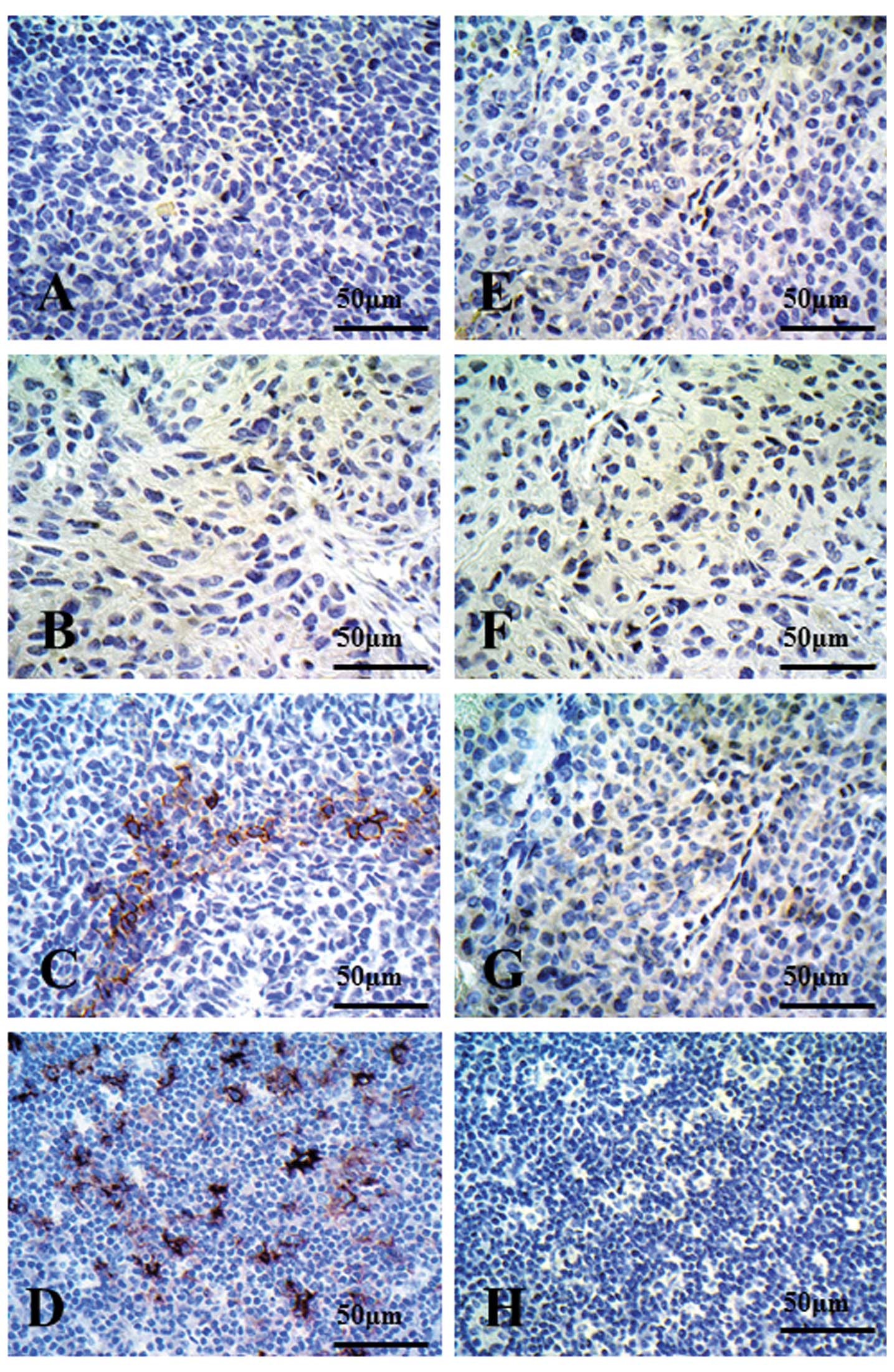
CD40 immunostaining was not detected in the ABC-1
xenograft (Fig. 6A) or in the LC-1
xenograft (Fig. 6B). On the other
hand, CD40 staining was strongly detected in the LK2 xenograft with
heterogeneity (Fig. 6C) and in
human lymph nodes (Fig. 6D). There
were no CD40-positive cells in any of the xenografts or in human
lymph nodes when control IgG was used as a primary antibody
(Fig. 6E-H).
