Introduction
Human cervical carcinoma is the second most common
cancer among women worldwide, with about 500,000 new cases and
250,000 related deaths occurring every year, primarily in
developing countries (1). Cervical
cancer can be cured by radical surgery or radiotherapy for the
patients diagnosed with cervical cancer in the early stages, while
chemotherapy or neoadjuvant chemotherapy is the primary option for
patients with advanced cervical cancer (2). However, the available chemotherapeutic
agents are not completely effective in patients with advanced
cervical cancer due to the lower chemosensitivity of the cervical
cancer cells. Therefore, effective chemotherapeutic agents are
required to improve the 5-year survival rate of these patients
(3). Cancer is a disease of
uncontrolled cell growth or proliferation and a lack of apoptosis;
therefore, any agent that can block the cell proliferation or
induce apoptosis in the cancer cells could prove to be a potent
anticancer agent. To date, several anticancer drugs (such as,
paclitaxel, doxorubicin, etoposide and cisplatin) already in use in
the clinical setting have been proven to be apoptosis-inducing
agents (4–7). Thus, apoptosis induction is a
promising direction in the development of new anticancer
agents.
Several sources from plants, marine organisms and
microorganisms are used to produce anticancer agents. In
microorganisms, it has been shown that both bacteria and fungi are
valuable sources of bioactive compounds. However, most anticancer
drugs developed from microorganisms currently used in the clinical
setting are from bacteria (8). The
anticancer properties of metabolites from fungi have yet to be
fully elucidated. Terrein
(C8H10O3) is a bioactive, fungal,
secondary metabolite which was first isolated from Aspergillus
terreus in 1935 (9). The
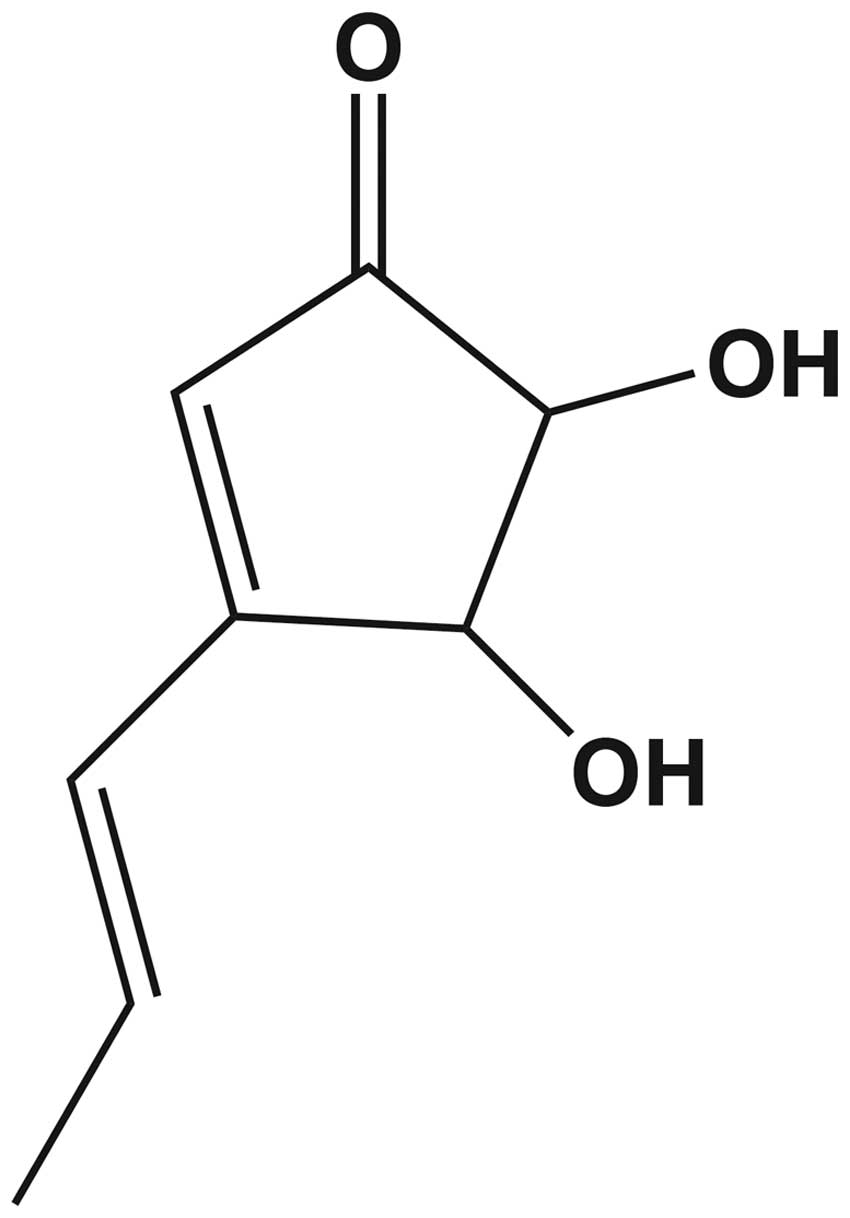
chemical structure of terrein contains free hydroxyl groups at
positions 4 and 5 of the cyclopentenone ring (Fig. 1) (10,11).
Terrein has been reported to have several biological activities. It
has been shown that terrein functions as a melanogenesis inhibitor
by reducing the tyrosinase production in the spontaneously
immortalized mouse melanocyte cell line of Mel-Ab (11,12).
In lipopolysaccharide (LPS)-induced inflammation of human dental
pulp cells, terrein has been shown to function as an
anti-inflammatory agent (13). In
MC3T3-E1 fibroblast cells grown on a titanium surface,
biocompatible material, terrein was found to reduce the oxidative
stress demonstrating anti-oxidant activity (14). Aside from the activities mentioned,
terrein has also been shown to suppress the proliferation of human
skin keratinocyte cells (15).
Markedly, terrein has been shown to inhibit the growth of several
types of cancer cells. In prostate cancer cells, terrein has been
reported to work as an angiogenesis inhibitor (16). In lung cancer, terrein has been
shown to function as a proteasome inhibitor that promotes cell
death by apoptosis (10).
Additionally, terrein has suppressive growth effects in
ABCG2-expressing breast cancer cells by inducing the apoptosis
mechanism (17). Thus, terrein is a
promising compound, particularly for its anticancer properties; it
may provide a new option in cancer therapeutics. In this study, we
further examined the anticancer properties of terrein in cervical
cancer cells (HeLa), as well as the signaling induced through ERK,
p53 and caspase-3, -8 and -9, which have yet to be reported for
terrein function.
Materials and methods
Chemicals and reagents
Dulbecco’s modified Eagle’s medium (DMEM), Medium
199, fetal bovine serum (FBS), 0.25% Trypsin-EDTA,
penicillin-streptomycin, TRIzol Reagent, Taq DNA Polymerase,
SuperScript® VILO™ cDNA Synthesis kit and Hoechst 33342
were purchased from Gibco (Gaithersburg, MD, USA). Dimethyl
sulfoxide (DMSO), RNase and propidium iodide (PI) were obtained
from Sigma-Aldrich (St. Louis, MO, USA). Sodium citrate,
dithiothreitol (DTT) and ethidium bromide were purchased from Sigma
Chemical Co., (St. Louis, MO, USA). Material
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was obtained from USB Corp., (Cleveland, OH, USA) and agarose from
Vivantis (Oceanside CA, USA). The JC-1 mitochondrial membrane
potential assay kit was purchased from Biotium, Inc. (Hayward, CA,
USA). The caspase colorimetric assay kit was from Calbiochem Merck
KGaA (Darmstadt, Germany). Power SyBR® Green Master Mix
was purchased from Applied Biosystems (Foster City, CA, USA).
Preparation of terrein
Terrein was extracted from the culture broth of
fungi Aspergillus terreus CRI301. The crude extract was
carried out using ethyl acetate as a solvent. The EtOAc extract was
concentrated in vacuo, and then the crude extract from the
broth was fractionated and purified by use of the Sephadex LH-20 (2
cm inner diameter and 125 cm long), using MeOH as an eluent.