Discussion
Immunohistochemical staining is associated with
several problems due to the sensitivity of the technical process.
In particular, results cannot be unified when there is no internal
positive control. We have repeatedly performed immunohistochemistry
and have often found the results to be inconsistent with in
vitro analysis data. Our aim is to unify the results from any
laboratory and to ensure that immunohistochemistry is
objective.
CD154 (CD40 ligand) is known to be expressed in
normal lymphocytes (3–5), while recent reports have found that
CD154 is expressed in various cancer cells (3,16,17).
We initially attempted immunostaining for CD154 in normal human
tonsils and lymph nodes, using C-20 (18) and TRAP1:IM1842 (17), respectively, as primary antibodies,
but no CD154 staining was seen (data not shown). Thus, we attempted
to establish a new positive control tissue section for CD154, and
we assessed the efficiency of these two primary antibodies for
CD154 recognition.
Using western blot analysis, rhsCD154 was used as a
positive control for CD154. CD154 was strongly detected at 36 kDa
as a homotrimer and at 16.3 kDa as a monomer (21). We expected CD154 expression to be
detected in PBMCs, as CD154 is expressed in normal lymphocytes
(3–5), but with western blot analysis, CD154
expression was not detected in PBMCs.
It has been reported that 0.2% of normal human
peripheral blood CD4-positive T lymphocytes are positive for CD154
(22,23). This suggests that CD154 is scarcely
detected at the protein level in lysates from PBMCs. C-20:sc978 is
able to detect rhsCD154 protein. These results suggest that normal
human tonsils or lymph nodes are not suitable as positive controls
for CD154 immunohistochemical staining. On the other hand, when
TRAP1:IM1842 was used as a primary antibody, there was no band
detected for rhsCD154 (data not shown). However, TRAP1:IM1842 is
only recommended for flow cytometric analysis, and is thus not
suitable for western blotting.
The PC10 xenograft immunostaining data indicated
that C-20:sc-978 was neutralized by rhsCD154 protein, and the
affinity between rhsCD154 and C-20:sc-978 is quite high (Fig. 2B and C). It is certain that the PC10
xenograft expresses CD154, while the LK2 xenograft immunostaining
data suggests that it could be used as the CD154-negative sample.
These findings suggest that the results of immunostaining are
consistent with the results of western blot analysis using
C-20:sc978 as a primary antibody.
These results (PC10 is CD154-positive and LK2 is
CD154-negative) should be preserved with other primary antibodies,
such as TRAP1:IM1842, in immunohistochemical staining for CD154. In
the present study, however, TRAP1:IM1842 was not suitable for
western blot analysis, as the PC10 xenograft exhibited no staining.
When TRAP1:IM1842 was used, the results of immunostaining for CD154
were consistent with those using C-20:sc978, but the staining level
was very weak in xenograft PC10 (Fig.
2F) when compared with the C-20:sc978 results (Fig. 2B). Although the action of
TRAP1:IM1842 is known for flow cytometric analysis, it is not
suitable for western blot analysis or immunohistochemical
staining.
The present observations suggest the following: i)
C-20:sc-978 is suitable for use as a primary antibody; ii) PC10
SCID xenografts may be used as a positive control tissue specimen
for CD154 immunostaining; and iii) LK2 SCID xenografts may be used
as a negative control tissue specimen for CD154 immunostaining.
These measures allow for simultaneous evaluation of reagent
controls, including primary antibodies, and tissue controls, such
as xenografts. Based on these findings, western blot analysis was
most suitable to confirm the target protein (CD154), and gave
results consistent with those of immunohistochemistry. The
advantage of this method is that the same antibody is used as the
primary antibody in both techniques, although different primary
antibodies could be used. In such a case, however, an alternative
experimental method should be used to confirm target gene
expression, such as RT-PCR or flow cytometric analysis.