Spectroscopic analysis was used for the compound
characteristics.
Cell culture and maintenance
The human cervical carcinoma cell line (HeLa) was
kindly provided by Dr Mathurose Ponglikitmongkol, Department of
Biochemistry, Faculty of Science, Mahidol University, Thailand. The
immortalized porcine epithelial glandular (PEG) cells were kindly
provided by Dr Chatsri Deachapunya, Srinakharinwirot University.
The HeLa cells were maintained in DMEM supplemented with 10% FBS
and with 1% penicillin-streptomycin. The PEG cells were cultured in
DMEM containing 5% FBS, 1% L-glutamine, 1% non-essential amino
acid, 0.1% insulin and 1% penicillin-streptomycin. Both specimens
were cultured at 37°C in a humidified atmosphere of 95% air and 5%
CO2.
Cytotoxicity assay
The cytotoxicity assay was performed by the MTT
method (18). The HeLa and PEG
cells at 1×104 cells/100 μl/well were seeded onto a
96-well plate and incubated overnight. The cells were then treated
with terrein at 5, 0.5, 0.05, 0.005, 0.0005 and 0.00005 mM for 24
h. An untreated group was combined with 1% DMSO and used as a
negative control. Following 24 h of cell treatment, the MTT dye
[Thiazolyl Blue Tetrazolium Bromide: (3-(4,
5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was added
at 0.5 mg/ml into each well and incubated for 3 h. The formazan
crystal products formed were dissolved by the addition of 100 μl of
DMSO. After 15 min, the amount of purple formazan was determined by
measuring the optical density (OD) using the ELISA microplate
reader at 595 nm. The experiment was performed in triplicate and
the percentage of cell viability was calculated as: % Viability =
[OD of treated cells/OD of control cells] × 100.
Nuclear morphological observation
The effect of terrein on the nuclear morphological
changes was investigated by Hoechst 33342 staining (19). Briefly, the HeLa cells at
4×105 cells/well were seeded onto a 12-well plate and
treated with terrein at 0, 0.3, 0.6 and 1.5 mM for 24 h. At the end
of the treatment, both the adherent and non-adherent cells were
collected. Then, the cells were fixed with 3.7% (vol/vol)
paraformaldehyde for 10 min at room temperature, permeated with
0.1% Triton X-100 for another 10 min at room temperature and
stained with Hoechst 33342 (1 mg/ml of phosphate-buffered saline;
PBS) at 37°C for 15 min. The nuclear morphology was observed with a
fluorescent microscope (Olympus, Tokyo, Japan).
Analysis of apoptotic sub-G0
population
The sub-G0 population was analyzed using flow
cytometry as previously described (20). The HeLa cells at 1×106
cells/well were plated on a 6-well plate and treated with terrein
at 0, 0.3, 0.6 and 1.5 mM. After 24 h, the treated cells were
trypsinized and washed twice with ice-cold PBS. The cell pellet was
resuspended in 1 ml PBS and gently fixed (drop by drop) with 4 ml
of absolute ethanol at −20°C for 5–15 min. Following
centrifugation, the ethanol was discarded and 5 ml of PBS was added
to the cell pellet which was then allowed to rehydrate for 15 min.
Subsequently, each sample was incubated with 500 μl of 100 μg/ml
RNase for 20 min at 37°C. After washing with PBS, the cell pellet
was gently resuspended in 500 μl of PI solution (50 μg/ml PI in
0.1% sodium citrate plus 0.1% Triton X-100) at 4°C in a darkened
environment overnight. Each sample was measured using flow
cytometry (BD FACSCanto, Becton-Dickinson, Lincoln Park, NJ, USA)
using the Consort 30 program (Becton-Dickinson).
Caspase activity assay
Caspase-3, -8 and -9 activities were measured using
fluorescent assay kit detection (Calbiochem Merck KGaA), according
to the manufacturer’s instructions. Briefly, HeLa cells at
1×106 cells/well were placed on a 6-well plate, treated
with terrein at 0, 0.3, 0.6 and 1.5 mM for 12 h. After the
treatment, supernatants from cell lysates were incubated with
fluorogenic substrates using DEVD-AFC (caspase-3-like), IETD-AFC
(caspase-8-like) and LEHD-AFC (caspase-9-like), at 37°C for 2 h
prior to monitoring with a fluorescent microplate reader with
excitation set at 400 nm, and emissions at 505 nm.
Analysis of mitochondrial transmembrane
potential
The changes in mitochondrial membrane potential
(ΔΨm) were detected using a
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolyl-carbocyanine
iodide (JC-1) dye (Biotium Inc.). In healthy cells, the JC-1
accumulates in the mitochondria as JC-1 aggregates (fluorescence is
red) and also in the cytoplasm as JC-1 monomers (fluorescence is
green). In early apoptosis, the ΔΨm collapses, making JC-1
aggregates unable to accumulate within the mitochondria and
dissipate into the JC-1 monomers leading to a loss of the red
fluorescence. Therefore, collapse of the ΔΨm is exhibited by a
decrease in the ratio of red to green fluorescence (21). In accordance with the terrein
treatment, HeLa cells at 1×104 cells/well were placed on
a 96-well plate, treated with terrein at 0, 0.3, 0.6 and 1.5 mM for
6 h. Following treatment, the cells were harvested and incubated
with a JC-1 reagent solution at 37°C for 20 min, then washed twice
with PBS and suspended once more in the PBS. The samples were
analyzed by a fluorescence microplate reader and measured with both
the red fluorescence (excitation 550 nm, emission 600 nm) and the
green fluorescence (excitation 485 nm, emission 535 nm). The ratio
of red fluorescence intensity vs. green fluorescence intensity was
calculated and presented as the means ± SD. This experiment was
performed in triplicate.
Real-time polymerase chain reaction
(real-time PCR)
To analyze the effect of terrein on the expression
of apoptosis-related genes (p53, Bax, Bcl-2,
p21 and ERK2), real-time PCR was used. HeLa cells at
1×106 cells/well were plated on a 6-well plate, treated
with terrein at 0, 0.3, 0.6 and 1.5 mM for 24 h. Following
treatment, the cells were harvested and washed with 500 μl of PBS.
The total RNA was extracted using a TRIzol Reagent (Invitrogen,
Carlsbad, CA, USA) and quantified by use of OD measurement at 260
and 280 nm using a spectrophotometer (Thermo Fisher Scientific,
Madison, WI, USA) (all RNA samples had an A260/A280 ratio
>1.8).
The isolated total RNA (2.5 μg) was
reverse-transcribed to cDNA with the SuperScript VILO cDNA
Synthesis kit (Invitrogen). The reaction mixture was composed of 4
μl of 5X VILO reaction mix, 2 μl of 10X SuperScript enzyme mixture,
and DEPC-treated water in a total volume of 25 μl. The reaction
mixture was incubated at 25°C for 10 min, at 42°C for 60 min and
the reaction was terminated by heating at 85°C for 5 min. The
resultant cDNA was stored at −20°C until further use. The PCR
primers were obtained from BioDesign Co., Ltd., Pathumthani,
Thailand. PCR primers were designed by Primer 3.0 and BLAST search
to check the specificity. The primer sequences used are listed in
Table I.
 | Table IOligonucleotides used in real-time
PCR. |
Table I
Oligonucleotides used in real-time
PCR.
| Gene | Forward primer | Reverse primer |
|---|
| p53 |
5′-ACTAAGCGAGCACTGCCCAA-3′ |
5′-ATGGCGGGAGGTAGACTGAC-3′ |
| p21 |
5′-TATGGGGCTGGGAGTAGTTG-3′ |
5′-AGCCGAGAGAAAACAGTCCA-3′ |
| Bax |
5′-GCGTCCACCAAGAAGCTGAG-3′ |
5′-ACCACCCTGGTCTTGGATCC-3′ |
| Bcl-2 |
5′-TGTGGCCTTCTTTGAGTTCG-3′ |
5′-TCACTTGTGGCCCAGATAGG-3′ |
| ERK2 |
5′-GCCTGGCCCGTGTTGCAGAT-3′ |
5′-CGCCCCTCCAAACGGCTCAA-3′ |
| GAPDH |
5′-GAAGGTGAAGGTCGGAGTCA-3′ |
5′-GACAAGCTTCCCGTTCTCAG-3′ |
Real-time quantitative-PCR was performed on an ABI
StepOnePlus (Applied Biosystems), using 96-well microtiter plates.