The differences in gene expression between cultured
cells in vitro and implanted cells were noteworthy. Although
the expression of CD154 in NSCLC cell lines did not vary,
confirmation of gene expression by western blot analysis both in
vitro and in vivo is critical.
We then determined whether these methods could be
applied to other target proteins, such as CD40. Markedly, CD40
expression in the lysates from SCID xenografts differed from that
in cultured cell lines. These phenomena suggest that cancer cell
implantation to the SCID mouse itself alters the gene profile.
Previous reports have shown a clear difference in methylation
pattern between cultured cancer cell lines and nude mice xenografts
(24). Variations in methylation
pattern were paralleled by variations in gene expression between
cancer cell lines and mouse xenografts (24). Expression of CD40 may also depend on
cytokines (25).
CD40 immunostaining was consistent with the results
of xenograft CD40 expression on western blot analysis. This
suggests that western blot analyses may exhibit different results
from immunostaining with cultured cell lines and xenografts, and
thus western blotting using lysates from xenografts should be
performed to assess consistency with the immunostaining results
using SCID xenografts. We established SCID xenografts and prepared
xenograft blocks as formalin-fixed, paraffin-embedded tissue, and
immunostaining may be performed using paraffin-embedded tissue,
cell blocks or acetone-fixed live cells. The most marked difference
between xenografts and cell lines was that the xenografts have the
morphological characteristics of a tumor. For accuracy, positive or
negative tissue controls should be prepared in the same manner as
patient samples.
We used anti-CD40 mouse monoclonal antibody (11E9)
for CD40 immunostaining in SCID xenografts and human lymph nodes.
The results suggested that mouse monoclonal antibody may be used as
a primary antibody against SCID xenograft samples without
background staining due to endogenous immunoglobulin. To our
knowledge, there have been few reports on the use of mouse
monoclonal antibodies as primary antibodies in immunohistochemistry
against SCID xenografts. The advantage of this method is that SCID
xenografts can be obtained using a simple procedure.
In conclusion, we propose a control concept for
immunohistochemistry based on western blot analysis, by
investigating CD154 and CD40 expression in NSCLC using SCID
xenograft models. The present results demonstrate that this method
is objectively suitable.
Acknowledgements
The authors are grateful for the immunohistochemical
technical support of Mr. Hiraku Shida. We would also like to thank
the many physicians who cared for the patients at the Departments
of Gastroenterological Surgery II of the affiliated hospitals.
References
|
1
|
Banchereau J, Bazan F, Blanchard D, et al:
The CD40 antigen and its ligand. Annu Rev Immunol. 12:881–922.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith CA, Farrah T and Goodwin RG: The TNF
receptor superfamily of cellular and viral proteins: activation,
costimulation, and death. Cell. 76:959–962. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Paulie S, Ehlin-Henriksson B, Mellstedt H,
Koho H, Ben-Aissa H and Perlman BA: p50 surface antigen restricted
to human urinary bladder carcinomas and B lymphocytes. Cancer
Immunol Immunother. 20:23–28. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aldderson MR, Armitage R, Tough TW,
Strockbine L, Fanslow WC and Spriggs M: CD40 expression by human
monocytes: regulation by cytokines and activation of monocytes by
the ligand for CD4. Exp Med. 178:669–674. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Caux C, Massacrier C, Vanbervliet B, et
al: Activation of human dendritic cells through CD40 cross-linking.
J Exp Med. 180:1263–1272. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yellin MJ, Winikoff S, Fortune SM, et al:
Ligation of CD40 on fibroblast induces CD54 (ICAM-1) and CD106
(VCAM-1) up-regulation and IL-6 production and proliferation. J
Leukocyt Biol. 58:209–216. 1995.PubMed/NCBI
|
|
7
|
Zong YS, Lin H, Choy DTK, et al:
Nasopharyngeal carcinoma and lymphoinfiltration. Oncology.
48:290–296. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galy AHM and Spitz H: CD40 is functionally
expressed on human thymic epithelial cells. J Immunol. 149:775–782.