The reaction was carried out in a total volume of 20 μl, containing
2.5 μl of the cDNA sample (equivalent to 75 ng), 1 μl of 0.5 μM
each of the primer and 10 μl of SYBR-Green Reaction Mix (Applied
Biosystems). PCR amplification was performed in duplicated wells.
The cycling conditions were: 10 min polymerase activation at 95°C
and 40 cycles at 95°C for 15 sec and 60°C for 60 sec. In addition,
the real-time reaction of the products was examined by analyzing
the melting point after each reaction. A sample without cDNA was
used as a negative control and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) was used as an internal control. The baseline
and the threshold were set automatically by the software. The
crossing point of the amplification curve with the threshold
represents the cycle threshold (Ct). The fluorescence threshold Ct
values were calculated, and the ΔCt values were determined using
the formula ΔCt = Cttarget gene − CtGAPDH.
The ΔΔCt values were then calculated based on the formula ΔΔCt =
ΔCt treated − ΔCt untreated. The expression level of the target
gene in the treated cells was measured relative to the level
observed in the untreated cells and was quantified using the
formula 2−ΔΔCT(22). The
PCR products were electrophoresed on a 2% agarose gel and stained
by ethidium bromide under UV light.
Statistical analysis
The results of each experiment were expressed as the
means ± standard deviation (SD, for each group n=3). The data were
processed with the GraphPad Prism 5 software. Statistical
significance was assessed by one-way ANOVA analysis of variance to
evaluate the significance of differences between the groups.
Results
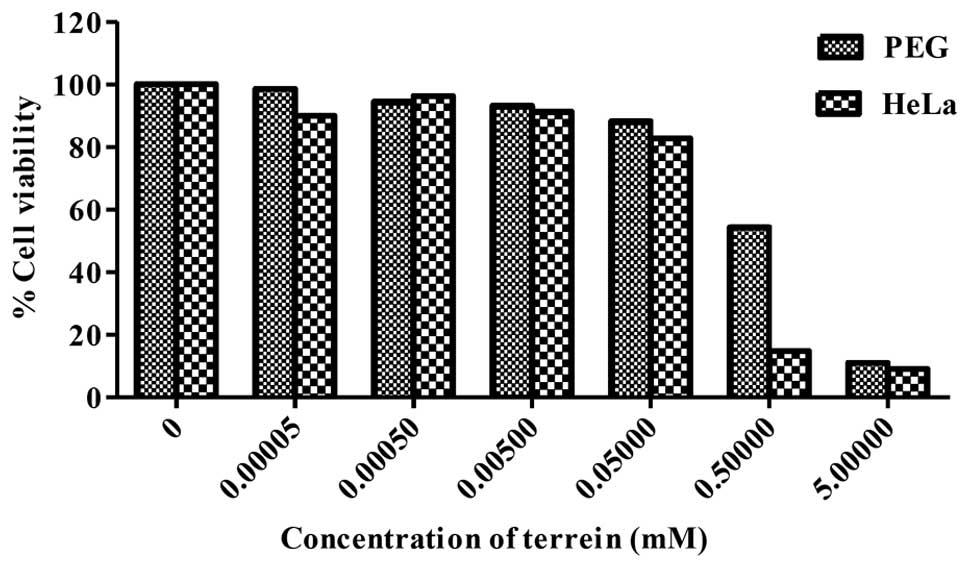
Effect of terrein on cell viability
Terrein was tested for its cytotoxicity against
human cervical cancer cells (HeLa) and normal cells (PEG) by the
MTT assay. As shown in Fig. 2,
terrein significantly inhibited the growth of PEG and HeLa cells in
relation to the concentration used. The IC50 values were
at 0.53 mM for PEG and 0.29 mM for HeLa. It is noteworthy that the
percentage of cell viability comparing the cancer and the normal
cells differed significantly when using terrein at a concentration
of 0.5 mM which was approximately 18 and 60%, respectively. The
results indicate a considerable potential of the cytotoxicity
effect on human cervical cancer HeLa cells with lower toxicity on
normal PEG cells.
Terrein induces apoptosis in HeLa
cells
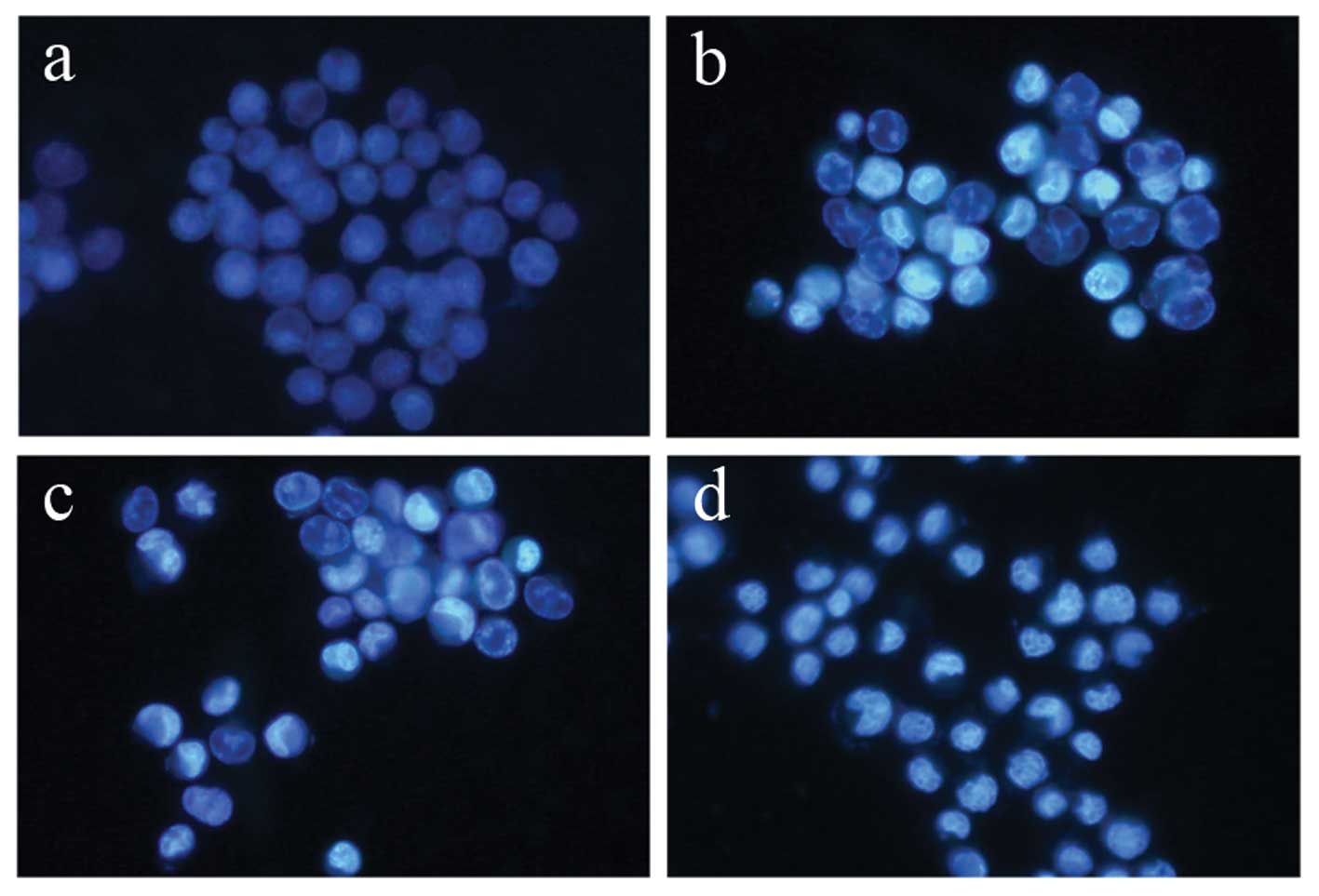
To evaluate the mode of cell death induced by
terrein in HeLa cells, the experiment was carried out by staining
cells with the DNA specific dye, Hoechst 33342. The cell samples
were compared between the terrein-treated cells and the untreated
control HeLa cells. The concentrations of terrein were 0, 0.3, 0.6
and 1.5 mM and were treated for 24 h. As depicted in Fig. 3, the untreated control cells
displayed normal, round nuclei (Fig.