1992.PubMed/NCBI
|
|
9
|
Pammer J, Weninger W, Mazal PR, Horvat R
and Tschachler E: Expression of the CD40 antigen on normal
endothelial cells and in benign and malignant tumours of vascular
origin. Histopathology. 29:517–524. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Armitage RJ, Fanslow WC, Stockbine L, et
al: Molecular and biological characterization of a murine ligand
for CD40. Nature. 357:80–82. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gauchat JF, Henchoz S, Mazzei G, et al:
Induction of human IgE synthesis in B cells by mast cells and
basophils. Nature. 365:340–343. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Banchereau J, de Paoli P, Valle A, et al:
Long-term human B cell lines dependent on interleukin-4 and
antibody to CD40. Science. 251:70–72. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu YJ, Mason DY, Johnson GD, et al:
Germinal center cells express bcl-2 protein after activation by
signals which prevent their entry into apoptosis. Eur J Immunol.
21:1905–1910. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi MSK, Boise LH, Gottschalk AR,
Quintans J, Thompson CB and Klaus GGB: The role of bcl-xl-mediated
rescue from anti-μ-induced apoptosis in WEHI-231 B lymphoma cells.
Eur J Immunol. 25:1352–1357. 1995.
|
|
15
|
Ludewig B, Graf D, Gelderblom HR, Becker
Y, Kroczek RA and Pauli G: Spontaneous apoptosis of dendritic cells
is efficiency inhibited by TRAP (CD40-ligand) and TNF-α, but
strongly enhanced by interleukin-10. Eur J Immunol. 25:1943–1950.
1995.PubMed/NCBI
|
|
16
|
Tong AW, Papayoti MH, Netto G, et al:
Growth-inhibitory effects of CD40 ligand (CD154) and its endogenous
expression in human breast cancer. Clin Cancer Res. 3:691–703.
2001.PubMed/NCBI
|
|
17
|
Costello A, Rey-Hipolito C, Patel A, et
al: Thyroid cancers express CD-40 and CD-40 ligand: cancers that
express CD-40 ligand may have a greater risk of recurrence in young
patients. Thyroid. 2:105–113. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Campean V, Neureiter D, Nonnast-Daniel B,
Garlichs C, Gross ML and Amann K: CD40-CD154 expression in
calcified and non calcified coronary lesions of patients with
chronic renal failure. Atherosclerosis. 190:155–166. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamada M, Shiroko T, Kawaguchi Y, et al:
CD40-CD40 ligand (CD154) engagement is required but not sufficient
for modulating MHC class I, ICAM-1 and Fas expression and
proliferation of human non-small cell lung tumors. Int J Cancer.
92:589–599. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ishikawa K, Miyamoto M, Yoshioka T, et al:
Up-regulation of CD40 with juxtacrine activity in human nonsmall
lung cancer cells correlates with poor prognosis. Cancer.
113:530–541. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bajorath J, Seyama K, Nonoyama S, Ochs HD
and Aruffo A: Classification of mutations in the human CD40 ligand,
gp39, that is associated with X-linked hyper IgM syndrome. Protein
Sci. 5:531–534. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujimoto T, Nakamura T, Nishimura Y, et
al: Up-regulation of interleukin-12 receptor expression in
peripheral blood mononuclear cells of patients with
HTLV-I-associated myelopathy/tropical spastic paraparesis. J Neurol
Sci. 196:21–26. 2002. View Article : Google Scholar
|
|
23
|
Balashov KE, Smith DR, Khoury SJ, Hafler
DA and Weiner HL: Increased interleukin 12 production in
progressive multiple sclerosis: Induction by activated
CD4+ T cells via CD40 ligand. Proc Natl Acad Sci USA.
94:599–603. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eriksson T, Frisk T, Gray SG, et al:
Methylation changes in the human IGF2 p3 promoter parallel IGF2
expression in the primary tumor, established cell line, and
xenograft of a human hepatoblastoma. Exp Cell Res. 270:88–95. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hellings PW, Kasran A, Bullens D, et al:
IL-10- and IL-12-independent down-regulation of allergic
sensitization by stimulation of CD40 signaling. J Immunol.
177:5138–5144. 2006. View Article : Google Scholar : PubMed/NCBI
|