3a), while the cells treated with terrein exhibited
characteristics of apoptosis, such as cell shrinkage, nuclear
condensation and fragmentation in a dose-dependent manner (Fig. 3b–d).
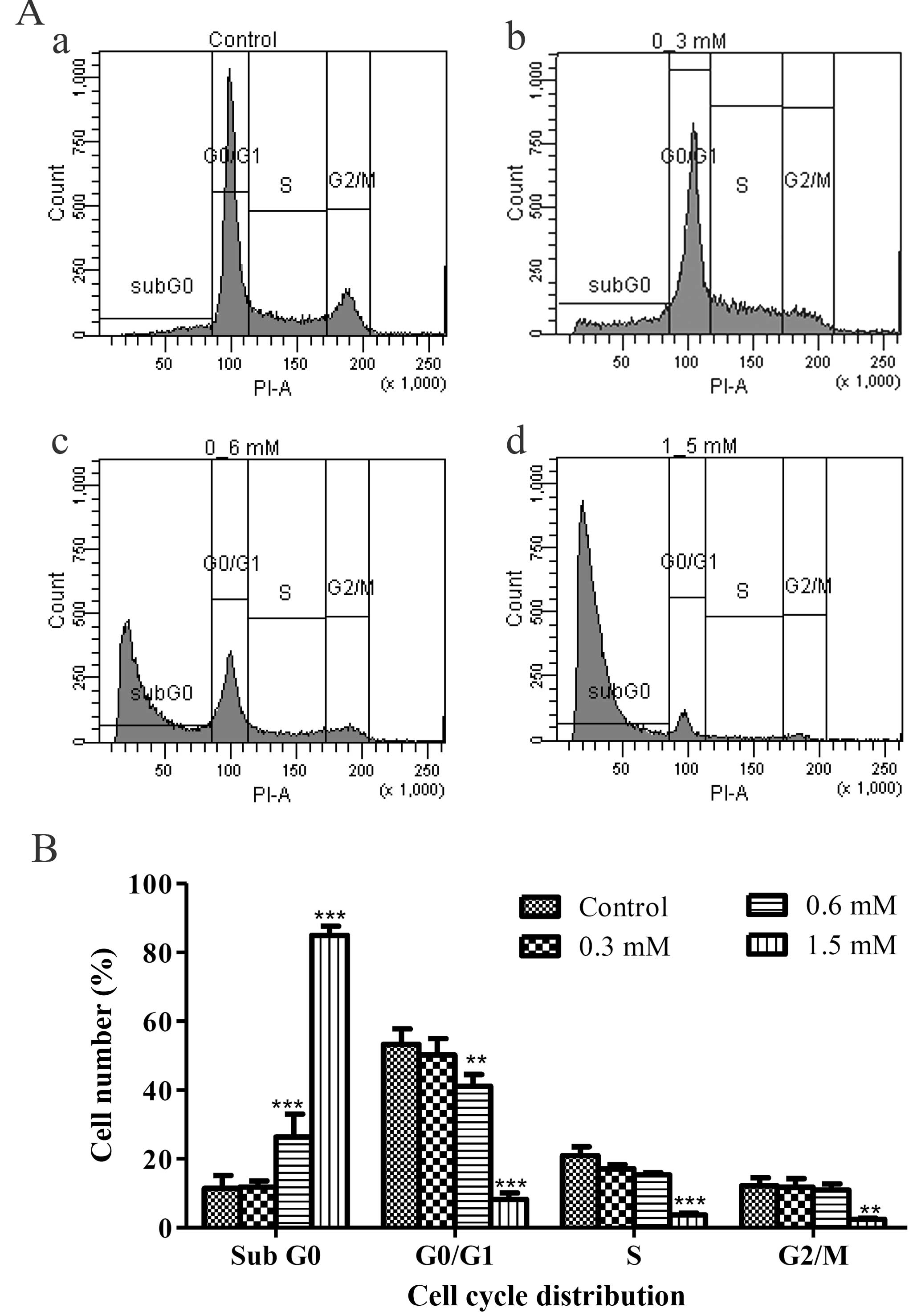
As the apoptotic cells with fragmented nuclei appear
as cells with hypodiploid DNA content and could be detected at
sub-G0 peak with flow cytometry, the numbers of the apoptotic
sub-G0 population were quantified. The result demonstrated that the
terrein-treated HeLa samples had significantly increased in the
sub-G0 phase as compared to the untreated sample (Fig. 4Aa–d). The statistics of each phase
of the cell cycle showed that the sub-G0 populations increased as
the doses of terrein increased from 11.90 to 26.37 and 84.93%.
(Fig. 4B). These results suggest
that apoptosis is the mode of cell death used by terrein against
HeLa cells.
Induction of apoptotic signaling is
triggered by the death receptor and the mitochondrial pathway
Apoptosis is triggered by sequential activation of
caspases, a group of cysteine proteases, and proceeds primarily
through two pathways. The extrinsic or death receptor pathway
involves activation of caspase-8 and is initiated by ligand
interaction with death receptors. Second, the intrinsic or
mitochondrial pathway is activated by an imbalance between
pro-apoptotic and anti-apoptotic proteins from the Bcl-2 family at
the mitochondria and cytosol, resulting in the release of
cytochrome c from the mitochondria, which in turn activates
caspase-9. Both caspase-8 and caspase-9 activate caspase-3 which
acts as a common downstream part of the two major apoptosis
pathways resulting in apoptosis (23). To address the apoptotic pathway in
the terrein-treated HeLa cells, measuring of the fluorogenic
substrate cleavage was performed. The result of the fluorescence
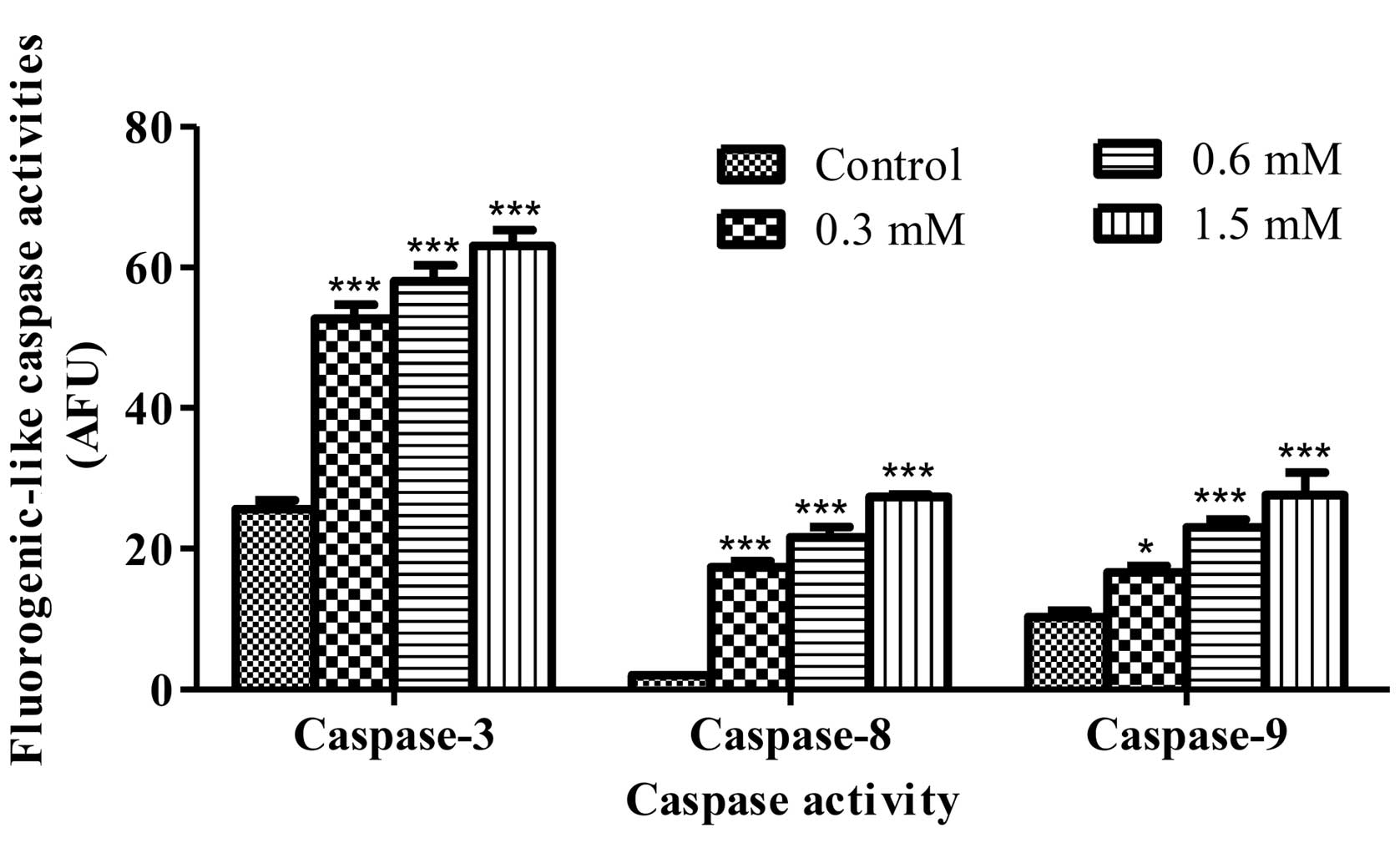
intensity showed that terrein significantly activated caspase-8,
caspase-9 and caspase-3 function after 12 h of treatment. Caspase
activity increased significantly when compared to the control group
of untreated cells in a concentration-dependent manner (Fig. 5). In addition, the activity of each
caspase was inhibited by their specific inhibitor provided by the
kit (data not shown). These results suggest that terrein activates
the signaling of both the death receptor and mitochondrial
pathways.
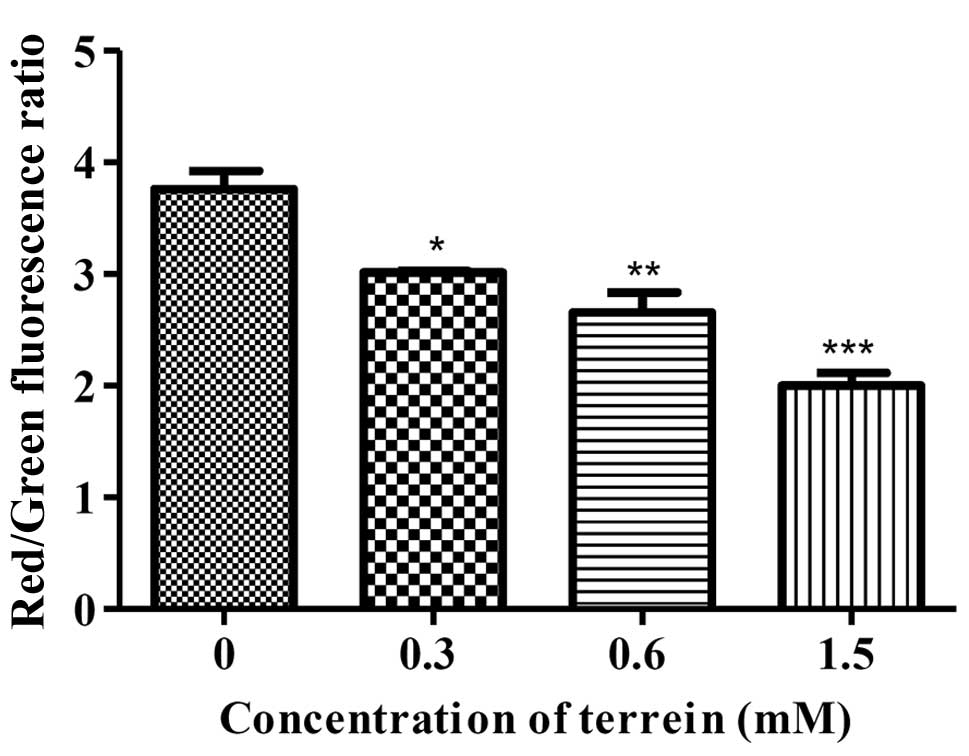
To confirm the cascade, the damage to the
mitochondria was analyzed using a specific dye for mitochondrial,
JC-1, staining. Upon quantification by flow cytometry, the HeLa
cells treated with terrein at 6 h presented with decreasing ΔΨm as
compared to the control group of untreated cells in a
dose-dependent manner (Fig. 6).
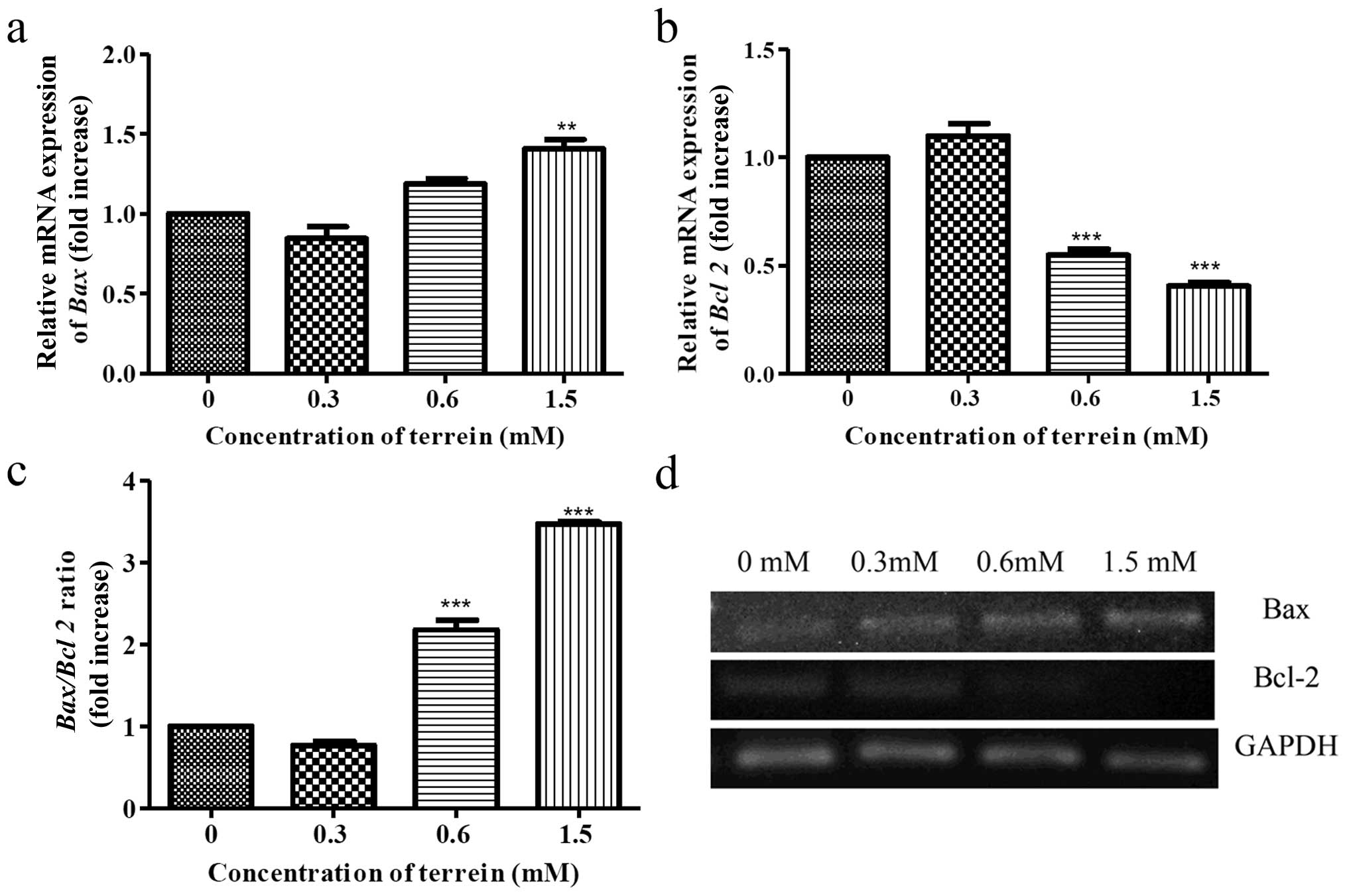
Then, we investigated the expression of Bcl-2 family proteins and
whether they were involved with the damage to the mitochondria. The
expression of Bax (pro-apoptotic) and Bcl-2
(anti-apoptotic) was selected for investigation. As a result,
terrein increased the expression of Bax (Fig. 7a) and decreased the expression of
Bcl-2 (Fig. 7b) in a
dose-dependent manner by real-time PCR. An increase in the
Bax/Bcl-2 ratio (Fig. 7c)
indicates that upregulation of these Bcl-2 family proteins are
upstream events causing damage to the mitochondria.
Apoptotic signaling is mediated by p53
and ERK activation
The tumor suppressor gene, p53, is known to
be responsible for the inhibition of cell growth and/or the
commitment to apoptosis. Meanwhile, p53 protein regulates the
expression of the downstream effector p21, a potent inhibitor of
cell cycle kinases, in which both are in response for DNA damage.
In addition, p53 regulates apoptosis via upregulation of the
expression of Bax and blocks the function of Bcl-2. Thus, it
is possible that a substantial increase in the Bax/Bcl-2
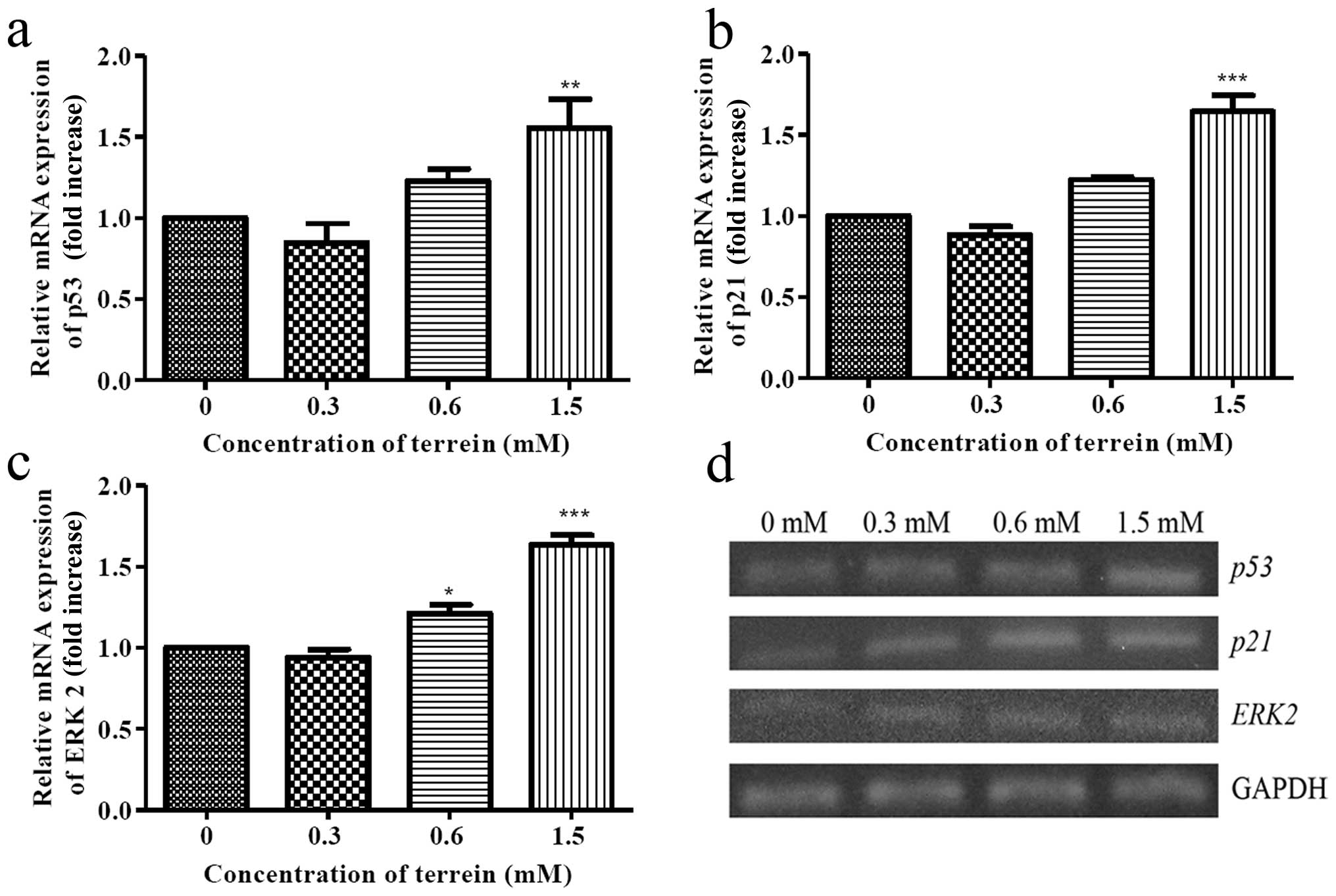
ratio may have resulted from p53 function. The level of the
expression of p53 and p21 were then examined. As
shown in Fig. 8a and b, the
expression of mRNA from both p53 and p21 was
upregulated suggesting that the upstream signaling of the
mitochondrial pathway was induced by terrein. To investigate this
signaling triggered by terrein, further upstream mediators of p53
were evaluated. As is well known, several protein kinases may
function to activate p53 and ERK2 may be the kinase that is
responsible for the DNA damage. Therefore, we selected ERK2 to
study the level of expression in response to the terrein treatment.
As depicted in Fig. 8c, the mRNA
expression of ERK2 increased following terrein treatment in
a dose-dependent manner indicating the involvement of ERK
signaling.
Discussion
The present study is the first to demonstrate that
terrein, a fungal metabolite, induces apoptosis in cervical cancer
cells via p53 and ERK signaling. As previously shown, terrein has a
variety of effects including anti-inflammatory (13), anti-oxidant (14), anti-proliferative (15) and skin-whitening properties
(11–12). The effects of terrein on cancer
cells have also been reported. In androgen-dependent prostate
cancer cells (LNCaP-CR), terrein demonstrates angiogenesis
inhibition by blocking the secretion of angiogenin with an
IC50 of 13 μM (16). In
human lung tumoral cell lines (NCI-H292), terrein acts as
proteasome inhibitor by suppressing the chymotrypsin- and
trypsin-like activities with the IC50 of 0.3 mM. Also,
in these lung tumor cells, terrein was able to induce apoptotic
cell death at concentrations of 0.15 mM and 0.3 mM (10). In breast cancer cells (MCF-7),
terrein markedly inhibited cell proliferation in IC50 of
1.1 nM (17). Meanwhile, for normal
cells, it has been shown that terrein has of non-cytotoxic effects
in human keratinocyte at the concentration of 1–50 μM (15). Comparing these data, our study found
that the IC50 for cervical cancer cells was at 0.29 mM,
while in normal porcine epithelial glandular (PEG) cells it was at
0.53 mM (Fig. 2). These data
suggest that the dose of terrein to induce cancer cell death is
cell type-dependent. The concentration appears to be high (mM
range) but this effective dose has almost the same value exhibited
in lung tumor cells. The inhibition concentration at 50% of terrein
treatment in HeLa cells did not differ significantly from normal
PEG cells. This indicates that terrein also exhibits cytotoxic
action on normal cells. However, at approximately 0.5 mM of
terrein, the percentage of cell viability of HeLa cells was
approximately 18%, while in the normal PEG cells it was
approximately 60% which was represented by the difference in the
sensitivity.
The evaluation of the mechanism used by terrein to
trigger cervical cancer cell death is implicated via apoptosis. As
shown in Figs. 2–5, chromatin condensation, DNA
fragmentation and caspase activation were clearly demonstrated.
These are distinct characteristics of the apoptosis mechanism
(24). To develop an anticancer
agent, apoptosis is the preferable mechanism as it does not trigger
the inflammation process observed in necrosis, another form of cell
death. As previously shown, several anticancer drugs use apoptosis
as their target mechanism, therefore, terrein is another promising
compound for development as an anticancer agent (4–7).
The pathway to induce apoptosis is initiated by two
major pathways, the extrinsic or death receptor pathway and the
intrinsic or mitochondrial pathway. The extrinsic pathway
integrates extracellular signals through the binding of external
ligands to death receptors located at the plasma membrane such as
the Fas/FasL interaction. Engagement of these receptors by their
specific ligand induces their trimerization and leads to the
assemblage of the death-inducing signaling complex (DISC). In this
complex, procaspase-8 is activated and in turn cleaves and
activates executioner caspases including caspase-3, caspase-6 or
caspase-7 (25,26). The intrinsic pathway is triggered by
the activation of the pro-apoptotic Bcl-2 family proteins known as
Bax or Bak. These proteins have shown the ability to form pores in
the mitochondrial outer membranes, thereby allowing
permeabilization of cytochrome c release to cytosol.
Cytochrome c binds to the adaptor apoptotic protease
activating factor-1 (Apaf-1) forming a large multi-protein
structure known as the apoptosome. The apoptosome then recruits and
activates procaspase-9 into the active form which further activates
the downstream effector caspases for the death receptor pathway,
finally resulting in cell death (27,28).
However, several reports have shown that the cascade
of the extrinsic and intrinsic pathway is not fully separated in
some cases. Caspase-8 can initiate death via caspase-3 directly or
it can trigger the mitochondrial pathway via Bid cleavage (25). Cells that perform directly to the
cascade from caspase-8 to caspase-3 are called type I cells, while
the cells relative to the cascade initiated from caspase-8 and that
have death enhancement via the mitochondrial pathway are called
type II cells (29). As shown in
this study, terrein activates both caspase-8 and caspase-9
(Fig. 5). Also, the changes in the
ratio of Bax/Bcl-2 expression and the dissipation of the ΔΨm
were detected (Figs. 6 and 7). These data suggested that
terrein-induced apoptosis in cervical cancer cells may display as
type II signaling, which is consistent with the reports that HeLa
cells triggered by apoptosis-inducing agents usually perform as
type II cells (30,31).
p53 plays an important role in several cellular
processes. It controls the cell cycle, cell senescence and cell
apoptosis. To regulate the apoptosis mechanism, p53 mediates the
expression of several proteins that are involved in the release of
cytochrome c from the mitochondria, and Bax, Noxa, Puma,
AIP1 and APAF1 are also included (32). We also demonstrated that Bax
is upregulated upon treatment with terrein, and this may be due to
the function of the transcriptional activation by p53. As shown by
our results, the level of p53 expression increased upon
treatment with terrein (Fig. 8a).
In addition, the level of p21, the cyclin-dependent kinase 2
inhibitor that is transcriptionally activated by p53, was also
upregulated (Fig. 8b). These data
support the critical role of p53 in terrein-mediated cervical
cancer cell death which correlates with previous studies of
bioactive agents, such as capsaicin (33), eurycomanone (34), flavonoid quercetin (35), kaempferol-7-O-β-D-glucoside
(36) and cisplatin (37).
Our study also analyzed the role of extracellular
signal-regulated kinase (ERK) signaling and whether or not it is
involved in terrein-induced apoptotic cell death. As previously
described, ERK2 is involved in cell death by interaction with
phosphorylated p53 (38). Thus, we
determined the level of ERK2 expression in response to
terrein treatment. As depicted in Fig.
8c, the level of the expression of ERK2 increased in a
dose-dependent manner. These data suggest that ERK may act upstream
of p53 and that consequently leads to cell death by apoptosis. In
addition, it has been reported in HeLa cells that ERK activation is
associated with the upregulation of p53 expression upon treatment
with shikonin (39) and
H2O2(40).
Otherwise, it is assumed that ERK may act upon the activation of
caspase-8. As it has been shown, the prolonged activation of ERK1/2
induces FADD-independent caspase-8 activation and cell death
(41,42). As is demonstrated by our study, the
upregulation of ERK2 is possibly an important mediator that
activates p53, caspase-8 and caspase-9, leading to the destruction
of the cancer cells.
In conclusion, our study demonstrated that terrein
is a potential candidate as an anticancer agent as it was shown to
induce cytotoxicity and apoptosis in cervical cancer cells. The
apoptosis pathway may be type II signaling which mediates through
ERK signaling. ERK acts as a mediator to regulate the activation of
both caspase-8 and p53. The downstream effect of the p53,
particularly Bax, was upregulated and significantly leads to the
dissipation of the ΔΨm. Consequently, caspase-9 and caspase-3 are
activated finally initiating the cleavage of all cellular
substrates and genetic materials.
Acknowledgements
This study was supported by the Commission on Higher
Education, Ministry of Education and Faculty of Medicine,
Srinakharinwirot University, Thailand.
References
|
1
|
Jit M, Demarteau N, Elbasha E, Ginsberg G,
Kim J, Praditsitthikorn N, Sinanovic E and Hutubessy R: Human
papillomavirus vaccine introduction in low-income and middle-income
countries: guidance on the use of cost-effectiveness models. BMC
Med. 54:2–9. 2011.PubMed/NCBI
|
|
2
|
Thomas GM: Improved treatment for cervical
cancer-concurrent chemotherapy and radiotherapy. N Engl J Med.
340:1198–1200. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ren G, Zhao YP, Yang L and Fu CX:
Anti-proliferative effect of clitocine from the mushroom
Leucopaxillus giganteus on human cervical cancer HeLa cells
by inducing apoptosis. Cancer Lett. 262:190–200. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park SJ, Wu CH, Gordon JD, Zhong X, Emami
A and Safa AR: Taxol induces caspase-10-dependent apoptosis. J Biol
Chem. 279:51057–51067. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang S, Konorev EA, Kotamraju S, Joseph J,
Kalivendi S and Kalyanaraman B: Doxorubicin induces apoptosis in
normal and tumor cells via distinctly different mechanisms. J Biol
Chem. 279:25535–25543. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Day TW, Wu CH and Safa AR: Etoposide
induces protein kinase Cδ- and caspase-3 dependent apoptosis in
neuroblastoma cancer cells. Mol Pharmacol. 76:632–640. 2009.
|
|
7
|
Tanida S, Mizoshita T, Ozeki K, Tsukamoto
H, Kamiya T, Kataoka H, Sakamuro D and Joh T: Mechanisms of
cisplatin-induced apoptosis and of cisplatin sensitivity: potential
of BIN1 to act as a potent predictor of cisplatin sensitivity in
gastric cancer treatment. Int J Surg Oncol.
2012:8628792012.PubMed/NCBI
|
|
8
|
Newman DJ and Cragg GM: Microbial
antitumor drugs: natural products of microbial origin as anticancer
agents. Curr Opin Investig Drugs. 10:1280–1296. 2009.PubMed/NCBI
|
|
9
|
Raistrick H and Smith G: Studies in the
biochemistry of micro-organisms: the metabolic products of
Aspergillus terreus Thom. A new mould metabolic
product-terrein. Biochem J. 29:606–611. 1935.PubMed/NCBI
|
|
10
|
Demasi M, Felicio AL, Pacheco AO, Leite
HG, Lima C and Andrade LH: Studies on terrein as a new class of
proteasome inhibitors. J Braz Chem Soc. 21:299–305. 2010.
View Article : Google Scholar
|
|
11
|
Park SH, Kim DS, Kim WG, Ryoo IJ, Lee DH,
Huh CH, Youn SW, Yoo ID and Park KC: Terrein: a new melanogenesis
inhibitor and its mechanism. Cell Mol Life Sci. 61:2878–2885. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim DS, Lee S, Lee HK, Park SH, Ryoo IJ,
Yoo ID, Kwon SB, Baek KJ, Na JI and Park KC: The hypopigmentary
action of KI-063 (a new tyrosinase inhibitor) combined with
terrein. J Pharm Pharmacol. 60:343–348. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee JC, Yu MK, Lee R, Lee YH, Jeon JG, Lee
MH, Jhee EC, Yoo ID and Yi HK: Terrein reduces pulpal inflammation
in human dental pulp cells. J Endod. 34:433–437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee YH, Lee NH, Bhattarai G, Oh YT, Yu MK,
Yoo ID, Jhee EC and Yi HK: Enhancement of osteoblast
biocompatibility on titanium surface with Terrein treatment. Cell
Biochem Funct. 28:678–685. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim DS, Lee HK, Park SH, Lee S, Ryoo IJ,
Kim WG, Yoo ID, Na JI, Kwon SB and Park KC: Terrein inhibits
keratinocyte proliferation via ERK inactivation and G2/M cell cycle
arrest. Exp Dermatol. 17:312–317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arakawa M, Someno T, Kawada M and Ikeda D:
A new terrein glucoside, a novel inhibitor of angiogenin secretion
in tumor angiogenesis. J Antibiot. 61:442–448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liao WY, Shen CN, Lin LH, Yang YL, Han HY,
Chen JW, Kuo SC, Wu SH and Liaw CC: Asperjinone, a nor-neolignan,
and terrein, a suppressor of ABCG2-expressing breast cancer cells,
from thermophilic Aspergillus terreus. J Nat Prod.
75:630–635. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Uthaisang W, Reutrakul V, Krachangchaeng
C, Wilairat P and Fadeel B: VR-3848, a novel peptide derived from
Euphobiaceae, induces mitochondria-dependent apoptosis in
human leukemia cells. Cancer Lett. 208:171–178. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oubrahim H, Stadtman ER and Chock PB:
Mitochondria play no roles in Mn(II)-induced apoptosis in HeLa
cells. Proc Natl Acad Sci USA. 98:9505–9510. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng Q, Cao HL, Xu W, Li XR, Ren YQ and Du
LF: Apoptosis induced by genipin in human leukemia K562 cells:
involvement of c-Jun N-terminal kinase in G2/M arrest. Acta
Pharmacol Sin. 31:519–527. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cossarizza A, Baccarani CM, Kalashnikova G
and Franceschi C: A new method for the cytofluorimetric analysis of
mitochondrial membrane potential using the J-aggregate forming
lipophilic cation
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine
iodide (JC-1). Biochem Biophys Res Commun. 197:40–45.
1993.PubMed/NCBI
|
|
22
|
Gomez-Lazaro M, Galindo MF, Concannon CG,
Segura MF, Fernandez-Gomez FJ, Llecha N, Comella JX, Prehn JH and
Jordan J: 6-Hydroxydopamine activates the mitochondrial apoptosis
pathway through p38 MAPK-mediated, p53-independent activation of
Bax and PUMA. J Neurochem. 104:1599–1612. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Puerto HLD, Martins AS, Milsted A,
Souza-Fagundes EM, Braz GF, Hissa B, Andrade LO, Alves F, Rajão DS,
Leite RC and Vasconcelos AC: Canine distemper virus induces
apoptosis in cervical tumor derived cell lines. Virol J. 334:2–7.
2011.PubMed/NCBI
|
|
24
|
Elmore S: Apoptosis: a review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ashkenazi A: Targeting the extrinsic
apoptosis pathway in cancer. Cytokine Growth Factor Rev.
19:325–331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gu Q, Wang JD, Xia HHX, Lin MC, He H, Zou
B, Tu SP, Yang Y, Liu XG, Lam SK, Wong WM, Chan AO, Yuen MF, Kung
HF and Wong BC: Activation of the caspase-8/Bid and Bax pathways in
aspirin-induced apoptosis in gastric cancer. Carcinogenesis.
26:541–546. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jin Z, Li Y, Pitti R, Lawrence D, Pham VC,
Lill JR and Ashkenazi A: Cullin3-based polyubiquitination and
p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis
signaling. Cell. 137:721–735. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim SH, Kim SH, Lee SC and Song YS:
Involvement of both extrinsic and intrinsic apoptotic pathways in
apoptosis induced by genistein in human cervical cancer cells. Ann
NY Acad Sci. 1171:196–201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hougardy BM, van der Zee AG, van den
Heuvel FA, Timmer T, de Vries EG and de Jong S: Sensitivity to
Fas-mediated apoptosis in high-risk HPV-positive human cervical
cancer cells: relationship with Fas, caspase-8, and Bid. Gynecol
Oncol. 97:353–364. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gross A, Jockel J, Wei MC and Korsmeyer
SJ: Enforced dimerization of BAX results in its translocation,
mitochondrial dysfunction and apoptosis. EMBO J. 17:3878–3885.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang W, Gong X, Zhao X, An W, Wang X and
Wang M: Capsaicin induces apoptosis in HeLa cells via Bax/Bcl-2 and
caspase-3 pathways. Asian J Traditional Med. 1:3–4. 2006.
|
|
34
|
Mahfudh N and Pihie AHL: Eurycomanone
induces apoptosis through the up-regulation of p53 in human
cervical carcinoma cells. J Cancer Mol. 4:109–115. 2008.
|
|
35
|
Priyadarsini RV, Murugan RS, Maitreyi S,
Ramalingam K, Karunagaran D and Nagini S: The flavonoid quercetin
induces cell cycle arrest and mitochondria-mediated apoptosis in
human cervical cancer (HeLa) cells through p53 induction and NF-κB
inhibition. Eur J Pharmacol. 649:84–91. 2010.PubMed/NCBI
|
|
36
|
Xu W, Liu J, Li C, Wu HZ and Liu YW:
Kaempferol-7-O-β-D-glucoside (KG) isolated from Smilax
china L. rhizome induces G2/M phase arrest and
apoptosis on HeLa cells in a p53-independent manner. Cancer Lett.
264:229–240. 2008.
|
|
37
|
Wang X, Martindale JL and Holbrook NJ:
Requirement for ERK activation in cisplatin-induced apoptosis. J
Biol Chem. 275:39435–39443. 2000. View Article : Google Scholar
|
|
38
|
Yeh PY, Chuang SE, Yeh KH, Song YC, Chang
LLY and Cheng AL: Phosphorylation of p53 on Thr55 by ERK2 is
necessary for doxorubicin-induced p53 activation and cell death.
Oncogene. 23:3580–3588. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu Z, Wu LJ, Tashiro S, Onodera S and
Ikejima T: Phosphorylated extracellular signal-regulated kinase
up-regulated p53 expression in shikonin-induced HeLa cell
apoptosis. Chin Med J. 118:671–677. 2005.PubMed/NCBI
|
|
40
|
Singh M, Sharma H and Singh N: Hydrogen
peroxide induces apoptosis in HeLa cells through mitochondrial
pathway. Mitochondrion. 7:367–373. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhuang S and Schnellmann RG: A
death-promoting role for extracellular signal-regulated kinase. J
Pharmacol Exp Ther. 319:991–997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cagnol S and Chambard JC: ERK and cell
death: mechanisms of ERK-induced cell death-apoptosis, autophagy
and senescence. FEBS J. 277:2–21. 2010. View Article : Google Scholar : PubMed/NCBI
|